Introduction
Acid fibroblast growth factor 1 (aFGF) is a
mitogenic factor that has been associated with peroxisome
proliferator-activated receptors (PPARs) (1,2), has
been reported to be a critical therapeutic regulator in numerous
chronic metabolic disorders. aFGF-knockout mice develop insulin
resistance when stressed with a high-fat diet (HFD), suggesting
that aFGF has a beneficial effect on nutrient homeostasis (1). aFGF additionally has therapeutic
potential for the treatment of non-alcoholic fatty liver disease
(2).
Atherosclerosis is the principal cause of coronary
artery disease, and is therefore a principal cause of mortality and
morbidity globally (3). It is
noteworthy that atherosclerosis is additionally recognized as a
lipid-driven chronic metabolic disease (4). Previous studies have reported
increased aFGF expression in atherosclerotic plaques in human
(5) and swine (6) models. However, there is no direct
evidence indicating whether aFGF serves a therapeutic role in
atherosclerosis. Recent studies demonstrated that PPARα is a key
regulator of atherosclerosis and is involved in HFD-induced
atherosclerosis in apolipoprotein E (ApoE)-null mice (7).
In the present study, the role of increased aFGF and
PPARα expression in atherosclerotic lesion development was verified
by examining ApoE-null mice. Furthermore, it was identified that
parenteral delivery of aFGF increased the expression of PPARα, the
induction of inflammatory cytokines, and the subsequent development
of atherosclerotic plaques.
Materials and methods
Animal experiments
The protocols used for all animal studies were
approved by the Wenzhou Medical University Animal Policy and
Welfare Committee (Wenzhou, China; approval no. wydw2014-0058).
Male ApoE−/− mice (n=28; 18–20 g; 8 weeks old) with a
C57BL/6 background were purchased from Beijing HFK Bioscience Co.,
Ltd., (Beijing, China). Mice were housed at 22±2.0°C with 50±5%
humidity in a 12 h light/dark cycle with free access to food and
water. To induce atherosclerosis, the mice were fed a HFD
containing 60% kcal from fat, 20% kcal from protein and 20% kcal
from carbohydrate (MediScience Diets Co., Ltd., Yangzhou, China;
cat. no. MD12033) for 16 weeks (n=7; ApoE HFD), while the control
animals were fed a normal-fat diet (NFD) containing 10% kcal from
fat, 20% kcal from protein and 70% kcal from carbohydrate
(MediScience Diets Co., Ltd.; cat. no. MD12031; n=7; ApoE NFD).
In the second set of experiments, mice that were fed
a HFD for 8 weeks were randomly divided into the following two
groups: ApoE HFD treated with vehicle via intraperitoneal (IP)
injection (PBS for 8 weeks; n=7) and ApoE HFD treated with aFGF
(Key Laboratory of Biotechnology and Pharmaceutical Engineering,
Zhejiang, China) via IP injection [0.5 mg/kg/2 days for 8 weeks, as
described in a previous paper (2);
n=7]. The mice were sacrificed by increasing CO2
inhalation, in accordance with Schedule 1 of the Animals
(Scientific Procedures) Act (1986) as previously described
(8), and blood was collected into
a syringe containing 4% trisodium citrate (1:10, v/v) via cardiac
puncture. Arterial tissues were fixed in 4% paraformaldehyde at a
room temperature for 24 h and embedded in optimum cutting
temperature compound. Tissues were snap-frozen in liquid nitrogen
and serial 10 µm-thick cryosections from the middle portion of the
tissues were collected for gene and protein expression
analysis.
Measurement of the expression levels
of serum lipids and biochemical indicators
The expression level of serum lipids was measured
using lipid-specific biochemical kits [Nanjing Jiancheng
Bioengineering Institute, Nanjing, China; cat. no. A110-1 for
triglycerides (TG); cat. no. A113-1 for low-density lipoprotein
(LDL); cat. no. A112-1 for high density lipoprotein (HDL); and cat.
no. F002-1 for total cholesterol (TC)].
