Introduction
Transmembrane cation channels include those for
movement of calcium, copper, and iron ions (1). Calcium channels have selective
permeability for calcium ions and regulate physiological responses
associated with contraction of muscle, hormone and neurotransmitter
release, activation of calcium-dependent enzymes, and
calcium-dependent gene transcription (2). Copper channels transport copper ions
into eukaryotic cells, bind copper ions, and exchange copper ions
for intercellular components. Copper ions are important in the
redox system as they act as cofactors for enzymes (3). Iron is notably involved in oxygen
transport, the redox system and numerous metabolic enzymes.
Transmembrane iron ion channels have roles in iron regulatory
pathways and contribute to iron homeostasis (4).
The protein sodium/potassium/calcium exchanger 3
(NCKX3) is a K+-dependent Na+-Ca2+
exchanger and a member of solute carrier family 24 (5). The primary role of this exchanger is
to control Ca2+ flux, through which it regulates
intracellular Ca2+ homeostasis. NCKX3 is expressed in a
number of organs and tissues, including the aorta and smooth muscle
(6). Transcription of NCKX3 occurs
principally in brain tissue, particularly in the thalamic nuclei,
hippocampal CA1 neurons, and layer IV of the cerebral cortex
(7). The calcium ion channel
protein transient receptor potential cation channel subfamily V
member 2 (TRPV2) is a member of the transient receptor potential
(TRP) channel family (8). TRP
channels serve to perceive various noxious mechanical, chemical and
thermal stimuli. In particular, TRPV2 is specialized in the
detection of thermal stimuli (9).
A number of TRP channels regulate non-selective cation flux
(including calcium and magnesium), and such flux may activate
cellular signaling pathways (10).
In addition, TRPV2 mediates calcium flow. TRPV2 is expressed in
many parts of the immune system (including liver Kupffer cells,
lung alveolar macrophages, mast cells and macrophages) and nervous
system (including dorsal root ganglia and spinal motor neurons in
developing mice) (11).
Copper transporter 1 (CTR1) is an adenosine
5′-triphosphate (ATP)-independent, high-affinity transporter, which
is primarily present in the cell membrane (12). The high affinity of CTR1 for copper
allows for an appropriate level of copper in the intracellular
space. CTR1 is expressed in the majority of organs, and
particularly in the liver, colon and intestine (13).
The copper-transporting ATPase 1 (ATP7A) copper
transporter is a member of a family of P1B-type ATPases that
transport heavy metals. ATP7A maintains copper homeostasis in
conjunction with other copper transporters, including
copper-transporting ATPase 2 (ATP7B) and CTR1 (14). ATP7A is present in a number of
organs including kidney and placenta (15). If copper levels are elevated,
ATP7A, which resides primarily in the trans-Golgi network, moves to
the cell membrane in order to export excess copper from cells,
prior to returning to its former position (16).
Iron-regulated transporter 1 (IREG1) is primarily
detected in enterocytes and macrophages, and it has a role in iron
efflux during intestinal iron absorption (17,18).
In addition, it has an iron recycling function in macrophages
(19). IREG1 is a transmembrane
protein that is regarded as a putative exporter of iron through the
basolateral membrane to the plasma (20). The multicopper ferroxidase
hephaestin (HEPH) converts Fe2+ into Fe3+
(21). HEPH is commonly expressed
in supranuclear compartments and in the basolateral membranes of
cells, including intestinal enterocytes (22). HEPH has a significant role in the
transfer of dietary iron from enterocytes to blood, and is
expressed in the intestinal tract, central nervous system, lung,
pancreas and heart (23).
Cation channels are physiologically important and
transmembrane channel dysfunction has been associated with a number
of diseases. Impaired divalent ion homeostasis may induce
neurodegenerative disorders including Alzheimer's and Parkinson's
diseases, kidney stones, cardiac arrhythmia, anemia and hepatic
disorders (24,25). Thus, a number of investigations
have been conducted into the expression and regulation of
transmembrane cation channel proteins in a variety of organisms
(5,10,19,5). In this regard, investigations
have been conducted to examine the expression and regulation of
divalent ion channel proteins in experimental animals and humans
(5,26,28,5). However, only a few studies have
been performed using canine models. Tanner and Beeton (33) demonstrated that human
channelopathies cannot fully be replicated in animal models,
particularly mice or rats; however, canine models are quite similar
to the human organ physiology (34,35).
In particular, comparisons between the mRNA and protein expression
of divalent ion channels in canine organs have not been made. Thus,
in the present study, the expression and localization of NCKX3,
TRPV2, CTR1, ATP7A, IREG1 and HEPH proteins were investigated in
canine organs. The organ-specific mRNA and protein expression of
these factors was measured in the dog duodenum, kidney, spleen and
liver by using reverse transcription (RT) polymerase chain reaction
(PCR), RT-quantitative PCR (RT-qPCR), and western blot analyses. In
addition, through immunohistochemical assessments, the localization
of these molecules in canine organs was confirmed.
Materials and methods
Experimental animal model
A total of three 3-year-old intact female beagle
dogs (average weight 20 kg; purchased from Koatech, Pyeongtaek,
Korea) were sacrificed. Prior to sacrifice, the dogs were fed with
tap water and a commercial diet, ad libitum (Natural Balance Pet
Foods, Inc., Burbank, CA, USA) and held in stainless steel cages.
The cages were in a controlled environment maintained on a 12-h
light/dark cycle (temperature, 23±2°C; relative humidity, 50±10%;
ventilation, 17±1 times/min). To collect samples from the duodenum,
kidney, spleen and liver, the dogs were sacrificed with an
injection of KCl and opened via a midline incision. All dissected
organ samples were washed in cold sterile saline (0.9% NaCl). All
procedures for organ collection were approved by the Ethics
Committee of Chungbuk National University (Cheongju, Republic of
Korea).
Total RNA extraction, RT-PCR and
qPCR
The whole organ samples were washed in cold sterile
saline (0.9% NaCl), placed in a volume of TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) that was proportional to the organ sample volume and
homogenized in an ULTRA-TURRAX homogenizer (IKA-Works;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). RNA extraction from
the homogenate was performed according to the manufacturer's
protocol. The total RNA concentration was determined by measuring
the absorbance at 260 nm. Subsequently, RNA (1 µg) was transcribed
using first-strand Moloney murine leukemia virus reverse
transcriptase (Intron Biotechnology, Inc., Sungnam, Korea), a
random 9-mer primer (Takara Bio, Inc., Otsu, Japan), and dNTPs
(Takara Bio, Inc.) with first strand buffer (Thermo Fisher
Scientific, Inc.) for 37°C for 1 h to synthesize cDNA. Random
primers are composed of 9-mer deoxyribonucleotide mixtures,
composed of a random sequence (up to 49 different
sequences) and a phosphorylated 5′-end.
