Introduction
Gastric cancer remains the leading cause of cancer
deaths worldwide and adds a significant health burden in many
high-risk areas of cancer, such as Eastern Asia (1). There are many treatments for gastric
cancer, such as surgery, surgery combined with chemotherapy and
radiotherapy. Even so, the survival rate and prognosis of gastric
cancer patients are still poor (2).
Acting as a growth factor, acetylcholine (Ach) can
stimulate cell growth in cholinergic autocrine loop (3,4).
Cancers derived from epithelial and endothelial cells can express a
cholinergic autocrine loop (5–7).
Therefore, Ach secreted by cancer or adjacent cells leads to
stimulate tumor growth when it interacts with M3 muscarinic
receptors, which were expressed on the cancer cells. Moreover,
muscarinic receptors (M1 and M5) can also increase cell
proliferation in majority of cancers derived from epithelial and
endothelial cells (6,8). Consistent with these, gastric cancer
can express muscarinic receptors.
Aclidinium bromide, used for maintenance treatment
of chronic obstructive pulmonary disease (COPD), is a long-acting
inhalable muscarinic antagonist (9) and has a similar affinity for M1 to M5
muscarinic receptor subtypes (10). It has been reported that blockade
of autocrine muscarinic cholinergic signaling with an M3 receptor
antagonist or darifenacin might inhibit the growth of small cell
lung cancer (SCLC) cells both in vitro and in vivo
(4,11). The ability of M3 antagonists to
inhibit tumor progression also has been clearly demonstrated in
colon cancer (12). Muscarinic
agonists have been found to stimulate tumor growth in melanoma
(13), pancreatic cancer (14), breast cancer (15), ovarian cancer (16) and prostate cancer (17). In contrast, muscarinic antagonists
will also inhibit growth of these tumors. However, the role and
mechanism of muscarinic antagonists in gastric cancer has not been
extensively studied.
In this study, a series of in vitro
experiments investigated the role of aclidinium bromide in gastric
cancer MKN-28 cells and its mechanism, in order to provide new
drugs and theoretical basis for the treatment of gastric cancer in
the future.
Materials and methods
Cell culture
Gastric cancer cell line MKN-28 and normal gastric
mucosa GES-1 were purchased from Chinese Academy of Sciences
Shanghai Branch cell bank. The cells were incubated in RPMI-1640
(HyClone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented
with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), 100 U/ml penicillin and 0.1 mg/ml
streptomycin at 37°C in a 5% CO2 atmosphere. Until the
cells grown to logarithmic phase, they were washed 3 times by PBS,
digested with trypsin and then repeatedly beat to single cell
suspension. The cells were then grown into a 6-well plate for
subsequent experiments.
Western blot analysis
The treated cells were placed on ice to extract
protein with RIPA lysis buffer (Beijing CWBIO Biotech Co., Ltd.,
Beijing, China) and heated at 95°C for 5 min. Protein concentration
was detected using BCA Protein Assay kit (Beijing CWBIO Biotech
Co., Ltd.). The cell lysates (20 µg protein/lane) were separated by
SDS-PAGE and then transferred onto PVDF membranes. After blocked
with 5% non-fat dry milk 1 h, the membranes were then incubated
with the following primary antibodies overnight at 4°C: AKT
(1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA), p-AKT
(1:1,000; Cell Signaling Technology, Inc.), mTor (1:1,000; Cell
Signaling Technology, Inc.), p-mTor (1:1,000; Cell Signaling
Technology, Inc.), p70S6K (PTG, 1:1,000), Bcl-2 (PTG, 1:1,000),
active-Caspase3 (1:1,000; ProteinTech Group, Inc., Chicago, IL,
USA), Cyclin D1 (1:1,000; ProteinTech Group, Inc.), GAPDH (1:5,000;
ProteinTech Group, Inc.). After washing 3–5 min by TBST, secondary
antibodies (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) were
incubated at room temperature and developed by ECL (ProteinTech
Group, Inc.) after washing membrane.
Quantity One was used to scan the gray scale values,
and GAPDH was used as the internal control, and the target
protein/internal reference was used to calculate the relative
expression of each protein.
