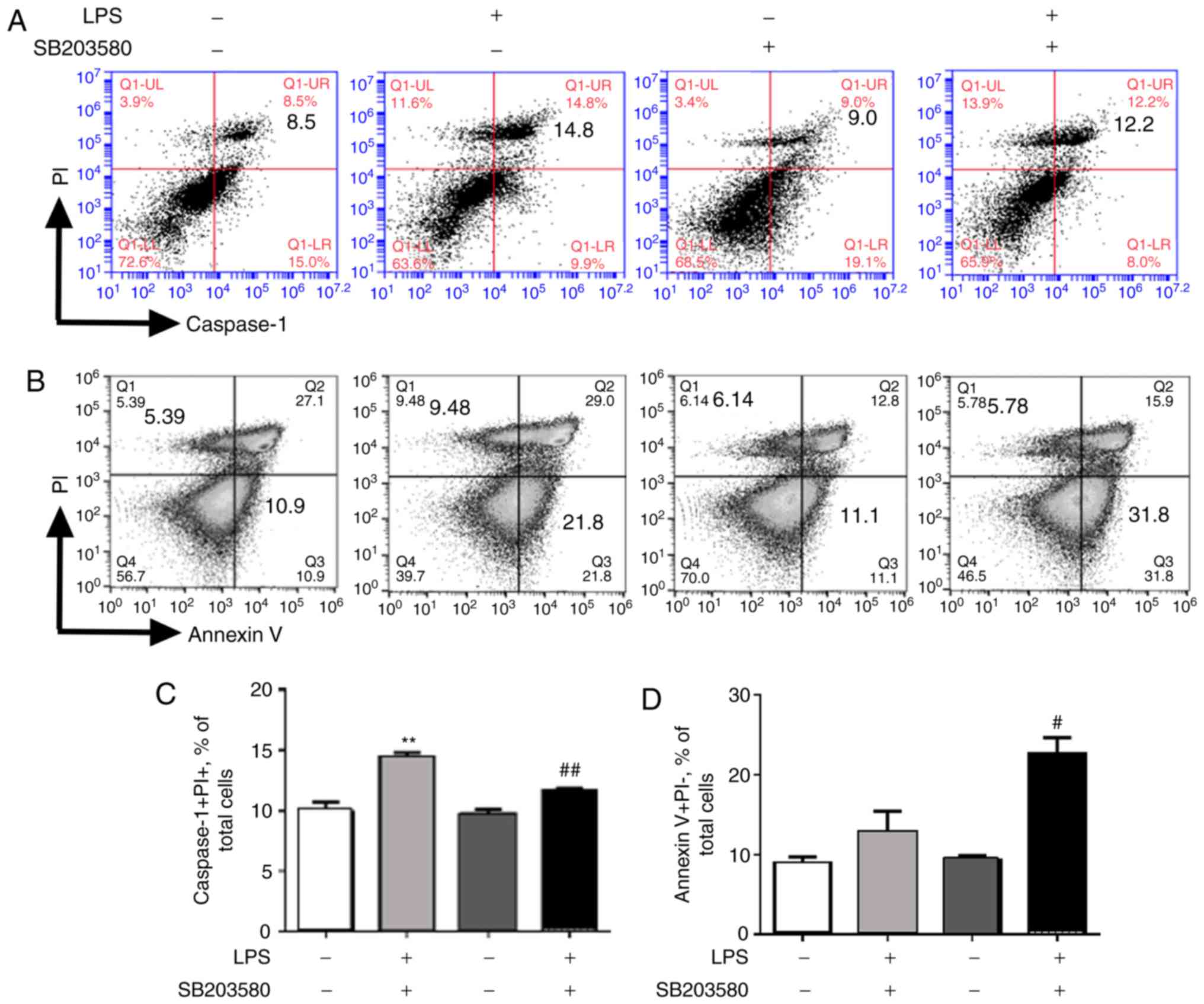
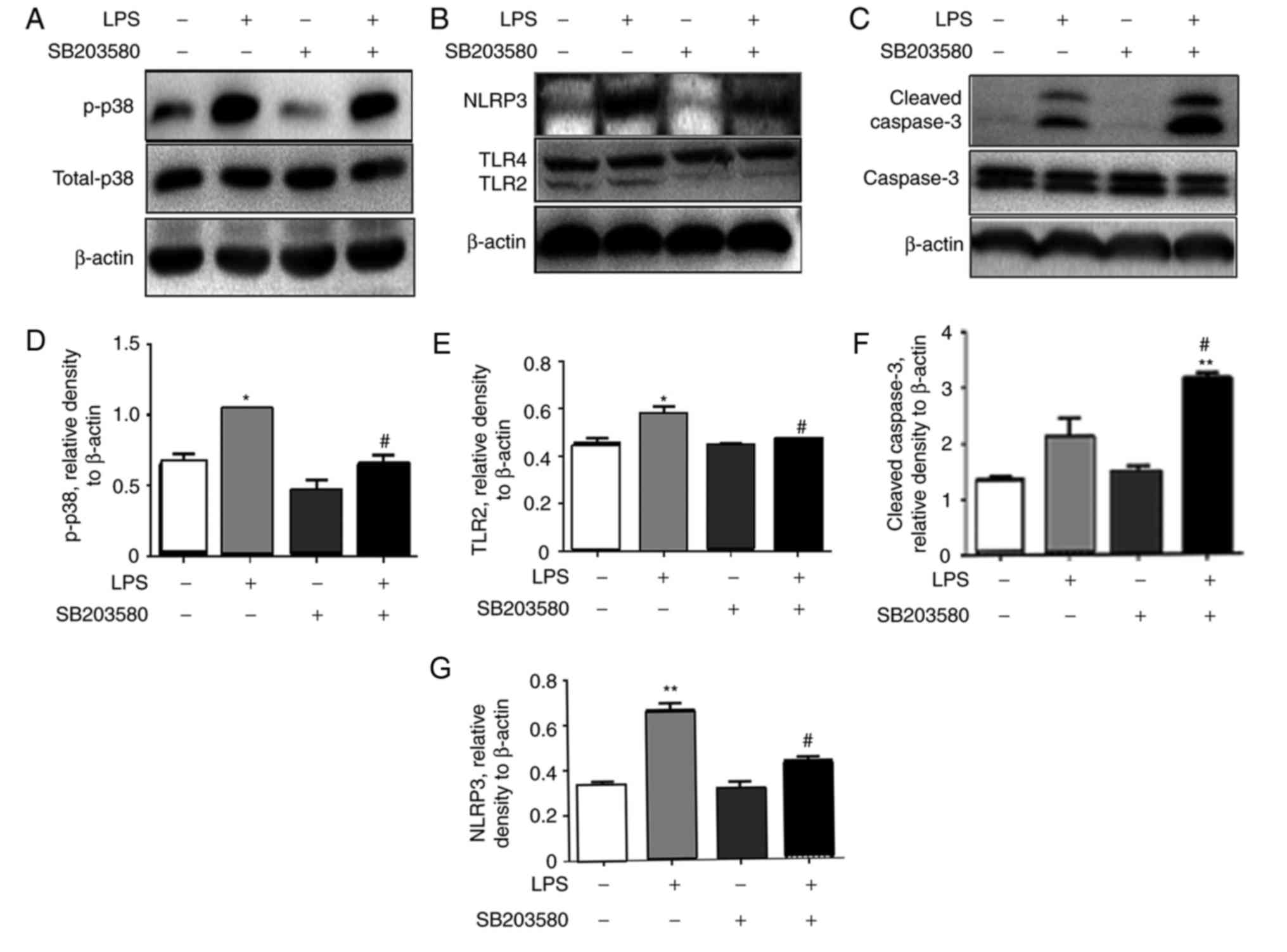
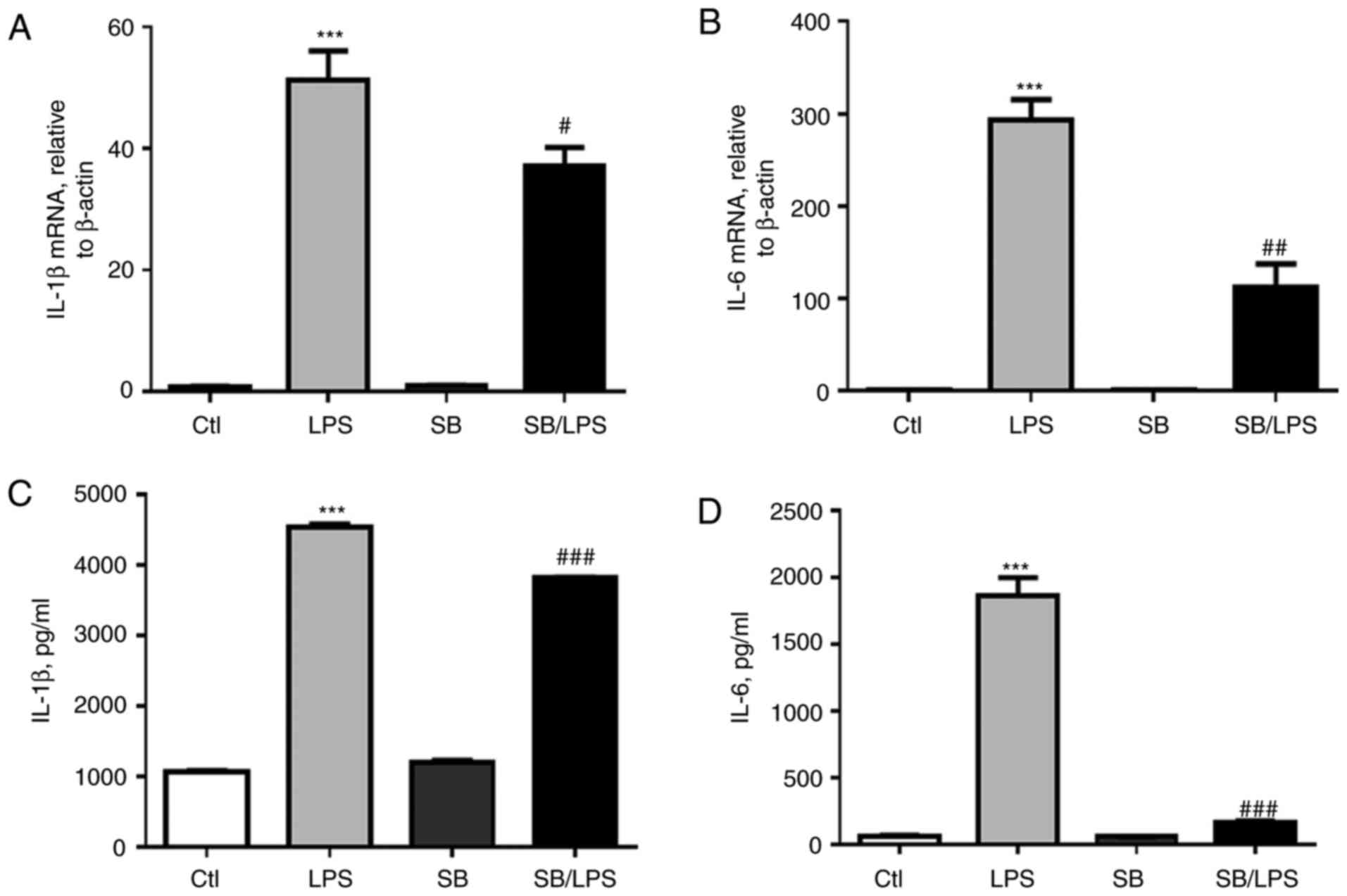
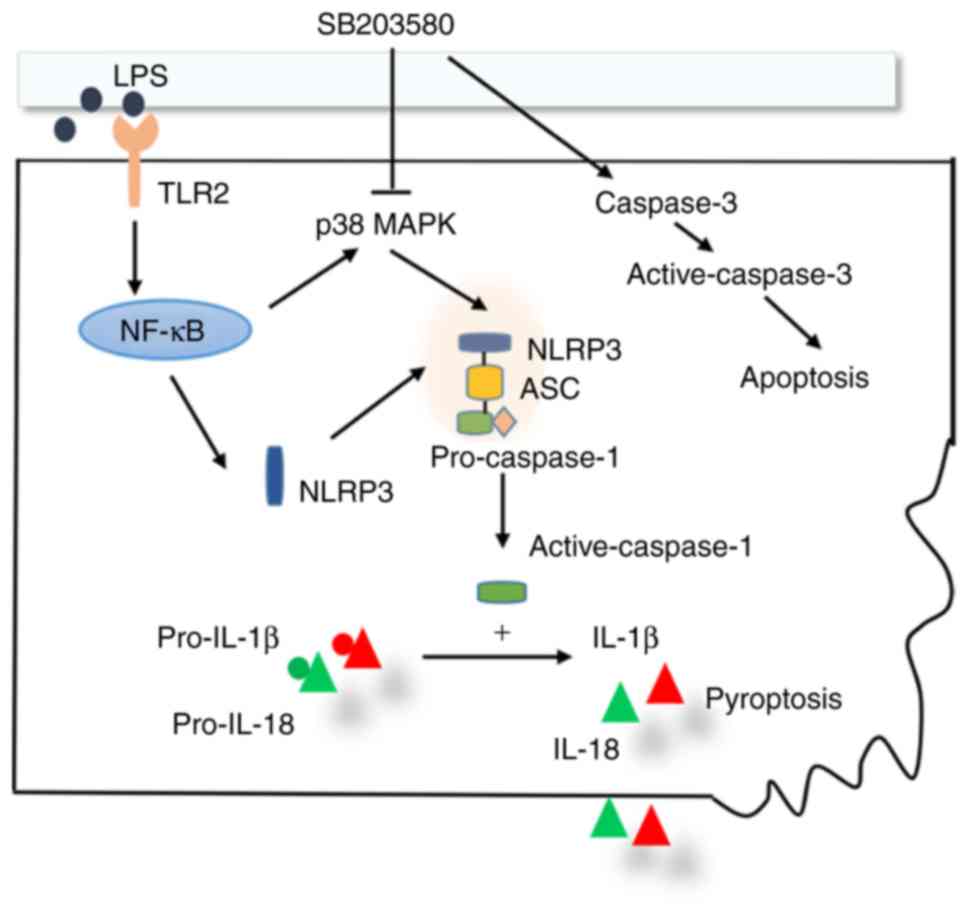
|
1
|
ARDS Definition Task Force, ; Ranieri VM,
Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E,
Camporota L and Slutsky AS: Acute respiratory distress syndrome:
The Berlin Definition. JAMA. 307:2526–2533. 2012.PubMed/NCBI
|
|
2
|
Rubenfeld GD, Caldwell E, Peabody E,
Weaver J, Martin DP, Neff M, Stern EJ and Hudson LD: Incidence and
outcomes of acute lung injury. N Engl J Med. 353:1685–1693. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Z, Chen L and Ni H: The
