Introduction
Osteoarthritis (OA) also known as degenerative
arthritis or proliferative arthritis is a joint disease
characterized by the cartilage progressive destruction, which
results from the excessive degradation of cartilage extracellular
matrix components (1). The risk
factors of OA is various, which can be classified as person-level
factors, such as sex, age, race, genetics, and diet, and
joint-level factors involving malalignment, injury, and abnormal
loading of the joints (2). Though
OA leads to great suffering to patients, seriously affects their
life qualities, and further raises a serious burden on the entire
social economy. However, there is no drug that is able to
effectively delay or prevent the progression of OA. Joint
replacement is the only medical treatment during the middle and
late stages of OA diease. However, studies have shown that synovial
inflammation plays a crucial role in the development and
progression of OA. Due to the high expression of inflammatory
mediators in early OA synovial tissues, acute synovitis may be the
origin of OA (3). Synovial
membrane is a special type of cementitious tissue that consists of
a lining layer, a lining under-layer, and an outer edge of the
lining fuses with the joint capsule (4). Synovial cells can be divided into
macrophages, fibroblasts, and dendritic cells. In addition,
synovial fibroblasts (SFBs) can secrete collagen, fibronectin,
osteonectin (ON), and hyaluronic acid (5). It has also been reported that SFBs
can generate multiple cytokines, involving osteoprotegerin (OPG)
and receptor activator of nuclear factor-κB ligand (RANKL)
(6,7). Hence, the SFBs from joints may become
the key in OA joint treatment.
As a prevalent membrane-bound glycoprotein, alkaline
phosphatase (ALP) promotes the hydrolysis of phosphate monoesters
at basic pH values (8). ALP is
expressed in several osteocytes, including osteoblasts,
osteoclasts, and bone marrow stromal cells (9–11).
Studies have found that the activity of ALP was closely associated
with the bone formation (12),
mineral bone disorder (13), and
osteogenic differentiation (14).
Although ALP is expressed in many mammalian tissues and has been
studied for several years, it is still little known. Moreover,
regulatory mechanisms of ALP in the ossification of SFBs in OA are
still little known to us.
A large number of co-receptors, receptors, ligands,
and regulatory components are involved in the complex Wnt pathway
(15) that proved to participate
in several evident signaling events, such as β-catenin signaling
activation (16). Researchers
found that Wnt pathway modulates the maturation, differentiation as
well as the apoptosis of osteoblasts and osteoclasts, therefore
maintaining the balance of organism's bone metabolism (17). There are usually two types of Wnt
pathways, which are the classical β-catenin-dependent pathway and
non-classical β-catenin-independent pathway (18). In the classical β-catenin-dependent
Wnt pathway, dishevelled acts as a key molecule that upregulates
the Wnt pathway (19). A recent
research indicates that the Wnt pathway may serve as a target for
OA therapy (20). SFBs, as one of
the bone progenitor cells, have a strong ability of reproduction
division in vitro in organizational engineering. Moreover,
FB could be directly turned into bone cells without OB cells, and
which was recognized as a most effective way in bone formation
(21). Therefore, it was important
to further explore the exact mechanisms of Wnt pathway in
osteogenic differentiation of SFBs.
Dishevelled (Dvl) is one of the cytoplasmic
proteins, and it servea as a pivotal hub in signaling intermediates
through a number of different signaling pathways of Wnt family
(22). Three dishevelled
homologs-Dvl-1/2/3 are expressed in human and mice. Dvl-2 has
effective impacts on the progressions of gliomas, prostate tumor
and esophageal squamous cell carcinoma (23–25).
Due to the osculating relationship between Dvl and Wnt pathway
(19), we thereby set out to
investigate the definite roles and mechanisms of Dvl-2 in the
ossification of SFBs in OA via regulating the Wnt pathway.
In the current study, we analyzed the correlation
between Dvl-2 and the ossification of SFBs in OA. Furthermore, it
was also fascinating to investigate the exact role and mechanisms
of Dvl-2 together with Wnt pathway in the ossification of SFBs in
OA.
Materials and methods
Cell culture, genes, plasmids, and
inhibitor
Human SFBs were obtained as previously described
(26). Synovial membranes were
obtained from 16 OA patients (mean age, 65±4.5 years) during
arthroplastic surgery with the informed consent from patient and
the approval of Ethics Committee of Jining No. 1 People's Hospital
(Jining, China). The synovial membranes collected from end-stage
joint narrowed space of hip and knee joints. The dissected tissues
were incubated in DMEM (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing 1 mg/ml collagenase (Wako Pure
Chemical Industries, Ltd., Osaka, Japan) with shaking for 90 min at
37°C. The cells were centrifuged at 400 × g at 20°C for 30 min.
Then, released SFBs were maintained in tissue culture flasks at
37°C for 1 h. Then, SFBs were incubated in DMEM supplemented with
10% heat inactivated FBS (Gibco; Thermo Fisher Scientific, Inc.),
10% penicillin/streptomycin in a 5% CO2 atmosphere at
37°C. Dvl-2 RNA and Dvl-2 siRNA were respectively cloned into 2
pcDNA3.1(+) empty vectors-Dvl-2 (Invitrogen; Thermo Fisher
Scientific, Inc.) and si-Dvl-2. IWR-1-endo (Beyotime, Shanghai,
China) was used as a Wnt inhibitor.
Grouping
Control group (SFBs), NC group (SFBs transfected
with empty vector), Dvl-2 group (SFBs transfected with Dvl-2), and
si-Dvl-2 group (SFBs transfected with si-Dvl-2) were prepared as
four treatment groups in this study. At least three independent
experiments were performed.
Cell viability analysis
Cell Counting Kit-8 (CCK-8; Beyotime) was used to
determine SFBs' cell viability. About 6×104 cells/ml of
SFBs in the logarithmic phase were sowed into the wells of 96-well
plates, and then maintained in a 5% CO2 atmosphere at
37°C for 12 h. Afterwards, SFBs were handled as described above.
Cells were then maintained for 12, 24, and 48 h respectively. 10 µl
of CCK reagent was then added into the wells. After that, cells
were maintained for 3 h. The absorbance at 450 nm was read by a
Microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Cell viability was evaluated by the percentage of cell
survival.