Cell proliferation assay
Cell Counting Kit-8 (CCK-8) assay was used to
evaluate the growth of the cell lines according to the
manufacturer's protocol. Cell suspension (1,000 cells/well) were
cultured in 96-well plates for 24 h, then incubated with different
concentrations of aclidinium bromide (0, 0.1, 1, 10, 20, 50 and 100
µM) and cultured for an additionally 72 h. For CCK-8 detection, 10
µl of CCK-8 solution was added into the wells and the cells were
incubated at 37°C for 1.5 h. The absorbance (optical density, OD)
of cells was determined at 450 nm using a microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The following
experiment drug concentration (10 µM) was chosen from the series of
the gradient concentration. Afterward, cells were incubated with 10
µM aclidinium bromide and vehicle (DMSO, 0.1% in culture media) for
24, 48, 72 h. Cells viabilities were measured by CCK-8 assay and OD
values were measured as above described.
Invasion and migration assay
Cell invasion was determined by using 24-well
transwell invasion chamber (EMD Millipore, Billerica, MA, USA). A
total of 100 µl matrigel (BD Biosciences, Franklin Lakes, NJ, USA)
were added to the upper chambers and stood 4–6 h at 37°C in a
CO2 incubator. The lower chambers were filled with
serum-free media and stood 0.5 h to hydrate basilar membrane. After
incubation for 24 h, cell suspension (1×105 cells/100 µl
serum-free MEM media) were added to the upper chambers and complete
culture solution (500 µl) were added to the lower chambers. To stay
overnight, cells were washed in PBS, fixed for 30 min in 4%
paraformaldehyde, stained for 20 min with 0.1% crystal violet.
After the indicated treatments, five fields were randomly selected
under the microscope and representative photographs of stained
cells were taken to observation and count.
The migration assay was similar to the invasion
assay, however, the transwell chamber was not required to use
matrigel matrix and the number of cells would be 5,000.
Flow cytometry analysis of cell
apoptosis
After the cells were treated for 24 h, the medium
was removed and replaced with serum-free medium to starvation for
24 h under normal conditions. Following the treatment, cells were
trypsinized without EDTA and collected in a centrifuge tube, then
the cells were centrifuged at 1,000 × g for 5 min. Afterwards cells
were resuspended in PBS pre-cooled at 4°C, centrifuged again and
carefully aspirated the supernatant. After that we added 1X binding
buffer to resuspend the cells, and regulated the cell density of
1–5×106/ml. 100 µl of the cells were gently mixed with 5
µl Annexin V-FITC/ PI (Beijing 4A Biotech Co., Ltd., Beijing,
China) and incubated in the dark for 5 min at room temperature. 10
µl of PI dye and 400 µl of PBS were added to the sample, and the
results were analyzed in a flow cytometer using Flowjo.
Statistical analysis
All statistical analyses were performed using SPSS
18.0 (SPSS, Inc., Chicago, IL, USA) software. The comparison
between two groups were performed by Student's t-test and for >2
groups used analysis of variance followed by Dunnett's post hoc
test. Data were expressed as mean ± standard deviation, and the
criteria for all statistical significances set as P<0.05.
Results
Aclidinium bromide inhibits the
proliferation of gastric cancer MKN-28 cells
CCK-8 was conducted to investigate the effect of
aclidinium bromide on the proliferation of gastric cancer cells. A
dose response in proliferation was observed as a decrease in MKN-28
cells from 0.1 to 100 µM of aclidinium bromide, while the
proliferation of GES-1 cells was inhibited by aclidinium bromide
beyond 50 µM (Fig. 1A).
Consequently, 10 µM of aclidinium bromide was selected to perform
following experiments. In addition, MKN-28 cells proliferation was
assessed by measuring the OD values at increasing time-points. As
shown in Fig. 1B, OD values of
MKN-28 cells were decreased with a time-dependent manner. At 48 and
72 h, the OD value was significant inhibited in aclidinium bromide
treated group (P<0.01). These results suggested that aclidinium
bromide impeded growth of gastric cancer cells.
