Introduction
Tumour vaccines have become the hot spots of
anticancer immunotherapy in recent decades, and tumour-specific
antigens (TSAs) have been used to induce specific cellular and
humoural immune responses to inhibit tumour occurrence and
development. TSAs can stimulate specific immune responses and are
the main components in tumour vaccines. As the first discovered
TSA, Melanoma-associated antigen A1 (MAGEA1) is expressed in most
tumours, including the testes and placenta (1–3).
MAGEA1 be processed and presented by human leukocyte antigen (HLA)
class I molecules on cancer cells, and MAGEA1-expressing cancer
cells can be rejected by host cytotoxic T lymphocytes (CTLs)
(4,5). Tumour vaccines based on MAGEA1 have
been used in patients with a variety of tumours, but most of these
vaccines do not exhibit particularly significant clinical effects
on cancer (6–10). Therefore, it is imperative to
explore more effective TSA-based tumour vaccines.
As a molecular chaperone, heat shock protein 70
(HSP70) participates in processing and presenting tumour antigens,
and HSP70 fused with TSA can significantly enhance cellular and
humoural immune responses against cancer cells (11). Moreover, previous studies have
shown that the HSP70-MAGEA1 fusion protein can stimulate
MAGEA1-specific antitumour immunity (11–13).
However, the immunoactivity of HSP70 fusion proteins needs further
improvement for their clinical application. In recent years,
gas-filled microbubbles (MBs) have been used in the clinic as
intravenously administered ultrasound-based contrast agents
(14,15) due to their high acoustic impedance
mismatch between gases and blood (16,17).
The delivery of DNA/RNA/protein in target cells by the application
of ultrasound, a process known as sonoporation, has successfully
been applied (18,19) and demonstrated as a good candidate
for lipid-based TSA delivery. Gas-filled ultrasound microbubbles
can transport drugs or genes across cell membranes and vessel walls
and are considered effective transport carriers in vivo.
Moreover, gas-filled ultrasound microbubbles are efficiently
phagocytosed and presented bydendritic cells (DC) and activate
stronger immune responses (20,21).
In the present study, we used gas-filled ultrasound
microbubbles as carriers for the controllable release of
HSP70-MAGEA1 fusion proteins at a specific location and evaluated
the immunoactivity of antitumours to explore a new strategy for
future tumour immunotherapy.
Materials and methods
Animals and cell lines
C57BL/6 mice (8 weeks old) were provided from the
Laboratory Animal Center of the Fourth Military Medical University
and fed in a specific pathogen-free (SPF) animal house. All
experiments were conducted according to the guidelines for the Care
and Use of Laboratory Animals and approved by the Animal Ethical
Committee of the Fourth Military Medical University. B16 cell lines
overexpressing MAGEA1 (B16-MAGEA1) were previously established
(22).
Preparation and identification of
gas-filled ultrasound microbubbles (MBs)
The MBs were prepared as previously reported
(23). Briefly, a mixture of the
nonionic surfactant Span 60 (3 wt%), Tween 80 (5 wt%), and
polyethylene glycol (PEG; 3 wt%) with different polymerization
degrees were sonicated in PBS solution using a high-intensity
ultrasonic processor (VCX-750; Sonix, USA) for 1 min at 400 W in
the presence of sulfur hexafluoride (SF6) gas. Then, the
mixture was incubated in a separating funnel for 30 min, and the
middle layer, comprising the microbubbles, was separated and washed
with PBS. To prepare the fusion protein-loaded microbubbles
(MB-FP), 200 µl of HSP70-MAGEA1 was incubated with 1 ml of MBs for
10 min at 4°C, and the fusion proteins were adsorbed onto the MBs
via electrostatic adsorption self-assembly. Unloaded protein was
removed after three centrifugal washes with PBS buffer solution.
After washing, the size distribution of the protein-loaded
microbubbles was measured using a Coulter counter (Multisizer III,
Beckman-Coulter, Pasadena, CA, USA) (15). After extraction, the protein
concentration was measured using the Bradford method. The prepared
gas-filled ultrasound microbubbles were stored in sealed glass
vials under a headspace filled with SF6 gas at 4°C.
Immunization regime
C57BL/6 mice were subcutaneously (s.c.) immunized in
the foreleg with 10 µg/100 µl/mouse gas-filled ultrasound
microbubbles carrying HSP70-MAGEA1 fusion protein (MB-FP group),
and HSP70-MAGEA1 fusion protein alone (FP group) or gas-filled
ultrasound microbubbles (MB group) alone were used as controls.
After administration, the MBs were destroyed near the inguinal
lymph nodes with a mechanical index (MI=0.7). Three administrations
at one-week intervals were performed, and the sera and spleen were
collected for experiments at two weeks after the last
injection.
Immunoblotting analysis
The lymph nodes from vaccinated mice were used for
immunoblotting to detect HSP70 and MAGEA1 proteins. The total
proteins were extracted from the spleens using RIPA buffer, and the
cell debris was removed by centrifugation at 12,000 × g for 20 min
at 4°C. After quantification, 10–50 µg/lane of the spleen proteins
were separated by SDS-PAGE. The MAGEA1 and HSP70 proteins were
detected using anti-human MAGEA1 and anti-HSP70 (both Abcam,
Cambridge, UK) antibodies, respectively.
IFNγ enzyme-linked immunosorbent spot
(ELISpot) assay
The mouse IFNγ ELISpot assay was performed as
previously described (22) in
PVDF-bottomed 96-well plates (Millipore, USA) using a commercial
kit (Diaclone, France). Briefly, the plates were covered with an
anti-IFNγ antibody overnight at 4°C and blocked with 5% non-fat
skimmed milk. The splenocytes (2×105 cells/well) were
then inoculated together with the indicated number of lethally
irradiated B16-MAGEA1 cells (2×104/well, respectively).