Western blot analysis
A total of 12% sodium dodecyl sulfate polyacrylamide
gel electrophoresis (SDS-PAGE) was used to segregate proteins
lysates of cultured SFBs, which were then transferred to a PVDF
membrane (EMD Millipore, Billerica, MA, USA). Blotting was carried
out by using specific antibodies (anti-Dvl-2, dilution, 1:1,000,
cat. no. ab22616, rabbit anti-human; anti-OPG, dilution, 1:1,000,
cat. no. ab73400, rabbit anti-human; anti-RANKL, dilution, 1:1,000,
cat. no. ab9957, rabbit anti-human; anti-ALP, dilution, 1:1,000,
cat. no. ab83259, rabbit anti-human; anti-ON, dilution, 1:1,000,
cat. no. ab8448, rabbit anti-human; anti-osteocalcin (OCN),
dilution, 1:500, cat. no. ab93876, rabbit anti-human; anti-osterix,
dilution, 1:1,000, cat. no. ab22552, rabbit anti-human; anti-Wnt3a,
dilution, 1:1,000, cat. no. ab28472, rabbit anti-human;
anti-β-catenin, dilution, 1:5,000, cat. no. ab32572, rabbit
anti-human; anti-Runx-2, dilution, 1:1,000, cat. no. ab23981,
rabbit anti-human; anti-β-actin, dilution, 1:2,000, cat. no.
ab8227, rabbit anti-human; all from Abcam, Cambridge, UK).
Horseradish peroxidase-conjugated secondary antibodies (dilution,
1:5,000, cat. no. ab205718, goat anti-rabbit; Abcam) were
supplemented and incubated for 1 h at room temperature. Enhanced
chemiluminescent reagents (EMD Millipore) using an ECL system
(Amersham; GE Healthcare, Chicago, IL, USA) were performed on the
evaluation of results.
Reverse transcription-quantitative
reverse transcription PCR (RT-qPCR) analysis
Total RNA was extracted from cultured SFBs by TRIzol
reagent (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China). RNA was reverse transcribed to cDNA by Reverse
Transcription kit (Beijing Solarbio Science & Technology Co.,
Ltd.) according to the direction. RT-qPCR analysis was performed on
ABI 7500 Thermocycler (Applied Biosystems Thermo Fisher Scientific,
Inc.). PCR cycling conditions were as follows: One pretreatment at
95°C for 10 min, 94°C for 15 sec, 62°C for 45 sec (45 cycles), 94°C
for 15 sec, 62°C for 1 min, 95°C for 15 sec, a final extension at
75°C for 10 min and was held at 4°C. Relative expressions of target
genes were calculated by 2−ΔΔCT method (27). The primers were purchased from
Shanghai Invitrogen Biotechnology Co., Ltd. (Shanghai, China):
Dvl-2 forward, 5′-CATCCAGCCAATTGACCCTG-3′ and reverse,
5′-AGGGATGGTGATCTTGAGCC-3′ (product, 241 bp); OPG forward,
5′-GGCACCAAAGTAAACGCAGA-3′ and reverse, 5′-TCCCGGTAAGCTTTCCATCA-3′
(product, 228 bp); RANKL forward, 5′-CGCTCGTGTTTCTGGACATC-3′ and
reverse, 5′-GGGGCTGCAGTATAGACACT-3′ (product, 233 bp); ALP forward,
5′-CCTCTTCCCCTTCCTGGTG-3′ and reverse, 5′-GATGCCACAAGTGTCAGGAC-3′
(product, 196 bp); ON forward, 5′-CAACGAAAGCCATGACCACA-3′ and
reverse, 5′-ACCTCGGCCATCATATGTGT-3′ (product, 247 bp); OCN forward,
5′-GCAGAGTCCAGCAAAGGTG-3′ and reverse, 5′-TCACAGTCCGGATTGAGCTC-3′
(product, 161 bp); osterix forward, 5′-TCTCTGGACATGACACACCC-3′ and
reverse, 5′-AGGGGAGCAAAGTCAGATGG-3′ (product, 233 bp); Wnt3a
forward, 5′-ATCGAGTTTGGTGGGATGGT-3′ and reverse,
5′-CGCTGTCGTACTTGTCCTTG-3′ (product, 238 bp); β-catenin forward,
5′-AGTTCCTTACCGTCCCCAAG-3′ and reverse, 5′-CAGACACGCCTGTTTCGAAT-3′
(product, 249 bp); Runx-2 forward, 5′-ATTCTGCTGAGCTCCGGAAT-3′ and
reverse, 5′-AGCTTCTGTCTGTGCCTTCT-3′ (product, 211 bp); and β-actin
forward, 5′-GTTACAGGAAGTCCCTCACCC-3′ and reverse,
5′-CAGACCTGGGCCATTCAGAAA-3′ (product, 194 bp). β-actin was used as
the control of the input RNA level.
Statistical analysis
Results are shown as the mean ± SD. Following
Dunnet's test, all experimental data were analyzed by one-way
analysis of variance followed by Dunnett's multiple comparisons
post hoc test. GraphPad Prism version 6.0 (GraphPad Software, Inc.,
La Jolla, CA, USA) was used to perform the statistical analysis.
The statistical significance was defined as P<0.05.
