Introduction
Mechanical ventilation (MV) is an irreplaceable
therapy for patients in intensive care units and emergency
departments. Although the techniques for MV have significantly
improved in the past 20 years (1),
the risk of aggravating lung injury in patients with acute
respiratory distress syndrome (ARDS) (2), and damaging healthy lung tissue,
remains (3,4). Lung injury caused by MV is also known
as ventilator-induced lung injury (VILI), and is characterized by
tissue disruption, lung edema and pulmonary inflammation (5–7). A
recent study from the ARDS Network indicated that VILI may increase
the mortality of critically ill patients by ~10% (8). However, in addition to the protective
pulmonary ventilation strategy, there are few specific treatments
for VILI, as the potential mechanism remains poorly understood.
Therefore, it is imperative to identify the pathogenesis of VILI to
provide an experimental foundation for clinical treatment.
In the transcriptome, numerous protein-coding mRNAs,
as well as non-protein coding transcripts, are closely associated
with the pathogenesis of several diseases. Long non-coding RNAs
(lncRNAs) are defined as non-protein coding transcripts >200
nucleotides long (9). In recent
years, a growing body of evidence has indicated that lncRNAs are
involved in various important biological processes, including
proliferation (10), apoptosis
(11), brain development (12,13),
inflammation (14,15) and immunoregulation (16,17),
leading to numerous diseases (18–20);
lncRNAs may therefore provide alternative targets for the treatment
of these diseases (19,21).
Although the crucial roles of lncRNAs have been
investigated in numerous diseases, to the best of the authors'
knowledge, no study has been conducted to investigate the profile
and function of lncRNAs in VILI. In the present study,
differentially expressed lncRNAs and mRNAs were investigated by
high-throughput sequencing, and bioinformatics analysis was
employed to predict their functions in VILI.
Materials and methods
Animals
Experiments were performed on 16 male ICR mice (age,
7–9 weeks, 25–30 g; Shanghai SLAC Laboratory Animal Co., Shanghai,
China). Mice were housed in a temperature-controlled room (22±2°C)
under a 12-h light/dark cycle, and were provided ad libitum
access to food and water. The humidity was maintained at 50–60%.
All experimental protocols and animal handling procedures were
approved by the Ethics Committee on Experimental Animals of
Shanghai Jiaotong University School of Medicine (Shanghai, China).
Mice were divided into two groups: Sham-operated group and VILI
group (n=8/group). A total of eight pairs of right lungs were used
for RNA-sequencing (RNA-Seq) analysis, whereas eight pairs of left
lungs were used for reverse transcription-quantitative polymerase
chain reaction (RT-qPCR).
Establishment of a VILI mouse
model
Following anesthesia with 100 mg/kg ketamine and 8
mg/kg xylazine (intraperitoneal), mice were fixed in a supine
position, endotracheal intubation was performed and the tube was
connected to a rodent ventilator (Inspira; Harvard Apparatus Ltd.,
Holliston, MA, USA). Ventilation was performed at a tidal volume of
30 ml/kg and a respiratory rate of 70 breaths/min for 4 h as
previously described (22,23). Mice in the sham group underwent
intubation but were allowed to breathe freely. Gas with fraction of
inspired oxygen at 21% was used, and the inspiratory/expiratory
ratio was 2:1. At the end of the experiment, animals were
sacrificed by anesthetic overdose and the lung tissues were
harvested for further investigation.
RNA-Seq analysis
Total RNA was extracted from lung tissues in the
VILI and sham groups with TRIzol® (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol. RNA concentration was determined using
NanoDrop 2000 (NanoDrop; Thermo Fisher Scientific, Inc.,
Wilmington, DE, USA), and RNA quality was evaluated with
Bioanalyzer 2200 (Agilent Technologies, Inc., Santa Clara, CA, USA)
and by 1% agarose gel electrophoresis. RNA with RNA integrity
number >8.0 was used to construct a cDNA library. A total of
five pair of lungs met the criterion and were used for RNA-Seq
analysis.
The cDNA libraries were subsequently constructed
with Ion Total RNA-Seq kit version 2.0 (Thermo Fisher Scientific,
Inc.). Subsequently, proton sequencing was performed using the cDNA
libraries and Ion PI Sequencing 200 kit version 2.0 (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Briefly, samples were mixed and processed on the Ion OneTouch 2
System (Thermo Fisher Scientific, Inc.). Subsequently, they were
enriched on the OneTouch 2 ES station for the preparation of
template-positive Ion PI Ion Sphere Particles. Finally, the mixed
samples were loaded onto 1 P1v2 Proton Chip (Thermo Fisher
Scientific, Inc.) for sequencing. Data analysis was performed by
NovelBio Bio-Pharm Technology Co., Ltd. (Shanghai, China).
RNA-seq mapping and identification of
differentially expressed genes
The Mapsplice v2.2.0 program was used for RNA-seq
data mapping (24). Per kilobase
per million was used to measure the expression level of each gene
(25). The expression of different
genes between two groups was evaluated with the EB-Seq (26) algorithm. When the absolute fold
change (FC) was >2 and the false discovery rate (FDR) was
<0.05, the gene was defined as differentially expressed
(26).
Gene ontology (GO) and pathway
analysis
GO analysis was performed to analyze the biological
functions of differentially expressed genes (27). GO annotations were obtained from
gene in NCBI (https://www.ncbi.nlm.nih.gov/gene/) and the Gene
Ontology (http://www.geneontoloy.org/). Kyoto
Encyclopedia of Genes and Genomics (KEGG) database (http://www.genome.jp.kegg/) was employed to analyze
the pathways associated with these differentially expressed genes
(28). In addition, differentially
expressed genes enriched in ≥2 biological pathways were selected to
build the ‘path-act network’ a graphical representation of pathways
of the interaction of these pathway terms using cytoscape 3.5.1
(29).
Gene co-expression network
analysis
Gene co-expression network analysis, analyzed by R
software 3.3.3, was employed to predict the key lncRNAs that
function in the important biological processes and signal
transduction pathways based on the correlation between
differentially expressed lncRNAs and mRNAs (30). The significant correlation pairs
(Pearson's correlation coefficient >0.95) were selected to build
the network. The core regulatory genes were determined by k-core
scoring in the network (31). The
k-core indicates the hub or nodal status of a gene having
interactions with other genes in the network. The higher the k-core
score of a gene, the more central the gene location in the gene
co-expression network.
RT-qPCR analysis
To confirm the veracity of RNA-Seq analysis, the
expression of three lncRNAs and three mRNAs was measured by RT-qPCR
using the SYBR-Green method (FastStart Universal SYBR Master; Roche
Diagnostics, Basel, Switzerland) and a MiniOpticon real-time PCR
detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Total RNA was extracted from lung tissues using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and cDNA was
synthesized (RevertAid First Strand cDNA Synthesis kit; Thermo
Fisher Scientific, Inc.) with the reaction conditions: 25°C 10 min,
42°C 1 h, 72°C 10 min and 4°C hold. All of the primers (Table I) used for RT-qPCR were designed
and synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). PCR
was performed under the following conditions: Predenaturation for 2
min at 95°C with cycle, denaturation for 15 sec at 95°C, annealing
for 15 sec at 60°C and extension for 1 min at 72°C with 40 cycles
from denaturation to extension. The expression of each gene was
calculated using the 2−ΔΔCq (32) method and normalized to that of
β-actin. The experiments were repeated twice.
 | Table I.Primers used for the amplification of
various genes (long non-coding RNAs and mRNAs) in mouse lung
tissues. |
Table I.
