Introduction
Hyperoxia liquids (HOS) is medical liquid containing
high concentration of dissolved oxygen (DO). HOS is mainly prepared
using the electrochemistry and rapid physical replacement of DO
mechanisms. A large amount of O2 and little
O3 are dissolved into the commonly used medical liquid
in clinic. The oxygen partial pressure in the liquid is 153.7 mmHg
and the DO content is 3.8 mmg before oxygen dissolving. However,
the oxygen partial pressure has reached 795 mmHg after oxygen
dissolving. Meanwhile, its O2 content is 5.3-fold of
that of conventional liquid, as well as 8.3-fold and 21.3-fold of
that in arterial and venous blood, respectively. The O3
concentration in the hyperoxic fluid measured immediately after
oxygen dissolving using iodimetry is 26.5 µg/ml. 20 min later,
O3 is completely reduced to the dissolved O2.
HOS can directly improve the blood oxygen partial pressure and
blood DO content after it enters the blood. Meanwhile, it can
indirectly enhance the chemically bonded oxygen (oxygen
saturation). Currently, HOS is used in auxiliary oxygen supply
treatment for multiple diseases with hypoxic symptoms. They include
the first-aid in critical patients, cardiopulmonary-cerebral
resuscitation, central respiration repression, as well as severe
head and face combined injury. In addition, it is also used in
respiratory tract burn, pulmonary interstitial fibrosis, coal
pneumoconiosis, pneumoconiosis, respiratory failure, one-lung
ventilation during thoracic surgery, cerebral ischemia, myocardial
ischemia, severe acute respiratory syndrome and high altitude
hypoxia. Notably, favorable auxiliary effects have been
attained.
Cardiopulmonary bypass (CPB) has been applied in
cardiac and major vascular surgery for over half a century.
Understanding towards CPB professional theoretical knowledge has
been enhanced in recent years (1).
In addition, medical materials, management level and surgical
techniques have been improved (1).
Therefore, the postoperative mortality and incidence of
complications have been greatly reduced (2). This contributes to saving the lives
of millions of patients. However, the CPB injury to the heart
remains a research hotspot (2).
According to literature report, the incidence of arrhythmia after
CPB is related to underlying disease, surgical type and
intraoperative myocardial protection (3). The incidence after surgery for
congenital heart disease is 40–60%, while that after valve
replacement for valvular heart disease is 70–84%. It has extended
the length of stay in patients (3). Moreover, it will add to the incidence
of complications and risk of death (4). Thus, it will cause tremendous
economic burdens on both the family and the society. The mechanism
of CPB in cardiac injury is quite complicated. It is mainly induced
by cardiac ischemic damage, ventricular hyperinflation or
emptiness, ascending aorta blocking and ischemia/reperfusion (I/R)
injury (4).
Myocardial ischemia injury refers to abnormal
myocardial energy metabolism, myocardial structural and functional
changes derived (5) from reduced
cardiac blood supply and hypoxidosis. Its pathogenesis is related
to multi-layer and multi-target factors. Clinical and experimental
studies suggest that oxidative stress plays a vital role in the
genesis and development of myocardial ischemic injury diseases
(6). Myocardial ischemia is
accompanying with excessive production and accumulation of reactive
oxygen species (ROS). It will induce endogenous oxidative stress
and lipid peroxidation of unsaturated fatty acid on myocardial cell
membrane (6). On the one hand, it
will promote myocardial cell apoptosis and myocardial tissue
necrosis. On the other hand, it will lead to changed cell membrane
permeability and fluidity. Therefore, it will induce leakage of
excessive intracellular myocardial enzymes and aggravate myocardial
ischemia injury (7). Thus,
effective intervention of the endogenous oxidative stress is an
important pathway for improving myocardial ischemia injury and
protecting myocardial tissue (7).
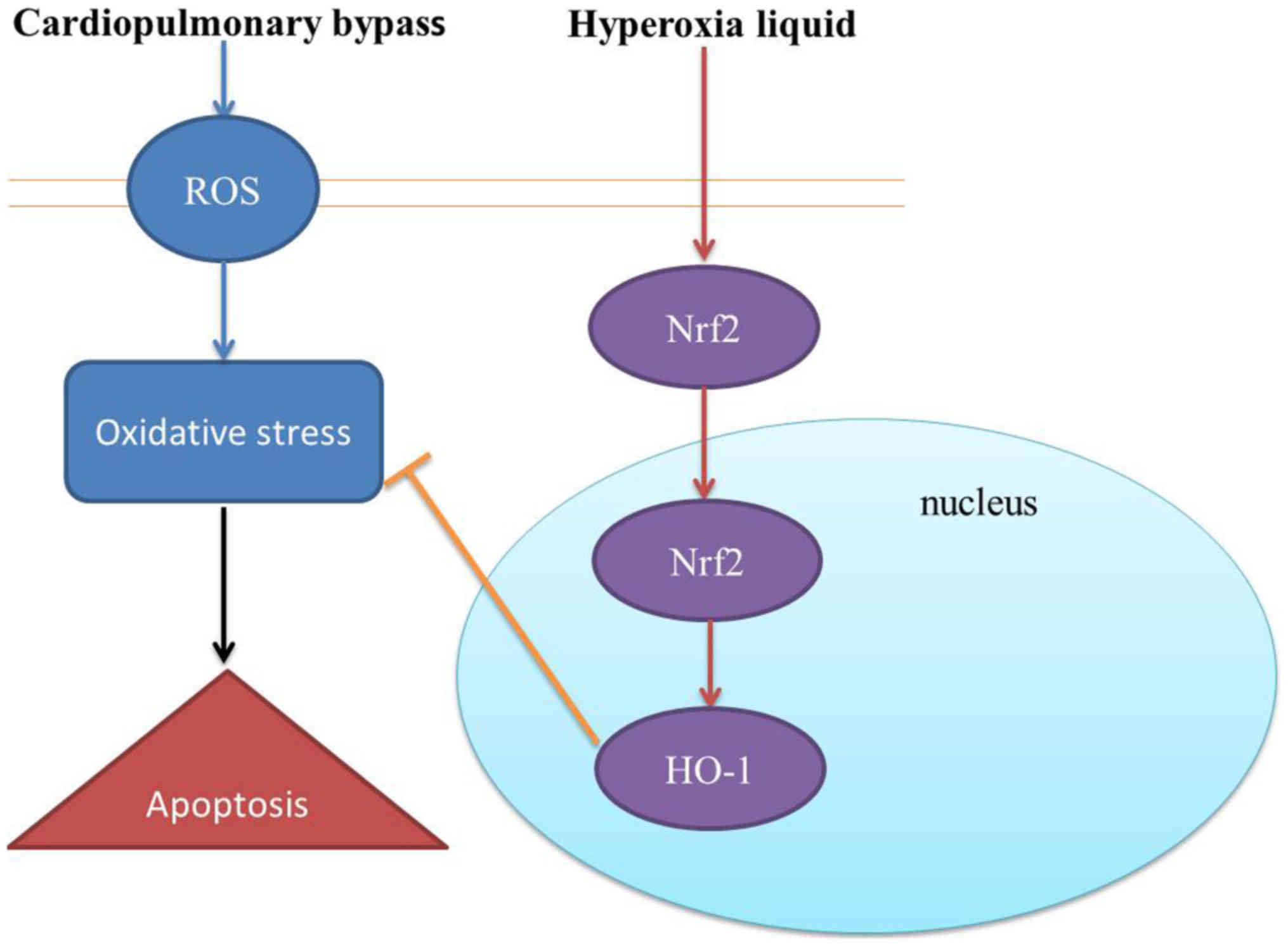
The nuclear factor erythroid 2-related factor 2
(Nrf2) pathway is the most important endogenous anti-oxidative
stress pathway discovered recently (8). It plays a pivotal role in response to
oxidative stress. Moreover, it is extensively distributed in the
cardiovascular system (8). It is
also closely related to improving myocardial ischemia injury.
