Introduction
Type 2 diabetes mellitus (T2DM) is a widespread
chronic metabolic disorder, which can adversely affect numerous
tissues due to its long-term complications. In addition to common
complications in the peripheral nervous system of patients with
T2DM (1), emerging evidence had
indicated that T2DM exerts negative effects on the central nervous
system (CNS), with cognitive impairment the most common symptom
(2). ‘Diabetes-associated
cognitive decline (DACD)’ has been proposed as a novel term to
strengthen research in associated fields and to facilitate
recognition of this disorder (3).
Based on clinical, epidemiological and experimental evidence,
numerous studies have suggested a causal association between T2DM
and cognitive impairment (4–6).
Therefore, DM-induced cognitive impairment has received increasing
attention; however, the pathophysiological alterations and
pathogenesis remain to be investigated. Cognitive dysfunction in DM
appears to be caused by various factors (7). Recent studies demonstrated that T2DM
could result in autophagic dysfunction in neuronal cells, thus
aggravating cerebral vascular disease and the process of vascular
dementia, eventually resulting in memory dysfunction (8,9).
Autophagy serves an important role in maintaining normal tissue and
cellular homeostasis (10). A
previous study revealed the crucial role of autophagy in DM, in
which autophagy functions to ameliorate or exacerbate DM-induced
cognitive dysfunction (9,11). Therefore, regulation of autophagy
in the hippocampus may be considered an essential method to
ameliorate neuronal damage.
Considering the pathogenesis of T2DM and brain
dysfunction, antidiabetic drugs may also exert beneficial effects
on neuronal metabolism, which could be clinically significant for
the treatment of CNS complications in DM and other neurological
diseases (12). Glucagon-like
peptide-1 (GLP-1) receptor agonists are a novel type of
hypoglycemic drug used in clinical practice, which have been
reported to possess numerous potential benefits in improving
capacity in various animal models of neurodegeneration (13–15).
In the Guangxi Diabetes and Metabolic Disorders (GDMD) Study in
China, clinical studies demonstrated that plasma dipeptidyl
peptidase 4 (DPP4) activity, which is associated with the
degradation of GLP-1, had a positive association with mild
cognitive impairment in elderly patients with T2DM (16). In addition, it has been revealed
that DPP4 inhibitors may improve cognitive deficits through
inhibiting oxidative stress or the inflammatory reaction in diverse
mouse models (17,18). Among the GLP-1 receptor agonists,
liraglutide is a long-acting GLP-1 receptor agonist, which has a
97% amino acid sequence similarity with human GLP-1 and exhibits an
increased half-life of ~13 h (19). Liraglutide has been reported to
prevent memory impairments in object recognition and water maze
tasks. Simultaneously it prevents synaptic loss and impairment of
synaptic plasticity in the hippocampus of mice with Alzheimer's
disease (AD) (20). Furthermore, a
previous study suggested that liraglutide acts to increase neuronal
density and improve cognitive function in the hippocampus of a
senescence-accelerated mouse model of AD (21). Another study confirmed that
liraglutide can activate autophagic pathways in diabetic rats
(22). However, the possibility of
administrating liraglutide for improving cognitive function and the
potential underlying mechanism warrants further investigation. In
the present study, GK rats were used to investigate the effect of
numerous doses of liraglutide on cognitive improvement, and to gain
deeper insight into the molecular mechanisms underlying autophagy.
In combination with other evidence (23), the present study hypothesized that
liraglutide is a candidate cognitive enhancer that acts by
increasing mammalian target of rapamycin (mTOR) expression via the
AMP-activated protein kinase (AMPK) and phosphoinositide 3 kinase
(PI3K)/protein kinase B (Akt) pathways. The present study may
provide novel evidence to suggest liraglutide exerts therapeutic
effects on T2DM-induced cognitive deficits.
Materials and methods
Animals
The animal experiments were conducted in compliance
with the guidelines for the Care and Use of Laboratory Animals
(24). The present study was
approved by the experimental ethics committee of the North China
University of Science and Technology (Tangshan, China). A total of
30 male Goto-Kakizaki (GK) rats (age, 32 weeks; weight, 300–350 g)
and 10 male Wistar rats (age, 32 weeks; weight, 300–350 g) were
obtained from the Animal Center of North China University of
Science and Technology. The rats were maintained in plastic cages
(n=5 rats/cage) with a temperature range of 20–24°C and humidity of
50±10%, under a 12-h light/dark cycle. The rats were permitted free
access to food and water.
Preparation of animal models
A total of 30 GK rats were randomly divided into
three groups (n=10 rats/group): DM group, DM + low dose of
liraglutide group [DM + Lira-L, 75 µg/kg subcutaneous (sc.)] and DM
+ high dose of liraglutide group (DM + Lira-H, 200 µg/kg sc.).
Liraglutide was administered once a day between days 1 and 28. The
liraglutide dosing was gradually increased from 37.5 to 75 µg/kg in
the DM + Lira-L group (37.5, 75, 75 and 75 µg/kg respectively), and
from 37.5 to 200 µg/kg in the DM + Lira-H group (37.5, 75, 150 and
200 µg/kg respectively) over the first 4 days. GK rats in the DM
group and 10 Wistar rats in the control group were administered
0.9% sterile saline (75 µg/kg sc.). Following treatment, behavioral
tests and biochemical experiments were performed.
Morris water maze (MWM) test
The spatial learning and memory of all rats were
tested in the MWM 28 days following liraglutide and saline
treatments. Rats were placed in a black circular pool filled with
water (diameter, 180 cm; depth, 45 cm), which was virtually divided
into four equivalent quadrants: North, south, east and west; the
water temperature was maintained at 24–26°C. The reference objects
around the water tank were considered to be visual hints and
remained unaltered throughout the MWM test. During the process of
testing, the rats were randomly placed into the water in any of the
four quadrants facing the wall of the pool. The test was conducted
for 4 days, with one test performed in each quadrant every morning
for 60 sec. The rats that could not find the platform or failed to
escape within 90 sec were placed on the platform and allowed to
stand for 15 sec, and the measurement was recorded as 90 sec. Those
that found the platform were also permitted to stand on it for 15
sec. On day 5, the platform was removed and the rats were allowed
to swim for 1 min. Maze performance was recorded using a video
camera located above the tank, which was interfaced with a video
tracking system (HVS Image Software Ltd., Hampton, UK). The average
escape latency of a total of five trials and the time rats remained
in the target quadrant were calculated.
Histology
Following the behavioral tests, all rats were
anesthetized with sodium pentobarbital (60 mg/kg) and euthanized by
transcardiac perfusion with cold PBS and then perfused with cold 4%
paraformaldehyde containing 0.2% saturated picric acid in PBS. The
hippocampus was removed and collected from the two hemispheres.
Some samples were frozen at −80°C. The remaining samples were
post-fixed overnight at 4°C in the same fixative solution. The
post-fixed samples were embedded in paraffin and cut along the
sagittal plane at a thickness of 5 µm using a microtome.