Primers used for the amplification of
various genes (long non-coding RNAs and mRNAs) in mouse lung
tissues.
| Description | Sequence
(5′-3′) | Product size
(bp) | Accession no. |
|---|
| Map2k3os, F |
AGCAAAGCAACAGCCTCACT | 1,267 | NR_027800.1 |
| Map2k3os, R |
CACGGGCTCTCTGTGCTTAT |
|
|
| Dnm3os, F |
GCCTGGCTGGACAGAGTTGT | 7,928 | NR_002870.2 |
| Dnm3os, R |
TCAATGGCTGGTGGTCATTC |
|
|
| Abhd11os, F |
CAGTCACCAGGCCTTGACTC | 519 | NR_026688.1 |
| Abhd11os, R |
CGCTTCTTAGCAATGGCTTC |
|
|
| Gadd45a, F |
CTGCAGAGCAGAAGACCGAA | 1,224 | NM_007836.1 |
| Gadd45a, R |
GGGTCTACGTTGAGCAGCTT |
|
|
| Cldn4, F |
GGCGTCTATGGGACTACAGG | 1,827 | NM_009903.2 |
| Cldn4, R |
GAGCGCACAACTCAGGATG |
|
|
| Tbxa2r, F |
GCTCATCTACCTGCGTGTGG | 1,809 | NM_001277265.1 |
| Tbxa2r, R |
CAGCCTGGAGCTGTGAACTG |
|
|
| β-actin, F |
CTGTATGCCTCTGGTCGTAC | 214 | NM_007393.3 |
| β-actin, R |
TGATGTCACGCACGATTTCC |
|
|
Cell culture and treatment
To determine the function of differentially
expressed lncRNAs, the effects of Map2k3os were measured on the
release of inflammatory cytokines from alveolar epithelial cells
induced by cyclic stretch. The MLE12 [Obio Technology (Shanghai)
Corp., Ltd., Shanghai, China] murine lung epithelial cell line was
cultured with Dulbecco's modified Eagle's medium (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) supplemented with 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C
in an atmosphere containing 5% CO2 (33). Map2k3os small interfering RNA
(siRNA) was synthesized by Shanghai GenePharma Co., Ltd. (Shanghai,
China), as was the negative control siRNA. The sequence of mouse
Map2k3os siRNA was: 5′-CCCAUGGGAAGCAAAGCAATT-3′. The sequence of
negative control siRNA was: 5′-UUCUCCGAACGUGUCACGUTT-3′. MLE-12
cells were seeded onto Collagen I-coated Bioflex 6-well culture
plates (BFI products, Inc., Hollywood, FL, USA). When the cells
reached 80% coverage of one well, they were transfected with siRNA
(60 pmol/ml) using Xfect siRNA transfection reagents (Takara Bio,
Inc., Otsu, Japan). Following transfection for 24 h, the medium was
renewed and the cells were subjected to cyclic stretch (20% linear
elongation, sinusoidal wave, 30 cycles/min) for 4 h, as previously
described (34), using the
Flexcell-FX-5,000 Tension system (Flexcell International
Corporation, Burlington, NC, USA). Control cells were placed in the
same culture plates and incubated next to the stretched cells at
37°C in an atmosphere containing 5% CO2. Following
cyclic stretch, the cells were harvested to analyze the expression
of interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α by
RT-qPCR conducted as previously described in the RT-qPCR
subsection. The primer sequences (Sangon Biotech Co., Ltd.) were as
follows: IL-1β forward, 5′-TCGCAGCAGCACATCAACAAGAG-3′ and reverse,
5′-TGCTCATGTCCTCATCCTGGAAGG-3′; IL-6 forward,
5′-ACTTCCATCCAGTTGCCTTCTTGG-3′ and reverse,
5′-TTAAGCCTCCGACTTGTGAAGTGG-3′; and TNF-α forward,
5′-GCGACGTGGAACTGGCAGAAG-3′ and reverse,
5′-GCCACAAGCAGGAATGAGAAGAGG-3′.
ELISA
Following cyclic stretch, the conditioned medium was
collected. IL-1β (cat. no. F10770), IL-6 (cat. no. F10830) and
TNF-α (cat. no. F11630) concentrations were determined using ELISA
kits (Shanghai Westang Biotechnology Co., Ltd., Shanghai, China)
for mice, according to the manufacturer's protocols.
Statistical analysis
All data were analyzed with SPSS version 20.0 (IBM
Corp., Armonk, NY, USA). Student's t-test was applied to identify
the differently expressed lncRNAs and mRNAs following RNA-Seq and
RT-qPCR. According to previous studies (35,36),
Fisher's exact test and χ2 test were used to examine the
significance of GO category and pathway analysis. FDR was used to
correct the multiple testing to minimize the error of P-value
(37,38), and in RNA-Seq to identify the
differentially expressed genes (FDR<0.05). One-way analysis of
variance followed by Dunnett's test for multiple comparisons was
use to compare the difference between multiple groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
Differentially expressed lncRNAs and
mRNAs
LncRNA and mRNA expression between the VILI and sham
groups was analyzed using the DE-Seq algorithm, and differentially
expressed genes were identified. A total of 104 lncRNAs and 809
mRNAs were identified as differentially expressed between the
groups by high-throughput sequencing. Among the 104 lncRNAs, 74
were upregulated and 30 were downregulated. Of the 809 mRNAs, 521
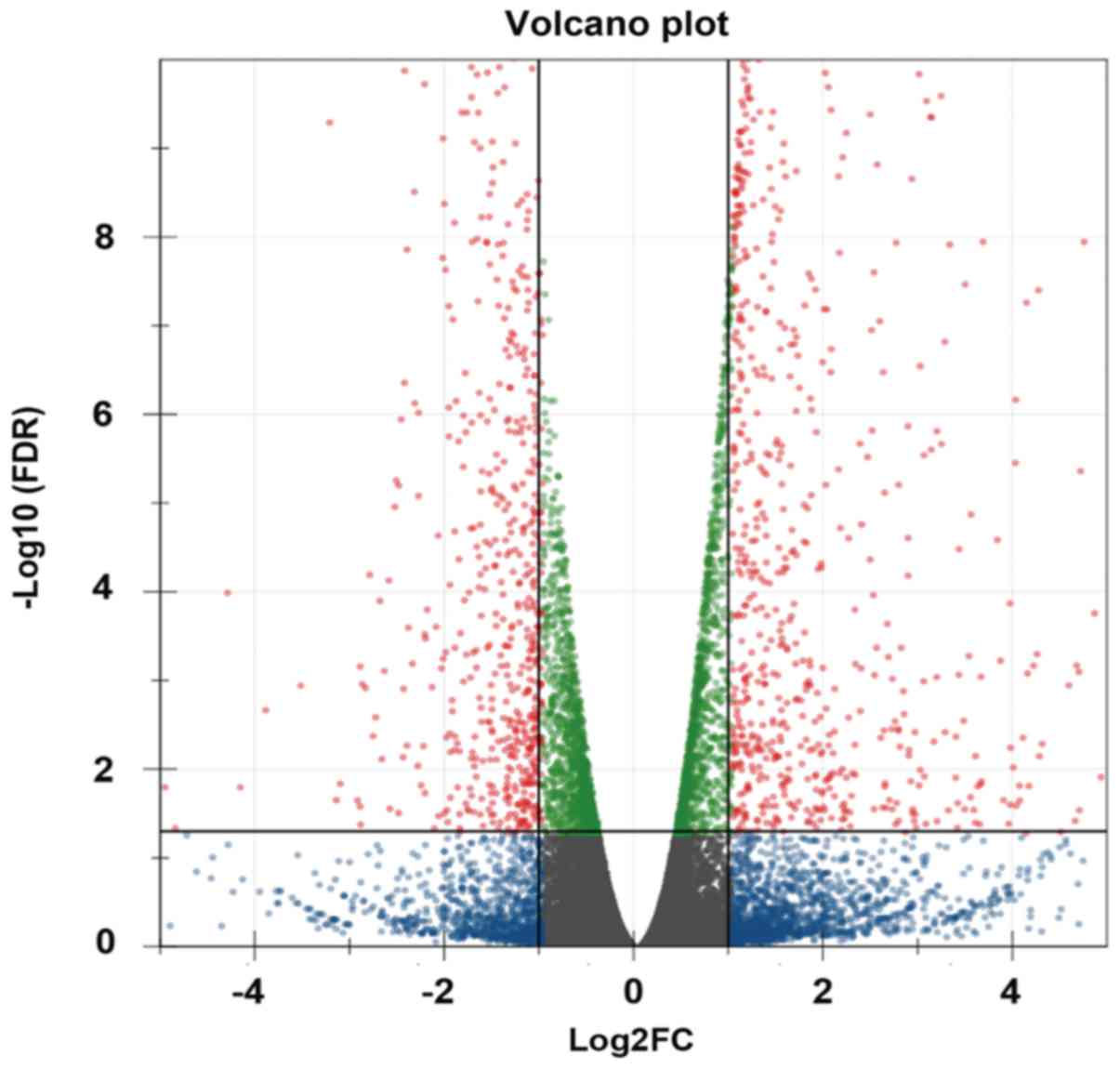
were upregulated and 288 were downregulated. Volcano plot was used
to identify differentially expressed genes (lncRNAs and mRNAs)
between the two groups; when the expression was similar, the genes
were marked blue, whereas the differentially expressed genes
(FC>2 and FDR<0.05) were marked red (Fig. 1). The top 20 differentially
expressed genes are presented in Table II (10 upregulated and 10
downregulated lncRNAs). To validate the results from RNA-Seq, the
expression levels of three lncRNAs (Map2k3os, Dnm3os and Abhd11os)
and three mRNAs (Gadd45a, Cldn4 and Tbxa2r) were detected by
RT-qPCR. The results demonstrated that the change in the expression
of these lncRNAs and mRNAs determined by RT-qPCR was similar to
that from RNA-Seq (Fig. 2A and
B).