Generally, it exerts its anti-oxidative effect through two aspects
(9). On the one hand, oxidative
stress can promote Nrf2 mRNA transcription and increase Nrf2
protein synthesis (8). On the
other hand, Nrf2 dissociates with its chaperonin Keap1 in the case
of electrophilic substance, ROS or upstream signaling pathway
stimulation (8). As a result,
increased amount of Nrf2 transfers from cytoplasm to cell nucleus.
Subsequently, it binds with antioxidant response element (ARE) in
the form of heterodimer with tendon fibrosarcoma protein (8,10).
Later, it up-regulates the expression of the downstream phase II
detoxifying enzyme and antioxidase genes NQO1 and heme oxygenase-1
(HO-1). Thus, it can alleviate oxidative stress injury. HO-1
reduced prooxidant and attenuate cardiac hypertrophy through ROS
production. Nrf2 upregulates HO-1 in different cell models to
suppress oxidative stress in different cell. The protective role of
hyperoxia liquid regulates CPB-induced myocardial damage and its
possible mechanism.
Materials and methods
Animals and treatment
Thirty adult male Sprague-Dawley rats (200–230 g)
were purchased from the Center of Experimental Animal in Shandong
Provincial Hospital Affiliated to Shandong University and were
housed in individual cages with free access to food and water and
maintained at an ambient temperature of 22±2°C, on a 12-h
light/dark cycle (8:00 a.m.-8:00 p.m.). All rats (n=18) were
randomly allocated into three groups: control (n=6), model (n=6)
and Hyperoxia liquids group (n=6). Pre-treatment with 15 ml/kg
hyperoxia liquids for 7 day was intravenous injected into rat of
hyperoxia liquids group. After 7 day, rat of model or hyperoxia
liquids were anesthetized i.p. with 30 mg/kg sodium pentobarbital
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Heparin (150 U)
and vecuronium bromide (2 mg/kg IV) was induced by switching off
the ventilator for 30 min. Resuscitation was started with the
initiation of CPB flow and resumption of ventilation. The slipknot
was released and rat was received 120 min of reperfusion.
Next, all rats were randomly allocated into four
groups (n=24): Control (n=6), model (n=6), Hyperoxia liquids group
(n=6) and ML385 (n=6). In ML385 group, pre-treatment with 15 ml/kg
Hyperoxia liquids and 5 mg/kg of ML385 for 7 day was intravenous
injected into rat of hyperoxia liquids group. Other experimental
procedure was invariability. The present study was approved by the
Ethical Approval Committee of Shandong Provincial Hospital
Affiliated to Shandong University (Shandong, China).
Detection of cardiac function
Rats were anesthetized with 30 mg/kg sodium
pentobarbital and a catheter was inserted into the left ventricle,
and which was used to measure left ventricular ejection fraction
(LVEF) and left ventricular internal dimension systole (LVIDs).
Measurement of serum oxidative stress
and tissue caspase-3/8/9 activity
Serum was collected by centrifugation at 1,000 × g
for 10 min at 4°C and used to analyze the levels of malondialdehyde
(MDA; S0131), superoxide dismutase (SOD; S0101), glutathione (GSH;
S0053), glutathione-peroxidase (GSH-PX; S0056) using ELISK kits
(Beyotime Institute of Biotechnology, Nanjing, China). Total
proteins of the cell were extracted by using RIPA assay and Protein
concentration was estimated by BCA assay. Protein (10 µg) was used
to analyze caspase-3/8/9 activity using caspase-3/8/9 activity kits
(C1116, C1152, C1158; Beyotime Institute of Biotechnology).
Western blot analysis
Total proteins of the cell were extracted by using
RIPA assay and Protein concentration was estimated by BCA assay.
Protein (30 µg) was loaded for 10% SDS-polyacrylamide gel
electrophoresis and transferred onto polyvinylidene difluoride
membranes. The membranes were blocked with 5% non-fat milk in TBS
and incubated with Nrf2 (sc-722, 1:1,000; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), HO-1 (sc-10789, 1:1,000; Santa Cruz
Biotechnology, Inc.) and GAPDH (sc-25778, 1:1,000; Santa Cruz
Biotechnology, Inc.) at 4°C overnight. The membranes were washed
with TBS containing 0.1% Tween-20 and incubated with horseradish
peroxidase-conjugated secondary antibodies (sc-2004, 1:5,000; Santa
Cruz Biotechnology, Inc.) for 1 h. Protein expression were detected
by Amersham ECL Plus Western Blotting Detection kit (GE Healthcare
Life Sciences, Piscataway, NJ, USA) and analyzed using
Image-ProPlus 6.0 software (Media Cybernetics, Inc., Rockville, MD,
USA).
Statistical analysis
Results are expressed as the mean ± standard error
of the mean. Statistical analysis was performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). Differences between groups
were calculated using one-way analysis of variance with Tukey's
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
The effects of hyperoxia liquid
regulated heart cell apoptosis in CPB induced rat model
Our study investigated the effects of hyperoxia
liquid in regulating heart cell apoptosis in CPB induced rat model.
H&E staining showed that heart cell apoptosis in CPB induced
rat model was higher than that of control group (Fig. 1A). The effects of hyperoxia liquid
inhibited heart cell apoptosis in CPB rat, compared with CPB
induced rat model group (Fig. 1A).
The LVEF was inhibited, and LVIDs level was increased in CPB
induced rat model, compared with CPB induced rat model group
(Fig. 1B and C). Hyperoxia liquid
enhanced LVEF and reduced LVIDs level in CPB induced rat model,
compared with CPB rat (Fig. 1B and
C). Caspase-3/8/9 activity levels in CPB were elevated,
compared with control group (Fig.
