Introduction
Casein kinase 2 (CK2) is a ubiquitous
serine/threonine protein kinase that is involved in numerous
cellular processes, including proliferation and apoptosis, and has
been revealed to be overexpressed in a number of human cancer cell
lines (1,2). A previous study suggested that CK2
overexpression creates a favorable environment for the development
of the tumor phenotype (3). CK2
has been associated with mammary tumorigenesis, as the levels of
CK2 protein and activity are increased in breast cancer tissue
compared with normal breast tissue (1,4).
Furthermore, overexpression of CK2 in the mammary glands of
transgenic mice can cause hyperplasia and breast carcinoma, thus
demonstrating the oncogenic potential of CK2 (4).
Invasion and metastasis, the fundamental properties
of cancer cells, are the primary causes of poor prognosis in
patients with breast cancer. The mechanisms of cancer cell invasion
and metastasis comprise a multistep biophysical process (5). In the early stages, the proteolytic
degradation of extracellular matrix (ECM) components is facilitated
by matrix metalloproteinases (MMPs) (6,7).
Apigenin is abundant in common fruits and vegetables, and has
notable anti-inflammatory, anti-oxidant and anti-carcinogenic
properties (8–13). Emodin is an active constituent
isolated from Rheum palmatum, a Chinese herb (14) and has been revealed to lead to the
inhibition of cell proliferation, cell cycle arrest, inhibition of
cell division, and decreased cell motility and invasion (15). TBBz is one of the most efficient
inhibitors of CK2 (16), and has
been demonstrated to induce apoptosis in cancer cells (17,18).
MMP-9 has an important role in the degradation of the ECM during
breast cancer cell invasion (19,20).
Considering its importance in cancer development and progression,
MMP-9 can be suggested to represent an early target in the
treatment of breast cancer metastasis (21,22),
and the inhibition of MMP-9 expression, activity and/or upstream
regulatory activity may represent a potential therapeutic strategy.
12-O-tetradecanoylphorbol-13-acetate (TPA), a selective activator
of protein kinase C (PKC) (23),
induces breast cancer cell invasion by stimulating MMP-9 synthesis
and secretion (21,24) via the activation of transcription
factors, including nuclear factor-κB (NF-κB) and activator
protein-1 (25–27).
Considering that CK2 has been previously revealed to
induce nuclear factor-κB (NF-κB) activation, the present study
aimed to investigate whether the inhibition of CK2 affected TPA
induced invasion and MMP-9 expression in MCF-7 human breast cancer
cells and determine the associated underlying molecular
mechanisms.
Materials and methods
Cells and materials
Human breast cancer MCF-7 cells were obtained from
the American Type Culture Collection (Manassas, VA, USA) and were
maintained in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS) (cat. no. 16000-044;
Gibco; Thermo Fisher Scientific., Inc. Waltham, MA, USA) and 1%
Anti-Anti (antibiotic-antimycotic) 100X (cat. no. 15240-062; Gibco;
Thermo Fisher Scientific, Inc.) at 37°C in a 5% CO2
incubator. CK2 inhibitors [apigenin,
1,3,8-trihydroxy-6-methylanthraquinone (emodin) and
2-dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole (TBBz)],
dimethyl sulfoxide used as the solvent of the CK2 inhibitors and
TPA (cat. no. P1585), and anti-β-actin (cat. no. A5441) antibodies
were purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Matrigel was obtained from Corning Incorporated (Corning, NY, USA).
Primary antibodies against CK2α (cat. no. 2656), p38 kinase (p38;
cat. no. 9212), c-Jun N-terminal kinase (JNK; cat. no. 9252),
extracellular signal-regulated kinase (ERK; cat. no. 9102), as well
as the phosphorylated forms of p38 (cat. no. 9211), JNK (cat. no.
9261) and ERK (cat. no. 9101), were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Antibodies specific to MMP-9
(cat. no. 12759), transcription factor p50 (cat. no. 7178) and
transcription factor p65 (cat. no. 372) and proliferating cell
nuclear antigen (PCNA; cat. no. 7907), as well as horseradish
peroxidase (HRP)-conjugated secondary immunoglobulin G (IgG; cat.
no. SC-2004, SC-2005), were all purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA).
RNA interference
CK2α specific small interfering (si)RNA and negative
control siRNA (cat. no. SN-1003) were obtained from Bioneer
Corporation (Daejeon, Korea). The siRNA used were as follows: CK2α
sense, CAU UUA GUU ACU GGG CAU A(dTdT) and antisense, UAU GCC CAG
UAA CUA AAU G(dTdT). Briefly, human breast cancer MCF-7 cells
(5×105) were transfected with 100 pmol siRNA using
RNAiMAX Transfection Reagent for 24 h at 37°C (Thermo Fisher
Scientific, Inc.), according to the manufacturer's
instructions.
