Introduction
Osteosarcoma is a common type of primary malignant
bone tumor in children and adolescents (1). Previously, the treatment of
osteosarcoma was primarily amputation; however, chemotherapeutic
drugs containing Adriamycin, methotrexate, ifosfamide and cisplatin
(2,3), surgery and radiotherapy have
increased the 5-year survival rate of patients to ~70% (4). However, high local recurrence rate,
early distant metastasis and poor chemotherapy sensitivity provide
large mental and economic burdens to patients and their families
(5). Therefore, it is important to
investigate the molecular mechanism of biological activity of
osteosarcoma.
A number of genes associated with tumor invasion and
metastasis have been reported in the investigation into the
occurrence, development and metastasis of osteosarcoma (6,7). In
particular, the discovery of microRNA (miRNA) provides a novel
direction for the study of osteosarcoma gene regulation. miRNA is a
small non-coding RNA molecule (containing ~22 nucleotides), which
inhibits the translation of target mRNA and is involved in the
regulation of protein biosynthesis at the post-transcriptional
level via the complete and incomplete complementation of the
terminal 3′untranslated region of a downstream gene. A previous
study demonstrated that ~3% of human genes encode miRNAs and
>30% human genes may be regulated by miRNA (8). Abnormal miRNA structure or expression
can cause the occurrence of tumors. Studies have revealed that a
variety of miRNAs exert different effects as oncogenes or tumor
suppressor genes on osteosarcoma; miR-32, miR-29b, miR-145 and
miR-194 exhibit an inhibitory effect (9–13),
but miR-27a and miR-128 exert promoting effects on tumor
proliferation, metastasis and invasion (14,15).
MiR-184, as a novel miRNA in recent years, exhibits abnormal
expression in numerous tumors, including hepatocellular carcinoma
(miR-184 functions as an oncogenic regulator in hepatocellular
carcinoma) and renal cell carcinoma (microRNA-184 functions as
tumor suppressor in renal cell carcinoma), and serves carcinogenic
or anticancer roles (16–20). A recent study verified the
promoting effect of miR-184 in proliferation and invasion of
osteosarcoma, but the precise mechanism remains unknown (21).
The Wnt signaling pathways are a group of signal
transduction pathways that transfer extracellular signals to cells
(22). The Wnt/β-catenin signaling
pathway is one of three Wnt signaling pathways and can
phosphorylate disheveled (Dv1) protein in the cytoplasm by binding
to Wnt proteins (23). Thus,
β-catenin is free from the Axin/adenomatous polypolis
coli/casein kinase 1α/glycogen synthase kinase 3 β/β-catenin
complex, and enters into the nucleus, binds to transcription factor
lymphoid enhancer-binding factor/T-cell factor in the nucleus, and
finally activates downstream target genes c-Myc and cyclin D1
(24). Abnormal initiation of the
Wnt/β-catenin signaling pathway may lead to unlimited cell
proliferation, thereby leading to tumorigenesis (25). Activation of the Wnt signaling
pathway regulates osteosarcoma invasion and metastasis (26), and interferes with Wnt/β-catenin
signals and inhibits growth and metastasis of osteosarcoma cells
(27). However, scholars have also
reported opposing findings that the activation of Wnt/β-catenin
signaling pathway suppresses osteosarcoma cell proliferation
(28). MiRNA regulation of the
Wnt/β-catenin signaling pathway contains two categories: Activation
and inhibition, involving 29 miRNAs, including miR-344 and miR-181,
as well as 18 miRNAs that inhibit the Wnt signaling pathway,
including miR-148a and miR-496 (29). However, its mechanism in the
occurrence and development of osteosarcoma remains unclear. The
present study was designed to investigate the effects of miR-184 on
osteosarcoma growth, development and metastasis, and to further
explore the effect of miR-184 on proliferation, invasion and
metastasis of osteosarcoma cells via regulation of the Wnt
signaling pathway.
Materials and methods
Culture of human osteosarcoma U-2OS
cells and 143B cells
Human osteosarcoma U-2OS cells and 143B cells were
purchased from the Type Culture Collection of the Chinese Academy
of Sciences (Shanghai, China). U-2OS cells were incubated with
RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), and 143B cells were incubated with Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) at
37°C in a 5% CO2 incubator. In addition, 0.25%
EDTA-trypsin and PBS were purchased from Gibco (Thermo Fisher
Scientific, Inc.).
MiR-184 transfection into U-2OS cells
and 143B cells
MiR-184 analogue sequence,
5′-GGCAUUCUGUAUACAUCGGAG-3′ and miR-184 inhibitor sequence,
5′-GAUCGGAGGUGCAUUCUA-3′ were synthesized by Shanghai GenePharma
Co., Ltd. (Shanghai, China) following the manufacturer's protocol.
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used for transfection. A total of
1×106 infection units/ml lentivirus (miR-184) were used
for transfection. Cells were washed three times with PBS and
incubated with RPMI-1640 and DMEM at 37°C in a 5% CO2
incubator for 6 h, and were then used for subsequent analysis.
U-2OS and 143B cells transfected with empty vectors (Shanghai
GenePharma Co., Ltd.) were used as control groups.
MiR-184 expression levels detected by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) prior to and post-transfection
RNA was extracted from U-2OS and 143B cells using
TRIzol reagent (Thermo Fisher Scientific, Inc.). To generate cDNA,
RT was performed using a Mir-X™ miRNA First-Strand Synthesis kit
(cat. no. 638313; Takara Biotechnology Co., Ltd., Dalian, China),
according to the manufacturer's protocol. An SYBR-Green Master mix
(RR820A; Takara Biotechnology Co., Ltd., Dalian, China) was used
for qPCR. All primers were designed and synthesized with Primer
Premier 6.0 (Premier Biosoft International, Palo Alto, CA, USA).
Primer sequences were as follows: MiR-184 sense,
5′-TACGACTATGTGACCTGCCTG-3′ and antisense,
5′-TGGTTCAACTCTCCTTTCCA-3′; U6 (sequence unavailable). The reaction
conditions were: 95°C for 30 sec, 95°C for 5 sec, 60°C for 30 sec,
and the fluorescence signal was detected from 40 cycles. MiR-184
expression levels were calculated using the 2−ΔΔCq
method (30). MiR-184 expression
levels were detected in the control group, miR-184 analogue group
and miR-184 inhibitor group.
Cell viability measured via an MTT
assay
U-2OS cells and 143B cells in the logarithmic phase
were seeded on a 96-well plate, 4,000 cells/well, at 37°C and 5%
CO2 for 24 h. Until stable growth, cells were harvested
at 12, 24 and 36 h. A total of 20 µl MTT (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) was added in each well for 4 h at 37°C.