After incubation for 24 h, the cells were removed, and a
biotinylated IFNγ detection antibody was added for 2 h and
incubated with streptavidin-alkaline phosphatase for another 1 h at
37°C. After washing with PBST, the spots were visualized by the
addition of the alkaline phosphatase substrate BCIP/NBT and counted
using a dissection microscope. The number of MAGEA1-specific T-cell
precursors in the splenocytes was calculated by subtracting the
IFNγ+ spots of splenocytes on B16-stimulating cells from that on
B16-MAGEA1 cells.
ELISA
Irradiated B16-MAGEA1 cells (5×105) were
cocultured with splenocytes (5×106 cells) from mice at 2
weeks after the last vaccination, and the cells were cultured in 2
ml of DMEM supplemented with 10% foetal bovine serum (FBS), 2 mM
L-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin in
24-well tissue culture plates for 72 h. Murine IL-2 ELSIA kits
(Diaclonek, France) were used to assay the presence of IL-2 in the
supernatants according to the manufacturer's instructions. The
titre of anti-MAGEA1 antibody in the sera was determined as
previously described (22).
Tumour treatmente
The mice were s.c. challenged with B16-MAGEA1 tumour
cells (1×106 cells/mouse, respectively) in the hind legs
(D0) as previously described (22). One week later (day 7), the mice
were s.c. vaccinated with 10 µg/100 µl/mouse MB-FP (gas-filled
ultrasound microbubbles with HSP70-MAGEA1 fusion protein) in the
forelegs. The control groups were s.c. injected with 10 µg/100
µl/mouse HSP70-MAGEA1 fusion protein alone or gas-filled
microbubbles alone. Every group included 10 mice. At one week (day
14) and 2 weeks (day 21) later, these mice were boosted with the
same regimes. The tumour volumes (length × width2x π/6)
and weight were measured twice a week after the tumours became
palpable and were plotted as the mean tumour volume of the group
(means ± SEM) vs. the number of days after the tumour was planted.
The survival time of the mice was also recorded.
Tumour challenge experiments
The mice were vaccinated (day 21) with MB-FP,
HSP70-MAGEA1 fusion protein or gas-filled microbubbles (10 mice per
group), as previously described (12,13).
At one week (day 14) and 2 weeks later (day 7), the immunization
regime was boosted twice. On the 7th day after the last
immunization (day 0), the mice were s.c. challenged with B16-MAGEA1
tumour cells (1×105 cells/mouse, respectively) in the