Determination of cell viability
Cell viability assays were performed using the
EZ-Cytox Enhanced Cell Viability Assay kit (DOGEN, Seoul, Korea;
www.dogenbio.com/shop/item.php?it_id=1490923054),
according to the manufacturer's instructions. Briefly,
3×104 cells/well were seeded into 96-well plates and
incubated at 37°C for 24 h to allow the cells to adhere. The cells
were then treated with apigenin (20 µM), emodin (20 µM) or TBBz (2
µM) for 24 h at 37°C. Following this, all cells were incubated with
EZ-CytoxReagent (10 µl) for 30 min at 37°C. Absorbance at 450 nm
was determined using an ELISA plate reader (Sunrise™; Tecan Group,
Ltd., Mannedorf, Switzerland).
Western blot analysis
MCF-7 cells (7×105) pretreated with CK2
inhibitors [20 µM apigenin, 20 µM emodin, or 2 µM TBBz] for 1 h,
and cells (3.5×105) transfected with CK2 were
subsequently incubated with TPA for 24 h at 37°C. The cells were
lysed with ice-cold radioimmunoprecipitation assay buffer (Thermo
Fisher Scientific, Inc.), and the protein concentration of the
resulting lysates was determined using a BioSpec-nano (Shimadzu
Corporation, Kyoto, Japan). Total protein samples were resolved via
10% SDS-PAGE analysis and then transferred to polyvinylidene
fluoride membranes (GE Healthcare Life Sciences, Little Chalfont,
UK). The membranes were blocked with 5% bovine serum albumin (cat.
no. 160069; MP biomedicals Inc.,) or 5% skimmed milk in TBS with
0.5% Tween-20 at 4°C for 2 h, and then incubated overnight at 4°C
with primary antibodies. All antibodies used were diluted 1:2,000.
HRP-conjugated IgGs were used as secondary antibodies and incubated
at 4°C for 1 h. Protein expression levels were visualized using a
Mini HD6 image analyzer using Alliance 1D software (UVItec
Cambridge; Cleaver Scientific Ltd., Rugby, UK).
Gelatin zymography assay
MMP-9 activity in the conditioned medium (serum-free
DMEM) was determined by gelatin zymography. MCF-7 cells
(7×105) were pretreated with CK2 inhibitors [apigenin
(20 µM), emodin (20 µM) or TBBz (2 µM)] for 1 h, and then incubated
with TPA for 24 h at 37°C. The conditioned medium was mixed with
non-reducing sample buffer (0.5M Tris-HCl (pH 6.8), Glycerol 2 ml,
10% SDS, 0.1% Bromophenol blue, D.W 1 ml up to 10 ml), and
subjected to 10% SDS-PAGE analysis containing 0.1% [weight
(w)/volume (v)] gelatin. The gel was then incubated in a renaturing
buffer (2.5% Triton™ X-100; Sigma-Aldrich; Merck KGaA) with gentle
agitation to remove the SDS at room temperature for 30 min.
Following this, gels were incubated in a developing buffer (5 mM
CaCl2, 0.02% Brij, pH 7.5 and 50 mM Tris-HCl) overnight
at 37°C. The gel was stained for 30 min at room temperature with
0.25% (w/v) Coomassie brilliant blue R-250 at room temperature
followed by destaining with washing buffer (10% acetic acid and 10%
methanol). Proteolysis was visualized as a white zone in a dark
blue field using a digital imaging system (FluorChem R by
ProteinSimple; Cell Biosciences, Palo Alto, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cultured cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Complementary DNA was
synthesized from total RNA using a PrimeScript™ RT Reagent kit
(Takara Biotechnology Co., Ltd., Dalian, China). mRNA expression
levels were determined by qPCR analysis using the StepOnePlus™
Real-Time PCR System and SYBR®-Green (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The primers used were
as follows: MMP-9 sense, 5′-CCTGGAGACCTGAGAACCAATCT-3′ and
antisense, 5′-CCACCCGAGTGTAACCATAGC-3′; GAPDH (NM_002046) sense,
5′-ATGGAAATCCCATCACCATCTT-3′ and antisense,
5′-CGCCCCACTTGATTTTGG-3′. qPCR was performed with a preliminary
incubation at 95°C for 10 min, followed by 40 cycles of 95°C for 15
sec and 60°C for 1 min. MMP-9 mRNA expression levels were
quantified relative to GAPDH mRNA expression using the comparative
2−∆∆Cq method (28).
Preparation of nuclear extracts
MCF-7 cells (2×106) were transfected with
CK2 siRNA in the presence of 20 nM TPA for 3 h at 37°C and then
washed twice with PBS, scraped, resuspended in 1.5 ml ice-cold PBS
(pH 7.5), and then centrifuged at 1,500 × g for 4 min at 4°C.
Isolation of the nuclear and cytoplasmic extracts was performed
using NE-PER Nuclear and Cytoplasmic Extraction Reagents kit (cat.
no. 78835; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol.