Immunofluorescence staining
The expression of aFGF and PPARα was measured by
immunofluorescence staining. Frozen sections were used for
immunofluorescence analysis. The slides were blocked using 1%
bovine serum albumin for 30 min at room temperature and incubated
overnight at 4°C with an aFGF antibody (1:200; cat. no. ab169748)
or a PPARα antibody (1:200; cat. no. ab119416). A
tetramethylrhodamine-conjugated secondary antibody (1:200; cat. no.
ab6786; all Abcam, Cambridge, UK) was used for detection at 4°C for
1 h. The slides were additionally stained with DAPI at 4°C for 5
min (5 mg/ml; Beyotime Institute of Biotechnology, Haimen, China;
cat. no. C1005). Slides were viewed under a fluorescence microscope
(magnification, ×200). The images were analyzed with Image-Pro Plus
(version 6.0 Media Cybernetics, Inc., Rockville, MD, USA).
Histology and analysis of
atherosclerotic lesions
Atherosclerotic lesions were measured as described
in a previous paper (9). The whole
aorta, including the aortic arch and the thoracic and abdominal
segments, was dissected, gently cleaned of adventitial tissue and
stained with Oil Red O at room temperature for 15 min (5 mg/ml;
Nanjing Jiancheng Bioengineering Institute; cat. no. D027). The
surface lesion area was quantified with ImageJ software (version
1.6.2; National Institutes of Health, Bethesda, MD, USA). To
measure lesions in the aortic root, the heart and proximal aorta
were excised, and the apex and lower half of the ventricles were
removed and stained with Oil Red O for 15 min (5 mg/ml) at room
temperature. The surface lesion area was quantified with ImageJ
software.
Five frozen sections were also stained with
hematoxylin and eosin at room temperature (eosin for 2 min and
hematoxylin for 5 min) for histopathological observation.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from arterial tissues using
TRIzol® (cat. no. 15596026). RT and qPCR were performed
using a two-step Moloney Murine Leukemia Virus kit (cat. no.
28025013) and a Platinum SYBR Green qPCR SuperMix-uracil DNA
glycosylase kit (cat. no. 11733046; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) in an Eppendorf Mastercycler ep RealPlex
detection system (Eppendorf, Hamburg, Germany). PCR quantification
was performed using the 2−ΔΔCq method (10). Primers were obtained from Thermo
Fisher Scientific, Inc. The primer sequences are listed in Table I. mRNA expression levels of the
target genes were normalized to β-actin.
 | Table I.Sequences of primers for the reverse
transcription quantitative polymerase chain reaction assay used in
the present study. |
Table I.
Sequences of primers for the reverse
transcription quantitative polymerase chain reaction assay used in
the present study.
| Gene | Species | Forward (5′-3′) | Reverse (3′-5′) |
|---|
| Acid fibroblast
growth factor | Mouse |
CTCATCCGGCAAAAGAGACAA |
TTGGAGCCAAAGAGTTTGACC |
| Peroxisome
proliferator-activated receptor α | Mouse |
ACTACGGAGTTCACGCATGTG |
TTGTCGTACACCAGCTTCAGC |
| IL-1β | Mouse |
ACTCCTTAGTCCTCGGCCA |
CCATCAGAGGCAAGGAGGAA |
| IL-6 | Mouse |
GAGGATACCACTCCCAACAGACC |
AAGTGCATCATCGTTGTTCATACA |
| β-actin | Mouse |
CCGTGAAAAGATGACCCAGA |
TACGACCAGAGGCATACAG |
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Differences between the groups were determined using the
Student's t-test, as appropriate, in GraphPad Prism 5.01 (GraphPad
Software Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Increased expression of aFGF and PPARα
in the aortas of HFD-fed ApoE−/− mice
A classical paradigm of the HFD-induced
ApoE−/− atherosclerosis model is the alteration of serum
lipid expression levels, as elevated LDL has been demonstrated to
be strongly associated with the development of atherosclerosis
(8). ApoE−/− mice fed a
HFD exhibited significantly increased serum expression levels of
TG, TC and LDL (Fig. 1A-C;
P<0.001) and significantly reduced expression levels of
high-density lipoprotein (HDL) compared with control mice fed an
NFD (Fig. 1D; P<0.05).
