Introduction
Atherosclerosis (AS) is a common consequence of
cardiovascular disease that includes abnormal lipid metabolism
(1) and foam cell formation, which
indicate early AS (2). Thus,
blocking foam cell formation may be a key to reducing AS (3). ATP-binding cassette transporter A1
(ABCA1), a critical protein for reverse cholesterol transport
(RCT), promotes macrophage cholesterol efflux and prevents foam
cell formation. ATP-binding cassette sub-family G member (ABCG1) is
an active lipid transporter and a member of the ABC family. Both of
them highly expressed in many tissues and can be activated by the
liver X receptors (LXRs) (4). It
contributes to RCT. Proprotein convertase subtilisin/kexin type 9
(PCSK9) is a lipid regulatory protein involved in lipid metabolism
and apoptosis (5). Previous
studies confirm that PCSK9 is upregulated in macrophages, causing
an inflammatory response and cholesterol accumulation via
inhibition of RCT (6). However,
inhibition of PCSK9 can intervene in AS. Also, PCSK9 inhibitors can
promote macrophage cholesterol efflux via upregulation of ABCA1
(7).
Quercetin is a flavonoid reported to have
anti-inflammatory, anti-oxidant, and lipid metabolic functions
(8). It may prevent AS by
regulating lipid metabolism, enhancing expression of ABCA1 in
RAW264.7 cells, promoting macrophage cholesterol efflux and
blocking foam cell formation (9).
Studies suggested that quercetin inhibited PCSK9 expression in
hepatocytes and promoted macrophage cholesterol efflux (10).
Thus, we assessed whether quercetin prevented
ox-LDL-induced lipid deposition in macrophages via increased
expression of ABCA1 and LXR-α and decreased expression of PCSK9.
RAW264.7 macrophages were induced with ox-LDL to create a cell
injury model, and cell viability was assessed. Lipid deposition and
expression of ABCAl, ABCG1, LXR-α, PCSK9, P53, P21 and P16 were
also measured.
Materials and methods
Materials
Mouse macrophage RAW264.7 cells were purchased from
the Shanghai Institute of Biochemistry and Cell, Shanghai, China.
Dulbecco's modified eagle medium (DMEM) was purchased from Gibco
(Thermo Fisher Scientific, Inc., Waltham, MA, USA), FBS was
purchased from Invitrogen (Thermo Fisher Scientific, Inc.)
Quercetin was purchased from Shanghai Yuanye Bio-Technology Co.,
Ltd. (Shanghai, China). Oxidized low density lipoprotein (ox-LDL)
was purchased from Shanghai Yuanye Bio-Technology Co., Ltd. The
protein ladder was from Thermo Fisher Scientific, Inc. We used
anti-ABCA1 (Thermo Fisher Scientific, Inc.), anti-ABCG1 anti-PCSK9,
anti-LXR-α, anti-P53, anti-P21, anti-P16 antibodies were all
purchased from Abcam (Cambridge, MA, USA), anti-β-actin (Cell
Signaling Technology, Inc., Danvers, MA, USA), goat anti-rabbit IgG
(H+L) secondary antibody, goat anti-mouse IgG (H+L) secondary
antibody (both LI-COR, Lincoln, NE, USA); FITC-labeled goat
anti-mouse IgG (H+L) (Shanghai Beyotime, Shanghai, China), and
FITC-labeled goat anti-rabbit IgG (H+L) (A0562; Shanghai
Beyotime).
A CCK8 kit was purchased from Shanghai Beyotime. An
enhanced BCA protein assay kit was used as was an SDS-PAGE
preparation kit (both from Shanghai Beyotime). We used an oil red O
staining kit Shanghai Yeasen (Shanghai, China) and a senescence
β-galactosidase (β-gal) staining kit (Shanghai Beyotime). SOD and
MDA were measured using assay kit (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China). A Filipin staining kit was purchased
from Shanghai Genmed (Shanghai, China), and DAPI staining solution
was purchased from Shanghai Beyotime.
Macrophage cell culture
RAW264.7 cells were cultured in DMEM medium
containing 10% FBS in a humidified atmosphere at 37°C, 5%
CO2. Then the cells were passaged three times. When the
cells were confluent, they were randomized into four groups:
Control (carrier medium), ox-LDL treatment, ox-LDL + quercetin
treatment, and quercetin treatment using at least three replicates
for each group. Cells were cultured with ox-LDL for 24 h at 100
mg/l or quercetin (20 µmol/l) or an ox-LDL/quercetin co-culture.
Subsequently, cells were harvested and extracted for analysis.
Cell proliferation assay
RAW264.7 cells were seeded into 96-well plates
(103/well), and 100 µl medium was added into each well.
Plates were cultured for 24 h in an incubator containing 5%
CO2 at 37°C. Then cells were randomized to ox-LDL (0,
10, 50, 100, or 200 mg/l), quercetin (10, 20, 40, or 60 µmol/l),
control (vehicle medium), or ox-LDL treated, ox-LDL + quercetin, or
quercetin. After culture for 24 h, cell proliferation was measured
using a CCK8 kit and a microplate reader (490 nm).
Oil red O and Filipin staining
RAW264.7 cells were randomized to control, ox-LDL,
ox-LDL + quercetin or quercetin, as described above. Cell cultures
were rinsed once with PBS and fixed with 10% (v/v) formaldehyde for
20 min at room temperature. After three washings with distilled
water, cells were incubated with filtered 60% (v/v) isopropyl
alcohol for 1 min and then fixed with oil red O solution for 15 min
at room temperature. Cell cultures were rinsed in distilled water
and counterstained with hematoxylin for 30 sec, rinsed with
distilled water and sealed with glycerin gelatin. Intracellular
lipid droplets were red, and nuclei were blue under a microscope.