Invasion assay
Invasion of MCF-7 cells was assessed using 24-well
chambers with 8 µm pore membranes coated with 20 µl Matrigel. The
Matrigel coating was rehydrated in 0.5 ml DMEM for 2 h at 37°C
immediately prior to experiments. Cell growth medium (0.5 ml) with
suspensed cells (3×105) and cells (2×105)
transfected with CK2 siNRA were added to the upper chambers, and
cell growth medium (0.5 ml) with TPA, alone or with CK2 inhibitors,
were added to the bottom well. The upper chambers and bottom well
were incubated with DMEM supplemented 10% FBS and 1% antibiotic for
24 h. Following incubation, cells on the upper membrane surface
were removed using cotton swabs, and cells that had migrated to the
lower membrane surface were fixed by formaldehyde solution (3.6%)
at room temperature, stained with crystal violet, and counted in
five random fields per chamber using a Leica DM ILLED Inverted Lab
microscope (Leica, Wetzlar, Germany) used magnification, ×10.
Statistical analysis
Data are expressed as the mean ± standard error.
Statistical significance was determined using one-way analysis of
variance followed by Scheffe post hoc test in Excel (Microsoft
Excel 2013). P<0.005 was considered to indicate a statistically
significant difference. All experiments were performed in
triplicate.
Results
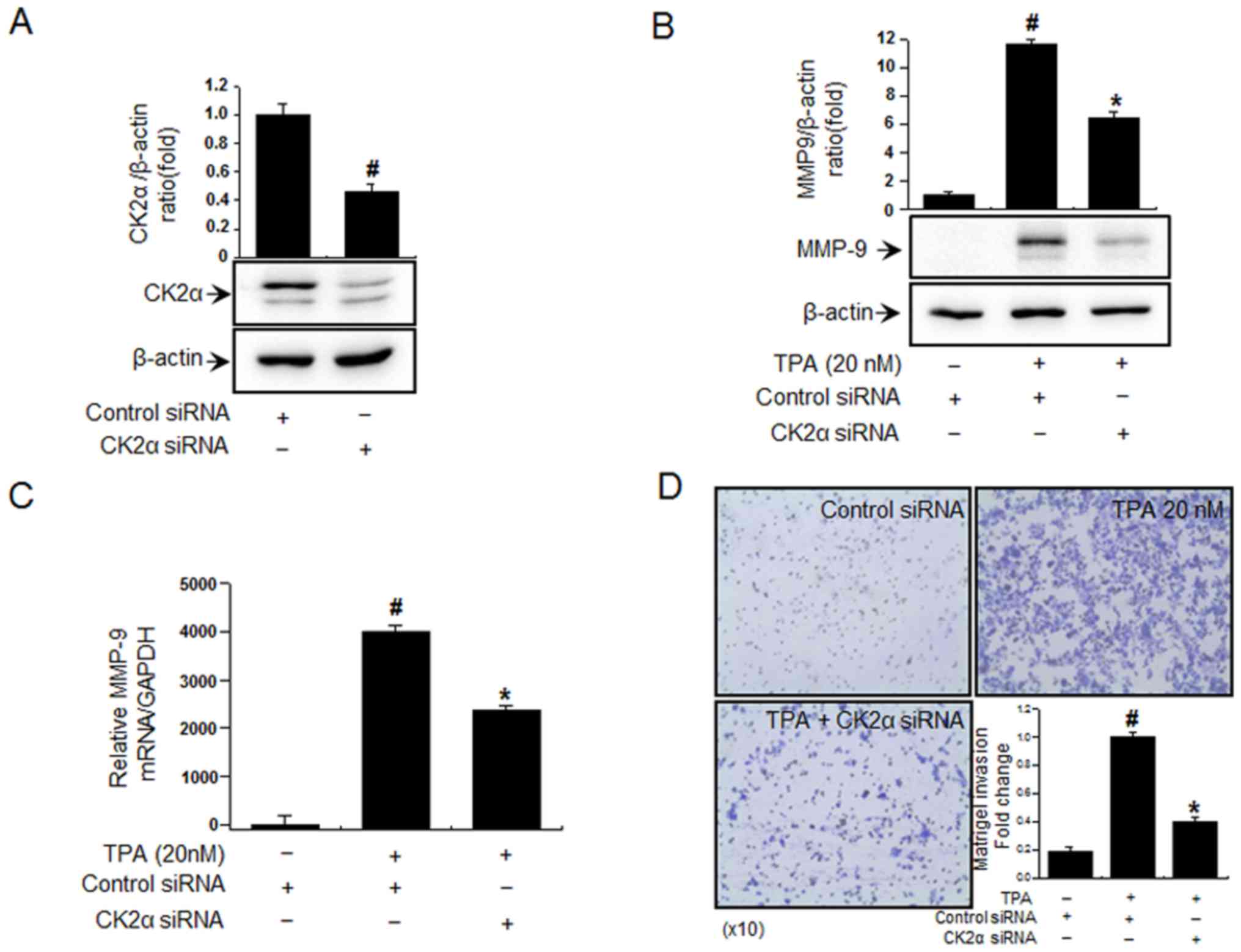
Inhibition of CK2α inhibits
TPA-induced MMP-9 expression in MCF-7 cells
In order to investigate the effects of CK2α
inhibition on TPA-induced cell invasion and MMP-9 expression,
intracellular CK2α expression was suppressed via transfection with
siRNA (Fig. 1A). The
siRNA-mediated inhibition of CK2α significantly suppressed the
increase in MMP-9 mRNA/protein expression compared with the TPA
group (Fig. 1B and C).