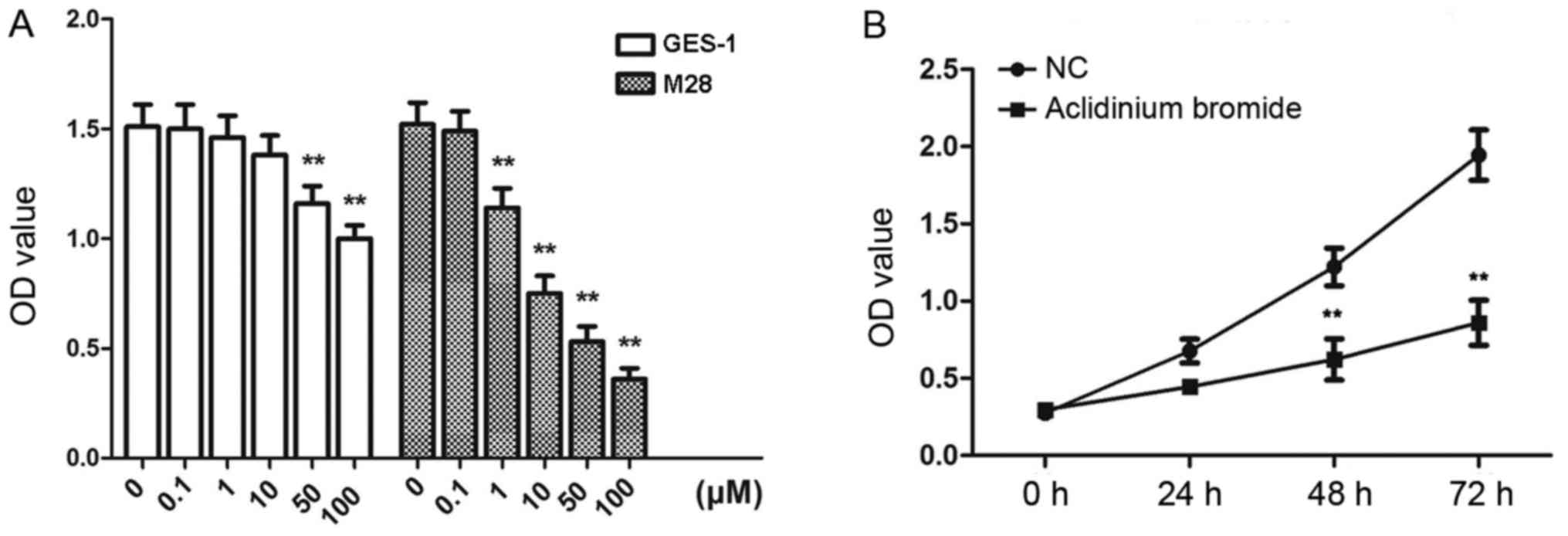 | Figure 1.Suppressive effects of aclidinium
bromide on gastric cancer MKN-28 cell proliferation. (A) The OD
values of MKN-28 and GES-1 cells treated with aclidinium bromide at
0, 0.1, 1, 10, 50, 100 µM. **P<0.01 vs. 0 µM group. (B) The OD
values of MKN-28 cells treated with aclidinium bromide following 0,
24, 48 and 72 h. **P<0.01 vs. NC group. The data are presented
as the mean ± standard deviation. OD, optical density; NC, negative
control; M28, MKN-28 cells. |
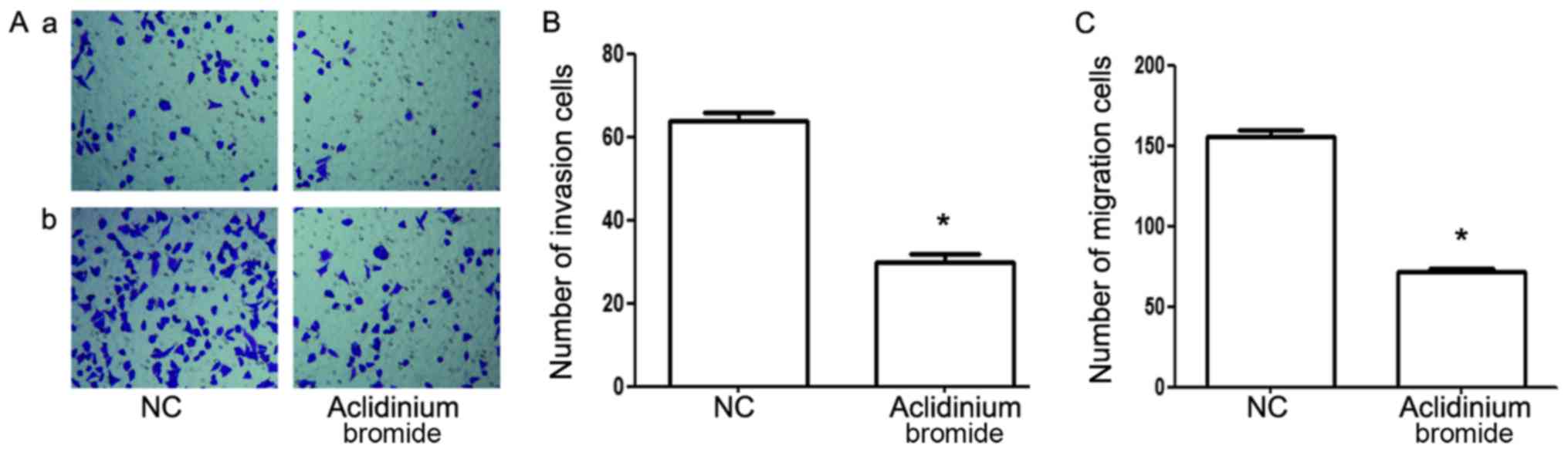
Aclidinium bromide inhibits the
invasion and migration of gastric cancer MKN-28 cells
To explore the effect of aclidinium bromide on
metastasis of gastric cancer MKN-28 cells, transwell assay and
matrigel invasion assay were carried out. As shown in Fig. 2, matrigel invasion analysis showed