Following the removal of the medium, 100 µl dimethyl sulfoxide was
added to each well (Sigma-Aldrich; Merck KGaA) and agitated at a
low speed for 10 min. The absorbance value of each well was
measured at 570 nm with a microplate reader. The effect on cell
proliferation was determined in the control group, miR-184 analogue
group and miR-184 inhibitor group. Experiments were conducted in
triplicate and each group had six parallel wells.
Cell invasion detected by Transwell
assay
A Transwell assay (Hyclone; GE Healthcare, Chicago,
IL, USA) was used to detect cell invasion. The Transwell chamber
polycarbonate film was coated with 100 µl diluted Matrigel. A total
of 100 µl cell suspension and 200 µl serum-free RPMI-1640 (for
U-2OS cells) and DMEM (for 143B cells) were added to the upper
chamber. A total of 500 µl RPMI-1640 and DMEM containing 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) was added to
the lower chamber. Following incubation for 24 and 48 h at 37°C,
samples were fixed with anhydrous ethanol for 60 min at 4°C,
stained with 0.1% crystal violet for 30 min at room temperature,
and then analyzed with a microscope (NE950; Leica Microsystems,
Inc., Buffalo Grove, IL, USA). A total of 5 fields of view were
observed. The effect on cell invasion was determined in the control
group, miR-184 analogue group and miR-184 inhibitor group.
Experiments were performed in triplicate and the average value was
calculated.
Animals
A total of 36, 4-week-old female Balb/c nude mice
(23–25 g) were purchased from the Experimental Animal Department of
China Medical University (Shenyang, China). Mice were provided with
access to food and water ad libitum, and the housing
conditions were as follows: A temperature of 20–26°C, a 40–70%
relative humidity and a 12/12 h light/dark cycle. The present study
was approved by the China Medical University Institutional Animal
Care and Use Committee (IACUC no. 2016024).
Establishment of nude mouse models of
tibial orthotopic metastasis from osteosarcoma
Osteosarcoma U-2OS cells and 143B cells in the
logarithmic phase were used to establish nude mouse models of
tibial orthotopic metastasis. A total of 36 nude mice were randomly
assigned to six experimental groups (six mice per group): i) U-2OS
cells transfected with empty vectors (control), ii) 143B cells
transfected with empty vectors (control), iii) U-2OS cells
transfected with miR-184 analogues, iv) 143B cells transfected with
miR-184 analogues, v) U-2OS cells transfected with miR-184
inhibitors and vii) 143B cells transfected with miR-184 inhibitors.
The cell density was 2×106/ml; 30 µl cell suspension was
infused into the bone marrow cavity of the right proximal tibia of
nude mice of each group. The growth of orthotopic metastatic tumor
was observed following injection every other day for 28 days.
Tumor growth rate and tumor
volume
A total of 2 weeks post-orthotopic transplantation,
nude mice bearing tumors were anesthetized with 1% pentobarbital
via intraperitoneal injection (45 mg/kg) and then euthanized by
cervical dislocation. Following dissection, tumor growth and volume
were grossly observed. The long and short diameters of the
transplanted tumor were calculated, and tumor volume was calculated
in each group according to Steel formula: V=1/2a × b × b (‘V’
represents the volume, ‘a’ represents the length, and ‘b’
represents the width) (31). Tumor
volume was compared among different groups.
Analysis of expression levels of Wnt
and β-catenin protein in tumor tissue via western blotting
Tumor tissue of nude mice with metastatic tumors was
collected from the control groups, the miR-184 analogue group and
the miR-184 inhibitor group. Total protein was extracted from tumor
tissues using radioimmunoprecipitation assay lysate buffer (Thermo
Fisher Scientific, Inc.) and a bicinchoninic acid kit (BD
Biosciences) was used to quantify protein. Protein samples (20 µg)
were subjected to 10% SDS-PAGE and then transferred onto
polyvinylidene fluoride membranes. The membranes were blocked with
5% non-fat milk powder for 2 h at room temperature, and incubated
with primary antibodies at 4°C overnight. Primary antibodies were
as follows: Anti-Wnt (cat. no. ab15251; 1:1,000), anti-β-catenin
(cat. no. ab32572; 1:3,000), anti-phosphorylated-β-catenin (cat.
no. ab27798; 1:500) and anti-GAPDH (cat. no. ab181602; 1:1,000; all
Abcam, Cambridge, UK). Secondary antibodies (cat. no. bs-0295G;
1:1,000; BIOSS, Beijing, China) were added and incubated with
membranes at room temperature for 2 h. Samples were detected with
enhanced chemiluminescence (Amersham; GE Healthcare). The gray
value was measured using Quantity One software (version 4.62;
Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Expression levels of Wnt and β-catenin
mRNA in tumor tissue of nude mice detected by RT-qPCR
RT-qPCR was employed to determine β-catenin mRNA
expression levels in control groups, miR-184 analogue groups and
miR-184 inhibitor groups. RNA was extracted from the tumor tissues
using TRIzol reagent (Thermo Fisher Scientific, Inc.). cDNA was
synthesized using a High-Capacity RNA-to-cDNA™ kit (Invitrogen;
Thermo Fisher Scientific, Inc.). A SYBR® Premix Ex
Taq™ II (cat. no. RR820A; Takara Biotechnology Co. Ltd.)
qPCR kit was used for detection. All primers were designed and
synthesized with Primer Premier 6.0 (Premier Biosoft
International). Primer sequences were as follows: Wnt sense,
5′-CCCGACGCTTCGCTTGAAT-3′, and antisense,
5′-GACCGCTGGAGGTGCCATG-3′; β-catenin sense,
5′-GAGCTGCCATGTTCCCTGAG-3′ and antisense,
5′-CAGTTGTCAATTTGATTAAC-3′; and GAPDH sense,
5′-ATCTGGAGTTTACCGCTGG-3′ and anti-sense,
5′-TACCGATGTCTGGTAGACGAT-3′. The thermocycling conditions were as
follows: Initial denaturation at 95°C for 30 sec; followed by 40
cycles of annealing and elongation at 95°C for 5 sec and 60°C for
30 sec. Wnt and β-catenin mRNA expression levels were
quantitatively analyzed using the 2−ΔΔCq method
(30).
