Myocardial ischemia was one of the main causes of
sudden cardiac death in the past decades (1). Acute myocardial infarction is a
leading cause of death worldwide (2). Strategies for reducing
ischemia/reperfusion (I/R)-induced injury in cardiomyocytes are
receiving considerable attention due to the failure of
cardiomyocytes to regenerate (1).
Coronary reperfusion is the most effective treatment for ischemic
diseases. However, it may initially aggravate cellular damage
during the ischemic period (3).
Cardiac ischemic preconditioning (IPC), which is
achieved through repeated brief I/R periods, is one of the
well-known protective strategies of the myocardium against I/R
injury (4). Recently, autophagy
has been linked to IPC-mediated cardioprotection (5). In addition, a number of
pharmaceutical therapies targeting I/R injury have been developed
to orchestrate multiple protein complexes and signaling pathways in
autophagy (6,7). In this review, we aim to draw
attention to the role of autophagy in cardioprotection.
Autophagy is a self-protective mechanism of living
cells under various stress conditions (8). During autophagy, cellular cytoplasm
constituents are delivered to lysosomes for degradation and
recycling (9). Autophagy limits
the production of reactive oxygen species and excessive protein
aggregation to maintain intracellular or extracellular homeostasis
(10). Autophagy has emerged as a
potential drug target for numerous diseases including cancer,
neurodegenerative diseases, and cardiovascular disease (11,12).
Myocardial I/R injury is a complex process that
destroys proteins, DNA, and plasma membrane, thereby resulting in
cell death and decreased cardiac output (20,21).
Many studies have reported an increase in the number of
autophagosomes in the heart during I/R in animal models (5,22).
Autophagy induced by ischemia was subsequently enhanced by
reperfusion in isolated rabbit hearts (23) and in mouse hearts (24). The activation of autophagy is
reflected in the abundance of autophagy-related protein pathways,
such as light chain 3 (LC3), Beclin-1, autophagy-related gene (ATG)
5–12 complex, and p62 (25–27).
Hu et al reported that approximately 20 min of aortic
clamping with hyperkalemic cold blood cardioplegia to achieve total
autophagy, which in accordance with previous evidence (28). The abundance of autophagic proteins
will actually decrease with the progress of autophagy because of
self-degradation (25,26). In particular, in biopsies from the
right atrial appendage of patients undergoing valve surgery or
coronary artery bypass grafting, the expression of
autophagy-related proteins, including LC3-I, LC3-II, ATG5-12,
Beclin-1, and p62, is reduced during reperfusion (26).
Until recently, the debate continues whether
autophagy plays a protective or deleterious role in the I/R injury
process. On the one hand, modest levels of autophagy triggered by
mild to moderate hypoxia/ischemia are protective and seem to
prevent the activation of apoptosis (23,29).
On the other hand, high levels of autophagy induced by severe
hypoxia or I/R may cause self-digestion and eventual cell death
(30). Therefore, autophagic flux
induced by ischemia during the early stage of I/R has been
speculated to be beneficial; however, it is harmful during
reperfusion at the later stage of I/R (15,19).
Autophagy may play an alternative role in I/R, which
determines cell fate. The extent of autophagy in response to
ischemia is considered based on the severity and duration of
ischemic insults (31). Nutrient
and oxygen deprivation in the heart threatens cellular survival
during I/R, and increased autophagy may provide at least a
temporary reprieve for a threatened myocardium by serving as a
source of intracellular nutrients (32). Oxidative stress, calcium overload,
endoplasmic reticulum (ER) stress, and mitochondrial dysfunction
maintain a high level of autophagy during reperfusion (33). However, high levels or long-term
upregulation of autophagy can lead to excessive degradation of
essential proteins and organelles (34). If intracellular energy sources
become inadequate, then autophagic processes will be a particular
form of cell death, called type II or autophagic cell death
(35). In fact, aware that
necrosis and apoptosis are not the only mechanisms of cell death is
increasing (36). Autophagic cell
death has been identified as a cell death phenotype via electron
microscope observations; it has a morphological term characterized
by abundant autophagic vacuoles in the cytoplasm (37,38).
Moreover, increased autophagy after I/R is not due
to increased autophagosome formation, but instead, to impaired
clearance of autophagosomes (39);
this assumption is derived from the concept of autophagic flux
(40). Furthermore, a rapid
decline induced by reperfusion in LAMP2, which is a critical
protein for autophagosome-lysosome fusion, can impair autophagosome
processing and mitochondrial permeabilization, thereby increasing
ROS generation and triggering cardiomyocyte death (41). In addition, when the engulfed
targets or autophagosomes cannot fuse with lysosomes and digest
their contents, a cell may eject the autophagosomes as a response,
which induces an acute and significant inflammatory response
(42).
Autophagy is a complex and dynamic multi-step
process that depends on strict regulation and coordination through
multiple signaling pathways (43).
To date, several cellular signaling pathways are considered to
trigger autophagy in I/R. In addition, autophagy has been shown to
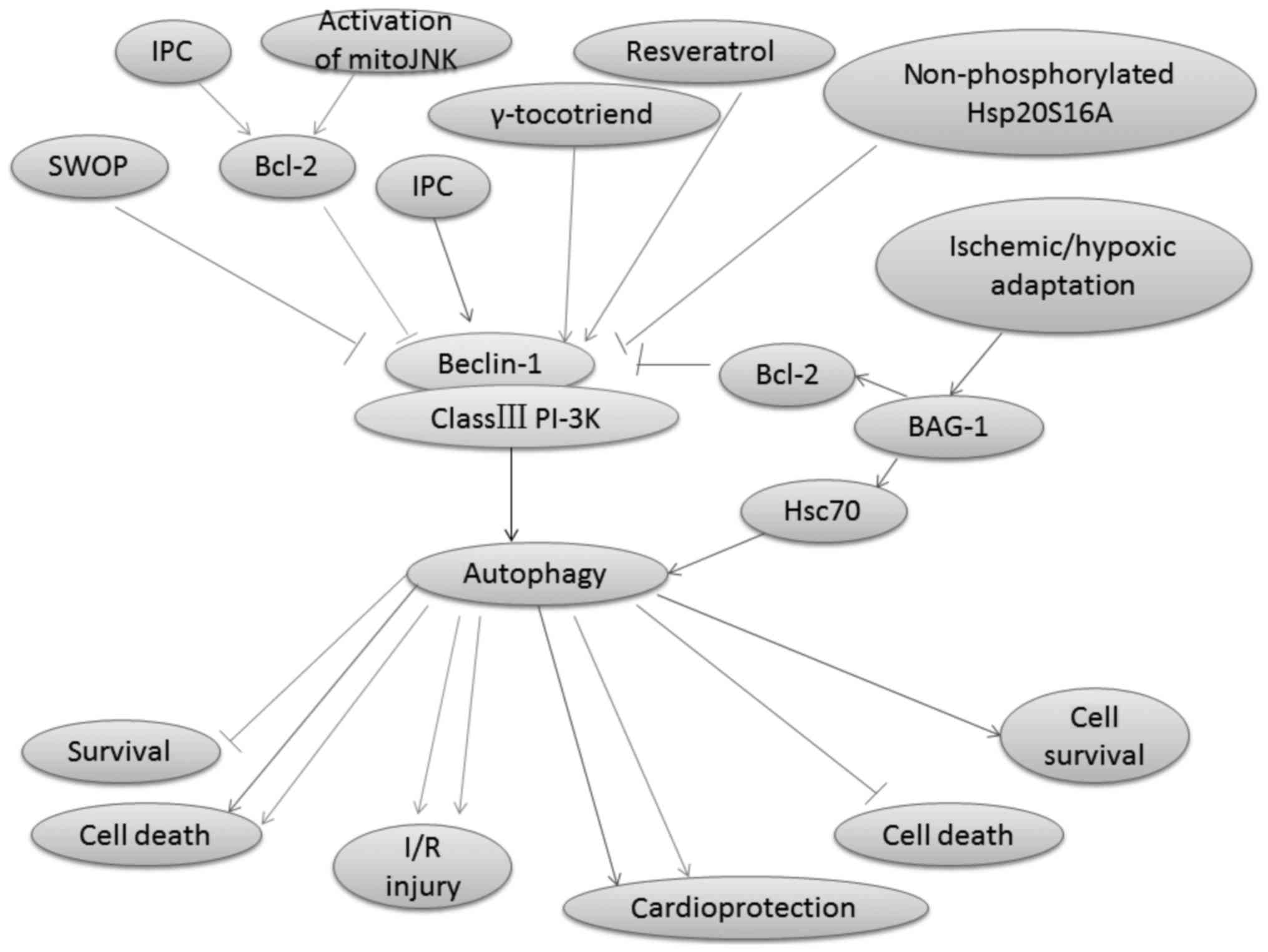
be regulated by several signaling pathways (44), including Beclin-1/class III
phosphatidylinositol-3 kinase (PI-3K), AMPK/mammalian target of
rapamycin (mTOR), and PI-3K/Akt/mTOR pathways.