Results
Over-expression and interference of
Dvl-2 in SFBs
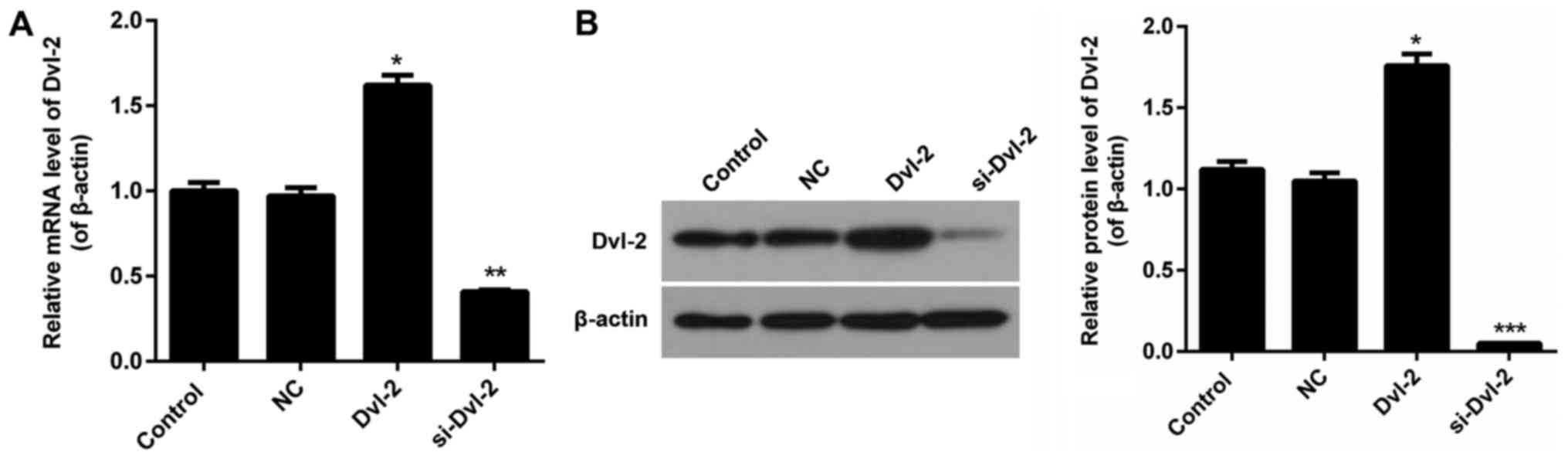
RNA and siRNA vectors targeting Dvl-2 gene named
Dvl-2 and si-Dvl-2 were constructed in our study. After being
transfecting with Dvl-2, the expression level of Dvl-2 in SFBs was
clearly upregulated, and the knockdown efficiency was about 60% in
SFBs after being stably transfected with si-Dvl-2 (P<0.05;
Fig. 1A and B).
Dvl-2 silence reduced the cell
viability of SFBs
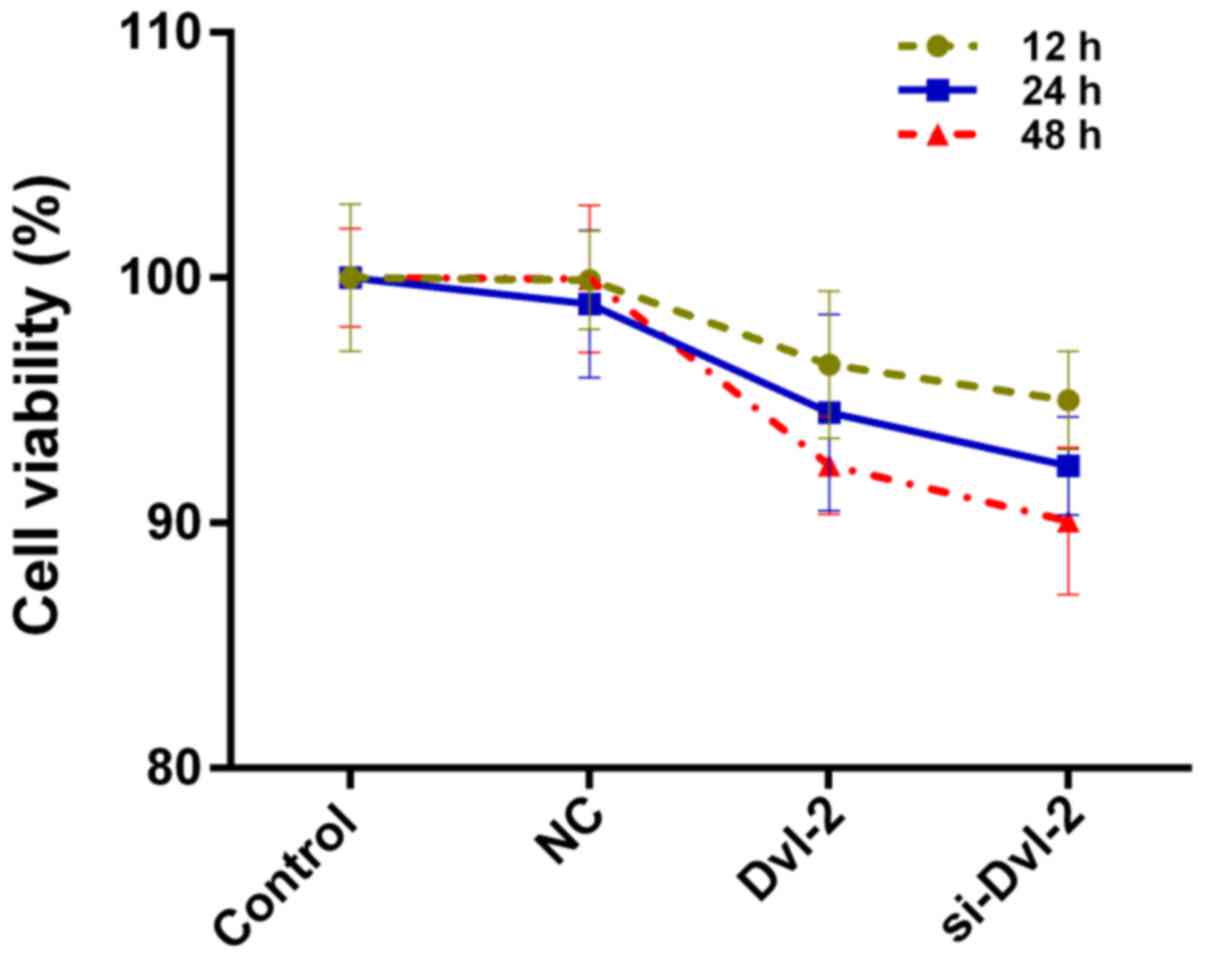
CCK-8 assay was carried out to determine the cell
viability of SFBs coped with the treatment groups as previously
described. The results shown that both the over-expression of Dvl-2
over-expression and depletion of Dvl-2 slightly reduced the cell
viability of SFBs (Fig. 2). No
significant difference was found among these groups.
