Introduction
It is reported that ~250,000,000 individuals
worldwide currently have chronic hepatitis B (CHB) infection and
are at risk of developing liver cirrhosis, hepatic decompensation
and hepatocellular carcinoma (1–3).
Furthermore, CHB infection can be life-threatening in certain cases
(1–3). Viral replication and host immune
responses have been shown to determine the clinical outcomes and
viral persistence in patients with CHB (4,5). The
eradication of HBV infection and life-long anti-HBV immunity can be
monitored following the acute infection period of HBV, which occurs
through the development of a robust innate or adaptive immune
response (6,7). CD4+ T cell priming and
subsequent development of effective CD8+ cytotoxic T
lymphocyte (CTL) responses contribute to viral clearance (8). However, patients with CHB have weak
or functionally impaired CD4+ and CD8+ T cell
immune responses (4).
Consequently, novel immunotherapeutic strategies are required to
enhance HBV-specific T cell responses.
Dendritic cell (DC)-based therapeutic vaccines are
essential in linking DCs and T lymphocytes and provide approaches
to reactivate HBV-specific host immunity against HBV. As the most
dominant antigen-presenting cells (APCs) (9), DCs are the main cells involved in
antigen presentation and activation of T cells, which are important
in the development of adaptive immune responses. DCs can prime
naïve T helper (Th) cells to polarize into Th1 or Th2 cells.
Various methods have been designed to load DCs with viral antigen
genes in order to induce specific immune responses, for example,
peptide- or protein-pulsed DCs (10,11).
However, there are certain limitations of these types of vaccine
strategies, including the short duration in antigenic epitope
presentation and the limited effect of the resulting immune
responses. Lentiviral vectors (LVs) are mostly based on human
immunodeficiency virus-l and represent effective gene carriers. LVs
can stably integrate large antigenic genes into DCs, leading to the
continuous expression of the related genes. Gene-modified DCs may
mediate enhanced antigen-specific immune responses (12).
Therapeutic vaccines that are based on hepatitis B
core antigen (HBcAg) can polarize Th1 cells and elicit high levels
of HBcAg-specific CTLs in HBV transgenic mice (13,14).
HBcAg-specific CTLs have been shown to suppress HBV replication and
alleviate liver damage (13,14).
Ubiquitin (Ub) is a conserved protein in eukaryotic cells, which
serves as a signal for the target protein to be recognized and
degraded through the ubiquitin-proteasome system (UPS) during
proteolysis (15). In our previous
study, LVs were used to encode the ubiquitinated HBcAg
(LV-Ub-HBcAg), and it was demonstrated that LV-Ub-HBcAg promoted
the maturation of DCs, which induced T cell polarization to Th1
cells and the production of HBV-specific CTLs in vitro
(16). The present study aimed to
investigate whether DCs transduced with LV-Ub-HBcAg can enhance the
polarization of Th1 cells and elicit HBV-specific T cell responses
in HBV transgenic mice.
Materials and methods
Mice
H-2Kd HBV-transgenic BALB/c mice (half
male and half female) were obtained from the Key Liver Army
Laboratory of No.458 Hospital (Guangzhou, China). They were 6–8
weeks old (20–23 g) and their characteristics were as described
previously (17,18). All mice were housed under specific
pathogen-free conditions (22–24°C; humidity 50–55%; 12 h light/dark
cycle), with free access to food and water. in the Experimental
Animal Centre of the Sixth Hospital affiliated to Shanghai Jiao
Tong University (Shanghai, China). The study protocol was performed
according to the guidelines established by the Laboratory Animal
Ethics Committees of Shanghai Jiao Tong University.
Recombinant lentiviral vector
preparation and DC transduction
The recombinant lentiviral vectors encoding Ub-HBcAg
and/or HBcAg were constructed as described previously (19). The lentiviral particles
(LV-Ub-HBcAg and LV-HBcAg or LV) were prepared in 293T cells
(American Type Culture Collection, Manassas, VA, USA) and tittered
as described previously (19).