hind legs. Once the tumours became palpable, observations were
taken twice a week, and the ratio of tumour-free mice was
recorded.
Statistical analysis
All statistical analyses were carried out using
Graphpad Prism 5 software (GraphPad, USA). Results are presented as
the means ± SEM. Data were analyzed by one-way analysis of variance
(ANOVA) with Tukey's Multiple Comparison test and two-way ANOVA
with Bonferroni post-test. Kaplan-Meier survival plots and the
log-rank test were used to evaluate differences in animal survival
among experimental groups (P-value).
Results
Gas-filled microbubbles effectively
delivered HSP70-MAGE-A1 fusion proteins to subcutaneous lymph
nodes
The gas-filled ultrasound microbubbles (MBs) were
generated as described in the Methods, and a representative
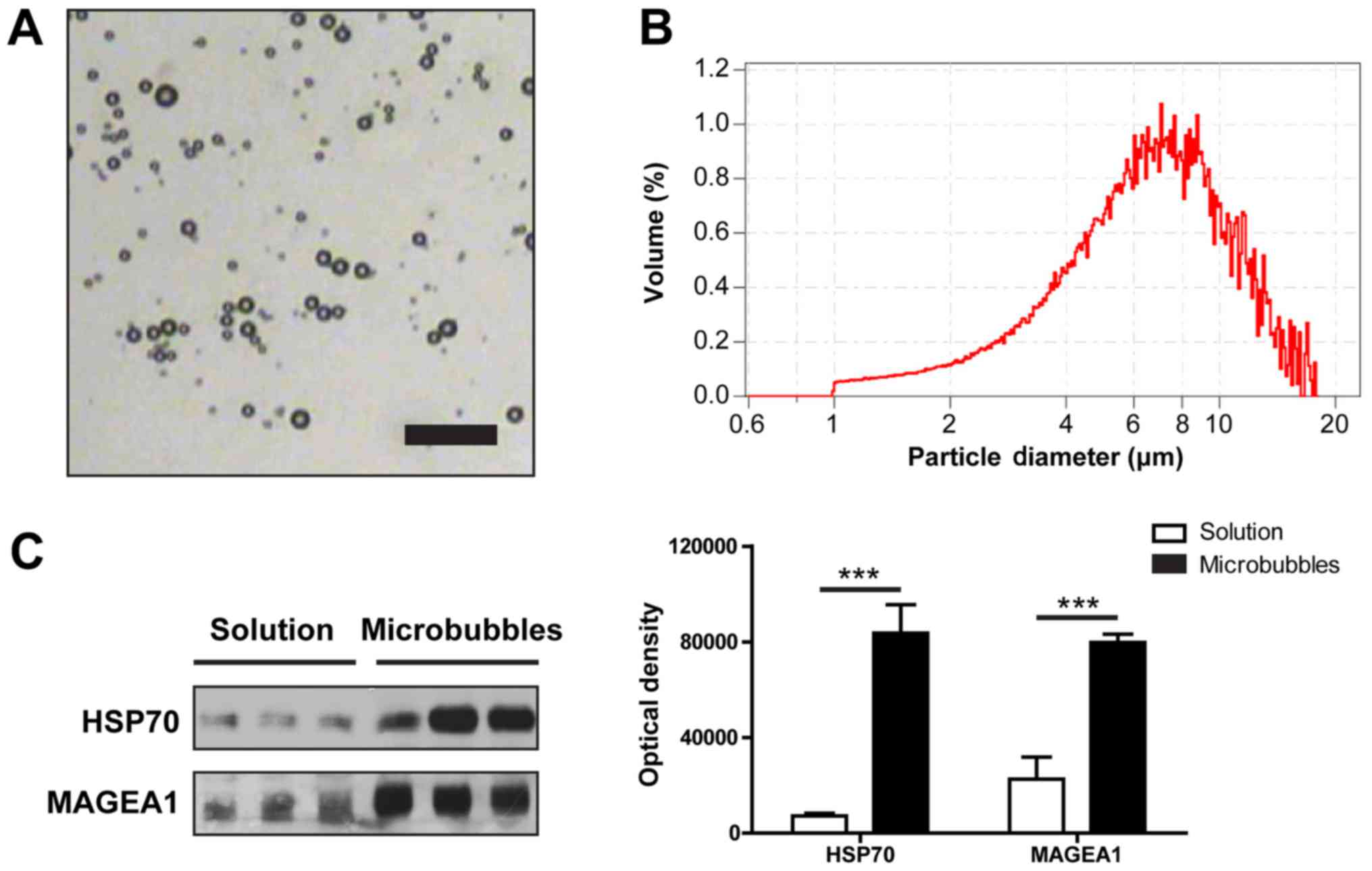
microscopic picture of the MBs is shown in Fig. 1A. The mean diameter of the
microbubbles was 6.02 µm, and approximately 90% microbubbles were
smaller than 13.86 µm in diameter (Fig. 1B). Through electrostatic
attraction, HSP70-MAGEA1 fusion proteins were easily adsorbed onto
the shells of these MBs. Immunoblotting and densitometric analysis
indicated that the vast majority of MBs were combined with the
HSP70-MAGEA1 fusion protein (Fig.
1C). The concentration of MBs was approximately
3.2×108/ml, and the protein concentration was
approximately 100 µg/ml. Prior to injection, the sample was further
diluted with PBS solution (pH=7.4).
As microbubbles are primarily used as ultrasonic
contrast agents, we therefore followed the kinetics of MBs
persistence in vivo after s.c. injection in the forelegs.
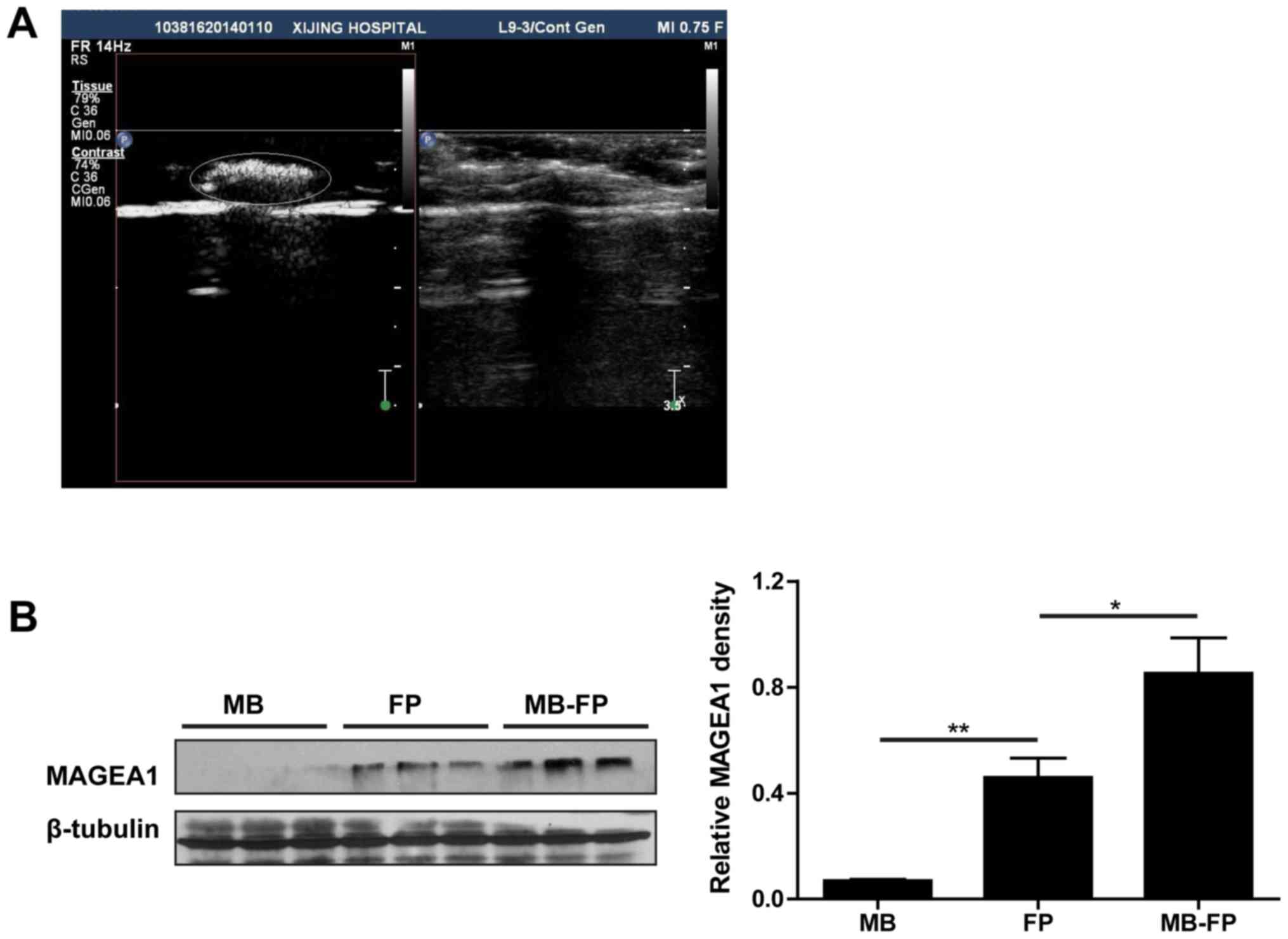
Under contrast-enhanced ultrasound-mediated visualization of the
low mechanical index ultrasound pulse signal persistence, the MBs
were clearly detected in the lymph nodes (Fig. 2A). After destroying the MBs with a
mechanical index (MI=0.75), the lymph nodes were dissected and the
levels of HSP70-MAGEA1 fusion proteins were analysed. The
immunoblotting and densitometric results indicated that both HSP70
and MAGEA1 proteins could be detected after immunization. However,
the antigens to MAGEA1 were higher in the lymph nodes of mice
immunized with MB-FP than in those immunized with FP alone
(Fig. 2B). These results showed
that gas-filled ultrasound microbubbles could improve the delivery
of HSP70-MAGEA1 antigen to the lymph tissues.