Dvl-2 affected the activity of ALP in
SFBs
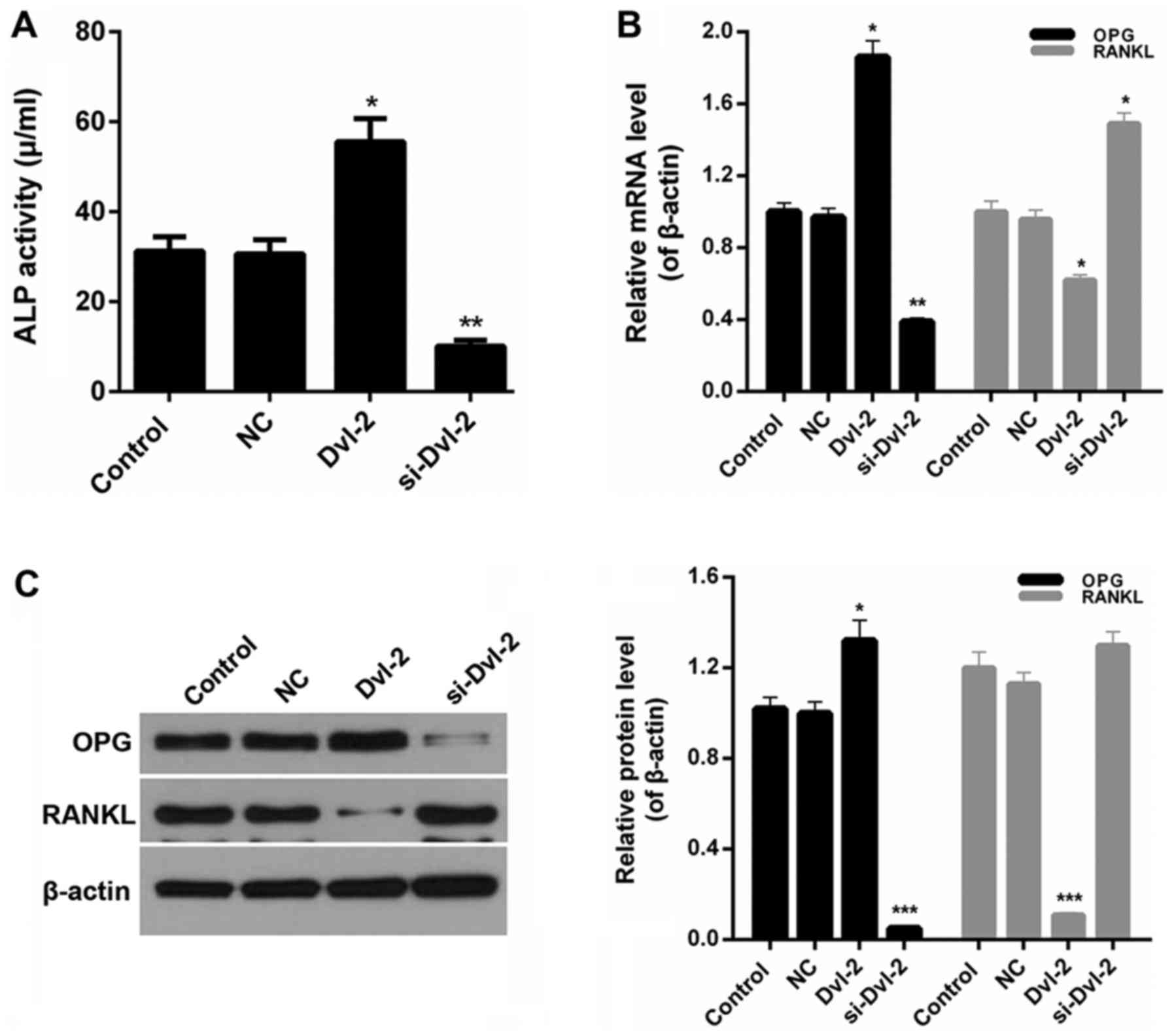
We also evaluated the activity of ALP in SFBs coped
with the treatment groups as described above. An obvious increase
of ALP activity was observed in Dvl-2 group compared with NC
(P<0.05; Fig. 3A). However, the
ALP activity in SFBs was markedly reduced by si-Dvl-2 (P<0.01;
Fig. 3A). Therefore, it was
determined that Dvl-2 silence lessened the activity of ALP in
SFBs.
Dvl-2 regulated the osteogenic
differentiation in SFBs
To further investigate the funtion of Dvl-2 in the
osteogenic differentiation of SFBs, we assessed the expression of
osteogenic factors in SFBs that were transfected with Dvl-2 or
si-Dvl-2, using OPG, RANKL, ALP, ON, OCN, and osterix. We found
that a rise in the OPG/RANKL ratio in Dvl-2 group, which is in
contrast to a decline in the si-Dvl-2 group (P<0.05; Fig. 3B and C). Moreover, compared to
control group, the expression levels of ON, OCN, and osterix in
SFBs transfected with si-Dvl-2 were significantly upregulated
However, a sharp decrease of ALP expression was observed in
si-Dvl-2 group; and an increase in Dvl-2 group (P<0.05; Fig. 4A and B).