Statistical analysis
Data were analyzed using SPSS 19.0 software (IBM
Corp., Armonk, NY, USA). Measurement data are expressed as the mean
± standard deviation. Comparisons between groups were made using a
Student's t-test (two-tailed) or analysis of variance with a
Tukey's post hoc test or Kruskal-Wallis test. P<0.05 was
considered to indicate a statistically significant difference.
Results
MiR-184 expression levels in U-2OS and
143B cell lines
MiR-184 analogue and inhibitor were transfected into
U-2OS cells and 143B cells using Lipofectamine® 2000.
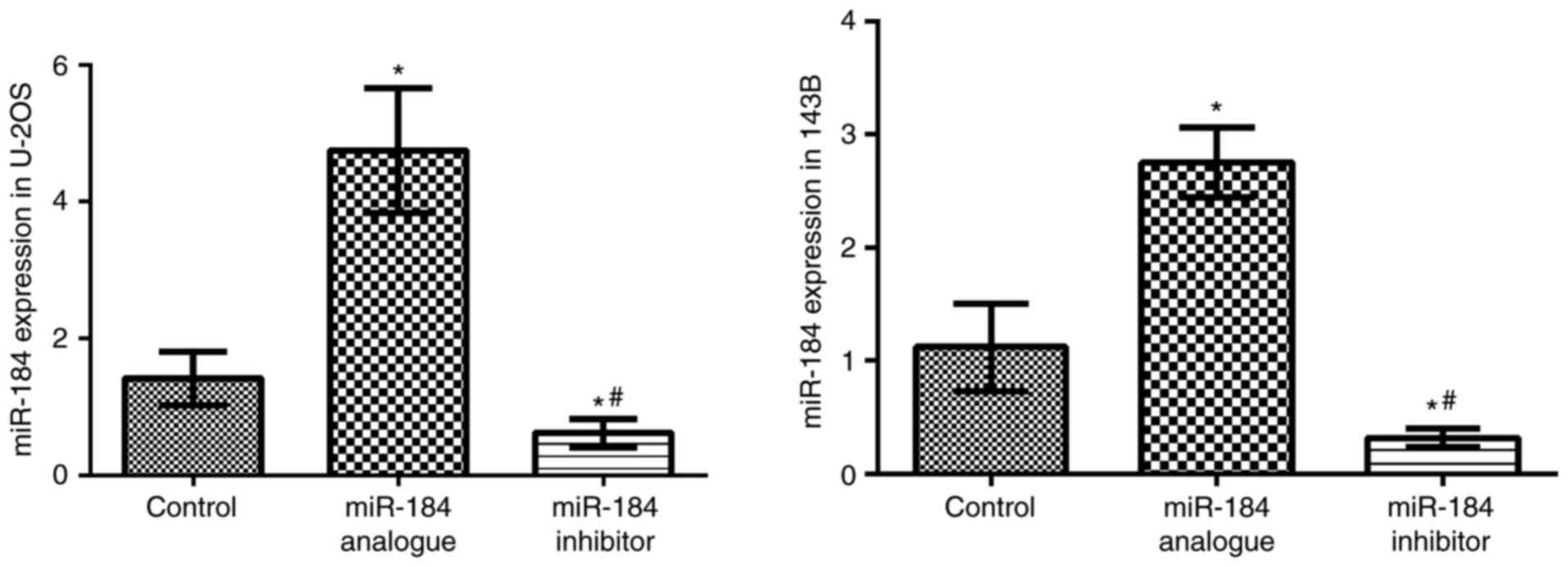
RT-qPCR was applied to detect miR-184 expression levels. Compared
with the control groups, miR-184 expression levels were
significantly higher in the miR-184 analogue groups (P<0.05),
but significantly lower in the miR-184 inhibitor groups (P<0.05;
Fig. 1). This result suggests that
miR184 was successfully transfected into both cell lines.
Effects of miR-184 on U-2OS and 143B
cell viability
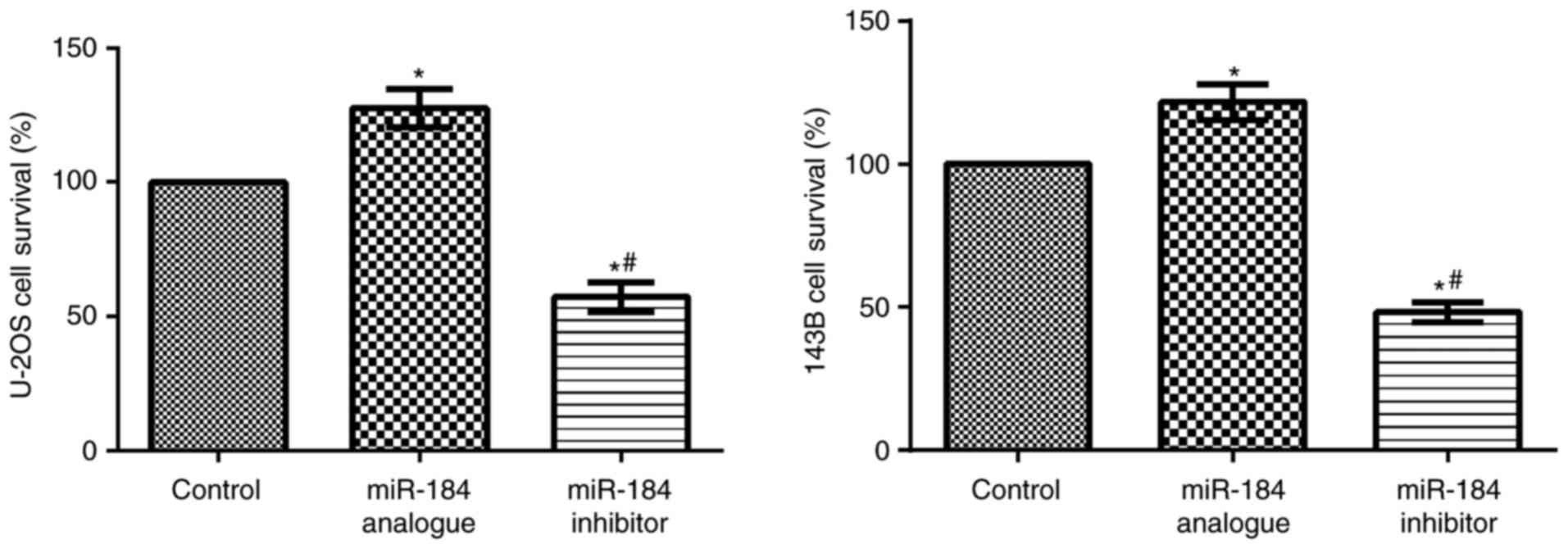
An MTT assay was used to determine cell
proliferation. Compared with the control groups, U-2OS cell and
143B cell proliferation were significantly increased in the
miR-184 analogue groups (P<0.05), but significantly reduced in
the miR-184 inhibitor groups (P<0.05; Fig. 2). This result demonstrated that
transfection with miR-184 enhances the stability of both cell
lines.