the number of invasive cells were significantly decreased in
aclidinium bromide treated group (30±2) compared with that in the
NC group (64±2) (P<0.05; Fig. 2Aa
and B). Furthermore, aclidinium bromide led to the remarkable
reduction of the number of MKN-28 cells passing though the
microwells of the transwell chamber (156±4>72±2, P<0.05;
Fig. 2Ab and C). Collectively, the
results showed that aclidinium bromide could significantly inhibit
the invasion and migration potential of MKN-28 cells.
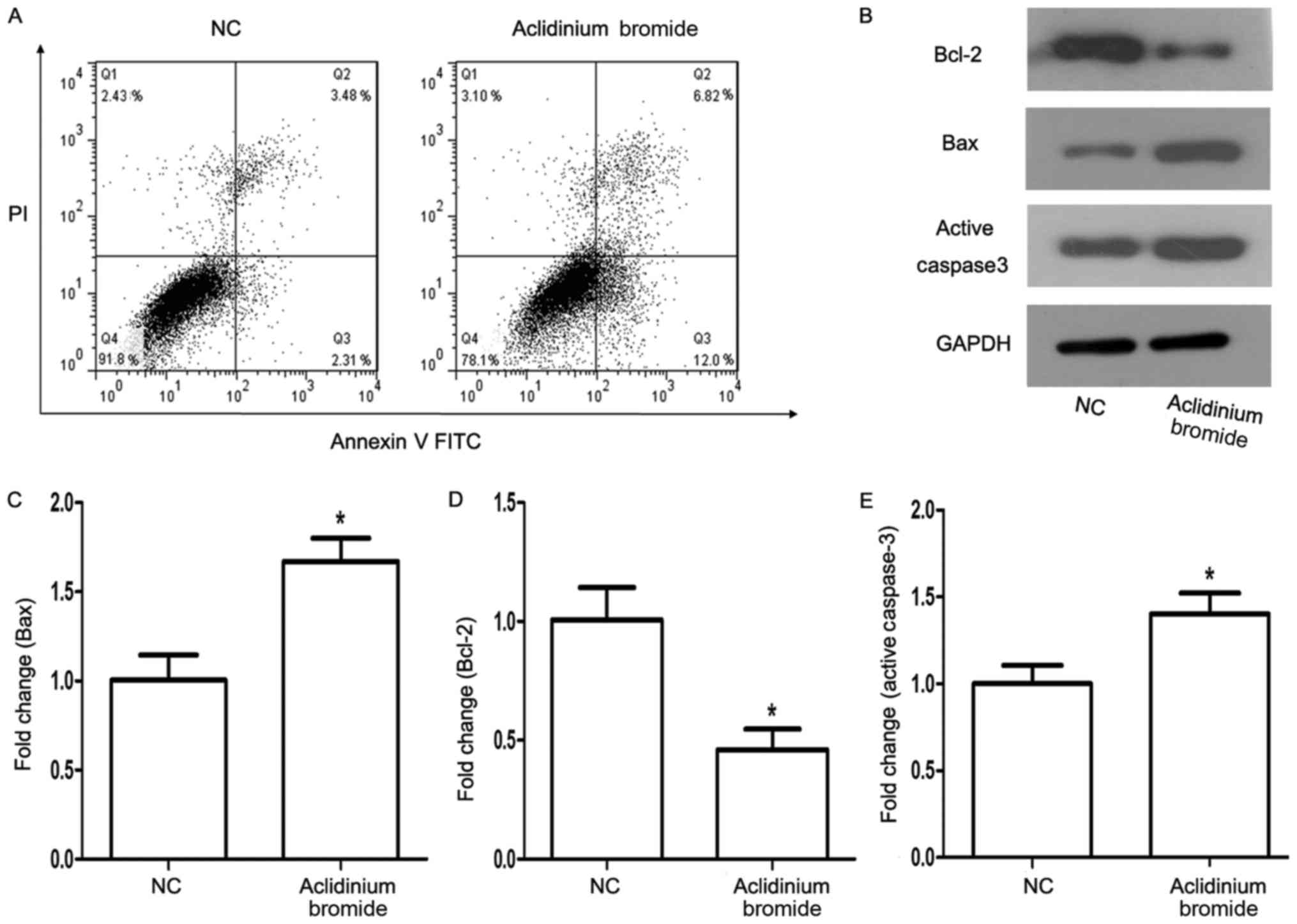
Aclidinium bromide induces apoptosis
of gastric cancer MKN-28 cells
To explore whether the role of aclidinium bromide in
MKN-28 cells was correlated with the apoptosis, flow cytometry
after Annexin V-FITC/PI staining and western blot assay were used.