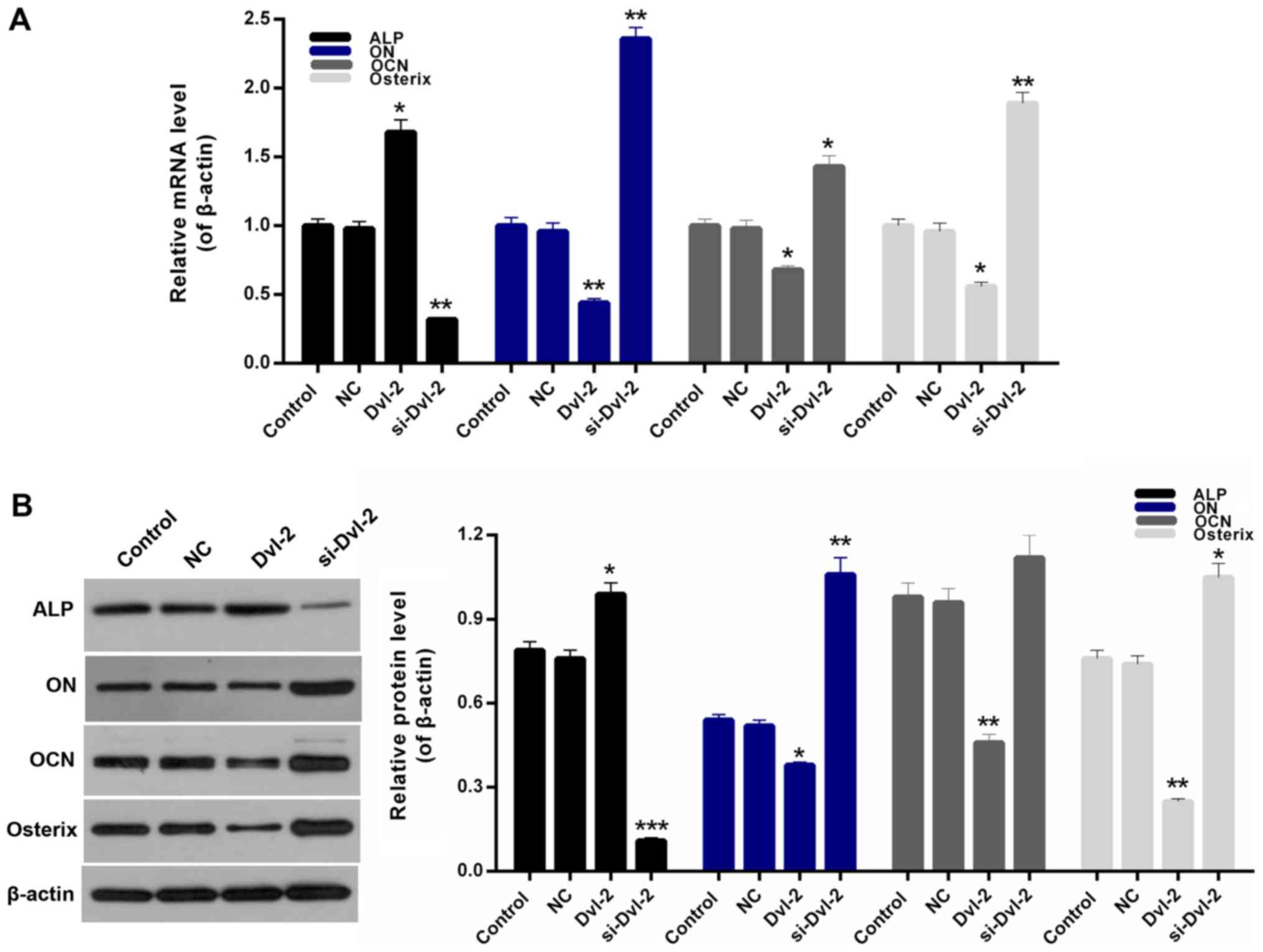 | Figure 4.Effect of Dvl-2 on the osteogenic
differentiation of SFBs. (A) Reverse transcription-quantitative
polymerase chain reaction and (B) western blot analysis were
performed to determine the mRNA and protein expression levels of
ALP, ON, OCN and osterix in SFBs, SFBs transfected with empty
vector, SFBs transfected with Dvl-2 and SFBs transfected with
si-Dvl-2. *P<0.05, **P<0.01 and ***P<0.001 vs. NC. Dvl-2,
dishevelled-2; SFB, synovial fibroblasts; si, short interfering;
ALP, alkaline phosphatase; ON, osteonectin; OCN, osteocalcin; NC,
negative control. |
Dvl-2 affected the activity of
Runx-2
For the purpose of exploring the mechanisms of Dvl-2
in the osteogenic differentiation of SFBs, we therefore measured
the expression levels of Runx-2 in SFBs from all treatment groups.
The results indicate that compared to control group, the expression
levels of Runx-2 was upregulated by Dvl-2 and downregulated by
si-Dvl2. Moreover, though the expression of Wnt3a and β-catenin
increased at the presence of Dvl-2, the depletion of Dvl-2
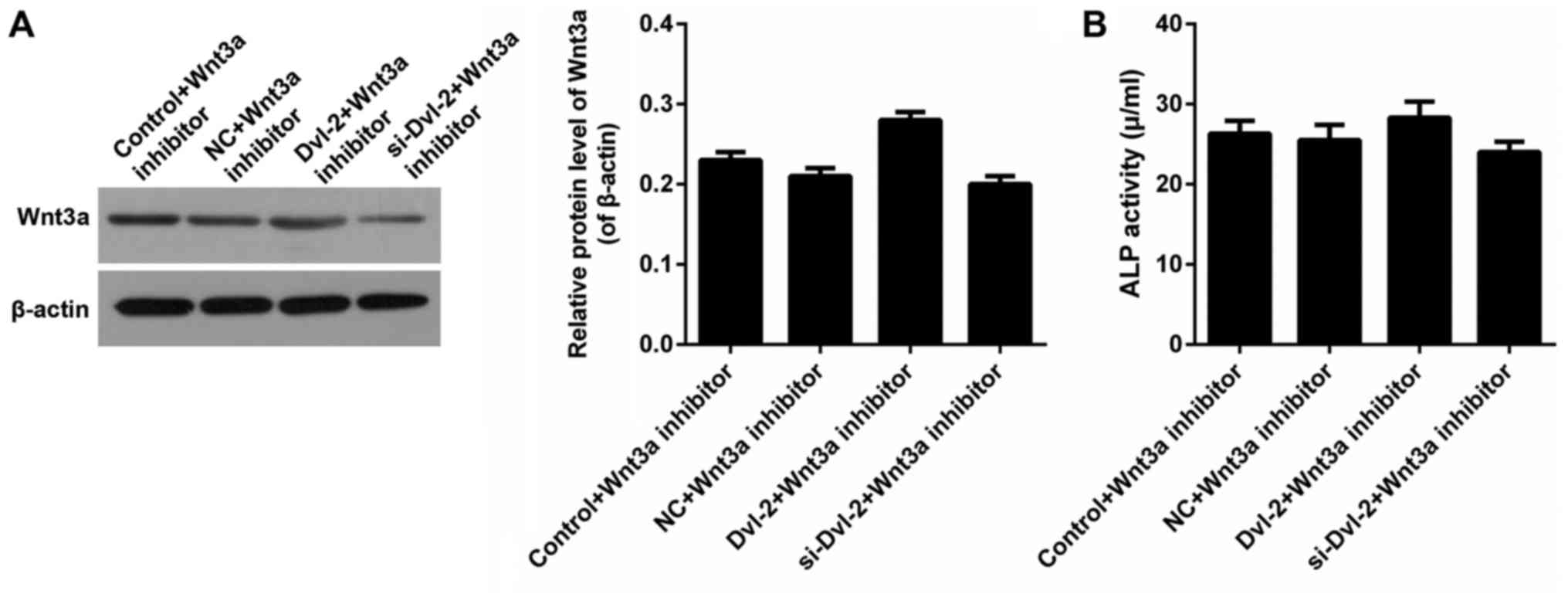
decreased the levels of Wnt3a and β-catenin (P<0.05; Fig. 5A and B). Furthermore, Wnt3a was
inhibited to estimate the effect of Dvl-2. The expression of Wnt3a
was shown in Fig. 6A. After the
inhibition of Wnt signaling by IWR-1-endo, there was no significant
difference of ALP activity in SFBs from all of the treatment groups
(Fig. 6B).
