Introduction
Ischemia/reperfusion (I/R) is a process that may
damage tissues or organs that depend on aerobic metabolism
(1). I/R is widely considered to
be a contributing factor in liver injury; it may lead to hepatic
failure following major hepatic resection, as portal vein occlusion
is a necessary step. In addition, I/R injury is attributed to
approximately 10% of acute graft dysfunction cases post-liver
transplantation (2,3). Thus, mitigating the adverse effects
of I/R injury is an important clinical issue. Researchers have
developed myriad therapeutic strategies as potential solutions to
prevent hepatic I/R injury, including surgical interventions,
pharmacotherapy or preconditioning with medication (4–6). In
addition, the benefits of mesenchymal stem cells (MSCs) in liver
I/R injury have received considerable attention (7).
As demonstrated previously, MSCs exhibited promising
efficacy in reducing I/R injury in different organs, including the
brain, myocardium, kidney, small bowel and liver (8–12).
Bone marrow MSCs (BM-MSCs) are a type of MSCs derived from bone
marrow. Studies have revealed that BM-MSCs have protective effects
against liver I/R injury; they are able to attenuate liver injury
by promoting liver regeneration or paracrine actions, including
cytokines, growth factors or chemokines. However, the precise
mechanisms through which BM-MSCs confer protection against I/R
injury have not been completely elucidated. Recently, autophagy has
been reported to be a potential mechanism underlying the protective
effects of BM-MSCs against I/R injury (13).
Autophagy, including macroautophagy, microautophagy
and chaperone-mediated autophagy, is regarded as a rudimentary
cellular response to injury; it removes macromolecules and
organelles via lysosomes, restricts cell death, enables cells to
withstand diverse insults and prevents irreversible organ damage
(14). In addition, a growing body
of evidence has indicated that autophagy has positive effects in
liver diseases (15,16). In liver I/R injury, the promotion
of autophagy may ameliorate liver damage (17). Furthermore, BM-MSCs are able to
alleviate damage in CCl4-injured livers in addition to
I/R-induced lung injury by autophagy (13,18).
To the best of our knowledge, autophagy has not yet been evaluated
in BM-MSC-mediated protection against liver I/R injury.
Heme oxygenase-1 (HO-1) is a stress-inducible enzyme
that has anti-inflammatory, anti-apoptotic, pro-survival and
antioxidant potential. HO-1 has been confirmed to serve a role in
protecting against liver I/R injury by regulating oxidative stress
or inflammation (19–21). Furthermore, recent studies have
demonstrated that HO-1 attenuates liver I/R injury through
autophagy (22). However, it
remains unclear as to whether HO-1 mediated autophagy is a
mechanism through which BM-MSCs protect against liver I/R
injury.
Based on these findings, it was hypothesized that
BM-MSCs may protect against liver I/R injury by promoting
autophagy. In addition, the present study aimed to investigate the
association between autophagy and HO-1 in experimental I/R injury
in vivo.
Materials and methods
Isolation, culture, identification,
and differentiation of BM-MSCs
BM-MSCs were obtained from the femurs and tibias of
4-week-old Wistar rats by flushing the bone marrow cavity with
complete culture medium as previously described (23). The extract was centrifuged,
resuspended and cultured in a 75-cm2 culture flask
containing Dulbecco's modified Eagle's medium with low glucose
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), and 1%
penicillin/streptomycin at 37°C and 5% CO2. One-half of
the medium was replaced following 24 h of culture, and the entire
culture medium every 2–3 days. Cells were subcultured when the
adherent cells reached 70–80% confluence.
Cells were identified as BM-MSCs by their
morphology, adherence and surface markers. BM-MSCs of 3–4 passages
were identified by antibodies against cluster of differentiation
(CD)29-phycoerythrin (PE)-A, CD34-PE-A, CD44-PE-A, CD45-fluorescein
isothiocyanate (FITC)-A, and CD90-PE-A (eBioscience; Thero Fisher
Scientific, Inc.). Briefly, resuspended cells were incubated with
CD29-PE-A (1:2; cat. no. 12-0291-82), CD34-PE-A (1:40; cat. no.
MA1-10205), CD44-PE-A (1:40; cat. no. MA5-16908), CD45-FITC-A
(1:20; cat. no. 11-0461-82) and CD90-PE-A (1:40; cat. no.
MA1-80650) at room temperature. After 20 min, the cells were washed
with three times PBS and a flow cytometer was used (FACS Aria II;
BD Biosciences, San Jose, CA, USA). Software was used for data
analysis (FlowJo software 7.6; FlowJo LLC, Ashland, OR, USA).
Cells were additionally identified by their
multilineage differentiation potential, as previously reported
(24). For adipogenic
differentiation, adipogenic induction medium (Cyagen Biosciences,
Co., Ltd., Suzhou, China) was added to the BM-MSCs at 37°C for 3
days, followed by maintenance medium (Cyagen Biosciences, Co.,
Ltd.) at 37°C for 1 day. Following three cycles, the cells were
cultured in maintenance medium for a further 7 days. The cells were
subsequently stained with Oil Red O (Cyagen Biosciences, Co., Ltd.)
at room temperature for 30 min. The sections were washed and
examined using a light microscope (magnification, ×40, Olympus
Corporation, Tokyo, Japan). For osteogenic differentiation, the
BM-MSCs were cultured with rat BM-MSC osteogenic differentiation
medium (Cyagen Biosciences, Co., Ltd.) when subconfluent at 37°C.
Subsequent to 3 weeks of differentiation, the calcium depositions
were stained with Alizarin red (Cyagen Biosciences, Co., Ltd.) at
room temperature for 3–5 min. The sections were washed and examined
using a light microscope (magnification, ×40, Olympus
Corporation).
Animals and treatment
A total of 30 healthy 4–6-week-old male Wistar rats
weighing ~200 g were purchased from the Laboratory Animal Center of
The Affiliated Drum Tower Hospital of Nanjing University Medical
School (Nanjing, China), and housed under specific pathogen-free
conditions. All animal experiments were approved by the
Institutional Animal Care and Use Committee of The Affiliated Drum
Tower Hospital of Nanjing University Medical School, under the
National Institutes of Health (Bethesda, MD, USA) Guide for the
Care and Use of Laboratory Animals. All experiments were conducted
under isoflurane anesthesia, and all efforts were made to minimize
suffering.
The rats were randomly divided into five groups as
follows: The sham group, the I/R group, the I/R+MSCs group, the
3-methyladenine (3-MA) group and the zinc protoporphyrin IX (ZnPP)
group. A hepatic warm I/R model was used as previously described
(25). The rats were anesthetized
with isoflurane followed by laparotomy. A sterile microvascular
clamp was placed around all structures in the portal triad for the
left and median liver lobes to interrupt the blood supply in all
groups except the sham group, which underwent the same procedure
without vascular occlusion. Reperfusion was initiated via removal
of the clamp after 1 h. In addition, 1×106 MSCs
suspended in 0.5 ml PBS were injected via the penis dorsal vein 30
min prior to hepatic warm I/R (26) in all the groups, except the sham
group and the I/R group. In the 3-MA group, the autophagy inhibitor
3-MA was administered (30 mg/kg; intraperitoneal; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) 0.5 h prior to ischemia. The rats
in the ZnPP group were treated twice with ZnPP (10 mg/kg;
intraperitoneal; Sigma-Aldrich; Merck KGaA) at 16 and 3 h prior to
ischemia to inhibit HO-1 in vivo. All the rats were
sacrificed following 6 h of reperfusion, and blood samples and
liver tissues were obtained. Blood samples were stored at 4°C
overnight. A portion of the liver tissues were fixed in 10%
formalin solution at room temperature for ≥24 h, and then prepared
for HE staining and immunohistochemistry (5 µm) as described below.