For RT-PCR and RT-qPCR, β-actin was used as the
endogenous reference for normalization and the determination of
relative gene expression levels. The dog-specific primers were:
NCKX3 sense, 5′-GGGCTCTGCAGTGTTCAATA-3′ and antisense,
5′-GACTCCCACCAGGAAACTTG-3′; TRPV2 sense, 5′-GTGACTGGGGACTCCAT-3′
and antisense, 5′-GACCAGGAAGAGCAGTTCAAA-3′; CTR1 sense,
5′-CCAGGTTACCTCCTATTC-3′ and antisense, 5′-TCATGTGCATTCCCTCG-3′;
ATP7A sense, 5′-CCCATAGCTGGAGTTTT-3′ and antisense,
5′-TTCCGAAGGCCTTTTCTGTC-3′; IREG1 sense, 5′-GCCAGACTTAAAGTGGCTCA-3′
and antisense), 5′-TGCAACATCGGCAATAGTGA-3′; HEPH sense,
5′-ACTGAAAGGGGTCAGGGTAA-3′ and antisense,
5′-CCTTGGGAGCATAGTTCCAC-3′; and β-actin sense,
5′-AAGTCCAGCTTCTGTTTCCTC-3′ and antisense,
5′-GCAGTGATCTCCTTCTGCAT-3′.
For RT-PCR, the NCKX3, TRPV2, CTR1, ATP7A, IREG1,
HEPH, and β-actin were amplified in a PCR reaction (20 µl)
containing 1 U i-StarTaq™ DNA polymerase (Intron Biotechnology,
Inc.), 1.5 mM MgCl2, 2 mM dNTP and 20 pmol of the
appropriate primers. PCR sequence parameters were 35 cycles of
denaturation at 95°C for 30 sec, annealing at 57°C for 30 sec, and
extension at 72°C for 30 sec. The obtained PCR products (10 µl)
were separated on 2.3% agarose gel and stained with ethidium
bromide. Gel images were taken under ultraviolet illumination and
were scanned using a Gel Doc EQ system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
For RT-qPCR, a 1-µl amplicon of cDNA was assayed
using SYBR (Takara Bio, Inc.) or TaqMan (Applied Biosystem; Thermo
Fisher Scientific, Inc.) kits and following the manufacturers'
protocols. The qPCR was performed using the following sequence: 30
cycles of denaturation at 95°C for 30 sec, annealing at 58°C for 30
sec (and 60°C for HEPH), and extension at 72°C for 30 sec. Data for
each sample were analyzed by comparing cycle quantification (Cq)
values at a constant fluorescence intensity. The amount of
transcript was inversely associated with the observed Cq, and for
every two-fold dilution of the transcript the Cq was expected to
increase by one increment. Relative expression (R) was calculated
as R=2−(ΔCq sample-ΔCq control) (36).
Western blot analysis
Whole organ samples obtained by sacrificing the
mice, were washed in cold sterile saline (0.9% NaCl), placed in 500
µl or 1,000 µl Pro-prep (Intron Biotechnology, Inc.) depending on
organ sample volume, and homogenized by using an ULTRA-TURRAX
homogenizer. Protein samples were acquired from the suspension by
centrifuging the homogenate at 17,800 × g at 4°C for 10 min. Each
50-µg sample was treated, in the following order, by mixing with
SDS sample buffer, heating at 60°C for 10 min, and centrifuging at
15,300 × g at 4°C for 3 min. The 5–10% SDS acrylamide gel was
prepared and electrophoresis was accomplished. The gel was
transferred to a polyvinylidene fluoride membrane (PerkinElmer,
Inc., Waltham, MA, USA), and the membrane was blocked at room
temperature for 1 h using 5% dry fat milk dissolved in TBS with
Tween-20 (TBS-T). The membrane was incubated overnight at 4°C with
primary antibodies against the following factors: NCKX3 (cat. no.
Sc-50129; 1:1,000; goat polyclonal), TRPV2 (cat. no. Sc-22520;
1:500; goat polyclonal; both Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), CTR1 (cat. no. NB100-402; 1:2,000; rabbit
polyclonal; Novus Biologicals, LLC, Littleton, CO, USA), ATP7A
(cat. no. Sc-32900; 1:2,000; rabbit monoclonal; Santa Cruz
Biotechnology, Inc.), IREG1 (cat. no. ab85370; 1:2,000; rabbit
polyclonal; Abcam, Cambridge, UK), HEPH (cat. no. ab56729, 1:2,000;
mouse monoclonal; Abcam) and β-actin (cat no. Sc-130656; 1:1,000,
mouse monoclonal, Santa Cruz Biotechnology, Inc.) diluted in bovine
serum albumin (BSA; Sigma-Aldrich; Merck KGaA). Subsequently, the
membrane was washed four times for 7 min each with TBS-T. Secondary
antibody conjugated with horseradish peroxidase (mouse polyclonal;
cat. no. 7076S; or rabbit polyclonal; cat. no. 7074S; both Cell
Signaling Technology, Inc., Danvers, MA, USA; or goat polyclonal;
cat. no. sc-2004; Santa Cruz Biotechnology, Inc.) diluted
2,000-fold in 2.5% non-fat dry milk dissolved in TBS-T for 1 h at
room temperature. The membrane was washed as described above.
Following washing, the blots were developed by incubation with an
enhanced chemiluminescence reagent (Merck KGaA) and exposed to
Biomax™ Light Film (Kodak, Rochester, NY, USA) for 1–5 min. Signal
specificity was confirmed by blotting without a primary antibody.
Experimental bands were normalized to those of β-actin. The signal
intensity of each band was measured using ImageJ software (v 1.50b;
National Institutes of Health, Bethesda, MD, USA). To determine
mean band density, the background signal from an area near each
lane was subtracted from each band.
Immunohistochemistry
The organ-specific localization of cation channels
was investigated by performing immunohistochemical assessment. The
canine duodenum (upper part of the duodenum, 3 cm from gastric
pylorus), kidney (borderline of the cortex and medulla), spleen
(borderline of the white and red pulp) and liver (quadrate lobe of
the liver) were examined. Each part of the tissue (duodenum,
kidney, spleen and liver) was identified by macroscopic necropsy.
The excised organ samples (duodenum, kidney, spleen and liver) were
immediately washed in sterile saline. Tissues were processed with
70% ethanol for 1 h, 95% ethanol (95% ethanol/5% methanol) for 1 h,
pure ethanol for 1 h, a second time with pure ethanol for 90 min, a
third time with pure ethanol for 90 min, a fourth time with pure
ethanol for 2 h, twice with clearing agent for 1 h, twice with wax
at 58°C for 1 h, and embedded in paraffin. Embedded blocks were
sectioned to 4 µm, and the sections were mounted on glass slides
(Muto Pure Chemicals, Tokyo, Japan) precoated with aminosilane,
deparaffinized using xylene, and hydrated in a descending
concentration gradient of ethanol solutions (100, 95, 75 and 60%; 5
min for each solution). Subsequently, the slide was boiled in
antigen retrieval solution buffered with Tris for 20 min, cooled at
room temperature for 30 min, and washed with TBS-T for 5 min. To
block endogenous peroxidase, the slide was placed in 3% hydrogen
peroxide for 30 min at room temperature and washed three times for
10 min each with TBS-T. To avoid non-specific reactions, the
sections were incubated with 1X BSA in PBS for 30 min at room
temperature, followed by washing three times for 10 min each with
TBS-T. Following washing, the slides were incubated with primary
antibodies (as detailed above for western blotting) overnight at
room temperature in a moist chamber. Each primary antibody was
diluted 250-fold in BSA. Slides were rinsed with TBS-T three times
for 10 min each to remove antibody. Biotinylated secondary
antibodies [1:500; Goat (cat. no. BA-9500), Mouse (cat. no.