Furthermore, MCF-7 cells treated with TPA exhibited significantly
increased invasion compared with the untreated control cells.
However, the inhibition of CK2α significantly suppressed TPA
induced MCF-7 cell invasion (Fig.
1D). These results suggest that CK2α may be involved in the
underlying mechanism resulting in the TPA-induced increase of cell
invasion and MMP-9 expression.
Inhibition of CK2α suppresses
TPA-induced mitogen-activated protein kinase (MAPK) and NF-κΒ
activation in MCF-7 cells
The role of MAPKs (ERK, p38 and JNK) as upstream
modulators of NF-κΒ in the activation of MMP-9 expression has been
well established (25,26,29).
The effects of CK2α inhibition on the TPA-induced phosphorylation
of MAPKs was investigated. Following suppression of CK2α expression
via siRNA knockdown, the phosphorylation levels of p38 and JNK, but
not of ERK, were significantly suppressed (Fig. 2A). As revealed in Fig. 2B, treatment with TPA significantly
enhanced the level of NF-κΒ (p65 and p50 subunits) compared with
the control group. However, following suppression of intracellular
CK2α expression, the expression levels of NF-κΒ were significantly
inhibited (Fig. 2B). These results
suggest that CK2α is an upstream regulator of p38 and JNK in the
PKC induced MAPK-NF-κΒ signaling pathway responsible for the
regulation of MMP-9 expression.
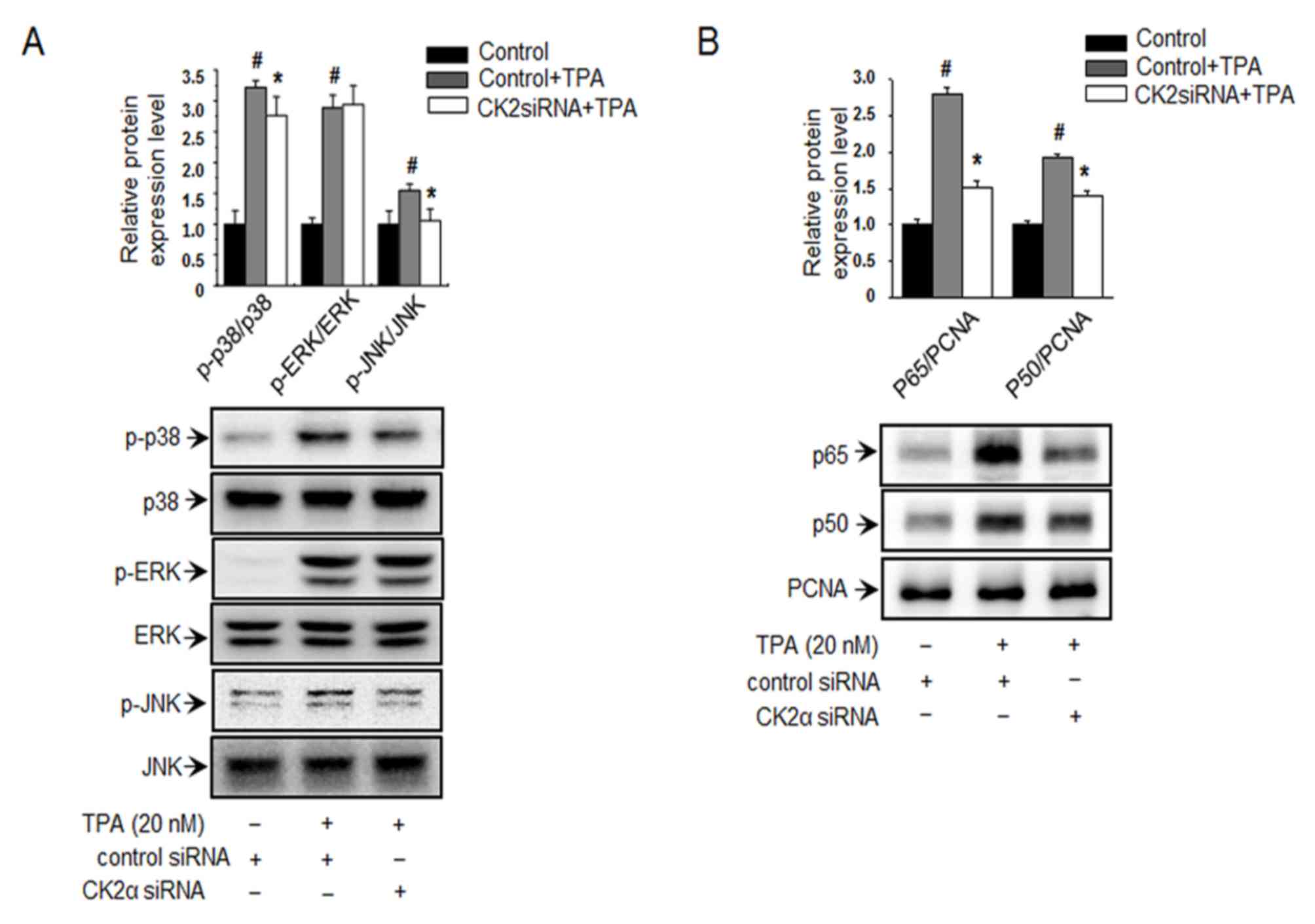 | Figure 2.Inhibition of CK2α suppresses
TPA-induced activation of mitogen activated protein kinase
signaling pathways and NF-κB expression in MCF-7 cells. (A) Western
blot analysis of p38, JNK, ERK, and their phosphorylated forms in
MCF-7 cells following transfection with CK2α siRNA and treatment
with TPA. (B) Nuclear lysates of cells transfected with CK2α siRNA
were subjected to western blot analysis using anti-p65 and -p50
antibodies. #P<0.005 vs. control; *P<0.005 vs.