The remaining liver tissues were stored at −80°C for further
use.
In order to detect the implantation of BM-MSCs in
the liver following I/R injury, the cells were stained with
lipophilic membrane dyes (DiO; Invitrogen; Thermo Fisher
Scientific, Inc.) prior to intravenous administration of BM-MSCs.
The BM-MSCs were suspended at a concentration of 106
cells/ml and incubated with 10 mM DiO for 20 min at 37°C. Frozen
sections were fixed at 4°C for 10 min in acetone and air-dried. The
nuclei were stained with DAPI (Invitrogen; Thermo Fisher
Scientific, Inc.). The sections were washed and examined using a
Leica TCS SP8 confocal laser-scanning microscope (magnification,
×200; Leica Microsystems GmbH, Wetzlar, Germany).
Blood biochemistry
Blood samples was harvested via the postcava and
centrifuged at 3,000 × g at 4°C for 10 min. Serum levels of alanine
aminotransferase (ALT) and aspartate aminotransferase (AST) were
measured using an automatic analyzer (Fujifilm Global, Tokyo,
Japan), as previously described (27).
Histological analysis and
immunohistochemistry
Following fixation of the liver tissues in 10%
buffered formalin at room temperature for at least 24 h, paraffin
embedding was performed using a standard protocol. Paraffin
sections (5 µm) were stained with hematoxylin at room temperature
for 3–5 min and stained with eosin at room temperature for 2 min,
then examined under a light microscope (magnification, ×100) for
histological evidence of liver injury. Immunohistochemical staining
for HO-1 was performed on the paraffin sections which were blocked
with blocking serum (OriGene Technologies, Inc., Beijing, China) at
room temperature for 10 min, incubated with primary antibodies
against HO-1 (1:200; cat. no. ab13243, Abcam, Cambridge, UK)
overnight at 4°C and developed using a horseradish
peroxidase-conjugated secondary antibody (PV-6000, undiluted,
OriGene Technologies, Inc., Beijing, China) at 37°C for 30 min and
a 3,3-diaminobenzidine substrate kit (ZLI-9018, undiluted; OriGene
Technologies, Inc.) at room temperature for 5–20 min, according to
standard methods in routine pathology. The sections were washed and
examined using a light microscope (×100; Leica Microsystems GmbH,
Wetzlar, Germany).
Gel electrophoresis and western
blotting
Western blotting was conducted as previously
described (22). Equal amounts (40
µg) of the proteins were separated on 15% SDS-PAGE gels and
transferred to polyvinylidene difluoride membranes. The membranes
were incubated overnight at 4°C with primary antibodies against
HO-1 and microtubule-associated proteins 1A/1B light chain 3B
(LC3B; 1:2,000; cat. no. ab192890; Abcam). GAPDH (1:1,000; cat. no.
ab9484; Abcam; incubated at 4°C overnight) expression served as a
loading control. The membrane was treated with horseradish
peroxidase-conjugated goat anti-mouse secondary antibody (1:8,000;
KGAA37; Nanjing KeyGen Biotech Co,. Ltd., Nanjing, China) or
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (1:8,000; KGAA35; Nanjing KeyGen Biotech Co,. Ltd.) at
room temperature for 2 h. Blots were developed using enhanced
chemiluminescence (ECL) western blotting substrate (EMD Millipore,
