Introduction
Osteosarcoma (OS) is the most common malignant tumor
of the skeletal system, with high rates of local invasion and early
metastasis. OS mostly occurs among children and adolescents, with a
reported incidence rate of 4–5 per million (1). The overall survival rate of patients
with OS is reported to be 60–80% (2–4).
However, despite the advances in multiagent chemotherapy and
surgical technique, the survival rate of patients with locally
advanced or metastatic tumors at diagnosis is still very low
(<20%) (5–9). Thus, there is urgent need to reveal
detailed signal pathways involved in OS pathogenesis and the
molecular mechanism of its metastasis, which may provide novel
therapeutic targets for the clinical management of OS.
Human trophoblast cell surface antigen 2 (TROP2) is
a 36-kDa single-pass transmembrane protein encoded by the
tumor-associated calcium signal transducer 2 (Tacstd2) gene, with
low to no expression in normal tissues (10–13).
Accumulating studies have demonstrated TROP2 to be a candidate
tumor prognostic marker, which was highly expressed by various
tumors, such as pancreatic cancer (14,15),
gastric cancer (11,16), lung cancer (17,18),
ovarian cancer (19), and
colorectal cancers (20). TROP2
overexpression correlates with increased tumor recurrence,
invasiveness, and poor clinical outcome (21–23).
Although TROP2 has mostly been reported to be highly expressed in
epithelial cancers, previous studies have also found TROP2
expression in stem cells in various tissue types, such as human and
mouse prostate (10,24). In addition, TROP2 is also expressed
in bone tissues and regulates the proliferation and differentiation
of bone marrow stromal cells (25–27).
However, so far the potential role and the molecular mechanisms of
TROP2 in OS remain largely unclear.
The present study aimed to investigate the role of
TROP2 in OS. We evaluated the expression of TROP2 in human OS
tissues and cell lines. Furthermore, the specific effects of TROP2
on OS cells proliferation, cell migration, together with the
possible mechanism involved in this process were also explored for
the first time. The results of the present study suggest that TROP2
may be a potential prognostic biomarker and a potential therapeutic
target for OS.
Materials and methods
Clinical tissue samples
This study was approved by the Medical Ethics
Committee of The First Affiliated Hospital of Xinjiang Medical
University (Urumqi, China). Ten OS specimens and paired adjacent
normal bone tissues were collected during surgery from OS patients
without prior chemotherapy or radiotherapy (Table I). Written informed consent was
obtained from each patient. Tissue samples were immediately stored
in liquid nitrogen for further analysis.
 | Table I.Clinical profiles of the 10 patients
with osteosarcoma. |
Table I.
Clinical profiles of the 10 patients
with osteosarcoma.
| Clinical
variable | No. of patients
(n=10) |
|---|
| Sex |
|
|
Female | 5 |
| Male | 5 |
| Age, years |
|
|
Median | 27.7 |
|
Range | 9-64 |
| Tumor location |
|
|
Femur/tibia | 8 |
|
Humerus | 2 |
| Tumor size, cm |
|
| ≤5 | 4 |
|
>5 | 6 |
| Distant
metastasis |
|
|
Yes | 0 |
| No | 10 |
Immunohistochemistry (IHC)
TROP2 expression was detected by IHC in
paraffin-embedded specimens, using the standard immunoperoxidase
staining procedure. The 5 µm thick slides were incubated overnight
with anti-TROP2 antibody (1:50; Cell Signaling Technology, Inc.,
Danvers, MA, USA). Sections were then stained with Diaminobenzidine
(DAB) and hematoxylin after the application of a secondary antibody
for 30 min at room temperature. The degree of immunostaining was
reviewed and scored independently by two observers as previously
described (6,28).
Cell culture
The normal osteoblast cells hFOB1.19 and OS cell
lines U2OS, MG63, and MNNG/HOS were purchased commercially from
Academia Sinica Cell Bank (Shanghai, China). HFOB1.19 cells were
cultured in DMEM/F-12 (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) according to American Type Culture Collection (ATCC; Manassas,
VA, USA) protocols. MNNG/HOS and MG63 cells were cultured in
Eagle's minimum essential medium (Thermo Fisher Scientific, Inc.)
containing 10% FBS (15140-122; Gibco; Thermo Fisher Scientific,
Inc.). The U2OS cells were cultured in Roswell Park Memorial
Institute-1640 (RPMI-1640; Thermo Fisher Scientific, Inc.) medium
containing 10% FBS (15140-122; Gibco; Thermo Fisher Scientific,
Inc.). Cells were cultured at 37°C in a humidified atmosphere of 5%
CO2.
Lentiviral and retroviral
infection
A lentiviral short hairpin RNA (shRNA) construct
targeting TROP2 was obtained from Jikai Corporation (Shanghai,
China), with a sequence as follows:
5′-GCGGCAGAACACGTCTCAGAACTCGAGTTCTGAGACGTGTTCTGCCGC-3′. The
oligonucleotides were phosphorylated, annealed, and cloned into the
pLKO.1 vector according to the manufacturer's instructions.
Lentiviral overexpression particles were prepared by GenePharma
(Shanghai, China). Lentiviral and retroviral infection of MG63,
U2OS and MNNG/HOS cells were performed according to the
manufacturer's protocols. The expression of TROP2 was determined by
western blot analyses and quantitative polymerase chain reaction
(qPCR).
Cell proliferation
To evaluate the effects of TROP2 on OS cells, MG63,
U2OS and MNNG/HOS cells were seeded into 96-well plates on days 1,
2, 3, and 4 post-infection (3,000 cells/well). After 24 h, the cell
proliferation rate of MG63 and MNNG/HOS cells was detected with the
CCK-8 Assay kit. Experiment was performed with three
replicates.
Wound healing assay
For wound healing assay, MG63 and MNNG/HOS cells
were seeded into six-well plates and cultured to 100% confluence.