control + TPA. CK2α, casein kinase 2α; si, small interfering; TPA,
12-O-tetradecanoylphorbol-13-acetate; MMP-9, matrix
metalloproteinase-9; p-, phosphorylated; p38, p38 kinase; JNK,
c-Jun N-terminal kinase; NF-κB, nuclear factor-κB; ERK,
extracellular signal-regulated kinase; PCNA, proliferating cell
nuclear antigen. |
CK2 inhibitors suppress TPA-induced
cell invasion and MMP-9 expression in MCF-7 cells
To further elucidate the therapeutic potential of
CK2 inhibition for the prevention of PKC induced breast cancer cell
invasion, three CK2 inhibitors were investigated. The cytotoxicity
of these three CK2 inhibitors was investigated using a EZ-cytox
assay. As revealed in Fig. 3A,
treatment with 20 µM apigenin, 20 µM emodin and 2 µM TBBz did not
cause any significant changes in cell viability. Furthermore, the
CK2 inhibitors were revealed to suppress TPA-induced increase in
MMP-9 mRNA/protein expression and cell invasion (Fig. 3B-D). These results suggest that CK2
inhibitors have the ability to inhibit cell invasion via regulating
MMP-9 expression.
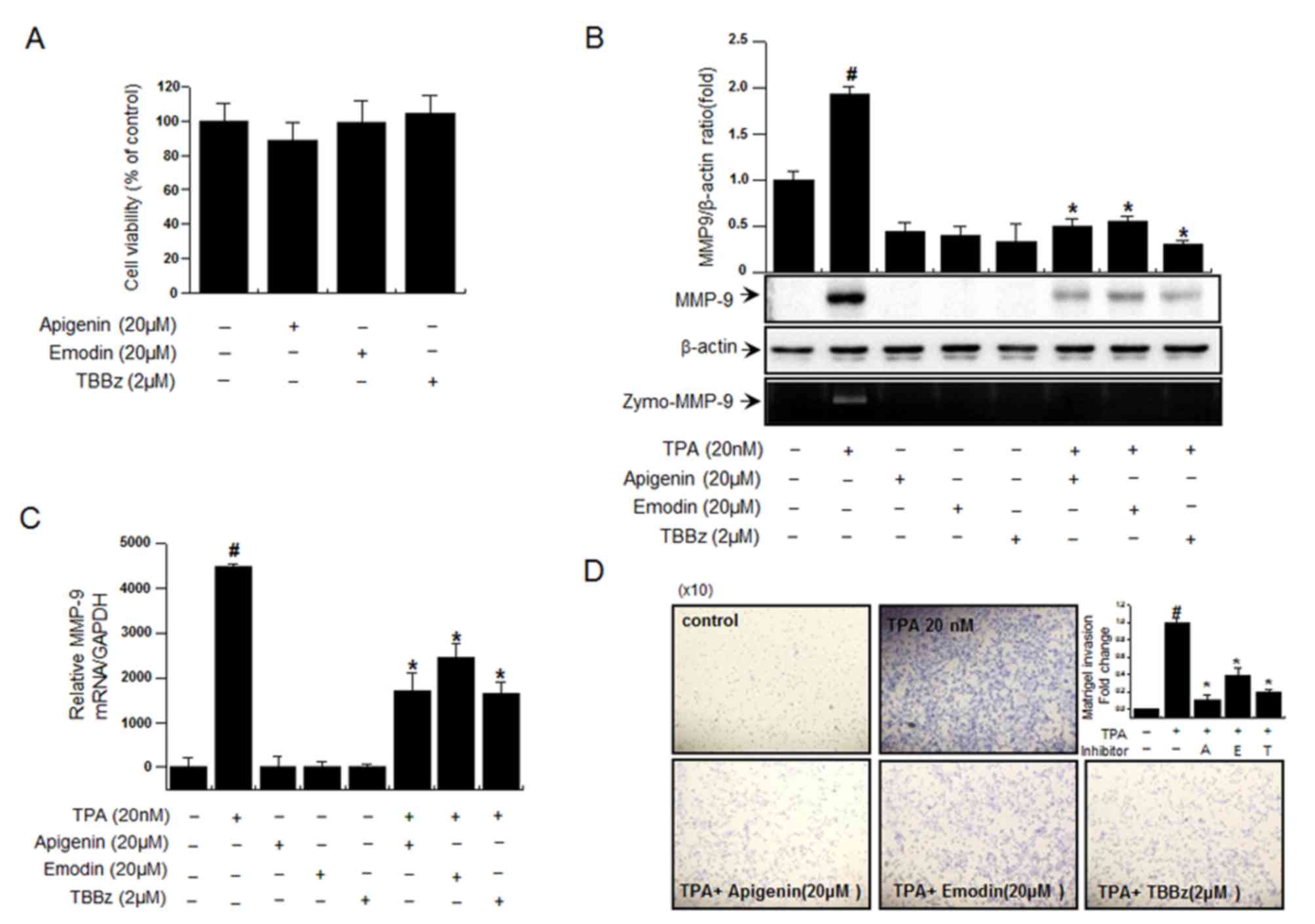 | Figure 3.Inhibition of CK2 suppresses
TPA-induced invasion and MMP-9 expression in MCF-7 cells. (A) To
investigate the cytotoxic effects of the CK2 inhibitors, cell
viability following incubation with CK2 inhibitors was determined.