Oil red O imaging was manually performed with the ×40 objective
lens, and from the images, five areas were randomly selected for
analysis using integrated optical density (IOD) and ImageJ software
(National Institutes of Health, Bethesda, MD, USA) to quantify
intracellular lipid droplets.
RAW264.7 cells were treated as described and washed
once with PBS and then stained with Filipin according to kit
instructions. Intracellular unesterified cholesterol was stained by
Filipin and fluoresced blue (340 nmex; 430
nmem).
Total cholesterol (TC) assay
RAW264.7 cells were treated as described above. TC
was measured according to kit instructions. Briefly, after
treatment, cells were washed once with PBS and lysed using an
ultrasonic homogenizer and heated for 10 min at 70°C. Then, the
cells were centrifuged at 2,000 rpm/min for 5 min at room
temperature. The supernatant was removed, and TC was measured using
a microplate reader. Protein was quantified using a BCA kit, and TC
was normalized to the protein content.
SOD measurement
Cells were scraped, and SOD was assayed for 10 min.
Cells were then centrifuged for 10 min at 1,500 rpm/min and
precipitated. Buffer was added to the cells, and ultrasonic lysis
was carried out. Reagent was added to the cells according to the
SOD assay instructions and incubated at 37°C for 20 min. Absorption
was read at 450 nm.
MDA measurement
Cells were treated as they were for the SOD assays
and heated at 95°C for 20 min. Reagents were prepared according to
MDA kit instructions, and the pore plate was treated as instructed.
The absorption was read at 530 nm.
Immunofluorescent assay
RAW264.7 cells were randomized to control, ox-LDL,
ox-LDL + quercetin, or quercetin. After cell routine treatment,
cells were washed three times with PBS and then fixed in 4%
paraformaldehyde for 10 min at room temperature. Cells were washed
three times with PBS and then saturated with 10% BSA for 30 min.
Samples were incubated with anti-ABCA1, anti-ABCG1, anti-LXR-α,
anti-PCSK9, anti-P53, anti-P21, anti-P16 overnight at 4°C. After
washing twice with PBS, samples were incubated with secondary
antibody FITC-labeled goat anti-rabbit IgG (1:50) for 30 min at
room temperature. After staining for quantification, samples were
incubated with 10 µg/ml DAPI for 5 min for visualization of the
cell nuclei. Finally, cells were washed three times with PBS and
sealed. The average integral optical density of green fluorescence
was analyzed using ImageJ software (National Institutes of Health)
to reflect the relative protein expression.
Western blot assay
RAW264.7 cells were treated as described and washed
three times with PBS, and cell lysates were generated using RIPA
lysis buffer with PMSF. Cells were scraped and removed to a new
tube. The solution was lysed on ice for 30 min and centrifuged at
12,000 rpm/min for 30 min at 4°C. Then, the supernatant was assayed
for protein with a BCA kit. The protein concentration was adjusted
with RIPA lysate, and 5× loading buffer was added and boiled for 5
min. Then, cells were stored at −80°C. In total, 30 µg protein was
resolved by SDS-PAGE and transferred to PVDF membranes, which were
blocked in TBST with 5% BSA, and incubated with anti-ABCA1,
anti-ABCG1, anti-LXR-α, anti-PCSK9, anti-P53, anti-P21, anti-P16
and anti-β-actin overnight at 4°C. Membranes were incubated with
appropriate horseradish peroxidase-conjugated secondary antibodies
for 1 h and then washed to remove unbound antibodies. Finally,
membranes were incubated with ECL HRP substrate and imaged with an
ECL system. Protein bands were visualized using ImageJ software
(National Institutes of Health), and the target protein was
quantified.
β-gal
Expression of pH-dependent senescence-associated
β-gal in RAW264.7 cells was analyzed using a β-gal staining kit
according to the manufacturer's instructions. Briefly, RAW264.7
cells grown in a microwell plate were washed and incubated in a
fixative solution. After one wash with PBS, 1 ml β-gal staining
solution was added to each well. The plate was sealed with Parafilm
to prevent evaporation and incubated at room temperature for 15
min. Then the fixative solution was removed, and cells were washed
with PBS 3 times. Finally, cells were incubated with special dyeing
fluid at 37°C overnight. Cells were examined under a light
microscope. Cells were counted, and those that were blue were
considered positive.
Statistical analyses
Data are presented as the mean + standard deviation.
An unpaired Student's t-test was used to compare two independent
groups and one-way ANOVA with Tukey HSD post hoc test was used to
compare multiple independent groups with SPSS17.0 software. For all
tests, P<0.05 was considered to indicate a statistically
significant difference.
