Introduction
For small cell lung cancer (SCLC) patients, the
two-year survival rate is below 5%, with a five-year survival rate
below 2%, and curative resection is currently the main therapy
(1). SCLC has a significant early
propensity to metastasize and is very sensitive to initial systemic
cytotoxic chemotherapy. Therefore, systemic chemotherapy combined
with radiotherapy and surgery is the main treatment for SCLC
(2). At the genetic level, SCLC is
a heterogeneous disease associated with a large number of genetic
changes. Many cancer-associated genes are likely prone to somatic
mutations, including oncogenes, tumor suppressor genes, enzymes
involved in chromatin modification, tyrosine kinase receptors and
their downstream signaling components (3). As a result, more and more targeted
drugs are expected to be developed for the treatment of SCLC. For
example, anti-angiogenesis drugs have been previously used to treat
extensive-stage SCLC (ES-SCLC). In a phase II clinical trial, basic
chemotherapy using bevacizumab combined with platinum was adopted
for the treatment of ES-SCLC patients. The results illustrated that
progression-free survival (PFS) was significantly increased in the
experimental group compared to the control group, suggesting that
bevacizumab had a curative effect on ES-SCLC patients (4). External environmental factors also
have an important impact on the occurrence of SCLC. Smoking is
recognized as the most important risk factor for SCLC, and most
SCLC patients have a history of smoking or being in a smoking
environment. So far, many studies have (5–7)
confirmed the correlation between the progression of SCLC and gene
methylation, providing a theoretical and experimental foundation
for further research to better understand the occurrence and
development of SCLC.
Lysine-specific demethylase 2 (LSD2) is encoded by a
gene on human chromosome 6p22, which has an extremely high
incidence of genetic abnormalities in cancer patients, such as
substitutions, deletions, and DNA amplifications. Therefore, LSD-2
is likely involved in the occurrence and development of
carcinogenesis. Similar to its homologue LSD-1, LSD-2 promotes the
demethylation of mono- and dimethylated H3K4 and H3K9 (8,9). It
has been found that the overexpression of LSD2 in breast cancer
promotes cancer cell growth and endows cancer cells with similar
characteristics to stem cells, while the inhibition of LSD2 blocks
the growth of breast cancer cells (10). Research has also found that
inhibiting the mRNA expression of LSD2 suppresses clonal formation
and migration of MDA-MB-231 cells, while LSD2 knock-down (LSD2 KD)
promotes the expression of tumor proliferation-related genes (such
as CLDN1, CDH11, CASP5) and tumor suppressor genes (such as
ERBB2IP, PR, ERα) and can also enhance the sensitivity of breast
cancer cells to DNA methyltransferase (DNMT) inhibitors (11). In non-small cell lung cancer
(NSCLC), LSD2 was found to have E3 ubiquitin ligase function, and
it directly promoted the ubiquitination and protein degradation of
O-GlcNAc transferase (OGT). Moreover, the inhibitory effects of
LSD2 on NSCLC cell line A549 depended on this E3 ligase activity
(12). However, related research
on the role of LSD2 in SCLC has not yet been reported.
We found that LSD2 presented high expression in SCLC
tissue and cell lines. Importantly, we show that LSD2 can
indirectly regulate tissue factor pathway inhibitor-2 (TFPI-2)
expression by mediating DNMT3B expression or by regulating the
demethylation of H3K4me2 in the promoter region of the TFPI-2
gene.
Materials and methods
SCLC clinical samples and cell
lines
From 2012 to 2016, 40 patients with SCLC were chosen
to undergo surgical treatment, during which cancer and
cancer-adjacent tissues (>3 cm from the edge of the tumor) were
collected. These SCLC patients comprised 30 males and 10 females
with an average age of 57 years, and most of them (32 cases) had a
history of smoking. The inclusion criteria for SCLC consisted of
the following: Cytologically or histologically diagnosed as SCLC,
age of at least 18 years, complete clinical data. The exclusion
criteria included mixed cancers, patients without clear
postoperative pathological diagnosis and patients with detectable
inflammatory disease or liver disease. All cases were diagnosed and
divided by their stages according to the 7th TNM staging system
proposed by the International Association for the Study of Lung
Cancer. All of the patients signed informed consent forms. All
experiments were approved by the Medical Ethics Association of
Shandong Provincial Qianfoshan Hospital (Jinan, China).
The normal human bronchial epithelial cell line
BEAS-2B and SCLC cell lines (H69, DMS-114 and H1417) were purchased
from the American Type Culture Collection (ATCC). After cell
recovery, all cells were seeded in RPMI-1640 medium containing 10%
fetal bovine serum (FBS; both Sigma-Aldrich, St. Louis MO, USA),
and then cultured in a humidified chamber with 5% CO2 at
37°C. The medium was replaced, and cells were passaged once every
3–4 days.
Vector construction for gene silencing
and overexpression
Based on the sequences of the LSD2 and TFPI-2 genes,
primers were designed to amplify their full-length cDNA (Table I). The cDNA of each gene was
subcloned into the pCDH-CopGFP vector (System Biosciences, Mountain
View, CA, USA) to construct overexpression plasmids, which were
then transfected into the cell lines using Lipofectamine 3000
(Invitrogen Inc., Carlsbad, CA, USA). Empty vectors were used as
controls. Subsequently, the cells were cultured for 48 h and then
screened with DMEM supplemented with 400 µg/ml G418
(Sigma-Aldrich). After 1 month of screening, stable gene silencing
and overexpression strains (LSD1, LSD2, and DNMT3B) were obtained,
cultured in DMEM with 10% FBS+P.S. medium and cryopreserved.
Meanwhile, short interfering (si) RNA to target LSD2 (si-LSD2) and
corresponding control siRNA were designed according to their
sequences (Table I), and
transfected into the cell lines using Lipofectamine 3000.
 | Table I.Primers for the clone and q-PCR of
genes. |
Table I.
