Introduction
Premature rupture of membranes (PROM) is a common
complication during pregnancy (1).
According to a previous study, PROM complicates 24.3% of all
pregnancies in Beijing, China (2).
PROM, particularly preterm PROM (PPROM), frequently results in
maternal and perinatal morbidity. The complications of PROM include
premature labor, chorioamnionitis, respiratory distress syndrome,
cord compression, placental abruption and antepartum fetal
mortality (3). Pregnant women with
vaginitis, a history of premature labor or twin pregnancies, have a
higher risk of developing PROM (4). The etiology of PROM is unknown and
its occurrence is unpredictable (5). Therefore, accurate and timely
diagnosis may improve maternal and perinatal outcomes.
In the past 80 years, several techniques to
clinically diagnose PROM have been developed, including microscopic
fetal cell identification, vaginal fluid pH determination,
examination of amniotic fluid (AF) crystallization, intra-amniotic
dye injection and protein marker detection in the vaginal fluid.
The most accurate method to diagnose PROM is with an intra-amniotic
dye injection, using a dye such as indigo carmine. However, this
method is invasive and may cause infection or abortion (5). Fetal cell identification or AF
crystallization examination with a microscope are time-consuming
processes and false positive rates are high. Therefore, vaginal pH
tests and a number of specific PROM marker-based bedside test
products are more popular at present due to their safety,
efficiency and convenience (3,6–9).
However, during clinical practice, patients with suspected PROM
frequently experience vaginal bleeding and excessive blood in the
cervical-vaginal fluid (VF) that may interfere with rapid tests and
result in false positive outcomes, which is concerning for
clinicians.
In order to improve the diagnostic accuracy of PROM,
the present focused on screening for novel PROM biomarkers. As PROM
occurs, AF disseminates into the vagina, and the detection of
unique fetal proteins in the VF may aid in the diagnosis of PROM.
Therefore, in the present study, the proteome profiles of AF and
maternal plasma of pregnant women was assessed by liquid
chromatography coupled with a tandem liquid chromatography-mass
spectrometry (LC-MS/MS)-based proteomic technique. Unique proteins
in AF were screened and preliminarily evaluated for their potential
diagnostic ability in PROM by using ELISA and a Magnetic
Luminex® screening assay to determine a potential
biomarker for PPROM.
Materials and methods
Subjects
The present study was approved by the Ethics
Committees of West China Second University Hospital of Sichuan
University (Sichuan, China) and Shuangliu District Maternal and
Child Health Hospital (Sichuan, China). All women (≥18 years old)
enrolled in the study signed consent forms. In the PPROM/PROM
group, the inclusion criteria were defined as follows: Leaking of
AF observed prior to labor; pH test positive; AF crystallization
test positive and sICAM-1 strip test positive. For intact membrane
group, women who met the following criteria were recruited in the
study: No leaking of AF observed prior to labor; pH test negative;
AF crystallization test negative and sICAM-1 strip test negative
(9,10). Women who had been administered
drugs vaginally in the last 72 h were excluded from both groups. A
total of 133 maternal plasma, 133 AF and 133 VF samples were
collected from January 2015 to October 2016. Among them, 100
maternal plasma and 100 AF samples were collected separately from
pregnant women in their third trimester, and 33 maternal plasma and
33 AF samples were provided by women who selected amniocentesis in
their second trimester. All 133 women were without any complex
pregnancy-associated diseases. A total of 71 VF samples were
obtained from 14 patients with PPROM (<37 weeks; five patients
with vaginal bleeding) and 57 patients with PROM (≥37 weeks; 11
patients with vaginal bleeding). The remaining 62 VF samples were
collected from pregnant women at ≥37 weeks (53 women, five of which
experienced vaginal bleeding) or <37 weeks (nine women, one of
whom experienced vaginal bleeding) with intact membranes. The
clinical characteristics of all subjects are presented in Table I.
 | Table I.Clinical characteristics of the
subjects from whom the AF, maternal plasma and VF samples were
collected. |
Table I.
Clinical characteristics of the
subjects from whom the AF, maternal plasma and VF samples were
collected.
|
| AF | Plasma | VF of PROM | VF of healthy
control |
|---|
|
|
|
|
|
|
|---|
|
| <37 weeks | ≥37 weeks | <37 weeks | ≥37 weeks | <37 weeks | ≥37 weeks | <37 weeks | ≥37 weeks |
|---|
| Maternal age, y | 25.9±3.6 | 27.4±3.8 | 25.9±3.6 | 27.8±4.8 | 26.7±5.6 | 25.7±3.3 | 25.4±3.6 | 27.2±4.8 |
| Mean ± SD
(range) | (19–33) | (22–34) | (19–33) | (19–39) | (19–38) | (20–35) | (20–31) | (20–38) |
| Gestational age at
sample collection, d | 145.8±10.4 |
276.7±6.2a | 145.8±10.4 |
276.9±6.4a | 221.3±34.9 |
274.8±6.7a | 227.6±39.3 |
275.8±8.4a |
| Mean ± SD
(range) | (127–163) | (260–286) | (127–163) | (260–286) | (174–258) | (265–286) | (147–258) | (260–288) |
| Gravidity, n | 2.0±1.0 | 2.3±1.3 | 2.0±1.0 | 2.9±1.6 | 2.9±2.0 | 2.2±1.3 | 1.9±1.4 | 1.8±0.7 |
| Mean ± SD
(range) | (1–4) | (1–5) | (1–4) | (1–6) | (1–7) | (1–5) | (1–5) | (1–3) |
| Parity, n | 0.2±0.4 | 0.4±0.5 | 0.2±0.4 | 0.6±0.7 | 0.6±0.5 | 0.3±0.5 | 0.1±0.3 | 0.4±0.5 |
| Mean ± SD
(range) | (0–1) | (0–1) | (0–1) | (0–2) | (0–1) | (0–1) | (0–1) | (0–1) |
Sample collection
Maternal plasma and AF samples were obtained from
the women during their third trimester by cesarean section. Samples
from women in their second trimester were collected during
amniocentesis. A total of 3 ml EDTA-anticoagulated peripheral whole
blood and 10 ml uncontaminated AF was collected from each woman. VF
samples were collected from the posterior fornix using vaginal
swabs. The swabs were inserted in 5 ml microcentrifuge tubes
containing 1 ml sterile 0.01 M PBS, and rotated for 30 sec. All
samples were centrifuged at 400 × g for 10 min at 4°C, and the
supernatant was removed, aliquoted and stored at −80°C.