1D-F). In addition, hyperoxia liquid reduced the CPB-induced
Caspase-3/8/9 activity levels in rat, compared with CPB induced rat
model (Fig. 1D-F).
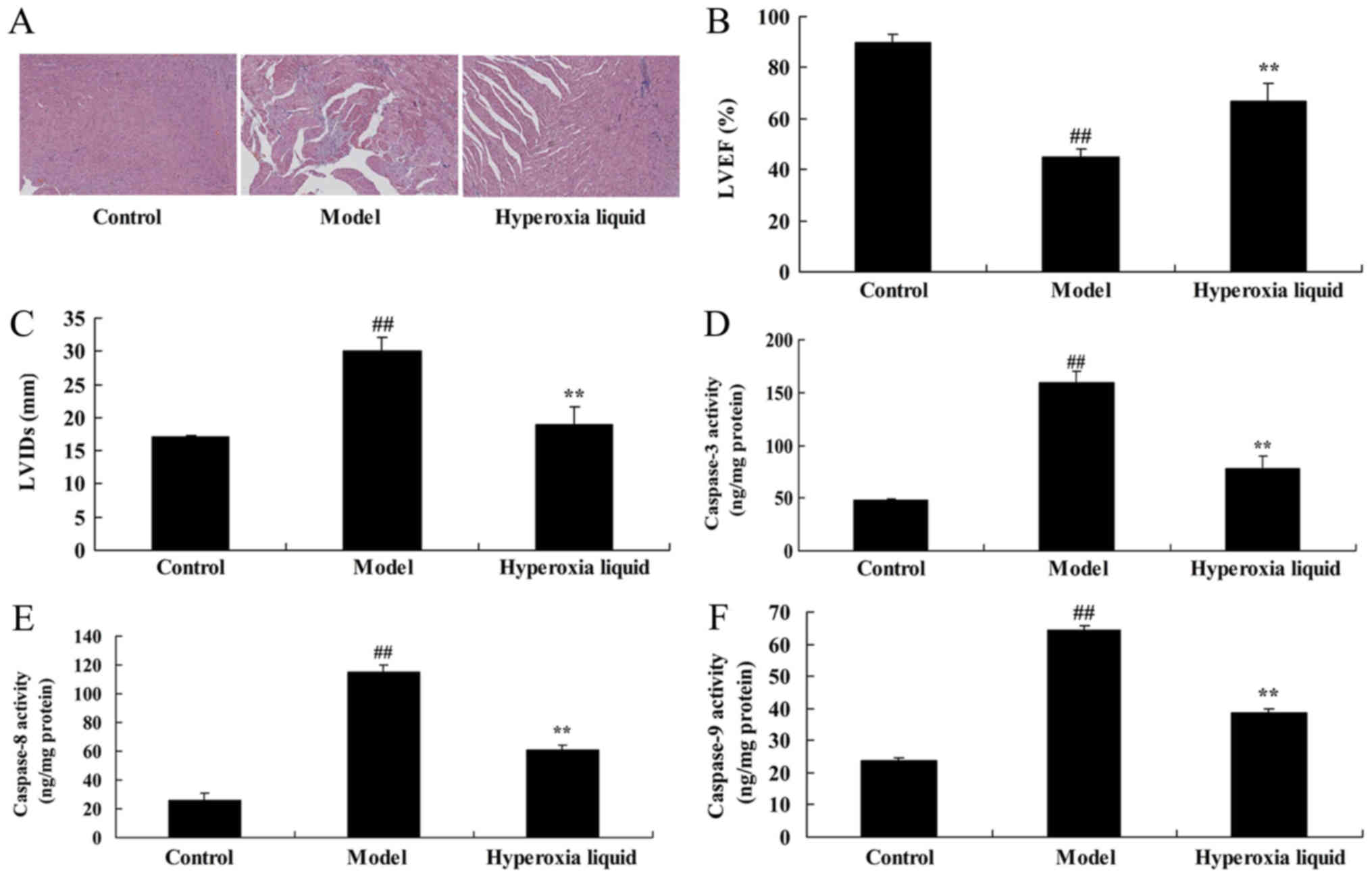 | Figure 1.Effects of hyperoxia liquid regulation
on heart cell apoptosis in a CPB-induced rat model. (A) Hematoxylin
and eosin staining of heart tissues (magnification, ×5). Levels of
(B) LVEF, (C) LVIDs, and (D) caspase-3, (E) caspase-8 and (F)
caspase-9 activity. ##P<0.01 vs. control group;
**P<0.01 vs. CPB induced rat model group. CPB, cardiopulmonary
bypass; Control, control normal rat group; Model, CPB induced rat
model group; Hyperoxia liquid, treatment with hyperoxia liquid
group; LVEF, left ventricular ejection fraction; LVIDs, left
ventricular internal dimension systole. |
The effects of hyperoxia liquid
regulated oxidative stress in CPB induced rat model
We analyzed the effects of hyperoxia liquid on the
changes of oxidative stress in CPB induced rat model. As shown in
Fig. 2, MDA level was increased,
and SOD, GSH, GAH-PX levels were decreased in CPB, compared with
control group. Moreover, the promotion of MDA level, and the
inhibition of SOD, GSH, GAH-PX levels were reversed after
administration with hyperoxia liquid, compared with CPB induced rat
model (Fig. 2).