Beclin-1, which is a phylogenetically conserved
protein, the mammalian homologue of the yeast Atg6, and the
interacting protein of the anti-apoptotic protein Bcl-2, is a key
molecule involved in mediating autophagy (45,46).
It plays a crucial role in engaging class III PI-3K to positively
modulate autophagy in mammalian cells (47,48).
Autophagy in mammalian cells is reported to be activated by the
class III PI-3K complex, which contains Vps34 and Beclin-1
(29,49). Moreover, a coiled-coil domain (aa
140–268) is present in this 450 amino acid-long protein in
Beclin-1; this domain can mediate binding to class III PI-3K Vps34
by interacting with an evolutionarily conserved domain (ECD; aa
244–337) (50). RNA interference
of Beclin-1, which inhibits autophagy, will subsequently enhance
cardiac cell survival (51).
Autophagy is involved in delayed cardioprotection
induced by sevoflurane preconditioning (52). Sevoflurane preconditioning reduces
the autophagy induced by H/R by decreasing the Beclin-1 expression
(52). Accordingly, IPC protects
the rat heart against MI/R injury by inhibiting Bcl-2 dissociation
from Beclin-1 during the reperfusion phase in vivo, although
IPC-induced autophagy reflects a compensatory pro-survival response
to I/R injury (53). Bcl-2 is the
prototype of a protein family, which contains at least one Bcl-2
homology (BH) region (54). Bcl-2
binding molecules have been recently shown to regulate autophagy
activation (55). Transgenic mice
with a cardiac-specific overexpression of Bcl-2 are protected from
I/R injury (56,57). Autophagy is disrupted when Bcl-2
binds to Beclin-1 (58). In
addition, when a mutant of Beclin-1 that lacks the Bcl-2 binding
domain is overexpressed in cells, excessive autophagy and cell
death are induced (47). Bcl-2 can
also inhibit Beclin-1/Vsp34 PI-3K complex formation and the
activity of Beclin-1-associated class III PI-3K (53). Furthermore, the class III PI-3K
autophagic pathway is inhibited by combining the BH3 hydrophobic
groove in Bcl-2 and the BH3-like amphipathic α-helix in Beclin-1
(59). However, the interaction
with Bcl-2 (and Bcl-xL) in the ER, rather than in the mitochondria,
inhibits the Beclin-1 activity in autophagy (60). The interaction between Bcl-2 and
Beclin-1 maintains autophagy at levels (47). Blocking the interaction between the
BH3 domains of Beclin-1 and Bcl-2 increases autophagic activity
(53). Recent studies indicate
that the increase in the interaction between Beclin-1 and Bcl-2 is
caused by IPC (53). C-Jun
N-terminal kinase (JNK), which is a member of an evolutionarily
conserved subfamily of mitogen-activated protein kinases, is
critical for the cellular responses of multiple environmental and
cellular stimuli (61,62). I/R can trigger Bcl2-regulated
autophagy by inducing a dominant increase in mitoJNK activation,
which causes cell death (63). Xu
et al reported that mitoJNK activation, and not JNK
mitochondrial localization, induced autophagy, which further
aggravates I/R injury (63). In
addition, the mitoJNK phosphorylate Bcl2, which antagonizes Bcl2
anti-apoptotic and anti-autophagic activities, may contribute to
the deleterious role of mitoJNK in I/R injury (64,65).
Heat shock protein (Hsp20) is the only member of the
sHsps family that contains the consensus peptide motif RRAS for
protein kinase A-/protein kinase G-dependent phosphorylation at
Ser16 (66). Qian et al
demonstrated that non-phosphorylated Hsp20S16A is detrimental in
I/R injury because it suppresses autophagy and further increases
cell death (36). Ischemic/hypoxic
adaptation improves cardiac cell survival by suppressing the BAG-1
protein expression (67). BAG-1
can bind with both Bcl-2 and Hsc70 molecules (67). Autophagosomal membrane contains a
significantly higher amount of Hsc70 proteins (68). BAG-1 has been shown exhibit
numerous functions through its interaction with Hsc70 (69). The treatment of rats with
wortmannin, an inhibitor of class III PI-3K, has been used to
suppress autophagy in many studies (70,71),
and attenuates both the LC3-II and BAG-1 protein expressions
(67). Zheng et al
(72) reported that the activated
PI3K/Akt pathway contributes to the berbamine
postconditioning-induced cardioprotection through modulating
autophagy. The Beclin-1/class III PI-3K pathway-regulated autophagy
and autophagy-mediated function in I/R injury are shown in Fig. 1.