Results
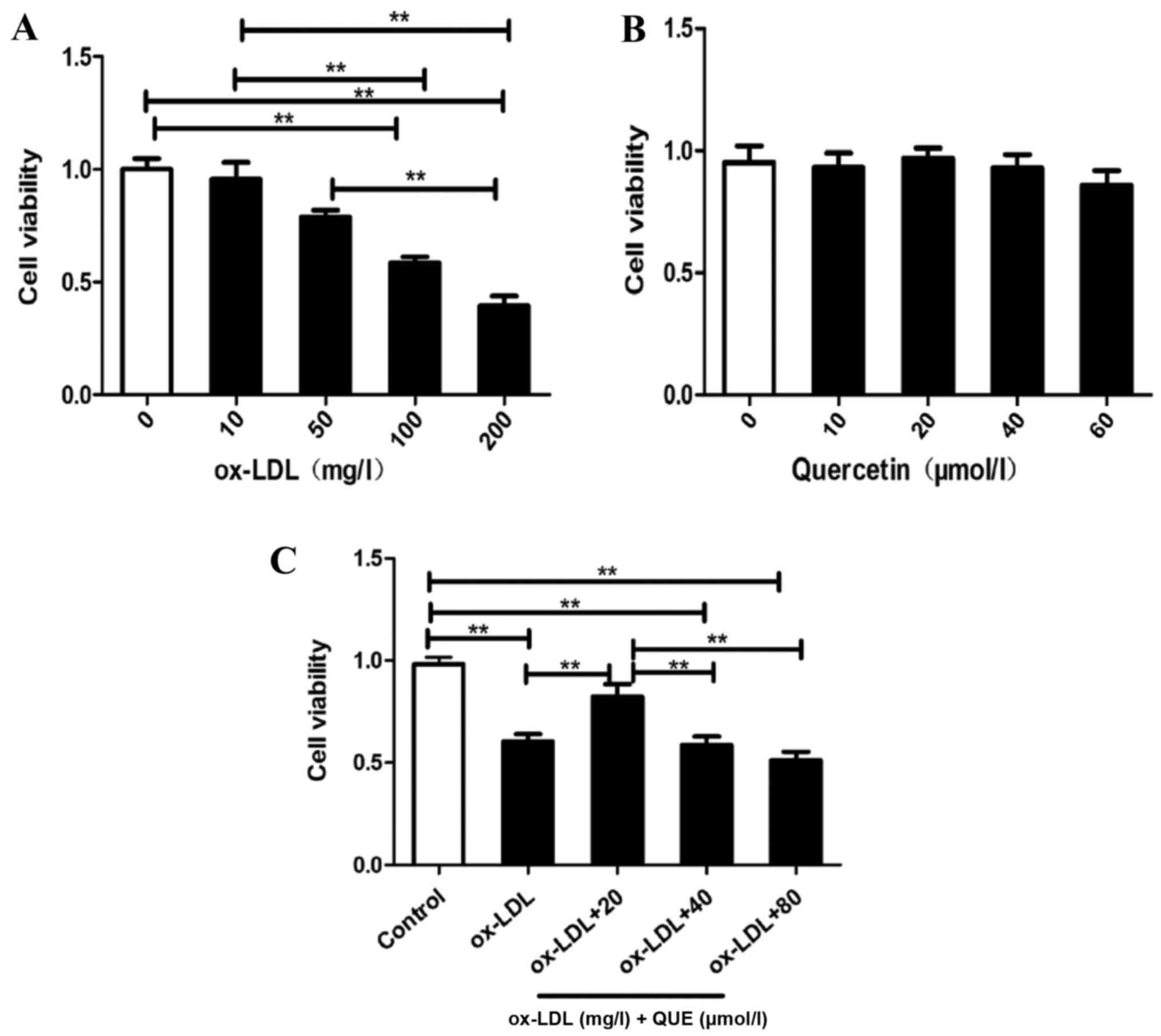
Quercetin improved RAW264.7 cells
viability induced by ox-LDL
RAW264.7 cells viability were assessed, and Fig. 1A shows that ox-LDL reduced RAW264.7
cell viability in a concentration-dependent manner. Half of the
cells were viable with 100 mg/ml ox-LDL, quercetin was not
cytotoxic to RAW264.7 cells at 60 µmol/l (Fig. 1B). Fig. 1C shows that treatment of
ox-LDL-induced RAW 264.7 cells with 20 µmol/l quercetin
significantly improved viability. Thus, quercetin blocked ox-LDL
reductions in RAW264.7 cells proliferation.
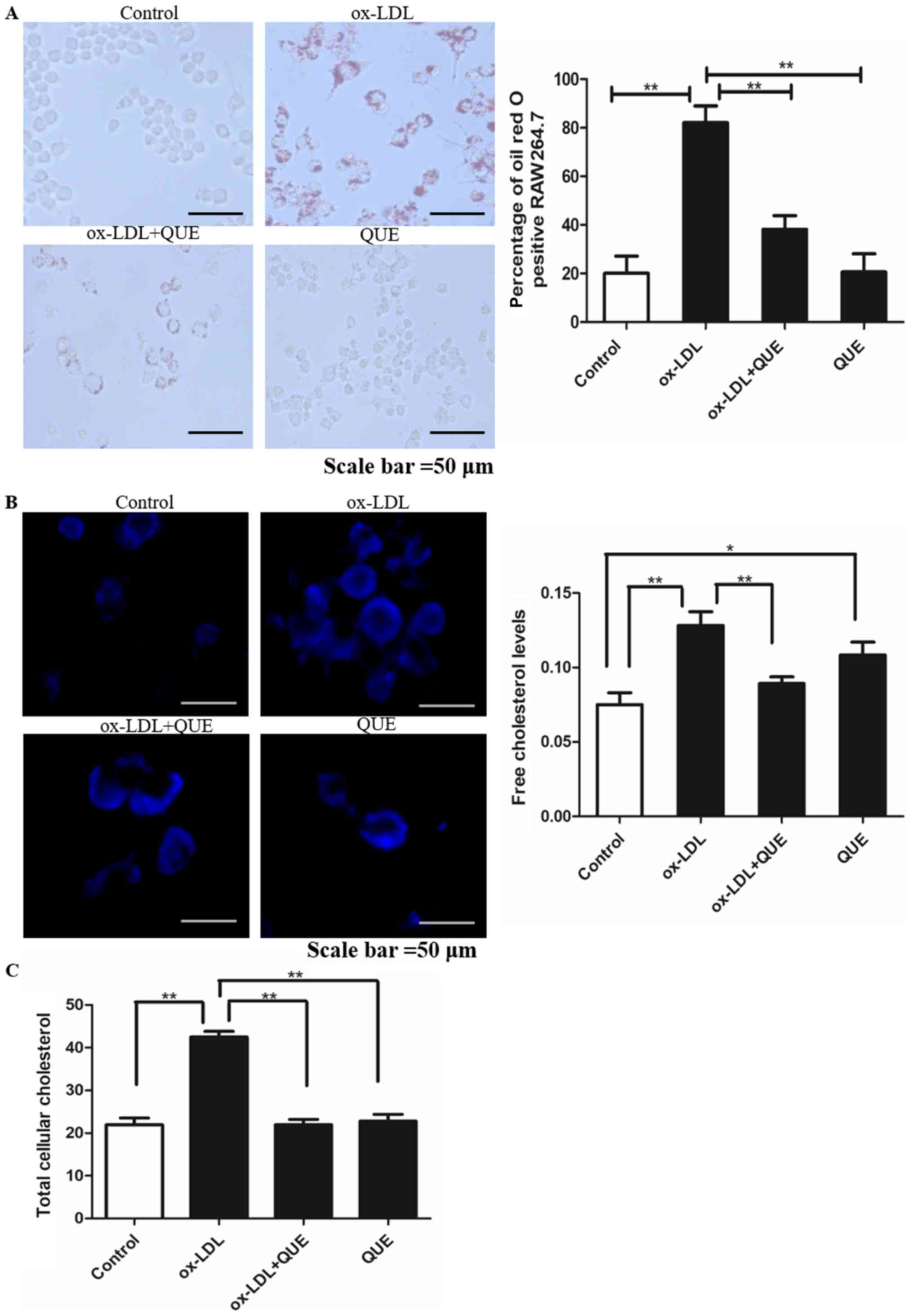
Quercetin inhibited lipid accumulation
in RAW264.7 cells induced by ox-LDL
We measured macrophage lipid accumulation in
RAW264.7 cells at 24 h and oil red staining showed that lipid
droplets in the ox-LDL + quercetin group were significantly less
than in the ox-LDL group (P<0.05). There was no significant
difference between controls and ox-LDL + quercetin groups