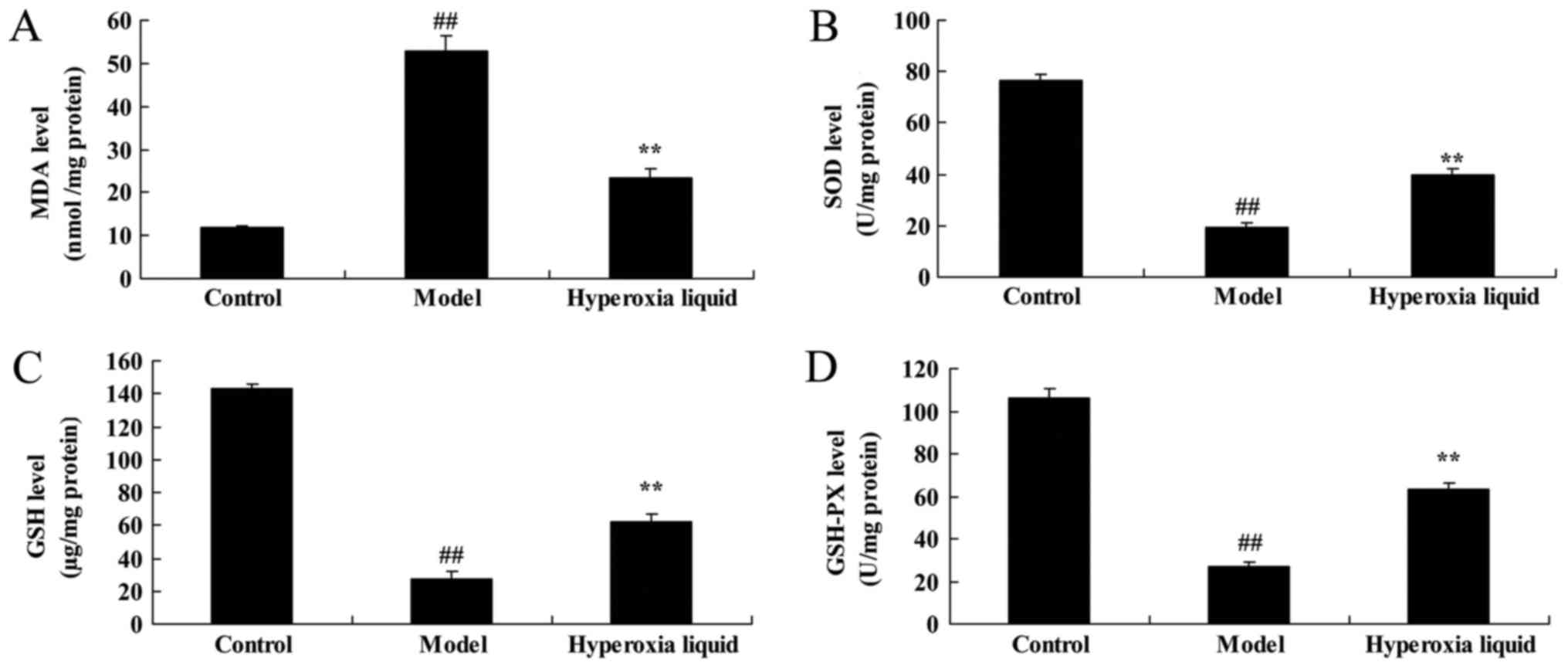 | Figure 2.Effects of hyperoxia liquid on the
regulation of oxidative stress in the CPB-induced rat model. Levels
of (A) MDA, (B) SOD, (C) GSH and (D) GAH-PX. ##P<0.01
vs. control group; **P<0.01 vs. CPB induced rat model group.
CPB, cardiopulmonary bypass; Control, control normal rat group;
Model, CPB induced rat model group; Hyperoxia liquid, treatment
with hyperoxia liquid group; MDA, malondialdehyde; SOD, superoxide
dismutase; GSH, glutathione; GSH-PX, glutathione-peroxidase. |
The effects of hyperoxia liquid
induced Nrf2-ARE signaling pathway in CPB induced rat model
To confirm the mechanism of hyperoxia liquid on
oxidative stress in CPB induced rat model, Nrf2-ARE signaling
pathway was analyzed using Western blot. As was shown in Fig. 3, Nrf2 and HO-1 protein expressions
were suppressed, while Bax protein expression was induced by
hyperoxia liquid in CPB induced rat model, compared with control
group. Administration with hyperoxia liquid induced Nrf2 and HO-1
protein expressions, and suppressed Bax protein expression in CPB
induced rat model, compared with CPB induced rat model (Fig. 3).
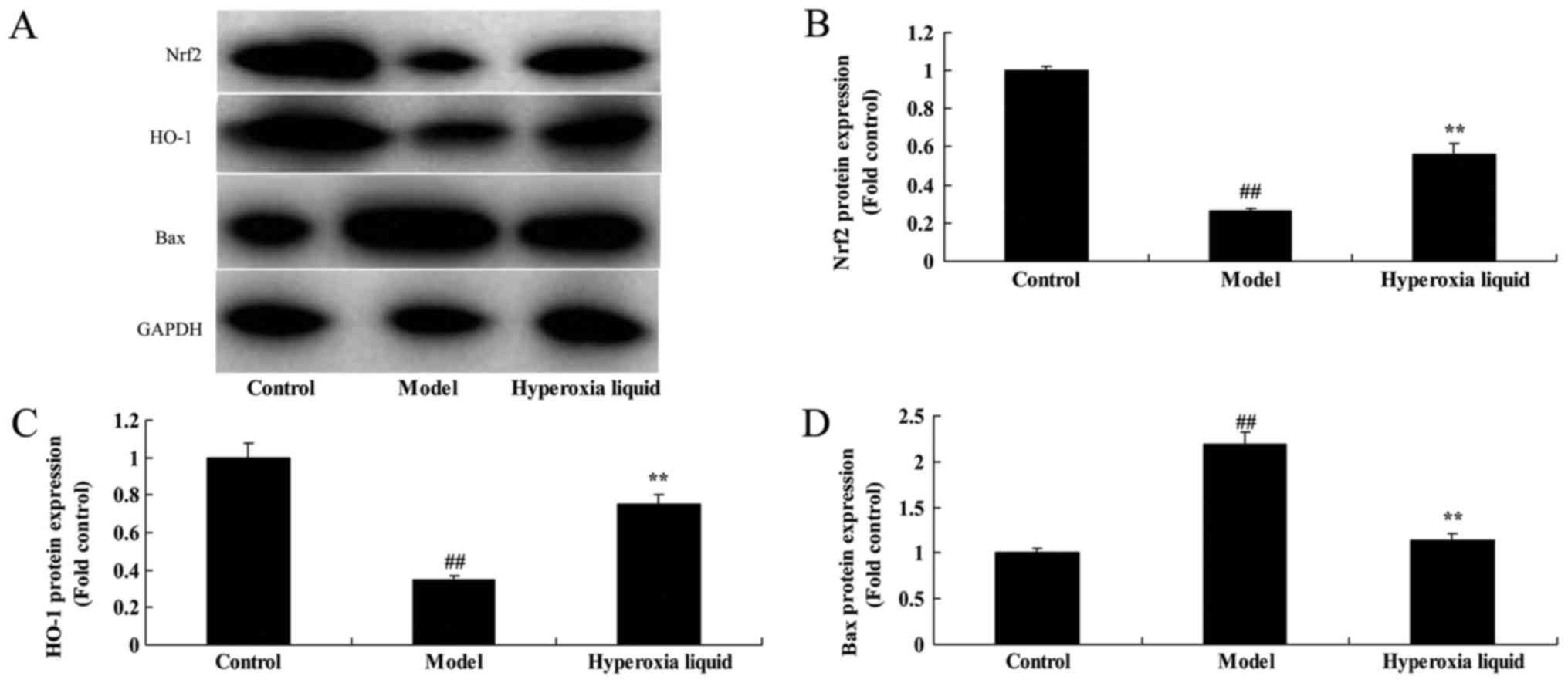 | Figure 3.Effects of hyperoxia liquid on the
Nrf2-ARE signaling pathway in the CPB-induced rat model. (A)
Western blotting for Nrf2, HO-1, Bax and GAPDH protein expression,
and the statistical analysis of (B) Nrf2, (C) HO-1 and (D) Bax
protein expression. GAPDH was used as the internal control.