Paraffin-embedded brain sections were treated with xylene to remove
paraffin and were rehydrated, after which they were stained with
0.1% (w/v) cresyl violet for 10 min at 37°C. The severity of
neuronal damage was evaluated by counting the number of surviving
neurons under an optical microscope (Olympus Corporation, Tokyo,
Japan). The mean number of morphologically intact neurons was
calculated and recorded per 100 µm in the CA1 hippocampal area, in
order to accurately estimate the degree of neuronal damage.
Western blot analysis
Frozen hippocampal samples were obtained and lysed
in Tissue Protein Lysis Solution (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) which contained 5% Proteinase Inhibitor Cocktail
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Protein
concentration was determined by the BCA reagent method (Wuhan
Boster Biological Technology Ltd., Wuhan, China). Equivalent
quantities of protein (30 µg) were separated by 12% SDS-PAGE
(Beyotime Institute of Biotechnology, Haimen, China) and underwent
western blot analysis. Following electrophoresis, the proteins were
transferred onto polyvinylidene difluoride membranes (Beyotime
Institute of Biotechnology) using a wet transfer system, the
membranes were then blocked in 5% fat-free dry milk for 2 h at room
temperature and washed three times in PBS with 0.1% Tween 20 at
room temperature. The membrane was incubated overnight at 4°C with
the following primary antibodies: Rabbit polyclonal anti-PI3K p110
antibody (1:3,000; cat. no. 4255; Cell Signaling Technology, Inc.,
Danvers, MA, USA), rabbit polyclonal anti-p-Akt antibody (1:1,000;
cat. no. ab38449; Abcam, Cambridge, UK), rabbit polyclonal anti-Akt
antibody (1:1,000; cat. no. ab8805; Abcam), rabbit polyclonal
anti-p-AMPK antibody (1:1,000; cat. no. ab23875; Abcam), rabbit
polyclonal anti-AMPK antibody (1:1,000; cat. no. ab131512; Abcam),
rabbit polyclonal anti-p-mTOR antibody (1:1,000; cat. no. ab84400;
Abcam), rabbit polyclonal anti-mTOR antibody (1:1,000; cat. no.
ab2732; Abcam), rabbit polyclonal anti-caspase-3 antibody (1:1,000;
cat. no. ab13847; Abcam), rabbit monoclonal anti-B-cell lymphoma 2
(Bcl-2)-associated X protein (Bax) antibody (1:1,000; cat. no.
ab32503; Abcam), rabbit polyclonal anti-Bcl-2 antibody (1:1,000;
cat. no ab59348; Abcam), rabbit polyclonal anti-Beclin-1 antibody
(1:1,000; cat. no. ab62557; Abcam), rabbit polyclonal
anti-microtubule-associated protein 1 light chain 3 I (LC-3 I)
antibody (1:1,000; cat. no. ab62720; Abcam), rabbit polyclonal
anti-microtubule-associated protein 1 light chain 3 II (LC-3 II)
antibody (1:1,000; cat. no. ab51520; Abcam) and rabbit anti-β-actin
monoclonal antibody (1:1,000; cat. no. ab6276; Abcam). Following
incubation overnight at 4°C with primary antibodies, the membranes
were washed with PBST three times for 10 min and were then
incubated with mouse anti-rabbit monoclonal secondary antibody
(1:10,000; cat. no. 5127; Cell Signaling Technology, Inc.) for 2 h
at room temperature. Following development with an enhanced
chemiluminescence (ECL) detection system, the western blot analysis
results were analyzed using Image 1.41 software (National
Institutes of Health).
Statistical analysis
All data are presented as the means ± standard
deviation and each experiment was repeated a ≥3 times and data
shown are representative experiments. The statistical analyses were
performed using SPSS 21.0 software (IBM Corp., Armonk, NY, USA).
Differences between three or more groups were analyzed by one-way
analysis of variance, followed by the Student-Newman-Keuls post-hoc
test for multiple comparisons. P<0.05 was considered to indicate
a statistically significant difference.
Results
Liraglutide improves spatial learning
and memory in the DM group
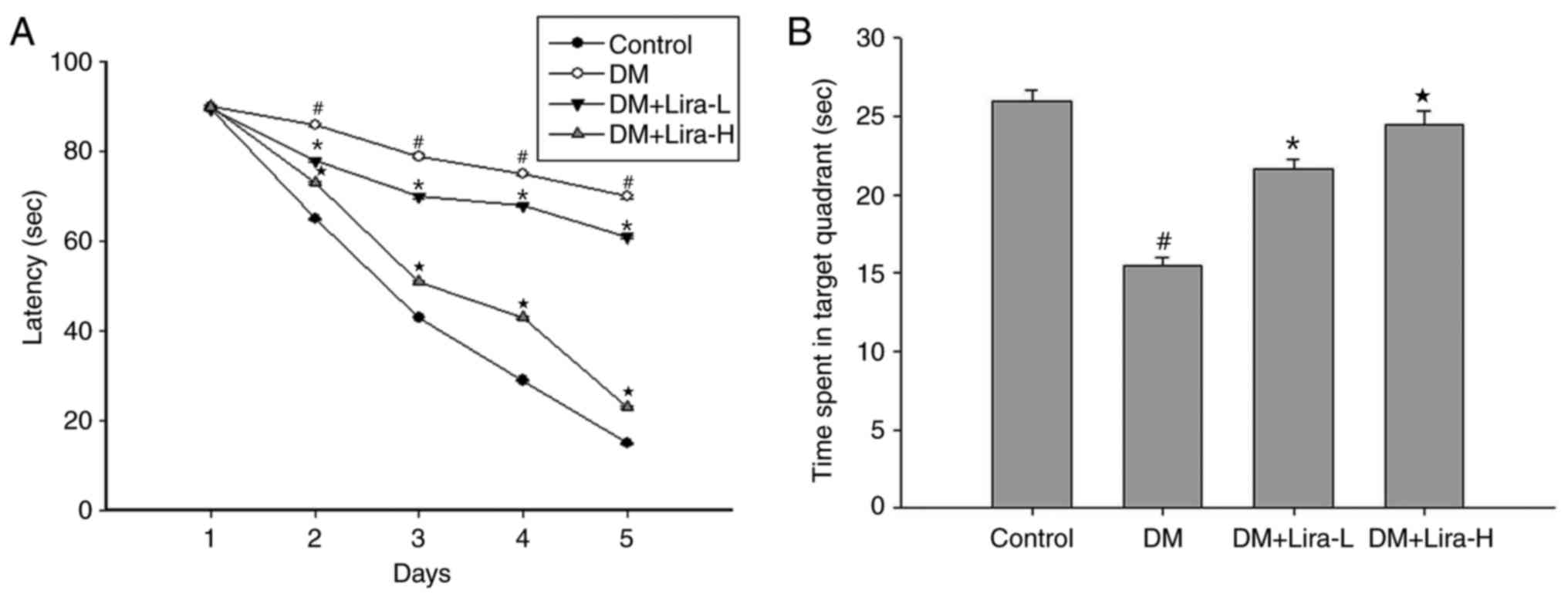
In the present study, the MWM test was used to
assess alterations in spatial learning and memory in rats. As shown
in Fig. 1A, T2DM induced spatial
learning impairment compared with in the control group, whereas
administration of liraglutide caused a decrease in escape latency
compared with in the DM group, particularly in the DM + Lira-H
group. After 4 days, the platform was removed, and time spent in
the target quadrant was reduced in the DM group compared with in
the control group (Fig. 1B).