Effects of miR-184 on U-2OS and 143B
cell invasion
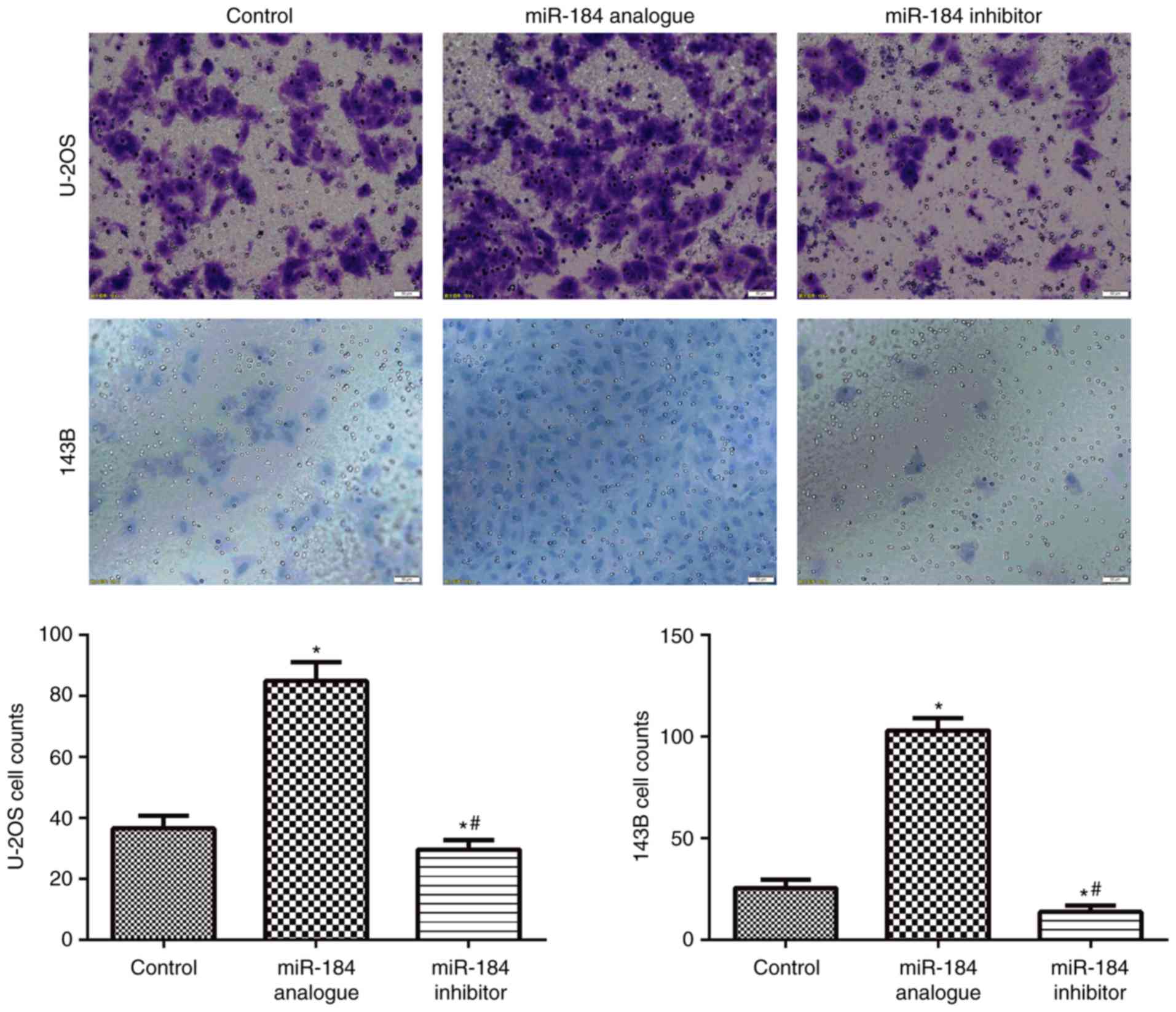
A Transwell assay was used to determine cell
invasion ability. Compared with the control groups, cell invasion
ability was significantly increased in the miR-184 analogue groups
(P<0.05), but significantly reduced in the miR-184 inhibitor
groups (P<0.05; Fig. 3). This
result suggested that miR-184 enhances the invasion ability of
U-2OS and 143B cells.