##P<0.01 vs. control group; **P<0.01 vs. CPB
induced rat model group. CPB, cardiopulmonary bypass; Control,
control normal rat group; Model, CPB induced rat model group;
Hyperoxia liquid, treatment with hyperoxia liquid group; Nrf2,
nuclear factor erythroid 2-related factor 2; ARE, antioxidant
response element; HO-1, heme oxygenase 1; Bax, B-cell
lymphoma-associated X protein. |
The inhibition of Nrf2 reduced the
function of hyperoxia liquid on Nrf2-ARE signaling pathway in CPB
induced rat model
Next, ML385, an Nrf2 inhibitor, was utilized to
reduce the function of hyperoxia liquid on Nrf2-ARE signaling
pathway in CPB induced rat model. As shown in Fig. 4, Nrf2 inhibitor suppressed Nrf2 and
HO-1 protein expressions, and induced Bax protein expression in CPB
induced rat, compared with CPB induced rat model. Treatment with
Nrf2 inhibitor suppressed the function of hyperoxia liquid on heart
cell apoptosis, the inhibition of LVEF level, promotion of LVIDs
level, and caspase-3/8/9 activity levels in CPB induced rat,
compared CPB induced rat model (Fig.
5). Moreover, the inhibition of Nrf2 reduced the function of
hyperoxia liquid on the promotion of MDA, and the inhibition of
SOD, GSH, GAH-PX levels in CPB induced rat, compared with CPB
induced rat model (Fig. 6).
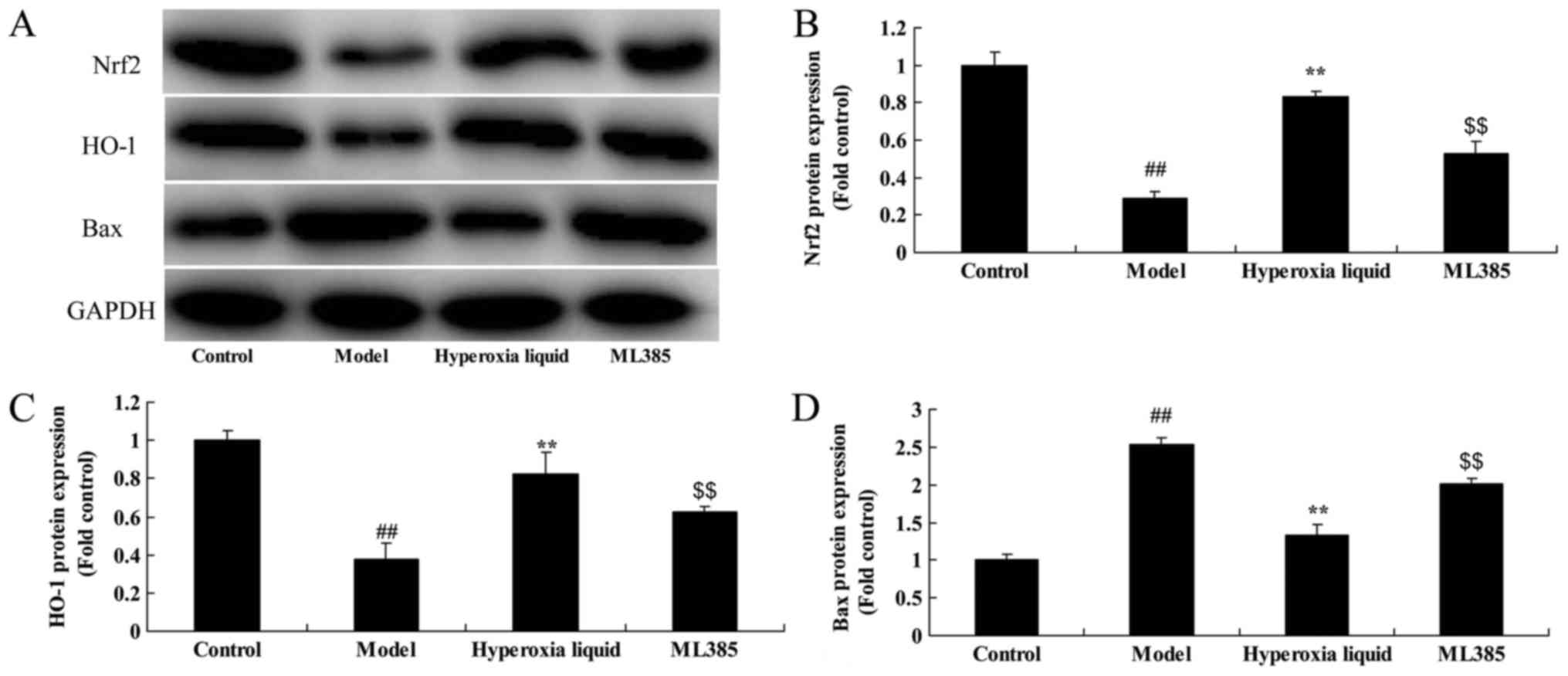 | Figure 4.Inhibition of Nrf2 reduces the effect
of hyperoxia liquid on the Nrf2-ARE signaling pathway in the
CPB-induced rat model. (A) Western blotting for Nrf2, HO-1, Bax and
GAPDH protein expression, and the statistical analysis of (B) Nrf2,
(C) HO-1 and (D) Bax protein expression, following treatment with
ML385. GAPDH was used as the internal control.