effectiveness of corticosteroids on mortality in patients with
acute respiratory distress syndrome or acute lung injury: A
secondary analysis. Sci Rep. 5:176542015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Frank JA, Wray CM, McAuley DF, Schwendener
R and Matthay MA: Alveolar macrophages contribute to alveolar
barrier dysfunction in ventilator-induced lung injury. Am J Physiol
Lung Cell Mol Physiol. 291:L1191–L1198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aggarwal NR, King LS and D'Alessio FR:
Diverse macrophage populations mediate acute lung inflammation and
resolution. Am J Physiol Lung Cell Mol Physiol. 306:L709–L725.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Machado-Aranda D, V Suresh M, Yu B,
Dolgachev V, Hemmila MR and Raghavendran K: Alveolar macrophage
depletion increases the severity of acute inflammation following
nonlethal unilateral lung contusion in mice. J Trauma Acute Care
Surg. 76:982–990. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Niesler U, Palmer A, Fröba JS, Braumüller
ST, Zhou S, Gebhard F, Knöferl MW and Seitz DH: Role of alveolar
macrophages in the regulation of local and systemic inflammation
after lung contusion. J Trauma Acute Care Surg. 76:386–393. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
D'Alessio FR, Craig JM, Singer BD, Files
DC, Mock JR, Garibaldi BT, Fallica J, Tripathi A, Mandke P, Gans
JH, et al: Enhanced resolution of experimental ARDS through
IL-4-mediated lung macrophage reprogramming. Am J Physiol Lung Cell
Mol Physiol. 310:L733–L746. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang Z, Zhou Q, Gu C, Li D and Zhu L:
Depletion of circulating monocytes suppresses IL-17 and HMGB1
expression in mice with LPS-induced acute lung injury. Am J Physiol
Lung Cell Mol Physiol. 312:L231–L242. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qiu Z, Hu J, Van den Steen PE and
Opdenakker G: Targeting matrix metalloproteinases in acute
inflammatory shock syndromes. Comb Chem High Throughput Screen.