Conversely, time in the quadrant in the DM + Lira-L group was
prolonged compared with in the DM group. Treatment with 200 µg/kg
liraglutide exhibited the most significant improvement in the
spatial probe test (P<0.05).
Treatment with liraglutide
demonstrates significant protective effects against neuronal cell
loss
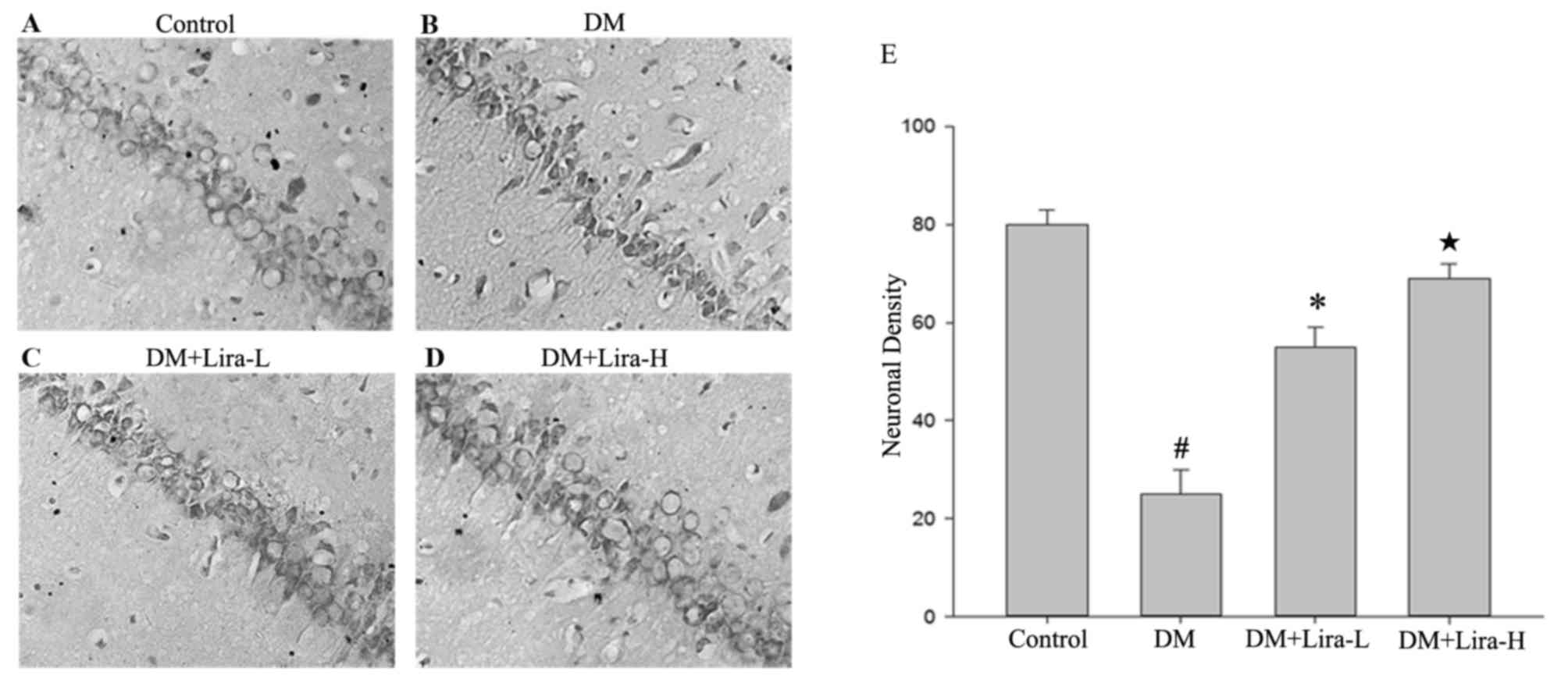
Nissl staining was conducted to investigate neuronal
alterations in the hippocampal CA1 region of rats in each group
(Fig. 2). Hippocampal neurons in
the DM group were characterized by marked shrinkage of neurocyte
bodies, the presence of pyknotic pyramidal cells and the loss of
nuclei (Fig. 2B). Conversely, in
the control group, neurons were neatly arranged, and were conically
shaped with a plump cytoplasm and round clear nucleus with
prominent nucleoli (Fig. 2A).
Treatment with liraglutide decreased DM-induced cell loss and
pyknosis; however, certain degenerative cells with morphological
alterations were still observed (Fig.
2C-E). These findings suggested that liraglutide treatment
exerted protective effects against DM-induced neurotoxicity.