Primers for the clone and q-PCR of
genes.
| Gene | Forward primer | Reverse primer |
|---|
| Clone |
|
|
|
LSD2+ |
CAGTAATCATATGGCAACTCCACGGGGGAGGACAAAG |
TAGTCTCGAGTTAAAATGCTGCAATCTTGCTTG |
|
si-LSD2 |
ATCGATGCGGTATGAAACCAA |
|
|
TFPI-2+ |
GCTTTCTCGGACGCCTTGC |
GAATACGACCCCAAGAAATGAGTGA |
| q-PCR |
|
|
| LSD2 |
CCTGGCTTTGAGAAACCTCATC |
TCTCCACTTCCTGAACGCATC |
|
TFPI-2 |
GTCGATTCTGCTGCTTTTCC |
CAGCTCTGCGTGTACCTGTC |
|
GAPDH |
GGTCGGTGTGAACGGATTTG |
GGGGTCGTTGATGGCAACA |
|
DNMT3B |
GTGCTCGCTTCGGCAGCACA |
TGGAACGCTTCACGAATTTG |
RNA extraction and fluorescence
quantitative polymerase chain reaction (Fq-PCR)
Total RNA was extracted from cells using TRIzol
(Invitrogen Inc.) according to the manufacturer's protocol.
Complementary DNA (cDNA) was synthesized from 1 µg of total RNA
using Superscript III reverse transcriptase (Invitrogen Inc.).
Fq-PCR was performed using an ABI StepOne real-time PCR system as
the following steps: 95°C for 10 min, followed by 50 cycles of 95°C
for 15 sec and 68°C for 45 sec. Primers used for quantitative
polymerase chain reaction (q-PCR) are shown in Table I. Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) was used as an internal reference.
Western blot analysis
The SCLC cells were washed 3 times with cold
phosphate buffered saline (PBS), and then lysed with RIPA lysis
buffer (both Sigma-Aldrich) to extract total protein. The total
protein concentration was determined using a BCA kit (Thermo Fisher
Scientific, Inc., San Jose, CA, USA). For each sample, 20 µg
protein was added to a gel, separated by 10% SDS-PAGE, and then
transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore
Corp., Bedford, MA, USA). Each membrane was blocked for 1 h with 5%
skim milk at room temperature, washed once with PBS, and incubated
overnight with primary antibodies at 4°C. The primary antibodies
were as follows: Anti-DNMT3B (ab16049; 1:1,000), anti-TFPI2
(ab86933; 1:1,000), and anti-GAPDH (1:2,500; Abcam, Cambridge, MA,
USA). Then, after being washed 2–3 times with TBS with Tween-20
(TBST), the membranes were incubated for 1 h with secondary
anti-rabbit IgG antibodies (ab205718; 1:2,000; Abcam) at room
temperature. Finally, an Odyssey Infrared Imaging System (LI-COR
Inc., Superior St. Lincoln, NE, USA) was used to detect immune
responses. GAPDH was used as an internal reference.
MTT assays
Cell proliferation was quantified using MTT assays.
Cells were plated into 96-well plates in triplicate using 100 µl
cell suspension fluid with a density of 2×104/ml and
cultured for 24, 48 and 72 h in 5% CO2 incubator at
37°C. At each time point, 50 µl MTT solution (1 g/l in normal
saline) was added into each well. After a 4-h incubation at 37°C,
the supernatant was removed and 100 µl dimethylformamide (DMSO) was
added. The plates were shaken for 10 min to fully resolve the MTT
pyrolysis products. Then, the optical density (OD) at 590 nm was
measured on an immunoassay analyzer, and the cell growth curve was
determined based on the OD values.
Chromatin immunoprecipitation
(ChIP)
ChIP was performed according to the method described
in a previous study (13). In
brief, 1×106 NSCLC cells were cross-linked with 1%
formaldehyde and washed with PBS in the presence of protease
inhibitors. Cells were homogenized and their chromatin was
subjected to ChIP using magnetic Dynabeads (Invitrogen Inc.) and
antibodies against dimethylated lysine 4 of histone H3 (H3K4)
(Millipore Corp., Temecula, CA, USA). The fold enrichment of the
DNAs amplified by ChIP was assessed using previously described
protocols. In accordance with the promoter region of the TFPI-2
gene, the specific primers were designed as follows: S1,
5′-ATAAAGCGGGTATTCGGGTC-3′ and AS1, 5′-CTCCGCCGATTAAAAAAA-3′.
Statistical analysis
Quantitative data are expressed as the mean ±
standard error. Statistical analysis was performed by using one-way
analysis of variance followed by the least significant difference
test or two-way analysis of variance followed by Tukey's multiple
comparisons test. Statistical analysis was performed with GraphPad
prism 7 software (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
LSD2 presented high expression SCLC
tissues and cell lines
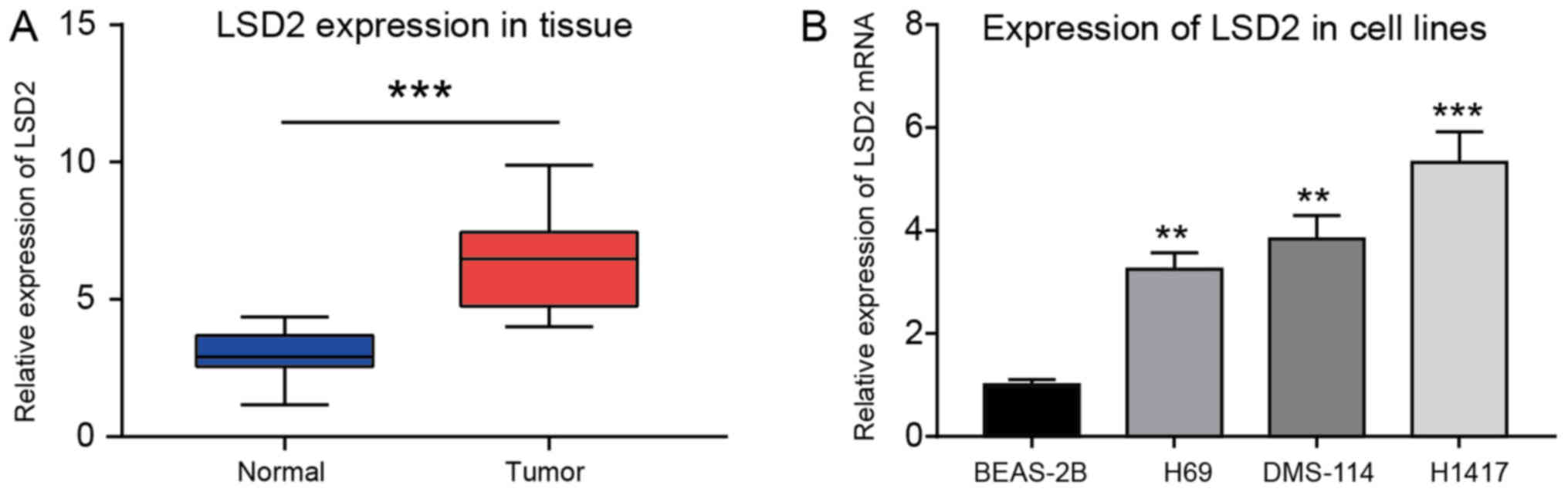
To investigate the role of LSD2 in SCLC, q-PCR was
performed to measure LSD2 expression in SCLC samples. The results
suggested that LSD2 is expressed relatively highly in cancer
tissues compared with normal cancer-adjacent tissues (P<0.05)
(Fig. 1A). Similarly, compared to
the normal cell line BEAS-2B, LSD2 expression was significantly
increased in H69, DMS-114, and particularly in H1417 cells
(Fig. 1B).