The cell layer was carefully scratched to create a wound using a
sterile 1,000 µl pipette tip. Then, the cells were washed twice
with PBS and treated with complete medium without FBS. The
evaluation of wound healing was done under a light microscope at 24
h after scratching. The percentage of wound closure was calculated
using Image J software (Rasband, W.S., Image J, U.S. National
Institutes of Health, Bethesda, Maryland, USA).
Transwell assay
Transwell assay was performed to determine the
effect of TROP2 on the migration of OS cells. Cells were suspended
in serum-free medium and added to the upper chamber (Corning
Costar, Rochester, NY, USA) and 10% FBS-medium was added in the
lower chamber. After incubation for 24 h, the non-migratory cells
were removed, while the migratory cells below the membrane were
fixed and stained with crystal violet. The number of migration
cells were counted under a light microscope (magnification, ×200)
using five randomly chosen visual fields.
RNA isolation and reverse
transcription (RT)-qPCR
Total cellular RNA was extracted from cell lines
using Trizol reagent (Takara Bio, Inc., Otsu, Japan) according to
the manufacturer's instructions. Total RNA was reverse-transcribed
into cDNA by using the reverse transcriptional kit (Takara Bio,
Inc.). The expression level of TROP2 was quantified by RT-qPCR
using SYBR Green PCR PrimeScript RT-PCR Kit (Takara Bio, Inc.) on
the ABI Step One Plus System. The following primers were used:
TROP2, forward: 5′-CCTCATCGCCGTCATCGT-3′ and reverse:
5′-CGGTTCCTTTCTCAACTCCC'; GAPDH, the internal control, were
forward: 5′-GACTCATGACCACAGTCCATGC-3′ and reverse:
5′-AGAGGCAGGGATGATGTTCTG-3′. The 2−∆∆Cq method was used
to calculate relative gene expression.
Western blot analysis
Total protein of tissue samples and cells was
extracted by using radioimmunoprecipitation assay buffer with a
protease inhibitor (Beyotime Institute of Biotechnology, Haimen,
China). After the protein concentration was measured by the BCA
Protein Assay Kit (Beyotime Institute of Biotechnology), equivalent
amounts of protein were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel and transferred onto a polyvinylidene
fluoride membrane (EMD Millipore, Billerica, MA, USA). After
blocking in 5% nonfat milk for 1 h, the polyvinylidene difluoride
membranes were incubated overnight at 4°C with antibodies specific
to TROP2 (1:1,000; Cell Signaling Technology, Inc.), β-actin
(1:2,000; Cell Signaling Technology, Inc.), p-phosphoinositide