Billerica, MA, USA) and visualized on a Tanon 5200 Multi Image
Station (Tanon Science & Technology Co., Ltd., Shanghai,
China). The results were quantified by Image Pro Plus 6.0 software
(Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
Statistical analysis was performed using SPSS
version 19.0 (IBM Corp., Armonk, NY, USA) and data are expressed as
the mean ± standard error of the mean; results were repeated in
triplicate. Differences between the groups were evaluated for
significance using one-way analysis of variance combined with
Bonferroni's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Culture, identification and detection
of BM-MSCs
At passage 3 or passage 4, the cells from the rat
bone marrow existed as a monolayer of typical fibroblastic,
spindle-shaped and plastic-adherent cells (Fig. 1A). As presented in Fig. 1B, identification of BM-MSCs was
performed using flow cytometry. These cells exhibited little or no
expression of hematopoietic markers (CD34 and CD45) and positive
expression of stromal markers (CD29, CD44 and CD90). In addition,
the BM-MSCs had the potential to differentiate into osteoblastic or
adipogenic lineages (Fig. 1C).
Based on these results, the cells from rat bone marrow were
confirmed to be BM-MSCs. The DiO-labeled BM-MSCs were used to
clarify whether BM-MSCs migrated to the liver following I/R.
BM-MSCs were observed in the I/R+MSCs group, although not in the
sham group or the I/R group (Fig.
1D).
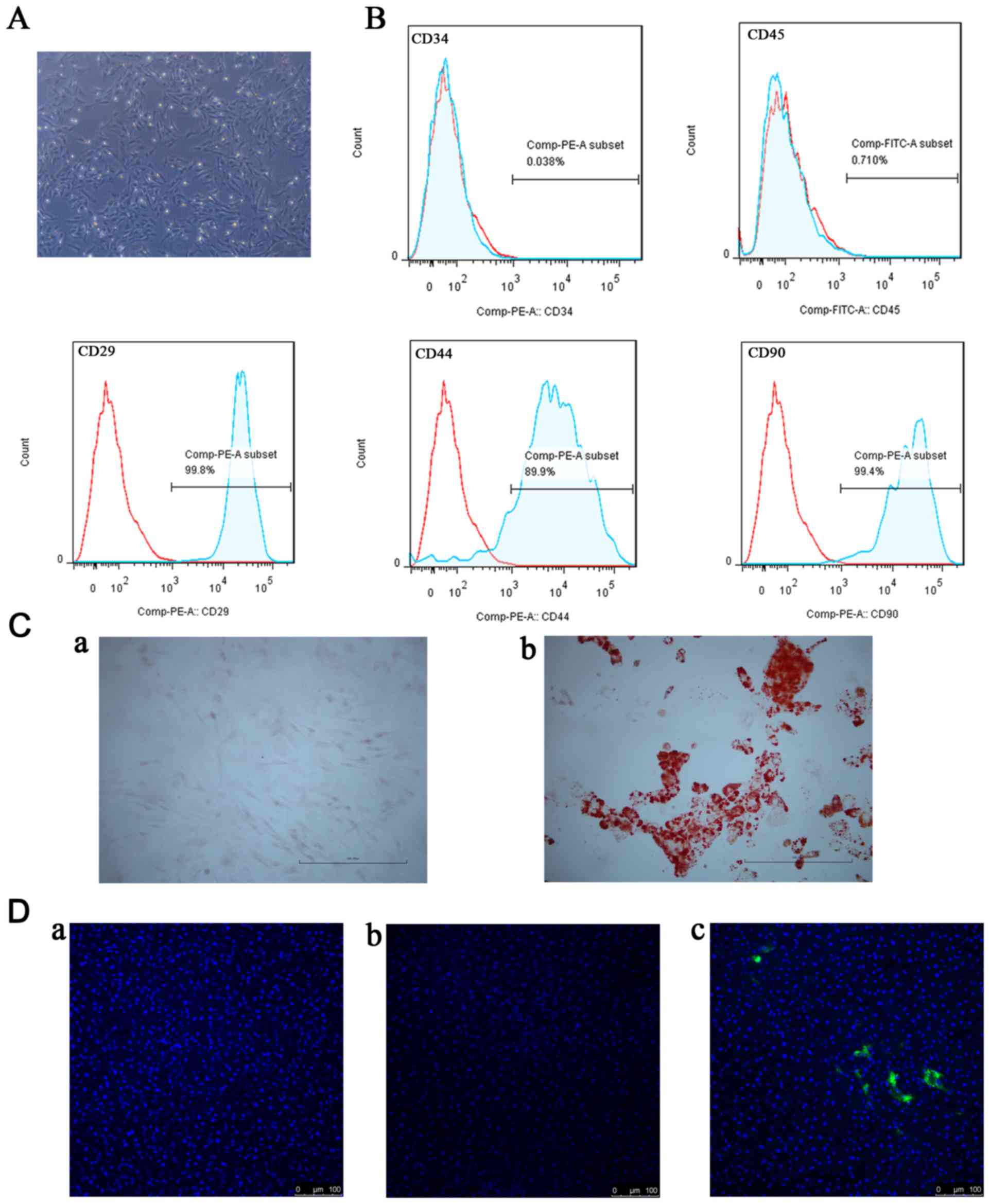 | Figure 1.Identification and detection of
BM-MSCs. (A) Morphological appearance of primary BM-MSCs expanded
in culture. At passage 3 or passage 4, the cells exhibited typical
fibroblastic, spindle-shaped morphology (magnification, ×200). (B)
Analysis of the surface markers of BM-MSCs by flow cytometry
demonstrated that they did not express CD34 or CD45, although they
positively expressed CD29, CD44 and CD90. (C) Multilineage
differentiation of BM-MSCs stained with (a) Alizarin red and (b)
Oil Red O. Scale bar, 100 µm. (D) Detection of DiO-labeled BM-MSCs
in the liver samples following reperfusion: (a) The sham group; (b)
the I/R group; (c) the I/R+MSCs group. Scale bar, 100 µm. BM-MSCs,
bone marrow mesenchymal stem cells; CD, cluster of differentiation;
I/R, ischemia/reperfusion. |
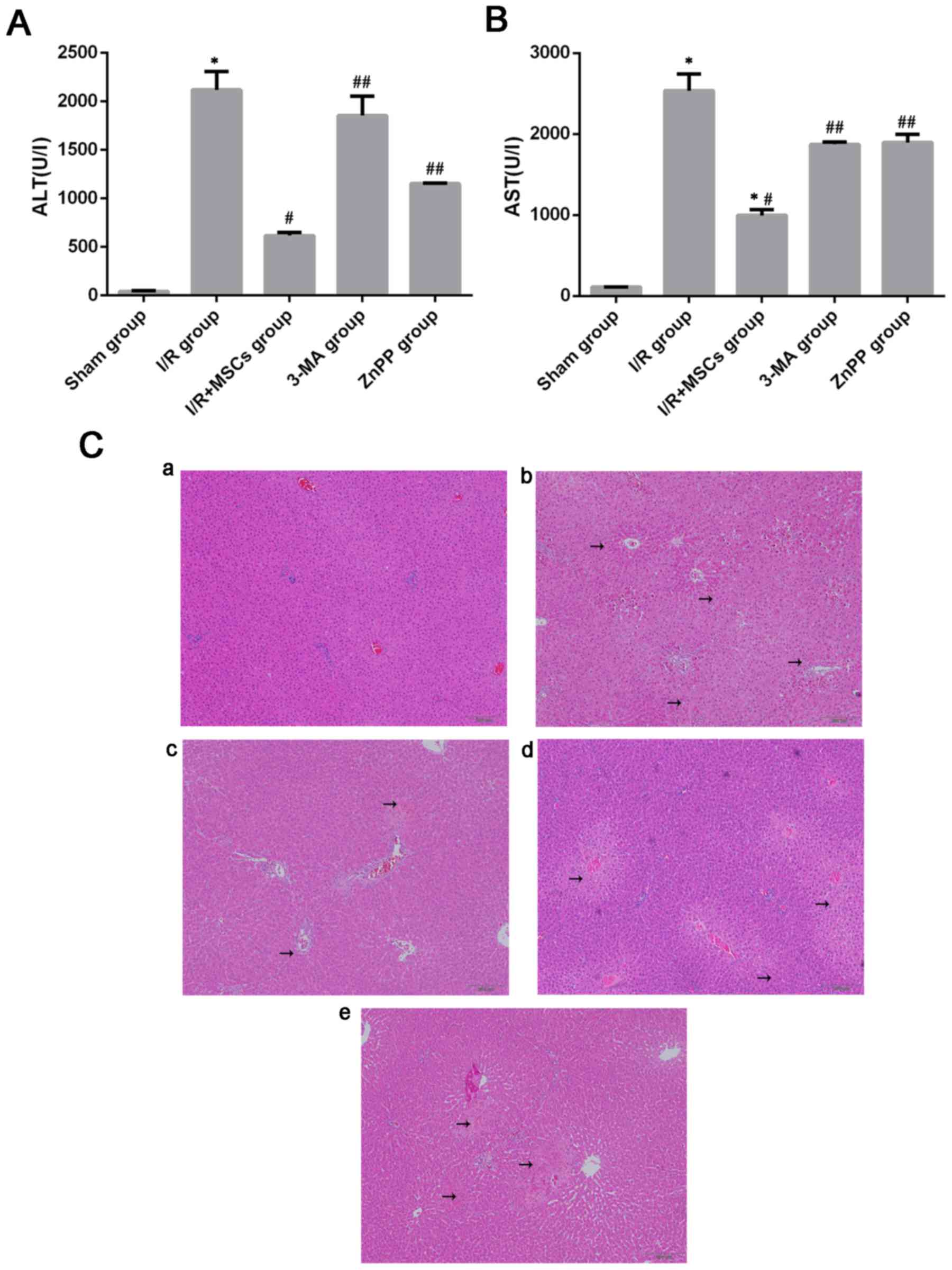
Pretreatment with BM-MSCs ameliorates
liver injury following I/R
In order to determine whether BM-MSCs attenuate
hepatic I/R injury, the rats were pretreated with BM-MSCs prior to
ischemia. Serious liver damage was observed following 6 h of
reperfusion, as previously reported (25). The rats were sacrificed 6 h
post-insult, and liver tissues and blood were collected for further
research. Compared with the rats in the I/R group, which had the
highest ALT and AST levels, a significant improvement was noted in
the I/R+MSCs group (Fig. 2A and
B). In addition, the liver samples from the I/R group exhibited
marked abnormalities, including severe hepatocellular necrosis and
cytoplasmic vacuolization, following 6 h of reperfusion, which were
improved in the I/R+MSCs group (Fig.
2C).