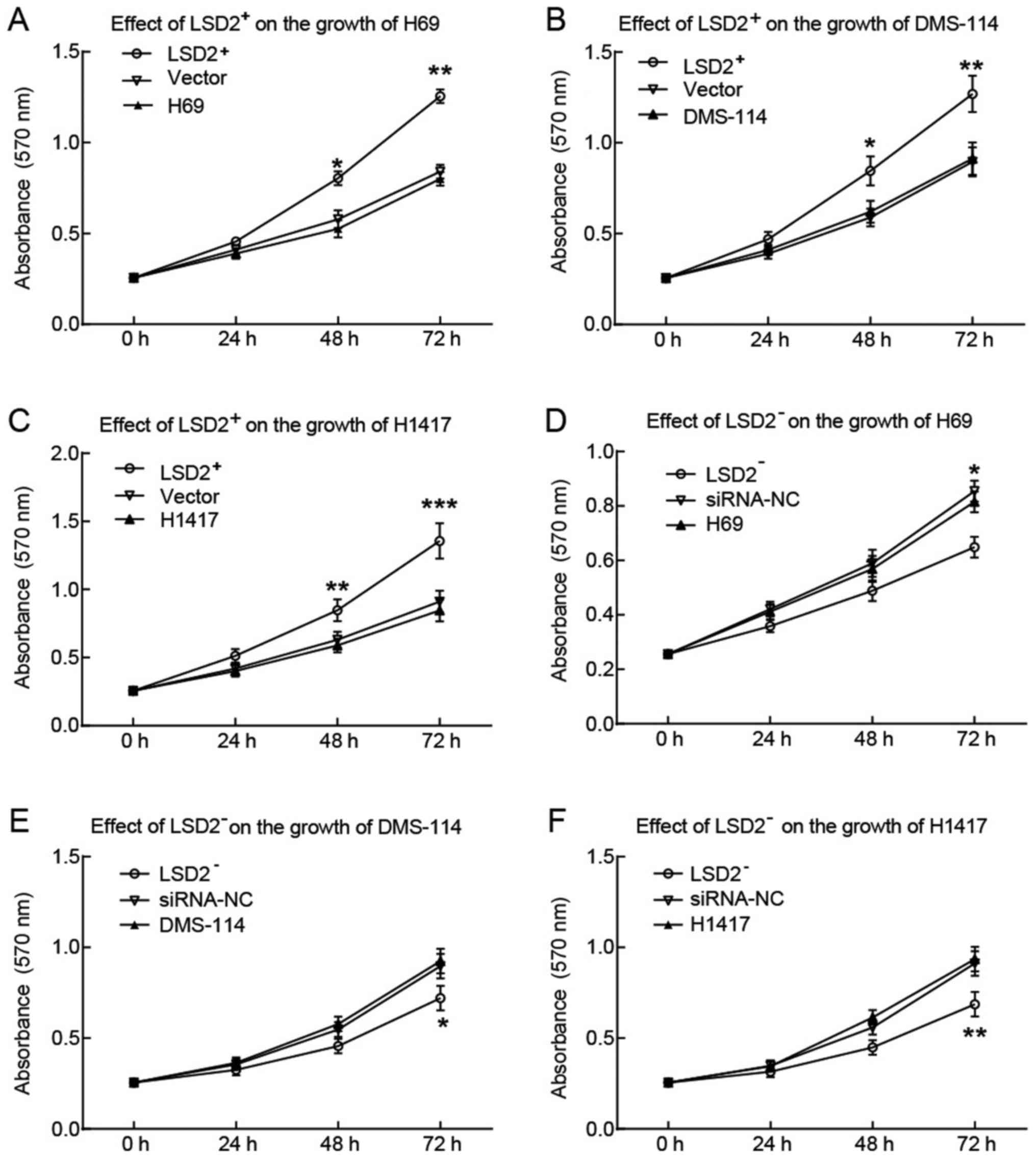
LSD2 overexpression promoted the
growth of SCLC cell lines
The high expression of LSD2 observed in SCLC
clinical samples and cell lines indicated that LSD2 may participate
in the progression of SCLC. Therefore, we assessed the effect of
LSD2 on the growth of H69, DMS-114 and H1417 cells by MTT assays.
The results demonstrated that, compared to that of control cells
transfected with empty vectors, the growth of H69, DMS-114 and
H1417 cells was significantly promoted by LSD2 overexpression
(Fig. 2A-C), while it was
inhibited by LSD2 silencing (Fig.
2D-F).
LSD2 negatively regulated the
expression of TFPI-2 through DNMT3B
Previous studies have shown that LSD2 regulates the
expression of DNMT3B and TFPI-2 in breast cancer (14,15),
and TFPI-2 also plays a tumor suppression role in a variety of
tumors (16,17). We first detected the expression of
TFPI-2 and DNMT3B in SCLC. The results of q-PCR demonstrated that
DNMT3B was significantly higher expressed in SCLC tissues (Fig. 3A) and cell lines (H69, DMS-114, and
H1417) (Fig. 3B) than in
cancer-adjacent tissues and the control cell line BEAS-2B,
respectively (P<0.05). In contrast, the expression of TFPI-2 was
significantly decreased in SCLC tissues (Fig. 3C) (P<0.01) and cell lines
(Fig. 3D) (P<0.05).