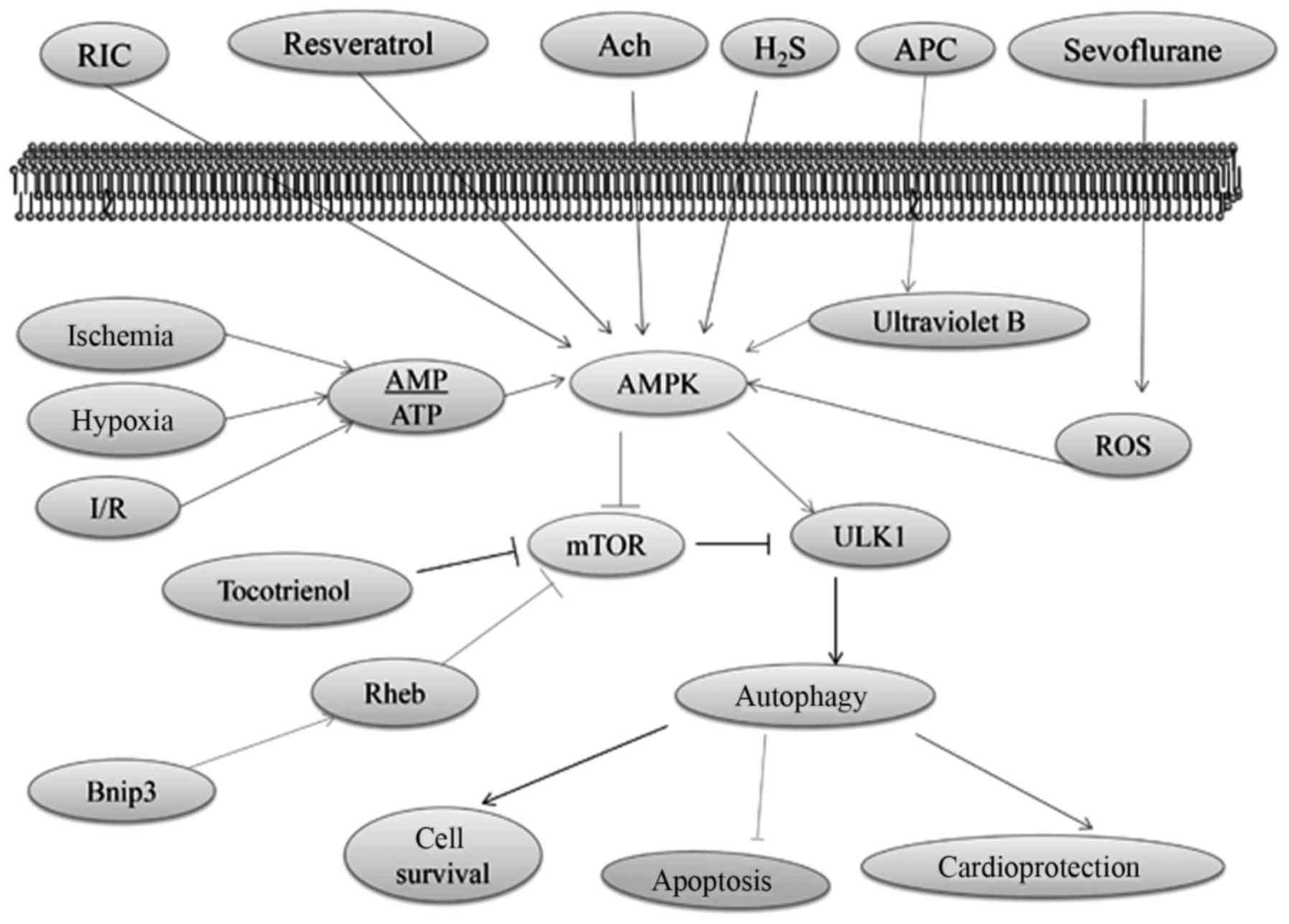
AMPK, which is activated in response to stress that
exhaust cellular ATP supplies, such as ischemia and hypoxia, plays
a crucial role as a master regulator of cellular energy homeostasis
(73). AMPK is ubiquitously
expressed in metabolically active tissues, such as cardiac muscles,
and activated upon the depletion of energy stores by functioning as
an intracellular fuel sensor (74). Ischemia has been proposed to
stimulate autophagy via an AMPK-dependent mechanism (53), which is one of the most significant
approaches in upregulating autophagy (75,76).
During I/R injury, intracellular ATP stores are rapidly consumed
and cannot be supplemented with decreasing glucose supply (77). AMPK signaling can positively
regulate autophagy by activating Ulk1 via the phosphorylation of
Ser 317 and Ser 777 or indirectly by inhibiting mTOR signaling
(78,79). Moreover, AMPK functions as a master
regulator of the autophagy pathway through inactivating mTOR
(80).
High mTOR activity negatively regulates autophagy by
inhibiting the activation of Ulk1, which is one of the mammalian
autophagy-initiating kinases that is important for membrane
nucleation via the phosphorylation of Ulk1 Ser 757 (1,81,82).
The association between ATG1 and ATG13 is negatively regulated by
mTOR, which inhibits autophagy (83). In addition, the activation of Ulk1
and its combination with other molecules, such as ATG13 and FIP200,
initiate the nucleation of the autophagic membrane (1). Lekli et al proposed that
tocotrienol could induce autophagy through the mTOR pathway, which
would consequently lead to cell survival and cardioprotection
(84). The overexpression of
Bnip3, a hypoxia-inducible Bcl-2 homology 3 domain-containing
protein (85) and the
pro-apoptotic molecule present in the mitochondrial membrane; can
upregulate autophagy and protect cardiac myocytes against I/R
injury-related apoptosis (86).
The high-mobility group box 1 protein (HMGB1)-mediated activation
of mTOR inhibits hypoxia and reoxygenation injury in rat
cardiomyocytes (87,88). Moreover, Bnip3 can inhibit the mTOR
pathway and induce autophagy by directly binding to the Ras homolog
that is enriched in the brain (Rheb), which is a Ras-related small
guanosine triphosphatase (85,89).
The cardioprotection effect of resveratrol has been
shown to induce autophagy by facilitating AMPK activation (90,91).
In addition, AMPK expression is elevated with ACh during H/R
(92). ACh activates
cytoprotective autophagy through the AMPK-mTOR-dependent pathway
that is activated by a muscarinic receptor (92). Xie et al found that the
post-reperfusion AMPK activation induced by a slow-releasing
organic H2S donor that could restore I/R impaired
autophagic flux; is critical to H2S cardioprotection
(93). Accordingly, studies have
proven that autophagy activation through the AMPK/mTOR pathway
plays a cardioprotection role (94). Recently, ultraviolet B-induced
autophagy has been found to activate AMPK by inhibiting the
phosphorylation of GSK3β (95),
which is a critical mediator of cardioprotection via anesthetic
preconditioning (96). Sevoflurane
provides cardioprotection against I/R injury via the ROS-mediated
upregulation of autophagy (97).
Hariharan et al reported that oxidative stress triggers
autophagic flux during MI/R injury (98). In addition, trimetazidine (99) and thioredoxin-2 (100) protect against
hypoxia/reoxygenation injury by promoting the AMPK-dependent
autophagic flux in H9c2 cardiomyocytes. The AMPK/mTOR
pathway-regulated autophagy and autophagy-mediated function in I/R
injury are illustrated in Fig.
2.