##P<0.01 vs. control group; **P<0.01 vs. CPB
induced rat model group; $$P<0.01 vs. Hyperoxia
liquid group. CPB, cardiopulmonary bypass; Control, control normal
rat group; Model, CPB induced rat model group; Hyperoxia liquid,
treatment with hyperoxia liquid group; ML385, treatment with
hyperoxia liquid and ML385 group; Nrf2, nuclear factor erythroid
2-related factor 2; ARE, antioxidant response element; HO-1, heme
oxygenase 1; Bax, B-cell lymphoma-associated X protein. |
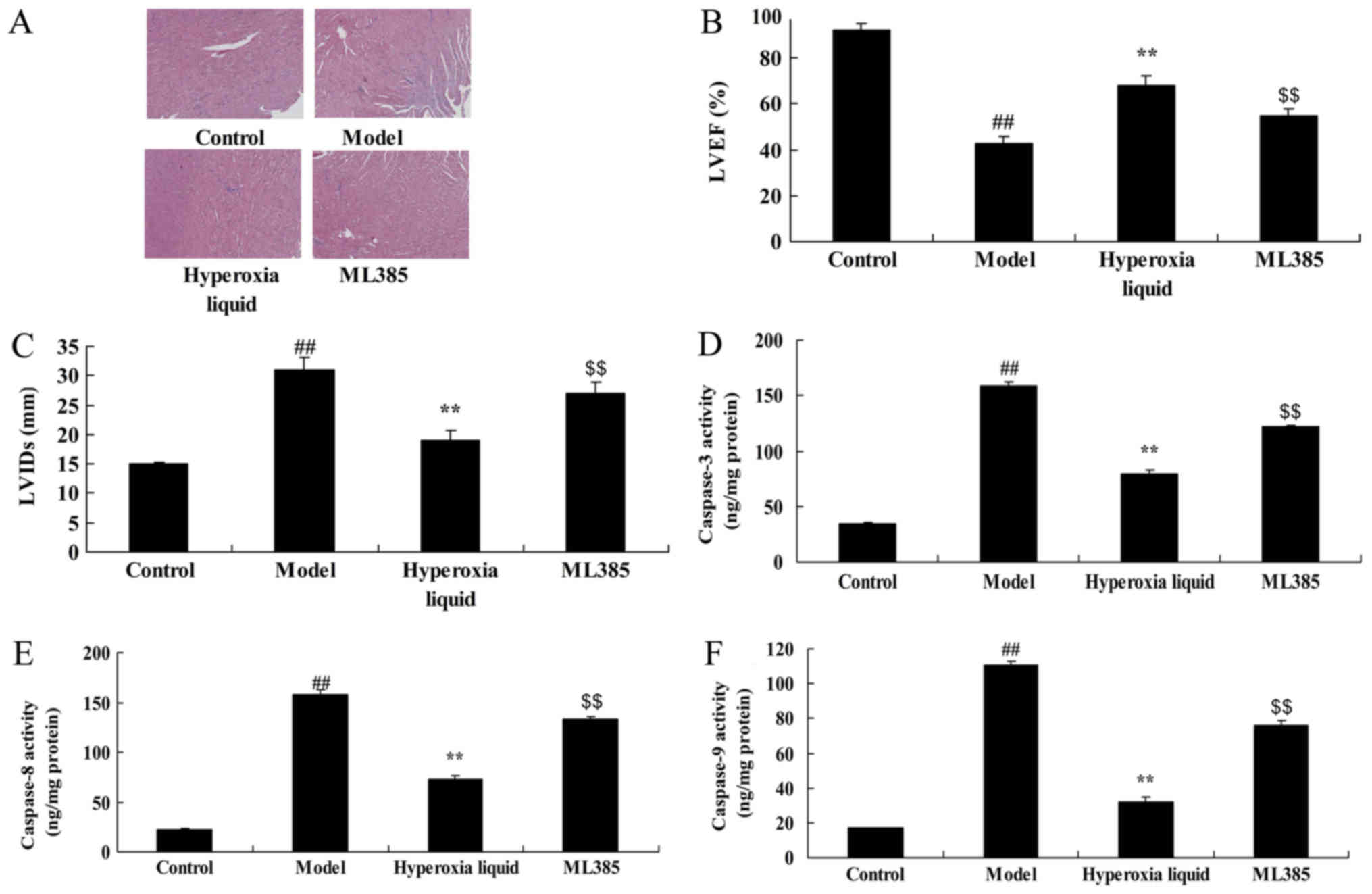 | Figure 5.Inhibition of nuclear factor erythroid
2-related factor 2 reduced the effect of hyperoxia liquid on heart
cell apoptosis in the CPB-induced rat model. (A) Hematoxylin and
eosin staining for heart tissue (magnification, ×5). Levels of (B)
LVEF, (C) LVIDs, and (D) caspase-3, (E) −8 and (F) −9 activity.
##P<0.01 vs. control group; **P<0.01 vs. CPB
induced rat model group; $$P<0.01 vs. Hyperoxia
liquid group. CPB, cardiopulmonary bypass; Control, control normal
rat group; Model, CPB induced rat model group; Hyperoxia liquid,
treatment with hyperoxia liquid group; ML385, treatment with
hyperoxia liquid and ML385 group; LVEF, left ventricular ejection
fraction; LVIDs, left ventricular internal dimension systole. |
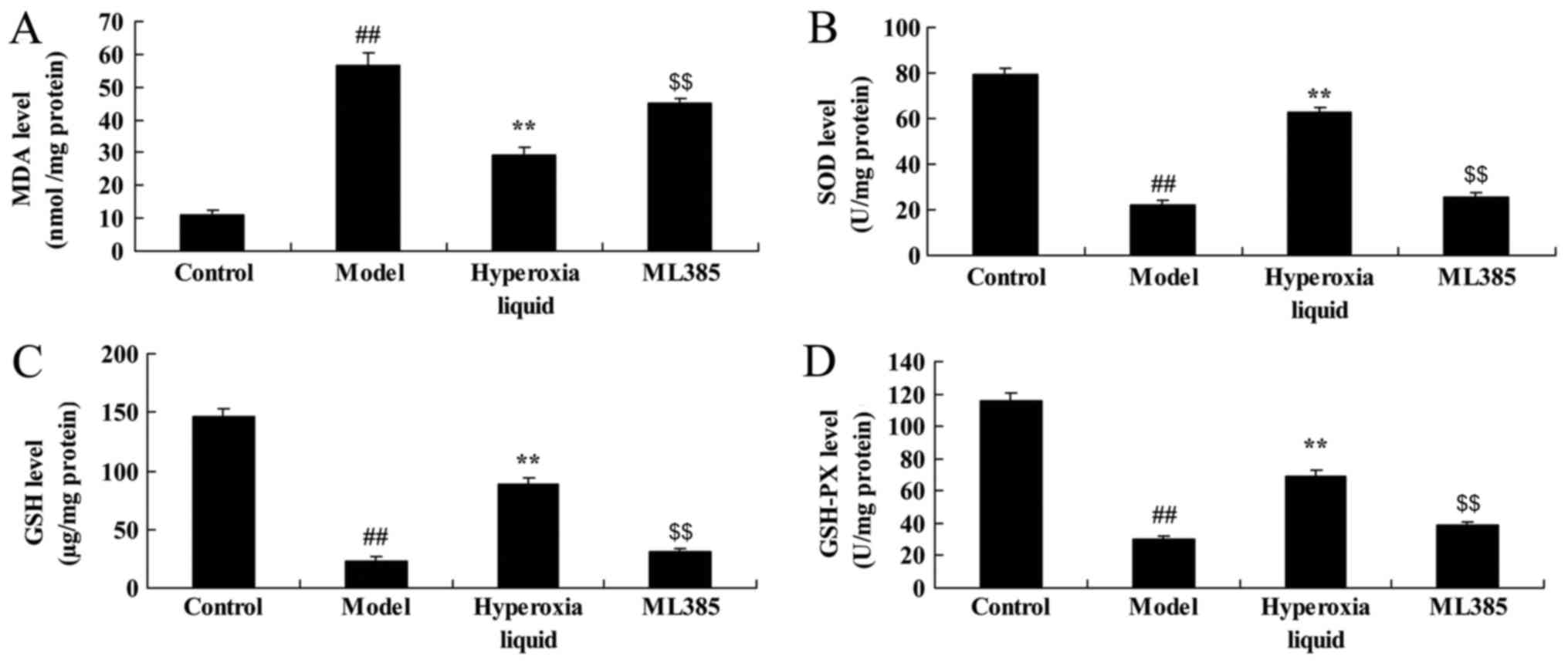 | Figure 6.Inhibition of Nrf2 reduces the effect
of hyperoxia liquid on oxidative stress in the CPB-induced rat
model. Levels of (A) MDA, (B) SOD, (C) GSH and (D) GAH-PX,
following ML385 treatment. ##P<0.01 vs. control
group; **P<0.01 vs. CPB induced rat model group;
$$P<0.01 vs. Hyperoxia liquid group. CPB,
cardiopulmonary bypass; Control, control normal rat group; Model,
CPB induced rat model group; Hyperoxia liquid, treatment with
hyperoxia liquid group; ML385, treatment with hyperoxia liquid and
ML385 group; MDA, malondialdehyde; SOD, superoxide dismutase; GSH,
glutathione; GSH-PX, glutathione-peroxidase. |
Discussion
The myocardial protection technique in hypothermic
CPB on-pump open-heart surgery has gradually emerged in recent
years (11). Favorable effects
have been achieved in clinic (12). Meanwhile, it is associated with
great drawbacks, such as poor exposure, increased blood return in
surgical field and elevated difficulty in surgical operation
(12). In addition, it will lead
to increased intracardiac suction, further aggravating the
destruction of visible blood components. It will also increase the
risk of gas embolism (12).
Meanwhile, ventricular fibrillation may be induced intraoperatively
due to the low temperature. CPB myocardial injury is associated
with high incidence of arrhythmia. Moreover, the damage mechanism
is complicated, which has involved diverse influence factors. These
have proposed higher challenges and requirements for clinical
prevention and treatment of cardiac surgery. The above measures
have been comprehensively applied. However, no expected clinical
effect can be attained (12).
Therefore, CPB perioperative myocardial protection remains a
research hotspot at present and in the future. In the present
study, we demonstrated that the effects of hyperoxia liquid
inhibited heart cell apoptosis in CPB induced rat model. Gao et