Effects of miR-184 on the growth of
tibial orthotopic metastasis from osteosarcoma in nude mouse
models
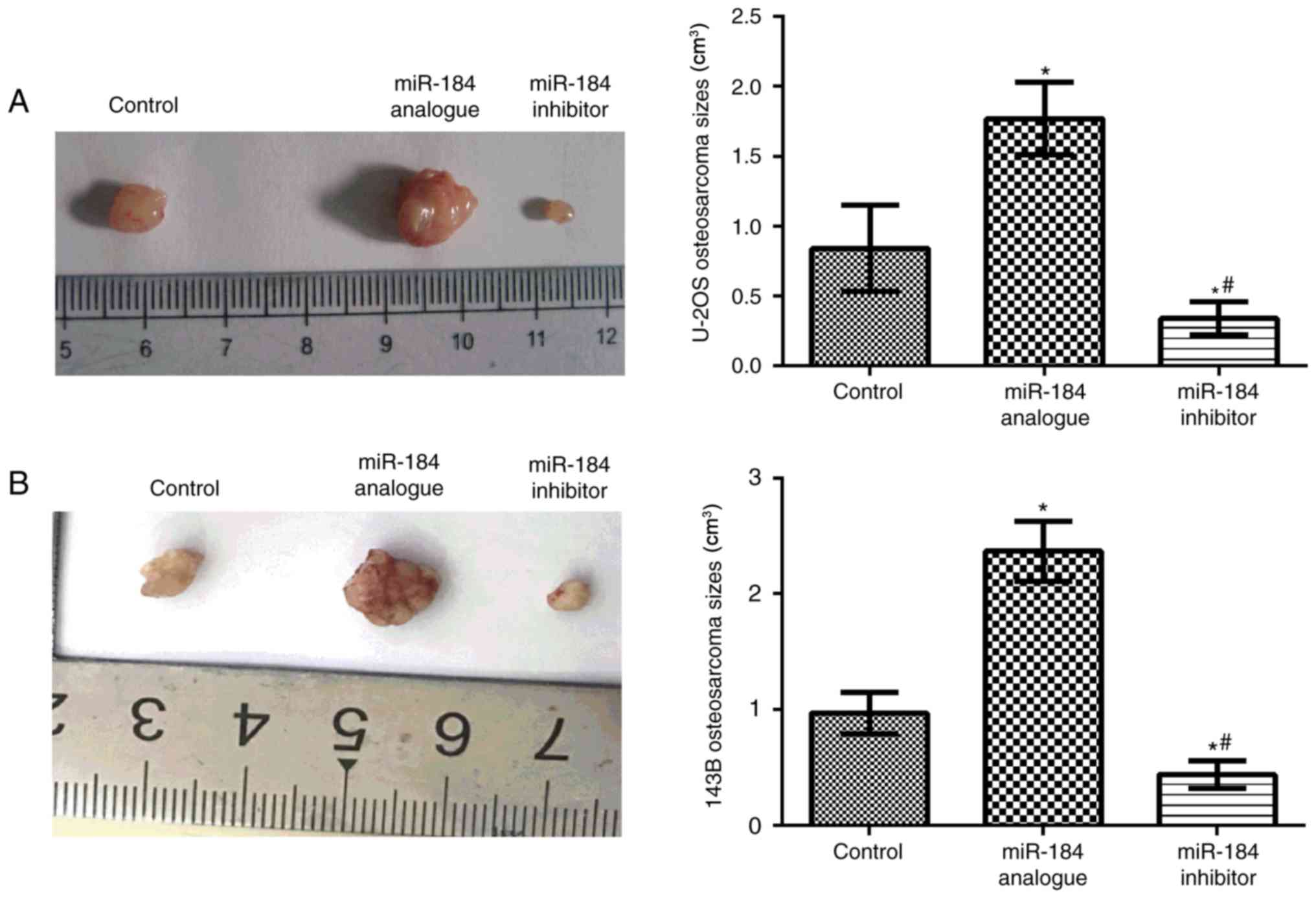
In vivo analysis demonstrated that compared
with the control groups, tumor volume significantly increased in
the miR-184 analogue groups in both cell lines (P<0.05), while
tumor volume was significantly decreased within the miR-184
inhibitor groups (P<0.05; Fig.
4). These results suggested that miR-184 promotes tumor growth
in nude mice.
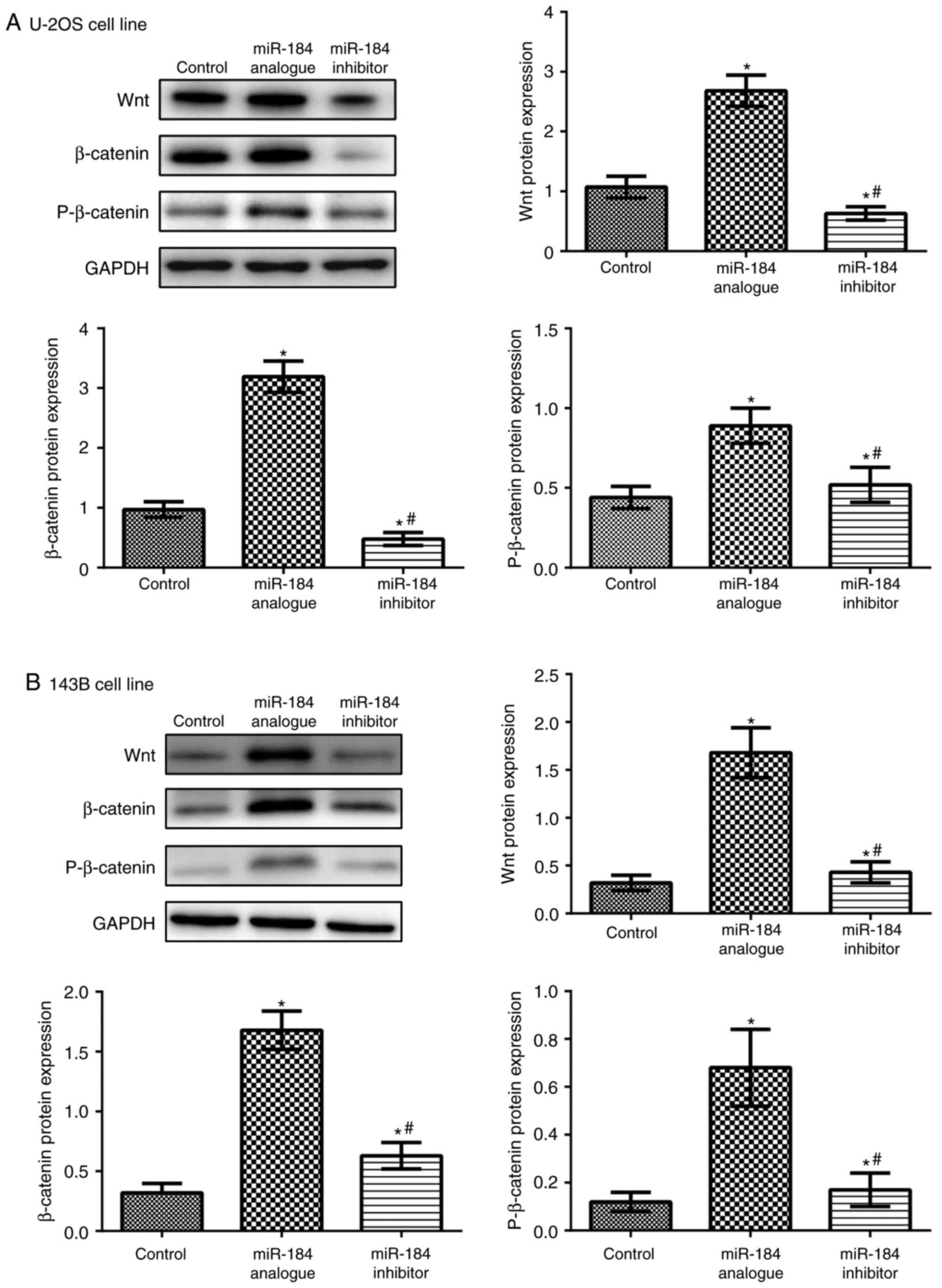
Wnt and β-catenin protein expression
levels in nude mouse models of tibial orthotopic metastasis from
osteosarcoma as detected by western blotting
Compared with the control groups, Wnt, β-catenin and
phosphorylated β-catenin levels were significantly increased in the
miR-184 analogue groups in both cell lines (P<0.05), but
significantly decreased in the miR-184 inhibitor groups (P<0.05;
Fig. 5). These results
demonstrated that miR-184 may positively regulate Wnt signaling
pathway-associated protein expression levels. Furthermore, the
results suggest that miR184 regulates the Wnt signaling pathway to
promote tumor growth.