The flow cytometry analysis showed that the apoptosis of cells
treated with aclidinium bromide was significantly increased (18.82
>5.79%, P<0.05, Fig. 3A).
Next, we detected the expression of apoptosis-related protein
Bcl-2, Bax, and Active Caspase3 after aclidinium bromide treatment
using western blotting (Fig. 3B).
As expected, the expression levels of anti-apoptotic protein BCL-2
decreased (P<0.05; Fig. 3D),
and the expression of pro-apoptotic protein Bax and Active Caspase3
increased concurrently (P<0.05; Fig. 3C and E). Therefore, we considered
that aclidinium bromide could induce the apoptosis of MKN-28
cell.
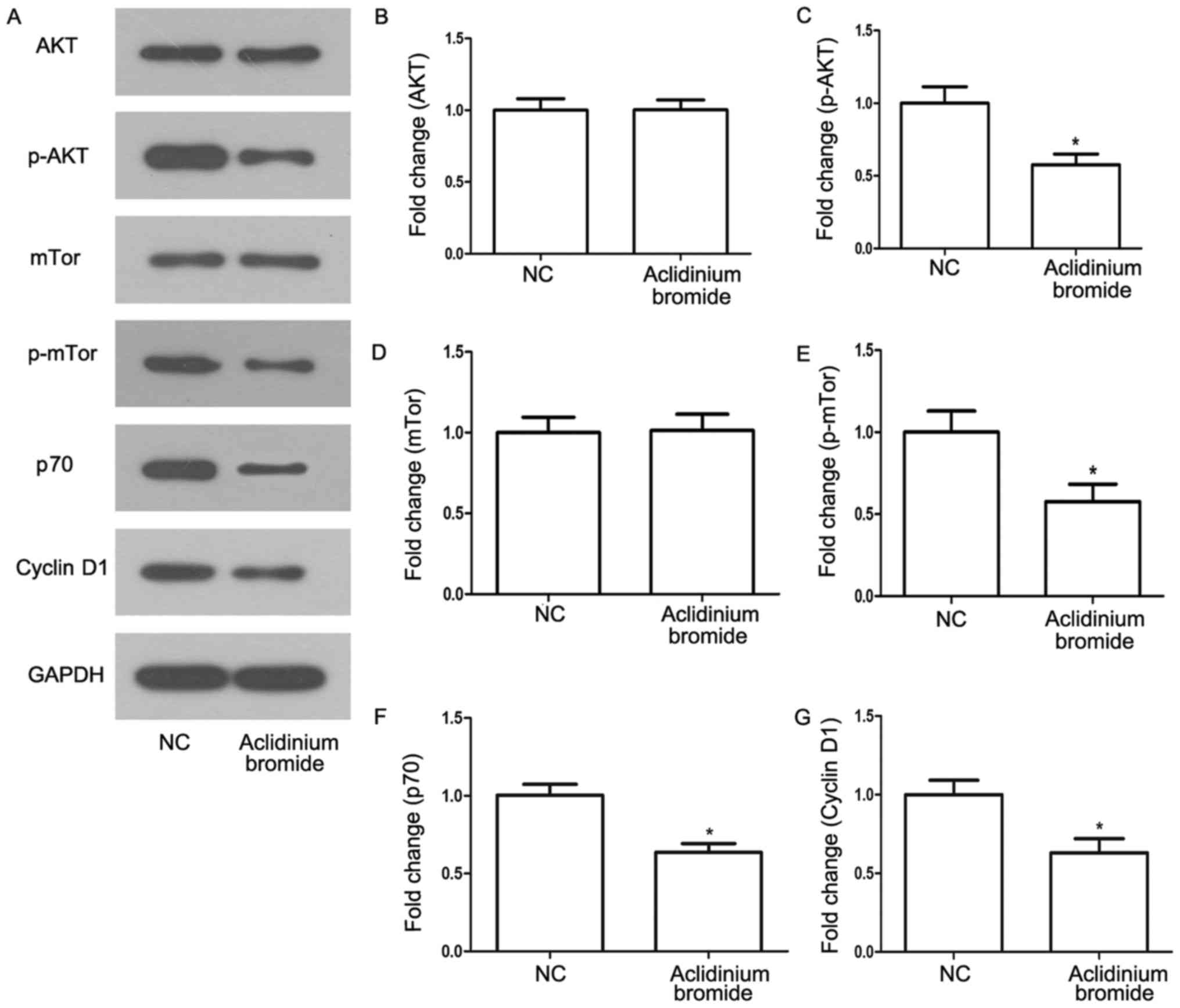
Aclidinium bromide inhibits the
phosphatidylinositol-3-kinase (PI3K) signaling pathway in gastric
cancer MKN-28 cells
PI3K/AKT/mTor signaling pathway is well-known
pathway associated with cell proliferation, survival and
metabolism. It is often activated in numerous types of tumors
(18–20). In order to know whether the effect
of aclidinium bromide on cells via the signaling pathway, we
determined the effects of aclidinium bromide on activation of
protein related with PI3K signaling pathway by western bolt assay
(Fig. 4A). Accordingly, we found
the phosphorylation of AKT and mTor were significantly inhibited in
MKN-28 cells treated with aclidinium bromide (Fig. 4C and E, P<0.05). Besides,
aclidinium bromide administration attenuated activity of the
downstream proteins such as p70 ribosomal protein S6 kinase
(p70S6K) and Cyclin D1 related to cell growth (P<0.05; Fig. 4F and G). Therefore, aclidinium
bromide inhibited the activation of PI3K signaling pathway in
gastric cancer MKN-28 cells.
Discussion
Recently, the ability of Ach and muscarinic
receptors to regulate tumor cell proliferation has been
significantly concerned (21). In
this study we focused on the growth inhibitory effect of aclidinium
bromide on gastric cancer cells and the possible mechanisms. In
MKN-28 cells, aclidinium bromide displayed significant inhibited