LC-MS/MS analysis
A total of 10 maternal plasma and 10 AF samples
which originated from five second trimester women and 10 third
trimester women were separately mixed into a plasma sample and an
AF sample pool. Protein identification in the two samples was
performed using the LC-MS/MS technique. The whole procedure may be
defined as follows. Firstly, the proteins in the two samples were
separately enriched and extracted using ProteoMiner™ kits (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) for LC-MS/MS analysis,
according to the manufacturer's protocol. Secondly, 100 µg total
protein/sample was digested into peptides using trypsin with a
protein/enzyme ratio of 30:1 (w/w) at 37°C for 16 h. Following
digestion, the mixture was acidified by the addition of 10 µl
formic acid. Thirdly, the peptides were fractionated using strong
cation exchange chromatography with a LC-20AB high performance
liquid chromatography pump system (Shimadzu Corporation, Kyoto,
Japan). Peptides were eluted at a flow rate of 1 ml/min with a
gradient of buffer A for 10 min, 5–60% buffer B (25 mM
NaH2PO4, 1 M KCl in 25% CAN; pH 2.7) for 27
min and 60–100% buffer B for 1 min at 25°C. The system was
subsequently maintained at 100% buffer B for 1 min before
equilibration with buffer A for 10 min prior to the next injection.
Elution was monitored by measuring the absorbance at 214 nm and
fractions were collected every 1 min. The eluted peptides were
pooled into 20 fractions, desalted with a Strata X C18 column
(Phenomenex, Inc., Tianjin, China) and vacuum dried. Each fraction
was subsequently resuspended in buffer C (5% ACN, 0.1% FA) and
centrifuged at 20,000 × g for 10 min at 4°C; the final
concentration of peptide was approximately 0.5 µg/µl. Supernatant
(10 µl) was loaded on a LC-20AD nanoHPLC (Shimadzu Corporation) by
the autosampler onto a 2 cm C18 trap column. Peptides were eluted
onto a 10 cm analytical C18 column (inner diameter, 75 µm) packed
in-house. The samples were loaded at 8 µl/min for 4 min. The
gradient was then run at 300 nl/min for 35 min, starting from 2 to
35% buffer D (95% ACN, 0.1% FA), followed by a 5 min linear
gradient to 60% and a 2 min linear gradient to 80%/Buffer D was
maintained at 80% for 4 min, and finally returned to 5% in 1 min.
Data acquisition was performed with a TripleTOF 5600 System
(Shanghai AB SCIEX Analytical Instrument Trading Co., Shanghai,
China) fitted with a Nanospray III source (Shanghai AB SCIEX
Analytical Instrument Trading Co.) and a pulled quartz tip as the
emitter (New Objective, Inc., Woburn, MA, USA). Data were acquired
using a positive-ion spray voltage of 2.5 kV, curtain gas of 30
psi, nebulizer gas of 15 psi and an interface heater temperature of
150°C. MS analysis was performed in a high sensitivity scan mode.
The MS scan range was 350–1,500 m/z. The top 30 precursor ions were
selected into the MS2 scan, and the MS/MS scan range was
350–1,250 m/z. The data were analyzed using the Mascot search
engine (Matrix Science, Ltd., London, UK; version 2.3.02) for
protein identification. Protein function methods were described by
the Gene Ontology (GO) system and the Cluster of Orthologous Groups
of Proteins (COGs) database.
ELISA and Magnetic Luminex®
screening assay
A total of 14 proteins were detected. Sandwich ELISA
kits purchased from RayBiotech, Inc. (Norcross, GA, USA) and
Cloud-Clone Corp. (Houston, TX, USA) were used to analyze AF and
maternal plasma samples for the concentrations of EGF-containing
fibulin-like extracellular matrix protein 1 (EFEMP1; cat. no.
SEF422Hu), keratin type II cytoskeletal 4 (KRT4; cat. no.
SEA489Hu), keratin type II cytoskeletal 6A (KRT6A; cat. no.
SED234Hu), keratin type II cytoskeletal 8 (KRT8; cat. no.
SEC025Hu), keratin type I cytoskeletal 15 (KRT15; cat. no.
SEA517Hu), keratin type I cytoskeletal 17 (KRT17; cat. no.
SEB822Hu), keratin type I cytoskeletal 19 (KRT19; cat. no.
ELH-CYT19-1), BPI fold-containing family A member 1 (BPIFA1 or
PLUNC; cat. no. ELH-PLUNC-1), pulmonary surfactant-associated
protein B (SFTPB; cat. no. SEB622Hu) and zymogen granule protein 16
homolog B (ZG16B; cat. no. SES158Hu). The assays were performed
according to the manufacturers' protocols. Dilutions (100 µl each)
of standard, blank and diluted samples was added into a 96 well
plate in duplicate. Plates were incubated for 2.5 h at room
temperature (RT) followed with gentle for 2 h at 37°C Following
washing, biotinylated antibodies were incubated for 1 h at RT with
gentle shaking at 37°C. Horseradish peroxidase-stretavidin solution
was incubated for 45 min at RT followed by gentle shaking for 30
min at 37°C. Tetramethylbenzidine dihydrochloride substrates were
added into each well for 30 min in the dark. The reactions were
terminated by adding 0.2 M sulfuric acid. The absorbance of each
well was recorded at a wavelength of 450 nm.
The concentrations of insulin-like growth
factor-binding protein 2 (IGFBP2), mesothelin, placental protein 14
(PP14), and serpin family B member 3 (serpin B3) in AF, maternal
plasma and VF samples were determined by Magnetic
Luminex® Screening Assay multiplex kits (R&D
Systems, Inc., Minneapolis, MN, USA). The kits were used according
to the manufacturer's protocol. The microparticle cocktail (50 µl)
was added into a 90 well plate, followed by 50 µl standard and
diluted samples. The microplates were incubated for 2 h at RT with
gentle shaking. A volume of 50 µl diluted biotin antibody cocktail
and diluted streptavidin-phycoerythin were added and incubated at
RT with shaking. The microparticles were re-suspended with 100 µl
wash buffer. Following incubation for 2 min, the microplates were
read using the Luminex® Liquid Chip.
Lateral flow assay development
A lateral flow assay based on colloidal gold
immunochromatography technology was developed to qualitatively
detect PP14. A concentration of 1 mg/ml mouse monoclonal to
anti-pp14 antibody (cat. no. ab17247; Abcam, Cambridge, UK) was
conjugated with 40 nm colloidal gold particles, and the
antibody-gold conjugate was atomized into a glass fiber pad with an
AirJet dispenser (BioDot, Inc., Irvine, CA, USA). A concentration
of 1 mg/ml rabbit anti-pp14 polyclonal antibody (cat. no.
abs124712; Absin, Shanghai, China) and 0.5 mg/ml goat-anti-mouse
immunoglobulin G (cat. no. TA130072; OriGene Technologies, Inc.,
Beijing, China) were atomized as a test line and control line
(BioDot, Inc.), respectively. Recombinant human PP14 protein
(Abcam) was diluted to 0.04, 0.02, 0.01, 0.008, 0.005 and 0.004
µg/ml with 0.01 M PBS. Diluted samples (80 µl) were dropped into
the sample wells of the lateral flow strip, and the results were
subsequently observed within 10 min. A positive result was judged
when the test line and the control line appeared. A negative result
was determined when only the control line was visible.