(P<0.05) (Fig. 2A).
Intracellular free cholesterol was measured using Filipin staining
and it was less in the ox-LDL + quercetin group (Fig. 2B). TC was measured and quercetin
reduced ox-LDL-induced TC (Fig.
2C). Thus, quercetin inhibits lipid accumulation in RAW264.7
cells induced by ox-LDL.
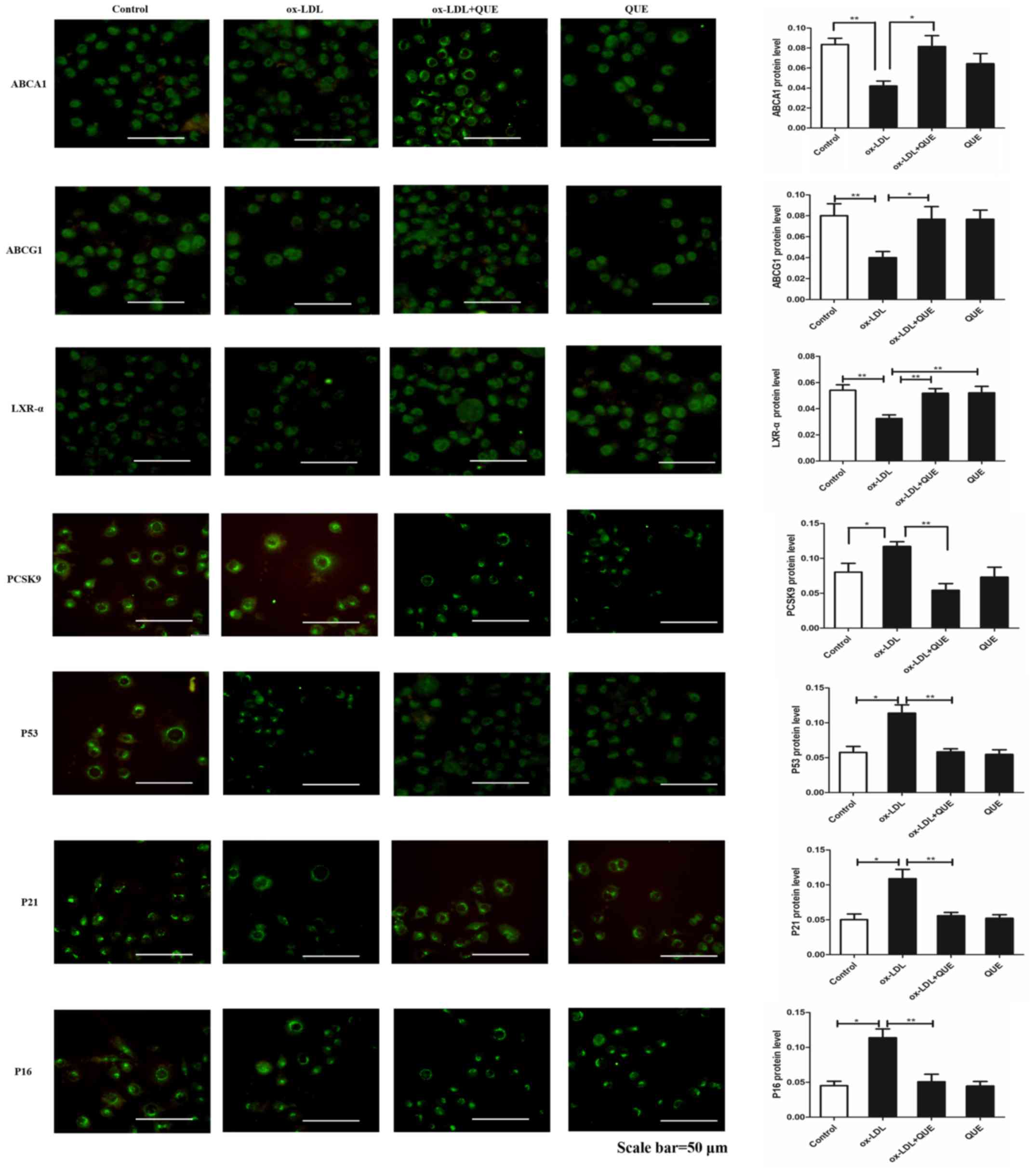
Quercetin effectively regulated the
expression of ABCAl, ABCG1, LXR-α, PCSK9, P53, P21, P16 in RAW264.7
cells
The effects of quercetin on ABCA1, LXR-α and PCSK9
expression were measured and immunofluorescent data show that
ox-LDL decreased expression of ABCA1, ABCG1 and LXR-α, but
increased PCSK9, P53, P21 and P16 expression. After treatment with
quercetin, ABCA1, ABCG1 and LXR-α expression were upregulated, and
expression of PCSK9, P53, P21 and P16 were downregulated (Fig. 3). We verified that quercetin
modulated ABCA1, ABCG1, LXR-α, PCSK9, P53, P21 and P16 expression
by Western Blot, and data agreed with immunofluorescent results.