proliferation, invasion and migration ability. Next, we found
aclidinium bromide could promote apoptosis. In addition, PI3K
signaling pathway was also involved in the progression of gastric
cancer cells, by which aclidinium bromide might inhibit the
migration and proliferation of these cells.
A large number of studies have shown that many
components of cholinergic signaling including Ach are present in a
variety of non-neuronal tissues, such as lung cancer (21,22),
colon (23), breast, ovarian
carcinomas (24,25) and other common cancer cells.
Interestingly, cholinergic signaling were found to increase further
in the local tumor environment, representing a potential new
pathway to target tumor growth (26). As can be seen, cholinergic
antagonists may provide a directed pathway to prevent tumor
proliferation by interrupting the upregulated autocrine signaling.
As we found in MKN-28 cells that aclidinium bromide displayed
significant inhibition of proliferation, invasion and migration
activity. Cell cycle arrest is a major mechanism preventing tumor
growth. Cesario et al (27)
reported that inhibition of a7-nAChR with a powerful high affinity
antagonist a-CbT induced antitumor activity in NSCLC and pleural
mesothelioma by triggering apoptosis. Our results from flow
cytometric analysis showed that aclidinium bromide markedly
suppressed the expression of anti-apoptotic protein BCL-2 and
increased the expression of pro-apoptotic protein BAX and Active
Caspase3, which meant that aclidinium bromide could promote
apoptotic of MKN-28 cells.
As a classical and important signaling pathway,
PI3K/Akt is often activated in many human cancer types, and is
thought to be associated with the characteristics of
carcinogenesis, including cell proliferation, apoptosis and
metabolism (28,29). ACh can rapidly increases the amount
of intracellular calcium in lung cancer cell lines, leading to the
activation of M3 receptors, eventually resulting in activation of
Akt and MAPK (19). Besides, this
activation then inhibit cell proliferation by M3 antagonists.