Statistical analysis
The normality of distribution of continuous
variables was tested by a one-sample Kolmogorov-Smirnov test in
SPSS version 13.0 for Windows (SPSS Inc., Chicago, IL, USA).
Continuous variables with a normal distribution are presented as
the mean ± standard deviation; non-normal variables were reported
as median (interquartile range). The means of two and three or more
groups of continuous normally distributed variables were compared
by independent sample Student's t-test or one-way analysis of
variance (ANOVA), respectively. If the ANOVA was significant,
Holm-Sidak's post-hoc test was used to analyze the difference
between two groups. The Mann-Whitney U-test or Kruskal-Wallis test
was used to compare the means of two and three or more groups of
variables not normally distributed, and Dunn's post hoc test was
performed to determine the difference between two groups if the
P-value of the Kruskal-Wallis test was <0.05. The diagnostic
values of each detected protein were evaluated via Receiver
Operating Characteristic (ROC) curve and the area under the curve
(AUC). Statistical analysis was performed using GraphPad Prism 7
(GraphPad Software Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Protein identification by
LC-MS/MS
Two pooled samples were analyzed by LC-MS/MS. A
total of 648,074 identified spectra [1.09% false discovery rate
(FDR)] and 625,162 identified spectra (0.89% FDR) were obtained in
AF and maternal plasma samples, respectively. 4,343 peptides and
896 proteins were identified in AF samples, and 3,788 peptides and
681 proteins were identified in maternal plasma samples.
COGs analysis illustrated 21 classifications of
proteins in AF and maternal plasma samples. For AF samples,
proteins focused on the functional class O (post-translation
modification, protein turnover and chaperones), class R (general
function prediction) and class G (carbohydrate transport and
metabolism). However, for maternal plasma samples, a greater number
of proteins focused on Class O, Class R and Class Z (cytoskeleton)
(data not shown).
GO analysis demonstrated that there was similar
functional grouping and protein localization for proteins in AF and
maternal plasma. However, the proteins in AF have a unique
molecular function called protein tag, compared with proteins in
maternal plasma (data not shown).
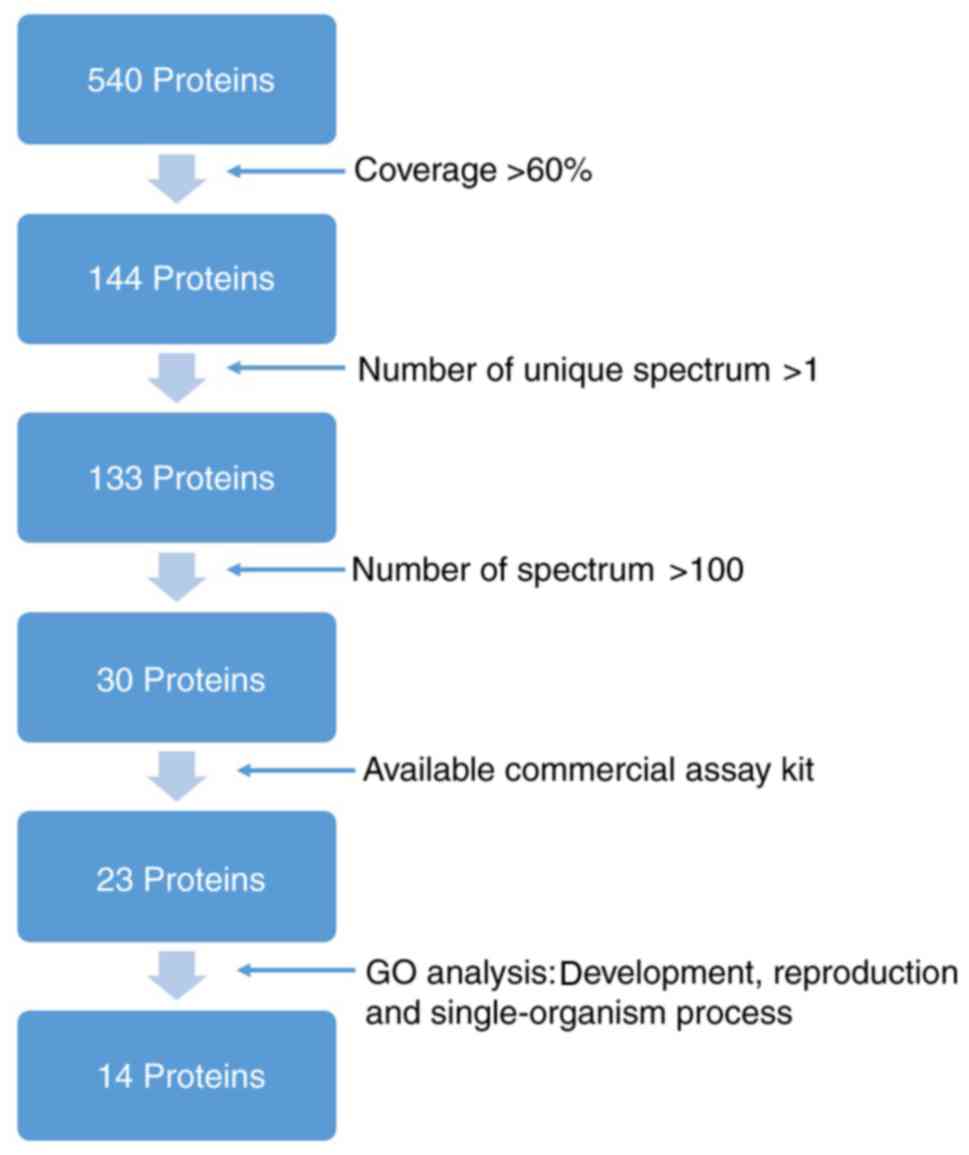
Unique proteins in AF sample
Subsequent to eliminating proteins identified in AF
and maternal plasma, 540 unique proteins were observed for AF (data
not shown). COG functional classification revealed that these
proteins were involved in post-translational modification, protein
turnover, chaperones, general function prediction, carbohydrate
transport and metabolism. The GO system analysis demonstrated that
these proteins were involved in ‘development’, ‘reproduction,
single-organism processes’, ‘organelle’ and ‘binding’ (data not
shown). Proteins EFEMP1, KRT4, KRT6A, KRT8, KRT15, KRT17, KRT19,
PLUNC, SFTPB, ZG16B, IGFBP2, mesothelin, PP14 and serpin B3 were
selected for further evaluation. Criteria for their selection is
presented in Fig. 1.