Thus, ox-LDL decreased expression of ABCA1, ABCG1 and LXR-α, but
increased PCSK9, P53, P21and P16 expression. After treatment with
quercetin, ABCA1, ABCG1 and LXR-α expression was upregulated, and
expression of PCSK9, P53, P21and P16 was downregulated (Fig. 4). Thus, quercetin can modify
expression of ABCAl, ABCG1, LXR-α, PCSK9, P53, P21 and P16 in
RAW264.7 cells.
 | Figure 4.ABCA1, ABCG1, LXR-α, PCSK9, p53, p21
and p16 expression changes following quercetin treatment in
ox-LDL-induced RAW264.7 cells. Western blot analysis quantification
of ABCA1, ABCG1, LXR-α, PCSK9, p53, p21 and p16 proteins. Data are
presented as the mean ± standard deviation. *P<0.05,
**P<0.01. ABCA1, ATP-binding cassette transporter 1; ABCG1,
ATP-binding cassette sub-family G member 1; LXR-α, liver X
receptor-α; PCSK9, proprotein convertase subtilisin/kexin type 9;
ox-LDL, oxidized low density lipoprotein; QUE, quercetin. |
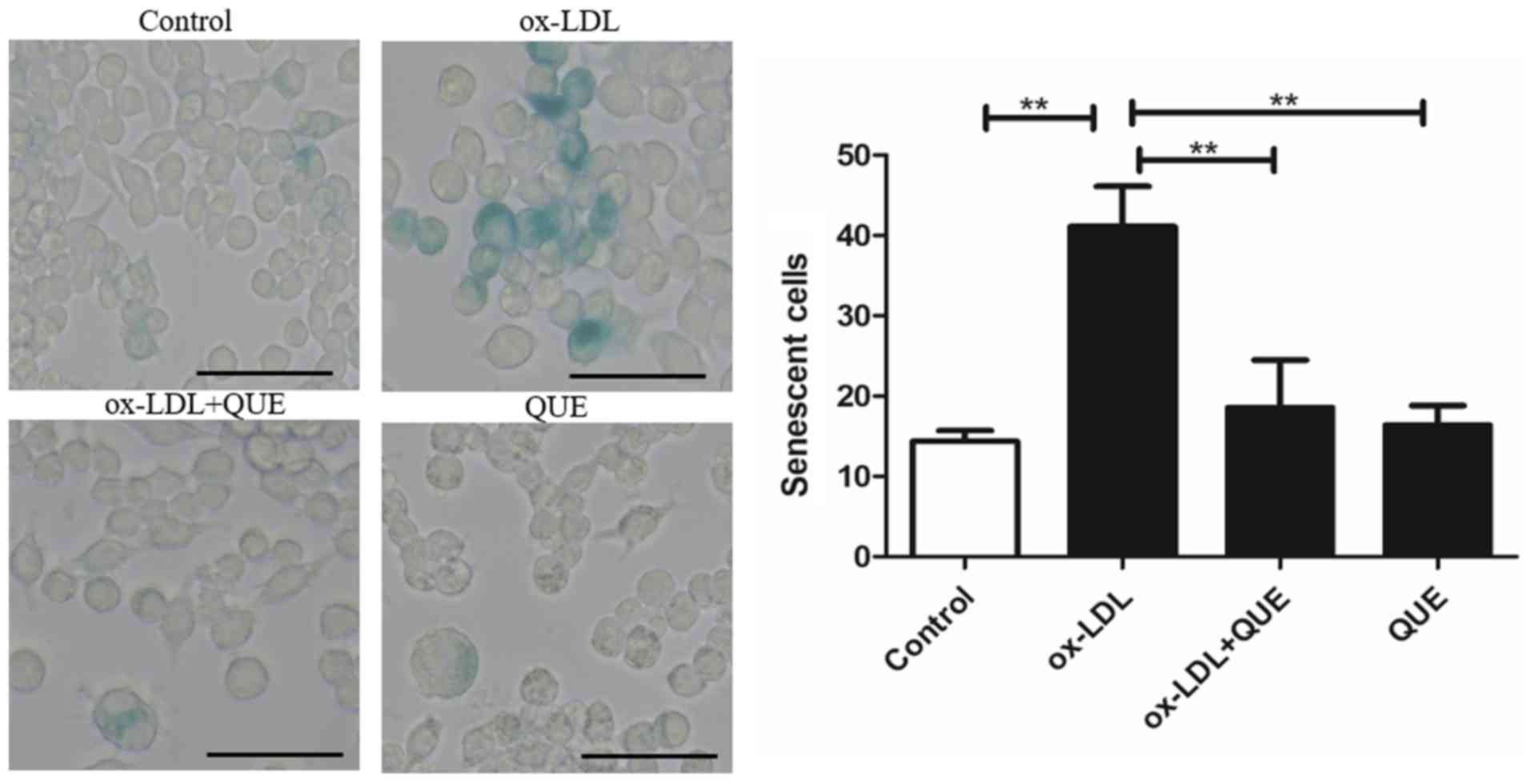
Quercetin slowed RAW 264.7 cells
senescence
We studied whether quercetin could reduce RAW264.7
cells senescence after ox-LDL intervention using β-gal staining.