Coincidentally, we found that the phosphorylation of Akt and mTOR
was significantly inhibited in MKN-28c ells treated with aclidinium
bromide, so we believed aclidinium bromide inhibited the PI3K
signaling pathway in gastric cancer MKN-28 cells. In this study, we
also found that activity of p70S6K and Cyclin D1 was also inhibited
and we identified they were the downstream targets of AKT that
mediated acilidinium-inhibition. In summary, we found that PI3K
signaling pathway may be involved in the aclidinium bromide-induced
inhibition of MKN-28 cell proliferation, invasion and migration
ability, meanwhile, the apoptosis of MKN-28 cells treated with
aclidinium bromide may be achieved via the signaling pathway.
Unfortunately, given the limited experimental tools,
we only select the MKN-28 cell line for the present study, and
other gastric cancer cell lines should be further studied. Even so,
we found that aclidinium bromide could inhibit proliferation,
invasion and migration, and induce apoptosis in gastric cancer
MKN-28 cells. In addition, we further demonstrated that aclidinium
bromide caused the above effects via the PI3K signaling pathway.
This novel finding provides a useful strategy for chemoprevention
and/or treatment of gastric cancer. Next, we will conduct a series
of in vivo experiments about this drug in patients with
gastric cancer as an area of future research.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZW contributed to the conception and design of the
study and provided administrative support. PC and JJL contributed
the study materials. JJL, HXW and JM performed data collection and
assembly. HXW, PC and JM analyzed and interpreted the data. All
authors contributed to the writing and editing of the manuscript,
and all authors gave final approval of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lochhead P and El-Omar EM: Molecular
predictors of gastric neoplastic progression. Cancer Cell. 33:9–11.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cazzola M, Calzetta L, Page CP, Rogliani
P, Facciolo F, Gavaldà A and Matera MG: Pharmacological
characterization of the interaction between aclidinium bromide and
formoterol fumarate on human isolated bronchi. Eur J Pharmacol.
745:135–143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ami N, Koga K, Fushiki H, Ueno Y, Ogino Y
and Ohta H: Selective M3 muscarinic receptor antagonist inhibits
small-cell lung carcinoma growth in a mouse orthotopic xenograft
model. J Pharmacol Sci. 116:81–88. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang L, Zhi X, Zhang Q, Wei S, Li Z, Zhou
J, Jiang J, Zhu Y, Yang L, Xu H and Xu Z: Muscarinic receptor M3
mediates cell proliferation induced by acetylcholine and
contributes to apoptosis in gastric cancer. Tumour Biol.
37:2105–2117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang Y and Le W: Differential roles of M1
and M2 microglia in neurodegenerative diseases. Mol Neurobiol.
53:1181–1194. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gentry PR, Kokubo M, Bridges TM, Cho HP,
Smith E, Chase P, Hodder PS, Utley TJ, Rajapakse A, Byers F, et al:
Discovery, synthesis and characterization of a highly muscarinic
acetylcholine receptor (mAChR)-selective M5-orthosteric antagonist,
VU0488130 (ML381): A novel molecular probe. ChemMedChem.
9:1677–1682. 2014.PubMed/NCBI
|
|
8
|
Tully BT, Li M, Sun Y, Berkowitz J and
Chai TC: Defects in muscarinic receptor cell signaling in bladder
urothelial cancer cell lines. Urology. 74:467–473. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bateman ED, Chapman KR, Singh D, D'Urzo
AD, Molins E, Leselbaum A and Gil EG: Aclidinium bromide and
formoterol fumarate as a fixed-dose combination in COPD: Pooled
analysis of symptoms and exacerbations from two six-month,
multicentre, randomised studies (ACLIFORM and AUGMENT). Respir Res.