al (13) showed that
therapeutic effects of hyperoxia liquid improve systemic
oxygenation during one-lung ventilation.
Free radical scavenger refers to substance that can
delay, inhibit and block the active oxygen/oxygen radical oxidative
injury (14). It can bind with and
scavenge oxygen radicals (15).
ATP reserve in myocardial tissue is reduced during the early
myocardial ischemia. Subsequently, it will provide energy through
anaerobic metabolism (14). In
early reperfusion, blood flow oxygen cannot be sufficiently used
due to the recovery of coronary blood flow (14). This results in the production of
oxygen radicals with potential damage by various oxidase
substrates. It leads to excessive accumulation of free radicals.
This will then attach the unsaturated fatty acid in membrane
phospholipid (16). Later, it will
induce changes in fluidity and permeability of myocardial cell
membrane and subcellular organelle membrane (15). Then, multiple lipid peroxides will
be produced, affecting the myocardial cell integrity and function.
In addition, it will attack the structural protein of myocardial
cell, rendering cleavage of peptide-chain (17). Oxidative stress is particularly
important in CPB and myocardial damage. In the present study,
resveratrol was suggested the effects of hyperoxia liquid reduced
oxidative stress in CPB induced rat model. Zhao et al
(18) suggested that hyperoxia
liquid protects against acute hypobaric hypoxia-induced oxidative
damage. Karu et al (19)
reported that the effects of 60 min of hyperoxia followed by
normoxia before coronary artery reduced myocardial injury and
inflammatory response profile.
The Nrf2-ARE signaling pathway is recognized to be
the most important endogenous anti-oxidative stress pathway
(20). Activation of this pathway
can induce the production of antioxidant enzyme and phase II drug
metabolic enzyme (21). It plays a
vital role in maintaining intracellular redox state, reducing
oxidative damage and protecting cell function. Research finds that
the Nrf2-ARE signaling pathway is closely related to improving
myocardial I/R injury. Meanwhile, Nrf2 is an important
transcription factor in cell to reduce ROS (22). Our results suggest that the effects
of hyperoxia liquid induced Nrf2-ARE signaling pathway in CPB
induced rat model. Nrf2 inhibitor, reduced the function of
hyperoxia liquid in CPB induced rat model. Karu et al
(23) showed that pre-treatment
with hyperoxia before coronary artery bypass grafting - effects on
myocardial injury and inflammatory response. Nagato et al
(24) showed that Hyperoxia
promotes polarization of the immune response, and induced Nrf2 and
iNOS protein expression. Šarić et al (25) showed that this model of hyperoxia
as a useful tool to assess sex differences in adaptive response to
acute stress conditions through activation of HO-1 and Nrf2 protein
expression. These experiments are three independent experiments,
tendency is correct.
Taken together, the results of this study suggest
that the effects of hyperoxia liquid inhibited oxidative stress to
open heart apoptosis in CPB-induced myocardial damage through
Nrf2-ARE signaling pathway (Fig.