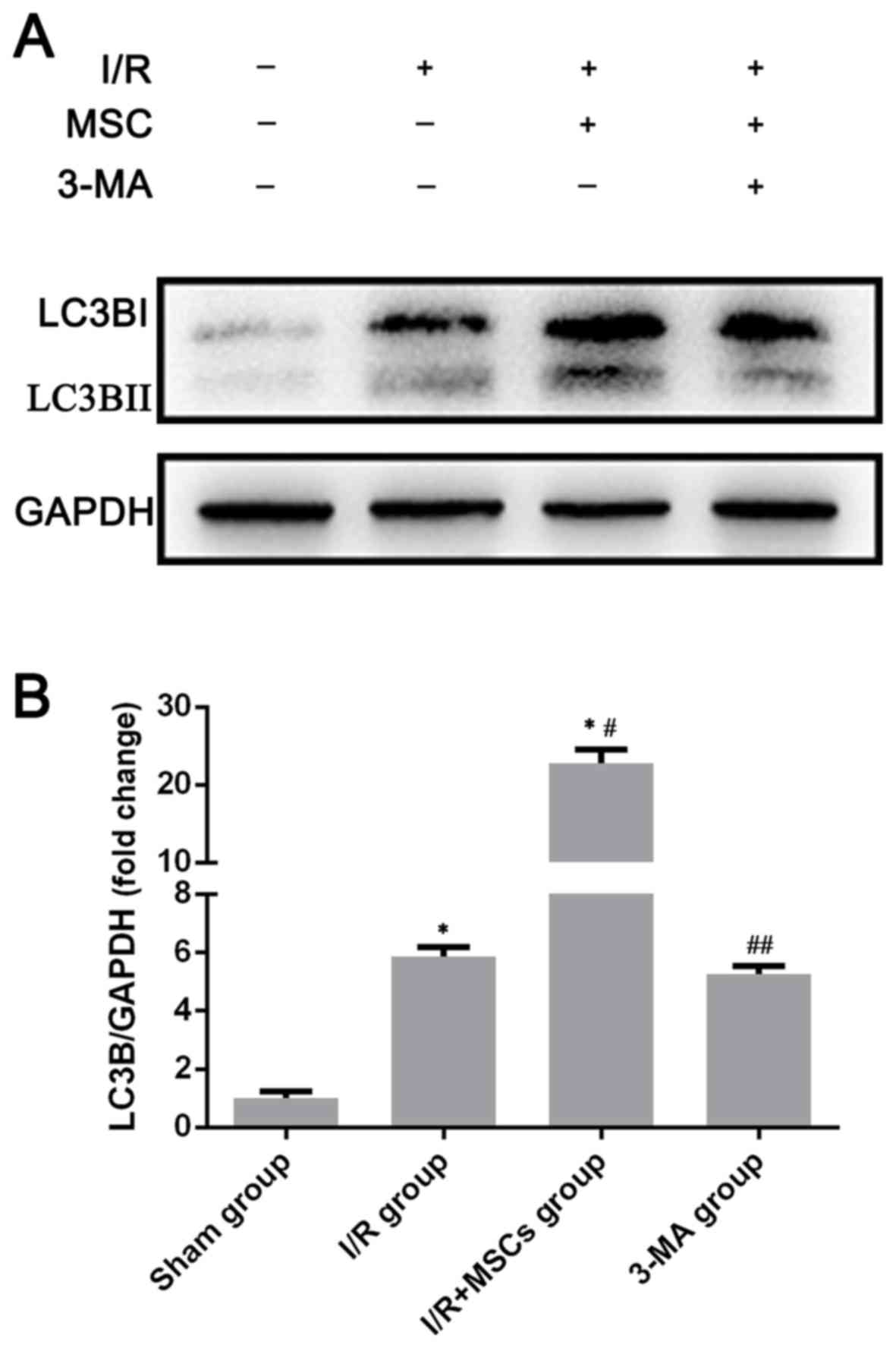
Autophagy is promoted by pretreatment
with BM-MSCs
Autophagy is part of the adaptive stress response to
infection or lipopolysaccharide (LPS) exposure to support cell
survival. In order to clarify the effects of pretreatment with
BM-MSCs on autophagy, the expression of the autophagy associated
marker LC3B in the rat liver was examined in the sham group, the
I/R group and the I/R+MSCs group by western blotting. Upon the
induction of autophagy, LC3B is a terminal autophagic protein used
as a classic marker to monitor alterations in autophagy (28). As presented in Fig. 3, the expression of the autophagic
signaling factor LC3B was markedly increased in the I/R+MSCs group
compared with the other study groups. These findings confirmed that
autophagy increased following pretreatment with BM-MSCs.
BM-MSCs protect against liver I/R
injury via the induction of autophagy
To evaluate whether autophagy contributes to
BM-MSC-mediated protection against I/R injury, the rats were
pretreated with 3-MA prior to ischemia, which is widely regarded as
an inhibitor of autophagy. The expression of autophagy was measured
using western blotting to determine the presence of LC3B. Compared
with the I/R+MSCs group, treatment with 3-MA resulted in a decrease
in LC3B expression in response to liver I/R injury (Fig. 3). Notably, compared with the
I/R+MSCs group, the serum levels of AST and ALT and the
histological abnormalities were reversed in the 3-MA group
(Fig. 2). Based on the results
following pretreatment with 3-MA, it was concluded that autophagy
served a notable role in BM-MSC-mediated protection against cell
death following I/R injury.
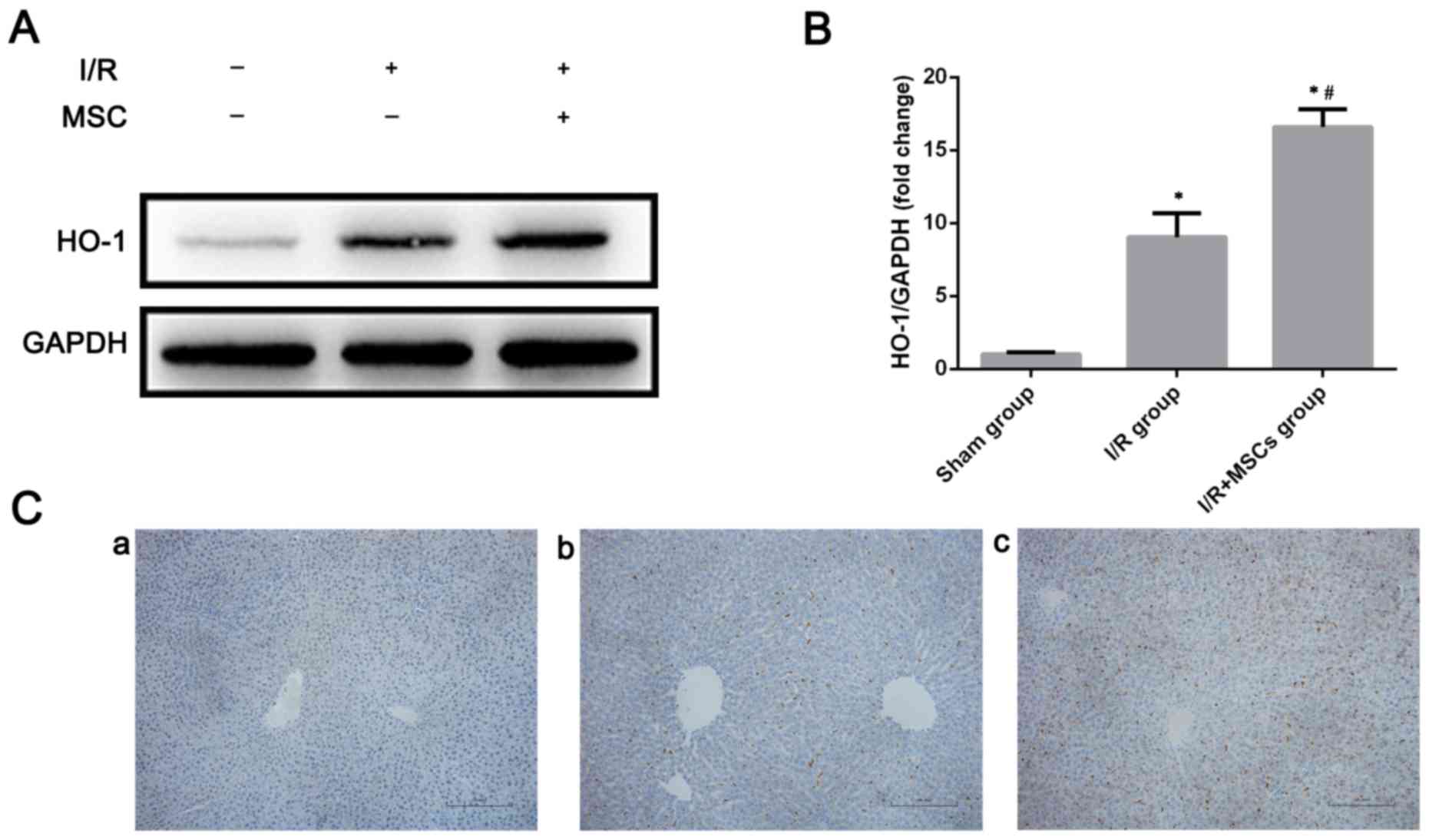
BM-MSC-promoted autophagy is dependent
on HO-1
The present study subsequently aimed to analyze how
BM-MSCs promote autophagy following I/R injury. A previous study
reported that upregulation of HO-1 was able to mitigate I/R injury
(29). Furthermore, HO-1 has been
demonstrated to be associated with autophagic activity (22,30).