Liraglutide treatment promotes
autophagy in the hippocampus of DM rats
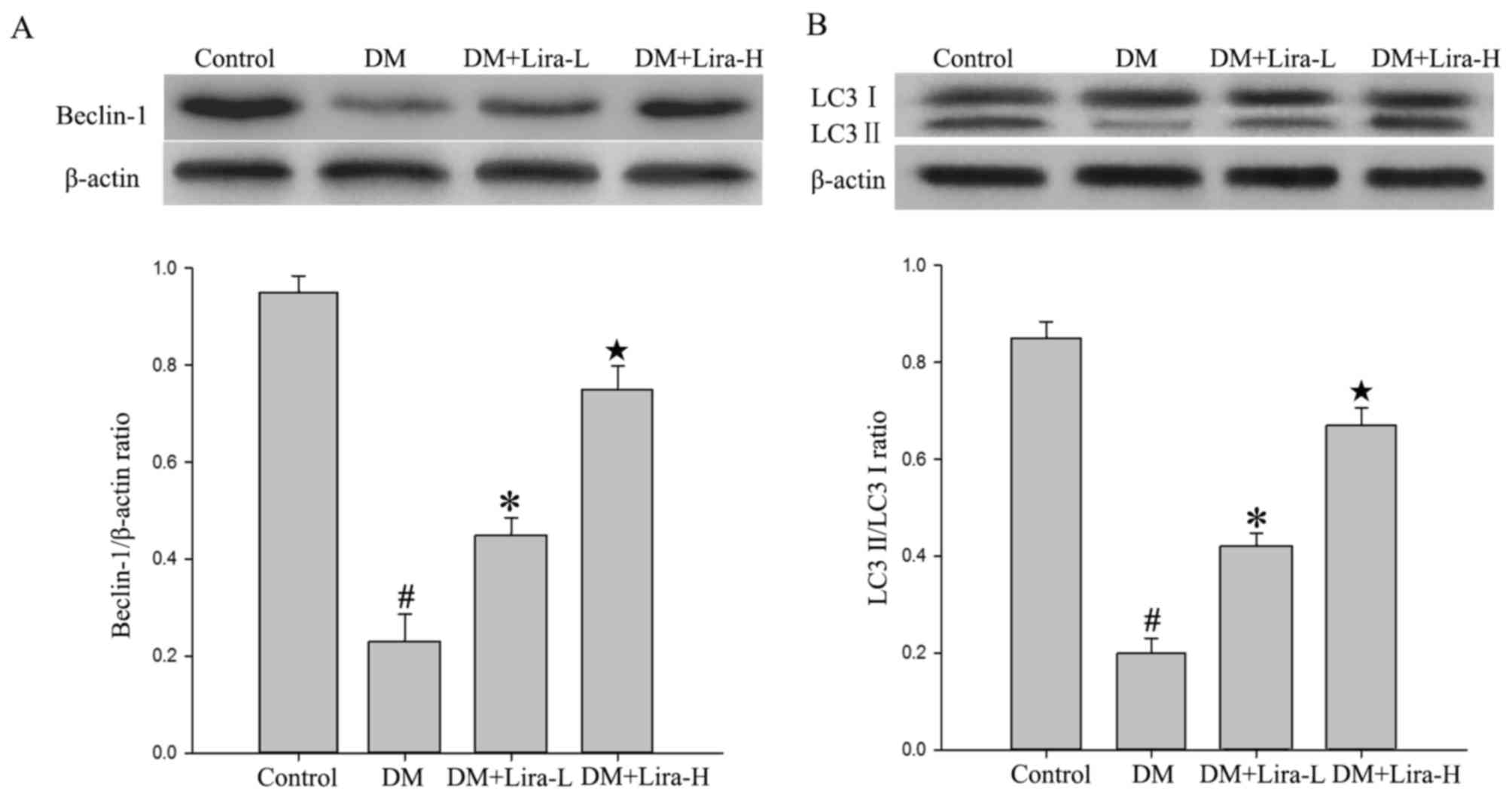
The expression levels of autophagy-associated
proteins, including Beclin-1, LC-3 II and LC-3 I, were detected in
the hippocampus of DM rats in the present study. As shown in
Fig. 3, a significant decrease
occurred in the hippocampal levels of Beclin-1 and LC-3 II/LC-3 I
in the DM group compared with in the control group (P<0.01).
Furthermore, treatment with liraglutide significantly increased the
expression levels of Beclin-1 and LC-3 II/LC-3 I compared with in
the DM group, thus suggesting that autophagy may be involved in its
effects (P<0.05). In addition, treatment with high dose of
liraglutide mildly increased the protein expression levels of
Beclin-1 and LC-3 II compared with in the Lira-L group
(P<0.05).
Liraglutide improves activation of the
PI3K/Akt pathway in GK rats
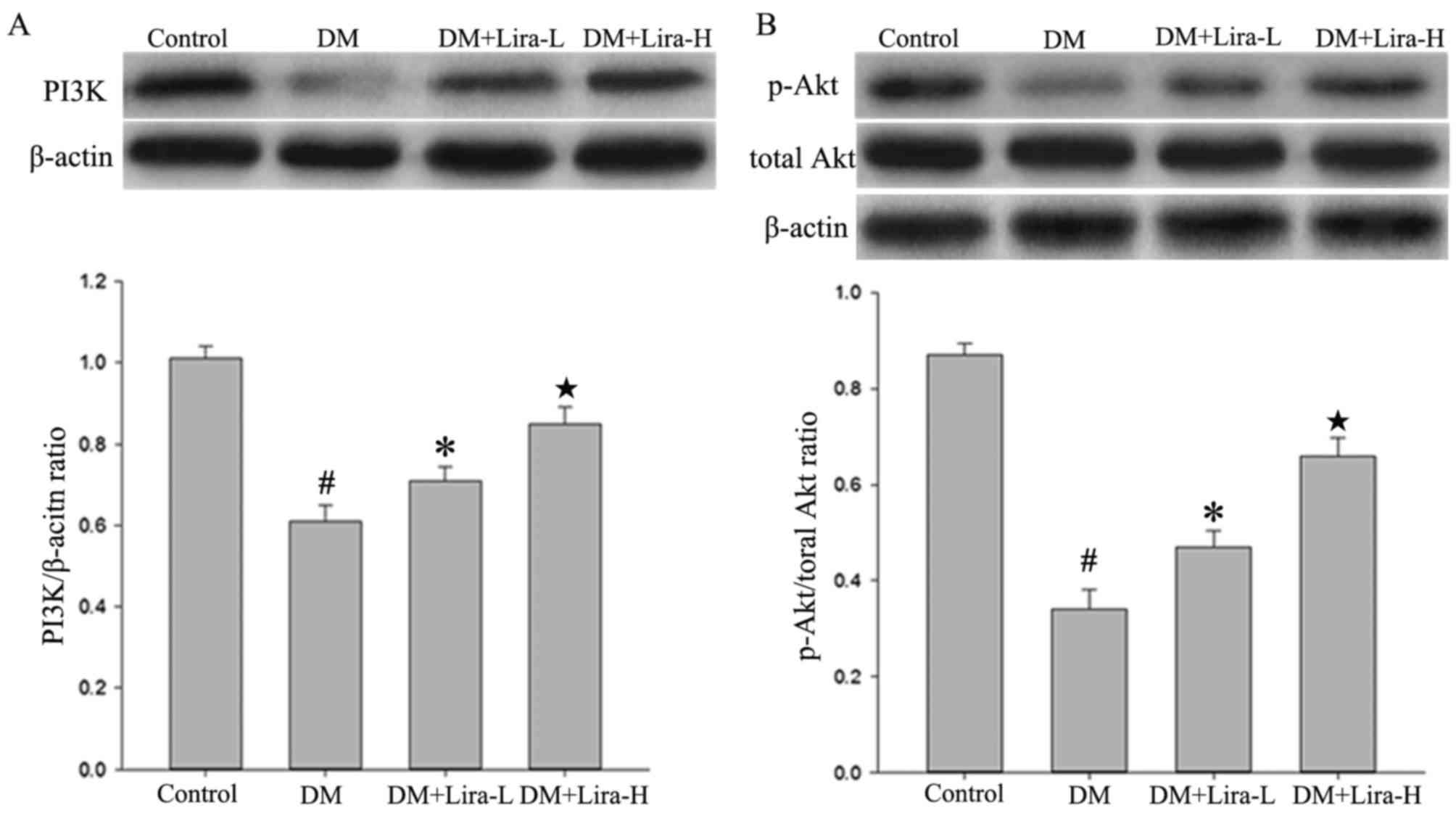
The results of the present study indicated that the
expression levels of PI3K and p-Akt were regulated by liraglutide.