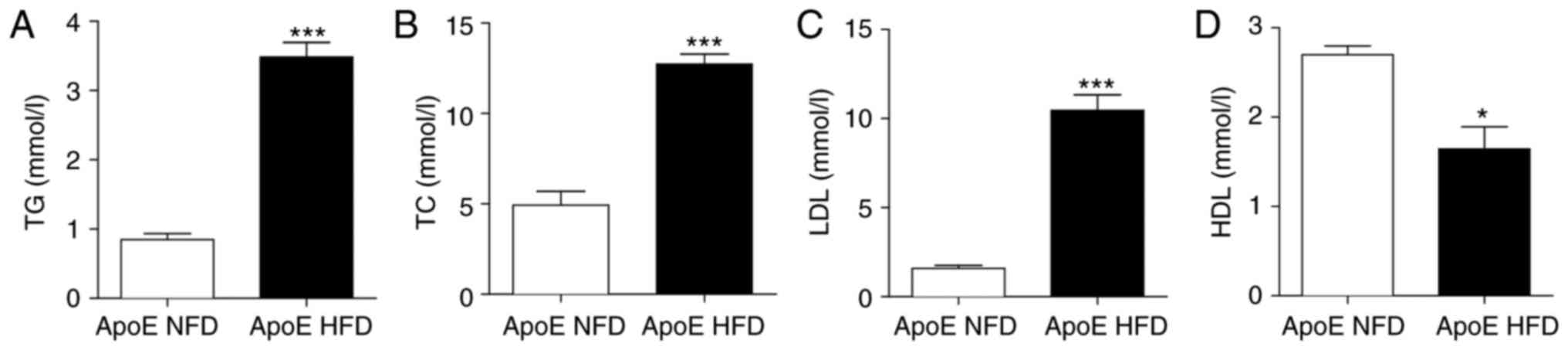 | Figure 1.ApoE−/− mice were placed on
a HFD and examined following 16 weeks. Serum levels of (A) TG, (B)
TC, (C) LDL and (D) HDL were analyzed. n=7/group; *P<0.05,
***P<0.001 vs. respective ApoE NFD. ApoE, apolipoprotein E; HFD,
high-fat diet; TG, triglycerides; TC, total cholesterol; LDL,
low-density lipoprotein; HDL, high-density lipoprotein; NFD,
normal-fat diet. |
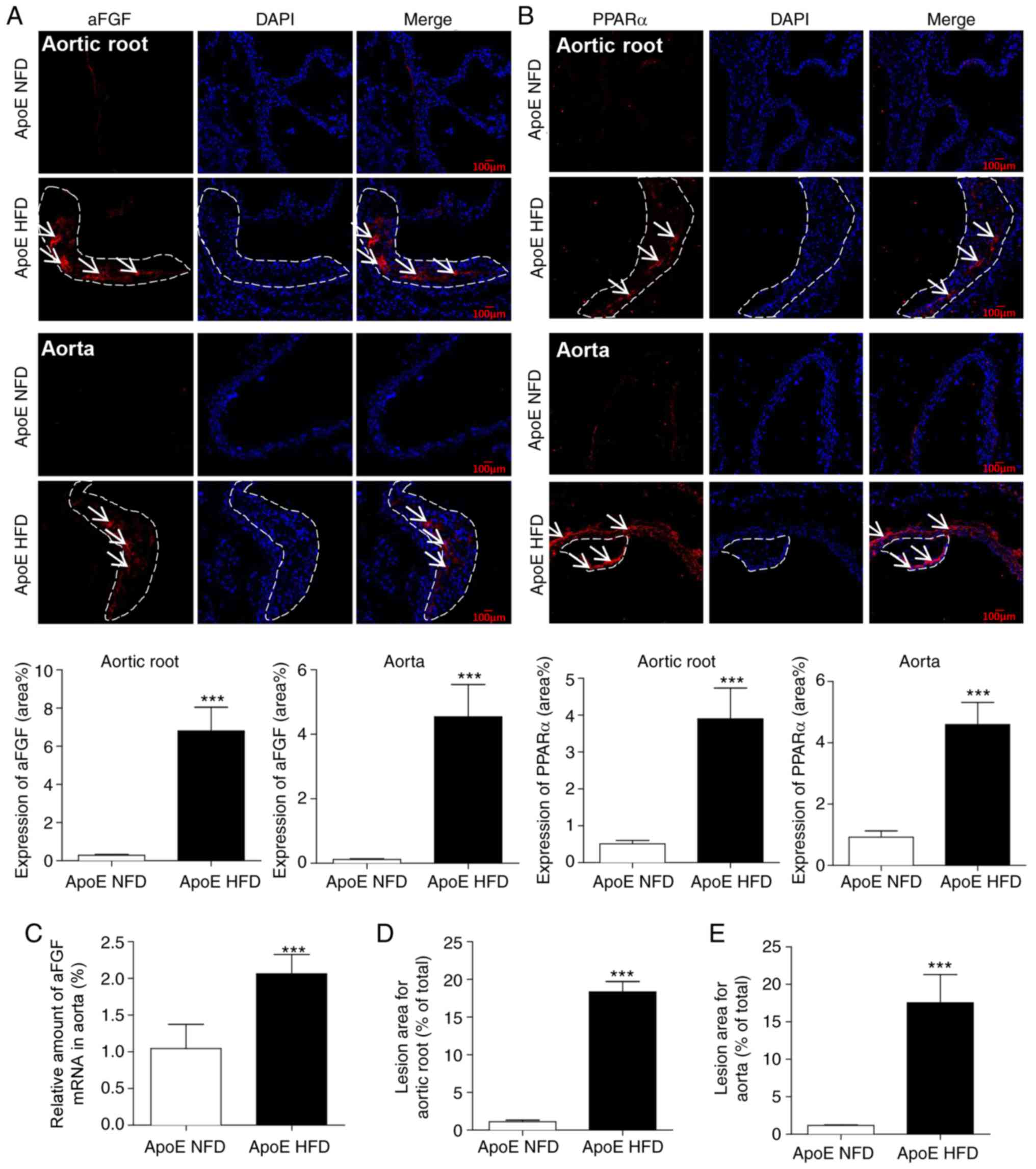
The aortic tissues from the mice were subsequently
assessed by immunofluorescence staining to determine whether aFGF
and PPARα are involved in the progression of atherosclerotic
plaques. The expression levels of aFGF (Fig. 2A) and PPARα (Fig. 2B) were increased in HFD-fed mice
compared with NFD-fed mice in atherosclerotic lesions of the aortic
root and aorta. The mRNA isolated from aortic tissues confirmed
that the expression levels of aFGF were increased in HFD-fed mice
(Fig. 2C). These increased