Expression levels of 14 proteins in AF
and maternal plasma
Expression levels of the 14 proteins in small panel
samples were detected initially. The results demonstrated that the
expression levels of KRT6A, KRT8, KRT15 and KRT17 in maternal
plasma samples were higher compared with in AF samples, and thus
these proteins were excluded for further analysis (data not shown).
The expression levels of SFTPB, PLUNC, ZG16B, EFEMP1, KRT4 and
KRT19 were detected by ELISA (Fig.
2). Additionally, the expression levels of PP14, IGFBP2,
mesothelin and serpin B3 were determined (Fig. 2) using a magnetic
Luminex® screening assay for the AF and maternal plasma
samples. The expression levels of SFTPB and PLUNC in AF
significantly decreased, and PP14 expression levels significantly
increased during the second trimester, compared with those of the
third trimester (P<0.05). In maternal plasma samples, there was
no detection of KRT19 via ELISA. The ELISA kit detected SFTPB in 28
maternal plasma samples (8 from the second trimester and 20 from
the third trimester). For EFEMP1, the expression levels of 18
maternal plasma samples from the second trimester and 95 maternal
plasma samples from the third trimester were undetected. As the
number of samples that were detected was insufficient, for the
purpose of the statistical analysis, the undetected samples were
assigned a value with the minimum corresponding detectable levels
stated by the ELISA kit. The results revealed that no significant
differences were observed between the second and third trimester
groups in the expression levels of all proteins in the maternal
plasma samples (P>0.05). For SFTPB, the expression levels in AF
during the second trimester were not statistically significant
different from the maternal plasma of the second trimester
(P>0.05).
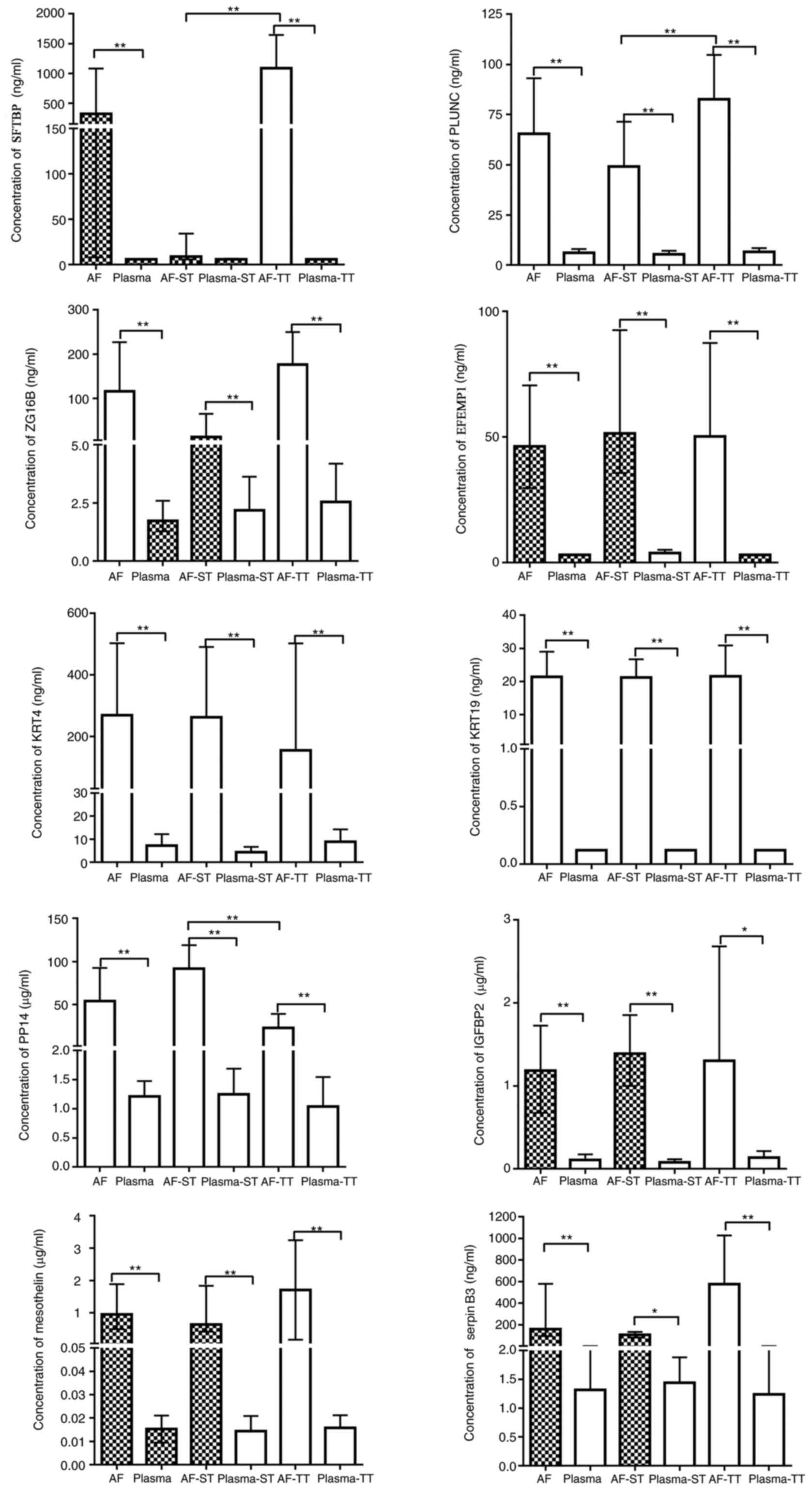 | Figure 2.Concentrations of SFTPB, PLUNC, ZG16B,
EFEMP1, KRT4, KRT19, PP14, IGFBP2, mesothelin and serpin B3 in AF
and maternal plasma. Blank bars mean continuous variables are
normally distributed. Filled bars mean continuous variables are
non-normally distributed. The normal variables are presented as the
mean ± standard deviation (above error bar); the non-normal
variable error bars are presented as the median (interquartile
range). *P<0.05, **P<0.001. SFTPB, pulmonary
surfactant-associated protein B; PLUNC, BPI fold-containing family
A member 1; ZG16B, zymogen granule protein 16 homolog B; EFEMP1,
EGF-containing fibulin-like extracellular matrix protein 1; KRT4,
keratin, type II cytoskeletal 4; KRT19, keratin, type I
cytoskeletal 19; PP14, placental protein 14; IGFB2, insulin-like
growth factor-binding protein 2; serpin B3, serpin family B member
3; AF, amniotic fluid; ST, second trimester; TT, third
trimester. |
Expression levels of KRT19, PP14,
IGFBP2, mesothelin and serpin B3 in VF samples
As the expression level of SFTPB in AF of the second
trimester was not significantly different compared with that in
maternal plasma in the same trimester, PLUNC, ZG16B, EFEMP1, KRT4,
KRT19, PP14, IGFBP2, mesothelin and serpin B3 were considered more
valuable for evaluating VF samples. Unfortunately, since the
expression levels of the majority of samples were below the minimum
detectable limit of the ELISA kit, no valid data were obtained for
the expression levels of PLUNC, ZG16B, EFEMP1 and KRT4 in VF
samples. The results (Fig. 3)
demonstrated that the expression levels of KRT19 and serpin B3 were
not significantly different in the overall PROM groups compared
with the control groups, PROM for ≥37 weeks vs. control for ≥37
weeks, or PPROM for <37 weeks vs. control for <37 weeks
(P>0.05). Expression levels of mesothelin and IGFBP2 in the PROM
group were significantly increased compared with those in the
control group (P<0.05). However, following <37 weeks and ≥37
weeks classification, the expression levels of mesothelin and
IGFBP2 in the PPROM groups were not significantly different
compared with those in the control for <37 weeks (P>0.05),
but were significantly different at ≥37 weeks compared with the
control group (P<0.05). Therefore, mesothelin and IGFBP2 were
observed to be more suitable for PROM diagnosis, occurring at ≥37
weeks of pregnancy. As for PP14, the expression level was
significantly increased in the PROM group compared with the control
group at either <37 weeks or ≥37 weeks (P<0.05).