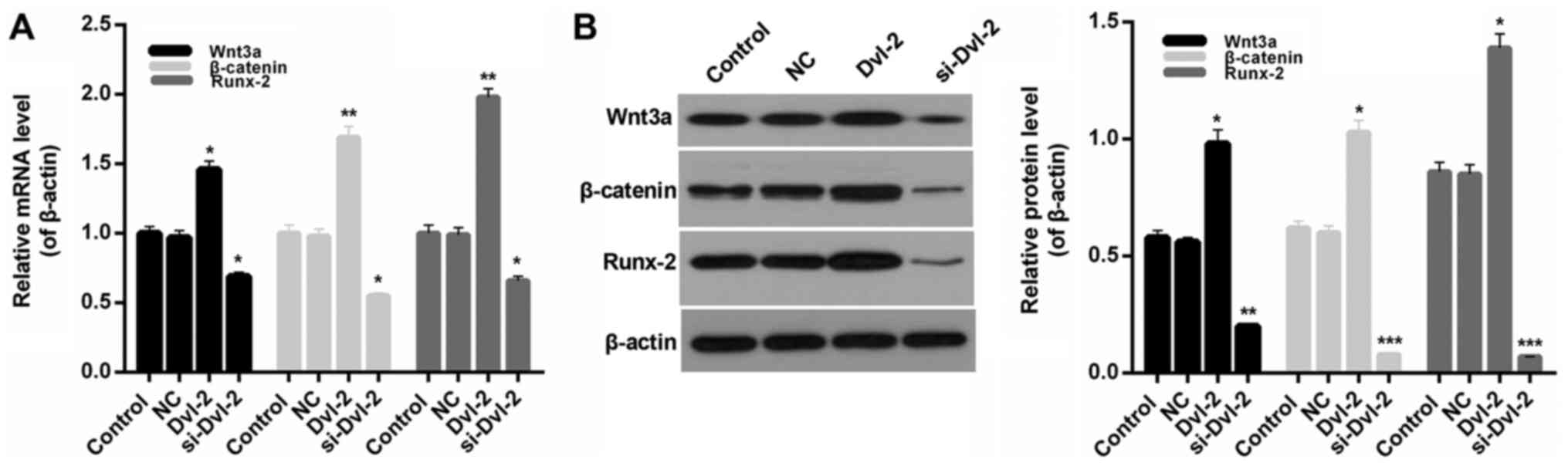 | Figure 5.Effect of Dvl-2 on the expression of
Runx-2, Wnt3a and β-catenin. (A) Reverse transcription-quantitative
polymerase chain reaction and (B) western blot analysis were
performed to determine the mRNA and protein expression levels of
Wnt3a, β-catenin and Runx-2 in SFBs, SFBs transfected with empty
vector, SFBs transfected with Dvl-2 and SFBs transfected with
si-Dvl-2. *P<0.05, **P<0.01 and ***P<0.001 vs. NC. Dvl-2,
dishevelled-2; SFB, synovial fibroblasts; si, short interfering;
Runx-2, runt-related transcription factor 2; NC, negative
control. |
Discussion
OA is one of the articular degenerative disorders.
It can lead to bone destruction around the joint, ossification in
the surrounding tissues, subsequent loss of bony rigidity and even
joint activity (28). Based on the
pathological investigation of OA ossification sites, it was
considered that non-osteocytes at the attachment sites can
proliferate and gradually differentiates into chondrocytes. The
fiber textures at attachment sites gradually grow thicker and the
cartilage-based pathological nodules will occur as a consequence
(29). Furthermore, these
possessing secretion function cells releases a large number of ALP
matrix vesicles, which will cause the partial formation of
hydeoxyapatite crystal and then gradually calcification (30–32).
Multiple cell types are involved in the progress of osteogenic
differentiation. SFBs have been widely investigated in the previous
research about OA (33–36). And SFB palyed important roles both
in bone resorption and bone formation (37,38).
Thus, in this study, we selected SFBs as our research objects to
further explore the the osteogenic differentiation in OA.
Previous studies showed that the close connections
between Dvl-2 and Wnt pathway (39–41).
However, to the best of our knowledge, the roles and mechanisms of
Dvl-2 in the osteogenic differentiation of SFBs have not been
studied yet. Thus, we selected Dvl-2 as the study object, and
transfected the SFBs with Dvl-2 and si-Dvl-2. Over-expression and
silencing of Dvl-2 in SFBs were observed, and the knockdown
efficiency of Dvl-2 was about 60%. We first measured the cell
viability of SFBs, which are transfected with Dvl-2 and si-Dvl-2.
The results indicated that Dvl-2 silence could inhibit the cell
viability of SFBs, especially for 48 h-treatment. Then, we measured
the activity of ALP in SFBs, which were transfected with Dvl-2 and
si-Dvl-2. The results showed that Dvl-2 significantly influenced
the ALP activity in SFBs. In order to investigate the functions of
Dvl-2 in the osteogenic differentiation of SFBs, the expressions of
OPG and RANKL in SFBs were examined in reference to previous
studies (6,7). Based on the experimental results, we
found that the OPG/RANKL ratio was remarkably reducced by si-Dvl-2.
Moreover, over-expression of Dvl-2 significantly enhanced the
OPG/RANKL ratio in SFBs. Additionally, Dvl-2 silence significantly
reduced the ALP expression, while it upregulated the expression
levels of ON, OCN, and osterix in SFBs. It was confirmed that Dvl-2
suppressed bone absorption of SFBs in OA by regulating the
expression levels of OPG, RANKL, ALP, ON, OCN, and osterix.
In different stages of osteogenic differentiation,
signaling pathways involved/participated are not the same. It has
been proved that ossification was largely affected by BMP pathway
at an early stage, while the Wnt pathway impacts ossification in
the advanced ossification (42–44).
Among them, Wnt/β-catenin pathway plays an important role in stem
cell differentiation, bone formation, and the regulation of balance
from the embryonic period (45).