Gas-filled microbubbles could boost
the specific immune responses against MAGEA1
CTLs are one of the most important antitumour
effectors of the cell immune response in vivo. To determine
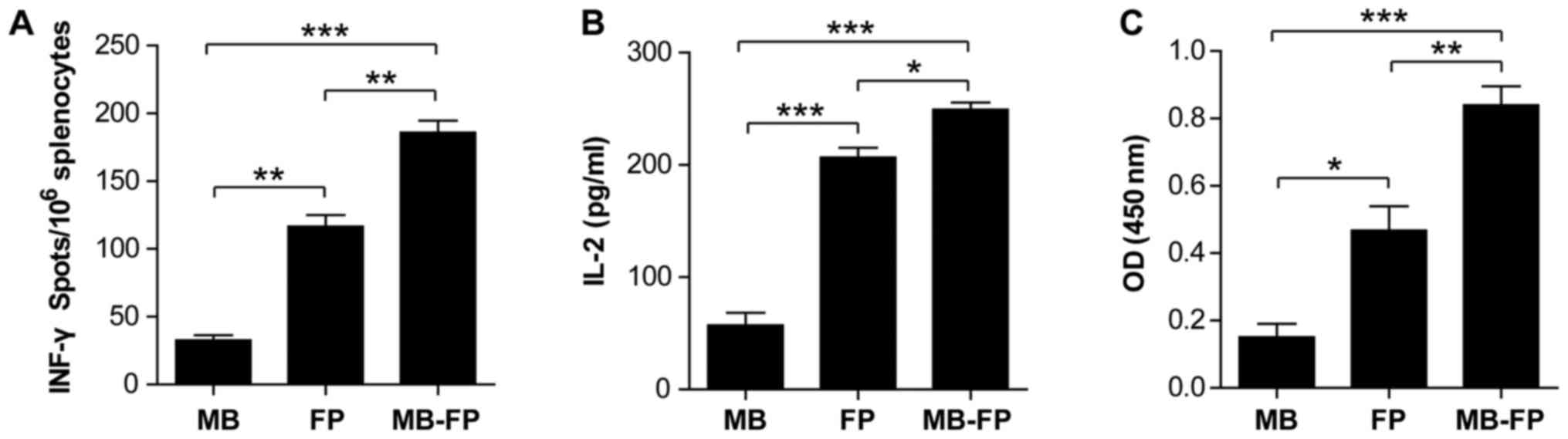
whether MBs enhanced the cellular immune response, the IFN-γ
ELISpot assay was used to determine the number of MAGEA1-specific
CD8+ T cells in the splenocytes from mice immunized with
gas-filled ultrasound microbubbles (MB), HSP70-MAGEA1 fusion
protein (FP) alone and gas-filled ultrasound microbubbles carrying
the HSP70-MAGEA1 fusion protein (MB-FP). The results showed that
the number of spot-forming T-cell precursors specific to
MAGEA1-expressing B16 cells were significantly higher in the
splenocytes of mice vaccinated with MB-FP compared with that in
mice vaccinated with FP or MB alone (Fig. 3A). These results suggested that MBs
could significantly enhance specific immune responses, although
HSP70-MAGEA1 fusion proteins alone also stimulated the generation
of INFγ-producing MAGEA1-specific T-cell precursors in vivo.
We then analysed the IL-2 secretion of splenocytes in mice treated
with various vaccines using ELISA to further determine the
T-cell-mediated antitumour immune responses. As shown in Fig. 3B, the IL-2 levels in the
supernatant of cocultured irradiated B16-MAGEA1 and splenocytes
from mice vaccinated with MB-FP were significantly higher than in
the cells from mice vaccinated with FP or MB alone. Combined with
the data from ELISpot assay, these results suggested that the
gas-filled microbubbles enhanced the generation of MAGEA1-specific
cellular immune responses by HSP70-MAGEA1 fusion proteins.