3-kinase (p-PI3K; 1:1,000; Cell Signaling Technology, Inc.), PI3K
(1:1,000; Cell Signaling Technology, Inc.), p-protein kinase B
(p-AKT; 1:1,000; Cell Signaling Technology, Inc.), and AKT
(1:1,000; Cell Signaling Technology, Inc.). Horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (1:1,000;
Beyotime Institute of Biotechnology) was applied for 2 h at room
temperature. An enhanced chemiluminescent detection reagent
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to detect
immunoreactive bands, and all protein expression was quantified
using Bio-Rad XRS chemiluminescence detection system (Bio-Rad
Laboratories, Inc.).
Statistical analysis
All experiments were repeated at least three times.
Results were presented as mean ± standard deviation and analyzed
using SPSS 23.0 software (IBM Corp., Armonk, NY, USA). Statistical
differences between the means of the various groups were analyzed
using one-way and two-way analysis of variance (for cell
proliferation rate and TROP2 mRNA levels at all time points)
followed by the post hoc Bonferroni's test. Differences between two
groups were evaluated by using the Student's t-test when
appropriate. For all analyses, P<0.05 was considered to indicate
a statistically significant difference.
Results
TROP2 was significantly up-regulated
in OS cell lines and clinical specimens
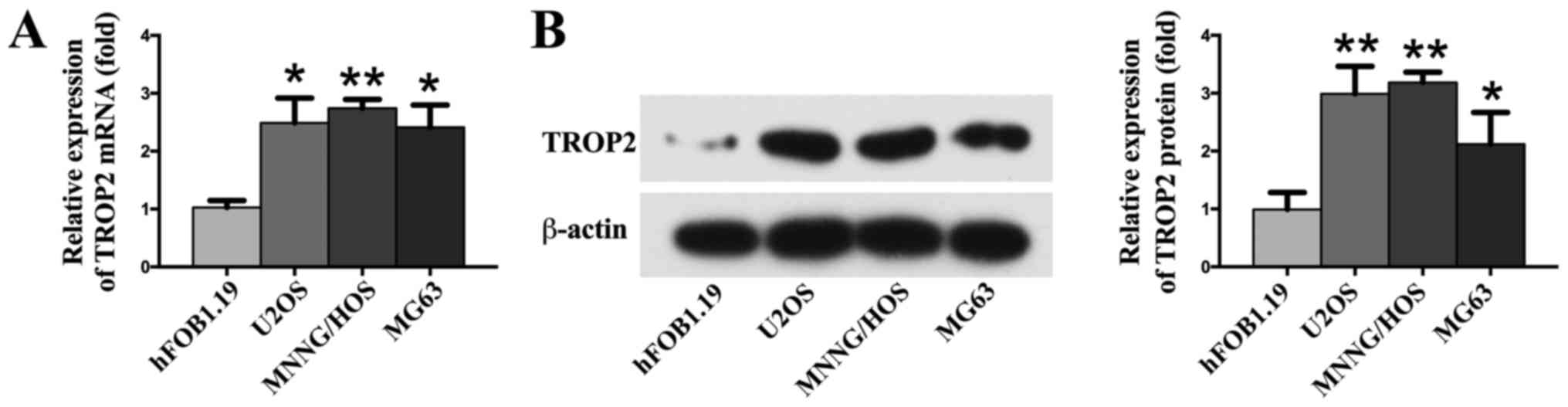
RT-qPCR and western blotting analysis were employed
to detect the expression of TROP2 in OS cell lines. The results
show that the expression of TROP2 was markedly up-regulated in
three different OS cell lines (U2OS, MG63, and MNNG/HOS) compared
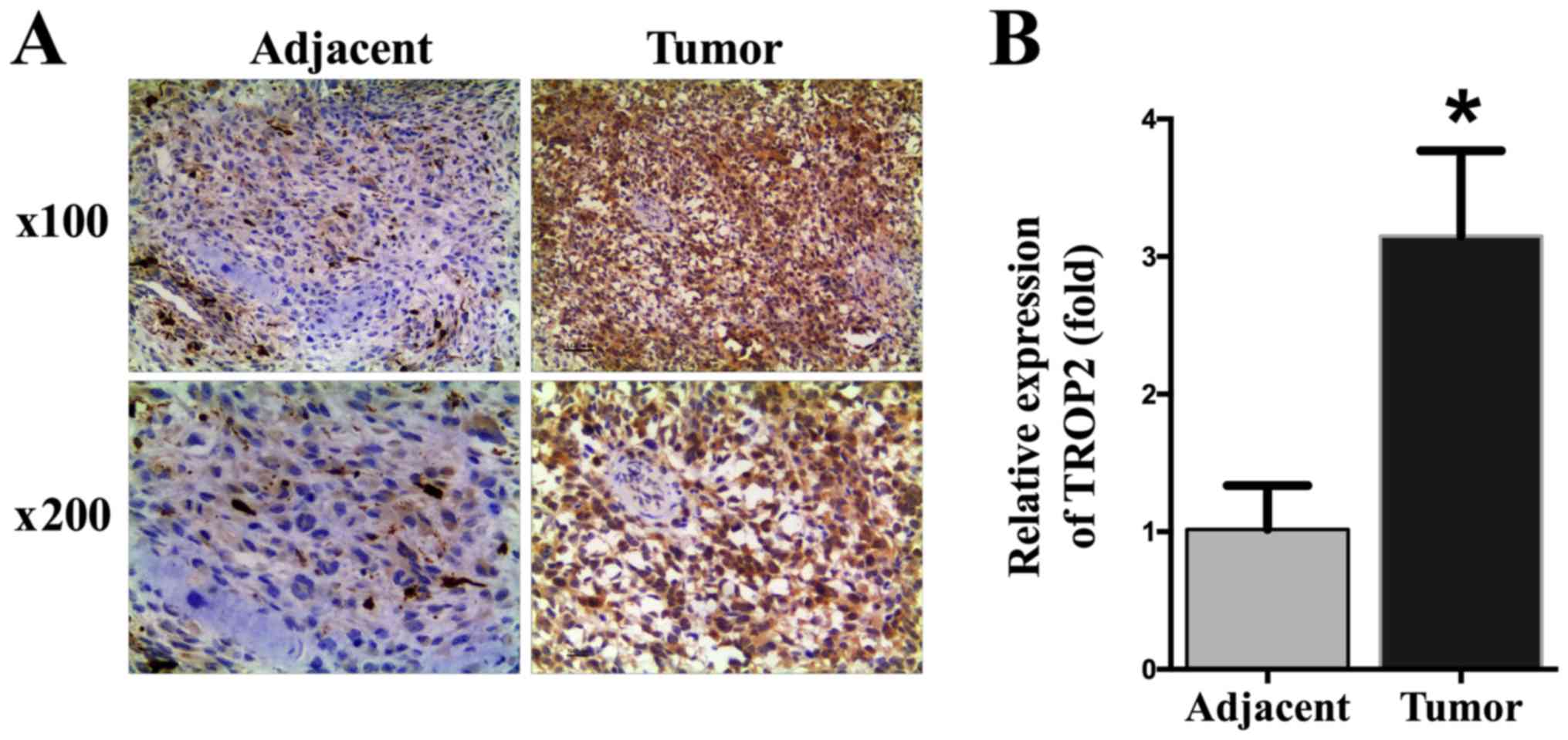
to normal osteoblast cells hFOB1.19 (P<0.05; Fig. 1). To determine the expression of
TROP2 in OS specimens and paired adjacent normal bone tissues, IHC
was performed. Significantly higher TROP2 levels were identified in
OS tissues than in adjacent normal bone tissues (P<0.05;
Fig. 2).
TROP2 promotes cell proliferation
The effects of TROP2 on the proliferation rate of OS
cells were identified by CCK-8 assay, which revealed the
overexpression of TROP2 significantly promoted the proliferation of
MG63, U2OS and MNNG/HOS cells in a time-dependent manner. When
TROP2 was knocked-down, the proliferation rate decreased
significantly (Fig. 3, S1 file).
In addition, to check the efficiency of TROP2 overexpression and
knockdown, TROP2 mRNA levels at all time points after infection
were detected and shown in S2 file.