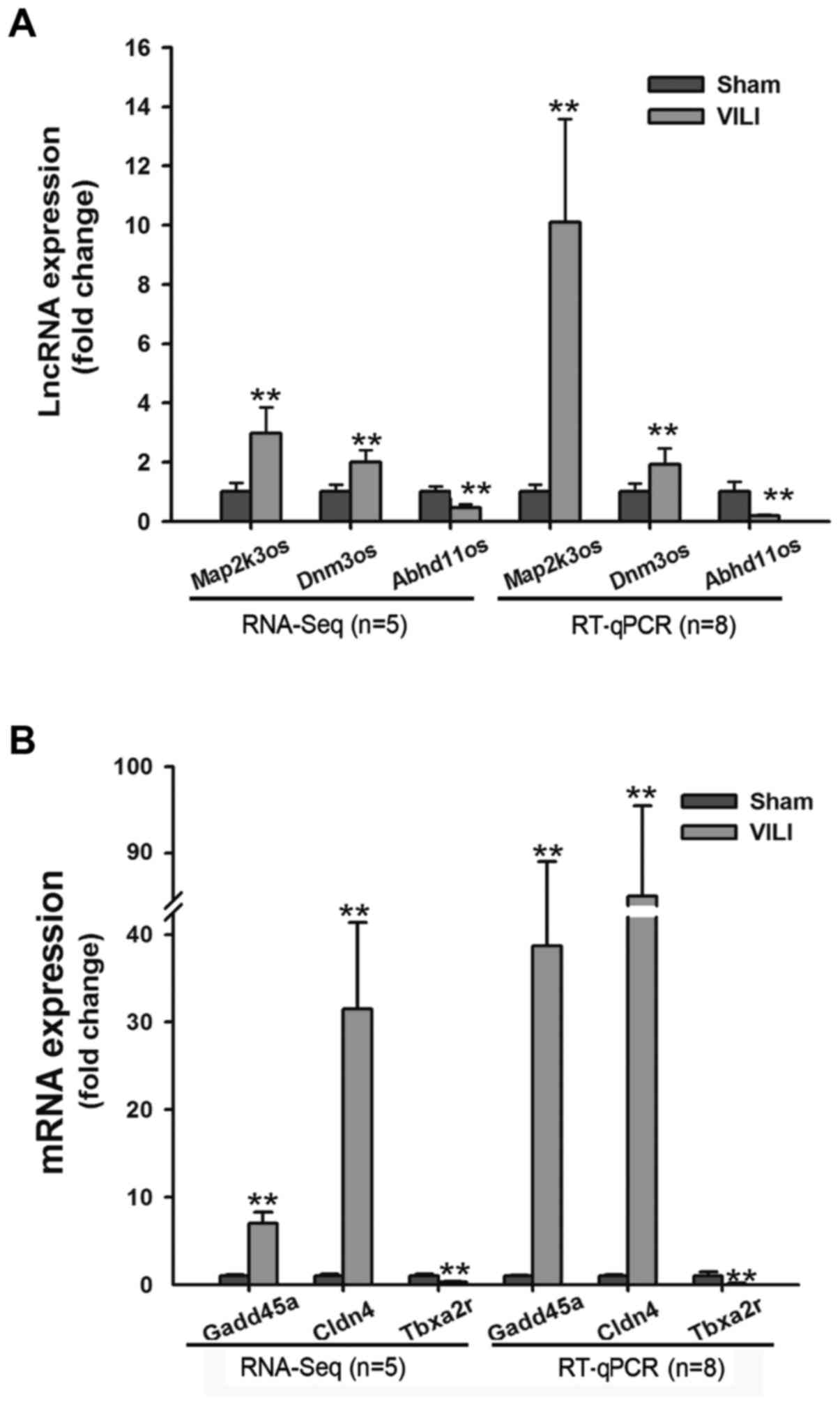 | Figure 2.(A) Three lncRNAs (Map2k3os, Dnm3os
and Abhd11os) identified by RNA-Seq were verified by RT-qPCR.
**P<0.01 vs. the sham group. (B) Three mRNAs (Gadd45a, Cldn4 and
Tbxa2r) identified by RNA-Seq were verified by RT-qPCR. **P<0.01
vs. the sham group. Abhd11os, abhydrolase domain containing 11,
opposite strand; Cldn4, claudin 4; Dnm3os, dynamin 3, opposite
strand; Gadd45a, growth arrest and DNA damage-inducible α; lncRNA,
long non-coding RNA; Map2k3os, mitogen-activated protein kinase
kinase 3, opposite strand; RNA-Seq, RNA-sequencing; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction;
Tbxa2r, thromboxane A2 receptor. |
 | Table II.Top 20 differentially expressed
lncRNAs (10 upregulated and 10 downregulated lncRNAs) between
ventilator-induced lung injury group and sham group. |
Table II.
Top 20 differentially expressed
lncRNAs (10 upregulated and 10 downregulated lncRNAs) between
ventilator-induced lung injury group and sham group.