The protein expression levels of total Akt did not vary
considerably among any of the groups. As shown in Fig. 4, western blotting demonstrated that
the expression levels of PI3K and p-Akt were significantly
decreased in hippocampal samples from the DM group compared with in
the control (both P<0.01). However, the administration of
liraglutide increased the expression levels of PI3K and p-Akt in a
dose-dependent manner compared with in the DM group.
Liraglutide affects the expression
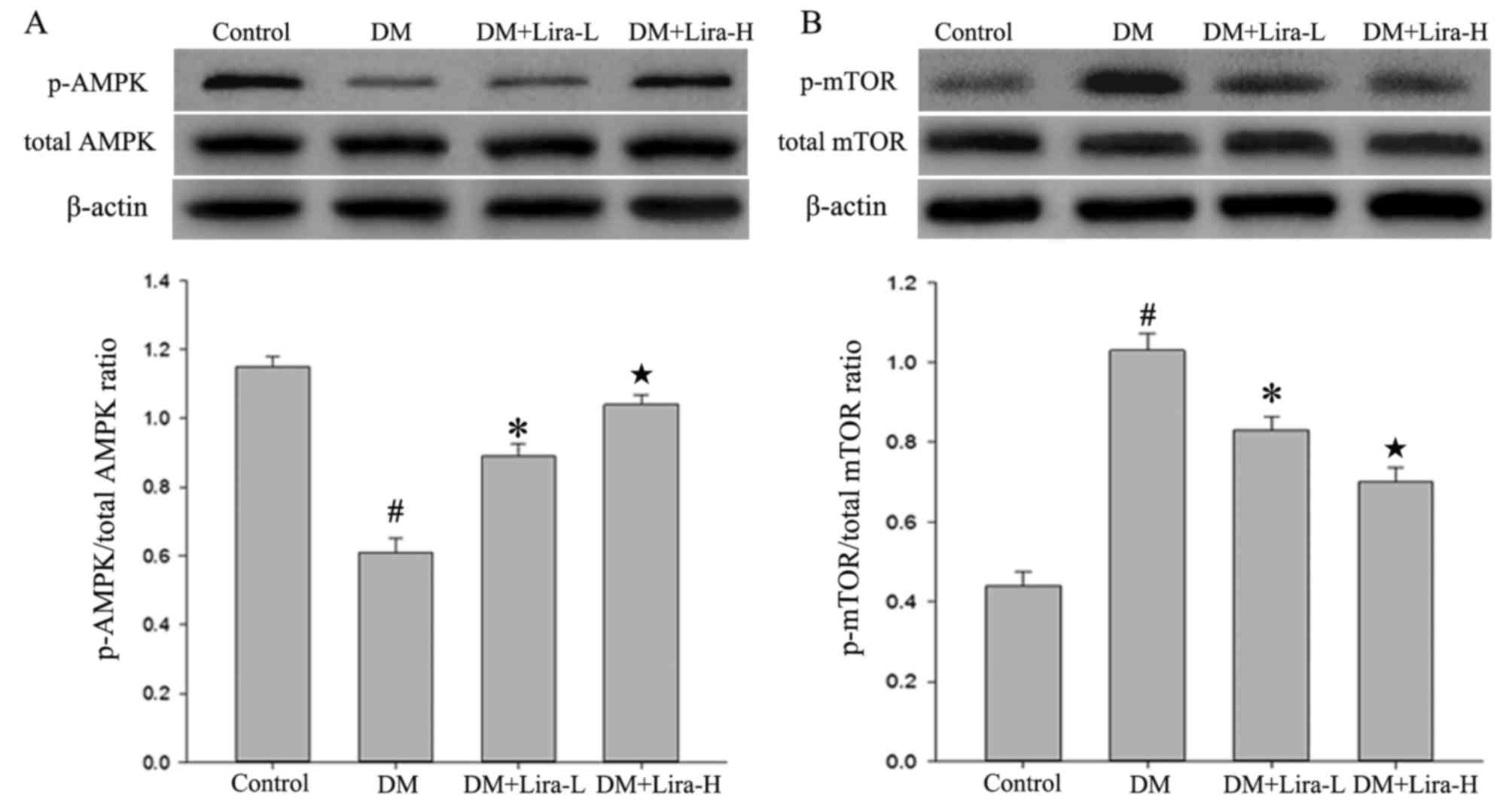
levels of p-AMPK and p-mTOR in the rat hippocampus
The expression levels of p-AMPK, AMPK, mTOR and
p-mTOR in the hippocampus were measured by western blotting.
Compared with in the control group, the expression levels of p-AMPK
were decreased in the DM group (Fig.
5A; P<0.01), whereas they were upregulated by liraglutide
treatment. Conversely, rats in the DM group exhibited significantly
increased levels of p-mTOR compared with in the control group
(P<0.01; Fig. 5B). Treatment
with liraglutide was also observed to attenuate the significantly
increased expression of p-mTOR in the hippocampus of GK rats,
particularly in the Lira-H group (P<0.05). These results
indicated that administration of liraglutide may reverse the
reduced expression of AMPK and overexpression of p-mTOR in T2DM in
a dose-dependent manner.