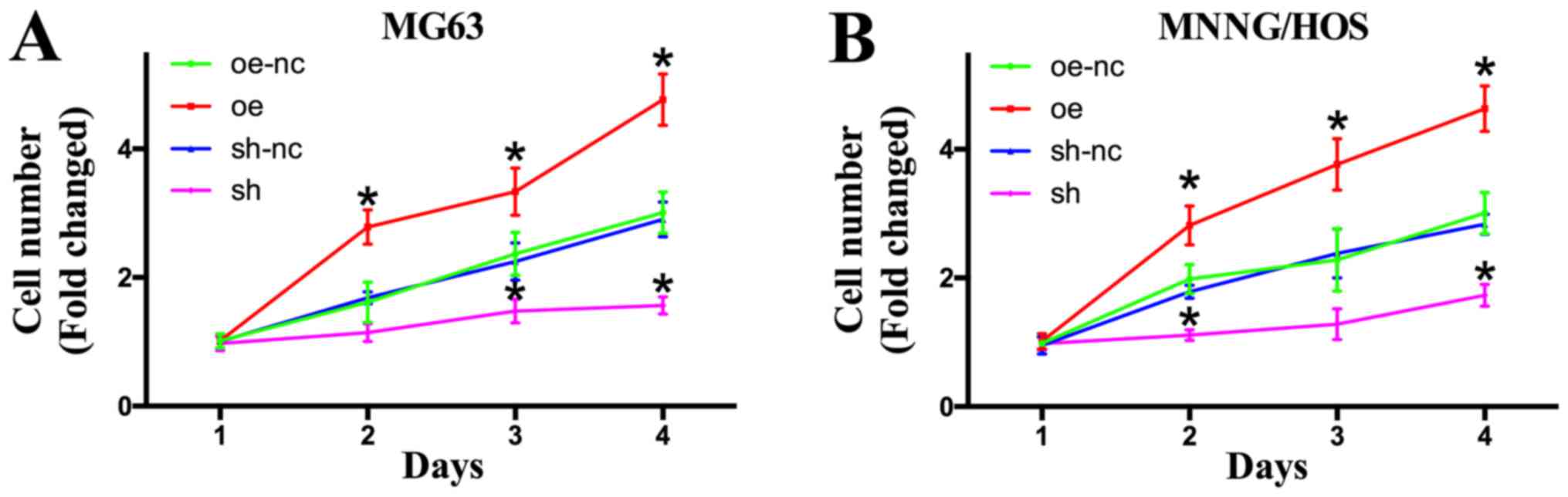 | Figure 3.Effects of TROP2 overexpression and
knockdown on OS cell proliferation. (A) Cell proliferation was
assessed in MG63 cell lines by CCK-8 assay on days 1, 2, 3 and 4
following lentiviral infection. (B) Cell proliferation was assessed
in MNNG/HOS cell lines by CCK8 assay on days 1, 2, 3 and 4
following lentiviral infection. *P<0.05 vs. oe-nc. TROP2,
trophoblast cell surface antigen 2; OS, osteosarcoma; Oe,
overexpression of TROP2; sh, short hairpin RNA knockdown of TROP2;
nc, negative control; oe-nc, the negative control group of TROP2
overexpression; sh-nc, the negative control group of TROP2
knockdown using nc short hairpin RNA; CCK-8, Cell Counting
Kit-8. |
TROP2 promotes cell migration
To explore the biological effects of TROP2 on OS
cell migration, a wound migration assay was performed. Relevant
photographs were taken at 0 and 24 h after wound induction. Western
blotting analysis showed successful upregulation and downregulation
of TROP2 levels in OS cell lines (Fig.
4A and B). The wound-healing abilities of TROP2-overexpressing
OS cells were significantly higher than those of the control group.
In contrast, TROP2 depletion markedly decreased the wound-closure
capacity (Fig. 4C and D). To
further explore the function of TROP2 in OS, a Transwell migration
assay was performed. As shown in Fig.
4E, migration was markedly increased in cells overexpressing
TROP2. In contrast, TROP2 downregulation inhibited the migration of
OS cells (Fig. 4E and F).
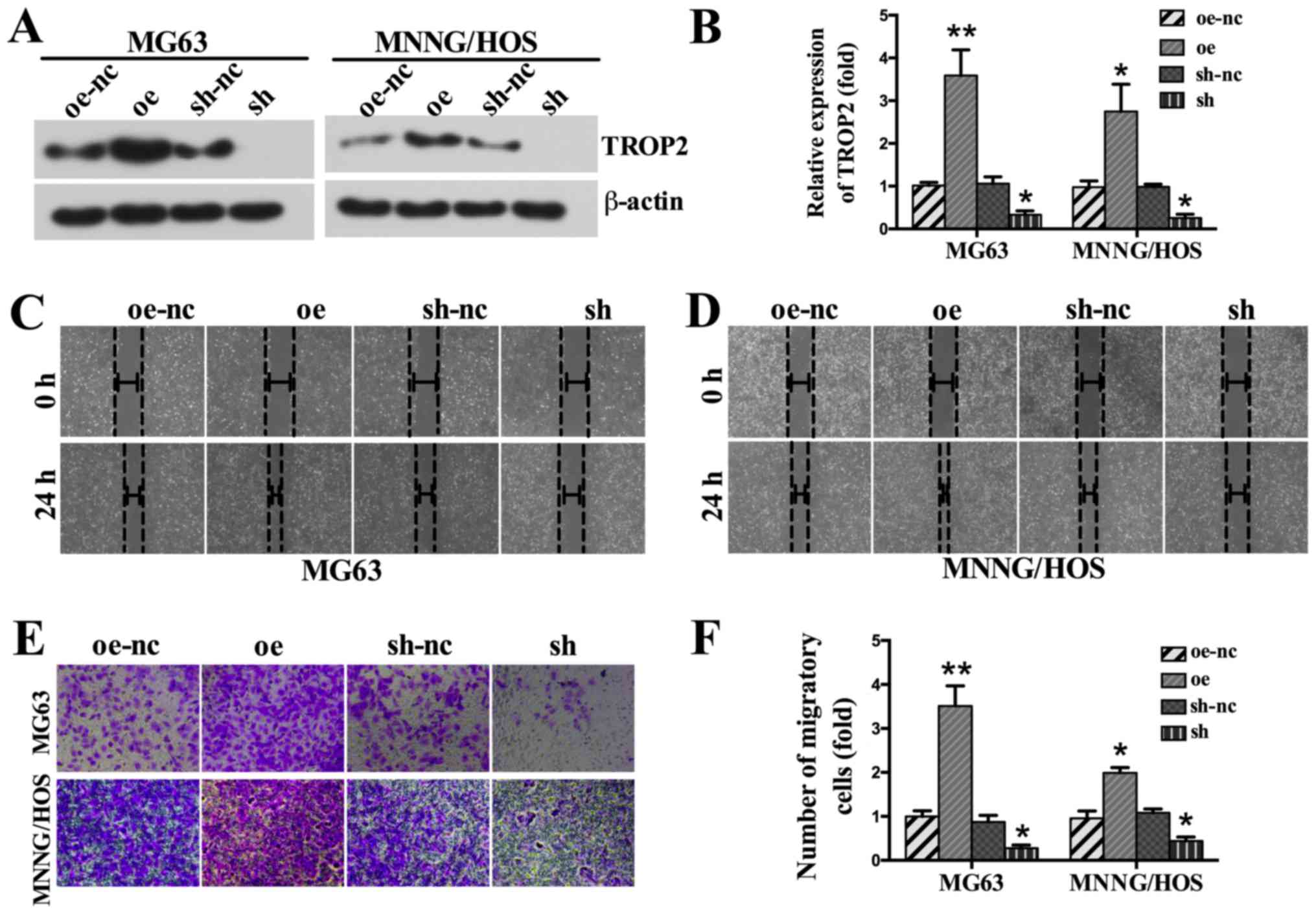 | Figure 4.Effects of TROP2 knockdown and
overexpression on the migration of OS cells. (A) Western blot
analysis was performed to (B) confirm the upregulation of TROP2
protein expression by lentiviral infection, and the downregulation