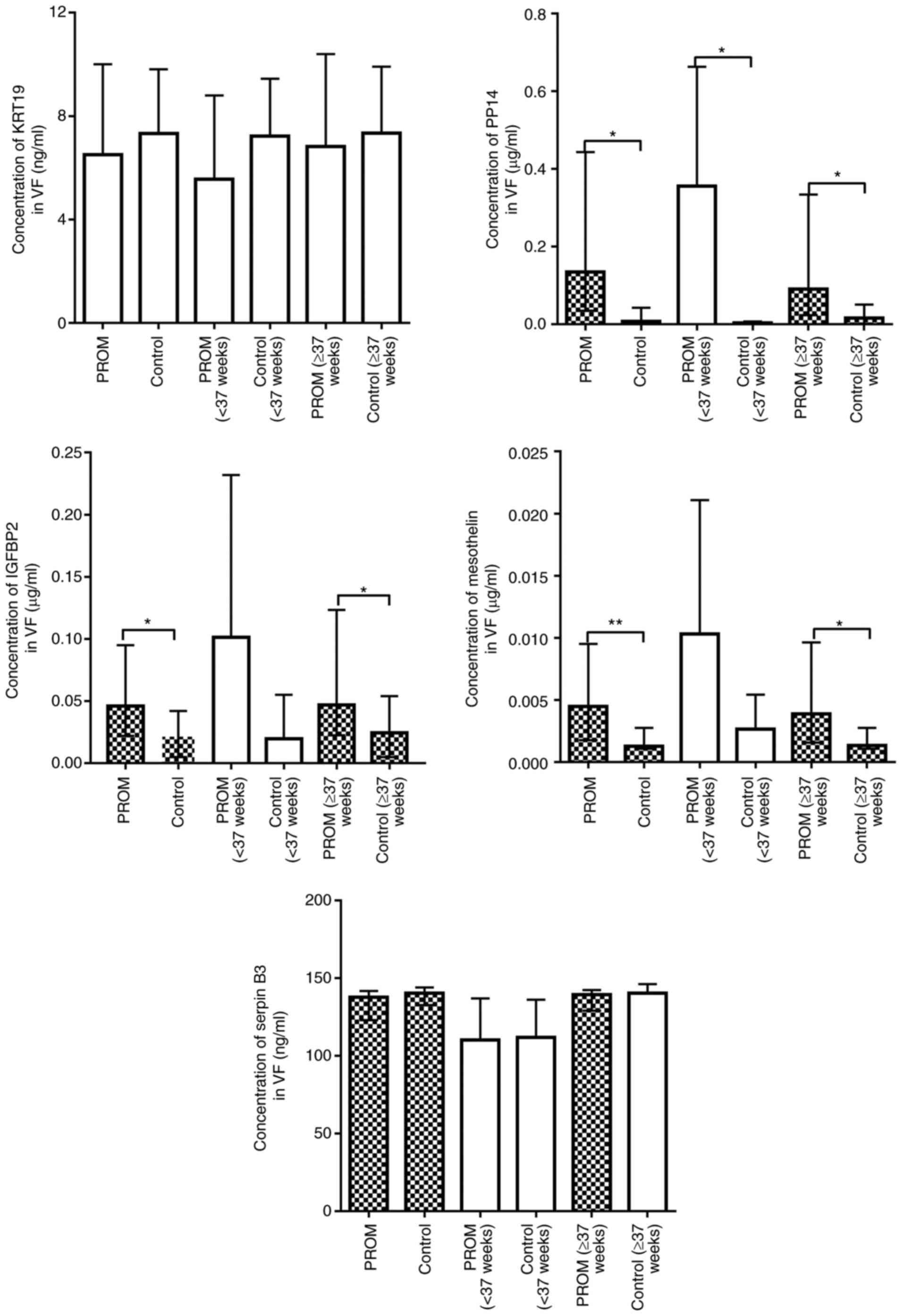 | Figure 3.Concentrations of KRT19, PP14, IGFBP2,
mesothelin and serpin B3 in the VF of women with overall PROM, PROM
(<37 weeks) and PROM (≥37 weeks) and overall control, control
(<37 weeks) and control (≥37 weeks). Blank bars mean continuous
variables are normally distributed. Filled bars mean continuous
variables are non-normally distributed. The normal variables are
presented as the mean ± standard deviation (above error bar); the
normal variables are presented as the median (interquartile range).
*P<0.05, **P<0.001. KRT19, keratin, type I cytoskeletal 19;
PP14, placental protein 14; IGFBP2, insulin-like growth
factor-binding protein 2; serpin B3, serpin family B member 3; VF,
vaginal fluid; PROM, premature rupture of membranes. |
To investigate whether the expression levels of
mesothelin, IGFBP2 and PP14 in VF samples may have been altered due
to blood contamination, analysis of the data in blood-contaminated
and blood-free VF samples was conducted (Table II). The results indicated that the
expression levels of mesothelin, IGFBP2 and PP14 in VF samples of
the PROM (either <37 weeks or ≥37 weeks) or control (≥37 weeks)
groups was not significantly different in blood-contaminated
samples compared with blood-free samples (P>0.05). There was
only one blood-contaminated VF sample in control (<37 weeks),
thus the data are not presented. These results indicated that
mesothelin, IGFBP2, and PP14 may be potential biomarkers for
diagnosing PROM.
 | Table II.Concentration of mesothelin and PP14
in blood-contaminated or blood-free vaginal VF samples. |
Table II.