| AccID | FDR | Regulation | Location | Description |
|---|
| Gm4610 |
9.71×10−27 | Up | chr3 | Predicted gene
4610 |
| LOC102636324 |
1.55×10−16 | Up | chr13 | Uncharacterized
LOC102636324 |
| Gm11827 |
2.74×10−15 | Up | chr4 | Predicted gene
11827 |
| LOC101056163 |
1.01×10−12 | Up | chr8 | 14-3-3 protein
sigma-like |
| Gm5582 |
3.60×10−12 | Up | chr6 | Predicted gene
5582 |
| Serpina3h |
5.07×10−12 | Up | chr12 | Serpina3h
protein |
| Krt8-ps |
9.79×10−10 | Up | chr7 | Keratin 8,
pseudogene |
| Gm9523 |
4.19×10−9 | Up | chr5 | Methionine
adenosyltransferase II, alpha pseudogene |
| LOC102633421 |
9.00×10−9 | Up | chr11 | 14-3-3 protein
sigma-like |
| Mir22hg |
3.83×10−8 | Up | chr11 | Mir22 host gene
(non-protein coding) |
| Gm16119 |
3.98×10−8 | Down | chr2 | Uridine-cytidine
kinase 1-like 1, opposite strand |
| LOC102632993 |
6.82×10−8 | Down | chr7 | Uncharacterized
LOC102632993 |
| LOC102635638 |
4.02×10−7 | Down | chr9 | Uncharacterized
LOC102635638 |
| 9630028I04Rik |
8.16×10−7 | Down | chr17 | RIKEN cDNA
9630028I04 gene |
| A330009N23Rik |
4.05×10−6 | Down | chr15 | RIKEN cDNA
A330009N23 gene |
| AI197445 |
3.76×10−5 | Down | chr13 | Expressed sequence
AI197445 |
| D930048N14Rik |
1.05×10−4 | Down | chr11 | Protein
D930048N14Rik |
| Panct2 |
1.57×10−4 | Down | chr1 |
Pluripotency-associated noncoding
transcript 2 |
| 5930430L01Rik |
3.56×10−4 | Down | chr5 | Katanin p60
ATPase-containing subunit A-like 1 |
| 2610507I01Rik |
5.23×10−4 | Down | chr11 | RIKEN cDNA
2610507I01 gene |
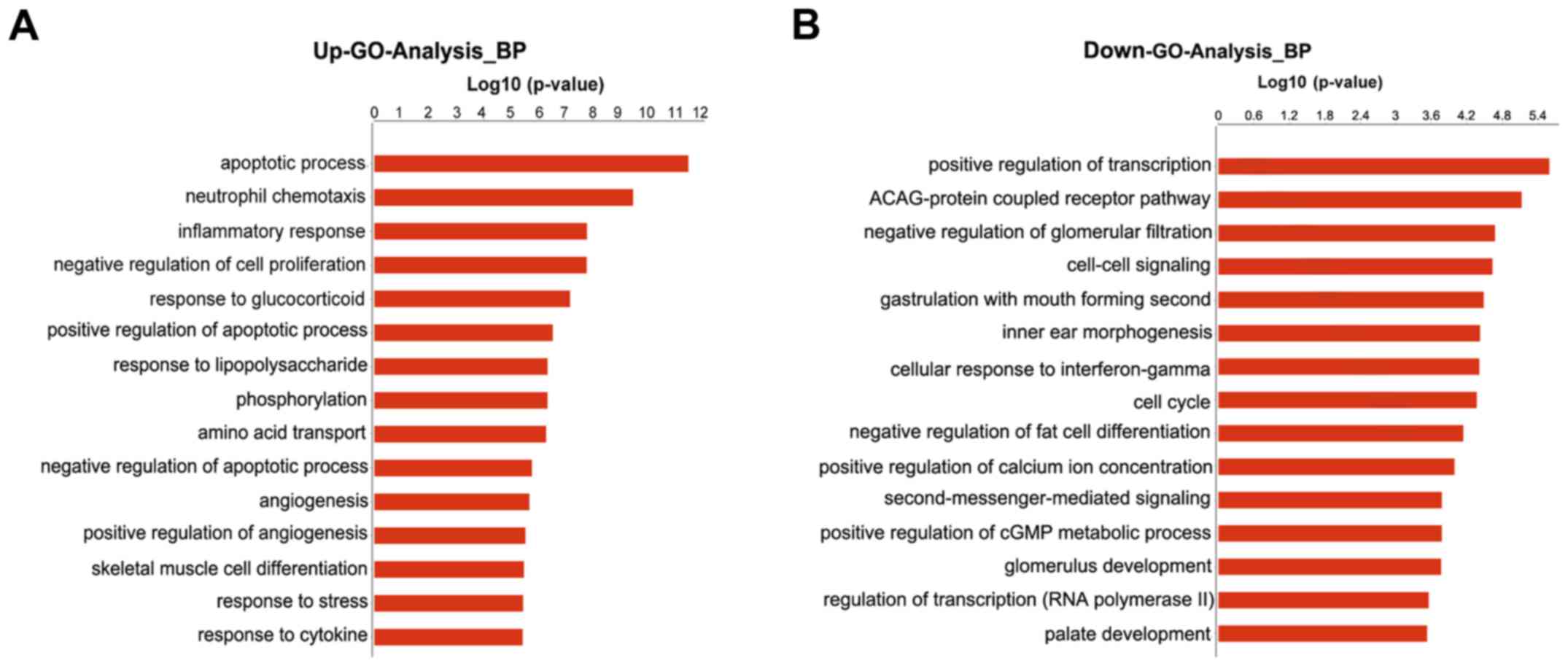
GO analysis
GO analysis indicated that the differentially
expressed mRNAs were associated with numerous important biological
processes, cellular components and molecular functions. The present
study indicated that 189 GO terms (147 upregulated and 42
downregulated) associated with biological processes were enriched
(P<0.01, FDR<0.05). The primary upregulated GO categories
were mainly involved in cell apoptosis, neutrophil chemotaxis,
inflammatory response, cell proliferation and response to
glucocorticoid (Fig. 3A), whereas
the downregulated categories were mainly associated with the
positive regulation of transcription (DNA-templated), cell-cell
signaling, cellular response to interferon-γ, cell cycle and
positive regulation of cytosolic calcium concentration (Fig. 3B).
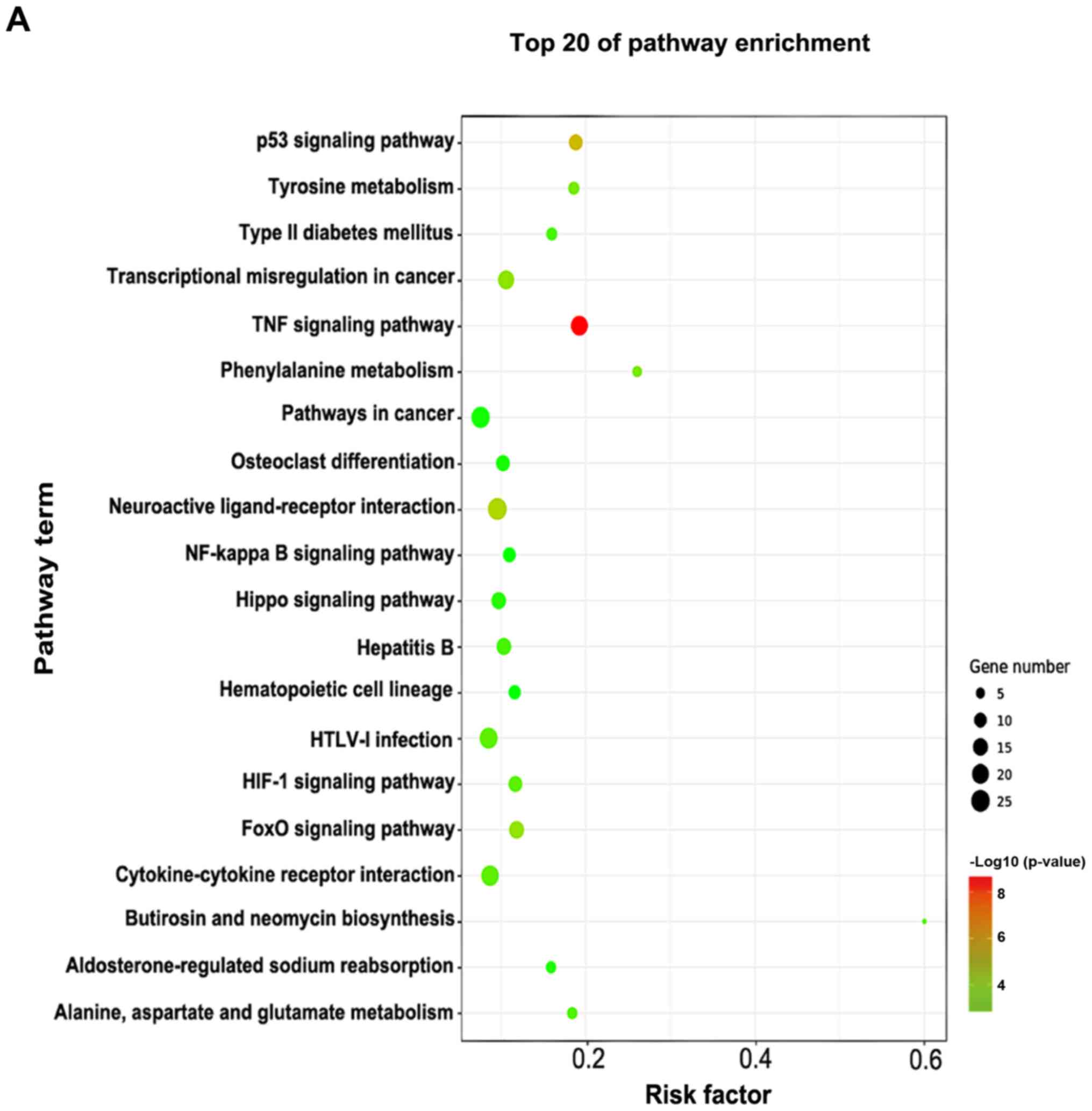
Pathway enrichment analysis
The pathway enrichment analysis was employed to
investigate the pathways associated with important differentially
expressed genes. -Lg P-value was used to describe the significance
level of pathway enrichment. There were 60 upregulated pathways and
21 downregulated pathways. The top 20 differentially expressed
pathways with upregulated expression were mainly associated with
the TNF, hypoxia-inducible factor (HIF)-1, p53 and Forkhead box O
signaling pathways, and those with downregulated expression were
associated with neuroactive ligand-receptor interaction, Hippo
signaling pathway, phenylalanine metabolism and calcium signaling
pathway (Fig. 4A). The
differentially expressed genes in these pathways provide evidence
for further investigations of the potential mechanisms underlying
the pathogenesis of VILI. The interactions between these pathways
are illustrated in Fig. 4B.