Fig. 5 shows that ox-LDL
accelerated cell senescence, and quercetin reduced senescent cells.
There was no statistically significant difference between the
ox-LDL + quercetin and quercetin groups. Data show that quercetin
significantly reduced RAW 264.7 cell senescence.
Effects of quercetin on SOD activity
and MDA in RAW 264.7 cells
SOD and MDA were measured after quercetin treatment
of RAW264.7 cells. Table I shows
that viability in the ox-LDL group was less than other groups and
this difference was statistically significant (P<0.01). Ox-LDL
group's MDA was greater than the other groups and this was
statistically significant (P<0.01). Thus, quercetin increased
SOD activity after ox-LDL treatment and blocked the effect of
quercetin on MDA.
 | Table I.Cell SOD activity and MDA. |
Table I.
Cell SOD activity and MDA.
| Group | SOD (µ/ml) | MDA (nmol/ml) |
|---|
| Control | 14.92±0.96 | 3.73±0.03 |
| ox-LDL |
12.54±0.89a |
2.31±0.08a |
| ox-LDL + QUE |
14.03±0.91b |
3.36±0.04b |
| QUE |
14.32±2.18c |
3.54±0.07c |
Discussion
AS contributes to many cardiovascular and
cerebrovascular diseases and lipid metabolic disorders contribute
to AS. When macrophages accumulate in vessels, macrophages
transform into foam cells and release intracellular cholesterol
which leads to AS development (11). In early AS, mononuclear cells
transform to macrophages which were induced by ox-LDL and
inflammatory mediators in the endothelial gap. Then macrophages
engulf ox-LDL to cause cholesterol ester accumulation and foam cell
formation. Foam cells contribute to initial pathological changes
leading to AS (3).
ABCA1 is an ABC transporter superfamily member
mainly expressed in macrophages. ABCA1, as an integrated membrane
protein, is necessary for lipid outflow transporters, and
contributes to clearance of excess cholesterol via consumption of
ATP and promotion of RCT (12,13).
As a major transporter of intracellular cholesterol efflux, ABCA1
promotes deposition of cholesterol in macrophages so it contributes
to AS (14). ABCG1 is expressed in
macrophages and ABCG1 expression contributes to RCT. ABCG1
expression can promote cholesterol outflow of macrophages, inhibit
excessive cholesterol accumulation, and reduce the formation of
foam cells (15). LXRs are
cholesterol metabolic receptors that regulate expression of key
genes in cholesterol metabolism, as well as participate in lipid
metabolism, innate immune of macrophages, inflammatory response and
other physiological activities (16). LXRs include two homologous
subtypes, LXR-α and LXR-β. LXR-α, a supranuclear receptor protein,
is an oxidized cholesterol-activated nuclear receptor that
regulates ABCA1 gene expression (17). PCSK9 is a subtilisin which
regulates lipid metabolism (18).
Studies of PCSK9 in AS are chiefly focused on regulation of lipid
metabolism, and PCSK9 can promote degradation of low density
lipoprotein receptor (LDLR) in hepatocytes and regulate lipid
metabolism to change LDL-C (19).
Moreover, PCSK9 can promote macrophage lipid accumulation and
expression of inflammatory factors (20). Some studies show that PCSK9
inhibitors have anti-AS effects because they activate the LXR-α
signaling pathway, and promote ABCA1 protein expression to
accelerate cholesterol efflux (7).
Studies indicate that P21 is involved in the regulation of cell
proliferation, differentiation, migration, senescence and
apoptosis. p21-mediated cell senescence may not be related to P53
(21). P 16 is a major inhibitor
of cyclin dependent kinase (CDKK), and upregulation of P16 is key
to cell senescence associated with cell growth arrest (22).
Quercetin may prevent AS (23) so it is often found in herbal
products (24,25) but these have not been studied for
efficacy. Studies suggest that quercetin may reduce inflammatory
factors and adhesion molecules of AS by regulating the TLR/NF-κB
signaling pathway (26) and
upregulating ABCA1 proteins associated with RCT in
apoE−/− mice as well as promoting cholesterol efflux
(9). Furthermore, quercetin may
activate the PPARγ-ABCA1 pathway to promote cholesterol efflux in
macrophages, thereby inhibiting the formation of foam cells
(27). Quercetin may improve
protein expression of ABCG1 in a concentration-dependent manner
in vitro (9), which is
consistent with in vivo data (28). Studies show that quercetin can
inhibit lipopolysaccharide-induced RAW264.7 macrophages and
downregulate the TLR4 signaling pathway to block inflammation
(29). Quercetin also upregulates
ABCA1 expression through p38 signaling pathway or activates the
LXR-α signaling pathway in THP-1 cells to promote cholesterol
efflux (30,31). Studies suggest that quercetin can
prevent apoptosis by regulating translational modification of P53
and P21 (32). In addition,
studies indicate that quercetin may decrease expression of PCSK9
and increase expression of ABCA1 in hepatocytes of
apoE−/− mice (10).