expression levels of aFGF and PPARα corresponded with morphological
alterations in HFD-fed ApoE−/− mice, including an
augmented atherosclerotic plaque lesion area in the aortic root
(Fig. 2D) and aorta (Fig. 2E) compared with ApoE NFD mice,
reinforcing the hypothesis that there is a positive association
between atherosclerotic plaque development and increased aFGF and
PPARα expression levels.
Treatment with aFGF aggravates
atherosclerotic plaque development in HFD-fed ApoE−/−
mice
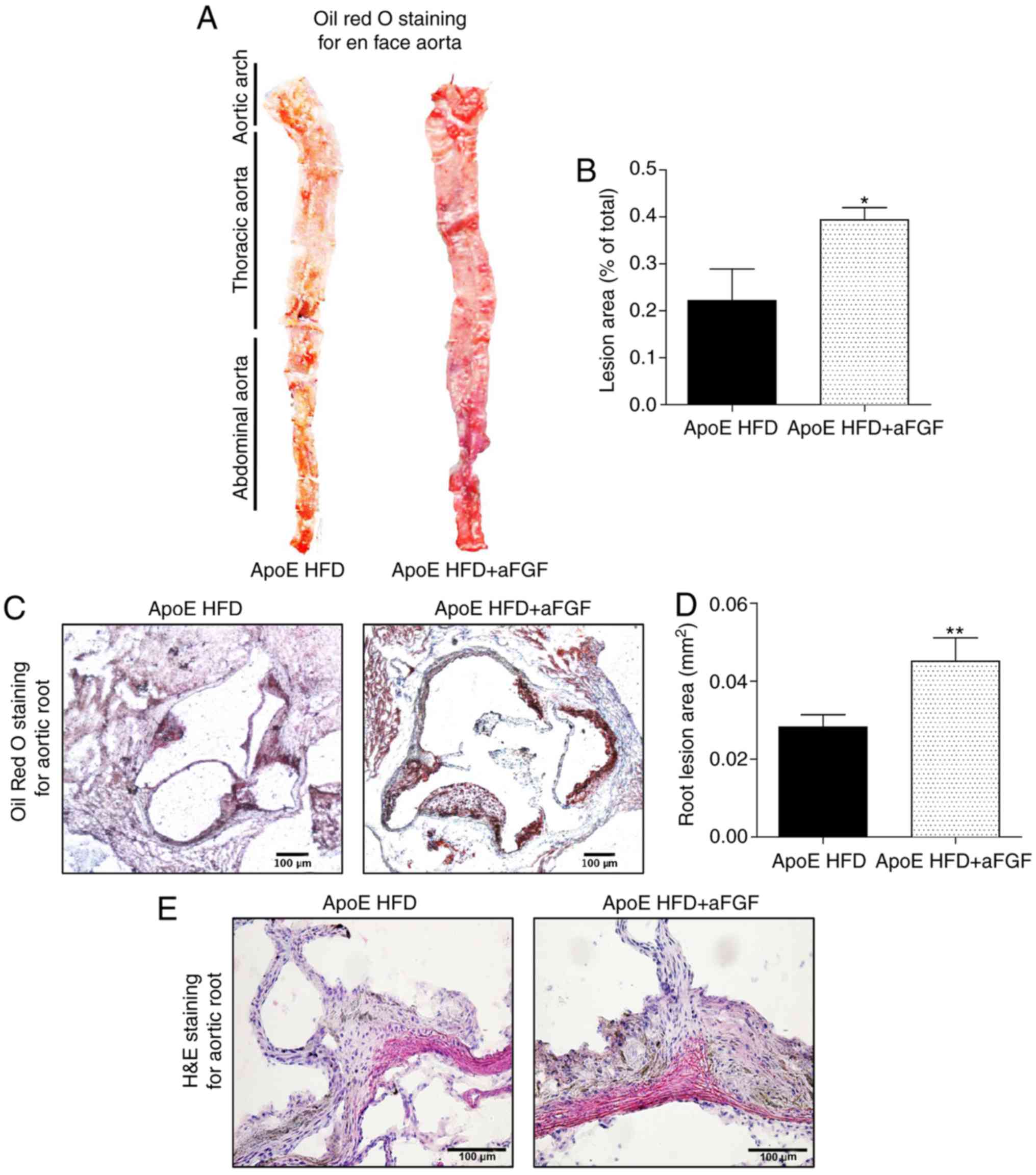
The second set of experiments aimed to determine
whether parenteral administration of aFGF was associated with
HFD-induced atherosclerotic development. Oil Red O staining of the
entire aorta was performed in the en face preparation and of the
aortic root to measure the severity of these lesions. Notably, the
present results demonstrated a significantly increased lesion area
in ApoE HFD mice treated with aFGF compared with ApoE HFD mice
treated with vehicle in the entire aorta (Fig. 3A and B) and the aortic root
(Fig. 3C and D). An additional
assessment by hematoxylin and eosin staining demonstrated that the
plaque areas in the aortic root of aFGF-treated mice were
aggravated in comparison with vehicle-treated HFD-fed mice
(Fig. 3E). The present results
indicated that the administration of aFGF accelerated the
progression of atherosclerotic plaques.
Treatment of mice with aFGF does not
affect the expression levels of serum lipids
The present study additionally aimed to determine
whether the administration of aFGF alters serum lipid expression
levels. Notably, the treatment of mice with aFGF for 8 weeks did
not affect the expression levels of serum lipids, including TG
(Fig. 4A), TC (Fig. 4B), LDL (Fig. 4C) and HDL (Fig. 4D), compared with vehicle-treated
HFD-fed mice, suggesting that the aFGF-facilitated progression of
atherosclerosis may be trigged or maintained via mechanisms that
are parallel to or independent of hyperlipidaemia.