BA-9200) or Rabbit (cat. no. BA-1000) IgG; Vector Laboratories,
Ltd., Peterborough, UK] were added and incubated at 37°C for 30
min. Slides were washed following the incubation with the primary
antibody. Subsequently, ABC Elite (Vector Laboratories, Ltd.) was
added to the slides, which were incubated at 37°C for 1 h and
washed three times for 10 min each with TBS-T. Diaminobenzidine
(DAB; Vector Laboratories, Ltd.) was used as a chromogen. Slides
were incubated with DAB at room temperature for 20 sec.
Counterstaining was achieved using Harris hematoxylin at room
temperature for 20 sec (Sigma-Aldrich; Merck KGaA). Images of the
tissue were obtained using a light microscope (BX51 Standard
Microscope; Olympus, Tokyo, Japan; magnification, ×200 and
×400).
Data analysis
The purpose of the present study was to present the
distribution of divalent ion channel mRNA/protein expression and
localization in canine organs, including the duodenum, kidney,
spleen and liver. Therefore, data are presented as the mean ±
standard deviation. Each experiment was repeated three times.
Statistical analyses were performed using GraphPad Prism software
v4.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Organ-specific mRNA expression of
canine divalent ion channels
Organ-specific mRNA expression of NCKX3 in the
canine duodenum, kidney, spleen and liver was analyzed by
performing RT-PCR and qPCR (Fig. 1A
and B) with β-actin as an internal control. The results
demonstrated that NCKX3 mRNA levels were high in the duodenum and
spleen, and low in the kidney and liver. The RT-PCR and qPCR
results for TRPV2 mRNA expression levels were different among the
sampled organs (Fig. 1C). The
TRPV2 mRNA expression was high in the spleen, moderately high in
the duodenum and liver, and low in the kidney. The expression of
CTR1 varied among the sampled organs. CTR1 mRNA expression levels
were the highest in the liver (Fig.
1D) followed, in descending order, by the duodenum, kidney and
spleen. The ATP7A mRNA levels were highest in the duodenum
(Fig. 1E) followed, in descending
order, by the kidney, spleen and liver. IREG1 mRNA expression was
high in the liver, moderately high in the duodenum and spleen, and
low in the kidney (Fig. 1F).
Finally, HEPH mRNA expression levels were notably low in the
duodenum and almost undetectable in the kidney, spleen and liver
(Fig. 1G).
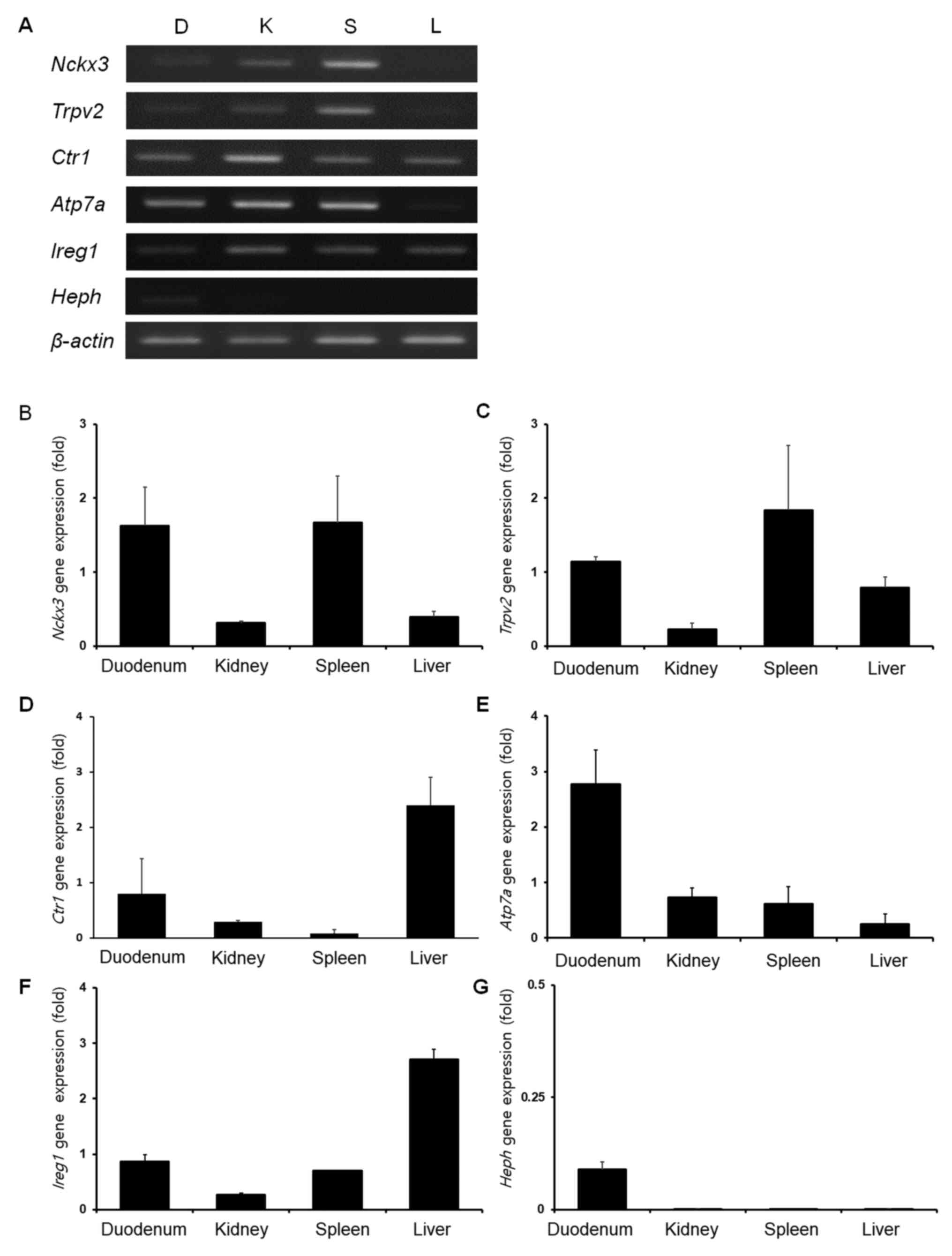 | Figure 1.Organ-specific mRNA expression of
NCKX3, TRPV2, CTR1, ATP7A, IREG1 and HEPH. (A) Representative image
of relative expression, and quantification of (B) NCKX3, (C) TRPV2,
(D) CTR1, (E) ATP7A, (F) IREG1 and (G) HEPH in the canine duodenum,
kidney, spleen and liver, as measured by RT-qPCR. D, duodenum; K,
kidney; S, Spleen; L, Liver; ATP7A, copper-transporting ATPase 1;
CTR1, copper uptake protein 1; HEPH, hephaestin; IREG1,
iron-regulated transporter 1; NCKX3, sodium/potassium/calcium
exchanger 3; TRPV2, transient receptor potential cation channel
subfamily V member 2. |
Organ-specific protein expression of
canine divalent ion channels
Western blot analysis was performed to identify
canine ion channel protein expression in the duodenum, kidney,
spleen and liver (Fig. 2A). The
NCKX3 protein expression was highest in the kidney, moderate in the
duodenum, and low in the spleen and liver (Fig. 2B). The TPRV2 protein expression
levels in the various organs were different from the mRNA levels
(Fig. 2C). In the kidney, duodenum
and liver, TRPV2 protein was highly expressed, whereas its
expression was low in the spleen. The CTR1 protein expression
levels were similar to the mRNA results, as its expression was the
highest in the liver, followed, in descending order, by the
duodenum, kidney, and spleen (Fig.