Consistent with these findings, alterations in HO-1 expression were
observed in the case of pretreatment with BM-MSCs in response to
I/R injury. HO-1 expression was increased in the rat livers
subjected to I/R injury, while in the I/R+MSCs group, HO-1
expression was even more significantly increased (Fig. 4). Since BM-MSCs increased HO-1
expression and autophagic activity, it was preliminarily suggested
that HO-1 may serve a role in the promotion of autophagy via
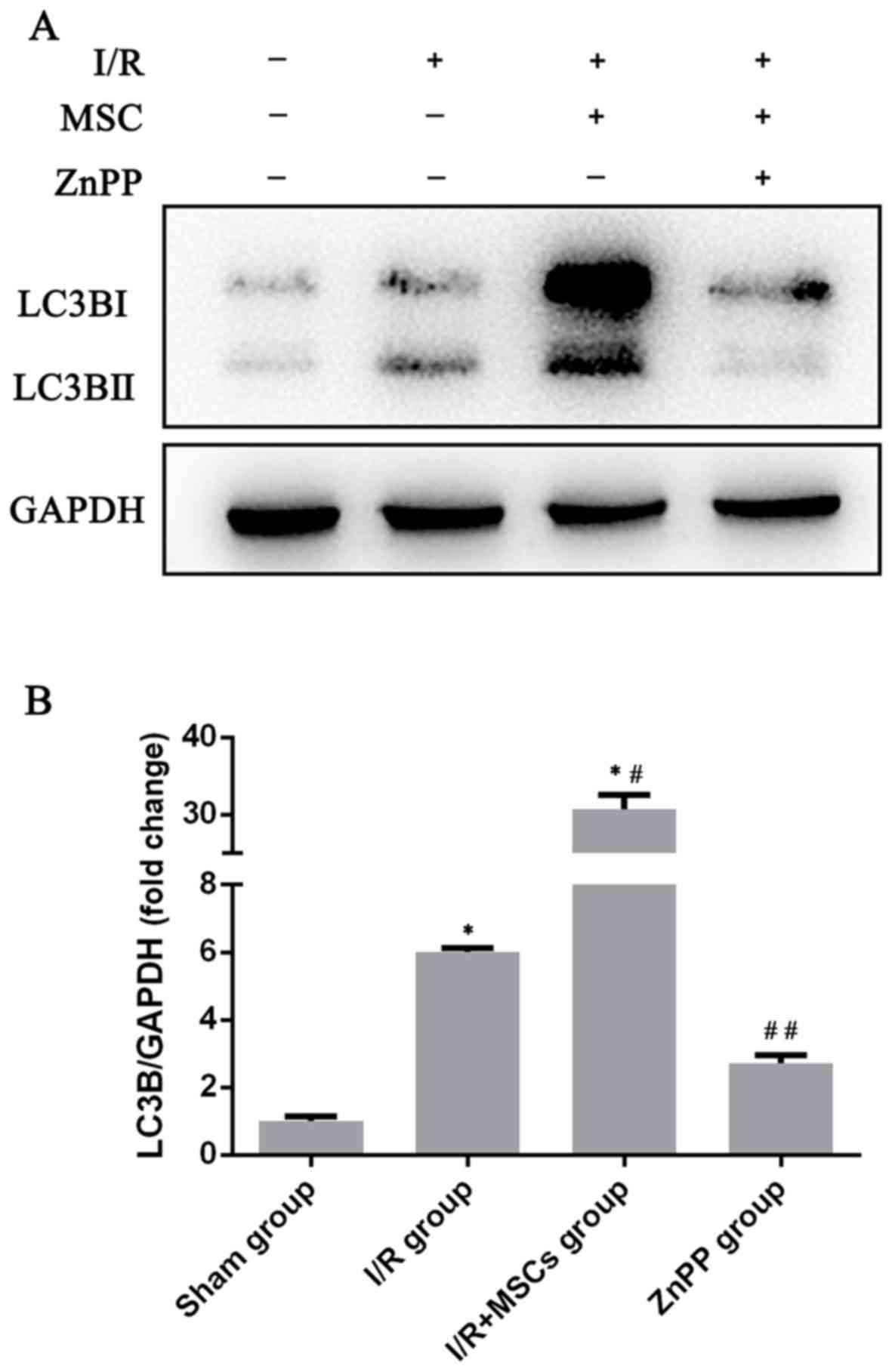
pretreatment with BM-MSCs. Therefore, in order to identify the
association between HO-1 and autophagy, HO-1 was inhibited by ZnPP.
ZnPP significantly decreased the expression of LC3B (Fig. 5). Furthermore, inhibition of HO-1
by ZnPP significantly inhibited the protective effect provided by
BM-MSCs following I/R insult. The serum levels of AST and ALT in
the ZnPP-treated rats were increased compared with those in the
I/R+MSCs group (Fig. 2). The I/R
injury-associated histopathological alterations in the livers of
rats were more severe in the ZnPP group compared with the I/R+MSCs
group (Fig. 2C). Taken together,
these data demonstrate that HO-1, increased by pretreatment with
BM-MSCs, promoted autophagy, which protected against I/R injury
in vivo.
Discussion
MSCs have been widely studied as anti-apoptotic and
pro-survival cells, and they have been demonstrated to successfully
mitigate liver injury caused by D-galactosamine/LPS or toxins, in
addition to organ I/R injury (31–34).
However, little is known about the role of autophagy in
BM-MSC-mediated protection and its detailed regulatory mechanism in
liver I/R injury is unclear. MSCs have recently been demonstrated
to ameliorate liver fibrosis by inducing autophagy (35), which is regarded as a protective
mechanism against I/R injury in the liver or other organs (22,36,37).
Therefore, the present study focused on evaluating whether BM-MSCs
protected against liver I/R injury by increasing the expression
level of HO-1. BM-MSCs are one of the most studied stem cells; they
have previously been identified by their morphology, surface
markers or multilineage differentiation potential (38,39).
Therefore, BM-MSCs were identified by the same method in the
present study. The results demonstrated that the cells we obtained
were BM-MSCs. BM-MSCs hold great potential in protecting organs
from I/R injury. Sheashaa et al (9) reported that adipose-derived MSCs were
able to significantly attenuate injury caused by I/R. Similarly, Lu
et al (40) reported that
BM-MSCs significantly reversed lung injury following I/R by
alleviating inflammation induced by I/R injury. The beneficial
effect of BM-MSCs was further confirmed by Fu et al
(25), who documented that the
hepatoprotection provided by BM-MSCs following I/R injury was
dependent on the inhibition of hepatocellular apoptosis and the
stimulation of N-acetyltransferase 8 regeneration in vivo or
in vitro. Consistent with these observations, the present
study confirmed that BM-MSCs had beneficial effects against liver
I/R injury in vivo by observing levels of ALT/AST and
alterations in histomorphology that were consistent with certain
published papers (41,42). Previous studies revealed that MSCs
attenuated I/R injury in solid organs through various complex
mechanisms, including an anti-inflammatory reaction (12,40),
angiogenesis (43), anti-oxidative
stress (44,45), and immunomodulation (46). The present study focused on whether
BM-MSCs mitigated liver I/R injury through autophagy.
Autophagy is regarded as a cellular self-digestion
process in response to a wide range of deleterious stimuli, through
which cytoplasmic materials or organelles integrate into lysosomes
for further degradation (47).