Briefly, the recombinant pLOV.UBC.Ub-HBcAg.EGFP.3FLAG vector
plasmid was constructed by inserting the Ub-HBcAg fragment into the
BamHI and NheI sites of the pLOV.UBC.EGFP.3FLAG plasmid
(Obio Technology Corp., Ltd., Shanghai, China). The control plasmid
was constructed by inserting the HBcAg fragment. Lentiviral
particles of LV-Ub-HBcAg, LV-HBcAg or LV were produced by triple
transfection of 80% confluent 293T cells with
pLOV.UBC.Ub-HBcAg.EGFP.3FLAG, pLOV.UBC.HBcAg.EGFP.3FLAG or
pLOV.UBC.EGFP.3FLAG using the pHelper 1.0 and pHelper 2.0 helper
plasmids (Obio Technology Corp., Ltd.) using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). Murine DCs were
generated according to the protocol described by Chen et al
(16). The DCs were transduced by
the LVs (LV-Ub-HBcAg, LV-HBcAg, or LV) at a multiplicity of
infection of 20 as described in our previous study (19).
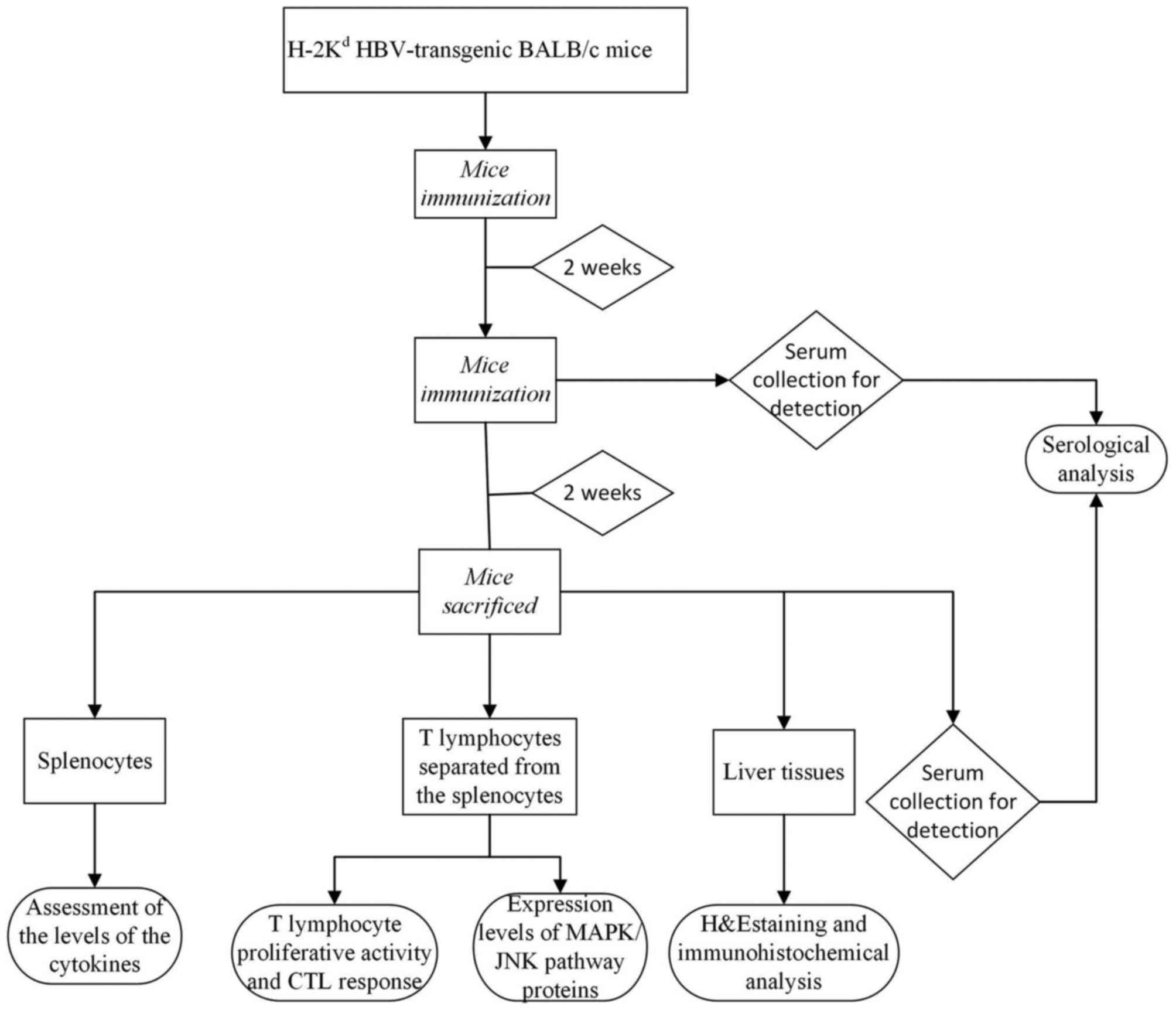
Mice immunization
The HBV transgenic mice were randomly divided into
six groups, with six mice in each group. Subcutaneous immunization
of the mice was performed twice at an interval of 2 weeks with
5×105 recombinant lentiviral-transduced DCs (LV/DC,
LV-HBcAg/DC, or LV-Ub-HBcAg/DC), 20,000 IU interferon (IFN)-α
(Roche Diagnostics, Basel, Switzerland) or 50 µg HBcAg
(CalBioreagents, Inc., San Mateo, CA, USA). Mice injected with PBS
and/or untransduced DCs served as the controls (Fig. 1).