To evaluate the humoural immune responses, the
titres of anti-MAGEA1 antibody in the sera of the vaccinated mice
were determined using ELISA after the last vaccination. As shown in
Fig. 3C, the levels of anti-MAGEA1
antibody were significantly boosted in the mice vaccinated with FP
or MB-FP, and the titres of anti-MAGEA1 were slightly higher in
mice vaccinated with MB-FP compared with those in mice vaccinated
with FP alone. These results indicated that the MB-FP could elicit
and boost MAGEA1-specific humoural immune responses (Fig. 3B). Collectively, these results
verified that HSP70-MAGEA1 fusion proteins delivered via gas-filled
ultrasound microbubbles could more effectively boost both cellular
and humoural immune responses against MAGEA1 compared with the
fusion protein alone.
HSP70-MAGEA1 fusion protein delivered
via gas-filled microbubbles delayed tumour growth and prolonged the
survival of mice
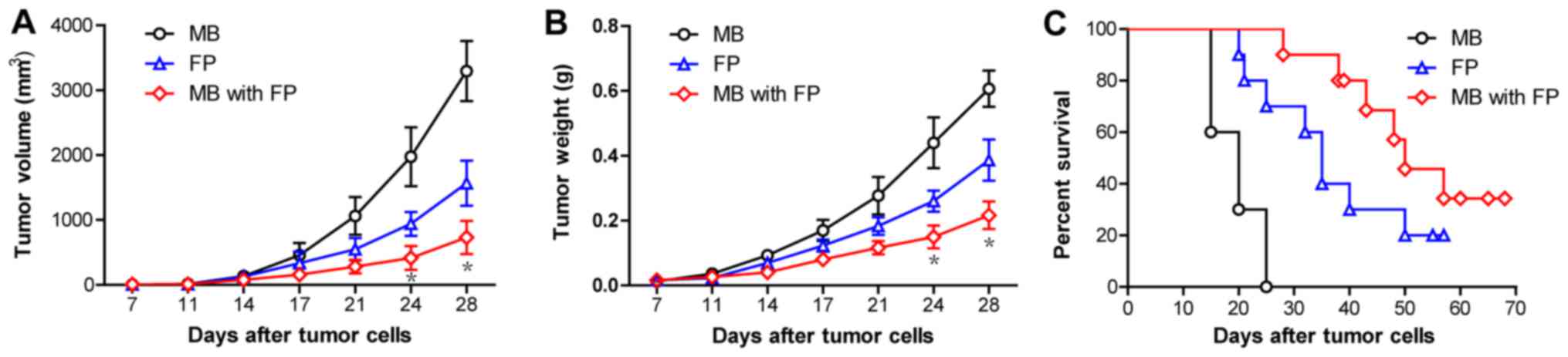
To investigate the clinical efficacy of MB-FP and FP
vaccines against MAGEA1-expressing tumours, the mice were s.c.
inoculated with MAGEA1-expressing B16 (B16-MAGEA1) cells
(106 cells/mouse) on day 0, and 10 µg/100 µl/mouse of
different vaccines was administered on days 7, 14 and 21. The
results showed that MB-FP vaccination significantly delayed the
growth of B16-MAGEA1 tumours compared with mice vaccinated with FP
and MB only (Fig. 4A & B).
Moreover, the survival times of mice with pre-existing B16-MAGEA1
tumours were significantly prolonged after vaccinated with MB-FP
compared with mice vaccinated with FP and MB only (Fig. 4C). These results suggested that the
use of gas-filled microbubbles significantly increases the
immunoactivity of HSP70-MAGEA1 fusion proteins and improves the
survival of mice bearing melanomas, suggesting that MB-FP is a
potent and efficient therapeutic vaccine against MAGEA1-expressing
tumours.
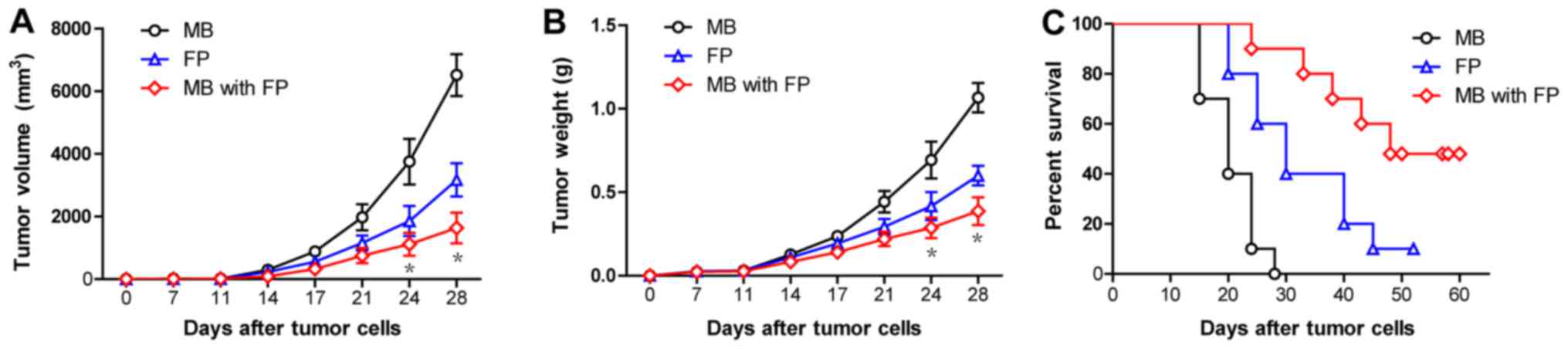 | Figure 4.The immunotherapy of pre-established
B16-MAGEA1 melanoma with gas-filled microbubbles with HSP70-MAGEA1
fusion protein. The mice were s.c. inoculated with
MAGEA1-expressing B16 (B16-MAGE-A1) cells (106
cells/mouse) on day 0 and were then administered MB, FP, and MB-FP
on days 7, 14 and 21. (A and B) Vaccination with MB-FP
significantly delayed the tumour growth of B16-MAGEA1 compared with
vaccination with FP and MB (n=10). *P<0.05 vs. the FP group.