of TROP2 protein expression by shRNA in MG63 and MNNG/HOS cell
lines. A wound-healing assay was performed to assess migration in
the (C) MG63 and (D) MNNG/HOS cell lines (magnification, ×100). (E)
Transwell assays were performed to assess migration in the MG63 and
MNNG/HOS cell lines (magnification, ×200). (F) Relative
quantitative comparison of migratory cells. Images are
representative of three independent experiments. All data are
expressed as means ± standard deviation. *P<0.05 and **P<0.01
vs. oe-nc. TROP2, trophoblast cell surface antigen 2; OS,
osteosarcoma; Oe, overexpression of TROP2; sh, short hairpin RNA
knockdown of TROP2; nc, negative control; oe-nc, the negative
control group of TROP2 overexpression; sh-nc, the negative control
group of TROP2 knockdown using nc short hairpin RNA. |
TROP2 knockdown inhibits the
progression of OS by regulating the PI3K/AKT pathway
To explore the possible molecular mechanism
underlying the effects of TROP2 on OS cells, we detected the
activation of PI3K/AKT signaling pathway, which is a key regulator
of OS development. The result showed that TROP2 overexpression
significantly upregulated the levels of p-PI3K and p-AKT. In
addition, p-PI3K and p-AKT levels were decreased in TROP2-knockdown
cells (Fig. 5A).
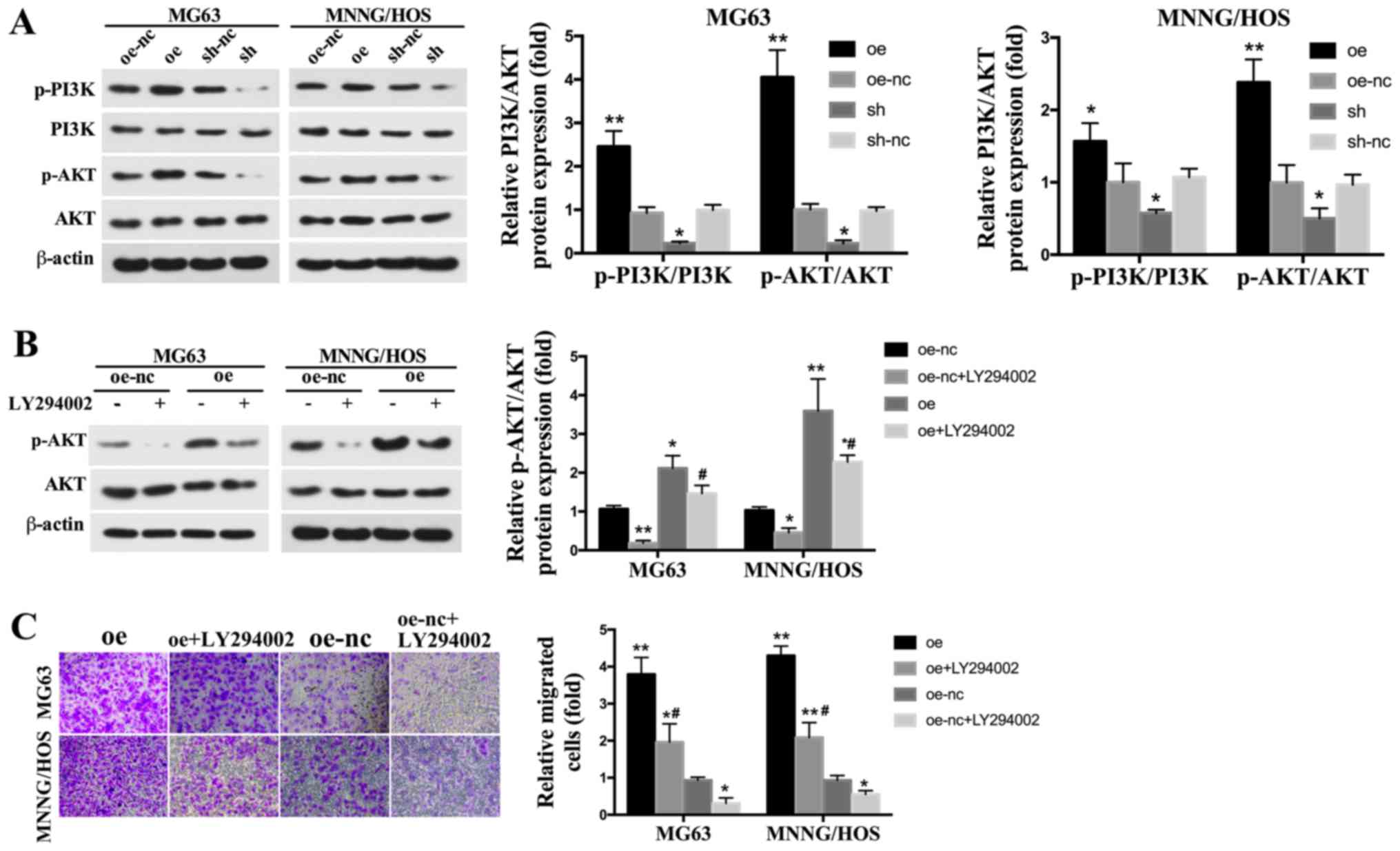 | Figure 5.Effects of TROP2 knockdown and
overexpression on the PI3K/AKT signaling pathway. (A) The protein
levels of p-PI3K, PI3K, p-AKT and AKT were assessed by western blot
analyses. (B) The effect of TROP2 overexpression on p-AKT
expression level following the application of LY294002. (C) The
effect of TROP2 overexpression on MG63 and MNNG/HOS cell migration
following the application of LY294002 (magnification, ×200).