2D). Compared with the other transmembrane cation channel
factors examined, the ATP7A protein expression levels were
relatively low in all organs (Fig.
2E). Similarly, the ATP7A protein expression levels were the
highest in duodenum; however, the lowest expression level was in
the spleen. Western blotting results demonstrated that the IREG1
protein expression level was the highest in the liver followed, in
descending order, by the kidney, duodenum and spleen (Fig. 2F). The HEPH protein expression
level results demonstrated a high level in the liver, moderately
high levels in the duodenum and kidney, and a low level in spleen
(Fig. 2G).
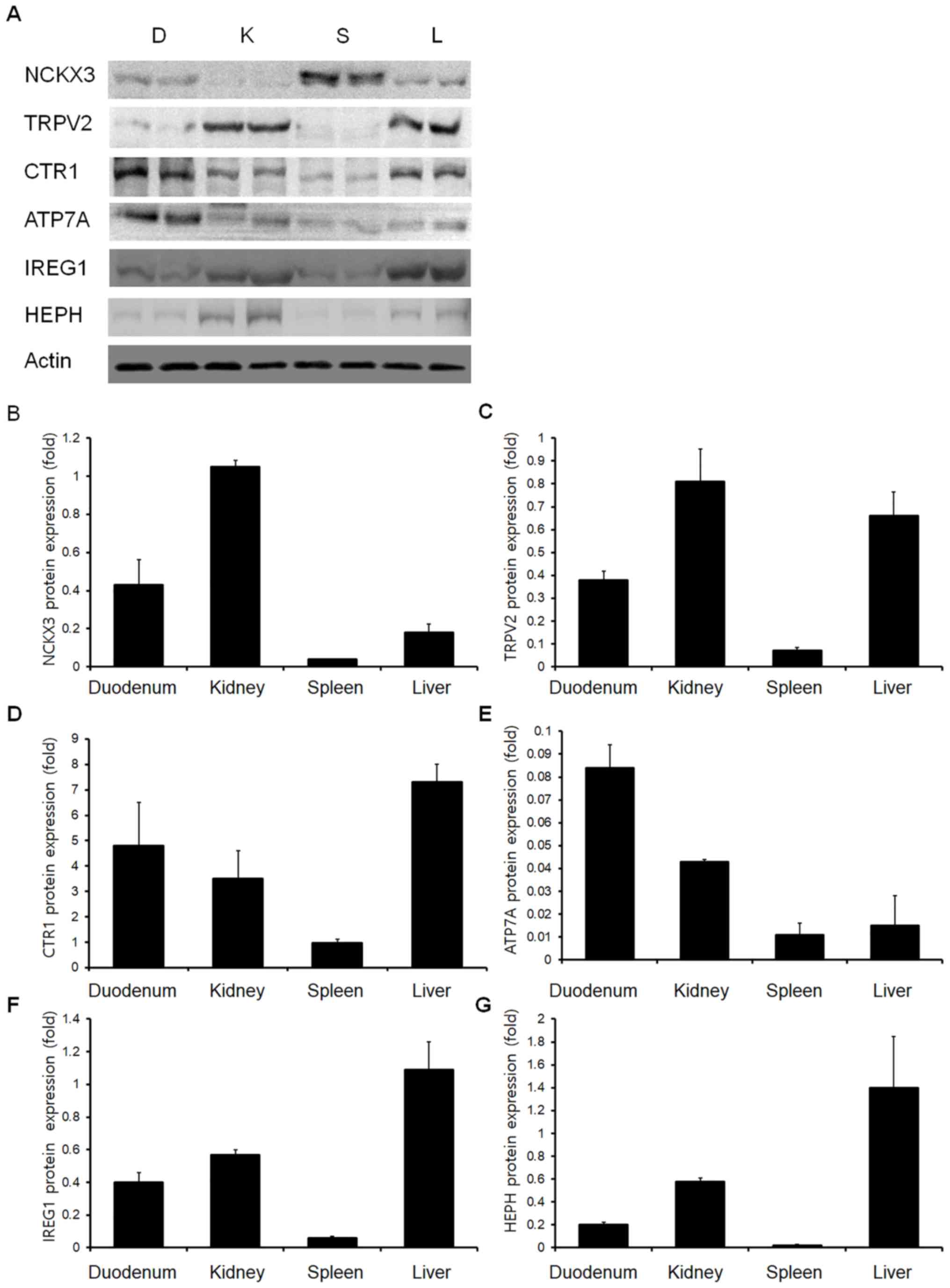 | Figure 2.Western blot analysis of NCKX3,
TRPV2, CTR1, ATP7A, IREG1 and HEPH. (A) Representative western
blotting images and quantification of (B) NCKX3, (C) TRPV2, (D)
CTR1, (E) ATP7A, (F) IREG1 and (G) HEPH protein expression levels
in the canine duodenum, kidney, spleen and liver. D, duodenum; K,
kidney; S, Spleen; L, Liver; ATP7A, copper-transporting ATPase 1;
CTR1, copper uptake protein 1; HEPH, hephaestin; IREG1,
iron-regulated transporter 1; NCKX3, sodium/potassium/calcium
exchanger 3; TRPV2, transient receptor potential cation channel
subfamily V member 2. |
Organ-specific localization of canine
divalent ion channels
Organ-specific localization of cation channels was
investigated by immunohistochemistry (Fig. 3). Immunohistochemical analysis
revealed that NCKX3 was expressed in the intestinal villi and
hepatocytes, in addition to tubules, glomeruli, and Henle's loops
of the kidney (Fig. 3A).