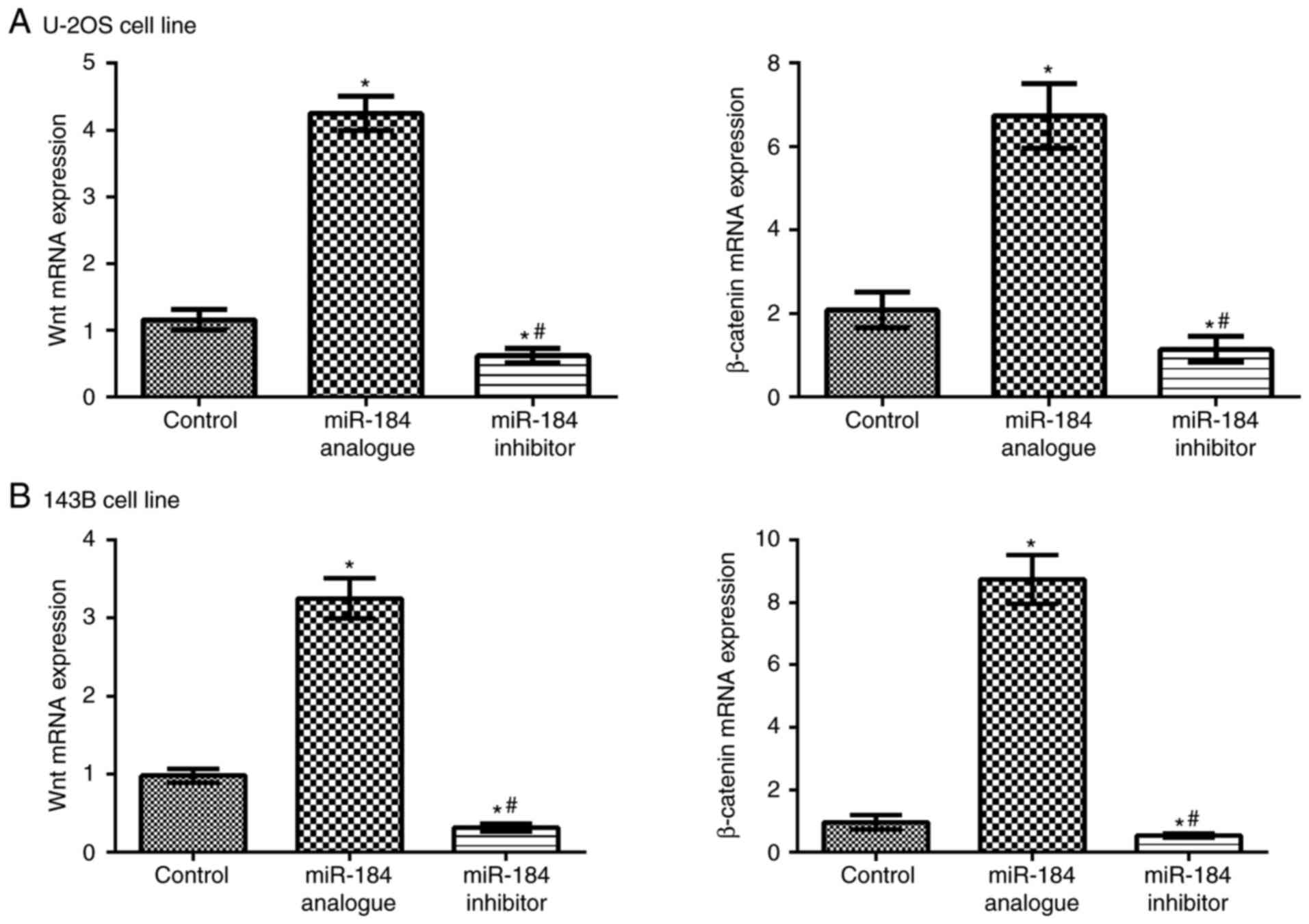
Wnt and β-catenin mRNA expression
levels in nude mouse models of tibial orthotopic metastasis from
osteosarcoma as measured by RT-qPCR
Wnt and β-catenin mRNA expression levels were
determined in the metastatic tumors of nude mice in the control,
miR-184 analogue and miR-184 inhibitor groups. Overexpressed
miR-184 could significantly upregulate Wnt and β-catenin mRNA
expression levels compared with the control groups (P<0.05).
Following the inhibition of miR-184 expression, Wnt and β-catenin
mRNA expression levels were significantly decreased compared with
the control (P<0.05; Fig. 6).
These findings indicated that miR-184 may positively regulate Wnt
and β-catenin mRNA expression levels. These results further suggest
that miR-184 promotes tumor growth via the regulation of the Wnt
signaling pathway.
Discussion
Osteosarcoma is the most common type of malignant
bone tumor, which is characterized by a high degree of malignancy,
distant metastasis easily occurring in the early stage, and poor
prognosis in adults, adolescents and children (32). It was recently reported that
osteosarcoma accounts for 3–5% cases of cancer in children and
adolescents, and the pathogenesis had significant heterogeneous
genetic characteristics (33).
Therefore, investigating and identifying the pathogenesis of
osteosarcoma is of great significance and may provide a novel
target for treatment.
MiRNAs, as endogenous small molecule RNAs, are
widely involved in the development and progression of numerous
tumors. Numerous miRNAs are implicated in the diagnosis, treatment
and prognosis of osteosarcoma (34,35).
In highly aggressive osteosarcoma cell lines, miR-199b-5p and
miR-100-3p expression levels were downregulated, but miR-155b-5p,
miR-135-5p and miR-146a-5p expression levels were upregulated;
among them, miR-135-5p and miR-146a-5p were strongly associated
with tumor cell invasion and metastasis (36). Further study on the role of miRNA
in osteosarcoma may clarify the mechanism of miRNA involved in the
proliferation and invasion of osteosarcoma cells and provide a
novel method for the treatment of osteosarcoma.