15:555–570. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gustot T: Multiple organ failure in
sepsis: Prognosis and role of systemic inflammatory response. Curr
Opin Crit Care. 17:153–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kovarova M, Hesker PR, Jania L, Nguyen M,
Snouwaert JN, Xiang Z, Lommatzsch SE, Huang MT, Ting JP and Koller
BH: NLRP1-dependent pyroptosis leads to acute lung injury and
morbidity in mice. J Immunol. 189:2006–2016. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liang J, Jung Y, Tighe RM, Xie T, Liu N,
Leonard M, Gunn MD, Jiang D and Noble PW: A macrophage
subpopulation recruited by CC chemokine ligand-2 clears apoptotic
cells in noninfectious lung injury. Am J Physiol Lung Cell Mol
Physiol. 302:L933–L940. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Q, Imamura R, Motani K, Kushiyama H,
Nagata S and Suda T: Pyroptotic cells externalize eat-me and
release find-me signals and are efficiently engulfed by
macrophages. Int Immunol. 25:363–372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Compan V, Martín-Sánchez F, Baroja-Mazo A,
López-Castejón G, Gomez AI, Verkhratsky A, Brough D and Pelegrín P:
Apoptosis-associated speck-like protein containing a CARD forms
specks but does not activate caspase-1 in the absence of NLRP3
during macrophage swelling. J Immunol. 194:1261–1273. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lamkanfi M, Sarkar A, Vande Walle L,
Vitari AC, Amer AO, Wewers MD, Tracey KJ, Kanneganti TD and Dixit
VM: Inflammasome-dependent release of the alarmin HMGB1 in
endotoxemia. J Immunol. 185:4385–4392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kamo N, Ke B, Ghaffari AA, Shen XD,
Busuttil RW, Cheng G and Kupiec-Weglinski JW: ASC/caspase-1/IL-1β
signaling triggers inflammatory responses by promoting HMGB1
induction in liver ischemia/reperfusion injury. Hepatology.
58:351–362. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Allam R, Kumar SV, Darisipudi MN and
Anders HJ: Extracellular histones in tissue injury and
inflammation. J Mol Med (Berl). 92:465–472. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haggadone MD, Grailer JJ, Fattahi F,
Zetoune FS and Ward PA: Bidirectional crosstalk between C5a
receptors and the NLRP3 inflammasome in macrophages and monocytes.
Mediators Inflamm. 2016:13401562016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiong LL, Tan Y, Ma HY, Dai P, Qin YX,
Yang RA, Xu YY, Deng Z, Zhao W, Xia QJ, et al: Administration of
SB239063, a potent p38 MAPK inhibitor, alleviates acute lung injury
induced by intestinal ischemia reperfusion in rats associated with
AQP4 downregulation. Int Immunopharmacol. 38:54–60. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma L, Zhao Y, Wang R, Chen T, Li W, Nan Y,
Liu X and Jin F: 3,5,4′-Tri-O-acetylresveratrol attenuates
lipopolysaccharide-induced acute respiratory distress syndrome via
MAPK/SIRT1 pathway. Mediators Inflamm. 2015:1430742015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pearson G, Robinson F, Beers Gibson T, Xu
BE, Karandikar M, Berman K and Cobb MH: Mitogen-activated protein
(MAP) kinase pathways: Regulation and physiological functions.
Endocr Rev. 22:153–183. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sui X, Kong N, Ye L, Han W, Zhou J, Zhang
Q, He C and Pan H: p38 and JNK MAPK pathways control the balance of
apoptosis and autophagy in response to chemotherapeutic agents.
Cancer Lett. 344:174–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McGuigan RM, Mullenix P, Norlund LL, Ward
D, Walts M and Azarow K: Acute lung injury using oleic acid in the
laboratory rat: Establishment of a working model and evidence
against free radicals in the acute phase. Curr Surg. 60:412–417.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Geng Y, Ma Q, Liu YN, Peng N, Yuan FF, Li
XG, Li M, Wu YS, Li BL, Song WB, et al: Heatstroke induces liver
injury via IL-1β and HMGB1-induced pyroptosis. J Hepatol.
63:622–633. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Galluzzi L, Vitale I, Abrams JM, Alnemri
ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry
WS, Fulda S, et al: Molecular definitions of cell death
subroutines: Recommendations of the nomenclature committee on cell
death 2012. Cell Death Differ. 19:107–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lamkanfi M and Dixit VM: Mechanisms and
functions of inflammasomes. Cell. 157:1013–1022. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ren WY, Zhu L, Hua F, Jin JJ and Cai YY:
The effect of lipopolysaccharide on gene expression of TLR4 and
MD-2 in rat alveolar macrophage and its secretion of inflammation
cytokines. Zhonghua Jie He He Hu Xi Za Zhi. 33:367–371. 2010.(In
Chinese). PubMed/NCBI
|
|
30
|
Ren W, Hu L, Hua F, Jin J, Wang Y and Zhu
L: Myeloid differentiation protein 2 silencing decreases
LPS-induced cytokine production and TLR4/MyD88 pathway activity in
alveolar macrophages. Immunol Lett. 141:94–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Netea MG, Simon A, van de Veerdonk F,
Kullberg BJ, Van der Meer JW and Joosten LA: IL-1beta processing in
host defense: Beyond the inflammasomes. PLoS Pathog.