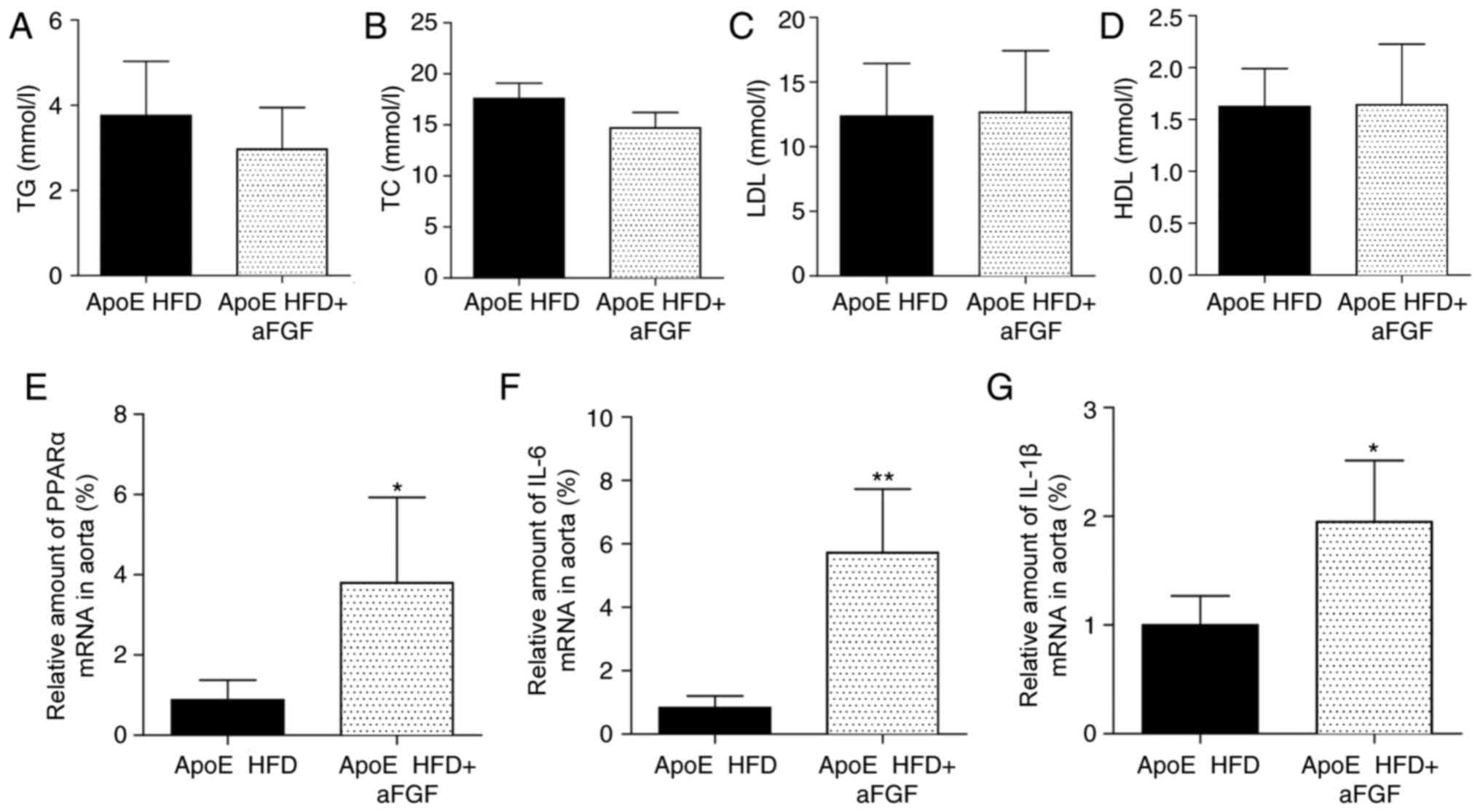 | Figure 4.Administration of aFGF increases the
mRNA expression levels of PPARα and inflammatory factors in HFD-fed
ApoE−/− mice. Serum expression levels of (A) TG, (B) TC,
(C) LDL and (D) HDL. Reverse-transcription-quantitative polymerase
chain reaction analysis of (E) PPARα, (F) IL-1β (G) and IL-6.
n=7/group. *P<0.05, **P<0.01 vs. respective ApoE HFD. TG,
triglycerides; TC, total cholesterol; LDL, low-density lipoprotein;
HDL, high-density lipoprotein; PPARα, peroxisome
proliferator-activated receptor α; IL, interleukin; ApoE,
apolipoprotein E; HFD, high-fat diet. |
Administration of aFGF increases the
mRNA expression levels of PPARα and inflammatory factors in HFD-fed
ApoE−/− mice
It has been established that PPARα (7) and inflammation (11) contribute to atherosclerotic
lesions. To determine whether the aggravating effect of aFGF on
atherogenesis is associated with PPARα and its downstream
inflammatory factors (12), the
mRNA expression levels of PPARα and associated inflammatory factors
were assessed, including interleukin (IL)-1β and IL-6, in
vivo. The present data demonstrated that the mRNA expression
levels of PPARα (Fig. 4E), IL-6
(Fig. 4F) and IL-1β (Fig. 4G) all significantly increased when
atherosclerotic mice were treated with aFGF (P<0.05).