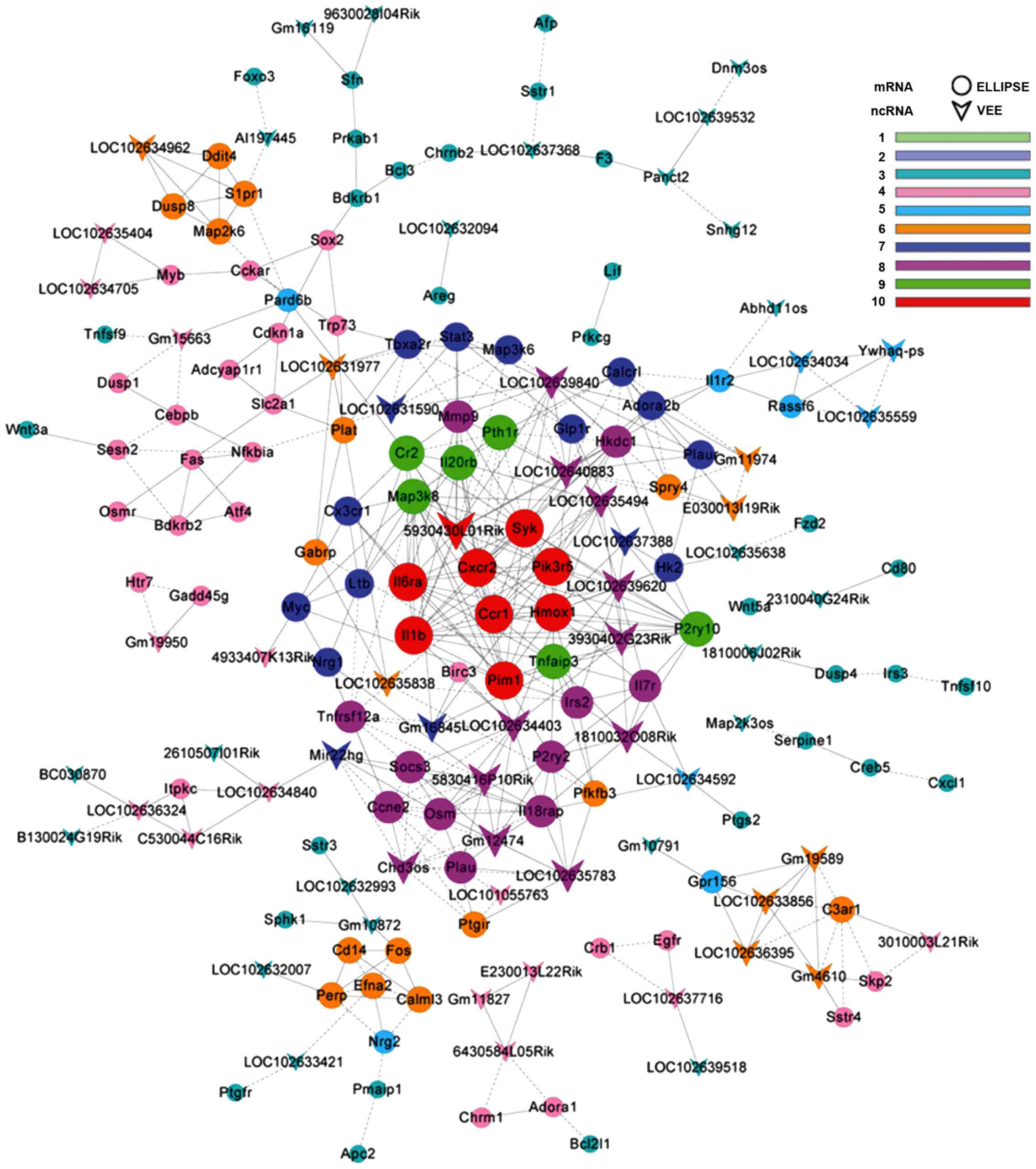
LncRNA-mRNA co-expression network
LncRNA-mRNA co-expression network was constructed
based on the differentially expressed genes detected between the
VILI and sham group lung tissues. There were 182 network nodes and
509 connections between genes in the VILI group. In this
co-expression network, 272 pairs had positive association and 237
pairs exhibited negative association (Fig. 5). The properties of the constructed
gene networks were determined by k-cores and the degree of
differences. In the co-expression network, lncRNAs with
differential degree ≥10 and k-core ≥8 included RIKEN cDNA
4933407K13 gene (4933407K13Rik), RIKEN cDNA E230013L22 gene
(E230013L22Rik), RIKEN cDNA 3010003L21 gene (3010003L21Rik), RIKEN
cDNA A330009N23 gene (A330009N23Rik), protein D930048N14Rik
(D930048N14Rik), predicted gene 11827 (Gm11827) and predicted gene
10872 (Gm10872). The top 20 differentially expressed lncRNAs, as
determined by the gene co-expression analysis, are presented in
Table III. These differentially
expressed lncRNAs may be responsible for the potential mechanism
underlying VILI.
 | Table III.Top 20 differentially expressed long
non-coding RNAs in the gene co-expression network. |
Table III.
Top 20 differentially expressed long
non-coding RNAs in the gene co-expression network.
| AccID | DifKcore | DifDegree | Description |
|---|
| D930048N14Rik | 10 | 19 | Protein
D930048N14Rik |
| Gm10872 | 9 | 14 | predicted gene
10872 |
| E230013L22Rik | 8 | 19 | RIKEN cDNA
E230013L22 gene |
| Gm11827 | 8 | 13 | predicted gene
11827 |
| 4933407K13Rik | 8 | 19 | RIKEN cDNA
4933407K13 gene |
| 3010003L21Rik | 8 | 17 | RIKEN cDNA
3010003L21 gene |
| A330009N23Rik | 8 | 17 | RIKEN cDNA
A330009N23 gene |
| B130024G19Rik | 7 | 14 | Putative
uncharacterized protein |
| Panct2 | 7 | 10 |
Pluripotency-associated noncoding
transcript 2 |
| Chd3os | 4 | 4 | Putative
uncharacterized protein RNF21 |
| AI197445 | 4 | 4 | Expressed sequence
AI197445 |
| Ywhaq-ps | 3 | 6 | Tyrosine
3-monooxygenase/tryptophan 5-monooxygenase activation protein,
theta polypeptide, pseudogene 1 |
| 9630028I04Rik | 3 | 6 | RIKEN cDNA
9630028I04 gene |
| LOC102635838 | 2 | 6 | Uncharacterized
LOC102635838 |
| Snhg12 | 2 | 3 | Small nucleolar RNA
host gene 12 |
| Map2k3os | 2 | 6 | Mitogen-activated
protein kinase kinase 3, opposite strand |
| 4930481A15Rik | 2 | 2 | MCG1045479, isoform
CRA_b |
| 1810006J02Rik | 1 | 3 | RIKEN cDNA
1810006J02 gene |
| LOC102632993 | 1 | 1 | Uncharacterized
LOC102632993 |
| LOC102637368 | 1 | 1 | Uncharacterized
LOC102637368 |
Effects of Map2k3os siRNA on
inflammatory cytokine release from MLE12 cells
Compared with in the control cells, it was
identified that Map2k3os siRNA decreased the expression of Map2k3os
by 89%, as determined by RT-qPCR (Table IV). Cyclic stretch significantly
increased the mRNA expression levels of TNF-α, IL-1β and IL-6 in
MLE12 cells, and protein levels in the supernatants. Conversely,
Map2k3os siRNA transfection inhibited the expression of these
stretch-induced cytokines (Fig.