Thus, quercetin may block the development of AS.
We used 100 mg/ml ox-LDL to transform RAW264.7 cells
to foam cells. Quercetin treatment improved cell viability of
ox-LDL-induced RAW264.7 cells and blocked lipid accumulation.
Furthermore, immunofluorescent and Western blot results showed that
quercetin regulated expression of ABCAl, ABCG1, LXR-α, PCSK9, P53,
P21 and P16, to promote RCT in RAW264.7 cells, inhibit foam cell
formation, and inhibit senescence. Finally, β-gal staining showed
that quercetin could significantly reduce β-gal positive cells, and
SOD and MDA data confirmed that quercetin reduced RAW264.7 cell
aging. Chinese kidney-tonifying drugs are reported to regulate
lipids in apoE−/− mice and CAS patients (33,34),
improving vascular elasticity and reducing senescence (35). These results above suggest that
Chinese kidney-tonifying drugs may have a good therapeutic prospect
for AS.
Thus, as one of the monomers of traditional Chinese
medicine for tonifying the kidney, quercetin blocked damage of
ox-LDL-induced RAW264.7 cells and improved viability, as well as
reduced lipid accumulation and senescence. This may be attributed
to effective regulation of ABCAl, ABCG1, LXR-α, PCSK9, P53, P21 and
P16 expression. Future studies will continue to study the effect of
quercetin on the expression of related proteins in RCT.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Shanghai Nature Science Fund (grant no. 16ZR1433900), the Shanghai
Health and Family Planning Commission Fund (grant no. 201640217)
and Shanghai University of Traditional Chinese Medicine graduate
‘innovation ability training’ special research projects (grant no.
Y201858).
Availability of data and materials
The datasets used or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DS, SL, HC, SLX and CC conceived and designed the
study. SL, HC and QJ performed the experiments. SL, QJ and HC made
substantial contributions to the acquisition, analysis and
interpretation of the data and wrote the paper. DS, CC and SLX
reviewed and edited the manuscript. All authors read and approved
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Getz GS and Reardon CA: The mutual
interplay of lipid metabolism and the cells of the immune system in
relation to atherosclerosis. Clin Lipidol. 9:657–671. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maranhão RC and Leite AC Jr: Development
of anti-atherosclerosis therapy based on the inflammatory and
proliferative aspects of the disease. Curr Pharm Des. 21:1196–1204.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu XH, Fu YC, Zhang DW, Yin K and Tang CK:
Foam cells in atherosclerosis. Clin Chim Acta. 424:245–252. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Voloshyna I, Seshadri S, Anwar K,
Littlefield MJ, Belilos E, Carsons SE and Reiss AB: Infliximab
reverses suppression of cholesterol efflux proteins by TNF-α: A
possible mechanism for modulation of atherogenesis. Biomed Res Int.
2014:3126472014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cui Q, Ju X, Yang T, Zhang M, Tang W, Chen
Q, Hu Y, Haas JV, Troutt JS, Pickard RT, et al: Serum PCSK9 is
associated with multiple metabolic factors in a large Han Chinese
population. Atherosclerosis. 213:632–636. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paciullo F, Fallarino F, Bianconi V,
Mannarino MR, Sahebkar A and Pirro M: PCSK9 at the crossroad of
cholesterol metabolism and immune function during infections. J
Cell Physiol. 232:2330–2338. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adorni MP, Cipollari E, Favari E, Zanotti
I, Zimetti F, Corsini A, Ricci C, Bernini F and Ferri N: Inhibitory
effect of PCSK9 on Abca1 protein expression and cholesterol efflux
in macrophages. Atherosclerosis. 256:1–6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lara-Guzman OJ, Tabares-Guevara JH,
Leon-Varela YM, Álvarez RM, Roldan M, Sierra JA, Londoño-Londoño JA
and Ramirez-Pineda JR: Proatherogenic macrophage activities are
targeted by the flavonoid quercetin. J Pharmacol Exp Ther.
343:296–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cui Y, Hou P, Li F, Liu Q, Qin S, Zhou G,
Xu X, Si Y and Guo S: Quercetin improves macrophage reverse
cholesterol transport in apolipoprotein E-deficient mice fed a
high-fat diet. Lipids Health Dis. 16:92017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mbikay M, Sirois F, Simoes S, Mayne J and
Chrétien M: Quercetin-3-glucoside increases low-density lipoprotein
receptor (LDLR) expression, attenuates proprotein convertase
subtilisin/kexin 9 (PCSK9) secretion and stimulates LDL uptake by
Huh7 human hepatocytes in culture. FEBS Open Bio. 4:755–762. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu Y and Jia Y: Quercetin upregulates
ABCA1 expression through liver X receptor alpha signaling pathway
in THP-1 macrophages. Eur Rev Med Pharmacol Sci. 20:3945–3952.