Concentration of mesothelin and PP14
in blood-contaminated or blood-free vaginal VF samples.
|
| PROM ≥37 weeks;
blood-contaminated | PROM ≥37 weeks;
blood-free | Control ≥37 weeks;
blood-contaminated | Control ≥37 weeks;
blood-free | PROM <37 weeks;
blood-contaminated | PROM <37 weeks;
blood-free |
|---|
| Mesothelin,
µg/ml | 0.006±0.008 |
0.007±0.007a | 0.002±0.001 | 0.001
(0.001,0.003) | 0.005±0.003 | 0.016±0.008 |
| IGFBP2, µg/ml | 0.044±0.059 | 0.047
(0.024,0.106)a | 0.069±0.091 | 0.030±0.033 | 0.035±0.030 | 0.168±0.163 |
| PP14, µg/ml | 0.197±0.254 | 0.063
(0.015,0.239)a | 0.115±0.095 | 0.015
(0.002,0.068) | 0.521±0.273 | 0.160±0.279 |
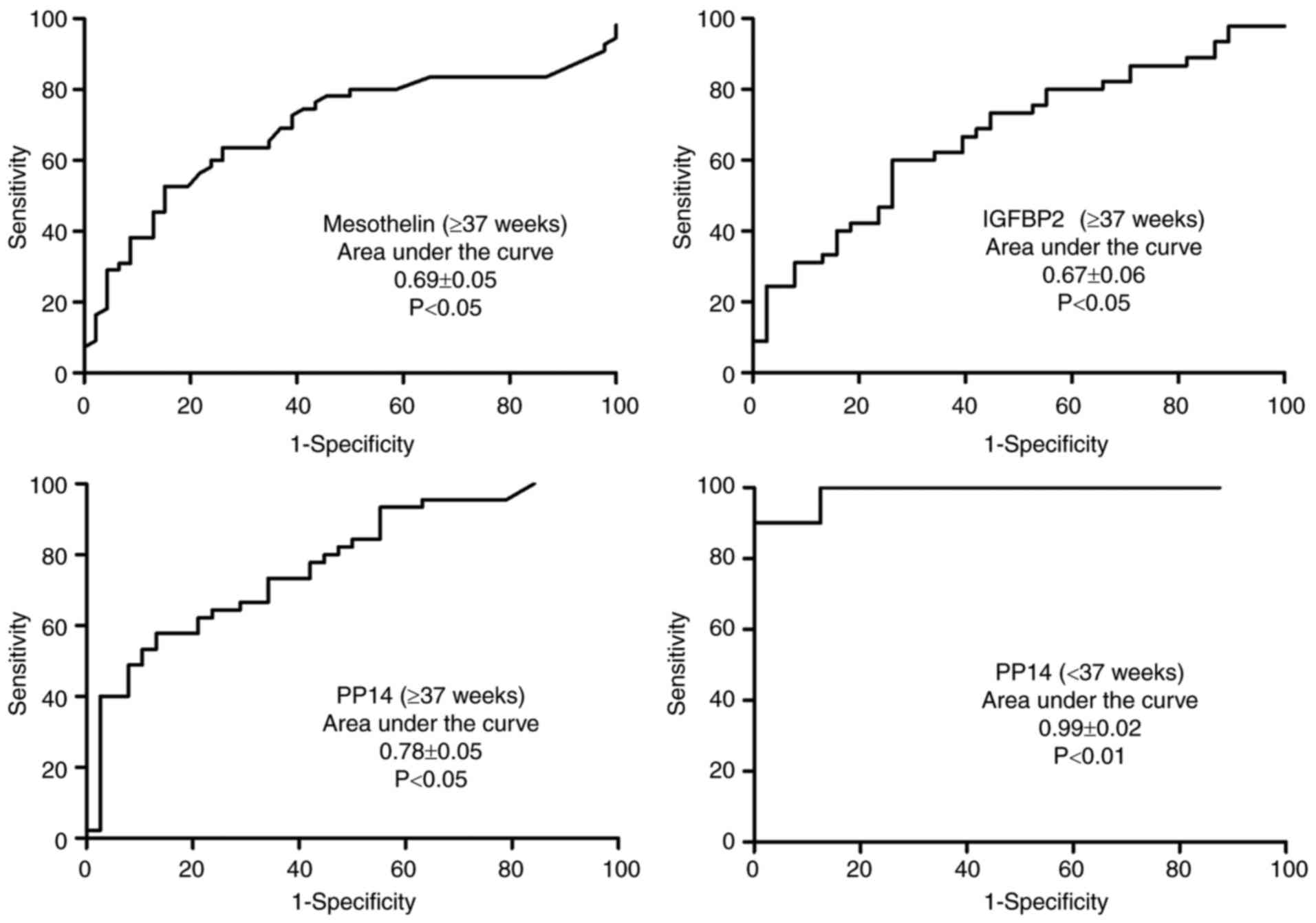
Diagnostic value of mesothelin,
IGFBP2, and PP14 for PROM
The ROC curve was used to evaluate the diagnostic
values of mesothelin, IGFBP2 and PP14 for PROM (Fig. 4). PP14 was observed to have an
excellent diagnostic accuracy for PPROM, with a respective
sensitivity and specificity of 100 and 87.5% for a cutoff value of
0.008 µg/ml (AUC of 0.99±0.02, P<0.001).