6). These results indicated the effects of Map2k3os on
epithelial secretion of cytokines in cyclic stretch-induced
VILI.
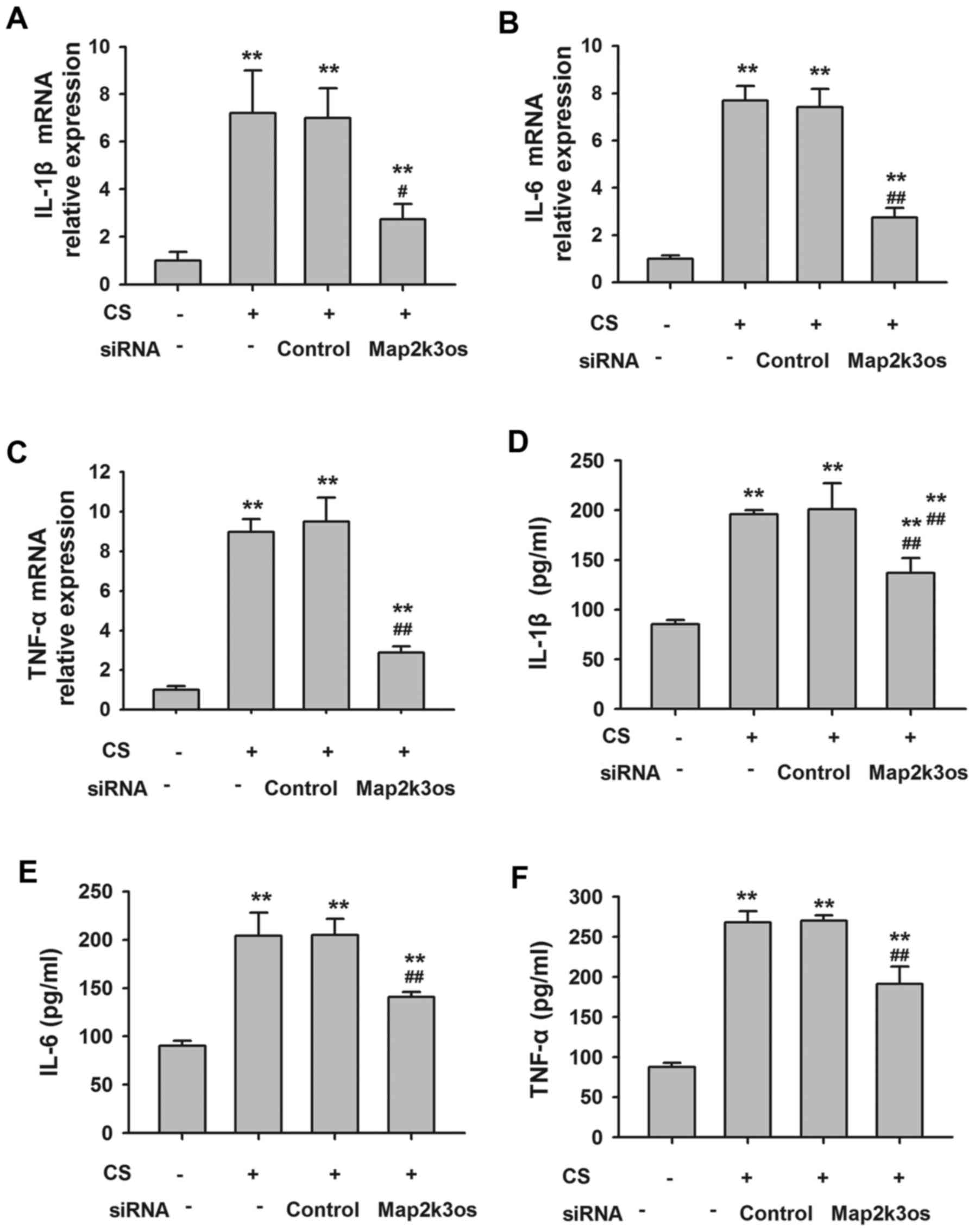 | Figure 6.mRNA expression levels of (A) IL-1β,
(B) IL-6 and (C) TNF-α in MLE12 cells, as determined by RT-qPCR.
Protein level of (D) IL-1β, (E) IL-6 and (F) TNF-α in the culture
medium, as determined by ELISA. (n=6). **P<0.01 compared with
control group without cyclic stretch or siRNA.
##P<0.01 compared with control siRNA group. CS,
cyclic stretch; IL, interleukin; Map2k3os, mitogen-activated
protein kinase kinase 3, opposite strand; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; siRNA, small
interfering RNA; TNF, tumor necrosis factor. |
 | Table IV.Effects of siRNA on long non-coding
RNA Map2k3os expression in MLE12 cells (n=4). |
Table IV.
Effects of siRNA on long non-coding
RNA Map2k3os expression in MLE12 cells (n=4).
| Group | Relative
expression | Relative reduction
(%) |
|---|
| Control siRNA | 1±0.184 | 0 |
| Map2k3os siRNA |
0.106±0.042a | 89 |
Discussion
In the present study, RNA-Seq analysis identified
104 lncRNAs and 809 mRNAs, which were differentially expressed in
lung tissues between the VILI group and the sham group.
Bioinformatics analysis predicted several lncRNAs that were likely
to alter the transcription of related mRNAs, and thus participate
in the development of VILI. To the best of the authors' knowledge,
the present study is the first to analyze the function of lncRNAs
in VILI using high-throughput sequencing and bioinformatics
analysis.
At present, there have been few studies regarding
the transcriptomics of VILI (39–41).
These previous studies used microarray analysis, and to the best of
the authors' knowledge, a previous study tested the lncRNAs. Ma
et al (39) focused on
early stress response genes in rodents (mouse and rat) following
VILI, and identified 41 upregulated genes and 7 downregulated genes
in the injured lungs. GO analysis revealed that these genes may be
involved in cell cycle arrest, immune response, inflammatory
response, blood coagulation, chemotaxis, apoptosis, cell-cell
signaling and negative regulation of cell proliferation (39). Another study (40) only reported inflammasome-related
gene expression, including IL-1α, caspase-activator domain-10, and
IL-1 receptor-1 and −2. Furthermore, a previous study (41) used microarray analysis to identify
the effects of non-muscle myosin light chain kinase isoform on
VILI. This study also identified alterations in the expression of
several genes in VILI, including thioredoxin domain-containing 9,
C-X-C motif chemokine ligand 2 and MYB proto-oncogene,
transcription factor. As in previous studies, all of the biological
processes reported and the majority of genes identified were also
observed in the present study. The difference in the differentially
expressed genes among available studies is possibly ascribed to the
difference in species (mouse and rat vs. mouse) and methodology
(microarray vs. high-throughput sequencing). In the present study,
not only mRNAs, but also lncRNAs, were analyzed in the lung
tissues, and GO analysis, pathway enrichment analysis, and
lncRNA-mRNA co-expression networks were employed to predict the
function of lncRNAs. Therefore, the findings may provide evidence
regarding the mechanism underlying VILI and aid the development of
novel targets to treat VILI.
The onset of VILI is associated with several
biological processes and signaling pathways. MV can damage the
lungs by direct mechanical injury and indirectly via the induction
of biotrauma. Several signaling pathways and mRNAs identified in
the present study have been identified in previous studies.
Hyperinflation of mouse lungs in vivo and in vitro
can induce the expression of nuclear factor (NF)-κB and IL-6 in
alveolar macrophages and alveolar epithelial type II cells
(42). In addition, chemokines
[chemokine (C-X-C motif) ligand 1, cytokine-induced neutrophil
chemoattractant-2α and macrophage inflammatory protein 2 (MIP-2)]
and their receptors are activated during lung injury (43). The neutrophil elastase inhibitor
sivelestat is able to inhibit neutrophil accumulation, and decrease
the release of MIP-2, IL-6 and TNF-α in VILI mice (44). The present study also confirmed the
important role of inflammatory response, neutrophil chemotaxis and
the NF-κB signaling pathway. In addition, apoptosis was identified
as the first key biological process in VILI by high-throughput
sequencing. Previous studies also identified that pathological
cyclic stretch could induce pulmonary epithelial apoptosis and
barrier dysfunction in alveolar epithelial cells (45,46).