16:922015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Melani AS: Long-acting muscarinic
antagonists. Expert Rev Clin Pharmacol. 8:479–501. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
von Rosenvinge EC, Cheng K, Drachenberg
CB, Fowler CB, Evers DL, Xie G and Raufman JP: Bedside to bench:
role of muscarinic receptor activation in ultrarapid growth of
colorectal cancer in a patient with pheochromocytoma. Mayo Clin
Proc. 88:1340–1346. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu ZP, Song Y, Yang K, Zhou W, Hou LN, Zhu
L, Chen HZ and Cui YY: M3 mAChR-mediated IL-8 expression through
PKC/NF-κB signaling pathways. Inflamm Res. 63:463–473. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Spindel ER: Muscarinic receptor agonists
and antagonists: Effects on cancer. Handb Exp Pharmacol. 451–468.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ehlert FJ, Pak KJ and Griffin MT:
Muscarinic agonists and antagonists: Effects on gastrointestinal
function. Handb Exp Pharmacol. 343–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lombardi MG, Negroni MP, Pelegrina LT,
Castro ME, Fiszman GL, Azar ME, Morgado CC and Sales ME:
Autoantibodies against muscarinic receptors in breast cancer: Their
role in tumor angiogenesis. PLoS One. 8:e575722013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Trkulja V, Crljen-Manestar V, Banfic H and
Lackovic Z: Involvement of the peripheral cholinergic muscarinic
system in the compensatory ovarian hypertrophy in the rat. Exp Biol
Med (Maywood). 229:793–805. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pagès G, Durivault J, Hannoun-Levi JM,
Viera A and Ortholan C: 45 POSTER VEGF targeting increases the
cystostatic effect of docetaxel on prostate and breast tumor cells;
a new interpretation of the therapeutic effect. Eur J Cancer
Supplements. 6:182008. View Article : Google Scholar
|
|
18
|
Li D, Wei X, Ma M, Jia H, Zhang Y, Kang W,
Wang T and Shi X: FFJ-3 inhibits PKM2 protein expression via the
PI3K/Akt signaling pathway and activates the mitochondrial
apoptosis signaling pathway in human cancer cells. Oncol Lett.
13:2607–2614. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu R, Shang C, Zhao J, Han Y, Liu J, Chen
K and Shi W: Activation of M3 muscarinic receptor by acetylcholine
promotes non-small cell lung cancer cell proliferation and invasion
via EGFR/PI3K/AKT pathway. Tumour Biol. 36:4091–4100. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang X, Shi H, Tang H, Fang Z, Wang J and
Cui S: miR-218 inhibits the invasion and migration of colon cancer
cells by targeting the PI3K/Akt/mTOR signaling pathway. Int J Mol
Med. 35:1301–1308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arrighi N, Bodei S, Zani D, Michel MC,
Simeone C, Cunico Cosciani S, Spano P and Sigala S: Different
muscarinic receptor subtypes modulate proliferation of primary
human detrusor smooth muscle cells via Akt/PI3K and map kinases.
Pharmacol Res. 74:1–6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cabadak H, Aydin B and Kan B: Regulation
of M2, M3, and M4 muscarinic receptor expression in K562 chronic
myelogenous leukemic cells by carbachol. J Recept Signal Transduct
Res. 31:26–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Raufman JP, Cheng K, Saxena N, Chahdi A,
Belo A, Khurana S and Xie G: Muscarinic receptor agonists stimulate
matrix metalloproteinase 1-dependent invasion of human colon cancer
cells. Biochem Biophys Res Commun. 415:319–324. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Parnell EA, Calleja-Macias IE, Kalantari
M, Grando SA and Bernard HU: Muscarinic cholinergic signaling in
cervical cancer cells affects cell motility via ERK1/2 signaling.
Life Sci. 91:1093–1098. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu Y, Wang S, Chan HF, Lu H, Lin Z, He C
and Chen M: Dihydromyricetin induces apoptosis and reverses drug
resistance in ovarian cancer cells by p53-mediated downregulation
of survivin. Sci Rep. 7:460602017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao Q, Gu X, Zhang C, Lu Q, Chen H and Xu
L: Blocking M2 muscarinic receptor signaling inhibits tumor growth
and reverses epithelial-mesenchymal transition (EMT) in non-small
cell lung cancer (NSCLC). Cancer Biol Ther. 16:634–643. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cesario A, Russo P, Nastrucci C and
Granone P: Is α7-nAChR a possible target for lung cancer and
malignant pleural mesothelioma treatment? Current drug targets.
13:688–694. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao R, Chen M, Jiang Z, Zhao F, Xi B,
Zhang X, Fu H and Zhou K: Platycodin-D induced autophagy in
non-small cell lung cancer cells via PI3K/Akt/mTOR and MAPK
signaling pathways. J Cancer. 6:623–631. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yuan L, Wei S, Wang J and Liu X:
Isoorientin induces apoptosis and autophagy simultaneously by
reactive oxygen species (ROS)-related p53, PI3K/Akt, JNK, and p38
signalin pathways in HepG2 cancer cells. J Agric Food Chem.
62:5390–5400. 2014. View Article : Google Scholar : PubMed/NCBI
|