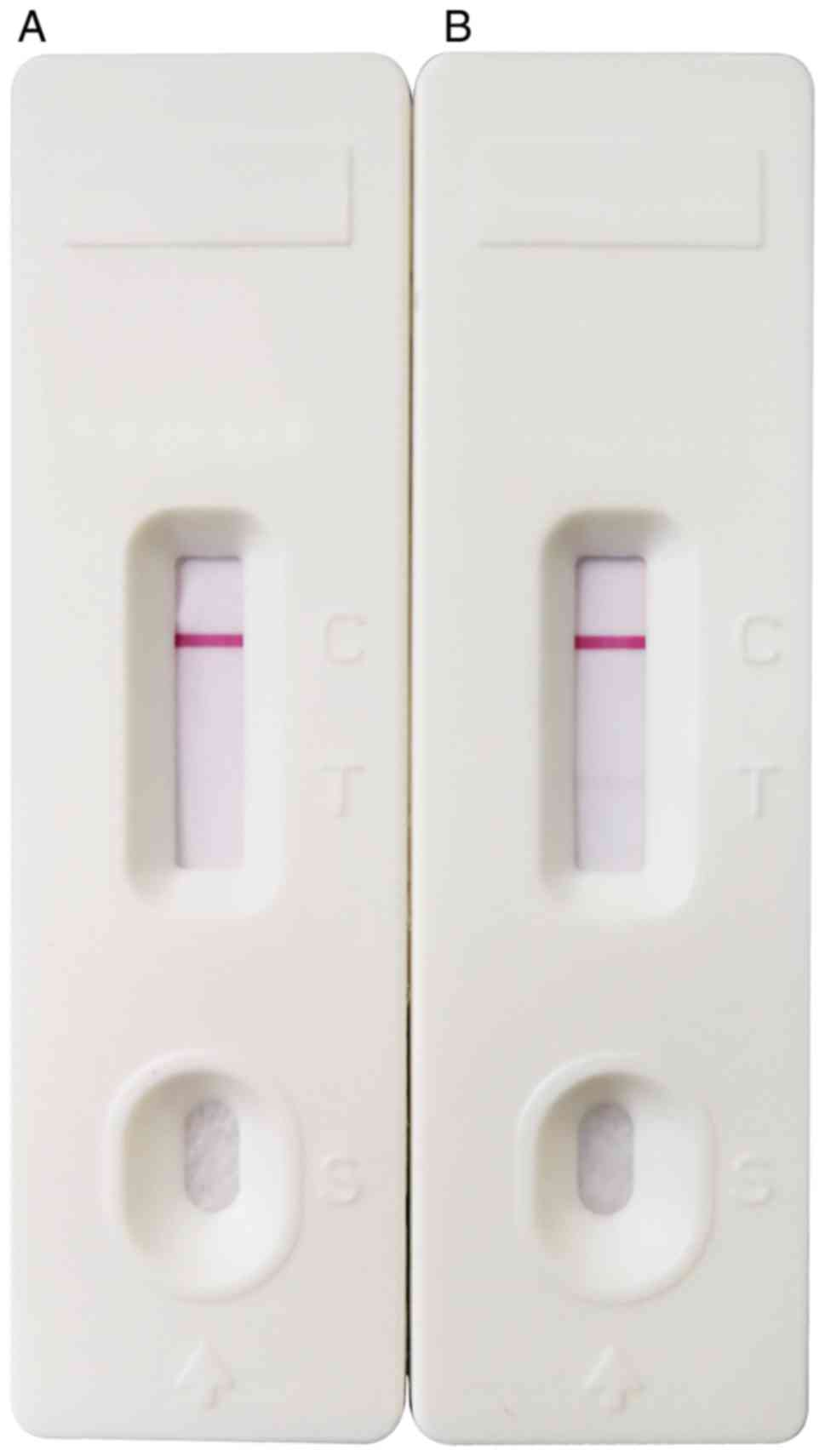
PP14-based lateral flow assay
The human recombinant PP14 protein was used to
determine the visible threshold of the PP14 strip. Different
concentrations (0.04, 0.02, 0.01, 0.008, 0.005 and 0.004 µg/ml) of
PP14 diluted with 0.01 M PBS were added to the test strip, and
detection was performed three times for each sample. The results
demonstrated that the PP14-based strip may be effective in clinical
use and had a detection threshold of 0.008 µg/ml (Fig. 5).
Discussion
In a previous study, a biomarker [soluble
intercellular adhesion molecule-1 (sICAM-1)] was identified for the
diagnosis of PROM, using a cytokine antibody array (9). A rapid test strip for clinical use
was developed, based on colloidal gold immunochromatography
technology. The validity of the strip was 95% (10). The product was approved for access
to the medical market by China's SFDA. However, a limitation of the
product is that the results may be false positive if the VF is
contaminated with blood. Therefore, an aim of the present study was
to discover novel biomarkers that are not influenced by blood
contamination using LC-MS/MS-based proteomic techniques.
As an excellent biomarker for diagnosing PROM,
sICAM-1 was observed to have high expression in AF and low
expression in VF with an intact fetal membrane throughout the whole
pregnancy. Once PROM occurred, AF was disseminated into the vagina
and diluted by VF; sICAM-1 was observed to have high detectable
expression levels in VF of PROM, regardless of the existence of
blood. AF contains numerous proteins; certain proteins are derived
from maternal blood and others from the fetus (11–13).
Proteins derived from the fetus are more suitable PROM biomarkers.
Therefore, a number of proteins were selected that primarily have
functions associated with development or reproduction, for further
quantitative detection and evaluation of diagnostic value.
In the present study, 14 unique proteins in AF were
examined according to the selection criteria. The results
demonstrated that the expression levels of these 14 unique proteins
could be detected in AF samples and maternal plasma samples, with
KRT6A, KRT8, KRT15 and KRT17 exhibiting higher expression levels in
maternal plasma samples compared with AF samples, which appeared to
contradict the results of the LC-MS/MS analysis. This may be
explained by the characteristics of ELISA. It known that false
positive signals from ELISA may be caused by heterophilic
antibodies, which in the case of the present study were the human
anti-mouse antibodies in the blood (14). The intensity of the signal is
easily influenced by ambient temperature, as higher temperatures
are able to increase the optical density value. The quality of the
ELISA kit may additionally interfere with the results.
The present results demonstrated that mesothelin,
IGFBP2 and PP14 had potential value for diagnosing PROM. Mesothelin
is a 40-kDa membrane-glycoprotein; it is reported to associate with
a number of types of cancer, although, its biological function in
normal conditions is unclear (15). IGFBP2 is a 36-kDa protein, which is
primarily involved in metabolic disease and cancer (16). PP14 is a secretory glycoprotein
produced by the endometrium during pregnancy and has a high
expression level in AF (17). The
results of the present study demonstrated that mesothelin and
IGFBP2 had insufficient ability to diagnose PPROM, and PP14 was the
superior biomarker for diagnosing PPROM, with a respective
sensitivity and specificity of 100 and 87.5% with a cutoff value of
0.008 µg/ml. The present study revealed that the expression level
of PP14 in AF during the second trimester was significantly higher
compared with in the third trimester. This was in agreement with
previous studies that the concentration of PP14 in AF reached its
peak at 18–20 weeks of gestation, and subsequently decreased
(18,19). This may explain why PP14 had a
higher accuracy in diagnosing PPROM compared with PROM (≥37 weeks).
Furthermore, the concentration of PP14 in VF samples was stable in
blood-contaminated VF and blood-free VF samples, which is an
advantage for the future clinical use of PP14.
As the cutoff value of PP14 for diagnosis of PPROM
was at the level of µg/ml, a colloid-gold lateral flow strip was
developed to rapidly test PP14. The strip was revealed to have a
detection threshold of 0.008 µg/ml, which was in accordance with
the cutoff value of PP14. This strip may become a useful and rapid
tool to supply indicatory information for diagnosing PPROM in
hospitals, particularly in vaginal bleeding-complicated cases.
However, there were five limitations to the present
study. First, no sufficient volume of VF samples for LC-MS/MS
analysis was available. As 50 ml VF samples was required, it was
impossible to obtain the proteome profile of VF samples. Second, a
total of 540 unique proteins were identified in AF samples.