6:e10006612010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bode JG, Ehlting C and Häussinger D: The
macrophage response towards LPS and its control through the
p38(MAPK)-STAT3 axis. Cell Signal. 24:1185–1194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu W, Jiang HL, Cai LL, Yan M, Dong SJ
and Mao B: Tanreqing injection attenuates
lipopolysaccharide-induced airway inflammation through MAPK/NF-κB
signaling pathways in rats model. Evid Based Complement Alternat
Med. 2016:52923462016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen CC, Lin MW, Liang CJ and Wang SH: The
anti-inflammatory effects and mechanisms of eupafolin in
lipopolysaccharide-induced inflammatory responses in RAW264.7
macrophages. PLoS One. 11:e01586622016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng DY, Zhou M, Jin J, He M, Wang Y, Du
J, Xiao XY, Li PY, Ye AZ, Liu J and Wang TH: Inhibition of P38 MAPK
downregulates the expression of IL-1β to protect lung from acute
injury in intestinal ischemia reperfusion rats. Mediators Inflamm.
2016:93480372016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bai X, Fan L, He T, Jia W, Yang L, Zhang
J, Liu Y, Shi J, Su L and Hu D: SIRT1 protects rat lung tissue
against severe burn-induced remote ALI by attenuating the apoptosis
of PMVECs via p38 MAPK signaling. Sci Rep. 5:102772015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Q, Wang J, Hu M, Yang Y, Guo L and Xu
J, Lei C, Jiao Y and Xu J: Uncoupling protein 2 increases
susceptibility to lipopolysaccharide-induced acute lung injury in
mice. Mediators Inflamm. 2016:91542302016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu X, Zhu Q, Niu F, Zhang R, Wang Y, Wang
W, Sun D, Wang X and Wang A: A2BAR activation attenuates acute lung
injury by inhibiting alveolar epithelial cell apoptosis both in
vivo and in vitro. Am J Physiol Cell Physiol. Jun 13–2018.(Epub
ahead of print). View Article : Google Scholar
|
|
39
|
Su WJ, Zhang Y, Chen Y, Gong H, Lian YJ,
Peng W, Liu YZ, Wang YX, You ZL, Feng SJ, et al: NLRP3 gene
knockout blocks NF-κB and MAPK signaling pathway in CUMS-induced
depression mouse model. Behav Brain Res. 322:1–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xiang P, Chen T, Mou Y, Wu H, Xie P, Lu G,
Gong X, Hu Q, Zhang Y and Ji H: NZ suppresses TLR4/NF-κB signalings
and NLRP3 inflammasome activation in LPS-induced RAW264.7
macrophages. Inflamm Res. 64:799–808. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Borges PV, Moret KH, Raghavendra NM,
Maramaldo Costa TE, Monteiro AP, Carneiro AB, Pacheco P, Temerozo
JR, Bou-Habib DC, das Graças Henriques M and Penido C: Protective
effect of gedunin on TLR-mediated inflammation by modulation of
inflammasome activation and cytokine production: Evidence of a
multitarget compound. Pharmacol Res. 115:65–77. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Robb CT, Regan KH, Dorward DA and Rossi
AG: Key mechanisms governing resolution of lung inflammation. Semin
Immunopathol. 38:425–428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Linkermann A, Stockwell BR, Krautwald S
and Anders HJ: Regulated cell death and inflammation: An
auto-amplification loop causes organ failure. Nat Rev Immunol.
14:759–767. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chollet-Martin S, Jourdain B, Gibert C,
Elbim C, Chastre J and Gougerot-Pocidalo MA: Interactions between
neutrophils and cytokines in blood and alveolar spaces during ARDS.
Am J Respir Crit Care Med. 154:594–601. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Williams AE and Chambers RC: The mercurial
nature of neutrophils: Still an enigma in ARDS? Am J Physiol Lung
Cell Mol Physiol. 306:L217–L230. 2014. View Article : Google Scholar : PubMed/NCBI
|