Previous studies have reported that, autophagy serves a crucial
role in the development, differentiation, survival and homeostasis
of cells (48–51). During organ I/R injury, autophagy
may help cells respond to injury. Liu et al (52) reported that autophagy is a critical
homeostatic mechanism in maintaining renal tubular cell integrity
during renal I/R. Zhang et al (37) suggested that autophagy evoked by
I/R is involved in the process of neuroprotection via
mitophagy-associated mitochondrial clearance and the inhibition of
downstream apoptosis. Autophagy was additionally suggested to be a
pro-survival mechanism in the field of liver I/R injury (53). Zhao et al (54) observed that increasing autophagy by
inhibiting calpain2 was able to decrease the sensitivity of fatty
liver to I/R injury. Wang et al (55) revealed that the restoration of
autophagy inhibited the activation of mitochondrial permeability
transition and attenuated damage in aged livers with I/R injury.
However, liver injury is aggravated following the suppression of
I/R-induced autophagy by chloroquine, which demonstrated the
benefit of autophagy for I/R injury of livers (56). These previous studies strongly
support the view that autophagy orchestrates a cytoprotective
mechanism in I/R injury. It has been demonstrated that MSCs are
associated with autophagy. Park et al (35) indicated that the mechanism of
BM-MSCs in the resolution of CCl4-induced liver fibrosis
was partially due to the accumulation of autophagy-associated
proteins. Shin et al (57)
reported that BM-MSCs were able to exert a neuroprotective effect
in Alzheimer's disease based on enhanced autophagy, which resulted
in the clearance of amyloid-β. Zhou and You (58) proved that BM-MSCs alleviated
LPS-induced acute lung injury by reducing the levels of microRNA
142a-5p and increasing Beclin1-mediated autophagy in pulmonary
endothelial cells.
The present study demonstrated that pretreatment
with BM-MSCs provided hepatoprotection against liver I/R injury
accompanied by the increased expression of autophagic signaling
molecules, including LC3B. To further determine the role of
autophagy in the protection mediated by BM-MSCs, the rats were
pretreated with BM-MSCs and concomitant 3-MA, a specific autophagy
inhibitor, prior to I/R injury. The results demonstrated that
pretreatment with 3-MA abrogated the increased in autophagy
mediated by BM-MSCs in vivo. This abrogation translated into
increased serum levels of AST and ALT, severe histological
abnormalities. From these investigations, it was confirmed that
BM-MSCs afforded hepatoprotection against liver I/R injury,
partially by promoting autophagy. In the present study, LC3B was
regarded as an indicator of autophagy, as reported previously
(28), which was a limitation of
the present study. In addition, there is a discrepancy between the
present results and the findings reported in several previous
investigations, which demonstrated that MSCs repaired I/R injury
via anti-autophagic mechanisms (59). Since autophagy is highly variable
during liver I/R, a potential explanation for this contradiction is
that it is likely that autophagy serves different roles at various
time points during reperfusion. Future research is required to
study the alterations in autophagic activity influenced by MSCs at
different time points following reperfusion.
The regulatory mechanisms of BM-MSCs on autophagy
during liver I/R injury remained unclear. HO-1 is an indispensable
protein in various organs, including the liver, and is involved in
restoring cellular homeostasis in response to multiple insults
(30). Previous studies have
demonstrated that autophagy is elevated by HO-1. Carchman et
al (30) revealed that
autophagy orchestrated protection against liver injury due to
sepsis, which was dependent on HO-1. In addition, HO-1-mediated
autophagy was the mechanism by which baicalein and remote ischemic
preconditioning prevented hepatocellular injury due to I/R
(5,22). Yun et al (60) confirmed that the HO-1 system was a
possible regulator of autophagy in liver I/R injury. Conversely,
Wang et al (61) used a
HO-1 inhibitor to demonstrate that HO-1 was a key inducer of
autophagy in response to liver I/R injury, thereby preventing
aggravation of liver damage. In the present study, the groups
pretreated with BM-MSCs exhibited increased HO-1 expression and
autophagic activity. Thus, it appears that MSC-promoted autophagy
occurred via HO-1. In order to elaborate on the role of HO-1 in
MSC-promoted autophagy, HO-1 induced by BM-MSCs was inhibited by
ZnPP prior to ischemia. The results of the present study
demonstrated that inhibition of HO-1 by ZnPP prevented autophagy
due to pretreatment with BM-MSCs; it additionally prevented the
protective effect of BM-MSCs on I/R injury in vivo.
From the results of previous studies and the present
observations, it was concluded that HO-1 was the regulator through
which the BM-MSCs increased autophagic activity in liver I/R
injury. However, the way in which treatment with BM-MSCs increased
in the expression of HO-1 in the liver remained unresolved. As
previously reported, HO-1 may be increased in the liver by the
phosphatidylinositol 3-kinase/RAC-α serine/threonine-protein
kinase/nuclear factor erythroid 2-related factor 2 (Nrf2) pathway
(62), the kelch-like
ECH-associated protein 1/Nrf2/thioredoxin 1/hypoxia inducible
factor-1α pathway (20), or the
cyclic AMP-dependent transcription factor ATF3-mediated Nrf2
pathway (63). Further study is
required to determine the mechanism of HO-1 expression increased by
BM-MSCs. In addition, HO-1 is an oxidation/antioxidation regulator.
A previous study revealed that BM-MSCs protect against liver I/R
injury by suppressing oxidative stress (7). Therefore, the present study did not
discuss whether BM-MSCs were able to attenuate I/R injury via
HO-1-mediated antioxidant action, which may merit further research.
Furthermore, the present study emphasized the influence of BM-MSCs
on the whole liver tissue as before (18). The present study aimed to
supplement the current protection mechanism of MSCs against liver
I/R injury; the origin of HO-1 or autophagy also requires further
investigation.
In conclusion, the present findings provide evidence
that the protective effects of BM-MSCs may be associated with the
promotion of autophagy by increasing the levels of HO-1 in
vivo.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81670566)
and the Natural Science Foundation of Jiangsu Province, China
(grant no. BK20131084).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW and SW conceived and designed the study,
conducted the experiments, acquired data, analyzed and interpreted
the data, and wrote the manuscript. YZ, HO, ZHZ, and BD conceived
and designed the study, collected data, and wrote the manuscript.
WZ and XLS conceived and designed the study, acquired financial
support and study materials, wrote and gave final approval of the
manuscript. All authors read and approved the manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the
Institutional Animal Care and Use Committee of The Affiliated Drum
Tower Hospital of Nanjing University Medical School, under the
National Institutes of Health (Bethesda, MD, USA) Guide for the
Care and Use of Laboratory Animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
de Groot H and Rauen U:
Ischemia-reperfusion injury: Processes in pathogenetic networks: A
review. Transplant Proc. 39:481–484. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ke B, Lipshutz GS and Kupiec-Weglinski JW:
Gene therapy in liver ischemia and reperfusion injury. Curr Pharm
Des. 12:2969–2975. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim YI: Ischemia-reperfusion injury of the
human liver during hepatic resection. J Hepatobiliary Pancreat
Surg. 10:195–199. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Selzner N, Rudiger H, Graf R and Clavien
PA: Protective strategies against ischemic injury of the liver.
Gastroenterology. 125:917–936. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu A, Huang L, Guo E, Li R, Yang J, Li A,
Yang Y, Liu S, Hu J, Jiang X, et al: Baicalein pretreatment reduces
liver ischemia/reperfusion injury via induction of autophagy in
rats. Sci Rep. 6:250422016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bahde R and Spiegel HU: Hepatic
ischaemia-reperfusion injury from bench to bedside. Br J Surg.