Conversely, a previous study reported that ventilation at low tidal
volumes results in a mild inflammatory response without increasing
apoptosis, which is accompanied by accelerated alveolar epithelial
cell proliferation (47). This
differs from the results of the present study, which may be
ascribed to the low tidal volume used in the previous study.
KEGG pathway analysis in the present study also
demonstrated that several signaling pathways were associated with
the pathogenesis of VILI. TNF is able to regulate pulmonary
alveolar permeability, alveolar fluid clearance, adhesion molecule
expression and leucocyte recruitment (48). TNF has been identified as a marker
of early activation of inflammation and can be rapidly released in
response to cyclic stretch (49,50).
In addition, HIF-1 can promote transcription of A2B adenosine
receptor (A2BAR), whereas deletion of the A2BAR gene could reduce
survival time and increase pulmonary albumin leakage following VILI
(51,52). Furthermore, although the specific
role of the P53 signaling pathway in VILI has not been reported,
microarray assay has revealed it is involved in the pathogenesis of
VILI (41). In the present study,
a number of genes were differentially expressed between the sham
group and the VILI group. Some genes have been verified by previous
studies (49,53), whereas others may be novel targets
with which to explore the mechanisms of VILI.
In the lncRNA-mRNA co-expression network,
4933407K13Rik, E230013L22Rik, 3010003L21Rik, A330009N23Rik,
D930048N14Rik, Gm11827 and Gm10872 were most likely to be
responsible for the potential mechanism underlying VILI. These
lncRNAs may directly and indirectly regulate the transcription of
related mRNAs, thus participating in the development of VILI. The
top 20 differentially expressed lncRNAs are main target genes for
future study. The present study confirmed that transfection with
siRNA targeting Map2k3os (one of the top 20 lncRNAs) inhibited
stretch-induced cytokine (TNF-α, IL-1β and IL-6) expression in
MLE12 cells. However, further experiments are required to study the
detailed function and mechanism of these lncRNAs in VILI. In
addition, next-generation sequencing technology itself has certain
limitations, including flux, which means the number of RNA samples
that can be measured by a sequenator at the same time, is not high
enough and the length of RNA reads remains relatively short
(54), which may be improved with
the development of improved technology.
In conclusion, the present study provided novel
information regarding the lncRNAs, signaling pathways and the
co-expression network involved in VILI. Numerous significant
lncRNAs are likely to participate in the pathogenesis of VILI,
which may be useful to guide further research into the mechanisms
and targeted therapy for this disease.
Acknowledgements
The authors wish to thank Dr Yan Wang and Dr Chufan
Xu (Department of Anesthesiology and SICU, Xinhua Hospital,
Shanghai Jiaotong University, School of Medicine) for their
technical assistance.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81100826,
81372100 and 81772108).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors participated in study design of the
research question. BX contributed to model building, performing the
ELISA and writing the manuscript. YW helped to perform RT-qPCR and
analyze data. XL performed cell culture and statistics. YM and XD
mainly designed the research and revised the manuscript.
Ethics approval and consent to
participate
All experimental protocols and animal handling
procedures were approved by the Ethics Committee on Experimental
Animals of Shanghai Jiaotong University School of Medicine
(Shanghai, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brower RG and Fessler HE: Mechanical
ventilation in acute lung injury and acute respiratory distress
syndrome. Clin Chest Med. 21(491–510): viii2000.
|
|
2
|
Belperio JA, Keane MP, Lynch JP III and
Strieter RM: The role of cytokines during the pathogenesis of
ventilator-associated and ventilator-induced lung injury. Semin
Respir Crit Care Med. 27:350–364. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Neto AS, Simonis FD, Barbas CS, Biehl M,
Determann RM, Elmer J, Friedman G, Gajic O, Goldstein JN, Linko R,
et al: Lung-protective ventilation with low tidal volumes and the
occurrence of pulmonary complications in patients without acute
respiratory distress syndrome: A systematic review and individual
patient data analysis. Crit Care Med. 43:2155–2163. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Neto Serpa A, Hemmes SN, Barbas CS,
Beiderlinden M, Biehl M, Binnekade JM, Canet J,
Fernandez-Bustamante A, Futier E, Gajic O, et al: Protective versus
conventional ventilation for surgery: A systematic review and
individual patient data meta-analysis. Anesthesiology. 123:66–78.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Choi WI, Quinn DA, Park KM, Moufarrej RK,
Jafari B, Syrkina O, Bonventre JV and Hales CA: Systemic
microvascular leak in an in vivo rat model of ventilator-induced
lung injury. Am J Respir Crit Care Med. 167:1627–1632. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Slutsky AS and Ranieri VM:
Ventilator-induced lung injury. N Engl J Med. 369:2126–2136. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Q, Ge YL, Li M, Fang XZ, Yuan YP, Liang
L and Huang SQ: miR-127 contributes to ventilator-induced lung
injury. Mol Med Rep. 16:4119–4126. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brower RG, Lanken PN, MacIntyre N, Matthay
MA, Morris A, Ancukiewicz M, Schoenfeld D and Thompson BT: National
Heart, Lung, and Blood Institute ARDS Clinical Trials Network:
Higher versus lower positive end-expiratory pressures in patients
with the acute respiratory distress syndrome. N Engl J Med.
351:327–336. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Su JC and Hu XF: Long non-coding RNA
HOXA11-AS promotes cell proliferation and metastasis in human
breast cancer. Mol Med Rep. 16:4887–4894. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang N, Chen J, Zhang H, Wang X, Yao H,
Peng Y and Zhang W: LncRNA OIP5-AS1 loss-induced microRNA-410
accumulation regulates cell proliferation and apoptosis by
targeting KLF10 via activating PTEN/PI3K/AKT pathway in multiple
myeloma. Cell Death Dis. 8:e29752017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Delás MJ and Hannon GJ: lncRNAs in
development and disease: From functions to mechanisms. Open Biol.
7:pii: 170121. 2017. View Article : Google Scholar
|
|
13
|
Ren C, Deng M, Fan Y, Yang H, Zhang G,
Feng X, Li F, Wang D, Wang F and Zhang Y: Genome-wide analysis
reveals extensive changes in LncRNAs during skeletal muscle
development in Hu sheep. Genes (Basel). 8:pii: E191. 2017.
View Article : Google Scholar
|
|
14
|
Zhang HJ, Wei QF, Wang SJ, Zhang HJ, Zhang
XY, Geng Q, Cui YH and Wang XH: LncRNA HOTAIR alleviates rheumatoid
arthritis by targeting miR-138 and inactivating NF-κB pathway. Int
Immunopharmacol. 50:283–290. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yi H, Peng R, Zhang LY, Sun Y, Peng HM,
Liu HD, Yu LJ, Li AL, Zhang YJ, Jiang WH and Zhang Z:
LincRNA-Gm4419 knockdown ameliorates NF-κB/NLRP3
inflammasome-mediated inflammation in diabetic nephropathy. Cell
Death Dis. 8:e25832017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang R, Tang J, Chen Y, Deng L, Ji J, Xie
Y, Wang K, Jia W, Chu WM and Sun B: The long noncoding RNA lnc-EGFR
stimulates T-regulatory cells differentiation thus promoting
hepatocellular carcinoma immune evasion. Nat Commun. 8:151292017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mumtaz PT, Bhat SA, Ahmad SM, Dar MA,
Ahmed R, Urwat U, Ayaz A, Shrivastava D, Shah RA and Ganai NA:
LncRNAs and immunity: Watchdogs for host pathogen interactions.