Immunohistochemical analysis demonstrated that TRPV2 was present in
all sampled organs (Fig. 3B) and
was detected in intestinal villi, convoluted tubules and Henle's
loops in the kidney, red pulp and macrophages in the spleen, and
Kupffer cells in the liver. In the magnified images, certain cells
in the spleen, which contained numerous nuclei were stained with
DAB and consequently identified as splenic macrophages. The CTR1
immunohistochemical staining results demonstrated its presence in
all sampled organs (Fig. 3C). In
the duodenum, CTR1 was present in intestinal villi. In addition, it
was observed in distal convoluted tubules in the kidney, red pulp
in the spleen, and canaliculi in the liver. The immunohistochemical
staining results demonstrated that ATP7A was expressed in
intestinal villi, tubules in the kidney, and macrophages in the
marginal zone of spleen (Fig. 3D).
The immunohistochemical staining results demonstrated that IREG1
was present in intestinal villi, the proximal and distal convoluted
tubules in the kidney, macrophages in the spleen, and Kupffer cells
in the liver (Fig. 3E). Finally,
the HEPH immunohistochemical staining results demonstrated that
HEPH protein was present in intestinal villi, the distal convoluted
tubules in the kidney, macrophages in the spleen, and hepatocytes
and Kupffer cells in the liver (Fig.
3F).
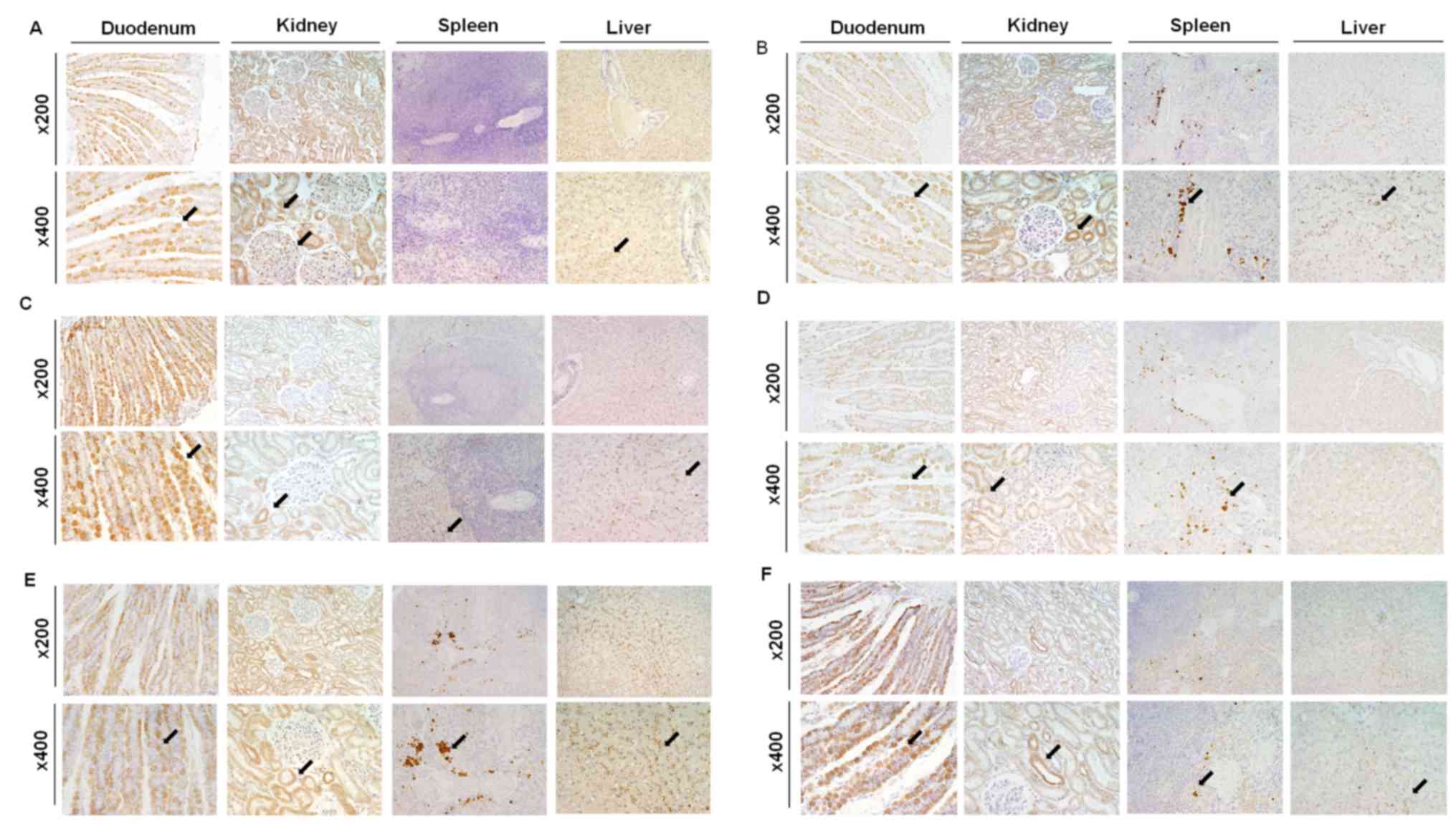 | Figure 3.Immunohistochemistry of NCKX3, TRPV2,
CTR1, ATP7A, IREG1 and HEPH. Organ-specific localization of (A)
NCKX3, (B) TRPV2, (C) CTR1, (D) ATP7A, (E) IREG1 and (F) HEPH in
the canine duodenum, kidney, spleen and liver. Magnification, ×200
and ×400. Black arrows indicate the immunopositive sites in each
tissue. ATP7A, copper-transporting ATPase 1; CTR1, copper uptake
protein 1; HEPH, hephaestin; IREG1, iron-regulated transporter 1;
NCKX3, sodium/potassium/calcium exchanger 3; TRPV2, transient
receptor potential cation channel subfamily V member 2. |
Discussion
Cation transmembrane channels, including those for
calcium, copper and iron ions, have important roles in the bodies
of humans and animals (5). The
functions of these channels are directly connected with the health
of the organism and include roles in homeostasis and metabolism
(5,31,37).
All aspects of metabolism, including hormone secretion, vitamin
synthesis, food digestion and the absorption of nutrients are
regulated via cation transmembrane channels (38). These channels are located in a
variety of organs and have the ability to function at metabolically
appropriate times (39). A number
of previous studies have reported on the expression and regulation
of various cation channel proteins; however, there are no similar
reports for such proteins in canine organs (2,31,39–41).