The optical density of the control was regarded as 100%. (B) The
activity of MMP-9 following treatment with CK2 inhibitors and TPA
was investigated using a zymography assay. (C) The mRNA expression
of MMP-9 was analyzed via reverse transcription-quantitative
polymerase chain reaction. (D) The invasive ability of cells
following treatment with CK2 inhibitors and TPA was determined
using a Matrigel invasion assay (magnification, ×10). Data are
presented as the mean ± standard error of the mean of three
independent experiments. #P<0.005 vs. control;
*P<0.005 vs. TPA only. CK2α, casein kinase 2α; si, small
interfering; TPA, 12-O-tetradecanoylphorbol-13-acetate; MMP-9,
matrix metalloproteinase-9; TBBz,
2-dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole; zymo,
zymography; A, apigenin; E, emodin; T, TBBz. |
Discussion
The stages of breast cancer are classified as 0–4.
Number staging systems usually use the TNM system to divide cancers
into stages. The majority of cancer types are classified into 4
stages, numbered from 1 to 4, which are important for diagnosis and
treatment (30). From stage 2
onwards, metastases develop and the 5 year survival rate begins to
decline (31). Mortality from
metastatic breast cancer are on the rise, and thus suppression of
metastasis has emerged as a major challenge in breast cancer
treatment (32,33). In the early stages of tumor
metastasis, individual tumor cells or clusters invade the ECM
surrounding the primary tumor (34,35).
The ECM consists of type IV collagen and other matrix proteins
(19). Type IV collagen is a major
component of the basement membrane and type IV collagenase MMP-9
has previously been revealed to be an important molecule in tumor
progression and invasion in mammary tumors (36).
Elevated MMP-9 levels are functionally associated
with breast cancer metastasis, and MMP-9 serves a role in TPA
induced invasion of MCF-7 cells (21,24,37).
Therefore, the suppression of MMP-9 expression may represent a
novel therapeutic strategy for the prevention of tumor metastasis.
TPA upregulates MMP-9 expression in MCF-7 cells; however, the
underlying molecular mechanisms have not been fully established.
Therefore, the present study aimed to investigate the role of CK2
in the regulation of MMP-9 expression in MCF-7 cells. The results
of the present study revealed that the inhibition of CK2α
suppressed TPA-induced MMP-9 expression and invasion in MCF-7
cells. Furthermore, the results of the present study revealed that
CK2α is a regulator of PKC-induced invasion in MCF-7 cells.
In addition, the inhibition of CK2 was demonstrated
to suppress the activation of NF-κB in MCF-7 cells. The MAPK/NF-κB
signaling cascade serves a role in PKC-mediated MMP-9 expression in
MCF-7 cells (23,24,38,39).
Furthermore, the MAPK signaling pathway has been previously
demonstrated to be important for the activation of NF-κB (40,41).
CK2 has been reported to induce NF-κB activation (27,42).
In the present study, it was revealed that CK2α inhibition markedly
suppresses TPA-induced activation of p38, JNK and NF-κB. In
addition, it was revealed that the treatment of MCF-7 cells with
CK2 inhibitors suppressed TPA-induced invasion and MMP-9 expression
in MCF-7 cells.
In conclusion, the results of the present study
revealed that suppression of CK2 exerts anti-invasive effects in
PKC-induction condition via the regulation of MMP-9 expression
levels. Therefore, CK2 may represent a novel anti-invasive target
for cancer therapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by a National
Research Foundation of Korea grant funded by the Korea Government
(grant nos. 2011-0030130 and 2013R1A1A2007181).
Availability of data and materials
The data sets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
JMK and EMN performed most of the experiments. HKS,
YOY and KBK analyzed the data and provided comments. SJ and JSK
helped with the experiments. HJY designed the project. YRL designed
the analysis. KBK, YRL and HJY wrote the manuscript. All authors
reviewed the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Authors declare that they have no competing
interests.