MiR-184, as a novel member of the miRNA family, is
involved in the development and metastasis of osteosarcoma. Lin
et al (37) suggested that
upregulated miR-184 may induce chemotherapy resistance by mediating
B-cell lymphoma-1 like 1 protein expression. The present study
reported that in vitro proliferation and invasive ability of
osteosarcoma U-2OS cells and 143B cells transfected with miR-184
analogue sequence were markedly enhanced, which was consistent with
a previous study regarding the regulatory effect of miR-184 on
osteosarcoma cell lines (21).
MiR-184 has been demonstrated to promote the proliferation and
invasion of glioblastoma (38). Su
et al (16) reported that
miR-184 may function as a tumor suppressor in renal cell carcinoma.
MiR-184 has various mechanisms of action in different tumors,
indicating tissue specificity. In the present study, an MTT assay
demonstrated that when miR-184 was overexpressed, the proliferation
of tumor cells was significantly higher compared with the control
group. By contrast, when miR-184 expression was suppressed, the
proliferation of tumor cells was inhibited. The present study
established nude mouse models of tibial orthotopic metastasis from
osteosarcoma and reported that compared with in the miR-184
inhibitor and control groups, tumor volume were markedly increased
within the miR-184 analogue group. The aforementioned results
confirmed that overexpression of miR-184 promoted osteosarcoma cell
proliferation in vivo and in vitro. Conversely,
inhibition of miR-184 expression may suppress the proliferation of
tumor cells.
The Wnt/β-catenin signaling pathway is strongly
associated with tumor invasion and metastasis, and has been
reported to be activated or inhibited by miRNA so as to induce
tumor cell proliferation, apoptosis and invasion (39,40).
MiR-184 is abnormally expressed in a variety of tumor tissues. In
addition to osteosarcoma, miR-184 is also involved in the
proliferation and invasion of various malignant tumor types,
including glioblastoma, renal cell carcinoma and central nervous
system lymphoma (19). However,
the existing mechanism of action requires further investigation. In
the present study, RT-qPCR and western blot analysis revealed that
high miR-184 expression may upregulate the expression levels of Wnt
and β-catenin mRNA and protein. Conversely, the expression levels
of Wnt and β-catenin mRNA and protein were decreased in the miR-184
inhibitor group. These results indicated that highly expressed
miR-184 was involved in the occurrence, development, invasion and
metastasis of osteosarcoma, possibly by upregulating Wnt/β-catenin
expression. Li et al (41)
confirmed that miR-184 promoted the proliferation and invasion of
pancreatic ductal adenocarcinoma cells, and inhibited cell
apoptosis by upregulating caspase-3 levels. In glioma and breast
cancer cells, miR-184 blocked cell cycle and cell adhesion by
upregulating P53 and P21 protein expression levels, caspase-3/8
activity, suppressing the protein kinase B/nuclear factor-κB
signaling pathway, and downregulating Staphylococcal nuclease and
tudor domain containing 1, matrix metalloproteinase-2/9 and cluster
of differentiation 44 expression levels (42). The results of the present study
indicated that miR-184 contributes to osteosarcoma cell
proliferation and invasion, and increases tumor volume in nude mice
bearing tumors by upregulating Wnt/β-catenin signaling
pathway-associated gene and protein expression levels. This
provides novel evidence for studying the mechanism of miR-184 in
osteosarcoma. However, Wnt signaling pathway inhibitors were not
employed to investigate specific mechanisms by which miR-184
regulates Wnt signaling, which was a limitation of the present
study.
Numerous genes and molecules are involved in the
genesis, development, invasion and metastasis of osteosarcoma.
Certain miRNAs exhibit carcinogenic effects on osteosarcoma, while
others exhibit inhibitory effects. The results of the present study
suggested that miR-184 may be a precancerous marker of
osteosarcoma, which may provide a basis for novel strategies for
the diagnosis and treatment of osteosarcoma. However, further
investigation into the precise mechanism and associated
interactions with other gene regulatory factors are required in the
future. Analyzing the biological mechanism of osteosarcoma and
investigating potential targets of molecular therapy may become the
focus and direction of future research.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZD and SX conceived and designed the study,
performed experiments, interpreted the results and drafted the
manuscript. FL and LW performed experiments. SX and HH analyzed the
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the China Medical
University Institutional Animal Care and Use Committee (IACUC no.
2016024).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Unni KK: Osteosarcoma of bone. J Orthop
Sci. 3:287–294. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Longhi A, Ferrari S, Bacci G and Specchia
S: Long-term follow-up of patients with doxorubicin-induced cardiac
toxicity after chemotherapy for osteosarcoma. Anticancer Drugs.
18:737–744. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meyers PA, Schwartz CL, Krailo MD, Healey
JH, Bernstein ML, Betcher D, Ferguson WS, Gebhardt MC, Goorin AM,
Harris M, et al: Osteosarcoma: The addition of muramyl tripeptide
to chemotherapy improves overall survival-a report from the
Children's Oncology Group. J Clin Oncol. 26:633–638. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hameed M and Dorfman H: Primary malignant
bone tumors-recent developments. Semin Diagn Pathol. 28:86–101.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the surveillance, epidemiology, and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Diao C, Xi Y and Xiao T: Identification
and analysis of key genes in osteosarcoma using bioinformatics.
Oncol Lett. 15:2789–2794. 2018.PubMed/NCBI
|
|
7
|
Liu Y, Sun W, Ma X, Hao Y, Liu G, Hu X,
Shang H, Wu P, Zhao Z and Liu W: Logistic regression analysis for
the identification of the metastasis-associated signaling pathways
of osteosarcoma. Int J Mol Med. 41:1233–1244. 2018.PubMed/NCBI
|
|
8
|
Miao J, Wu S, Peng Z, Tania M and Zhang C:
MicroRNAs in osteosarcoma: Diagnostic and therapeutic aspects.
Tumour Biol. 34:2093–2098. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu JQ, Zhang WB, Wan R and Yang YQ:
MicroRNA-32 inhibits osteosarcoma cell proliferation and invasion
by targeting Sox9. Tumour Biol. 35:9847–9853. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan K, Gao J, Yang T, Ma Q, Qiu X, Fan Q
and Ma B: MicroRNA-34a inhibits the proliferation and metastasis of
osteosarcoma cells both in vitro and in vivo. PLoS One.