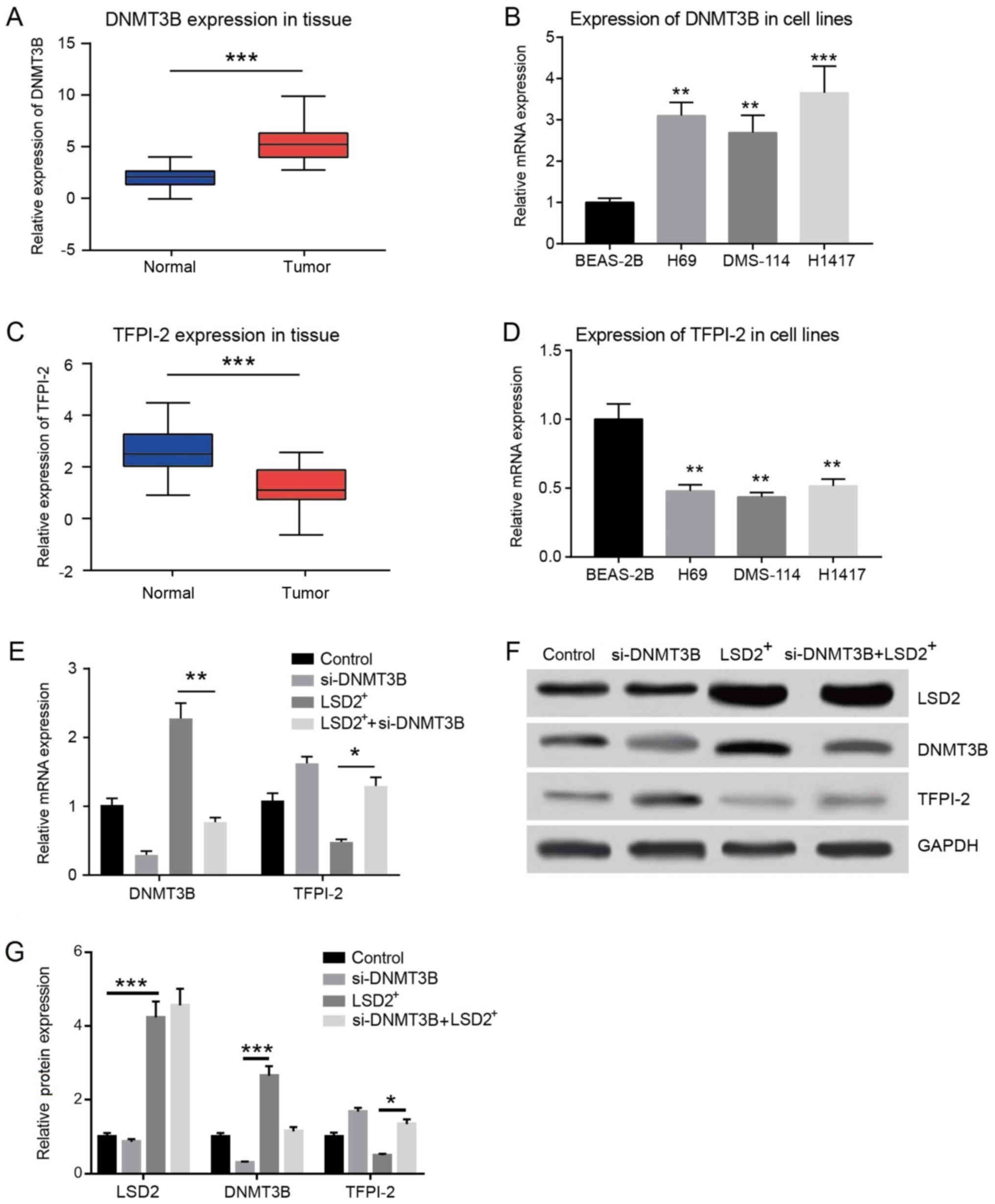 | Figure 3.LSD2 negatively regulates the
expression of TFPI-2 through DNMT3B. (A) Expression of DNMT3B mRNA
in 40 cases of clinical samples of SCLC. (B) Expression of DNMT3B
mRNA in SCLC cell lines. BEAS-2B was used as the control group. (C)
Expression of TFPI-2 mRNA in 40 cases of clinical samples of SCLC.
(D) Expression of TFPI-2 mRNA in SCLC cell lines. BEAS-2B was used
as the control group. (E) mRNA expression of DNMT3B and TFPI-2 in
LSD2+ and/or si-DNMT3B H1417 cells. (F and G) Effect of
LSD2+ and/or si-DNMT3B on the protein expression of
TFPI-2. Control, H1417 cells; si-DNMT3B, H1417 cells transfected
with si-DNMT3B; LSD2+, H1417 cells transfected with LSD2
overexpression plasmids; si-DNMT3B+LSD2+, H1417 cells
transfected with si-DNMT3B and LSD2 overexpression plasmids.
*P<0.05; **P<0.01 and ***P<0.001 vs. the BEAS-2B group or
as indicated. DNMT3B, DNA methyltransferase 3B; TFPI-2, tissue
factor pathway inhibitor-2; LSD2, lysine-specific demethylase 2;
SCLC, small cell lung cancer; siRNA, small interfering RNA;
si-DNMT3B, siRNA to target DNMT3B. |
We then investigated the effects of LSD2
overexpression or inhibition on the expression of DNMT3B and
TFPI-2. q-PCR and western blots suggested that compared with the
control cells, LSD2-overexpressing H1417 cells showed a remarkable
increase in the mRNA and protein expression of DNMT3B and a strong
decrease in the mRNA and protein expression of TFPI-2. After
LSD2-overexpressing H1417 cells were transfected with siRNA-DNMT3B,
the mRNA and protein expression of TFPI-2 were elevated to a
different degree than in the cells only overexpressing LSD2
(Fig. 3E and F). These results
suggested that LSD-2 indirectly represses the expression of TFPI-2
by mediating the expression of DNMT3B.