97:1461–1475. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun CK, Chang CL, Lin YC, Kao YH, Chang
LT, Yen CH, Shao PL, Chen CH, Leu S and Yip HK: Systemic
administration of autologous adipose-derived mesenchymal stem cells
alleviates hepatic ischemia-reperfusion injury in rats. Crit Care
Med. 40:1279–1290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang H, Xiang M, Meng D, Sun N and Chen
S: Inhibition of myocardial ischemia/reperfusion injury by exosomes
secreted from mesenchymal stem cells. Stem Cells Int.
2016:43283622016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sheashaa H, Lotfy A, Elhusseini F, Aziz
AA, Baiomy A, Awad S, Alsayed A, El-Gilany AH, Saad MA, Mahmoud K,
et al: Protective effect of adipose-derived mesenchymal stem cells
against acute kidney injury induced by ischemia-reperfusion in
Sprague-Dawley rats. Exp Ther Med. 11:1573–1580. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang CL, Sung PH, Sun CK, Chen CH, Chiang
HJ, Huang TH, Chen YL, Zhen YY, Chai HT, Chung SY, et al:
Protective effect of melatonin-supported adipose-derived
mesenchymal stem cells against small bowel ischemia-reperfusion
injury in rat. J Pineal Res. 59:206–220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin YC, Ko TL, Shih YH, Lin MY, Fu TW,
Hsiao HS, Hsu JY and Fu YS: Human umbilical mesenchymal stem cells
promote recovery after ischemic stroke. Stroke. 42:2045–2053. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Semedo P, Palasio CG, Oliveira CD, Feitoza
CQ, Gonçalves GM, Cenedeze MA, Wang PM, Teixeira VP, Reis MA,
Pacheco-Silva A and Câmara NO: Early modulation of inflammation by
mesenchymal stem cell after acute kidney injury. Int
Immunopharmacol. 9:677–682. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Zhou J, Zhang D, Song Y, She J and
Bai C: Bone marrow-derived mesenchymal stem cells enhance autophagy
via PI3K/AKT signalling to reduce the severity of
ischaemia/reperfusion-induced lung injury. J Cell Mol Med.
19:2341–2351. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rautou PE, Mansouri A, Lebrec D, Durand F,
Valla D and Moreau R: Autophagy in liver diseases. J Hepatol.
53:1123–1134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Denaes T, Lodder J, Chobert MN, Ruiz I,
Pawlotsky JM, Lotersztajn S and Teixeira-Clerc F: The cannabinoid
receptor 2 protects against alcoholic liver disease via a
macrophage autophagy-dependent pathway. Sci Rep. 6:288062016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu S, Wu Y, Ye X, Ma L, Qi J, Yu D, Wei
Y, Lin G, Ren G and Li D: FGF21 ameliorates nonalcoholic fatty
liver disease by inducing autophagy. Mol Cell Biochem. 420:107–119.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang D, Ma Y, Li Z, Kang K, Sun X, Pan S,
Wang J, Pan H, Liu L, Liang D and Jiang H: The role of AKT1 and
autophagy in the protective effect of hydrogen sulphide against
hepatic ischemia/reperfusion injury in mice. Autophagy. 8:954–962.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jung J, Choi JH, Lee Y, Park JW, Oh IH,
Hwang SG, Kim KS and Kim GJ: Human placenta-derived mesenchymal
stem cells promote hepatic regeneration in CCl4-injured rat liver
model via increased autophagic mechanism. Stem Cells. 31:1584–1596.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yun N, Eum HA and Lee SM: Protective role
of heme oxygenase-1 against liver damage caused by hepatic ischemia
and reperfusion in rats. Antioxid Redox Signal. 13:1503–1512. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ke B, Shen XD, Zhang Y, Ji H, Gao F, Yue
S, Kamo N, Zhai Y, Yamamoto M, Busuttil RW, et al: KEAP1-NRF2
complex in ischemia-induced hepatocellular damage of mouse liver
transplants. J Hepatol. 59:1200–1207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ke B, Shen XD, Ji H, Kamo N, Gao F,
Freitas MC, Busuttil RW and Kupiec-Weglinski JW: HO-1-STAT3 axis in
mouse liver ischemia/reperfusion injury: Regulation of TLR4 innate
responses through PI3K/PTEN signaling. J Hepatol. 56:359–366. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Shen J, Xiong X, Xu Y, Zhang H,
Huang C, Tian Y, Jiao C, Wang X and Li X: Remote ischemic
preconditioning protects against liver ischemia-reperfusion injury
via heme oxygenase-1-induced autophagy. PLoS One. 9:e988342014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xia X, Chen W, Ma T, Xu G, Liu H, Liang C,
Bai X, Zhang Y, He Y and Liang T: Mesenchymal stem cells
administered after liver transplantation prevent acute
graft-versus-host disease in rats. Liver Transpl. 18:696–706. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang X, Li J, Nie J, Jiang K, Zhen Z,
Wang J and Shen L: Differentiation character of adult mesenchymal
stem cells and transfection of MSCs with lentiviral vectors. J
Huazhong Univ Sci Technolog Med Sci. 30:687–693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fu J, Zhang H, Zhuang Y, Liu H, Shi Q, Li
D and Ju X: The role of N-acetyltransferase 8 in mesenchymal stem
cell-based therapy for liver ischemia/reperfusion injury in rats.
PLoS One. 9:e1033552014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saidi RF, Rajeshkumar B, Shariftabrizi A,
Bogdanov AA, Zheng S, Dresser K and Walter O: Human adipose-derived
mesenchymal stem cells attenuate liver ischemia-reperfusion injury
and promote liver regeneration. Surgery. 156:1225–1231. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang S, Shi XL, Feng M, Wang X, Zhang ZH,
Zhao X, Han B, Ma HC, Dai B and Ding YT: Puerarin protects against
CCl4-induced liver fibrosis in mice: Possible role of PARP-1
inhibition. Int Immunopharmacol. 38:238–245. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang YH, Yang YL, Tiao MM, Kuo HC, Huang
LT and Chuang JH: Hepcidin protects against
lipopolysaccharide-induced liver injury in a mouse model of
obstructive jaundice. Peptides. 35:212–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schmidt R, Tritschler E, Hoetzel A, Loop
T, Humar M, Halverscheid L, Geiger KK and Pannen BH: Heme
oxygenase-1 induction by the clinically used anesthetic isoflurane
protects rat livers from ischemia/reperfusion injury. Ann Surg.
245:931–942. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Carchman EH, Rao J, Loughran PA, Rosengart
MR and Zuckerbraun BS: Heme oxygenase-1-mediated autophagy protects
against hepatocyte cell death and hepatic injury from
infection/sepsis in mice. Hepatology. 53:2053–2062. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Y, Cai W, Huang Q, Gu Y, Shi Y,
Huang J, Zhao F, Liu Q, Wei X, Jin M, et al: Mesenchymal stem cells
alleviate bacteria-induced liver injury in mice by inducing
regulatory dendritic cells. Hepatology. 59:671–682. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee KC, Lin HC, Huang YH and Hung SC:
Allo-transplantation of mesenchymal stem cells attenuates hepatic
injury through IL1Ra dependent macrophage switch in a mouse model
of liver disease. J Hepatol. 63:1405–1412. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun L, Fan X, Zhang L, Shi G, Aili M, Lu
X, Jiang T and Zhang Y: Bone mesenchymal stem cell transplantation
via four routes for the treatment of acute liver failure in rats.