Discussion
Atherosclerosis is a systemic, chronic metabolic
disease of the principal arteries. The formation and progression of
atherosclerotic plaques involves hyperlipidemia, inflammation, foam
cell formation, smooth muscle cell proliferation and increased
matrix synthesis (13). In the
present study, it was observed that atherosclerosis was associated
with elevated aFGF expression levels, consistent with a previous
report (14).
The FGF family, which contains 22 members in
mammals, has diverse biological functions in the progression of
atherosclerotic plaques (15).
Basic (b)FGF has been detected in human atherosclerotic plaques
(16), and increased expression of
bFGF is associated with carotid atherosclerotic plaque instability
(17). By contrast, the depletion
of FGF21 in ApoE−/− mice results in a markedly increased
exacerbation of atherosclerosis, which may be reversed by
replenishment with exogenous mouse recombinant FGF21 (18). The key present results suggested
that aFGF facilitated the progression of atherosclerosis regardless
of alterations in serum lipid expression levels, which is
inconsistent with the protective role of aFGF in other chronic
metabolic diseases (1,2). However, FGFs exert their biological
effects by interacting with and activating FGF receptors (FGFRs)
(14). The present results are
consistent with those reported by Raj et al (14), who demonstrated that the inhibition
of FGFR tyrosine kinase activity reduced atherosclerotic plaque
development, suggesting that an active aFGF/FGFR1 signaling system
promotes atherosclerosis development.
Tordjman et al (7) demonstrated that PPARα deficiency
reduces insulin resistance and atherosclerosis in ApoE-null mice.
The present results additionally demonstrated that the expression
levels of PPARα were increased in aortic atherosclerotic lesions in
HFD-fed mice. Although certain evidence suggests a role for aFGF in
PPAR-γ-associated chronic metabolic disease (1,2), the
association between aFGF and PPARα remains unknown. The present
results demonstrated that treatment with aFGF increased the mRNA
expression levels of PPARα and inflammatory factors. Therefore, the
present results suggested that aFGF may be the upstream regulator
of PPARα and its associated inflammation, which requires validation
in future studies.
In conclusion, the present results demonstrated that
aFGF promotes the progression of atherosclerotic plaques via PPARα
and inflammatory mechanisms, which occurs independently from
alterations in serum lipid expression levels. The present results
suggested that targeting aFGF may have therapeutic potential for
preventing atherosclerosis.
Acknowledgements
The authors thank Dr Jibo Han and Dr Xiong Chen.
They are the guarantors of the present study and had full access to
all the data in the present study, and take responsibility for the
integrity of the data and the accuracy of the data analysis.
Funding
The present study was supported by Public Welfare
Science and Technology Program of Wenzhou City (grant no.
Y20160306; Wenzhou, China).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LJ, JX and YD performed the research. LW, XC and JH
designed the research study. XC and JH contributed essential
reagents or tools. LJ, JX, YD and JH analyzed the data. LW and JH
wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The protocols used for all animal studies in the
present study were approved by the Wenzhou Medical University
Animal Policy and Welfare Committee (Wenzhou, China; approval no.
wydw2014-0058).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jonker JW, Suh JM, Atkins AR, Ahmadian M,
Li P, Whyte J, He M, Juguilon H, Yin YQ, Phillips CT, et al: A
PPARγ-FGF1 axis is required for adaptive adipose remodelling and
metabolic homeostasis. Nature. 485:391–394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu W, Struik D, Nies VJ, Jurdzinski A,
Harkema L, de Bruin A, Verkade HJ, Downes M, Evans RM, van Zutphen
T and Jonker JW: Effective treatment of steatosis and
steatohepatitis by fibroblast growth factor 1 in mouse models of
nonalcoholic fatty liver disease. Proc Natl Acad Sci USA.