Two-way ANOVA with Bonferroni post-test. (C) MB-FP vaccination
could significantly increase the survival time compared with FP or
MB alone in B16-MAGEA1 tumour-bearing mice (n=10). Log-rank test,
FP vs. MB, P=0.0011; MB with FP vs. MB, P<0.001; MB with FP vs.
FP, P=0.0295. HSP70, heat shock protein 70; MAGEA1,
melanoma-associated antigen A1; MB, gas-filled ultrasound
microbubbles; FP, HSP70-MAGEA1 fusion protein; MB-FP, gas-filled
ultrasound microbubbles with HSP70-MAGEA1 fusion protein. |
HSP70-MAGE-A1 fusion protein delivered
via gas-filled microbubbles is an efficient protective vaccine
against MAGEA1-expressing tumours
To determine the protective effects of MB-FP
vaccines, the mice were vaccinated with MB-FP, MBs and FP as
described in the Materials and methods and were then challenged
with 1×106 B16-MAGEA1 melanoma cells. The mice were
monitored for evidence of tumour growth by palpation and
inspection. The results showed that MB-FP vaccination slowed the
tumour volumes and weight of B16-MAGEA1 cells (Fig. 5A & B) and prolonged the
survival times compared with those in the mice vaccinated with FP
and MBs alone (Fig. 5C). In
conclusion, these results showed that MB-FP exhibited protective
effects against MAGEA1-expressing tumours.
Discussion
As an important member of melanoma antigen, MAGEA1
is expressed in most tumours but not in normal tissues, except
testis and ovary. MAGEA1 encodes several antigenic peptides that
bind to HLA class I molecules and are recognized by T lymphocytes.
To improve the immunoactivity of MAGEA1, several adjuvant molecules
have been used. Heat shock protein 70 (HSP70) plays an important
role in protein trafficking, folding and antigen presentation. A
previous study showed that HSP70 could be exploited to enhance the
cellular and humoural immune responses against any attached
tumour-specific antigens (TSA) (11). Moreover, the fusion proteins of
HSP70 and TSA have been demonstrated to be powerful strategies to
increase immunogenicity (24–26).
However, a targeted delivery system could effectively increase the
immunoactivity and improve the clinical applications of HSP70
fusion proteins.
Recently, gas-filled ultrasound microbubbles (MBs)
have been used in the clinic as intravenously administered
ultrasound-based contrast agents (16,27–29),
as microbubbles oscillate and vibrate when a sonic energy field is
applied and may reflect ultrasound waves, distinguishing the
microbubbles from surrounding tissues. Moreover, gas-filled
microbubbles can be used as protein carriers when the shells of
microbubbles are composed of special molecules (23,30,31).
Compared with other carriers, the components of
gas-filled microbubbles are biodegradable materials, so these
compounds do not exhibit immunogenicity, and gas-filled ultrasound
microbubbles are small and relatively stable and can remain stable
in the body. Moreover, gas-filled ultrasound microbubbles could be
broken under ultrasonic irradiation in a targeted area, achieving
targeted drug release (32–34).
In addition, the ultrasound-mediated microbubble destruction could
also increase cell membrane permeability, thereby increasing the
drug concentration in the target cell. These advantages make
gas-filled microbubbles ideal delivery carriers (20,35).
Gas-filled microbubbles are used in therapeutic
applications based on the intrinsic sonoporation ability of the
encapsulated drugs. Microbubbles coupled with ultrasound exposure
have been studied for opening the blood-brain barrier (BBB) and
delivering drugs (36–38). Combining siRNA silencing
ABCG2-loaded mPEG-PLGA-PLL nanoparticles and ultrasound-targeted
Microbubbles destruction enhanced therapeutic effect of Adriamycin
on multidrug-resistant breast cancer (39). Thrombus-targeting Microbubbles,
which have fibrinolytic drugs on the surface reduced the size of
the thrombus via ultrasound imaging without prolonging bleeding
time (40).
In the present study, we presented a new strategy
for the targeted release of HSP70-MAGEA1 fusion proteins. The
proposed polymer gas-filled microbubbles were used as targeted
delivery carriers of the fusion protein HSP70-MAGEA1 for
subcutaneous delivery around superficial lymph nodes. After the
contrast agent into the specific lymph nodes, the gas-filled
ultrasound microbubbles carrying the HSP70-MAGEA1 fusion protein
released the immunizing antigen, which effectively increased the
generation of specific antitumour immune responses.
Consistent with the immune responses, the results of
tumour treatment and protection in vivo demonstrated that
gas-filled microbubbles could increase the antitumour effects of
HSP70-MAGEA1 fusion proteins. Tumour xenografts in mice from
B16-MAGE-A1 cells were immunized with MB-FP grew significantly
slower compared with those from mice immunized with the
HSP70-MAGEA1 fusion protein alone, and the survival times of the
mice were significantly prolonged, demonstrating that gas-filled
microbubbles could enhance the therapeutic immunization of
HSP70-MAGEA1 fusion protein. Furthermore, we showed that the
HSP70-MAGEA1 delivered via gas-filled microbubbles was more
proficient at protecting against tumour development when used
earlier in the progression of cancer, suggesting the preferential
application of this tumour vaccine to prevent tumour recurrence in
postoperative cancer patients.