LSD2 regulated TFPI-2 expression by
mediating the demethylation of H3K4me1 in the promoter region of
TFPI-2
To determine whether LSD2 regulates TFPI-2
expression by regulating the demethylation of H3K4me1 in the
promoter region of TFPI-2, ChIP was performed. The results showed
that H3K4me1 enrichment in the promoter region of TFPI-2 was
reduced in H1417 cells compared to that of BEAS-2B cells (Fig. 4A) (P<0.05). Compared to the
control cells transfected with empty vectors, the TFPI-2 promoter
region showed a lower level of H3K4me1 enrichment in H1417 cells
overexpressing LSD2 (Fig. 4B)
(P<0.05). Contrarily, the enrichment of H3K4mel in the TFPI-2
promoter region of H1417 cells was significantly increased after
LSD2 was inhibited (Fig. 4C)
(P<0.01). These findings indicated that in the H1417 cell line,
LSD2 positively regulates the demethylation of H3K4me1 in the
promoter region of TFPI-2 and thereby controls its
transcription.
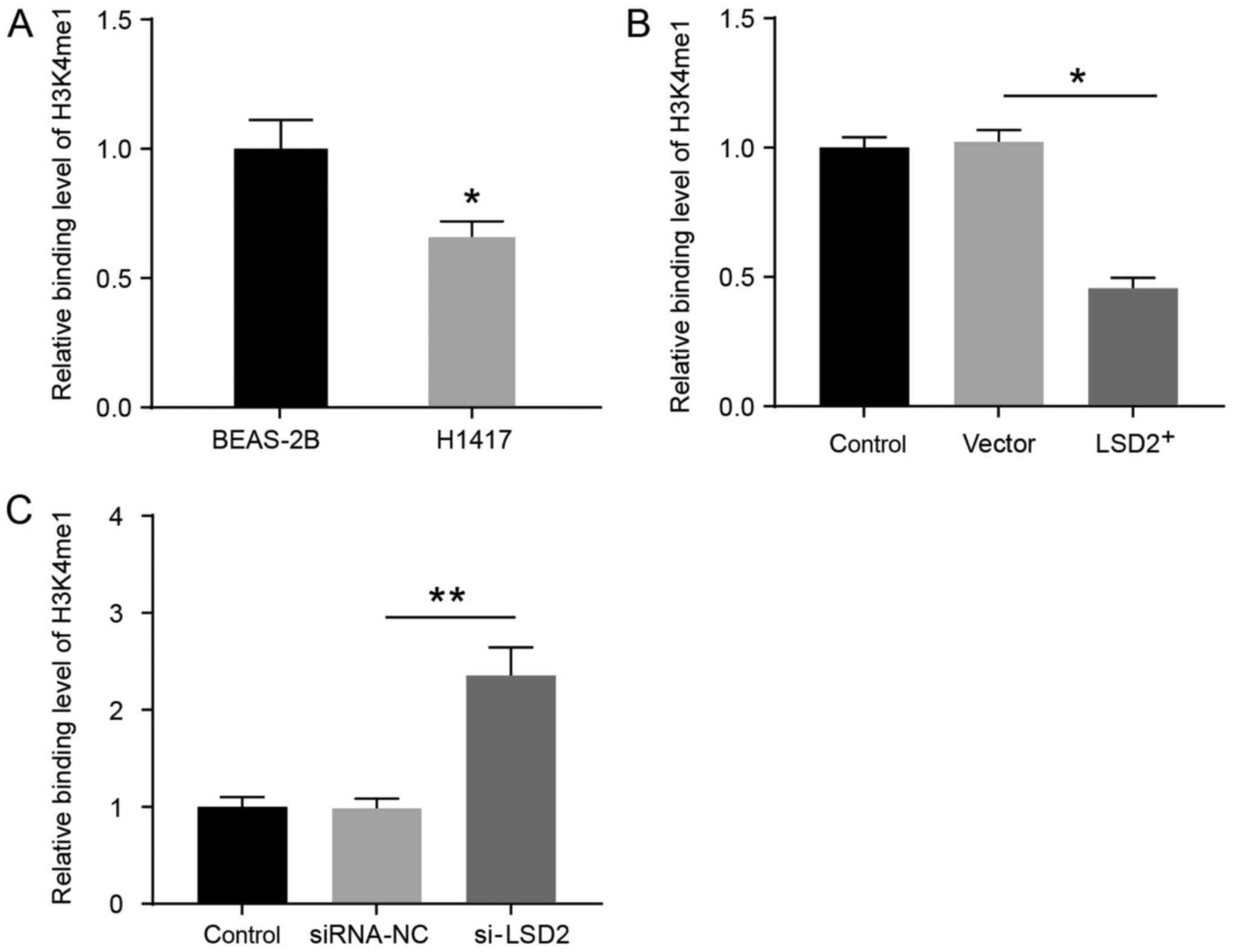 | Figure 4.Histone modification H3K4me1 at the
promoter region of TFPI-2 in SCLC cells. ChIP assays were performed
with antibodies against H3K4me1 in SCLC cells. Region of the TFPI-2
promoter for quantitative ChIP-PCR assay were indicated via primers
S1 and AS1. The binding of H3K4-me1 antibody to region of the
TFPI-2 promoter was measured by the quantification of ChIP DNA
against a standard curve generated from input DNA. (A) H3K4me1
enrichment level in the promoter region of TFPI-2 showed lower
degree in H1417 cells than that in BEAS-2B cells, *P<0.05 vs.
the BEAS-2B group. (B) TFPI-2 promoter region showed a
significantly lower enrichment level of H3K4me1 in the cell line
H1417 with LSD2 overexpression. (C) TFPI-2 promoter region showed a
significantly higher enrichment level of H3K4me1 in the cell line
H1417 with siRNA-LSD2. *P<0.05 and **P<0.01 as indicated.