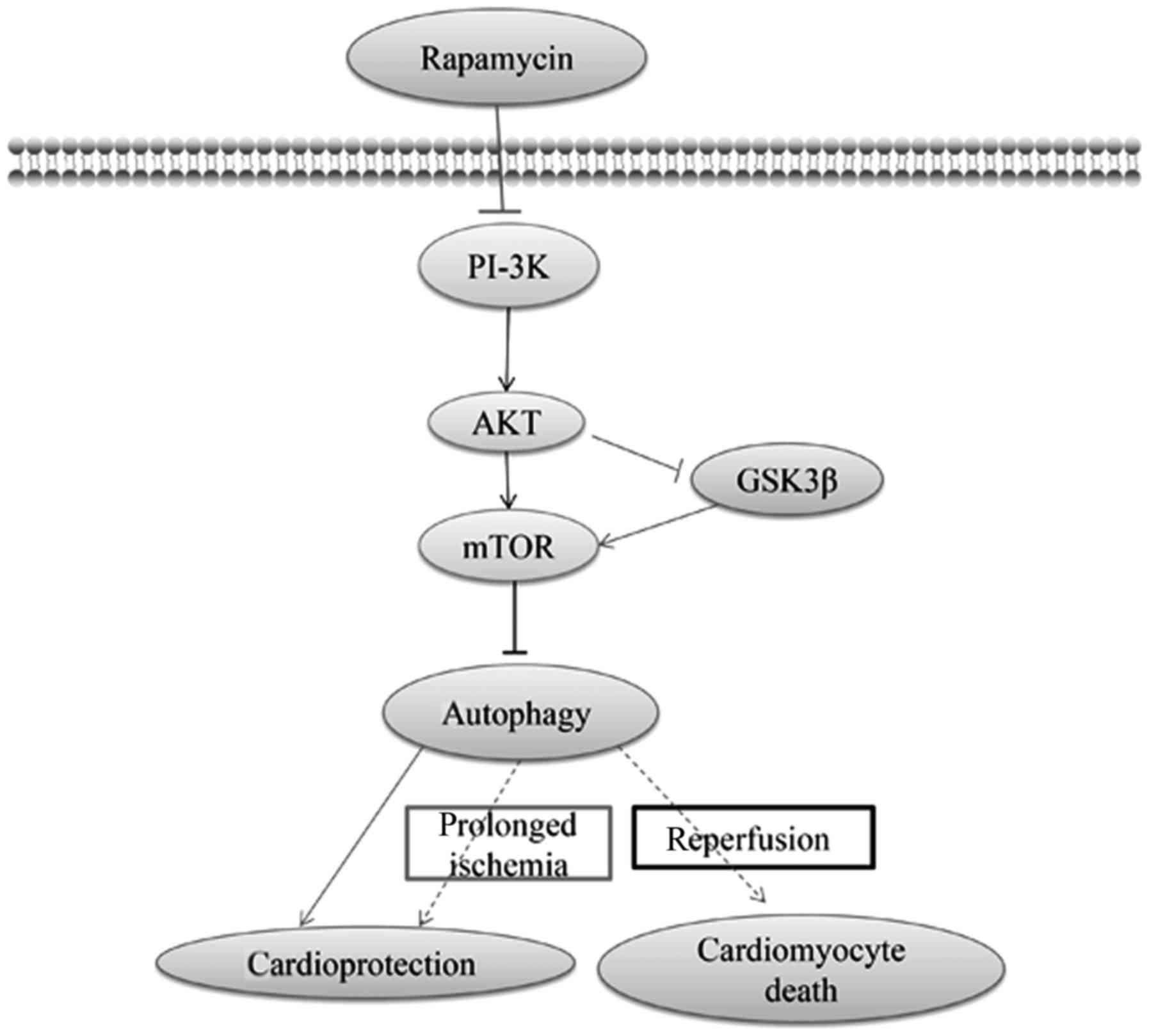
PI-3K/Akt/mTOR signaling may also provide an
additional benefit against I/R injury (101). In addition, class III PI-3K
focuses on the formation of autophagosomes, whereas class I PI-3K
will inhibit the induction of autophagy through the phosphorylation
of Akt and mTOR (70). Thus, the
interaction of Akt with mTOR is multifaceted and bidirectional
(101). Moreover, the
self-regulation of autophagy has been postulated to be regulated by
the autophagy-induced inhibition of mTOR (102). Furthermore, rapamycin provided a
strong beneficial effect against cardiomyocyte anoxia/reoxygenation
injury, which would mediate cardioprotection via autophagy that
probably depended on the PI-3K/Akt signaling pathway (103). During prolonged ischemia and I/R,
the differential effects of GSK-3β, which is the downstream of
PI-3K/Akt, on myocardial injury has been suggested to be determined
by changes in autophagy (104).
GSK-3β inhibition modulates mTOR-dependent attenuation of
autophagy, thereby causing the death of cardiomyocytes during
prolonged ischemia while mediating their survival during
reperfusion (104). In addition,
mTOR activation via GSK-3β has been suggested to provide
cardioprotection via autophagy (104). The PI-3K/Akt/mTOR
pathway-regulated autophagy and autophagy-mediated function in I/R
injury are illustrated in Fig.
3.
The p53 transcription factor is a major regulator of
cellular response to acute stress (105). Knockdown of p53 can activate
autophagy in cardiomyocytes, thereby protecting the myocardium
against ischemic injury (106).
Autophagy is inhibited with STAT1 to modulate stress response to
I/R in STAT1−/− null hearts (32). In I/R, STAT1 can interact directly
with p53 and regulate its functional activity (107), thereby suggesting that STAT1 can
act with p53 to modulate autophagy (32). The mitochondrial permeability
transition pore (MPTP) plays an important role in myocardial I/R
injury, and the opening of MPTP has also been shown to trigger
autophagy (108). Making the
heart more tolerant to subsequent I/R injury is a crucial step in
transient MPTP opening before prolonged ischemia (109,110). Moreover, PKC has been reported to
trigger the phosphorylation of a regulatory sub-unit of VPATPase,
which subsequently induces autophagy (111–113).
Microarray analysis showed that autophagy-associated
genes and the unfolded protein response were upregulated under the
condition of repetitive coronary occlusion achieved during chronic
local ischemic conditioning in mice (114,115). In another study, inhibiting mTOR
decreased infarct size in mice (116). Rapamycin (116), caloric restriction (117), exercise (118), nitric oxide (119), and lipopolysaccharide (120) has been identified as
cardioprotective interventions for triggering autophagy. Gurusamy
et al used isolated rat heart models and demonstrated that
the induction of IPC via repeated I/R cycles immediately enhanced
the expression of LC3-II and Beclin-1 (67). In vivo swine models, infarct
size was limited after chloramphenicol succinate was used before
ischemia (121). Han et al
found that cardioprotection induced by remote limb ischemic
postconditioning was associated with elevated autophagy 3 h
post-reperfusion (2). A similar
phenomenon was observed by Hamacher-Brady et al in HL-1
myocytes; they found that simulated I/R-mediated cell death was
prevented by strengthening autophagy, whereas its inhibition caused
cell death (40). Other
researchers have observed that blocking autophagy via
cell-permeable Tat-Atg5K130R concurrently increased infarct size in
hearts when treated with SUL (39). Moreover, Tibetan patients with
coronary heart disease resist I/R injury during cardiac surgery
better than patients living at sea level, which is possibly
correlated with the upregulation of basal autophagy resulting from
chronic hypoxia (28).
Autophagy has been determined as a significant
element of the endogenous defense mechanisms activated by various
preconditioning types. Induction of autophagy may represent a novel
therapeutic approach to myocardial protection in humans (39). The identification of agents that
can rapidly induce autophagy can contribute to the discovery of new
cardioprotective drugs (122). In
addition, induction of autophagy can preserve heart function during
I/R injury (91,121,123). Other studies have suggested that
autophagy is detrimental because it contributes to cell death
(15,124). The beneficial or detrimental role
of autophagy may be a consequence of balance, depending on the
extent of autophagy (7). Thus, for
autophagy to be effective, searching for a candidate
cardioprotective drug that can induce autophagy in a target
population is important, together with the appropriate timing and
response magnitude (16). Various
autophagy modulators on cardiac ischemia/injury are summarized in
Table I.
Recent studies have shown that autophagy plays an
important role in I/R injury. Moreover, evidence has emerged that
autophagy plays various roles in I/R through multiple mechanisms.
Autophagy can trigger a survival signal in the case of myocardial
ischemia, whereas defective autophagy during reperfusion is
detrimental. Although we have obtained substantial knowledge about
the function of autophagy in I/R injury, the autophagy pathway is
highly complex and remains far from being understood completely.
Additional studies are necessary to identify the molecular
components of the autophagy pathway, characterize the role of
autophagy in I/R injury, elucidate the diverse processes that
regulate autophagy expression and activity, and determine the
contribution of autophagy to myocardial infarction protection in
humans. Such studies will provide additional insights into the role
of autophagy in I/R injury and potentially discover novel
therapeutic strategies for treating the diseases.
Not applicable.