In conclusion, these results showed that the
gas-filled microbubble-mediated delivery of HSP70-MAGEA1 fusion
protein as a tumour vaccine could be used for targeted release at
lymph nodes, thereby effectively improving the antitumour efficacy
of the HSP70-MAGEA1 fusion protein. The present study not only
described a new strategy for targeted tumour biological treatments
using HSP70 fusion proteins but also provided a research basis for
controllable drug carriers for clinical application.
Acknowledgements
The present study was financially supported by
grants from the National Natural Science Foundation of China (nos.
81670792 and 31671416), and the Booster Programme of Xijing
Hospital.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HSP70
|
heat shock protein 70
|
|
MAGEA1
|
melanoma-associated antigen A1
|
|
MB
|
gas-filled ultrasound microbubbles
|
|
FP
|
HSP70-MAGEA1 fusion protein
|
|
MB-FP
|
gas-filled ultrasound microbubbles
with HSP70-MAGEA1 fusion protein
|
References
|
1
|
Fang JB and Wang L: The function of tumor
specific antigen (MAGE) in tumor immunotherapy. Sheng Li Ke Xue Jin
Zhan. 36:273–275. 2005.(In Chinese). PubMed/NCBI
|
|
2
|
Xiao J and Chen HS: Biological functions
of melanoma-associated antigens. World J Gastroenterol.
10:1849–1853. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xiao J and Chen HS: Biological functions
of melanoma-associated antigens (MAGEs) in cell activities. Ai
Zheng. 24:124–128. 2005.(In Chinese). PubMed/NCBI
|
|
4
|
Sudo T, Kuramoto T, Komiya S, Inoue A and
Itoh K: Expression of MAGE genes in osteosarcoma. J Orthop Res.
15:128–132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van der Bruggen P, Traversari C, Chomez P,
Lurquin C, De Plaen E, Van den Eynde BJ, Knuth A and Boon T: A gene
encoding an antigen recognized by cytolytic T lymphocytes on a
human melanoma. J Immunol. 178:2617–2621. 2007.PubMed/NCBI
|
|
6
|
van Baren N, Bonnet MC, Dréno B, Khammari
A, Dorval T, Piperno-Neumann S, Liénard D, Speiser D, Marchand M,
Brichard VG, et al: Tumoral and immunologic response after
vaccination of melanoma patients with an ALVAC virus encoding MAGE
antigens recognized by T cells. J Clin Oncol. 23:9008–9021. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Slingluff CL Jr, Petroni GR, Olson W,
Czarkowski A, Grosh WW, Smolkin M, Chianese-Bullock KA, Neese PY,
Deacon DH, Nail C, et al: Helper T-cell responses and clinical
activity of a melanoma vaccine with multiple peptides from MAGE and
melanocytic differentiation antigens. J Clin Oncol. 26:4973–4980.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mackensen A, Herbst B, Chen JL, Köhler G,
Noppen C, Herr W, Spagnoli GC, Cerundolo V and Lindemann A: Phase I
study in melanoma patients of a vaccine with peptide-pulsed
dendritic cells generated in vitro from CD34(+) hematopoietic
progenitor cells. Int J Cancer. 86:385–392. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chianese-Bullock KA, Pressley J, Garbee C,
Hibbitts S, Murphy C, Yamshchikov G, Petroni GR, Bissonette EA,
Neese PY, Grosh WW, et al: MAGE-A1-, MAGE-A10-, and gp100-derived
peptides are immunogenic when combined with granulocyte-macrophage
colony-stimulating factor and montanide ISA-51 adjuvant and
administered as part of a multipeptide vaccine for melanoma. J
Immunol. 174:3080–3086. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akiyama Y, Tanosaki R, Inoue N, Shimada M,
Hotate Y, Yamamoto A, Yamazaki N, Kawashima I, Nukaya I, Takesako
K, et al: Clinical response in Japanese metastatic melanoma
patients treated with peptide cocktail-pulsed dendritic cells. J
Transl Med. 3:42005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ge W, Sui YF, Wu DC, Sun YJ, Chen GS, Li
ZS, Si SY, Hu PZ, Huang Y and Zhang XM: MAGE-1/Heat shock protein
70/MAGE-3 fusion protein vaccine in nanoemulsion enhances cellular
and humoral immune responses to MAGE-1 or MAGE-3 in vivo. Cancer
Immunol Immunother. 55:841–849. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ge W, Li Y, Li ZS, Zhang SH, Sun YJ, Hu
PZ, Wang XM, Huang Y, Si SY, Zhang XM and Sui YF: The antitumor
immune responses induced by nanoemulsion-encapsulated
MAGE1-HSP70/SEA complex protein vaccine following peroral
administration route. Cancer Immunol Immunother. 58:201–208. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ge W, Hu PZ, Huang Y, Wang XM, Zhang XM,
Sun YJ, Li ZS, Si SY and Sui YF: The antitumor immune responses
induced by nanoemulsion-encapsulated MAGE1-HSP70/SEA complex
protein vaccine following different administration routes. Oncol
Rep. 22:915–920. 2009.PubMed/NCBI
|
|
14
|
Gkegkes ID and Iavazzo C: Contrast
Enhanced Ultrasound (CEU) using microbubbles for sentinel lymph
node biopsy in breast cancer: A systematic review. Acta Chir Belg.
115:212–218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stride E: Physical principles of
microbubbles for ultrasound imaging and therapy. Front Neurol
Neurosci. 36:11–22. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wilson SR and Burns PN:
Microbubble-enhanced US in body imaging: What role? Radiology.
257:24–39. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wan C, Li F and Li H: Gene therapy for
ocular diseases meditated by ultrasound and microbubbles (Review).