Assessment of the levels of the
cytokines
The splenocytes were harvested 2 weeks following the
final immunization, and were seeded in 24-well plates
(2×106 cells/ml) in the presence of 10 µg/ml HBcAg.
Following 72 h of incubation in RPMI 1640 culture medium
(Invitrogen; Thermo Fisher Scientific, Inc.) containing 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 g/ml streptomycin at 37°C in a humidified
atmosphere with 5% CO2, the levels of IFN-γ, interleukin
(IL)-2, tumor necrosis factor (TNF)-α, IL-4 and IL-10 in the
supernatants were measured using commercial mouse cytokine ELISA
kits (cat. nos. MIF00, M2000, MTA00B, M4000B and M1000B; R&D
Systems, Inc., Minneapolis, NN USA), according to the
manufacturer's protocols. The results are expressed as pg/ml.
Detection of T lymphocyte
proliferative activity and CTL response
The T lymphocytes were separated from the
splenocytes using nylon wool columns (Wako Pure Chemical
Industries, Ltd., Tokyo, Japan) according to the method described
previously by Chen et al (20). The Cell Counting Kit-8 (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) was utilized to
detect T lymphocyte proliferation. The T lymphocytes were seeded at
a density of 4×105 cells/well in a final volume of 200
µl in 96-well culture plates. The cells were subsequently
co-cultured with 1 µg of ConA solution at 37°C with 5%
CO2. Following 48 h of incubation, 20 µl of Cell
Counting Kit-8 reagent was added to the plates, and the plates were
incubated at 37°C with 5% CO2 for 4 h. The absorbance
was recorded at 450 nm.
P815/c cells (expressing HBcAg; Nanjing Medical
University, Nanjing, China) were maintained in our laboratory
(Department of Infectious Disease, Shanghai Jiao Tong University
Affiliated Sixth People's Hospital) and used as target cells. T
lymphocytes were used as the effector cells and incubated (RPMI1640
culture medium containing 10% FBS, 100 U/ml penicillin, and 100
g/ml streptomycin) with the P815/c cells at different effector and
target (E/T) ratios (5:1,10:1, and 20:1) at 37°C with 5%
CO2 for 4 h. HBcAg-specific CTL activity was determined
using a lactate dehydrogenase release assay according to the
protocol of the CytoTox 96® Non-radioactive cytotoxicity
kit (Promega Corporation, Madison, WI, USA). The absorbance values
of the sample supernatants were recorded at 490 nm. The
cytotoxicity (%) was calculated as follows: [(experimental
release-effector spontaneous release-target spontaneous
release)/(target maximum release-target spontaneous release)] ×100%
(16,21).
Serological analysis
The serum was harvested 2 weeks following the first
and final immunization. Serum HBsAg and HBV DNA levels were
measured respectively using Abbott kits (Abbott Diagnostics,
Chicago, IL, USA) and quantitative polymerase chain reaction (qPCR;
Terra PCR Direct Polymerase mix, Clontech Laboratories, Inc.,
Mountainview, CA, USA) (22).
Primer sequences (sequences unavailable) were synthesized and
provided by DaAn Gene Co., Ltd. of Sun Yat-sen University
(Guangzhou, China). Fluorescence was measured using a LightCycler
600 real-time fluorescence quantitative PCR instrument (Roche
Diagnostics, Basel, Switzerland). The thermocycling conditions were
as follows: 93°C for 7 min; 10 cycles of 93°C for 45 sec and 55°C
for 1 min; 30 cycles of 93°C for 30 sec and 55°C for 45 sec; then
40°C for 20 sec. The 2−ΔΔCq method was used to quantify
the results (23). Furthermore,
the levels of alanine aminotransferase (ALT) and aspartate
transaminase (AST) in the serum were detected using the ARCHITECT
Automatic Biochemistry Analyzer (Abbott Diagnostics, Abbott Park,
IL, USA).