The abnormal regulation of Wnt pathway is closely associated with
the bony ankylosis in OA, and was considered as one of the
important factors in osteogenic differentiation. In the present
study, the Wnt3a, β-catenin, and Runx-2 expressions in SFBs were
studied. Our results showed that Dvl-2 silence significantly
downregulated the expression levels of Wnt3a, β-catenin, and Runx-2
in SFBs. After downregulating the Wnt3a expression, we found that
there was no significant difference in the activities of ALP in
SFBs. Such results remind us of that Dvl-2 regulated the activity
of Runx-2 by affecting the Wnt pathway in SFBs. Thus, it can
concluded that Dvl-2 modulated the osteogenic differentiation of
SFBs in OA via Wnt/β-catenin/Runx-2 pathway, to some extent.
Taken together, our research demonstrated that the
Dvl-2 plays a critical role in osteogenic differentiation of SFBs
in OA, which was related to the Wnt pathway. Also, the results
provided a new thread for understanding the pathogenesis of OA and
put forward a fascinating approach for the therapy of OA.
In conclusoin, our study highlights that Dvl-2
silence modulates the ossification of SFBs in OA by downregulating
the Wnt pathway. The findings of our research are crucial to
unfolding the mechanisms of Dvl-2 in the ossification of SFBs. The
potential effects of Dvl-2 in the ossification of SFBs suggest that
Dvl-2 might be an effective target for OA therapies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LZ wrote the main manuscript. LL performed the
experiments. YM designed the study. LZ and LL performed data
analysis. LZ, LL and YM contributed to manuscript revisions and all
authors reviewed the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Jining No. 1 People's Hospital. Written informed
consent was obtained from all patients prior to their inclusion
within the study.
Consent for publication
Written informed consent was obtained from all
patients for the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Glyn-Jones S, Palmer AJ, Agricola R, Price
AJ, Vincent TL, Weinans H and Carr AJ: Osteoarthritis. Lancet.
386:376–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johnson VL and Hunter DJ: The epidemiology
of osteoarthritis. Best Pract Res Clin Rheumatol. 28:5–15. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Benito MJ, Veale DJ, FitzGerald O, van den
Berg WB and Bresnihan B: Synovial tissue inflammation in early and
late osteoarthritis. Ann Rheum Dis. 64:1263–1267. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lambert C, Dubuc JE, Montell E, Vergés J,
Munaut C, Noë A and Henrotin Y: Gene expression pattern of cells
from inflamed and normal areas of osteoarthritis synovial membrane.
Arthritis Rheumatol. 66:960–968. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iwanaga T, Shikichi M, Kitamura H, Yanase
H and Nozawa-Inoue K: Morphology and functional roles of
synoviocytes in the joint. Arch Histol Cytol. 63:17–31. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Danks L, Komatsu N, Guerrini MM, Sawa S,
Armaka M, Kollias G, Nakashima T and Takayanagi H: RANKL expressed
on synovial fibroblasts is primarily responsible for bone erosions
during joint inflammation. Ann Rheum Dis. 75:1187–1195. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miyashita T, Kawakami A, Nakashima T,
Yamasaki S, Tamai M, Tanaka F, Kamachi M, Ida H, Migita K, Origuchi
T, et al: Osteoprotegerin (OPG) acts as an endogenous decoy
receptor in tumour necrosis factor-related apoptosis-inducing
ligand (TRAIL)-mediated apoptosis of fibroblast-like synovial
cells. Clin Exp Immunol. 137:430–436. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sharma U, Pal D and Prasad R: Alkaline
phosphatase: An overview. Indian J Clin Biochem. 29:269–278. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Delgado-Calle J, Sanudo C, Sánchez-Verde
L, Garcia-Renedo RJ, Arozamena J and Riancho JA: Epigenetic
regulation of alkaline phosphatase in human cells of the
osteoblastic lineage. Bone. 49:830–838. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ek-Rylander B and Andersson G: Osteoclast
migration on phosphorylated osteopontin is regulated by endogenous
tartrate-resistant acid phosphatase. Exp Cell Res. 316:443–451.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prins HJ, Braat AK, Gawlitta D, Dhert WJ,
Egan DA, Tijssen-Slump E, Yuan H, Coffer PJ, Rozemuller H and
Martens AC: In vitro induction of alkaline phosphatase levels
predicts in vivo bone forming capacity of human bone marrow stromal
cells. Stem Cell Res. 12:428–440. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seo HJ, Cho YE, Kim T, Shin HI and Kwun
IS: Zinc may increase bone formation through stimulating cell
proliferation, alkaline phosphatase activity and collagen synthesis
in osteoblastic MC3T3-E1 cells. Nutr Res Pract. 4:356–361. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sardiwal S, Magnusson P, Goldsmith DJ and
Lamb EJ: Bone alkaline phosphatase in CKD-mineral bone disorder. Am
J Kidney Dis. 62:810–822. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gulseren G, Yasa IC, Ustahuseyin O, Tekin
ED, Tekinay AB and Guler MO: Alkaline phosphatase-mimicking peptide
nanofibers for osteogenic differentiation. Biomacromolecules.
16:2198–2208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chien AJ, Conrad WH and Moon RT: A Wnt
survival guide: From flies to human disease. J Invest Dermatol.
129:1614–1627. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
James RG, Conrad WH and Moon RT:
Beta-catenin-independent Wnt pathways: Signals, core proteins and
effectors. Methods Mol Biol. 468:131–144. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baron R and Kneissel M: WNT signaling in
bone homeostasis and disease: From human mutations to treatments.
Nat Med. 19:179–192. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sen M: Wnt signalling in rheumatoid
arthritis. Rheumatology (Oxford). 44:708–713. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao C and Chen YG: Dishevelled: The hub of
Wnt signaling. Cell Signal. 22:717–727. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Blom AB, van Lent PL, van der Kraan PM and
van den Berg WB: To seek shelter from the WNT in osteoarthritis?
WNT-signaling as a target for osteoarthritis therapy. Curr Drug
Targets. 11:620–629. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abraham DJ, Shiwen X, Black CM, Sa S, Xu Y
and Leask A: Tumor necrosis factor alpha suppresses the induction
of connective tissue growth factor by transforming growth
factor-beta in normal and scleroderma fibroblasts. J Biol Chem.