Control, H1417 cells; si-LSD2, H1417 cells transfected with
si-LSD2; TFPI-2, tissue factor pathway inhibitor-2; siRNA-NC, H1417
cells transfected with siRNA-NC; si, small interfering; SCLC, small
cell lung cancer; si-LSD2, siRNA to target LSD2; siRNA-NC, control
siRNA of LSD2; H3K4me1, monomethylated histone H3 lysine 4; ChIP,
chromatin immunoprecipitation. |
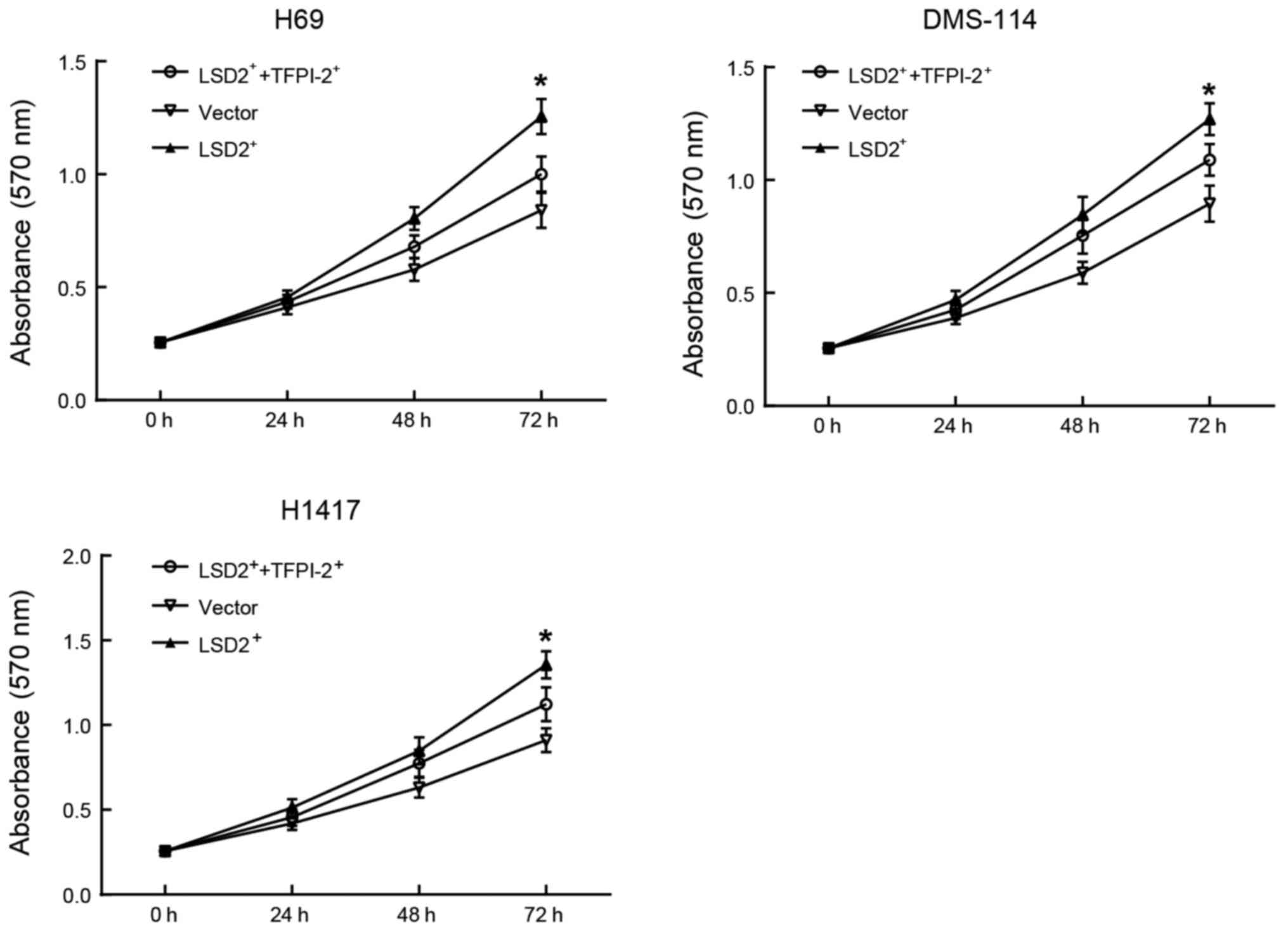
TFPI-2 overexpression inhibited the
promoting effect of LSD2 on the proliferation of SCLC cells
To further investigate whether the effect of LSD2 on
the proliferation of SCLC cells is related to the expression of
TFPI-2, a rescue experiment for TFPI-2 was conducted. MTT assays
revealed that, compared with cells only overexpressing LSD2, SCLC
cells (H69, DMS-144 and H1417) overexpressing both LSD2 and TFPI-2
showed significantly reduced proliferation (Fig. 5). This implied that the promoting
effect of LSD2 on the proliferation of SCLC cells may be partially
achieved through the inhibition of TFPI-2.
Discussion
DNA methylation is one of the major causes of
epigenetic changes in organisms. It exerts its effects by
differentially regulating gene expression based on the number and
distribution of methylated cytosines in the genome, without
changing the DNA sequence. Methylation plays a critical role in
embryonic development, cell differentiation, and in the occurrence
of a variety of human diseases (18). Although methylation levels are
generally reduced in cancer, hypermethylation occurs periodically
in the course of cancer progression, which is associated with the
activation of proto-oncogenes, thereby promoting the occurrence and
metastasis of cancer (19). In
addition, covalent modification of histones is a key mechanism for
regulating gene expression, including methylation, phosphorylation,
and acetylation. (20). Histone
modification has been recognized as being involved in
chromatin-related processes, including DNA replication, repair, and
transcription. The association between histone modification and
transcription has been intensively studied (21). Methylation of H3K4 is one type of
histone modification, which is generally believed to mediate the
transcriptional activation of genes (22).
Meanwhile, previous studies have claimed that
knocking out LSD2 can promote the expression of both tumor
proliferation-associated genes and tumor suppressor genes (23). LSD2 overexpression in MDA-MB-231
cells significantly altered the expression of key epigenetic
modifiers such as DNMT3B, HDAC1/2, and LSD1; augmented colony
formation in soft agar; and promoted cellular proliferation
(14). To investigate whether LSD2
plays a role in suppressing or promoting SCLC, we measured the
expression of LSD2 in SCLC and tested its effect on the growth of
cancer cells. The results demonstrated that LSD2, compared with
controls, was more highly expressed in SCLC clinical tissues or
SCLC cell lines (including H69, DMS-114 and H1417), while the
results of MTT assays revealed that the growth of SCLC cell lines
was promoted by LSD2 overexpression and inhibited by LSD2 gene
silencing. These results suggest that the up-regulation of LSD2 in
SCLC participates in the tumor progression of SCLC and that its
expression in SCLC may be beneficial for SCLC tumorigenesis. In
addition, a previous study claimed that knockout of LSD1 promotes
the expression of TFPI-2, which is accompanied by increased levels
of H3K4me2 in the promoter region of TFPI-2, suggesting that TFPI-2
expression can be regulated by LSD1-mediated transcriptional
initiation (24). Thus, we
hypothesized that the homologous gene, LSD2, may regulate H3K4
demethylation in the promoter region of TFPI-2 in SCLC. In the
present study, the level of H3K4me1 enrichment in the TFPI-2
promoter region was reduced in H1417 cells compared to that in
normal BEAS-2B lung cells. In H1417 cells, LSD2 overexpression
reduced the H3K4me2 enrichment level in the TFPI-2 promoter region.