Biol Proced Online. 19:32017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Misawa A, Takayama KI and Inoue S: Long
non-coding RNAs and prostate cancer. Cancer Sci. 108:2107–2114.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haemmig S, Simion V, Yang D, Deng Y and
Feinberg MW: Long noncoding RNAs in cardiovascular disease,
diagnosis, and therapy. Curr Opin Cardiol. 32:776–783. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhuang YT, Xu DY, Wang GY, Sun JL, Huang Y
and Wang SZ: IL-6 induced lncRNA MALAT1 enhances TNF-α expression
in LPS-induced septic cardiomyocytes via activation of SAA3. Eur
Rev Med Pharmacol Sci. 21:302–309. 2017.PubMed/NCBI
|
|
21
|
Wahlestedt C: Targeting long non-coding
RNA to therapeutically upregulate gene expression. Nat Rev Drug
Discov. 12:433–446. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li H, Su X, Yan X, Wasserloos K, Chao W,
Kaynar AM, Liu ZQ, Leikauf GD, Pitt BR and Zhang LM: Toll-like
receptor 4-myeloid differentiation factor 88 signaling contributes
to ventilator-induced lung injury in mice. Anesthesiology.
113:619–629. 2010.PubMed/NCBI
|
|
23
|
Li LF, Yang CT, Huang CC, Liu YY, Kao KC
and Lin HC: Low-molecular-weight heparin reduces
hyperoxia-augmented ventilator-induced lung injury via
serine/threonine kinase-protein kinase B. Respir Res. 12:902011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang K, Singh D, Zeng Z, Coleman SJ, Huang
Y, Savich GL, He X, Mieczkowski P, Grimm SA, Perou CM, et al:
MapSplice: Accurate mapping of RNA-seq reads for splice junction
discovery. Nucleic Acids Res. 38:e1782010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mortazavi A, Williams BA, McCue K,
Schaeffer L and Wold B: Mapping and quantifying mammalian
transcriptomes by RNA-Seq. Nat Methods. 5:621–628. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Anders S and Huber W: Differential
expression analysis for sequence count data. Genome Biol.
11:R1062010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The Gene
Ontology Consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kanehisa M, Goto S, Kawashima S, Okuno Y
and Hattori M: The KEGG resource for deciphering the genome.
Nucleic Acids Res. 32:(Database Issue). D277–D280. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang GT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pujana MA, Han JD, Starita LM, Stevens KN,
Tewari M, Ahn JS, Rennert G, Moreno V, Kirchhoff T, Gold B, et al:
Network modeling links breast cancer susceptibility and centrosome
dysfunction. Nat Genet. 39:1338–1349. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ravasz E, Somera AL, Mongru DA, Oltvai ZN
and Barabási AL: Hierarchical organization of modularity in
metabolic networks. Science. 297:1551–1555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun D, Wang J, Yang N and Ma H: Matrine
suppresses airway inflammation by downregulating SOCS3 expression
via inhibition of NF-κB signaling in airway epithelial cells and
asthmatic mice. Biochem Biophys Res Commun. 477:83–90. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Y, Xu CF, Liu YJ, Mao YF, Lv Z, Li
SY, Zhu XY and Jiang L: Salidroside attenuates ventilation induced
lung injury via SIRT1-dependent inhibition of NLRP3 inflammasome.
Cell Physiol Biochem. 42:34–43. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang W, Meng M, Zhang Y, Wei C, Xie Y,
Jiang L, Wang C, Yang F, Tang W, Jin X, et al: Global
transcriptome-wide analysis of CIK cells identify distinct roles of
IL-2 and IL-15 in acquisition of cytotoxic capacity against tumor.
BMC Med Genomics. 7:492014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin ZW, Gu J, Liu RH, Liu XM, Xu FK, Zhao
GY, Lu CL and Ge D: Genome-wide screening and co-expression network
analysis identify recurrence-specific biomarkers of esophageal
squamous cell carcinoma. Tumour Biol. 35:10959–10968. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Benjamini Y and Hochberg Y: Controlling
the false discovery rate: A practical and powerful approach to
multiple testing. J R Stat Soc Series B. 57:pp289–300. 1995.
|
|
38
|
Pawitan Y, Michiels S, Koscielny S,
Gusnanto A and Ploner A: False discovery rate, sensitivity and
sample size for microarray studies. Bioinformatics. 21:3017–3024.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ma SF, Grigoryev DN, Taylor AD, Nonas S,
Sammani S, Ye SQ and Garcia JG: Bioinformatic identification of
novel early stress response genes in rodent models of lung injury.
Am J Physiol Lung Cell Mol Physiol. 289:L468–L477. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dolinay T, Kim YS, Howrylak J, Hunninghake
GM, An CH, Fredenburgh L, Massaro AF, Rogers A, Gazourian L,
Nakahira K, et al: Inflammasome-regulated cytokines are critical
mediators of acute lung injury. Am J Respir Crit Care Med.
185:1225–1234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mirzapoiazova T, Moitra J, Moreno-Vinasco
L, Sammani S, Turner JR, Chiang ET, Evenoski C, Wang T, Singleton
PA, Huang Y, et al: Non-muscle myosin light chain kinase isoform is
a viable molecular target in acute inflammatory lung injury. Am J
Respir Cell Mol Biol. 44:40–52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Uhlig U, Fehrenbach H, Lachmann RA,
Goldmann T, Lachmann B, Vollmer E and Uhlig S: Phosphoinositide
3-OH kinase inhibition prevents ventilation-induced lung cell
activation. Am J Respir Crit Care Med. 169:201–208. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vanderbilt JN, Mager EM, Allen L, Sawa T,
Wiener-Kronish J, Gonzalez R and Dobbs LG: CXC chemokines and their
receptors are expressed in type II cells and upregulated following
lung injury. Am J Respir Cell Mol Biol. 29:661–668. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sakashita A, Nishimura Y, Nishiuma T,
Takenaka K, Kobayashi K, Kotani Y and Yokoyama M: Neutrophil
elastase inhibitor (sivelestat) attenuates subsequent
ventilator-induced lung injury in mice. Eur J Pharmacol. 571:62–71.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hammerschmidt S, Kuhn H, Grasenack T,
Gessner C and Wirtz H: Apoptosis and necrosis induced by cyclic
mechanical stretching in alveolar type II cells. Am J Respir Cell
Mol Biol. 30:396–402. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gao J, Huang T, Zhou LJ, Ge YL, Lin SY and
Dai Y: Preconditioning effects of physiological cyclic stretch on
pathologically mechanical stretch-induced alveolar epithelial cell
apoptosis and barrier dysfunction. Biochem Biophys Res Commun.
448:342–348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chess PR, Benson RP, Maniscalco WM, Wright
TW, O'Reilly MA and Johnston CJ: Murine mechanical ventilation
stimulates alveolar epithelial cell proliferation. Exp Lung Res.
36:331–341. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mukhopadhyay S, Hoidal JR and Mukherjee
TK: Role of TNFalpha in pulmonary pathophysiology. Respir Res.
7:1252006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wilson MR and Takata M: Inflammatory
mechanisms of ventilator-induced lung injury: A time to stop and
think? Anaesthesia. 68:175–178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wilson MR, Wakabayashi K, Bertok S, Oakley
CM, Patel BV, O'Dea KP, Cordy JC, Morley PJ, Bayliffe AI and Takata
M: Inhibition of TNF receptor p55 by a domain antibody attenuates
the initial phase of acid-induced lung injury in mice. Front
Immunol. 8:1282017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Eckle T, Kewley EM, Brodsky KS, Tak E,
Bonney S, Gobel M, Anderson D, Glover LE, Riegel AK, Colgan SP and
Eltzschig HK: Identification of hypoxia-inducible factor HIF-1A as
transcriptional regulator of the A2B adenosine receptor during
acute lung injury. J Immunol. 192:1249–1256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Eckle T, Grenz A, Laucher S and Eltzschig
HK: A2B adenosine receptor signaling attenuates acute lung injury
by enhancing alveolar fluid clearance in mice. J Clin Invest.
118:3301–3315. 2008.PubMed/NCBI
|
|
53
|
Wang T, Gross C, Desai AA, Zemskov E, Wu
X, Garcia AN, Jacobson JR, Yuan JX, Garcia JG and Black SM:
Endothelial cell signaling and ventilator-induced lung injury:
Molecular mechanisms, genomic analyses, and therapeutic targets. Am
J Physiol Lung Cell Mol Physiol. 312:L452–L476. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hert DG, Fredlake CP and Barron AE:
Advantages and limitations of next-generation sequencing
technologies: A comparison of electrophoresis and
non-electrophoresis methods. Electrophoresis. 29:4618–4626. 2008.
View Article : Google Scholar : PubMed/NCBI
|