References
|
1
|
Munstermann U, Fritz G, Seitz G, Lu YP,
Schneider HR and Issinger OG: Casein kinase II is elevated in solid
human tumours and rapidly proliferating non-neoplastic tissue. Eur
J Biochem. 189:251–257. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ahmed K, Davis AT, Wang H, Faust RA, Yu S
and Tawfic S: Significance of protein kinase CK2 nuclear signaling
in neoplasia. J Cell Biochem Suppl. Suppl 35:S130–S135. 2000.
View Article : Google Scholar
|
|
3
|
Ruzzene M and Pinna LA: Addiction to
protein kinase CK2: A common denominator of diverse cancer cells?
Biochim Biophys Acta. 1804:499–504. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Landesman-Bollag E, Romieu-Mourez R, Song
DH, Sonenshein GE, Cardiff RD and Seldin DC: Protein kinase CK2 in
mammary gland tumorigenesis. Oncogene. 20:3247–3257. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Christofori G: New signals from the
invasive front. Nature. 441:444–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chambers AF and Matrisian LM: Changing
views of the role of matrix metalloproteinases in metastasis. J
Natl Cancer Inst. 89:1260–1270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Woodhouse EC, Chuaqui RF and Liotta LA:
General mechanisms of metastasis. Cancer. 80 8 Suppl:S1529–S1537.
1997. View Article : Google Scholar
|
|
8
|
Patel D, Shukla S and Gupta S: Apigenin
and cancer chemoprevention: Progress, potential and promise
(review). Int J Oncol. 30:233–245. 2007.PubMed/NCBI
|
|
9
|
Shukla S and Gupta S: Apigenin-induced
cell cycle arrest is mediated by modulation of MAPK, PI3K-Akt, and
loss of cyclin D1 associated retinoblastoma dephosphorylation in
human prostate cancer cells. Cell Cycle. 6:1102–1114. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shukla S and Gupta S: Suppression of
constitutive and tumor necrosis factor alpha-induced nuclear factor
(NF)-kappaB activation and induction of apoptosis by apigenin in
human prostate carcinoma PC-3 cells: Correlation with
down-regulation of NF-kappaB-responsive genes. Clin Cancer Res.
10:3169–3178. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen D, Landis-Piwowar KR, Chen MS and Dou
QP: Inhibition of proteasome activity by the dietary flavonoid
apigenin is associated with growth inhibition in cultured breast
cancer cells and xenografts. Breast Cancer Res. 9:R802007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Way TD, Kao MC and Lin JK: Degradation of
HER2/neu by apigenin induces apoptosis through cytochrome c release
and caspase-3 activation in HER2/neu-overexpressing breast cancer
cells. FEBS Lett. 579:145–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim JS, Eom JI, Cheong JW, Choi AJ, Lee
JK, Yang WI and Min YH: Protein kinase CK2alpha as an unfavorable
prognostic marker and novel therapeutic target in acute myeloid
leukemia. Clin Cancer Res. 13:1019–1028. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang L and Hung MC: Sensitization of
HER-2/neu-overexpressing non-small cell lung cancer cells to
chemotherapeutic drugs by tyrosine kinase inhibitor emodin.
Oncogene. 12:571–576. 1996.PubMed/NCBI
|
|
15
|
Zou J, Luo H, Zeng Q, Dong Z, Wu D and Liu
L: Protein kinase CK2α is overexpressed in colorectal cancer and
modulates cell proliferation and invasion via regulating
EMT-related genes. J Transl Med. 9:972011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sarno S, Ruzzene M, Frascella P, Pagano
MA, Meggio F, Zambon A, Mazzorana M, Di Maira G, Lucchini V and
Pinna LA: Development and exploitation of CK2 inhibitors. Mol Cell
Biochem. 274:69–76. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Di Maira G, Brustolon F, Bertacchini J,
Tosoni K, Marmiroli S, Pinna LA and Ruzzene M: Pharmacological
inhibition of protein kinase CK2 reverts the multidrug resistance
phenotype of a CEM cell line characterized by high CK2 level.
Oncogene. 26:6915–6926. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pagano MA, Andrzejewska M, Ruzzene M,
Sarno S, Cesaro L, Bain J, Elliott M, Meggio F, Kazimierczuk Z and
Pinna LA: Optimization of protein kinase CK2 inhibitors derived
from 4,5,6,7-tetrabromobenzimidazole. J Med Chem. 47:6239–6247.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakajima M, Welch DR, Belloni PN and
Nicolson GL: Degradation of basement membrane type IV collagen and
lung subendothelial matrix by rat mammary adenocarcinoma cell
clones of differing metastatic potentials. Cancer Res.
47:4869–4876. 1987.PubMed/NCBI
|
|
20
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin CW, Hou WC, Shen SC, Juan SH, Ko CH,
Wang LM and Chen YC: Quercetin inhibition of tumor invasion via
suppressing PKC delta/ERK/AP-1-dependent matrix metalloproteinase-9
activation in breast carcinoma cells. Carcinogenesis. 29:1807–1815.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee SO, Jeong YJ, Im HG, Kim CH, Chang YC
and Lee IS: Silibinin suppresses PMA-induced MMP-9 expression by
blocking the AP-1 activation via MAPK signaling pathways in MCF-7
human breast carcinoma cells. Biochem Biophys Res Commun.