*P<0.05 and **P<0.01 vs. oe-nc; #P<0.05 vs. oe
group. TROP2, trophoblast cell surface antigen 2; OS, osteosarcoma;
Oe, overexpression of TROP2; sh, short hairpin RNA knockdown of
TROP2; nc, negative control; oe-nc, the negative control group of
TROP2 overexpression; sh-nc, the negative control group of TROP2
knockdown using nc short hairpin RNA. |
Blocking the PI3K/AKT signaling
pathway rescues the increased cell proliferation and migration
induced by TROP2 overexpression
To verify the role of the PI3K/AKT signaling
pathway, LY294002, a specific PI3K/AKT inhibitor, was used to
inhibit p-AKT expression. The concentration of LY294002 used was 10
µM, based on previous studies (29–31).
LY294002 significantly inhibited p-AKT expression (Fig. 5B). The migration ability of OS
cells exposed to LY294002 were markedly decreased. The increased
migration induced by TROP2 overexpression were also reduced by
LY294002 exposure (Fig. 5C).
Discussion
Although outstanding advances in multiagent
chemotherapy and surgical technique have been achieved in recent
decades, the prognosis for OS patients with locally advanced or
metastatic remains poor (2–4).
Thus, there is an urgent need to research the molecular mechanisms
and deregulated genes responsible for OS carcinogenesis. In this
study, we found that TROP2 was significantly upregulated in human
OS tissues. Overexpression of TROP2 increased the proliferation and
migration of OS cells, while TROP2 knockdown significantly
decreased cell growth and migration. Moreover, upregulation of
TROP2 activated PI3K/AKT pathway. The increased migration observed
after TROP2 overexpression were rescued by inhibition of PI3K/AKT.
These novel findings indicate that TROP2 promotes OS cell
proliferation and migration through PI3K/AKT signaling.
Previous studies have linked TROP2 to increased
tumor growth and demonstrated TROP2 to be a candidate tumor
prognostic marker in various tumors. TROP2 is found to be
overexpressed in the majority of human epithelial cancers,
including pancreatic cancer, gastric cancer, lung cancer, ovarian
cancer, and colorectal cancers (10,14,32).
Chen et al (33)
investigated the expression of TROP2 and epithelial-mesenchymal
transition (EMT) indicator proteins in 93 patients with gallbladder
cancer (GBC) and demonstrated that increased expression of TROP2 in
GBC is associated significantly with aggressive progression and
poor prognosis. In another study based on clinical samples, Guan
et al (34) found that
overexpression of TROP2 could be an independent predictor for poor
clinical outcome in nasopharyngeal carcinoma. In cervical cancer
cell line CaSki cells, Liu et al (35) found that Trop2 knockdown
significantly inhibited the proliferation and colony formation of
CaSki cells, and demonstrated that TROP2 might become a novel
target for cervical cancer treatment. Similarly, in our study, IHC
revealed higher TROP2 expression in OS samples, suggesting that
high TROP2 expression may be associated with a poor prognosis.
Overexpression of TROP2 increased the proliferation and migration
of two different OS cell lines, indicating that high TROP2 levels
may promote the generation, development, and metastasis of OS.
The PI3K/AKT pathway plays a crucial role in
tumorigenesis. Previous studies have shown that alteration of the
PI3K/AKT pathway is strongly implicated in OS pathogenesis.
Moreover, studies have suggested that inhibition of the PI3K/AKT
pathway disrupts functions essential for OS progression (36,37).
Graziano et al (38) found
that Wilms' tumor gene 1 (WT1) silencing inhibits OS cell
proliferation by downregulating PI3K/AKT pathway. Huang and Jin
(39) reported that the activation
of the PI3K/AKT signal pathway is essential for the effects of zinc
finger transcription factor ZIC2 on OS cells, and the effects of
ZIC2 on the OS cells were reversed by a PI3K/AKT inhibitor.
Interestingly, a previous study indicated that TROP2 promotes
PI3K/AKT activation (40).
Considering the critical role of PI3K/AKT in cell proliferation and
migration, we assessed the levels of PI3K/AKT signaling pathway in
OS cells. Being consistent with previous studies (26,40,41),
TROP2 increased the expression of total p-PI3K and p-AKT via
PI3K/AKT pathway. Furthermore, the increased migration and
proliferation induced by TROP2 overexpression was rescued by a
PI3K/AKT inhibitor. Thus, we inferred that up-regulating TROP2
causes aberrant activation of PI3K/AKT in OS cells.
To the best of our knowledge, this is the first
study of the effect of TROP2 on OS cells. However, some limitations
of the present study should be noted. First, this was an in
vitro study without in vivo evaluation, which may
decrease the robustness of the results. Thus, further in
vivo application of TROP2-related treatment, such as molecular
targeted drug for TROP2 should be used to verify these findings.
Second, as most of the findings in this study were obtained from
MG63 and MNNG/HOS cells, more OS cell lines should be studied in
the future. Third, due to the small patient population size, we
have limited ability to reveal the correlation between TROP2
expression and the prognosis of OS. In addition, the carcinogenesis
of OS is complicated, which means TROP2 may also target other
signaling pathways, further studies are therefore required.