The present study was supported by grants from the
National natural science foundation of china (grant no. 81600342)
and Graduate student research innovation project of Hunan province
(grant no. CX2013B397).
Not applicable.
X-LL and M-HL conceived and designed this review.
X-LL, L-LX and W-JX contributed the central idea, analyzed most of
the data, and wrote the initial draft of the paper. L-LX and M-HL
revised the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Yang K, Xu C, Li X and Jiang H:
Combination of D942 with curcumin protects cardiomyocytes from
ischemic damage through promoting autophagy. J Cardiovasc Pharmacol
Therap. 18:570–581. 2013. View Article : Google Scholar
|
|
2
|
Han Z, Cao J, Song D, Tian L, Chen K, Wang
Y, Gao L, Yin Z, Fan Y and Wang C: Autophagy is involved in the
cardioprotection effect of remote limb ischemic postconditioning on
myocardial ischemia/reperfusion injury in normal mice, but not
diabetic mice. PLoS One. 9:e868382014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murphy E and Steenbergen C: Mechanisms
underlying acute protection from cardiac ischemia-reperfusion
injury. Physiol Rev. 88:581–609. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Das DK and Maulik N: Preconditioning
potentiates redox signaling and converts death signal into survival
signal. Arch Biochem Biophys. 420:305–311. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aoyagi T, Kusakari Y, Xiao CY, Inouye BT,
Takahashi M, Scherrer-Crosbie M, Rosenzweig A, Hara K and Matsui T:
Cardiac mTOR protects the heart against ischemia-reperfusion
injury. Am J Physiol Heart Circ Physiol. 303:H75–H85. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Y, Xiang Y, Zhang S, Wang Y, Yang J,
Liu W and Xue F: Intramyocardial injection of thioredoxin
2-expressing lentivirus alleviates myocardial ischemia-reperfusion
injury in rats. Am J Transl Res. 9:4428–4439. 2017.PubMed/NCBI
|
|
7
|
Sasaki Y, Ikeda Y, Iwabayashi M, Akasaki Y
and Ohishi M: The impact of autophagy on cardiovascular senescence
and diseases. Int Heart J. 58:666–673. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang L, Wang H, Shen Q, Feng L and Jin H:
Long non-coding RNAs involved in autophagy regulation. Cell Death
Dis. 8:e30732017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Green DR, Galluzzi L and Kroemer G:
Mitochondria and the autophagy-inflammation-cell death axis in
organismal aging. Science. 333:1109–1112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sica V, Galluzzi L, Pedro Bravo-San JM,
Izzo V, Maiuri MC and Kroemer G: Organelle-specific initiation of
autophagy. Mol Cell. 59:522–539. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mowers EE, Sharifi MN and MacLeod KF:
Functions of autophagy in the tumor microenvironment and cancer
metastasis. FEBS J. 2018.doi: 10.1111/febs.14388. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pellacani C and Costa LG: Role of
autophagy in environmental neurotoxicity. Environ Pollut.
235:791–805. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang X, Cohen MV and Downey JM: Mechanism
of cardioprotection by early ischemic preconditioning. Cardiovasc
Drugs Ther. 24:225–234. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Levine B and Klionsky DJ: Development by
self-digestion: Molecular mechanisms and biological functions of
autophagy. Dev Cell. 6:463–477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma H, Guo R, Yu L, Zhang Y and Ren J:
Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial
ischaemia/reperfusion injury: Role of autophagy paradox and toxic
aldehyde. Eur Heart J. 32:1025–1038. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang C, Yitzhaki S, Perry CN, Liu W,
Giricz Z, Mentzer RM Jr and Gottlieb RA: Autophagy induced by
ischemic preconditioning is essential for cardioprotection. J
Cardiovasc Transl Res. 3:365–373. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shintani T and Klionsky DJ: Autophagy in
health and disease: A double-edged sword. Science. 306:990–995.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rubinsztein DC, Gestwicki JE, Murphy LO
and Klionsky DJ: Potential therapeutic applications of autophagy.
Nat Rev Drug Discov. 6:304–312. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sciarretta S, Hariharan N, Monden Y,
Zablocki D and Sadoshima J: Is autophagy in response to ischemia
and reperfusion protective or detrimental for the heart? Pediatr
Cardiol. 32:275–281. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Go AS, Mozaffarian D, Roger VL, Benjamin
EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, et al:
Executive summary: Heart disease and stroke statistics-2014 update:
A report from the American Heart Association. Circulation.
129:399–410. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bulluck H, Yellon DM and Hausenloy DJ:
Reducing myocardial infarct size: Challenges and future
opportunities. Heart. 102:341–348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hamacher-Brady A, Brady NR, Logue SE,
Sayen MR, Jinno M, Kirshenbaum LA, Gottlieb RA and Gustafsson AB:
Response to myocardial ischemia/reperfusion injury involves Bnip3
and autophagy. Cell Death Differ. 14:146–157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Decker RS and Wildenthal K: Lysosomal
alterations in hypoxic and reoxygenated hearts. I. Ultrastructural
and cytochemical changes. Am J Pathol. 98:425–444. 1980.PubMed/NCBI
|
|
24
|
Matsui Y, Takagi H, Qu X, Abdellatif M,
Sakoda H, Asano T, Levine B and Sadoshima J: Distinct roles of
autophagy in the heart during ischemia and reperfusion: Roles of
AMP-activated protein kinase and Beclin 1 in mediating autophagy.
Circ Res. 100:914–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kassiotis C, Ballal K, Wellnitz K, Vela D,
Gong M, Salazar R, Frazier OH and Taegtmeyer H: Markers of
autophagy are downregulated in failing human heart after mechanical
unloading. Circulation. 120 11 Suppl:S191–S197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jahania SM, Sengstock D, Vaitkevicius P,
Andres A, Ito BR, Gottlieb RA and Mentzer RM Jr: Activation of the
homeostatic intracellular repair response during cardiac surgery. J
Am Coll Surg. 216:719–729. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schiattarella GG and Hill JA: Therapeutic
targeting of autophagy in cardiovascular disease. J Mol Cell
Cardiol. 95:86–93. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu Y, Sun Q, Li Z, Chen J, Shen C, Song Y
and Zhong Q: High basal level of autophagy in high-altitude
residents attenuates myocardial ischemia-reperfusion injury. J
Thorac Cardiovasc Surg. 148:1674–1680. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hamacher-Brady A, Brady NR and Gottlieb
RA: The interplay between pro-death and pro-survival signaling
pathways in myocardial ischemia/reperfusion injury: Apoptosis meets
autophagy. Cardiovasc Drugs Ther. 20:445–462. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gustafsson AB and Gottlieb RA: Eat your
heart out: Role of autophagy in myocardial ischemia/reperfusion.
Autophagy. 4:416–421. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song X, Kusakari Y, Xiao CY, Kinsella SD,
Rosenberg MA, Scherrer-Crosbie M, Hara K, Rosenzweig A and Matsui
T: mTOR attenuates the inflammatory response in cardiomyocytes and
prevents cardiac dysfunction in pathological hypertrophy. Am J
Physiol Cell Physiol. 299:C1256–C1266. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
McCormick J, Suleman N, Scarabelli TM,
Knight RA, Latchman DS and Stephanou A: STAT1 deficiency in the
heart protects against myocardial infarction by enhancing
autophagy. J Cell Mol Med. 16:386–393. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gustafsson AB and Gottlieb RA: Autophagy
in ischemic heart disease. Circ Res. 104:150–158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
House SL, Branch K, Newman G, Doetschman T
and Jel Schultz J: Cardioprotection induced by cardiac-specific
overexpression of fibroblast growth factor-2 is mediated by the
MAPK cascade. Am J Physiol Heart Circ Physiol. 289:H2167–H2175.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Baehrecke EH: Autophagy: Dual roles in
life and death? Nat Rev Mol Cell Biol. 6:505–510. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qian J, Ren X, Wang X, Zhang P, Jones WK,
Molkentin JD, Fan GC and Kranias EG: Blockade of Hsp20
phosphorylation exacerbates cardiac ischemia/reperfusion injury by
suppressed autophagy and increased cell death. Circ Res.