Hematoxylin and eosin (H&E)
staining and immunohistochemical analysis of the liver
The liver tissues were fixed in paraformaldehyde
solution (4%), embedded in paraffin and sectioned (5 µm). For
histological analysis, the de-paraffinized sections were stained
with H&E (Beyotime Institute of Biotechnology, Jiangsu, China).
For immunohistochemical analysis, the de-paraffinized sections were
treated with 0.3% H2O2 for 10 min in order to
inactivate the endogenous peroxidase. The sections were blocked
with 2% goat serum (Beyotime Institute of Biotechnology) for 30 min
at room temperature and washed with PBS. Mouse anti-HBsAg (cat. no.
NB110-62652; 1:500 dilution) and/or anti-HBcAg (cat. no.
NB100-64452; 1:500 dilution) monoclonal antibodies (Novus
Biologicals, LLC, Littleton, CO, USA) were added overnight at 4°C.
Following washing with PBS, the sections were stained with
secondary antibodies (cat. no. BA1001; 1:1,000 dilution; Boster
Biological Technology, Wuhan China) for 30 min at 37°C and then
with streptavidin-biotin-peroxidase complex for 30 min. The
sections were subsequently visualized with diaminobenzidine (Boster
Biological Technology) and counterstained with hematoxylin under a
Nikon light microscope (Nikon Corporation, Tokyo, Japan).
Detection of expression levels of
MAPK/JNK pathway proteins
The T lymphocytes were lysed in RIPA lysis buffer
containing protease inhibitor mixture (Beyotime Institute of
Biotechnology, Jiangsu, China). A Pierce BCA protein assay reagent
kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used to
assess the protein concentration levels. The protein lysates (30
µg/ml) were separated by 10% SDS-PAGE and subsequently transferred
onto a PVDF membrane (EMD Millipore, Bedford, MA, USA). Rabbit
anti-p38MAPK (cat. no. 8690; 1:1,000 dilution), phosphorylated
(p)-p38MAPK (cat. no. 4511; 1:1,000 dilution), JNK (cat. no. 9258;
1:1,000 dilution) and p-JNK (cat. no. 4668; 1:1,000 dilution)
monoclonal antibodies were used as primary antibodies. Horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin-G (cat. no.
7074; 1:2,000 dilution) was used as secondary antibody. All
antibodies were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). The protein bands were visualized by enhanced
chemiluminescence (Beyotime Institute of Biotechnology) and
analyzed using Image Pro Plus version 6.0 (Media Cybernetics, Inc.,
Rockville, MD, USA).
Statistical analysis
The data are presented as the mean ± standard
deviation and each value was obtained from at least three
independent experiments. One-way analysis of variance and a
post-hoc least significant difference test were used to determine
the statistical significance compared with the control samples. The
data were analyzed using SPSS 19.0 software (IBM SPSS, Armonk, NY,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
LV-Ub-HBcAg/DC stimulates the
secretion of Th1-like cytokines
The present study assayed the splenocytes from
immunized animals for the secretion of cytokines, namely IFN-γ,
IL-2, TNF-α (Th1-like), IL-4 and IL-10 (Th2-like), upon
restimulation with HBcAg (10 µg/ml). T cells from the
LV-Ub-HBcAg/DC groups produced higher levels of IFN-γ (369.71±13.04
pg/ml), IL-2 (422.85±10.91 pg/ml) and TNF-α (171.02±14.68 pg/ml),
compared with the other groups (Fig.
2A-C). However, no significant difference was found in the
production of IL-4 or IL-10 (Th2-like) between the groups examined
(Fig. 2D and E).