In breast cancer, LSD2-KD led to accumulation of H3K4me1/2 without
changing the methylation levels of other key histone lysine
residues, indicating that LSD2 serves as a bona fide H3K4
demethylase (23). Therefore, the
results of this study indicated that LSD1/2 suppresses TFPI-2
expression by promoting the demethylation of H3K4me1 in the TFPI-2
promoter region.
TFPI-2 plays an important role as a tumor suppressor
gene. For instance, TFPI-2 acts as an independent prognostic factor
for NSCLC patients (25). In
cervical cancer, TFPI-2 expression shows a decreasing trend with
tumor progression and has a close association with tumor cell
apoptosis and angiogenesis (26).
The methylation level of TFPI2 is significantly increased in
colorectal tumor tissues compared with that in colorectal normal
tissues, and TCGA data also supported the hypothesis that TFPI2
hypermethylation is a promising diagnostic marker for CRC and GC
(27) In the present study, our
results suggested that TFPI-2 expression in SCLC tissues was
significantly lower than that in normal tissue. One of the primary
reasons for the low expression of TFPI-2 in tumors is
hypermethylation of its promoter region (28–31).
The depletion of DNMT1 or DNMT3B expression in lung, esophageal
carcinoma and malignant pleural mesothelioma cells increases the
expression of the tumor suppressor genes p16, p21 and TFPI-2. In
the present study, after DNMT3B in H1417 cells was inhibited by
siRNA-DNMT3B, the mRNA and protein expression of TFPI-2 were
significantly increased, suggesting that TFPI-2 expression was also
affected by the methylation of its promoter region. Further
experiments demonstrated that in H1417 cells overexpressing LSD2,
the mRNA and protein expression of DNMT3B were remarkably increased
compared with that in the control group, in contrast to the
expression of TFPI-2. This indicated that LSD2 indirectly represses
TFPI-2 expression by regulating DNMT3B expression. It has been
reported that LSD2 overexpression can significantly alter the
expression of epigenetic modifying enzymes such as LSD1, HDAC1/2
and DNMT3B in the breast cancer cell line MDA-MB-231, thereby
promoting cell proliferation and colony formation in soft agar
(14). Interestingly, in SCLC
cells overexpressing both LSD2 and TFPII-2, the growth of cancer
cells was reduced compared to that in cells only overexpressing
LSD2. This result indicated that the role of LSD2 in promoting the
proliferation of SCLC cells may partially be due to the inhibition
of TFPI-2 expression. In the future clinical experiments,
researchers should focus on the role of the LSD2/DNMT3B/TFPI-2 axis
in SCLC, and identify suitable drugs or inhibitors targeting them
to provide a new path for SCLC treatment.
Collectively, we have shown that LSD2 can indirectly
mediate TFPI-2 expression in SCLC by regulating DNMT3B. On the
other hand, LSD2 can negatively mediate TFPI-2 expression by
regulating the demethylation of H3K4me2 in the TFPI-2 promoter
region, further leading to the promotion of SCLC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YFC, CHG and YHY designed the present study and
conducted experiments, analysis and interpretation of data. XL and
LZ were involved in the experimental analysis and data acquisition.
All authors have read and approved the final submitted
manuscript.
Ethics approval and consent to
participate
All patients provided written informed consent. All
experiments were approved by the Medical Ethics Association of
Shandong Provincial Qianfoshan Hospital.
Consent for publication
All patients provided written informed consent for
the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Coleman MH and Bueno R: Role of adjuvant
chemotherapy in NSCLC (stages I to III). Surg Oncol Clin N Am.
20:757–767. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pillai RN and Owonikoko TK: Small cell
lung cancer: Therapies and targets. Semin Oncol. 41:133–142. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arcaro A: Targeted therapies for small
cell lung cancer: Where do we stand? Crit Rev Oncol Hematol.
95:154–164. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Spigel DR, Townley PM, Waterhouse DM, Fang
L, Adiguzel I, Huang JE, Karlin DA, Faoro L, Scappaticci FA and
Socinski MA: Randomized phase II study of bevacizumab in
combination with chemotherapy in previously untreated
extensive-stage small-cell lung cancer: Results from the SALUTE
trial. J Clin Oncol. 29:2215–2222. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hopkins-Donaldson S, Ziegler A, Kurtz S,
Kurtz S, Bigosch C, Kandioler D, Ludwig C, Zangemeister-Wittke U
and Stahel R: Silencing of death receptor and caspase-8 expression
in small cell lung carcinoma cell lines and tumors by DNA
methylation. Cell Death Differ. 10:356–364. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tanaka N, Toyooka S, Soh J, Kubo T,
Yamamoto H, Maki Y, Muraoka T, Shien K, Furukawa M, Ueno T, et al:
Frequent methylation and oncogenic role of microRNA-34b/c in
small-cell lung cancer. Lung Cancer. 76:32–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsou JA, Hagen JA, Carpenter CL and
Laird-Offringa IA: DNA methylation analysis: A powerful new tool
for lung cancer diagnosis. Oncogene. 21:5450–5461. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karytinos A, Forneris F, Profumo A,
Ciossani G, Battaglioli E, Binda C and Mattevi A: A novel mammalian
flavin-dependent histone demethylase. J Biol Che. 284:17775–17782.