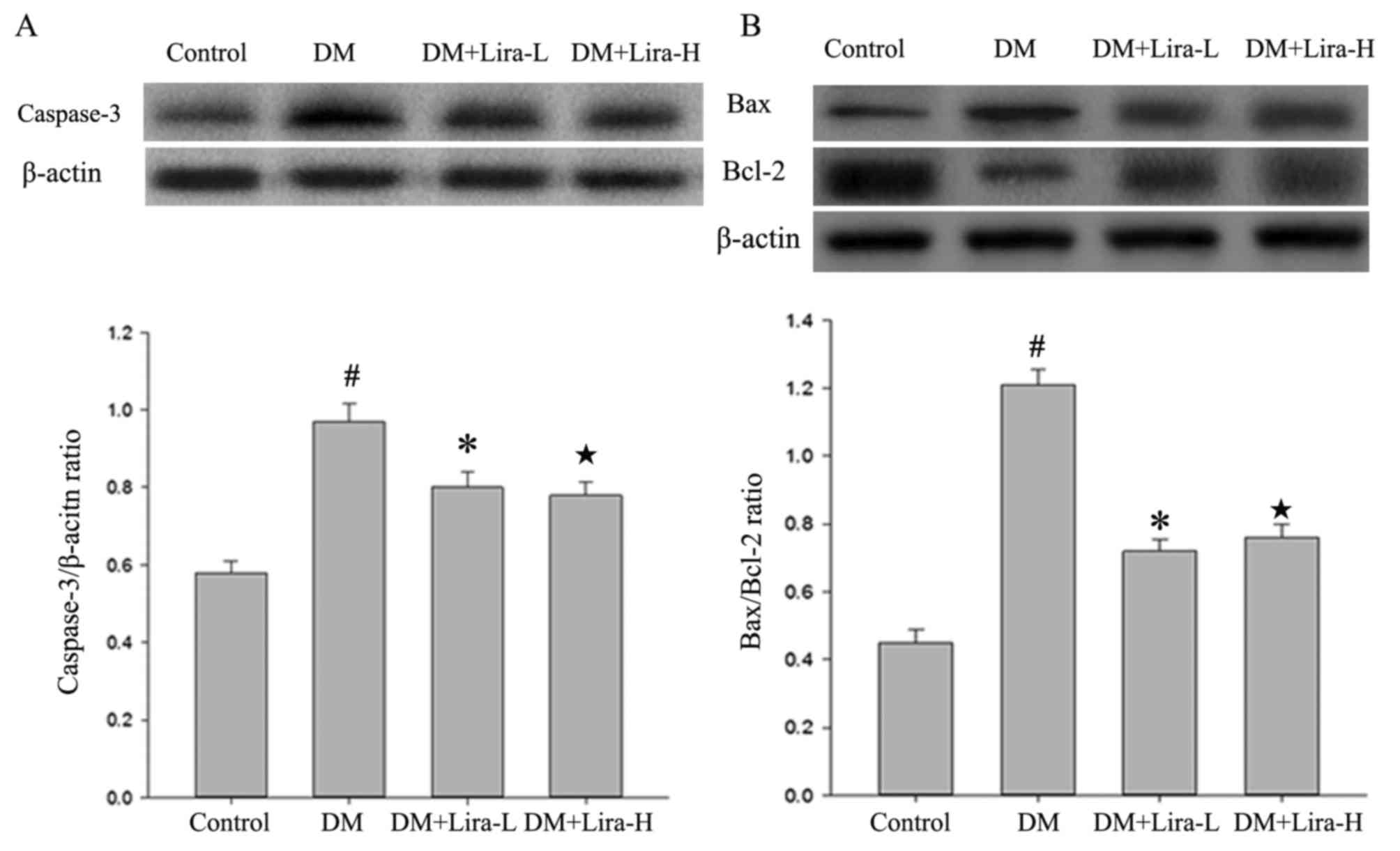
Treatment with liraglutide attenuates
the expression of apoptosis-associated proteins
The expression levels of caspase-3, Bax and Bcl-2
were detected in the hippocampus using western blot analysis.
Compared with in the control group, the expression levels of
caspase-3 and Bax were significantly increased in the DM group
(Fig. 6; P<0.01), whereas they
were downregulated by liraglutide administration. Conversely, the
protein expression levels of Bcl-2 were decreased in the DM group
compared with the control group (Fig.
6). In liraglutide-treated (75 and 200 µg/kg) rats, the
expression levels of Bcl-2 were increased compared with in the DM
group. Therefore, Lira-L (75 µg/kg) may markedly inhibit neuronal
apoptosis. In addition, Lira-H (200 µg/kg) did not exhibit a more
pronounced effect on the inhibition of apoptosis compared with
Lira-L.
Discussion
DACD is a neurodegenerative disease that
demonstrates a gradual progression of pathogenicity. The clinical
manifestations of DACD include memory and learning impairment,
disorientation, visual impairment, impairment of planning and
executive functions, and personality and behavioral alterations
(25). DACD is a common but easily
neglected CNS complication of DM. Although numerous animal studies
and clinical trials have been conducted regarding DACD (26,27),
the underlying pathogenesis remains unclear, and associated
interventions or treatments have yet to be identified.
In recent years, studies have reported that
autophagy serves an important role in the pathogenesis of
DM-induced cognitive deficits and other complications (28,29).
Autophagy is a highly conserved metabolic process that is present
in eukaryotic cells. Autophagy leads to the degradation of
cytoplasmic components in lysosomes and has recently attracted
considerable attention due to its association with DM-induced
cognitive deficits (30). Observed
autophagy impairments in the hippocampus of GK rats may result in a
reduction in hippocampal cell viability and dendritic spine density
(31). Numerous hypotheses have
been proposed to explain the molecular mechanisms by which
autophagy is modulated in diabetic models. For example, the AMPK
signaling pathway may exert a protective effect in high
glucose-induced neuronal apoptosis via the induction of autophagy
(32). Another study provided
evidence to suggest that disturbance to endoplasmic reticulum
stress may mediate downregulation of the Akt/tuberous sclerosis
complex/mTOR pathway, thus resulting in the deactivation of mTOR,
which causes downregulation of autophagy signaling (33). Hyperglycemia-associated oxidative
stress can upregulate the reactive oxygen species-extracellular
signal regulated kinase/c-Jun N-terminal kinase-p53 pathway, thus
resulting in activation of autophagic signaling, which may induce
mitochondrial loss in diabetic GK rats (34). The diversity of these examples
could be explained by the fact that the observed autophagic
alterations may be the net effect of numerous pathways.
GLP-1 receptor agonists are a novel class of
hypoglycemic agents used as monotherapy or in combination with
other antidiabetic compounds. GLP-1 receptor agonists directly
activate the GLP-1 receptor on the β cell membrane and promote its
binding to GLP-1, which is released from L cells of the small
intestine (35). It has previously
been reported that GLP-1 receptor agonists have been used to
improve cognitive functions in diabetic models. In a previous
study, administration of exenatide reduced Tau hyperphosphorylation
in the hippocampus of T2DM rats via insulin signaling and the
PI3K/Akt/glycogen synthase kinase-3 (GSK-3) pathway (13). In addition, liraglutide, which is
an efficient receptor agonist, can cross the blood-brain barrier,
restrain hippocampal neuronal death and impede the reduced
phosphorylation of Akt and p70S6K in the hippocampus of
streptozotocin-treated animals (36). Liraglutide may also elicit a
beneficial influence on synaptic plasticity in ob/ob mice with
severe obesity, and improve hippocampal neurogenesis and neuronal
survival partially by increasing the expression of achaete-scute
family BHLH transcription factor 1 (37).
In the present study, liraglutide was chosen as the
target therapeutic drug and diabetic GK rats were used to screen
the effective doses. The results of the present study demonstrated
that rats suffering from DM exhibited a decline in memory
performance. Treatment for 28 days with 75 µg/kg liraglutide
ameliorated cognitive deficits. In addition, the higher dose of
liraglutide (200 µg/kg) demonstrated a protective effect on the DM
model. These results displayed a dose-dependent effect of
liraglutide on memory. In addition, the results demonstrated that
liraglutide increased Beclin-1 and LC-3 II/LC-3 I expression
compared with the DM group, which represented activation of
autophagy. Research increasingly demonstrates that liraglutide can
effectively mediate the process of autophagy in diverse tissues or
cells affected by high glucose levels. In rat INS-1 β-cells,
liraglutide has been revealed to enhance autophagy, and suppression
of autophagy decreased the anti-apoptotic effects of liraglutide
under high glucose conditions (22). Furthermore, a recent study
demonstrated that liraglutide treatment protected cardiomyocytes
from high glucose-induced apoptosis, which is accompanied by a
notable increase in autophagy via activation of the Rap guanine
nucleotide exchange factor 3/Akt signaling pathway (38). Furthermore, in accordance with
potential liraglutide-induced autophagy in the hippocampus of T2DM
rats, the present study detected a slightly higher level of
PI3K/Akt and p-AMPK expression following liraglutide administration
in GK rats. In addition, liraglutide reversed the increased levels
of p-mTOR in the hippocampus of GK rats. These findings indicated
that liraglutide administration following neuronal damage may
inhibit expression of p-mTOR via activation of the PI3K/Akt and
AMPK signaling pathway, thus resulting in enhanced autophagic
signaling and improved cognitive function, which is similar to the
previously reported effects of Exendin-4 in a T2DM rat model
(23).