Thus, in the present study, the organ-specific expression and
localization of calcium (NCKX3, TRPV2), copper (CTR1, ATP7A), iron
(IREG1) and ferroxidase for iron transporting (HEPH) proteins and
mRNAs were investigated in the canine duodenum (upper part of the
duodenum), kidney (borderline of the cortex and medulla), spleen
(borderline of the white and red pulp) and liver (quadrate lobe of
the liver). Each tissue (duodenum, kidney, spleen and liver) is
composed of various cell types, resulting in heterogeneity in the
samples. Therefore, immunohistochemistry was performed to identify
the tissue-specific expression of divalent ion channel proteins to
address this limitation.
The expression of NCKX3 in the mouse kidney is
reported to be different between males and females, with the female
NCKX3 expression level being notably higher than that in the male
mouse (27). Through the action of
NCKX3, intracellular potassium and calcium ions are exchanged for
four extracellular sodium ions. In addition, it has been reported
that NCKX3 is important in calcium reabsorption in the kidney in
the presence or absence of another calcium-associated gene activity
(27). In the present results,
NCKX3 protein was highly expressed in the canine kidney and was
predominantly localized in distal convoluted tubules, with
moderately high localization in the proximal convoluted tubules. In
the kidney, NCKX3 in the renal tubular epithelium has a key role in
calcium reabsorption (6). In
another study, NCKX3 was demonstrated to be expressed in the rat
duodenum (27). NCKX3 in the
basolateral membrane of intestinal tissue transports calcium to the
blood, thus it has a key role in calcium absorption (41). Due to this function of NCKX3,
abnormal expression of NCKX3 can induce decreased calcium
absorption in the duodenum and calcium reabsorption in the kidney;
furthermore, it may result in hypocalcemia and osteoporosis
(42). In the present study, the
results demonstrated that NCKX3 mRNA levels were high in the
duodenum, spleen and kidney, and low in the liver. Based on these
results and those from previous studies, NCKX3 may have an
important role in maintaining calcium homeostasis in the canine
duodenum, spleen and kidney for preventing hypocalcemia and
osteoporosis.
The results of previous studies have demonstrated
that TRPV2 has a key role in macrophage phagocytosis, and one study
revealed that TRPV2 is involved in the earliest stages of
phagocytosis (37,43–45).
In addition, TRPV2 functions in intra- and extracellular
Ca2+ mobilization, and it has a role in
lipopolysaccharide-induced cytokine production in macrophages
(46). The present results
demonstrated that TRPV2 was present in the liver and spleen
macrophages. Another study demonstrated that TRP family members are
expressed in the gastrointestinal tract (31) and kidney, and they have roles in
mucosal function and Ca2+ homeostasis (47). TRPV2 also has a notable
immunomodulatory function (48),
and abnormalities in its expression create issues, including the
development of chemoattractant-elicited, motility-defective
macrophages (40). Based on the
present results and those of previous studies, TRPV2 may have an
important role in macrophage phagocytosis and cytokine production
in dogs. In addition, TRPV2 may be hypothesized to have a function
in Ca2+ homeostasis in dogs.
CTR1 protein expression levels in the present study
were high in the duodenum, kidney, and liver, and low in the
spleen. A previous study reported that CTR1 expression was high in
liver canaliculi, kidney convoluted tubules, and intestinal
enterocytes in mice (30). In
general, CTR1 regulates copper levels between the intra- and
extracellular spaces, in order to maintain homeostatic balance on
the two sides of the cell membrane (36–38).
In the intestine, CTR1 has been demonstrated to have an important
role in copper absorption and regulation. Furthermore, it was
revealed that CTR1 is expressed primarily on the apical surface of
intestinal epithelial cells (28).
Another study indicated that the liver is an important organ for
copper storage, as mouse hepatocytes lacking CTR1 had a low copper
concentration and their copper-dependent hepatic enzymes had
reduced activity (49). In
addition, a study suggested that CTR1 has a critical role in copper
reabsorption in the kidney, since mice lacking CTR1 exhibited a
high copper concentration in the urine (12). These results indicated that CTR1
may have a significant role in copper absorption and homeostatic
regulation in the canine duodenum and kidney. Copper storage and
liver enzyme activity may be hypothesized to be influenced by CTR1
expression in dogs.
A previous study demonstrated that ATP7A expression
in the intestine has a copper efflux function following dietary
copper absorption (50). Another
study demonstrated that ATP7A is expressed in the kidney and
contributes to renal copper homeostasis by acting in conjunction
with ATP7B. Furthermore, ATP7A has been demonstrated to have a
compensatory copper export function in order to maintain renal
copper homeostasis in ATP7B-lacking mice (51). Silencing of ATP7A has resulted in
attenuated macrophages, thus indicating its presence in
macrophages; additionally, ATP7A has weak bactericidal activity
(29). In addition, ATP7A has been
associated with certain diseases, including Menke's syndrome. The
ATP7A mutation that results in Menke's syndrome is an X-linked
recessive disorder associated with copper deficiency, resulting in
reduced functioning of copper-dependent enzymes (52). Based on these observations and
those in the present study, ATP7A may have important roles in
copper homeostasis and immunological function in dogs.
Previous studies have demonstrated that IREG1
protein, a principal modulator of iron homeostasis, is expressed in
the rat duodenum, liver and reticuloendothelial system macrophages
(19,20). It has also been reported that IREG1
protein is located in the basolateral plasma membrane of intestinal
cells and acts as an iron exporter (19,20).
Furthermore, IREG1 is expressed in the medullary portions of
nephrons and proximal tubules (32). Similarly, the results of the
present study demonstrated that IREG1 is expressed in canine kidney
tubules and intestinal cells. In the kidney, IREG1 is active in
exporting iron when iron levels increase. Mutations of IREG1 may
result in iron overload, as exhibited in type IV hemochromatosis
(17,19,20).
In addition, if there is an imbalance between IREG1 and hepcidin
levels, systemic iron homeostasis is lost, which may result in
tumors including breast cancer (53). Based on these results, IREG1 is
hypothesized to function as an iron exporter and in the maintenance
of iron homeostasis in dogs.
HEPH presence has been reported in intestinal
tissue, particularly in the basolateral membrane of human
enterocytes (26). In rats, HEPH
has been located in the duodenum, and, in conjunction with other
iron-associated proteins including hepcidin, is reported to be
active in the maintenance of iron homeostasis (54). The present results demonstrated
that HEPH mRNA levels were higher in the canine duodenum compared
with other organs. In addition, immunohistochemical staining
results demonstrated a marked presence of HEPH in duodenal
enterocytes. Based on results from other animal studies, it appears
that HEPH protein in the canine duodenum may have a notable role in
transporting dietary iron from enterocytes to the circulatory
system (21–23,26,54,55).
Other studies have reported HEPH presence in the rat liver
(56) and in the mouse spleen and
kidney (55). HEPH mutation has
been associated with systemic iron deficiency, and an absence of
HEPH may result in iron accumulation in duodenal enterocytes,
resulting in microcytic and hypochromic anemia in mouse (23). Based on the present results and
those of other studies, HEPH is hypothesized to have an important
role in maintaining iron homeostasis and preventing diseases
associated with iron imbalance in dogs.