7:e337782012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang K, Zhang C, Liu L and Zhou J: A key
role of microRNA-29b in suppression of osteosarcoma cell
proliferation and migration via modulation of VEGF. Int J Clin Exp
Pathol. 7:5701–5708. 2014.PubMed/NCBI
|
|
12
|
Li E, Zhang J, Yuan T and Ma B: MiR-145
inhibits osteosarcoma cells proliferation and invasion by targeting
ROCK1. Tumour Biol. 35:7645–7650. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han K, Zhao T, Chen X, Bian N, Yang T, Ma
Q, Cai C, Fan Q, Zhou Y and Ma B: microRNA-194 suppresses
osteosarcoma cell proliferation and metastasis in vitro and
in vivo by targeting CDH2 and IGF1R. Int J Oncol.
45:1437–1449. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pan W, Wang H, Jianwei R and Ye Z:
MicroRNA-27a promotes proliferation, migration and invasion by
targeting MAP2K4 in human osteosarcoma cells. Cell Physiol Biochem.
33:402–412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shen L, Chen XD and Zhang YH: MicroRNA-128
promotes proliferation in osteosarcoma cells by downregulating
PTEN. Tumour Biol. 35:2069–2074. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Su Z, Chen D, Li Y, Zhang E, Yu Z, Chen T,
Jiang Z, Ni L, Yang S, Gui Y, et al: microRNA-184 functions as
tumor suppressor in renal cell carcinoma. Exp Ther Med. 9:961–966.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu G, Liu J, Wu Z, Wu X and Yao X:
MicroRNA-184 inhibits cell proliferation and metastasis in human
colorectal cancer by directly targeting IGF-1R. Oncol Lett.
14:3215–3222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang YB, Zhao XH, Li G, Zheng JH and Qiu
W: MicroRNA-184 inhibits proliferation and promotes apoptosis of
human colon cancer SW480 and HCT116 cells by downregulating C-MYC
and BCL-2. J Cell Biochem. 119:1702–1715. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang XG, Meng WT, Hu LJ, Li L, Xing H,
Xie G, Wang AQ and Jia YQ: MicroRNA-184 modulates human central
nervous system lymphoma cells growth and invasion by targeting
iASPP. J Cell Biochem. 118:2645–2653. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao B, Gao K, Li L, Huang Z and Lin L:
miR-184 functions as an oncogenic regulator in hepatocellular
carcinoma (HCC). Biomed Pharmacother. 68:143–148. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yin GR, Wang Q, Zhang XB and Wang SJ:
Regulatory role of microRNA184 in osteosarcoma cells. Genet Mol
Res. 14:14246–14252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rapp J, Jaromi L, Kvell K, Miskei G and
Pongracz JE: WNT signaling-lung cancer is no exception. Respir Res.
18:1672017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bryja V, Gradl D, Schambony A, Arenas E
and Schulte G: Beta-arrestin is a necessary component of
Wnt/beta-catenin signaling in vitro and in vivo. Proc Natl Acad Sci
USA. 104:6690–6695. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Seki Y, Yamamoto H, Ngan CY, Yasui M,
Tomita N, Kitani K, Takemasa I, Ikeda M, Sekimoto M, Matsuura N, et
al: Construction of a novel DNA decoy that inhibits the oncogenic
beta-catenin/T-cell factor pathway. Mol Cancer Ther. 5:985–994.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lustig B and Behrens J: The Wnt signaling
pathway and its role in tumor development. J Cancer Res Clin Oncol.
129:199–221. 2003.PubMed/NCBI
|
|
26
|
Rubin EM, Guo Y, Tu K, Xie J, Zi X and
Hoang BH: Wnt inhibitory factor 1 decreases tumorigenesis and
metastasis in osteosarcoma. Mol Cancer Ther. 9:731–741. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
An JH, Yang JY, Ahn BY, Cho SW, Jung JY,
Cho HY, Cho YM, Kim SW, Park KS, Kim SY, et al: Enhanced
mitochondrial biogenesis contributes to Wnt induced osteoblastic
differentiation of C3H10T1/2 cells. Bone. 47:140–150. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cai Y, Mohseny AB, Karperien M, Hogendoorn
PC, Zhou G and Cleton-Jansen AM: Inactive Wnt/beta-catenin pathway
in conventional high-grade osteosarcoma. J Pathol. 220:24–33. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun X, He Y, Huang C, Ma TT and Li J:
Distinctive microRNA signature associated of neoplasms with the
Wnt/β-catenin signaling pathway. Cell Signal. 25:2805–2811. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Euhus DM, Hudd C, LaRegina MC and Johnson
FE: Tumor measurement in the nude mouse. J Surg Oncol. 31:229–234.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lindsey BA, Markel JE and Kleinerman ES:
Osteosarcoma overview. Rheumatol Ther. 4:25–43. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Durfee RA, Mohammed M and Luu HH: Review
of osteosarcoma and current management. Rheumatol Ther. 3:221–243.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang J, Yan YG, Wang C, Zhang SJ, Yu XH
and Wang WJ: MicroRNAs in osteosarcoma. Clin Chim Acta. 444:9–17.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang J and Zhang W: New molecular insights
into osteosarcoma targeted therapy. Curr Opin Oncol. 25:398–406.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lauvrak SU, Munthe E, Kresse SH, Stratford
EW, Namløs HM, Meza-Zepeda LA and Myklebost O: Functional
characterisation of osteosarcoma cell lines and identification of
mRNAs and miRNAs associated with aggressive cancer phenotypes. Br J
Cancer. 109:2228–2236. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lin BC, Huang D, Yu CQ, Mou Y, Liu YH,
Zhang DW and Shi FJ: MicroRNA-184 modulates doxorubicin resistance
in osteosarcoma cells by targeting BCL2L1. Med Sci Monit.
22:1761–1765. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang X, Ding H, Han Y, Sun D, Wang H and
Zhai XU: The significance of microRNA-184 on JAK2/STAT3 signaling
pathway in the formation mechanism of glioblastoma. Oncol Lett.
10:3510–3514. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ueno K, Hirata H, Hinoda Y and Dahiya R:
Frizzled homolog proteins, microRNAs and Wnt signaling in cancer.
Int J Cancer. 132:1731–1740. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schepeler T: Emerging roles of microRNAs
in the Wnt signaling network. Crit Rev Oncog. 18:357–371. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li H, Xiang H, Ge W, Wang H, Wang T and
Xiong M: Expression and functional perspectives of miR-184 in
pancreatic ductal adenocarcinoma. Int J Clin Exp Pathol.
8:12313–12318. 2015.PubMed/NCBI
|
|
42
|
Feng R and Dong L: Inhibitory effect of
miR-184 on the potential of proliferation and invasion in human
glioma and breast cancer cells in vitro. Int J Clin Exp Pathol.
8:9376–9382. 2015.PubMed/NCBI
|