105:1223–1231. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tsujimoto Y and Shimizu S: Another way to
die: Autophagic programmed cell death. Cell Death Differ. 12 Suppl
2:S1528–S1534. 2005. View Article : Google Scholar
|
|
38
|
Galluzzi L, Maiuri MC, Vitale I, Zischka
H, Castedo M, Zitvogel L and Kroemer G: Cell death modalities:
Classification and pathophysiological implications. Cell Death
Differ. 14:1237–1243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang C, Liu W, Perry CN, Yitzhaki S, Lee
Y, Yuan H, Tsukada YT, Hamacher-Brady A, Mentzer RM Jr and Gottlieb
RA: Autophagy and protein kinase C are required for
cardioprotection by sulfaphenazole. Am J Physiol Heart Circ
Physiol. 298:H570–H579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hamacher-Brady A, Brady NR and Gottlieb
RA: Enhancing macroautophagy protects against ischemia/reperfusion
injury in cardiac myocytes. J Biol Chem. 281:29776–29787. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huber SM, Misovic M, Mayer C, Rodemann HP
and Dittmann K: EGFR-mediated stimulation of sodium/glucose
cotransport promotes survival of irradiated human A549 lung
adenocarcinoma cells. Radiother Oncol. 103:373–379. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gottlieb RA and Mentzer RM: Autophagy
during cardiac stress: Joys and frustrations of autophagy. Annu Rev
Physiol. 72:45–59. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sciarretta S, Zhai P, Shao D, Zablocki D,
Nagarajan N, Terada LS, Volpe M and Sadoshima J: Activation of
NADPH oxidase 4 in the endoplasmic reticulum promotes cardiomyocyte
autophagy and survival during energy stress through the protein
kinase RNA-activated-like endoplasmic reticulum kinase/eukaryotic
initiation factor 2α/activating transcription factor 4 pathway.
Circ Res. 113:1253–1264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wei L, Wu RB, Yang CM, Zheng SY and Yu XY:
Cardioprotective effect of a hemoglobin-based oxygen carrier on
cold ischemia/reperfusion injury. Cardiology. 120:73–83. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Boya P and Kroemer G: Beclin 1: A BH3-only
protein that fails to induce apoptosis. Oncogene. 28:2125–2127.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zalckvar E, Berissi H, Eisenstein M and
Kimchi A: Phosphorylation of Beclin 1 by DAP-kinase promotes
autophagy by weakening its interactions with Bcl-2 and Bcl-XL.
Autophagy. 5:720–722. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pattingre S, Tassa A, Qu X, Garuti R,
Liang XH, Mizushima N, Packer M, Schneider MD and Levine B: Bcl-2
antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell.
122:927–939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Itakura E, Kishi C, Inoue K and Mizushima
N: Beclin 1 forms two distinct phosphatidylinositol 3-kinase
complexes with mammalian Atg14 and UVRAG. Mol Biol Cell.
19:5360–5372. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Martinet W, Knaapen MW, Kockx MM and De
Meyer GR: Autophagy in cardiovascular disease. Trends Mol Med.
13:482–491. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Furuya N, Yu J, Byfield M, Pattingre S and
Levine B: The evolutionarily conserved domain of Beclin 1 is
required for Vps34 binding, autophagy and tumor suppressor
function. Autophagy. 1:46–52. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Valentim L, Laurence KM, Townsend PA,
Carroll CJ, Soond S, Scarabelli TM, Knight RA, Latchman DS and
Stephanou A: Urocortin inhibits Beclin1-mediated autophagic cell
death in cardiac myocytes exposed to ischaemia/reperfusion injury.
J Mol Cell Cardiol. 40:846–852. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xie H, Liu Q, Qiao S, Jiang X and Wang C:
Delayed cardioprotection by sevoflurane preconditioning: A novel
mechanism via inhibiting Beclin 1-mediated autophagic cell death in
cardiac myocytes exposed to hypoxia/reoxygenation injury. Int J
Clin Exp Pathol. 8:217–226. 2015.PubMed/NCBI
|
|
53
|
Peng W, Liu Y, Xu WJ and Xia QH: Role of
Beclin 1-dependent autophagy in cardioprotection of ischemic
preconditioning. J Huazhong Univ Sci Technolog Med Sci. 33:51–56.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Levine B, Sinha S and Kroemer G: Bcl-2
family members: Dual regulators of apoptosis and autophagy.
Autophagy. 4:600–606. 2008. View Article : Google Scholar :
|
|
55
|
Shimizu S, Kanaseki T, Mizushima N, Mizuta
T, Arakawa-Kobayashi S, Thompson CB and Tsujimoto Y: Role of Bcl-2
family proteins in a non-apoptotic programmed cell death dependent
on autophagy genes. Nat Cell Biol. 6:1221–1228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Brocheriou V, Hagege AA, Oubenaissa A,
Lambert M, Mallet VO, Duriez M, Wassef M, Kahn A, Menasché P and
Gilgenkrantz H: Cardiac functional improvement by a human Bcl-2
transgene in a mouse model of ischemia/reperfusion injury. J Gene
Med. 2:326–333. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Imahashi K, Schneider MD, Steenbergen C
and Murphy E: Transgenic expression of Bcl-2 modulates energy
metabolism, prevents cytosolic acidification during ischemia and
reduces ischemia/reperfusion injury. Circ Res. 95:734–741. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liang XH, Kleeman LK, Jiang HH, Gordon G,
Goldman JE, Berry G, Herman B and Levine B: Protection against
fatal Sindbis virus encephalitis by beclin, a novel
Bcl-2-interacting protein. J Virol. 72:8586–8596. 1998.PubMed/NCBI
|
|
59
|
Ke J, Yao B, Li T, Cui S and Ding H: A2
Adenosine receptor-mediated cardioprotection against reperfusion
injury in rat hearts is associated with autophagy downregulation. J
Cardiovasc Pharmacol. 66:25–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Maiuri MC, Le Toumelin G, Criollo A, Rain
JC, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K,
Tavernarakis N, et al: Functional and physical interaction between
Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 26:2527–2539.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Weston CR and Davis RJ: The JNK signal
transduction pathway. Curr Opin Cell Biol. 19:142–149. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhao Y and Herdegen T: Cerebral ischemia
provokes a profound exchange of activated JNK isoforms in brain
mitochondria. Mol Cell Neurosci. 41:186–195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Xu J, Qin X, Cai X, Yang L, Xing Y, Li J,
Zhang L, Tang Y, Liu J, Zhang X and Gao F: Mitochondrial JNK
activation triggers autophagy and apoptosis and aggravates
myocardial injury following ischemia/reperfusion. Biochim Biophys
Acta. 1852:262–270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Madesh M, Antonsson B, Srinivasula SM,
Alnemri ES and Hajnoczky G: Rapid kinetics of tBid-induced
cytochrome c and Smac/DIABLO release and mitochondrial
depolarization. J Biol Chem. 277:5651–5659. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yang J, Liu X, Bhalla K, Kim CN, Ibrado
AM, Cai J, Peng TI, Jones DP and Wang X: Prevention of apoptosis by
Bcl-2: Release of cytochrome c from mitochondria blocked. Science.