In conclusion, the present results demonstrated that
NCKX3, TRPV2, CTR1, ATP7A, IREG1 and HEPH protein and mRNA are
expressed and localized in a tissue-specific manner in canines.
These results suggest that such transmembrane cation proteins have
characteristic functions in the tissues in which they are highly
expressed. The results from the present study provide a basis for
further studies into transmembrane cation channel proteins and
their functions in canine pathological and physiological
states.
Acknowledgements
Not applicable.
Funding
The present study was supported by a National
Research Foundation of Korea (grant no. 2017R1A2B2005031) grant
funded by the Korean government and the Global Research and
Development Center Program through the National Research Foundation
of Korea, funded by the Ministry of Education, Science and
Technology (grant no. 2017K1A4A3014959).
Availability of data and materials
All data generated or analyzed during this current
study are included in this published article.
Authors' contributions
E-BJ designed the study and analyzed the data. CA
and J-SC performed RNA/protein experiments and interpreted the
data. CA, J-SC and E-BJ wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All procedures for organ collection were approved by
the Ethics Committee of Chungbuk National University (Cheongju,
Republic of Korea).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Argüello JM, Raimunda D and
González-Guerrero M: Metal transport across biomembranes: Emerging
models for a distinct chemistry. J Biol Chem. 287:13510–13517.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Turner RW, Anderson D and Zamponi GW:
Signaling complexes of voltage-gated calcium channels. Channels
(Austin). 5:440–448. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maryon EB, Molloy SA, Ivy K, Yu H and
Kaplan JH: Rate and regulation of copper transport by human copper
transporter 1 (hCTR1). J Biol Chem. 288:18035–18046. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Anderson ER and Shah YM: Iron homeostasis
in the liver. Compr Physiol. 3:315–330. 2013.PubMed/NCBI
|
|
5
|
Jalloul AH, Szerencsei RT and Schnetkamp
PP: Cation dependencies and turnover rates of the human
K+-dependent Na+-Ca2+ exchangers
NCKX1, NCKX2, NCKX3 and NCKX4. Cell Calcium. 59:1–11. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang H, Lee GS, Yoo YM, Choi KC and Jeung
EB: Sodium/potassium/calcium exchanger 3 is regulated by the
steroid hormones estrogen and progesterone in the uterus of mice
during the estrous cycle. Biochem Biophys Res Commun. 385:279–283.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kraev A, Quednau BD, Leach S, Li XF, Dong
H, Winkfein R, Perizzolo M, Cai X, Yang R, Philipson KD and Lytton
J: Molecular cloning of a third member of the potassium-dependent
sodium-calcium exchanger gene family, NCKX3. J Biol Chem.
276:23161–23172. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nilius B and Owsianik G: The transient
receptor potential family of ion channels. Genome Biol. 12:2182011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park DJ, Kim SH, Nah SS, Lee JH, Kim SK,
Lee YA, Hong SJ, Kim HS, Lee HS, Kim HA, et al: Polymorphisms of
the TRPV2 and TRPV3 genes associated with fibromyalgia in a Korean
population. Rheumatology (Oxford). 55:1518–1527. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kunert-Keil C, Bisping F, Kruger J and
Brinkmeier H: Tissue-specific expression of TRP channel genes in
the mouse and its variation in three different mouse strains. BMC
Genomics. 7:1592006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Perálvarez-Marín A, Doñate-Macian P and
Gaudet R: What do we know about the transient receptor potential
vanilloid 2 (TRPV2) ion channel? FEBS J. 280:5471–5487. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsai CY, Liebig JK, Tsigelny IF and Howell
SB: The copper transporter 1 (CTR1) is required to maintain the
stability of copper transporter 2 (CTR2). Metallomics. 7:1477–1487.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Landon CD, Benjamin SE, Ashcraft KA and
Dewhirst MW: A role for the copper transporter Ctr1 in the
synergistic interaction between hyperthermia and cisplatin
treatment. Int J Hyperthermia. 29:528–538. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yi L and Kaler SG: Direct interactions of
adaptor protein complexes 1 and 2 with the copper transporter ATP7A
mediate its anterograde and retrograde trafficking. Hum Mol Genet.
24:2411–2425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
La Fontaine S and Mercer JF: Trafficking
of the copper-ATPases, ATP7A and ATP7B: Role in copper homeostasis.
Arch Biochem Biophys. 463:149–167. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Holloway ZG, Velayos-Baeza A, Howell GJ,
Levecque C, Ponnambalam S, Sztul E and Monaco AP: Trafficking of
the Menkes copper transporter ATP7A is regulated by clathrin-,
AP-2-, AP-1-, and Rab22-dependent steps. Mol Biol Cell.
24:1735–1748, S1-S8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McKie AT and Barlow DJ: The SLC40
basolateral iron transporter family (IREG1/ferroportin/MTP1).
Pflugers Arch. 447:801–806. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miret S, Simpson RJ and McKie AT:
Physiology and molecular biology of dietary iron absorption. Annu
Rev Nutr. 23:283–301. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aguirre P, Mena N, Tapia V, Arredondo M
and Núñez MT: Iron homeostasis in neuronal cells: A role for IREG1.
BMC Neurosci. 6:32005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kelleher T, Ryan E, Barrett S, Sweeney M,
Byrnes V, O'Keane C and Crowe J: Increased DMT1 but not IREG1 or
HFE mRNA following iron depletion therapy in hereditary
haemochromatosis. Gut. 53:1174–1179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yeh KY, Yeh M, Mims L and Glass J: Iron
feeding induces ferroportin 1 and hephaestin migration and
interaction in rat duodenal epithelium. Am J Physiol Gastrointest
Liver Physiol. 296:G55–G65. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee SM, Attieh ZK, Son HS, Chen H,
Bacouri-Haidar M and Vulpe CD: Iron repletion relocalizes
hephaestin to a proximal basolateral compartment in polarized MDCK
and Caco2 cells. Biochem Biophys Res Commun. 421:449–455. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fuqua BK, Lu Y, Darshan D, Frazer DM,
Wilkins SJ, Wolkow N, Bell AG, Hsu J, Yu CC, Chen H, et al: The
multicopper ferroxidase hephaestin enhances intestinal iron
absorption in mice. PLoS One. 9:e987922014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gaggelli E, Kozlowski H, Valensin D and
Valensin G: Copper homeostasis and neurodegenerative disorders
(Alzheimer's, prion, and Parkinson's diseases and amyotrophic
lateral sclerosis). Chem Rev. 106:1995–2044. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hubner CA and Jentsch TJ: Ion channel
diseases. Hum Mol Genet. 11:2435–2445. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hudson DM, Curtis SB, Smith VC, Griffiths
TA, Wong AY, Scudamore CH, Buchan AM and MacGillivray RT: Human
hephaestin expression is not limited to enterocytes of the
gastrointestinal tract but is also found in the antrum, the enteric
nervous system, and pancreatic {beta}-cells. Am J Physiol
Gastrointest Liver Physiol. 298:G425–G432. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee GS, Choi KC and Jeung EB: K+-dependent
Na+/Ca2+ exchanger 3 is involved in renal active calcium transport
and is differentially expressed in the mouse kidney. Am J Physiol
Renal Physiol. 297:F371–F379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nose Y, Wood LK, Kim BE, Prohaska JR, Fry
RS, Spears JW and Thiele DJ: Ctr1 is an apical copper transporter
in mammalian intestinal epithelial cells in vivo that is controlled
at the level of protein stability. J Biol Chem. 285:32385–32392.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
White C, Lee J, Kambe T, Fritsche K and
Petris MJ: A role for the ATP7A copper-transporting ATPase in
macrophage bactericidal activity. J Biol Chem. 284:33949–33956.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kuo YM, Gybina AA, Pyatskowit JW,
Gitschier J and Prohaska JR: Copper transport protein (Ctr1) levels
in mice are tissue specific and dependent on copper status. J Nutr.