However, it was impossible to examine all of them. Accordingly,
only 14 proteins of interest were selected for confirmation
analysis, and thus it is possible that some effective biomarkers
were missed. Third, the sample size of PPROM was small. A greater
number of samples are required for further verification that PP14
may be an excellent biomarker for PPROM, which is not influenced by
blood contamination. Fourth, the accuracy of the PP14 strip for
clinical samples of PPROM requires confirmation using large
samples, and multiple-center and single-blind clinical trials. As a
standard clinical trial must be approved by China SFDA, this may be
a future direction. Fifth, the expression levels of STFBP, KRT19
and EFEMP1 in maternal plasma samples, and PLUNC, ZG16B, EFEMP1 and
KRT4 levels in VF samples were undetectable, so accurate levels
could not be determined. This may have been caused by insufficient
ELISA kit sensitivity.
To the best of the authors' knowledge, the present
study was the first study to provide data on the expression levels
of SFTPB, PLUNC, ZG16B, EFEMP1, KRT4, KRT19, PP14, IGFBP2,
mesothelin and serpin B3 in AF and maternal plasma during the
second and third trimester of pregnancy. Expression levels of
KRT19, PP14, IGFBP2, mesothelin and serpin B3 in PROM and non-PROM
VF samples in the Chinese population may aid the understanding of
physiological processes experienced by pregnant women. Finally, the
present study suggested that PP14 may be a novel potential
biomarker for PPROM and that the PP14-based strip may be a helpful
bedside test for rapidly diagnosing PPROM.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81501261),
the Sichuan Provincial Science & Technology Project (grant nos.
2016SZ0013 and 2015SZ0054-2) and the Office of Science &
Technology of Chengdu (grant no. 2015-HM01-00431-SF).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW made substantial contributions to conception and
design of the study. HL made substantial contributions to data
acquisition and was involved in drafting the manuscript. GC, BZ, LG
and TW analyzed and interpreted the data. YL, JG and QY collected
samples and critically revised the manuscript for important
intellectual content. YuL was accountable for all aspects of the
work, in ensuring that questions related to the accuracy or
integrity of the research were appropriately investigated and
resolved, and analyzed the data. LZ made substantial contributions
to conception and design of the study, data acquisition, revised
the manuscript critically for important intellectual content, and
gave final approval of the version to be published. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committees of West China Second University Hospital of Sichuan
University (Sichuan, China) and Shuangliu District Maternal and
Child Health Hospital (Sichuan, China). All subjects signed consent
forms.
Consent for publication
All patients provided written informed consent for
the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mariona FG and Cabero L: Are we ready for
a new look at the diagnosis of premature rupture of membranes? J
Matern Fetal Neonatal Med. 25:403–407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang HL, Wang X and Zhang WY:
Investigation of premature rupture of membrane in Beijing area.
Chin J Clin. 74–76. 2015.(In Chinese).
|
|
3
|
Medina TM and Hill DA: Preterm premature
rupture of membranes: Diagnosis and management. Am Fam Physician.
73:659–664. 2006.PubMed/NCBI
|
|
4
|
Waters TP and Mercer B: Preterm PROM:
Prediction, prevention, principles. Clin Obstet Gynecol.
54:307–312. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
El-Messidi A and Cameron A: Diagnosis of
premature rupture of membranes: Inspiration from the past and
insights for the future. J Obstet Gynaecol Can. 32:561–569. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee SM, Lee J, Seong HS, Lee SE, Park JS,
Romero R and Yoon BH: The clinical significance of a positive
Amnisure test in women with term labor with intact membranes. J
Matern Fetal Neonatal Med. 22:305–310. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liang DK, Qi HB, Luo X, Xiao XQ and Jia
XY: Comparative study of placental α-microglobulin-1, insulin-like
growth factor binding protein-1 and nitrazine test to diagnose
premature rupture of membranes: A randomized controlled trial. J
Obstet Gynaecol Res. 40:1555–1560. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abdelazim IA, Abdelrazak KM, Al-Kadi M,
Yehia AH and Abdulkareem AF: Fetal fibronectin (Quick Check fFN
test) versus placental alpha microglobulin-1 (AmniSure test) for
detection of premature rupture of fetal membranes. Arch Gynecol
Obstet. 290:457–464. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang T, Zhou R, Zhang L, Wang Y, Song CP,
Lin W, Niu X, Lin Y and Hu H: Proteins in leaked amniotic fluid as
biomarkers diagnostic for prelabor rupture of membranes. Proteomics
Clin Appl. 5:415–421. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang T, Zhou R, Xiong W, Wang Y, Zhu C,
Song C, Gao L, Zhang L and Hu H: Clinical evaluation of soluble
intercellular adhesion molecule-1 and insulin like growth
factor-binding protein-1-based rapid immunoassays for the diagnosis
of prelabor rupture of membranes. J Perinat Med. 41:181–185. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tong XL, Wang L, Gao TB, Qin YG, Qi YQ and
Xu YP: Potential function of amniotic fluid in fetal
development-novel insights by comparing the composition of human
amniotic fluid with umbilical cord and maternal serum at mid and
late gestation. J Chin Med Assoc. 72:368–373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen WW: Studies on the origin of human
amniotic fluid cells by immunofluorescent staining of keratin
filaments. J Med Genet. 19:433–436. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tydén O, Bergström S and Nilsson BA:
Origin of amniotic fluid cells in mid-trimester pregnancies. Br J
Obstet Gynaecol. 88:278–286. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grebenchtchikov N, Sweep CG,
Geurts-Moespot A, Piffanelli A, Foekens JA and Benraad TJ: An ELISA
avoiding interference by heterophilic antibodies in the measurement
of components of the plasminogen activation system in blood. J
Immunol Methods. 268:219–231. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baldo P and Cecco S: Amatuximab and novel
agents targeting mesothelin for solid tumors. Onco Targets Ther.
10:5337–5353. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Russo VC, Azar WJ, Yau SW, Sabin MA and
Werther GA: IGFBP-2: The dark horse in metabolism and cancer.
Cytokine Growth Factor Rev. 26:329–346. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rachmilewitz J, Riely GJ, Huang JH, Chen A
and Tykocinski ML: A rheostatic mechanism for T-cell inhibition
based on elevation of activation thresholds. Blood. 98:3727–3732.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bischof P: Three pregnancy proteins (PP12,
PP14, and PAPP-A): Their biological and clinical relevance. Am J
Perinatol. 6:110–116. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chatzakis K, Wathen N, Campbell J, Iles R,
Dawnay A and Chard T: Dramatic increase in levels of placental
protein 14 in amniotic fluid at 10–15 weeks' pregnancy. Early Hum
Dev. 36:113–116. 1994. View Article : Google Scholar : PubMed/NCBI
|