275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Chu G, Egnaczyk GF, Zhao W, Jo SH, Fan GC,
Maggio JE, Xiao RP and Kranias EG: Phosphoproteome analysis of
cardiomyocytes subjected to beta-adrenergic stimulation:
Identification and characterization of a cardiac heat shock protein
p20. Circ Res. 94:184–193. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Gurusamy N, Lekli I, Gorbunov NV,
Gherghiceanu M, Popescu LM and Das DK: Cardioprotection by
adaptation to ischaemia augments autophagy in association with
BAG-1 protein. J Cell Mol Med. 13:373–387. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Overbye A, Fengsrud M and Seglen PO:
Proteomic analysis of membrane-associated proteins from rat liver
autophagosomes. Autophagy. 3:300–322. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Townsend PA, Stephanou A, Packham G and
Latchman DS: BAG-1: A multi-functional pro-survival molecule. Int J
Biochem Cell Biol. 37:251–259. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Petiot A, Ogier-Denis E, Blommaart EF,
Meijer AJ and Codogno P: Distinct classes of phosphatidylinositol
3′-kinases are involved in signaling pathways that control
macroautophagy in HT-29 cells. J Biol Chem. 275:992–998. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Gutierrez MG, Master SS, Singh SB, Taylor
GA, Colombo MI and Deretic V: Autophagy is a defense mechanism
inhibiting BCG and Mycobacterium tuberculosis survival in infected
macrophages. Cell. 119:753–766. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zheng Y, Gu S, Li X, Tan J, Liu S, Jiang
Y, Zhang C, Gao L and Yang HT: Berbamine postconditioning protects
the heart from ischemia/reperfusion injury through modulation of
autophagy. Cell Death Dis. 8:e25772017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Herzig S and Shaw RJ: AMPK: Guardian of
metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol.
19:121–135. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hardie DG and Sakamoto K: AMPK: A key
sensor of fuel and energy status in skeletal muscle. Physiology
(Bethesda). 21:48–60. 2006.PubMed/NCBI
|
|
75
|
Meley D, Bauvy C, Houben-Weerts JH,
Dubbelhuis PF, Helmond MT, Codogno P and Meijer AJ: AMP-activated
protein kinase and the regulation of autophagic proteolysis. J Biol
Chem. 281:34870–34879. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Samari HR and Seglen PO: Inhibition of
hepatocytic autophagy by adenosine, aminoimidazole-4-carboxamide
riboside and N6-mercaptopurine riboside. Evidence for involvement
of amp-activated protein kinase. J Biol Chem. 273:23758–23763.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Rohailla S, Clarizia N, Sourour M, Sourour
W, Gelber N, Wei C, Li J and Redington AN: Acute, delayed and
chronic remote ischemic conditioning is associated with
downregulation of mTOR and enhanced autophagy signaling. PLoS One.
9:e1112912014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kandadi MR, Hu N and Ren J: ULK1 plays a
critical role in AMPK-mediated myocardial autophagy and contractile
dysfunction following acute alcohol challenge. Curr Pharm Des.
19:4874–4887. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Park CW, Hong SM, Kim ES, Kwon JH, Kim KT,
Nam HG and Choi KY: BNIP3 is degraded by ULK1-dependent autophagy
via MTORC1 and AMPK. Autophagy. 9:345–360. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Przyklenk K, Undyala VV, Wider J,
Sala-Mercado JA, Gottlieb RA and Mentzer RM Jr: Acute induction of
autophagy as a novel strategy for cardioprotection: Getting to the
heart of the matter. Autophagy. 7:432–433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Dunlop EA, Hunt DK, Acosta-Jaquez HA,
Fingar DC and Tee AR: ULK1 inhibits mTORC1 signaling, promotes
multisite Raptor phosphorylation and hinders substrate binding.
Autophagy. 7:737–747. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Fiordaliso F, Li B, Latini R, Sonnenblick
EH, Anversa P, Leri A and Kajstura J: Myocyte death in
streptozotocin-induced diabetes in rats in angiotensin
II-dependent. Lab Invest. 80:513–527. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Lekli I, Ray D, Mukherjee S, Gurusamy N,
Ahsan MK, Juhasz B, Bak I, Tosaki A, Gherghiceanu M, Popescu LM and
Das DK: Co-ordinated autophagy with resveratrol and
gamma-tocotrienol confers synergetic cardioprotection. J Cell Mol
Med. 14:2506–2518. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Li Y, Wang Y, Kim E, Beemiller P, Wang CY,
Swanson J, You M and Guan KL: Bnip3 mediates the hypoxia-induced
inhibition on mammalian target of rapamycin by interacting with
Rheb. J Biol Chem. 282:35803–35813. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hamacher-Brady A, Brady NR, Gottlieb RA
and Gustafsson AB: Autophagy as a protective response to
Bnip3-mediated apoptotic signaling in the heart. Autophagy.
2:307–309. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Xu W, Jiang H, Hu X and Fu W: Effects of
high-mobility group box 1 on the expression of Beclin-1 and LC3
proteins following hypoxia and reoxygenation injury in rat
cardiomyocytes. Int J Clin Exp Med. 7:5353–5357. 2014.PubMed/NCBI
|
|
88
|
Ouyang F, Huang H, Zhang M, Chen M, Huang
F and Zhou S: HMGB1 induces apoptosis and EMT in association with
increased autophagy following H/R injury in cardiomyocytes. Int J
Mol Med. 37:679–689. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sciarretta S, Zhai P, Shao D, Maejima Y,
Robbins J, Volpe M, Condorelli G and Sadoshima J: Rheb is a
critical regulator of autophagy during myocardial ischemia:
Pathophysiological implications in obesity and metabolic syndrome.
Circulation. 125:1134–1146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Shi WY, Xiao D, Wang L, Dong LH, Yan ZX,
Shen ZX, Chen SJ, Chen Y and Zhao WL: Therapeutic metformin/AMPK
activation blocked lymphoma cell growth via inhibition of mTOR
pathway and induction of autophagy. Cell Death Dis. 3:e2752012.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Gurusamy N, Lekli I, Mukherjee S, Ray D,
Ahsan MK, Gherghiceanu M, Popescu LM and Das DK: Cardioprotection
by resveratrol: A novel mechanism via autophagy involving the
mTORC2 pathway. Cardiovasc Res. 86:103–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhao M, Sun L, Yu XJ, Miao Y, Liu JJ, Wang
H, Ren J and Zang WJ: Acetylcholine mediates AMPK-dependent
autophagic cytoprotection in H9c2 cells during
hypoxia/reoxygenation injury. Cell Physiol Biochem. 32:601–613.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Xie H, Xu Q, Jia J, Ao G, Sun Y, Hu L,
Alkayed NJ, Wang C and Cheng J: Hydrogen sulfide protects against
myocardial ischemia and reperfusion injury by activating
AMP-activated protein kinase to restore autophagic flux. Biochem
Biophys Res Commun. 458:632–638. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Takagi H, Matsui Y, Hirotani S, Sakoda H,
Asano T and Sadoshima J: AMPK mediates autophagy during myocardial
ischemia in vivo. Autophagy. 3:405–407. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Yang Y, Wang H, Wang S, Xu M, Liu M, Liao
M, Frank JA, Adhikari S, Bower KA, Shi X, et al: GSK3β signaling is
involved in ultraviolet B-induced activation of autophagy in
epidermal cells. Int J Oncol. 41:1782–1788. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Onishi A, Miyamae M, Kaneda K, Kotani J
and Figueredo VM: Direct evidence for inhibition of mitochondrial
permeability transition pore opening by sevoflurane preconditioning
in cardiomyocytes: Comparison with cyclosporine A. Eur J Pharmacol.