2009. View Article : Google Scholar
|
|
9
|
Fang R, Barbera AJ, Xu Y, Rutenberg M,
Leonor T, Bi Q, Lan F, Mei P, Yuan GC, Lian C, et al: Human
LSD2/KDM1b/AOF1 regulates gene transcription by modulating
intragenic H3K4me2 methylation. Mol Cell. 39:222–233. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen L, Vasilatos SN, Qin Y, Oesterreich
S, Davidson NE and Huang Y: Abstract 1385: New insights into the
roles of histone lysine-specific demethylase 2 (LSD2) in breast
cancer. AACR. 77:13852017.
|
|
11
|
Katz TA, Vasilatos SN, Oesterreich S,
Chandran U, Davidson NE and Huang Y: Abstract 1052: Synergy between
inhibition of novel histone demethylase (LSD2) and DNA
methyltransferase (DNMT) and histone deacetylase (HDAC) in
modulating gene expression and inhibiting growth in human breast
cancer cells. AACR. 72:10522012.
|
|
12
|
Yang Y, Yin X, Yang H and Xu Y: Histone
demethylase LSD2 acts as an E3 ubiquitin ligase and inhibits cancer
cell growth through promoting proteasomal degradation of OGT. Mol
Cell. 58:47–59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qin H, Chan MW, Liyanarachchi S, Balch C,
Potter D, Souriraj IJ, Cheng AS, Agosto-Perez FJ, Nikonova EV, Yan
PS, et al: An integrative ChIP-chip and gene expression profiling
to model SMAD regulatory modules. BMC Syst Biol. 3:732009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen L, Vasilatos SN, Qin Y, Katz TA, Cao
C, Wu H, Tasdemir N, Levine KM, Oesterreich S, Davidson NE and
Huang Y: Functional characterization of lysine-specific demethylase
2 (LSD2/KDM1B) in breast cancer progression. Oncotarget.
8:81737–81753. 2017.PubMed/NCBI
|
|
15
|
Mino K, Nishimura S, Ninomiya S, Tujii H,
Matsumori Y, Tsuchida M, Hosoi M, Koseki K, Wada S, Hasegawa M, et
al: Regulation of tissue factor pathway inhibitor-2 (TFPI-2)
expression by lysine-specific demethylase 1 and 2 (LSD1 and LSD2).
Biosci Biotechnol Biochem. 78:1010–1017. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Konduri SD, Tasiou A, Chandrasekar N and
Rao JS: Overexpression of tissue factor pathway inhibitor-2
(TFPI-2), decreases the invasiveness of prostate cancer cells in
vitro. Int J Oncol. 18:127–131. 2001.PubMed/NCBI
|
|
17
|
Cruickshanks HA, Vafadar-Isfahani N,
Dunican DS, Lee A, Sproul D, Lund JN, Meehan RR and Tufarelli C:
Expression of a large LINE-1-driven antisense RNA is linked to
epigenetic silencing of the metastasis suppressor gene TFPI-2 in
cancer. Nucleic Acids Res. 41:6857–6869. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kader F and Ghai M: DNA methylation-based
variation between human populations. Mol Genet Genomics. 292:5–35.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qu Y, Dang S and Hou P: Gene methylation
in gastric cancer. Clin Chim Acta. 424:53–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Karlić R, Chung HR, Lasserre J, Vlahovicek
K and Vingron M: Histone modification levels are predictive for
gene expression. Proc Natl Acad Sci USA. 107:2926–2931. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Heintzman ND, Hon GC, Hawkins RD,
Kheradpour P, Stark A, Harp LF, Ye Z, Lee LK, Stuart RK, Ching CW,
et al: Histone modifications at human enhancers reflect global
cell-type-specific gene expression. Nature. 459:108–112. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gupta J, Kumar S, Li J, Krishna Murthy
Karuturi R and Tikoo K: Histone H3 lysine 4 monomethylation
(H3K4me1) and H3 lysine 9 monomethylation (H3K9me1): Distribution
and their association in regulating gene expression under
hyperglycaemic/hyperinsulinemic conditions in 3T3 cells. Biochimie.
94:2656–2664. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Katz TA, Vasilatos SN, Harrington E,
Oesterreich S, Davidson NE and Huang Y: Inhibition of histone
demethylase, LSD2 (KDM1B), attenuates DNA methylation and increases
sensitivity to DNMT inhibitor-induced apoptosis in breast cancer
cells. Breast Cancer Res Treat. 146:99–108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schulte JH, Lim S, Schramm A, Friedrichs
N, Koster J, Versteeg R, Ora I, Pajtler K, Klein-Hitpass L,
Kuhfittig-Kulle S, et al: Lysine-specific demethylase 1 is strongly
expressed in poorly differentiated neuroblastoma: Implications for
therapy. Cancer Res. 69:2065–2071. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu D, Xiong L, Wu S, Jiang M, Lian G and
Wang M: TFPI-2 methylation predicts poor prognosis in non-small
cell lung cancer. Lung Cancer. 76:106–111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Q, Zhang Y, Wang SZ, Wang N, Jiang
WG, Ji YH and Zhang SL: Reduced expression of tissue factor pathway
inhibitor-2 contributes to apoptosis and angiogenesis in cervical
cancer. J Exp Clin Cancer Res. 31:12012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu H, Chen X, Wang C, Jiang Y, Li J, Ying
X, Yang Y, Li B, Zhou C, Zhong J, et al: The role of TFPI2
hypermethylation in the detection of gastric and colorectal cancer.
Oncotarget. 8:84054–84065. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sato N, Parker AR, Fukushima N, Miyagi Y,
Iacobuzio-Donahue CA, Eshleman JR and Goggins M: Epigenetic
inactivation of TFPI-2 as a common mechanism associated with growth
and invasion of pancreatic ductal adenocarcinoma. Oncogene.
24:850–858. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang S, Xiao X, Zhou X, Huang T, Du C, Yu
N, Mo Y, Lin L, Zhang J, Ma N, et al: TFPI-2 is a putative tumor
suppressor gene frequently inactivated by promoter hypermethylation
in nasopharyngeal carcinoma. BMC Cancer. 10:6172010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Konduri SD, Srivenugopal KS, Yanamandra N,
Dinh DH, Olivero WC, Gujrati M, Foster DC, Kisiel W, Ali-Osman F,
Kondraganti S, et al: Promoter methylation and silencing of the
tissue factor pathway inhibitor-2 (TFPI-2), a gene encoding an
inhibitor of matrix metalloproteinases in human glioma cells.
Oncogene. 22:4509–4516. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hubé F, Reverdiau P, Iochmann S, Rollin J,
Cherpi-Antar C and Gruel Y: Transcriptional silencing of the TFPI-2
gene by promoter hypermethylation in choriocarcinoma cells. Biol
Chem. 384:1029–1034. 2003. View Article : Google Scholar : PubMed/NCBI
|