2016.PubMed/NCBI
|
|
12
|
Yvan-Charvet L, Pagler T, Gautier EL,
Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW
and Tall AR: ATP-binding cassette transporters and HDL suppress
hematopoietic stem cell proliferation. Science. 328:1689–1693.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kolovou V, Marvaki A, Boutsikou M,
Vasilopoulos G, Degiannis D, Marvaki C and Kolovou G: Effect of
ATP-binding cassette transporter A1 (ABCA1) gene polymorphisms on
plasma lipid variables and common demographic parameters in Greek
nurses. Open Cardiovasc Med J. 10:233–239. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang S and Smith JD: ABCA1 and nascent HDL
biogenesis. Biofactors. 40:547–554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vaughan AM and Oram JF: ABCG1
redistributes cell cholesterol to domains removable by high density
lipoprotein but not by lipid-depleted apolipoproteins. J Biol Chem.
280:30150–30157. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oosterveer MH, Grefhorst A, Groen AK and
Kuipers F: The liver X receptor: Control of cellular lipid
homeostasis and beyond Implications for drug design. Prog Lipid
Res. 49:343–352. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ikhlef S, Berrougui H, Kamtchueng SO and
Khalil A: Paraoxonase 1-treated oxLDL promotes cholesterol efflux
from macrophages by stimulating the PPARγ-LXRα-ABCA1 pathway. FEBS
Lett. 590:1614–1629. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sharma K and Baliga RR: Genetics of
dyslipidemia and ischemic heart disease. Curr Cardiol Rep.
19:462017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Soutar AK: Unexpected roles for PCSK9 in
lipid metabolism. Curr Opin Lipidol. 22:192–196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu M, Wu G, Baysarowich J, Kavana M,
Addona GH, Bierilo KK, Mudgett JS, Pavlovic G, Sitlani A, Renger
JJ, et al: PCSK9 is not involved in the degradation of LDL
receptors and BACE1 in the adult mouse brain. J Lipid Res.
51:2611–2618. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Romanov VS, Pospelov VA and Pospelova TV:
Cyclin-dependent kinase inhibitor p21(Waf1): Contemporary view on
its role in senescence and oncogenesis. Biochemistry (Mosc).
77:575–584. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shih CT, Chang YF, Chen YT, Ma CP, Chen
HW, Yang CC, Lu JC, Tsai YS, Chen HC and Tan BC: The PPARγ-SETD8
axis constitutes an epigenetic, p53-independent checkpoint on
p21-mediated cellular senescence. Aging Cell. 16:797–813. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang M, Xie Z, Gao W, Pu L, Wei J and Guo
C: Quercetin regulates hepatic cholesterol metabolism by promoting
cholesterol-to-bile acid conversion and cholesterol efflux in rats.
Nutr Res. 36:271–279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mehrbani M, Choopani R, Fekri A and
Mehrabani M, Mosaddegh M and Mehrabani M: The efficacy of whey
associated with dodder seed extract on moderate-to-severe atopic
dermatitis in adults: A randomized, double-blind,
placebo-controlled clinical trial. J Ethnopharmacol. 172:325–332.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun X, Yamasaki M, Katsube T and Shiwaku
K: Effects of quercetin derivatives from mulberry leaves: Improved
gene expression related hepatic lipid and glucose metabolism in
short-term high-fat fed mice. Nutr Res Pract. 9:137–143. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bhaskar S, Sudhakaran PR and Helen A:
Quercetin attenuates atherosclerotic inflammation and adhesion
molecule expression by modulating TLR-NF-κB signaling pathway. Cell
Immunol. 310:131–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun L, Li E, Wang F, Wang T, Qin Z, Niu S
and Qiu C: Quercetin increases macrophage cholesterol efflux to
inhibit foam cell formation through activating PPARγ-ABCA1 pathway.
Int J Clin Exp Pathol. 8:10854–10860. 2015.PubMed/NCBI
|
|
28
|
Guo S, Tian H, Dong R, Yang N, Zhang Y,
Yao S, Li Y, Zhou Y, Si Y and Qin S: Exogenous supplement of
N-acetylneuraminic acid ameliorates atherosclerosis in
apolipoprotein E-deficient mice. Atherosclerosis. 251:183–191.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Byun EB, Yang MS, Choi HG, Sung NY, Song
DS, Sin SJ and Byun EH: Quercetin negatively regulates TLR4
signaling induced by lipopolysaccharide through Tollip expression.
Biochem Biophys Res Commun. 431:698–705. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang YC, Lee TS and Chiang AN: Quercetin
enhances ABCA1 expression and cholesterol efflux through a
p38-dependent pathway in macrophages. J Lipid Res. 53:1840–1850.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu Y and Jia YP: Quercetin upregulates
ABCA1 expression through liver X receptor alpha signaling pathway
in THP-1 macrophages. Eur Rev Med Pharmacol Sci. 20:3945–3952.
2016.PubMed/NCBI
|
|
32
|
Gong C, Yang Z, Zhang L, Wang Y, Gong W
and Liu Y: Quercetin suppresses DNA double-strand break repair and
enhances the radiosensitivity of human ovarian cancer cells via
p53-dependent endoplasmic reticulum stress pathway. Onco Targets
Ther. 11:17–27. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shen DZ, Xing SL, Chen C, et al: Effect of
shouqian granule on atherosclerosis in ApoE−/− mice
based on expression of TLR4, MCP-1 and ICAM-1 in mice. Chin Med
Emerg. 2:192–194+232. 2017.
|
|
34
|
Shen DZ, Chen C, Chen JL and Xing S:
Effect of shoushen granules on level of blood lipids and
inflammatory cytokines when treating carotid artherosclerosis. Chin
Arch Tradit Chin Med. 1:22–24. 2014.
|
|
35
|
Shen DZ, Xing SL, Chen C, Shen R and Lou
DF: Effect of Shoushen granule on arterial elasticity in patients
with carotid atherosclerosis: a clinical randomized controlled
trial. J Tradit Chin Med. 4:389–395. 2015.
|