113:2288–2293. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pearson-Stuttard J, Guzman-Castillo M,
Penalvo JL, Rehm CD, Afshin A, Danaei G, Kypridemos C, Gaziano T,
Mozaffarian D, Capewell S and O'Flaherty M: Modeling future
cardiovascular disease mortality in the United States: National
trends and racial and ethnic disparities. Circulation. 133:967–978.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Daugherty A, Tall AR, Daemen MJAP, Falk E,
Fisher EA, García-Cardeña G, Lusis AJ, Owens AP III, Rosenfeld ME,
Virmani R, et al: Recommendation on design, execution, and
reporting of animal atherosclerosis studies: A scientific statement
from the American heart association. Arterioscler Thromb Vasc Biol.
37:e131–e157. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brogi E, Winkles JA, Underwood R, Clinton
SK, Alberts GF and Libby P: Distinct patterns of expression of
fibroblast growth factors and their receptors in human atheroma and
nonatherosclerotic arteries. Association of acidic FGF with plaque
microvessels and macrophages. J Clin Invest. 92:2408–2418. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liau G, Winkles JA, Cannon MS, Kuo L and
Chilian WM: Dietary-induced atherosclerotic lesions have increased
levels of acidic FGF mRNA and altered cytoskeletal and
extracellular matrix mRNA expression. J Vasc Res. 30:327–332. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tordjman K, Bernal-Mizrachi C, Zemany L,
Weng S, Feng C, Zhang F, Leone TC, Coleman T, Kelly DP and
Semenkovich CF: PPARalpha deficiency reduces insulin resistance and
atherosclerosis in apoE-null mice. J Clin Invest. 107:1025–1034.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang L, Huang Z, Huang W, Chen X, Shan P,
Zhong P, Khan Z, Wang J, Fang Q, Liang G and Wang Y: Inhibition of
epidermal growth factor receptor attenuates atherosclerosis via
decreasing inflammation and oxidative stress. Sci Rep. 8:459172017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin Y, Bai L, Chen Y, Zhu N, Bai Y, Li Q,
Zhao S, Fan J and Liu E: Practical assessment of the quantification
of atherosclerotic lesions in apoE-/- mice. Mol Med Rep.
12:5298–5306. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sorci-Thomas MG and Thomas MJ:
Microdomains, inflammation, and atherosclerosis. Circ Res.
118:679–691. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pawlak M, Lefebvre P and Staels B:
Molecular mechanism of PPARα action and its impact on lipid
metabolism, inflammation and fibrosis in non-alcoholic fatty liver
disease. J Hepatol. 62:720–733. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Libby P, Ridker PM and Hansson GK:
Progress and challenges in translating the biology of
atherosclerosis. Nature. 473:317–325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Raj T, Kanellakis P, Pomilio G, Jennings
G, Bobik A and Agrotis A: Inhibition of fibroblast growth factor
receptor signaling attenuates atherosclerosis in apolipoprotein
E-deficient mice. Arterioscler Thromb Vasc Biol. 26:1845–1851.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Beenken A and Mohammadi M: The FGF family:
Biology, pathophysiology and therapy. Nat Rev Drug Discov.
8:235–253. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cordon-Cardo C, Vlodavsky I,
Haimovitz-Friedman A, Hicklin D and Fuks Z: Expression of basic
fibroblast growth factor in normal human tissues. Lab Invest.
63:832–840. 1990.PubMed/NCBI
|
|
17
|
Sigala F, Savvari P, Liontos M, Sigalas P,
Pateras IS, Papalampros A, Basdra EK, Kolettas E, Papavassiliou AG
and Gorgoulis VG: Increased expression of bFGF is associated with
carotid atherosclerotic plaques instability engaging the NF-κB
pathway. J Cell Mol Med. 14:2273–2280. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin Z, Pan X, Wu F, Ye D, Zhang Y, Wang Y,
Jin L, Lian Q, Huang Y, Ding H, et al: Fibroblast growth factor 21
prevents atherosclerosis by suppression of hepatic sterol
regulatory element-binding protein-2 and induction of adiponectin
in mice. Circulation. 131:1861–1871. 2015. View Article : Google Scholar : PubMed/NCBI
|