354:165–171. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Newton AC: Regulation of protein kinase C.
Curr Opin Cell Biol. 9:161–167. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee SO, Jeong YJ, Kim M, Kim CH and Lee
IS: Suppression of PMA-induced tumor cell invasion by capillarisin
via the inhibition of NF-kappaB-dependent MMP-9 expression. Biochem
Biophys Res Commun. 366:1019–1024. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chung TW, Moon SK, Chang YC, Ko JH, Lee
YC, Cho G, Kim SH, Kim JG and Kim CH: Novel and therapeutic effect
of caffeic acid and caffeic acid phenyl ester on hepatocarcinoma
cells: Complete regression of hepatoma growth and metastasis by
dual mechanism. FASEB J. 18:1670–1681. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hong S, Park KK, Magae J, Ando K, Lee TS,
Kwon TK, Kwak JY, Kim CH and Chang YC: Ascochlorin inhibits matrix
metalloproteinase-9 expression by suppressing activator
protein-1-mediated gene expression through the ERK1/2 signaling
pathway: Inhibitory effects of ascochlorin on the invasion of renal
carcinoma cells. J Biol Chem. 280:25202–25209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Woo MS, Jung SH, Kim SY, Hyun JW, Ko KH,
Kim WK and Kim HS: Curcumin suppresses phorbol ester-induced matrix
metalloproteinase-9 expression by inhibiting the PKC to MAPK
signaling pathways in human astroglioma cells. Biochem Biophys Res
Commun. 335:1017–1025. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ohashi Y, Tsuchiya Y, Koizumi K, Sakurai H
and Saiki I: Prevention of intrahepatic metastasis by curcumin in
an orthotopic implantation model. Oncology. 65:250–258. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Reyes-Ortiz CA, Eschbach K, Zhang DD and
Goodwin JS: Neighborhood composition and cancer among hispanics:
Tumor stage and size at time of diagnosis. Cancer Epidemiol
Biomarkers Prev. 17:2931–2936. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Howlader N, Noone AM, Krapcho M, Miller D,
Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z and Mariotto A:
SEER cancer statistics review, 1975–2014. National Cancer
Institute; Bethesda, MD: 2017
|
|
32
|
Polyak K: Heterogeneity in breast cancer.
J Clin Invest. 121:3786–3788. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Neophytou C, Boutsikos P and Papageorgis
P: Molecular mechanisms and emerging therapeutic targets of
triple-negative breast cancer metastasis. Front Oncol. 8:312018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li DM and Feng YM: Signaling mechanism of
cell adhesion molecules in breast cancer metastasis: Potential
therapeutic targets. Breast Cancer Res Treat. 128:7–21. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Scorilas A, Karameris A, Arnogiannaki N,
Ardavanis A, Bassilopoulos P, Trangas T and Talieri M:
Overexpression of matrix-metalloproteinase-9 in human breast
cancer: A potential favourable indicator in node-negative patients.
Br J Cancer. 84:1488–1496. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Park SK, Hwang YS, Park KK, Park HJ, Seo
JY and Chung WY: Kalopanaxsaponin A inhibits PMA-induced invasion
by reducing matrix metalloproteinase-9 via PI3K/Akt- and
PKCdelta-mediated signaling in MCF-7 human breast cancer cells.
Carcinogenesis. 30:1225–1233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Weng CJ, Chau CF, Hsieh YS, Yang SF and
Yen GC: Lucidenic acid inhibits PMA-induced invasion of human
hepatoma cells through inactivating MAPK/ERK signal transduction
pathway and reducing binding activities of NF-kappaB and AP-1.
Carcinogenesis. 29:147–156. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Eberhardt W, Huwiler A, Beck KF, Walpen S
and Pfeilschifter J: Amplification of IL-1 beta-induced matrix
metalloproteinase-9 expression by superoxide in rat glomerular
mesangial cells is mediated by increased activities of NF-kappa B
and activating protein-1 and involves activation of the
mitogen-activated protein kinase pathways. J Immunol.
165:5788–5797. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yao J, Xiong S, Klos K, Nguyen N, Grijalva
R, Li P and Yu D: Multiple signaling pathways involved in
activation of matrix metalloproteinase-9 (MMP-9) by heregulin-beta1
in human breast cancer cells. Oncogene. 20:8066–8074. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Madrid LV, Mayo MW, Reuther JY and Baldwin
AS Jr: Akt stimulates the transactivation potential of the RelA/p65
Subunit of NF-kappa B through utilization of the Ikappa B kinase
and activation of the mitogen-activated protein kinase p38. J Biol
Chem. 276:18934–18940. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Saja K, Babu MS, Karunagaran D and
Sudhakaran PR: Anti-inflammatory effect of curcumin involves
downregulation of MMP-9 in blood mononuclear cells. Int
Immunopharmacol. 7:1659–1667. 2007. View Article : Google Scholar : PubMed/NCBI
|