Nevertheless, our study provides useful insight into the effect of
TROP2 on cell proliferation and migration in OS cell lines. Taken
together, our data suggest that TROP2 promotes human OS cell
proliferation and migration via activation of PI3K/AKT pathway.
Acknowledgements
Not applicable.
Funding
The present study received financial support from
the Natural Science Foundation of China in Xinjiang Uygur
Autonomous Region (grant no. 2014211C034).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZT conceived and designed the present study. QG, AN
and XG performed the experiments and were also the predominant
contributors to the writing of the manuscript. KT and CL analyzed
and interpreted the data. XF made contributions to the
interpretation of data and critically revised the manuscript for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of The First Affiliated Hospital of Xinjiang Medical
University (Xinjiang, China). Written informed consent was obtained
from each patient.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zambo I and Vesely K: WHO classification
of tumours of soft tissue and bone 2013: The main changes compared
to the 3rd edition. Cesk Patol. 50:64–70. 2014.(In Czech).
PubMed/NCBI
|
|
2
|
Hu K, Wang Z, Lin P, Wen Z, Ren H, Sun L,
Li H, Li B, Wang S, Zhou X, et al: Three hematological indexes that
may serve as prognostic indicators in patients with primary,
high-grade, appendicular osteosarcoma. Oncotarget. 8:43130–43139.
2017.PubMed/NCBI
|
|
3
|
Li GL, Wu YX, Li YM and Li J: High
expression of long non-coding RNA XIST in osteosarcoma is
associated with cell proliferation and poor prognosis. Eur Rev Med
Pharmacol Sci. 21:2829–2834. 2017.PubMed/NCBI
|
|
4
|
Wu Y, Wu J, Dong QR and Guo NZ:
Association between expression of nuclear receptor co-activator 5
protein and prognosis in postoperative patients with osteosarcoma.
Oncol Lett. 15:1888–1892. 2018.PubMed/NCBI
|
|
5
|
Tao Y, Xin M, Cheng H, Huang Z, Hu T,
Zhang T and Wang J: TRIM37 promotes tumor cell proliferation and
drug resistance in pediatric osteosarcoma. Oncol Lett.
14:6365–6372. 2017.PubMed/NCBI
|
|
6
|
Lu J, Song G, Tang Q, Yin J, Zou C, Zhao
Z, Xie X, Xu H, Huang G, Wang J, et al: MiR-26a inhibits stem
cell-like phenotype and tumor growth of osteosarcoma by targeting
Jagged1. Oncogene. 36:231–241. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu X, Zhou X, Xu H, He Z, Shi X and Wu S:
SLC34A2 regulates the proliferation, migration, and invasion of
human osteosarcoma cells through PTEN/PI3K/AKT signaling. DNA Cell
Biol. 36:775–780. 2017.PubMed/NCBI
|
|
8
|
Cao J, Han X, Qi X, Jin X and Li X: TUG1
promotes osteosarcoma tumorigenesis by upregulating EZH2 expression
via miR-144-3p. Int J Oncol. 51:1115–1123. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao YX, Wang YS, Cai QQ, Wang JQ and Yao
WT: Up-regulation of HDAC9 promotes cell proliferation through
suppressing p53 transcription in osteosarcoma. Int J Clin Exp Med.
8:11818–11823. 2015.PubMed/NCBI
|
|
10
|
Kong JS, Kim HJ, Kim MJ, Kim A, Lee D, Han
K, Park S, Koh JS and Myung JK: The significance of TROP2
expression in predicting braf mutations in papillary thyroid
carcinoma. J Pathol Transl Med. 52:14–20. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao W, Zhu H, Zhang S, Yong H, Wang W,
Zhou Y, Wang B, Wen J, Qiu Z, Ding G, et al: Trop2 is overexpressed
in gastric cancer and predicts poor prognosis. Oncotarget.
7:6136–6145. 2016.PubMed/NCBI
|
|
12
|
Shvartsur A and Bonavida B: Trop2 and its
overexpression in cancers: Regulation and clinical/therapeutic
implications. Genes Cancer. 6:84–105. 2015.PubMed/NCBI
|
|
13
|
Lin H, Zhang H, Wang J, Lu M, Zheng F,
Wang C, Tang X, Xu N, Chen R, Zhang D, et al: A novel human Fab
antibody for Trop2 inhibits breast cancer growth in vitro and in
vivo. Int J Cancer. 134:1239–1249. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mao Y, Wang X, Zheng F, Wang C, Tang Q,
Tang X, Xu N, Zhang H, Zhang D, Xiong L, et al: The
tumor-inhibitory effectiveness of a novel anti-Trop2 Fab conjugate
in pancreatic cancer. Oncotarget. 7:24810–24823. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fong D, Moser P, Krammel C, Gostner JM,
Margreiter R, Mitterer M, Gastl G and Spizzo G: High expression of
TROP2 correlates with poor prognosis in pancreatic cancer. Br J
Cancer. 99:1290–1295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mühlmann G, Spizzo G, Gostner J, Zitt M,
Maier H, Moser P, Gastl G, Zitt M, Müller HM, Margreiter R, et al:
TROP2 expression as prognostic marker for gastric carcinoma. J Clin
Pathol. 62:152–158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Inamura K, Yokouchi Y, Kobayashi M,
Ninomiya H, Sakakibara R, Subat S, Nagano H, Nomura K, Okumura S,
Shibutani T and Ishikawa Y: Association of tumor TROP2 expression
with prognosis varies among lung cancer subtypes. Oncotarget.
8:28725–28735. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pak MG, Shin DH, Lee CH and Lee MK:
Significance of EpCAM and TROP2 expression in non-small cell lung
cancer. World J Surg Oncol. 10:532012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu B, Yu C, Zhou B, Huang T, Gao L, Liu T
and Yang X: Overexpression of TROP2 promotes proliferation and
invasion of ovarian cancer cells. Exp Ther Med. 14:1947–1952. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ohmachi T, Tanaka F, Mimori K, Inoue H,
Yanaga K and Mori M: Clinical significance of TROP2 expression in
colorectal cancer. Clin Cancer Res. 12:3057–3063. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sukhthankar M, Alberti S and Baek SJ:
(−)-Epigallocatechin-3-gallate (EGCG) post-transcriptionally and
post-translationally suppresses the cell proliferative protein
TROP2 in human colorectal cancer cells. Anticancer Res.