275:15220–15225. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wallingford JB and Habas R: The
developmental biology of Dishevelled: An enigmatic protein
governing cell fate and cell polarity. Development. 132:4421–4436.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pulvirenti T, Van Der Heijden M, Droms LA,
Huse JT, Tabar V and Hall A: Dishevelled 2 signaling promotes
self-renewal and tumorigenicity in human gliomas. Cancer Res.
71:7280–7290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang Y, Jiao L, Hou J, Xu C, Wang L, Yu Y,
Li Y, Yang C, Wang X and Sun Y: Dishevelled-2 silencing reduces
androgen-dependent prostate tumor cell proliferation and migration
and expression of Wnt-3a and matrix metalloproteinases. Mol Biol
Rep. 40:4241–4250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou G, Ye J, Sun L, Zhang Z and Feng J:
Overexpression of Dishevelled-2 contributes to proliferation and
migration of human esophageal squamous cell carcinoma. J Mol
Histol. 47:287–295. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sadouk MB, Pelletier JP, Tardif G, Kiansa
K, Cloutier JM and Martel-Pelletier J: Human synovial fibroblasts
coexpress IL-1 receptor type I and type II mRNA. The increased
level of the IL-1 receptor in osteoarthritic cells is related to an
increased level of the type I receptor. Lab Invest. 73:347–355.
1995.PubMed/NCBI
|
|
27
|
Arocho A, Chen B, Ladanyi M and Pan Q:
Validation of the 2-DeltaDeltaCt calculation as an alternate method
of data analysis for quantitative PCR of BCR-ABL P210 transcripts.
Diagn Mol Pathol. 15:56–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Loeser RF, Goldring SR, Scanzello CR and
Goldring MB: Osteoarthritis: A disease of the joint as an organ.
Arthritis Rheum. 64:1697–1707. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grenier S, Bhargava MM and Torzilli PA: An
in vitro model for the pathological degradation of articular
cartilage in osteoarthritis. J Biomech. 47:645–652. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Anderson HC, Sipe JB, Hessle L,
Dhanyamraju R, Atti E, Camacho NP, Millán JL and Dhamyamraju R:
Impaired calcification around matrix vesicles of growth plate and
bone in alkaline phosphatase-deficient mice. Am J Pathol.
164:841–847. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fallon MD, Whyte MP and Teitelbaum SL:
Stereospecific inhibition of alkaline phosphatase by L-tetramisole
prevents in vitro cartilage calcification. Lab Invest. 43:489–494.
1980.PubMed/NCBI
|
|
32
|
Yadav MC, Simão AM, Narisawa S, Huesa C,
McKee MD, Farquharson C and Millán JL: Loss of skeletal
mineralization by the simultaneous ablation of PHOSPHO1 and
alkaline phosphatase function: A unified model of the mechanisms of
initiation of skeletal calcification. J Bone Miner Res. 26:286–297.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Carrión M, Juarranz Y, Pérez-Garcia S,
Jimeno R, Pablos JL, Gomariz RP and Gutiérrez-Cañas I: RNA sensors
in human osteoarthritis and rheumatoid arthritis synovial
fibroblasts: Immune regulation by vasoactive intestinal peptide.
Arthritis Rheum. 63:1626–1636. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eisinger K, Bauer S, Schäffler A, Walter
R, Neumann E, Buechler C, Müller-Ladner U and Frommer KW: Chemerin
induces CCL2 and TLR4 in synovial fibroblasts of patients with
rheumatoid arthritis and osteoarthritis. Exp Mol Pathol. 92:90–96.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Haas S and Straub RH: Disruption of
rhythms of molecular clocks in primary synovial fibroblasts of
patients with osteoarthritis and rheumatoid arthritis, role of
IL-1beta/TNF. Arthritis Res Ther. 14:R1222012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qin Y, Chen Y, Wang W, Wang Z, Tang G,
Zhang P, He Z, Liu Y, Dai SM and Shen Q: HMGB1-LPS complex promotes
transformation of osteoarthritis synovial fibroblasts to a
rheumatoid arthritis synovial fibroblast-like phenotype. Cell Death
Dis. 5:e10772014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Galicka A, Surazynski A, Wołczyński S,
Palka J, Popko J and Gindzieński A: Phenotype variability in a
daughter and father with mild osteogenesis imperfecta correlated
with collagen and prolidase levels in cultured skin fibroblasts.
Ann Clin Biochem. 42:80–84. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bhattaram P and Chandrasekharan U: The
joint synovium: A critical determinant of articular cartilage fate
in inflammatory joint diseases. Semin Cell Dev Biol. 62:86–93.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gonzalez-Sancho JM, Greer YE, Abrahams CL,
Takigawa Y, Baljinnyam B, Lee KH, Lee KS, Rubin JS and Brown AM:
Functional consequences of Wnt-induced dishevelled 2
phosphorylation in canonical and noncanonical Wnt signaling. J Biol
Chem. 288:9428–9437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Smalley MJ, Signoret N, Robertson D,
Tilley A, Hann A, Ewan K, Ding Y, Paterson H and Dale TC:
Dishevelled (Dvl-2) activates canonical Wnt signalling in the
absence of cytoplasmic puncta. J Cell Sci. 118:5279–5289. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yokoyama N and Malbon CC: Dishevelled-2
docks and activates Src in a Wnt-dependent manner. J Cell Sci.
122:4439–4451. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kamiya N, Ye L, Kobayashi T, Mochida Y,
Yamauchi M, Kronenberg HM, Feng JQ and Mishina Y: BMP signaling
negatively regulates bone mass through sclerostin by inhibiting the
canonical Wnt pathway. Development. 135:3801–3811. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kawaguchi H: Regulation of osteoarthritis
development by Wnt-beta-catenin signaling through the endochondral
ossification process. J Bone Miner Res. 24:8–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yu YY, Lieu S, Lu C and Colnot C: Bone
morphogenetic protein 2 stimulates endochondral ossification by
regulating periosteal cell fate during bone repair. Bone. 47:65–73.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tamamura Y, Otani T, Kanatani N, Koyama E,
Kitagaki J, Komori T, Yamada Y, Costantini F, Wakisaka S, Pacifici
M, et al: Developmental regulation of Wnt/beta-catenin signals is
required for growth plate assembly, cartilage integrity, and
endochondral ossification. J Biol Chem. 280:19185–19195. 2005.
View Article : Google Scholar : PubMed/NCBI
|