There are numerous types of cell death, including
necrotic, apoptotic and autophagic cell death. A complex
interaction exists between autophagy and apoptosis, and both can be
activated by numerous stress stimuli, regulatory molecules, and may
even share common pathways that exert neuroprotective effects.
Under certain circumstances, autophagy is a stress adaptation that
inhibits apoptosis and prevents cell death; however, in other
cellular settings it constitutes another pathway of cell death. The
results of the present study demonstrated that a low dose of
liraglutide may be sufficient to reduce the expression of caspase-3
and Bax, and to increase the expression of Bcl-2, thus resulting in
the reduction of apoptosis, which is similar to the results of a
previous study by Badawi et al (39). According to the results of the
present study, administration of liraglutide may activate autophagy
while suppressing apoptosis. Regulation of autophagy,
PI3K/Akt/GSK3β activation and several other mechanisms are all
possible mechanisms underlying the decreased expression of
caspase-3 and Bax following liraglutide administration, which was
confirmed by previous studies (23,40,41).
However, the probable mechanism underlying the interaction of
autophagy and apoptosis in the present study was not determined. In
future research, the crosstalk between autophagy and apoptosis, and
its underlying molecular mechanism, should be investigated.
In conclusion, the present study demonstrated that
administration of liraglutide decreased the expression of
apoptosis-associated proteins. In addition, it activated the AMPK
and PI3K/Akt signaling pathways in the hippocampus of GK rats, and
promoted autophagy, ultimately providing protection against chronic
T2DM-associated learning and memory impairments. The present study
provided evidence that may facilitate the development of
liraglutide as a promising preventative or therapeutic strategy for
the treatment of DM-induced cognitive impairment.
Acknowledgements
The authors would like to thank all teachers in the
Animal Experiment Center of North China University of Science and
Technology (Tangshan, China) for their technical support for the
experiments.
Funding
The present study was supported by a Project of
Hebei Provincial Natural Science fund (grant no. H2015105083).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YY, HF and GX conceived and designed the study. YZhe
and YZha performed the experiments. YY wrote the manuscript. JT,
DZ, GZ and JX analyzed the data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The animal experiments were conducted in compliance
with the guidelines for the Care and Use of Laboratory Animals
(National Institutes of Health, Bethesda, MD, USA). The present
study was approved by the experimental ethics committee of North
China University of Science and Technology (Tangshan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dobretsov M, Romanovsky D and Stimers JR:
Early diabetic neuropathy: Triggers and mechanisms. World J
Gastroenterol. 13:175–191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McCrimmon RJ, Ryan CM and Frier BM:
Diabetes and cognitive dysfunction. Lancet. 379:2291–2299. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mijnhout GS, Scheltens P, Diamant M,
Biessels GJ, Wessels AM, Simsek S, Snoek FJ and Heine RJ: Diabetic
encephalopathy: A concept in need of a definition. Diabetologia.
49:1447–1448. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carvalho C, Santos MS, Oliveira CR and
Moreira PI: Alzheimer's disease and type 2 diabetes-related
alterations in brain mitochondria, autophagy and synaptic markers.
Biochim Biophys Acta. 1852:1665–1675. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feinkohl I, Price JF, Strachan MW and
Frier BM: The impact of diabetes on cognitive decline: Potential
vascular, metabolic and psychosocial risk factors. Alzheimers Res
Ther. 7:462015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vagelatos NT and Eslick GD: Type 2
diabetes as a risk factor for Alzheimer's disease: The confounders,
interactions and neuropathology associated with this relationship.
Epidemiol Rev. 35:152–160. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Muriach M, Flores-Bellver M, Romero FJ and
Barcia JM: Diabetes and the brain: Oxidative stress, inflammation
and autophagy. Oxid Med Cell Longev. 2014:1021582014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guan ZF, Tao YH, Zhang XM, Guo QL, Liu YC,
Zhang Y, Wang YM, Ji G, Wu GF, Wang NN, et al: G-CSF and cognitive
dysfunction in elderly diabetic mice with cerebral small vessel
disease: Preventive intervention effects and underlying mechanisms.
CNS Neurosci Ther. 23:462–474. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kong FJ, Ma LL, Guo JJ, Xu LH, Li Y and Qu
S: Endoplasmic reticulum stress/autophagy pathway is involved in
diabetes-induced neuronal apoptosis and cognitive decline in mice.
Clin Sci. 132:111–125. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qian M, Fang X and Wang X: Autophagy and
inflammation. Clin Transl Med. 6:242017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guan ZF, Zhou XL, Zhang XM, Zhang Y, Wang
YM, Guo QL, Ji G, Wu GF, Wang NN, Yang H, et al: Beclin-1-mediated
autophagy may be involved in the elderly cognitive and affective
disorders in streptozotocin-induced diabetic mice. Transl
Neurodegener. 5:222016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Palleria C, Leporini C, Maida F, Succurro
E, De Sarro G, Arturi F and Russo E: Potential effects of current
drug therapies on cognitive impairment in patients with type 2
diabetes. Front Neuroendocrinol. 42:76–92. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goto H, Nomiyama T, Mita T, Yasunari E,
Azuma K, Komiya K, Arakawa M, Jin WL, Kanazawa A, Kawamori R, et
al: Exendin-4, a glucagon-like peptide-1 receptor agonist, reduces
intimal thickening after vascular injury. Biochem Biophys Res
Commun. 405:79–84. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen S, Liu AR, An FM, Yao WB and Gao XD:
Amelioration of neurodegenerative changes in cellular and rat
models of diabetes-related Alzheimer's disease by exendin-4. Age.
34:1211–1224. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Solmaz V, Çınar BP, Yiğittürk G, Çavuşoğlu
T, Taşkıran D and Erbaş O: Exenatide reduces TNF-α expression and
improves hippocampal neuron numbers and memory in streptozotocin
treated rats. Eur J Pharmacol. 765:482–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng T, Qin L, Chen B, Hu X, Zhang X, Liu
Y, Liu H, Qin S, Li G and Li Q: Association of plasma DPP4 activity
with mild cognitive impairment in elderly patients with type 2
diabetes: Results from the GDMD study in China. Diabetes Care.