Mol Med Rep. 12:4803–4814. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suzuki R, Oda Y, Utoguchi N, Namai E,
Taira Y, Okada N, Kadowaki N, Kodama T, Tachibana K and Maruyama K:
A novel strategy utilizing ultrasound for antigen delivery in
dendritic cell-based cancer immunotherapy. J Control Release.
133:198–205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lemmon JC, McFarland RJ, Rybicka JM, Balce
DR, McKeown KR, Krohn RM, Matsunaga TO and Yates RM: In vitro and
in vivo transfection of primary phagocytes via microbubble-mediated
intraphagosomal sonoporation. J Immunol Methods. 371:152–158. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bioley G, Lassus A, Bussat P, Terrettaz J,
Tranquart F and Corthésy B: Gas-filled microbubble-mediated
delivery of antigen and the induction of immune responses.
Biomaterials. 33:5935–5946. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bioley G, Bussat P, Lassus A, Schneider M,
Terrettaz J and Corthésy B: The phagocytosis of gas-filled
microbubbles by human and murine antigen-presenting cells.
Biomaterials. 33:333–342. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ye J, Chen GS, Song HP, Li ZS, Huang YY,
Qu P, Sun YJ, Zhang XM and Sui YF: Heat shock protein 70/MAGE-1
tumor vaccine can enhance the potency of MAGE-1-specific cellular
immune responses in vivo. Cancer Immunol Immunother. 53:825–834.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li S, Zhu C, Fang S, Zhang W, He N, Xu W,
Kong R and Shang X: Ultrasound microbubbles enhance human
β-defensin 3 against biofilms. J Surg Res. 199:458–469. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma JH, Sui YF, Ye J, Huang YY, Li ZS, Chen
GS, Qu P, Song HP and Zhang XM: Heat shock protein 70/MAGE-3 fusion
protein vaccine can enhance cellular and humoral immune responses
to MAGE-3 in vivo. Cancer Immunol Immunother. 54:907–914. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suzue K and Young RA: Adjuvant-free hsp70
fusion protein system elicits humoral and cellular immune responses
to HIV-1 p24. J Immunol. 156:873–879. 1996.PubMed/NCBI
|
|
26
|
Mizukami S, Kajiwara C, Ishikawa H,
Katayama I, Yui K and Udono H: Both CD4+ and CD8+ T cell epitopes
fused to heat shock cognate protein 70 (hsc70) can function to
eradicate tumors. Cancer Sci. 99:1008–1015. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shen ZY, Wu MF, Zhang YX, Shen K and Xia
GL: Treatment of hepatic carcinoma by low-frequency ultrasound and
microbubbles: A case report. Oncol Lett. 9:1249–1253. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wallace N and Wrenn SP: Ultrasound
triggered drug delivery with liposomal nested microbubbles.
Ultrasonics. 63:31–38. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song S, Guo H, Jiang Z, Jin Y, Wu Y, An X,
Zhang Z, Sun K and Dou H: Self-assembled microbubbles as contrast
agents for ultrasound/magnetic resonance dual-modality imaging.
Acta Biomater. 24:266–278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin T, Cai XZ, Shi MM, Ying ZM, Hu B, Zhou
CH, Wang W, Shi ZL and Yan SG: In vitro and in vivo evaluation of
vancomycin-loaded PMMA cement in combination with ultrasound and
microbubbles-mediated ultrasound. Biomed Res Int. 2015:3097392015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kato S, Shirai Y, Kanzaki H, Sakamoto M,
Mori S and Kodama T: Delivery of molecules to the lymph node via
lymphatic vessels using ultrasound and nano/microbubbles.
Ultrasound Med Biol. 41:1411–1421. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Palekar-Shanbhag P, Chogale MM, Jog SV and
Gaikwad SS: Microbubbles and their applications in pharmaceutical
targeting. Curr Drug Deliv. 10:363–373. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Castle J and Feinstein SB:
Ultrasound-directed, site-specific gene delivery. Methods Mol Biol.
1141:67–76. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Byrn SR and Stowell JG: Drug targeting
using conjugates: The importance of pharmaceutical chemistry. J
Drug Target. 3:239–241. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jiang J, Xie D, Zhang W, Xiao G and Wen J:
Fusion of Hsp70 to Mage-a1 enhances the potency of vaccine-specific
immune responses. J Transl Med. 11:3002013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang FY, Fu WM, Chen WS, Yeh WL and Lin
WL: Quantitative evaluation of the use of microbubbles with
transcranial focused ultrasound on blood-brain-barrier disruption.
Ultrason Sonochem. 15:636–643. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hernot S and Klibanov AL: Microbubbles in
ultrasound-triggered drug and gene delivery. Adv Drug Deliv Rev.
60:1153–1166. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tung YS, Vlachos F, Feshitan JA, Borden MA
and Konofagou EE: The mechanism of interaction between focused
ultrasound and microbubbles in blood-brain barrier opening in mice.
J Acoust Soc Am. 130:3059–3067. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bai M, Shen M, Teng Y, Sun Y, Li F, Zhang
X, Xu Y, Duan Y and Du L: Enhanced therapeutic effect of Adriamycin
on multidrug resistant breast cancer by the ABCG2-siRNA loaded
polymeric nanoparticles assisted with ultrasound. Oncotarget.
6:43779–43790. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang X, Gkanatsas Y, Palasubramaniam J,
Hohmann JD, Chen YC, Lim B, Hagemeyer CE and Peter K:
Thrombus-targeted theranostic microbubbles: A new technology
towards concurrent rapid ultrasound diagnosis and bleeding-free
fibrinolytic treatment of thrombosis. Theranostics. 6:726–738.
2016. View Article : Google Scholar : PubMed/NCBI
|