30:2497–2503. 2010.PubMed/NCBI
|
|
22
|
Cubas R, Zhang S, Li M, Chen C and Yao Q:
Trop2 expression contributes to tumor pathogenesis by activating
the ERK MAPK pathway. Mol Cancer. 9:2532010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fang YJ, Lu ZH, Wang GQ, Pan ZZ, Zhou ZW,
Yun JP, Zhang MF and Wan DS: Elevated expressions of MMP7, TROP2,
and survivin are associated with survival, disease recurrence, and
liver metastasis of colon cancer. Int J Colorectal Dis. 24:875–884.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guan H, Guo Z, Liang W, Li H, Wei G, Xu L,
Xiao H and Li Y: Trop2 enhances invasion of thyroid cancer by
inducing MMP2 through ERK and JNK pathways. BMC Cancer. 17:4862017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chiang SF, Kan CY, Hsiao YC, Tang R, Hsieh
LL, Chiang JM, Tsai WS, Yeh CY, Hsieh PS, Liang Y, et al: Bone
marrow stromal antigen 2 is a novel plasma biomarker and
prognosticator for colorectal carcinoma: A secretome-based
verification study. Dis Markers. 2015:8740542015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang J, Zhu Z, Wang H, Li F, Du X and Ma
RZ: Trop2 regulates the proliferation and differentiation of murine
compact-bone derived MSCs. Int J Oncol. 43:859–867. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eisenwort G, Jurkin J, Yasmin N, Bauer T,
Gesslbauer B and Strobl H: Identification of TROP2 (TACSTD2), an
EpCAM-like molecule, as a specific marker for TGF-β1-dependent
human epidermal Langerhans cells. J Invest Dermatol. 131:2049–2057.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wen ZQ, Li XG, Zhang YJ, Ling ZH and Lin
XJ: Osteosarcoma cell-intrinsic colony stimulating factor-1
receptor functions to promote tumor cell metastasis through JAG1
signaling. Am J Cancer Res. 7:801–815. 2017.PubMed/NCBI
|
|
29
|
Gong C, Liao H, Wang J, Lin Y, Qi J, Qin
L, Tian LQ and Guo FJ: LY294002 induces G0/G1 cell cycle arrest and
apoptosis of cancer stem-like cells from human osteosarcoma via
down-regulation of PI3K activity. Asian Pac J Cancer Prev.
13:3103–3107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han G, Wang Y and Bi W: C-Myc
overexpression promotes osteosarcoma cell invasion via activation
of MEK-ERK pathway. Oncol Res. 20:149–156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Y, Cheng Z, Pan F and Yan W:
MicroRNA-373 promotes growth and cellular invasion in osteosarcoma
cells by activation of the PI3K/AKT-Rac1-JNK pathway: The potential
role in spinal osteosarcoma. Oncol Res. 25:989–999. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Son S, Shin S, Rao NV, Um W, Jeon J, Ko H,
Deepagan VG, Kwon S, Lee JY and Park JH: Anti-Trop2
antibody-conjugated bioreducible nanoparticles for targeted triple
negative breast cancer therapy. Int J Biol Macromol. 110:406–415.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen MB, Wu HF, Zhan Y, Fu XL, Wang AK,
Wang LS and Lei HM: Prognostic value of TROP2 expression in
patients with gallbladder cancer. Tumour Biol. 35:11565–11569.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guan GF, Zhang DJ, Wen LJ, Yu DJ, Zhao Y,
Zhu L, Guo YY and Zheng Y: Prognostic value of TROP2 in human
nasopharyngeal carcinoma. Int J Clin Exp Pathol. 8:10995–11004.
2015.PubMed/NCBI
|
|
35
|
Liu X, Li S and Yi F: Trop2 gene: A novel
target for cervical cancer treatment. J Cancer Res Clin Oncol.
140:1331–1341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma J, Huang H, Han Z, Zhu C and Yue B:
RLN2 Is a positive regulator of AKT-2-induced gene expression
required for osteosarcoma cells invasion and chemoresistance.
Biomed Res Int. 2015:1474682015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang H, Luo QF, Peng AF, Long XH, Wang TF,
Liu ZL, Zhang GM, Zhou RP, Gao S, Zhou Y and Chen WZ: Positive
feedback regulation between Akt phosphorylation and fatty acid
synthase expression in osteosarcoma. Int J Mol Med. 33:633–639.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Graziano AC, Cardile V, Avola R, Vicario
N, Parenti C, Salvatorelli L, Magro G and Parenti R: Wilms' tumor
gene 1 silencing inhibits proliferation of human osteosarcoma MG-63
cell line by cell cycle arrest and apoptosis activation.
Oncotarget. 8:13917–13931. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang S and Jin A: ZIC2 promotes viability
and invasion of human osteosarcoma cells by suppressing SHIP2
expression and activating PI3K/AKT pathways. J Cell Biochem.
119:2248–2257. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li X, Teng S, Zhang Y, Zhang W, Zhang X,
Xu K, Yao H, Yao J, Wang H, Liang X and Hu Z: TROP2 promotes
proliferation, migration and metastasis of gallbladder cancer cells
by regulating PI3K/AKT pathway and inducing EMT. Oncotarget.
8:47052–47063. 2017.PubMed/NCBI
|
|
41
|
Lin JC, Wu YY, Wu JY, Lin TC, Wu CT, Chang
YL, Jou YS, Hong TM and Yang PC: TROP2 is epigenetically
inactivated and modulates IGF-1R signalling in lung adenocarcinoma.
EMBO Mol Med. 4:472–485. 2012. View Article : Google Scholar : PubMed/NCBI
|