39:1594–1601. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen B, Zheng T, Qin L, Hu X, Zhang X, Liu
Y, Liu H, Qin S, Li G and Li Q: Strong association between plasma
dipeptidyl peptidase-4 activity and impaired cognitive function in
elderly population with normal glucose tolerance. Fron Aging
Neurosci. 9:2472017. View Article : Google Scholar
|
|
18
|
Zheng T, Liu H, Qin L, Chen B, Zhang X, Hu
X, Xiao L and Qin S: Oxidative stress-mediated influence of plasma
DPP4 activity to BDNF ratio on mild cognitive impairment in elderly
type 2 diabetic patients: Results from the GDMD study in China.
Metabolism. March 21–2018.(Epub ahead of print). View Article : Google Scholar :
|
|
19
|
Jacobsen LV, Hindsberger C, Robson R and
Zdravkovic M: Effect of renal impairment on the pharmacokinetics of
the GLP-1 analogue liraglutide. Br J Clin Pharmacol. 68:898–905.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McClean PL and Hölscher C: Liraglutide can
reverse memory impairment, synaptic loss and reduce plaque load in
aged APP/PS1 mice, a model of Alzheimer's disease.
Neuropharmacology. 76:57–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hansen HH, Fabricius K, Barkholt P,
Niehoff ML, Morley JE, Jelsing J, Pyke C, Knudsen LB, Farr SA and
Vrang N: The GLP-1 receptor agonist liraglutide improves memory
function and increases hippocampal CA1 neuronal numbers in a
senescence-accelerated mouse model of Alzheimer's disease. J
Alzheimers Dis. 46:877–888. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miao X, Gu Z, Liu Y, Jin M, Lu Y, Gong Y,
Li L and Li C: The glucagon-like peptide-1 analogue liraglutide
promotes autophagy through the modulation of 5′-AMP-activated
protein kinase in INS-1 β-cells under high glucose conditions.
Peptides. 100:127–139. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Candeias E, Sebastião I, Cardoso S,
Carvalho C, Santos MS, Oliveira CR, Moreira PI and Duarte AI: Brain
GLP-1/IGF-1 signaling and autophagy mediate exendin-4 protection
against apoptosis in type 2 diabetic rats. Mol Neurobiol.
55:4030–4050. 2018.PubMed/NCBI
|
|
24
|
National Research Council (US), .
Institute for Laboratory Animal Research: Guide for the care and
use of laboratory animals. The National Academies Press;
Washington, DC: 1996
|
|
25
|
Ryan CM, van Duinkerken E and Rosano C:
Neurocognitive consequences of diabetes. Am Psychol. 71:563–576.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stranahan AM: Models and mechanisms for
hippocampal dysfunction in obesity and diabetes. Neuroscience.
309:125–139. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yates KF, Sweat V, Yau PL, Turchiano MM
and Convit A: Impact of metabolic syndrome on cognition and brain:
A selected review of the literature. Arterioscler Thromb Vasc Biol.
32:2060–2067. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
de Faria JM, Duarte DA, Montemurro C,
Papadimitriou A, Consonni SR and de Faria Lopes JB: Defective
autophagy in diabetic retinopathy defective autophagy. Invest
Ophthalmol Vis Sci. 57:4356–4366. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma LY, Lv YL, Huo K, Liu J, Shang SH, Fei
YL, Li YB, Zhao BY, Wei M, Deng YN and Qu QM: Autophagy-lysosome
dysfunction is involved in Aβ deposition in STZ-induced diabetic
rats. Behav Brain Res. 320:484–493. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guan ZF, Zhou XL, Zhang XM, Zhang Y, Wang
YM, Guo QL, Ji G, Wu GF, Wang NN, Yang H, et al: Beclin-1-mediated
autophagy may be involved in the elderly cognitive and affective
disorders in streptozotocin-induced diabetic mice. Transl
Neurodegener. 5:222016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li XH, Xin X, Wang Y, Wu JZ, Jin ZD, Ma
LN, Nie CJ, Xiao X, Hu Y and Jin MW: Pentamethylquercetin protects
against diabetes-related cognitive deficits in diabetic
Goto-Kakizaki rats. J Alzheimers Dis. 34:755–767. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xue H, Ji Y, Wei S, Yu Y, Yan X, Liu S,
Zhang M, Yao F, Lan X and Chen L: HGSD attenuates neuronal
apoptosis through enhancing neuronal autophagy in the brain of
diabetic mice: The role of AMP-activated protein kinase. Life Sci.
153:23–34. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qin L, Wang Z, Tao L and Wang Y: ER stress
negatively regulates AKT/TSC/mTOR pathway to enhance autophagy.
Autophagy. 6:239–247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yan J, Feng Z and Liu J, Shen W, Wang Y,
Wertz K, Weber P, Long J and Liu J: Enhanced autophagy plays a
cardinal role in mitochondrial dysfunction in type 2 diabetic
Goto-Kakizaki (GK) rats: Ameliorating effects of
(−)-epigallocatechin-3-gallate. J Nutr Biochem. 23:716–724. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Holz GG IV, Kiihtreiber WM and Habener JF:
Pancreatic beta-cells are rendered glucose-competent by the
insulinotropic hormone glucagon-like peptide-1(7–37). Nature.
36:362–365. 1993. View
Article : Google Scholar
|
|
36
|
Palleria C, Leo A, Andreozzi F, Citaro R,
Lannone M, Spiga R, Sesti G, Constanti A, De Sarri G, Arturi F and
Russo E: Liraglutide prevents cognitive decline in a rat model of
streptozotocin-induced diabetes independently from its peripheral
metabolic effects. Behav Brain Res. 321:157–169. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Porter WD, Flatt PR, Hölscher C and Gault
VA: Liraglutide improves hippocampal synaptic plasticity associated
with increased expression of Mash1 in ob/ob mice. Int J Obes
(Lond). 37:678–684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu XM, Ou QY, Zhao W, Liu J and Zhang H:
The GLP-1 analogue liraglutide protects cardiomyocytes from high
glucose-induced apoptosis by activating the Epac-1/Akt pathway. Exp
Clin Endocrinol Diabetes. 122:608–614. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Badawi GA, Abd El Fattah MA, Zaki HF and
Sayed EI MI: Sitagliptin and liraglutide reversed nigrostriatal
degeneration of rodent brain in rotenone-induced Parkinson's
disease. Inflammopharmacology. 25:369–382. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Abbas NAT and Kabil SL: Liraglutide
ameliorates cardiotoxicity induced by doxorubicin in rats through
the Akt/GSK-3β signaling pathway (J). Archiv Für Exp Pathol Und
Pharmakol. 1–9. 2017.
|
|
41
|
Zhu H, Zhang Y, Shi Z, Lu D, Li T, Ding Y,
Ruan Y and Xu A: The neuroprotection of liraglutide against
ischaemia-induced apoptosis through the activation of the PI3K/AKT
and MAPK Pathways. Sci Rep. 6:268592016. View Article : Google Scholar : PubMed/NCBI
|