Int J Mol Med. 34:987–996. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
van Poll D, Parekkadan B, Cho CH,
Berthiaume F, Nahmias Y, Tilles AW and Yarmush ML: Mesenchymal stem
cell-derived molecules directly modulate hepatocellular death and
regeneration in vitro and in vivo. Hepatology. 47:1634–1643. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park M, Kim YH, Woo SY, Lee HJ, Yu Y, Kim
HS, Park YS, Jo I, Park JW, Jung SC, et al: Tonsil-derived
mesenchymal stem cells ameliorate CCl4-induced liver fibrosis in
mice via autophagy activation. Sci Rep. 5:86162015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li X, Zeng Z, Li Q, Xu Q, Xie J, Hao H,
Luo G, Liao W, Bin J, Huang X and Liao Y: Inhibition of
microRNA-497 ameliorates anoxia/reoxygenation injury in
cardiomyocytes by suppressing cell apoptosis and enhancing
autophagy. Oncotarget. 6:18829–18844. 2015.PubMed/NCBI
|
|
37
|
Zhang X, Yan H, Yuan Y, Gao J, Shen Z,
Cheng Y, Shen Y, Wang RR, Wang X, Hu WW, et al: Cerebral
ischemia-reperfusion-induced autophagy protects against neuronal
injury by mitochondrial clearance. Autophagy. 9:1321–1333. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zou Z, Cai Y and Chen Y, Chen S, Liu L,
Shen Z, Zhang S, Xu L and Chen Y: Bone marrow-derived mesenchymal
stem cells attenuate acute liver injury and regulate the expression
of fibrinogen-like-protein 1 and signal transducer and activator of
transcription 3. Mol Med Rep. 12:2089–2097. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao X, Shi X, Zhang Z, Ma H, Yuan X and
Ding Y: Combined treatment with MSC transplantation and neutrophil
depletion ameliorates D-GalN/LPS-induced acute liver failure in
rats. Clin Res Hepatol Gastroenterol. 40:730–738. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lu W, Si YI, Ding J, Chen X, Zhang X, Dong
Z and Fu W: Mesenchymal stem cells attenuate acute
ischemia-reperfusion injury in a rat model. Exp Ther Med.
10:2131–2137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu A, Fang H, Wei W, Dirsch O and Dahmen
U: Ischemic preconditioning protects against liver
ischemia/reperfusion injury via heme oxygenase-1-mediated
autophagy. Crit Care Med. 42:e762–e771. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li SP, He JD, Wang Z, Yu Y, Fu SY, Zhang
HM, Zhang JJ and Shen ZY: miR-30b inhibits autophagy to alleviate
hepatic ischemia-reperfusion injury via decreasing the Atg12-Atg5
conjugate. World J Gastroenterol. 22:4501–4514. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jiang YB, Zhang XL, Tang YL, Ma GS, Shen
CX, Wei Q, Zhu Q, Yao YY and Liu NF: Effects of heme oxygenase-1
gene modulated mesenchymal stem cells on vasculogenesis in ischemic
swine hearts. Chin Med J (Engl). 124:401–407. 2011.PubMed/NCBI
|
|
44
|
Nong K, Wang W, Niu X, Hu B, Ma C, Bai Y,
Wu B, Wang Y and Ai K: Hepatoprotective effect of exosomes from
human-induced pluripotent stem cell-derived mesenchymal stromal
cells against hepatic ischemia-reperfusion injury in rats.
Cytotherapy. 18:1548–1559. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen YT, Sun CK, Lin YC, Chang LT, Chen
YL, Tsai TH, Chung SY, Chua S, Kao YH, Yen CH, et al:
Adipose-derived mesenchymal stem cell protects kidneys against
ischemia-reperfusion injury through suppressing oxidative stress
and inflammatory reaction. J Transl Med. 9:512011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang B, Adesanya TM, Zhang L, Xie N, Chen
Z, Fu M, Zhang J, Zhang J, Tan T, Kilic A, et al: Delivery of
placenta-derived mesenchymal stem cells ameliorates ischemia
induced limb injury by immunomodulation. Cell Physiol Biochem.
34:1998–2006. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gracia-Sancho J, Guixé-Muntet S, Hide D
and Bosch J: Modulation of autophagy for the treatment of liver
diseases. Expert Opin Investig Drugs. 23:965–977. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Awan MU and Deng Y: Role of autophagy and
its significance in cellular homeostasis. Appl Microbiol
Biotechnol. 98:5319–5328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hale AN, Ledbetter DJ, Gawriluk TR and
Rucker EB III: Autophagy: Regulation and role in development.
Autophagy. 9:951–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mizushima N and Levine B: Autophagy in
mammalian development and differentiation. Nat Cell Biol.
12:823–830. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu Q, Guan JZ, Sun Y, Le Z, Zhang P, Yu D
and Liu Y: Insulin-like growth factor 1 receptor-mediated cell
survival in hypoxia depends on the promotion of autophagy via
suppression of the PI3K/Akt/mTOR signaling pathway. Mol Med Rep.
15:2136–2142. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu S, Hartleben B, Kretz O, Wiech T,
Igarashi P, Mizushima N, Walz G and Huber TB: Autophagy plays a
critical role in kidney tubule maintenance, aging and
ischemia-reperfusion injury. Autophagy. 8:826–837. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cursio R, Colosetti P and Gugenheim J:
Autophagy and liver ischemia-reperfusion injury. Biomed Res Int.
2015:4175902015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhao Q, Guo Z, Deng W, Fu S, Zhang C, Chen
M, Ju W, Wang D and He X: Calpain 2-mediated autophagy defect
increases susceptibility of fatty livers to ischemia-reperfusion
injury. Cell Death Dis. 7:e21862016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang JH, Ahn IS, Fischer TD, Byeon JI,
Dunn WA Jr, Behrns KE, Leeuwenburgh C and Kim JS: Autophagy
suppresses age-dependent ischemia and reperfusion injury in livers
of mice. Gastroenterology. 141:2188–2199.e6. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Fang H, Liu A, Dahmen U and Dirsch O: Dual
role of chloroquine in liver ischemia reperfusion injury: Reduction
of liver damage in early phase, but aggravation in late phase. Cell
Death Dis. 4:e6942013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shin JY, Park HJ, Kim HN, Oh SH, Bae JS,
Ha HJ and Lee PH: Mesenchymal stem cells enhance autophagy and
increase β-amyloid clearance in Alzheimer disease models.
Autophagy. 10:32–44. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhou Z and You Z: Mesenchymal stem cells
alleviate LPS-induced acute lung injury in mice by
MiR-142a-5p-Controlled pulmonary endothelial cell autophagy. Cell
Physiol Biochem. 38:258–266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yin F, Meng C, Lu R, Li L, Zhang Y, Chen
H, Qin Y and Guo L: Bone marrow mesenchymal stem cells repair
spinal cord ischemia/reperfusion injury by promoting axonal growth
and anti-autophagy. Neural Regen Res. 9:1665–1671. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yun N, Cho HI and Lee SM: Impaired
autophagy contributes to hepatocellular damage during
ischemia/reperfusion: Heme oxygenase-1 as a possible regulator.
Free Radic Biol Med. 68:168–177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang Y, Xiong X, Guo H, Wu M, Li X, Hu Y,
Xie G, Shen J and Tian Q: ZnPP reduces autophagy and induces
apoptosis, thus aggravating liver ischemia/reperfusion injury in
vitro. Int J Mol Med. 34:1555–1564. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Park J, Kang JW and Lee SM: Activation of
the cholinergic anti-inflammatory pathway by nicotine attenuates
hepatic ischemia/reperfusion injury via heme oxygenase-1 induction.
Eur J Pharmacol. 707:61–70. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Rao J, Qian X, Li G, Pan X, Zhang C, Zhang
F, Zhai Y, Wang X and Lu L: ATF3-mediated NRF2/HO-1 signaling
regulates TLR4 innate immune responses in mouse liver
ischemia/reperfusion injury. Am J Transplant. 15:76–87. 2015.
View Article : Google Scholar : PubMed/NCBI
|