7). Our present results represent useful tools for the
development of a new mode for treatment of CPB-induced myocardial
damage.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Research Award Fund for Outstanding Young-Middle Aged Scientists of
Shandong Province (grant no. BS2014YY004) and Natural Science
Foundation of Shandong Province (grant no. ZR2017BH017).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
BH designed the experiments. XM, CZ, AH, ZX, TZ and
WY performed the experiments. BH and XM analyzed the data, and BH
wrote the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Approval Committee of Shandong Provincial Hospital Affiliated to
Shandong University (Shandong, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ching T, Song MA, Tiirikainen M, Molnar J,
Berry M, Towner D and Garmire LX: Genome-wide hypermethylation
coupled with promoter hypomethylation in the chorioamniotic
membranes of early onset pre-eclampsia. Mol Hum Reprod. 20:885–904.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang G, Xiao S and Gao C: The effects of
cardiopulmonary bypass on pulmonary function during robotic cardiac
surgery. Perfusion. 30:213–218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang X, Ji B, Zhang Y, Zhu X, Liu J, Long
C and Zheng Z: Comparison of the effects of three cell saver
devices on erythrocyte function during cardiopulmonary bypass
procedure-a pilot study. Artif Organs. 36:931–935. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arsenault KA, Paikin JS, Hirsh J, Dale B,
Whitlock RP, Teoh K, Young E, Ginsberg JS, Weitz JI and Eikelboom
JW: Subtle differences in commercial heparins can have serious
consequences for cardiopulmonary bypass patients: A randomized
controlled trial. J Thorac Cardiovasc Surg. 144(944–950):
e32012.PubMed/NCBI
|
|
5
|
Lee D, Park S, Bae S, Jeong D, Park M,
Kang C, Yoo W, Samad MA, Ke Q, Khang G and Kang PM: Hydrogen
peroxide-activatable antioxidant prodrug as a targeted therapeutic
agent for ischemia-reperfusion injury. Sci Rep. 5:165922015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu H, Jing X, Dong A, Bai B and Wang H:
Overexpression of TIMP3 protects against cardiac
ischemia/reperfusion injury by inhibiting myocardial apoptosis
through ROS/Mapks pathway. Cell Physiol Biochem. 44:1011–1023.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hirji SA, Stevens SR, Shaw LK, Campbell
EC, Granger CB, Patel MR, Sketch MH Jr, Wang TY, Ohman EM, Peterson
ED and Brennan JM: Predicting risk of cardiac events among
ST-segment elevation myocardial infarction patients with
conservatively managed non-infarct-related artery coronary artery
disease: An analysis of the Duke Databank for Cardiovascular
disease. Am Heart J. 194:116–124. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang J, Hu X and Jiang H: ERS-PERK
signaling pathway-mediated Nrf2/ARE-HO-1 axis: A novel therapeutic
target for attenuating myocardial ischemia and reperfusion injury.
Int J Cardiol. 203:779–780. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee TM, Lin SZ and Chang NC:
Antiarrhythmic effect of lithium in rats after myocardial
infarction by activation of Nrf2/HO-1 signaling. Free Radic Biol
Med. 77:71–81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li W, Wu M, Tang L, Pan Y, Liu Z, Zeng C,
Wang J, Wei T and Liang G: Novel curcumin analogue 14p protects
against myocardial ischemia reperfusion injury through
Nrf2-activating anti-oxidative activity. Toxicol Appl Pharmacol.
282:175–183. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tashiro N, Takahashi S, Takasaki T,
Katayama K, Taguchi T, Watanabe M, Kurosaki T, Imai K, Kimura H and
Sueda T: Efficacy of cardiopulmonary rehabilitation with adaptive
servo-ventilation in patients undergoing off-pump coronary artery
bypass grafting. Circ J. 79:1290–1298. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Elgebaly AS and Sabry M: Infusion of
low-dose vasopressin improves left ventricular function during
separation from cardiopulmonary bypass: A double-blind randomized
study. Ann Card Anaesth. 15:128–133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao C, Zhang G, Sun X, Zhang H, Kuai J,
Zhao H, Yao L, Yu D, Yang Y, Xu L and Chai W: The effects of
intravenous hyperoxygenated solution infusion on systemic
oxygenation and intrapulmonary shunt during one-lung ventilation in
pigs. J Surg Res. 159:653–659. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eigel BN, Gursahani H and Hadley RW: ROS
are required for rapid reactivation of Na+/Ca2+ exchanger in
hypoxic reoxygenated guinea pig ventricular myocytes. Am J Physiol
Heart Circ Physiol. 286:H955–H963. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kayama Y, Raaz U, Jagger A, Adam M,
Schellinger IN, Sakamoto M, Suzuki H, Toyama K, Spin JM and Tsao
PS: Diabetic cardiovascular disease induced by oxidative stress.
Int J Mol Sci. 16:25234–25263. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yuan Y, Pan S, Yang SL, Liu YL and Xu QM:
Antioxidant and cardioprotective effects of Ilex cornuta on
myocardial ischemia injury. Chin J Nat Med. 15:94–104.
2017.PubMed/NCBI
|
|
17
|
Bashar T and Akhter N: Study on oxidative
stress and antioxidant level in patients of acute myocardial
infarction before and after regular treatment. Bangladesh Med Res
Counc Bull. 40:79–84. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao H, Chai W, Gao W, Xu L, Zhang H and
Yang Y: Hyperoxygenated solution: Effects on acute hypobaric
hypoxia-induced oxidative damage in rabbits. High Alt Med Biol.
10:283–291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karu I, Tähepõld P, Ruusalepp A, Zilmer K,
Zilmer M and Starkopf J: Effects of 60 min of hyperoxia followed by
normoxia before coronary artery bypass grafting on the inflammatory
response profile and myocardial injury. J Negat Results Biomed.
11:142012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao S, Zhan L, Yang Z, Shi R, Li H, Xia Z,
Yuan S, Wu QP, Wang T and Yao S: Remote limb ischaemic
postconditioning protects against myocardial ischaemia/reperfusion
injury in mice: Activation of JAK/STAT3-mediated Nrf2-antioxidant
signalling. Cell Physiol Biochem. 43:1140–1151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Donnarumma E, Bhushan S, Bradley JM,
Otsuka H, Donnelly EL, Lefer DJ and Islam KN: Nitrite therapy
ameliorates myocardial dysfunction via H2S and nuclear
factor-erythroid 2-related factor 2 (Nrf2)-dependent signaling in
chronic heart failure. J Am Heart Assoc. 5:pii: e003551. 2016.
View Article : Google Scholar
|
|
22
|
Zhang X, Hu H, Luo J, Deng H, Yu P, Zhang
Z, Zhang G, Shan L and Wang Y: A novel
danshensu-tetramethylpyrazine conjugate DT-010 provides
cardioprotection through the PGC-1α/Nrf2/HO-1 pathway. Biol Pharm
Bull. 40:1490–1498. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Karu I, Loit R, Zilmer K, Kairane C,
Paapstel A, Zilmer M and Starkopf J: Pre-treatment with hyperoxia
before coronary artery bypass grafting-effects on myocardial injury
and inflammatory response. Acta Anaesthesiol Scand. 51:1305–1313.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nagato AC, Bezerra FS, Talvani A,
Aarestrup BJ and Aarestrup FM: Hyperoxia promotes polarization of
the immune response in ovalbumin-induced airway inflammation,
leading to a TH17 cell phenotype. Immun Inflamm Dis. 3:321–337.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Šarić A, Sobočanec S, Šafranko Mačak Ž,
Hadžija Popović M, Bagarić R, Farkaš V, Švarc A, Marotti T and
Balog T: Diminished resistance to hyperoxia in brains of
reproductively senescent female CBA/H mice. Med Sci Monit Basic
Res. 21:191–199. 2015. View Article : Google Scholar : PubMed/NCBI
|