675:40–46. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Shiomi M, Miyamae M, Takemura G, Kaneda K,
Inamura Y, Onishi A, Koshinuma S, Momota Y, Minami T and Figueredo
VM: Sevoflurane induces cardioprotection through reactive oxygen
species-mediated upregulation of autophagy in isolated guinea pig
hearts. J Anesth. 28:593–600. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Hariharan N, Zhai P and Sadoshima J:
Oxidative stress stimulates autophagic flux during
ischemia/reperfusion. Antioxid Redox Signal. 14:2179–2190. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhong Y, Zhong P, He S, Zhang Y, Tang L,
Ling Y, Fu S, Tang Y, Yang P, Luo T, et al: Trimetazidine protects
cardiomyocytes against hypoxia/reoxygenation injury by promoting
AMP-activated protein kinase-dependent autophagic flux. J
Cardiovasc Pharmacol. 69:389–397. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Li YY, Xiang Y, Zhang S, Wang Y, Yang J,
Liu W and Xue FT: Thioredoxin-2 protects against oxygen-glucose
deprivation/reperfusion injury by inhibiting autophagy and
apoptosis in H9c2 cardiomyocytes. Am J Transl Res. 9:1471–1482.
2017.PubMed/NCBI
|
|
101
|
Shiomi M, Miyamae M, Takemura G, Kaneda K,
Inamura Y, Onishi A, Koshinuma S, Momota Y, Minami T and Figueredo
VM: Induction of autophagy restores the loss of sevoflurane cardiac
preconditioning seen with prolonged ischemic insult. Eur J
Pharmacol. 724:58–66. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
He C and Klionsky DJ: Regulation
mechanisms and signaling pathways of autophagy. Annu Rev Genet.
43:67–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Wang LQ, Cheng XS, Huang CH, Huang B and
Liang Q: Rapamycin protects cardiomyocytes against
anoxia/reoxygenation injury by inducing autophagy through the
PI3k/Akt pathway. J Huazhong Univ Sci Technolog Med Sci. 35:10–15.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhai P, Sciarretta S, Galeotti J, Volpe M
and Sadoshima J: Differential roles of GSK-3β during myocardial
ischemia and ischemia/reperfusion. Circ Res. 109:502–511. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Horn HF and Vousden KH: Coping with
stress: Multiple ways to activate p53. Oncogene. 26:1306–1316.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Hoshino A, Matoba S, Iwai-Kanai E,
Nakamura H, Kimata M, Nakaoka M, Katamura M, Okawa Y, Ariyoshi M,
Mita Y, et al: p53-TIGAR axis attenuates mitophagy to exacerbate
cardiac damage after ischemia. J Mol Cell Cardiol. 52:175–184.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Townsend PA, Scarabelli TM, Davidson SM,
Knight RA, Latchman DS and Stephanou A: STAT-1 interacts with p53
to enhance DNA damage-induced apoptosis. J Biol Chem.
279:5811–5820. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Halestrap AP, Clarke SJ and Javadov SA:
Mitochondrial permeability transition pore opening during
myocardial reperfusion-a target for cardioprotection. Cardiovasc
Res. 61:372–385. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Hausenloy D, Wynne A, Duchen M and Yellon
D: Transient mitochondrial permeability transition pore opening
mediates preconditioning-induced protection. Circulation.
109:1714–1717. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Saotome M, Katoh H, Yaguchi Y, Tanaka T,
Urushida T, Satoh H and Hayashi H: Transient opening of
mitochondrial permeability transition pore by reactive oxygen
species protects myocardium from ischemia-reperfusion injury. Am J
Physiol Heart Circ Physiol. 296:H1125–H1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Nanda A, Gukovskaya A, Tseng J and
Grinstein S: Activation of vacuolar-type proton pumps by protein
kinase C. Role in neutrophil pH regulation. J Biol Chem.
267:22740–22746. 1992.PubMed/NCBI
|
|
112
|
Nordstrom T, Grinstein S, Brisseau GF,
Manolson MF and Rotstein OD: Protein kinase C activation
accelerates proton extrusion by vacuolar-type H(+)-ATPases in
murine peritoneal macrophages. FEBS Lett. 350:82–86. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Voss M, Vitavska O, Walz B, Wieczorek H
and Baumann O: Stimulus-induced phosphorylation of vacuolar H
(+)-ATPase by protein kinase A. J Biol Chem. 282:33735–33742. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Shen YT, Depre C, Yan L, Park JY, Tian B,
Jain K, Chen L, Zhang Y, Kudej RK, Zhao X, et al: Repetitive
ischemia by coronary stenosis induces a novel window of ischemic
preconditioning. Circulation. 118:1961–1969. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Depre C, Park JY, Shen YT, Zhao X, Qiu H,
Yan L, Tian B, Vatner SF and Vatner DE: Molecular mechanisms
mediating preconditioning following chronic ischemia differ from
those in classical second window. Am J Physiol Heart Circ Physiol.
299:H752–H762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Khan S, Salloum F, Das A, Xi L, Vetrovec
GW and Kukreja RC: Rapamycin confers preconditioning-like
protection against ischemia-reperfusion injury in isolated mouse
heart and cardiomyocytes. J Mol Cell Cardiol. 41:256–264. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Marzetti E, Wohlgemuth SE, Anton SD,
Bernabei R, Carter CS and Leeuwenburgh C: Cellular mechanisms of
cardioprotection by calorie restriction: State of the science and
future perspectives. Clin Geriatr Med. 25:715–732. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Kavazis AN, Alvarez S, Talbert E, Lee Y
and Powers SK: Exercise training induces a cardioprotective
phenotype and alterations in cardiac subsarcolemmal and
intermyofibrillar mitochondrial proteins. Am J Physiol Heart Circ
Physiol. 297:H144–H152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Jones SP and Bolli R: The ubiquitous role
of nitric oxide in cardioprotection. J Mol Cell Cardiol. 40:16–23.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Ha T, Hua F, Liu X, Ma J, McMullen JR,
Shioi T, Izumo S, Kelley J, Gao X, Browder W, et al:
Lipopolysaccharide-induced myocardial protection against
ischaemia/reperfusion injury is mediated through a
PI3K/Akt-dependent mechanism. Cardiovasc Res. 78:546–553. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Sala-Mercado JA, Wider J, Undyala VV,
Jahania S, Yoo W, Mentzer RM Jr, Gottlieb RA and Przyklenk K:
Profound cardioprotection with chloramphenicol succinate in the
swine model of myocardial ischemia-reperfusion injury. Circulation.
122:S179–S184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Shirakabe A, Ikeda Y, Sciarretta S,
Zablocki DK and Sadoshima J: Aging and autophagy in the heart. Circ
Res. 118:1563–1576. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Gottlieb RA and Mentzer RM Jr:
Cardioprotection through autophagy: Ready for clinical trial?
Autophagy. 7:434–435. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Kanamori H, Takemura G, Goto K, Maruyama
R, Tsujimoto A, Ogino A, Takeyama T, Kawaguchi T, Watanabe T,
Fujiwara T, et al: The role of autophagy emerging in postinfarction
cardiac remodelling. Cardiovasc Res. 91:330–339. 2011. View Article : Google Scholar : PubMed/NCBI
|