136:21–26. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vennekens R, Owsianik G and Nilius B:
Vanilloid transient receptor potential cation channels: An
overview. Curr Pharm Des. 14:18–31. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wolff NA, Liu W, Fenton RA, Lee WK,
Thévenod F and Smith CP: Ferroportin 1 is expressed basolaterally
in rat kidney proximal tubule cells and iron excess increases its
membrane trafficking. J Cell Mol Med. 15:209–219. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tanner MR and Beeton C: Differences in ion
channel phenotype and function between humans and animal models.
Front Biosci (Landmark Ed). 23:43–64. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lin JH: Species similarities and
differences in pharmacokinetics. Drug Metab Dispos. 23:1008–1021.
1995.PubMed/NCBI
|
|
35
|
Chandler K: Canine epilepsy: What can we
learn from human seizure disorders? Vet J. 172:207–217. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Entin-Meer M, Levy R, Goryainov P, Landa
N, Barshack I, Avivi C, Semo J and Keren G: The transient receptor
potential vanilloid 2 cation channel is abundant in macrophages
accumulating at the peri-infarct zone and may enhance their
migration capacity towards injured cardiomyocytes following
myocardial infarction. PLoS One. 9:e1050552014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kiela PR and Ghishan FK: Physiology of
intestinal absorption and secretion. Best Pract Res Clin
Gastroenterol. 30:145–159. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kulbacka J, Choromańska A, Rossowska J,
Weżgowiec J, Saczko J and Rols MP: Cell membrane transport
mechanisms: Ion channels and electrical properties of cell
membranes. Adv Anat Embryol Cell Biol. 227:39–58. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Santoni G, Farfariello V, Liberati S,
Morelli MB, Nabissi M, Santoni M and Amantini C: The role of
transient receptor potential vanilloid type-2 ion channels in
innate and adaptive immune responses. Front Immunol. 4:342013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang H, An BS, Choi KC and Jeung EB:
Change of genes in calcium transport channels caused by hypoxic
stress in the placenta, duodenum, and kidney of pregnant rats. Biol
Reprod. 88:302013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kruger MC and Wolber FM: Osteoporosis:
Modern paradigms for last century's bones. Nutrients. 8:pii: E376.
2016. View Article : Google Scholar
|
|
43
|
Entin-Meer M, Cohen L, Hertzberg-Bigelman
E, Levy R, Ben-Shoshan J and Keren G: TRPV2 knockout mice
demonstrate an improved cardiac performance following myocardial
infarction due to attenuated activity of peri-infarct macrophages.
PLoS One. 12:e01771322017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hassan S, Eldeeb K, Millns PJ, Bennett AJ,
Alexander SP and Kendall DA: Cannabidiol enhances microglial
phagocytosis via transient receptor potential (TRP) channel
activation. Br J Pharmacol. 171:2426–2439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Link TM, Park U, Vonakis BM, Raben DM,
Soloski MJ and Caterina MJ: TRPV2 has a pivotal role in macrophage
particle binding and phagocytosis. Nat Immunol. 11:232–239. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yamashiro K, Sasano T, Tojo K, Namekata I,
Kurokawa J, Sawada N, Suganami T, Kamei Y, Tanaka H, Tajima N, et
al: Role of transient receptor potential vanilloid 2 in LPS-induced
cytokine production in macrophages. Biochem Biophys Res Commun.
398:284–289. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Holzer P: TRP channels in the digestive
system. Curr Pharm Biotechnol. 12:24–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sulk M, Seeliger S, Aubert J, Schwab VD,
Cevikbas F, Rivier M, Nowak P, Voegel JJ, Buddenkotte J and
Steinhoff M: Distribution and expression of non-neuronal transient
receptor potential (TRPV) ion channels in rosacea. J Invest
Dermatol. 132:1253–1262. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ohrvik H and Thiele DJ: How copper
traverses cellular membranes through the mammalian copper
transporter 1, Ctr1. Ann N Y Acad Sci. 1314:32–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Collins JF, Hua P, Lu Y and Ranganathan
PN: Alternative splicing of the Menkes copper Atpase (Atp7a)
transcript in the rat intestinal epithelium. Am J Physiol
Gastrointest Liver Physiol. 297:G695–G707. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Linz R, Barnes NL, Zimnicka AM, Kaplan JH,
Eipper B and Lutsenko S: Intracellular targeting of
copper-transporting ATPase ATP7A in a normal and Atp7b-/-kidney. Am
J Physiol Renal Physiol. 294:F53–F61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Vonk WI, de Bie P, Wichers CG, van den
Berghe PV, van der Plaats R, Berger R, Wijmenga C, Klomp LW and van
de Sluis B: The copper-transporting capacity of ATP7A mutants
associated with Menkes disease is ameliorated by COMMD1 as a result
of improved protein expression. Cell Mol Life Sci. 69:149–163.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Pinnix ZK, Miller LD, Wang W, D'Agostino R
Jr, Kute T, Willingham MC, Hatcher H, Tesfay L, Sui G, Di X, et al:
Ferroportin and iron regulation in breast cancer progression and
prognosis. Sci Transl Med. 2:43ra562010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kong WN, Chang YZ, Wang SM, Zhai XL, Shang
JX, Li LX and Duan XL: Effect of erythropoietin on hepcidin, DMT1
with IRE, and hephaestin gene expression in duodenum of rats. J
Gastroenterol. 43:136–143. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Petrak J and Vyoral D: Hephaestin-a
ferroxidase of cellular iron export. Int J Biochem Cell Biol.
37:1173–1178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Malik IA, Naz N, Sheikh N, Khan S,
Moriconi F, Blaschke M and Ramadori G: Comparison of changes in
gene expression of transferrin receptor-1 and other iron-regulatory
proteins in rat liver and brain during acute-phase response. Cell
Tissue Res. 344:299–312. 2011. View Article : Google Scholar : PubMed/NCBI
|

