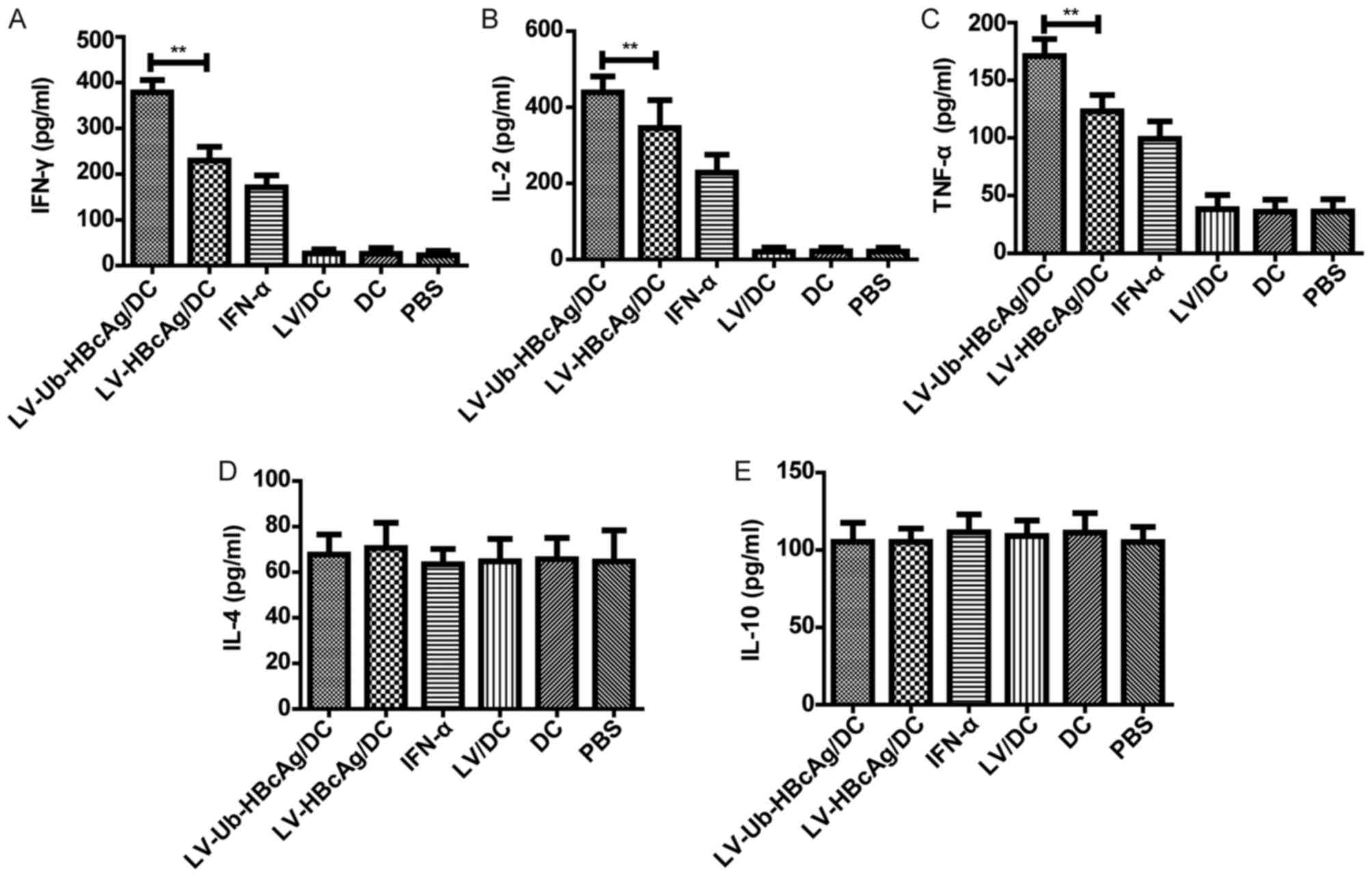 | Figure 2.Expression levels of the IFN-γ, IL-2,
TNF-α, IL-4, and IL-10 cytokines. The levels of (A) IFN-γ, (B) IL-2
and (C) TNF-α in the supernatants of the splenocytes from the group
of LV-Ub-HBcAg/DC-immunized HBV transgenic mice were significantly
higher than those detected in the other groups (**P<0.01). The
levels of (D) IL-4 and (E) IL-10 in the supernatants of splenocytes
from immunized mice did not differ significantly among the groups.
The data are representative from at least three independent
experiments, and presented as the mean ± standard deviation (n=6).
IFN, interferon; IL, interleukin; TNF, tumor necrosis factor; LV,
lentivirus; HBcAg, hepatitis B core antigen, Ub-HBcAg,
ubiquitinated HBcAg; DC, dendritic cell; PBS, phosphate-buffered
saline. |
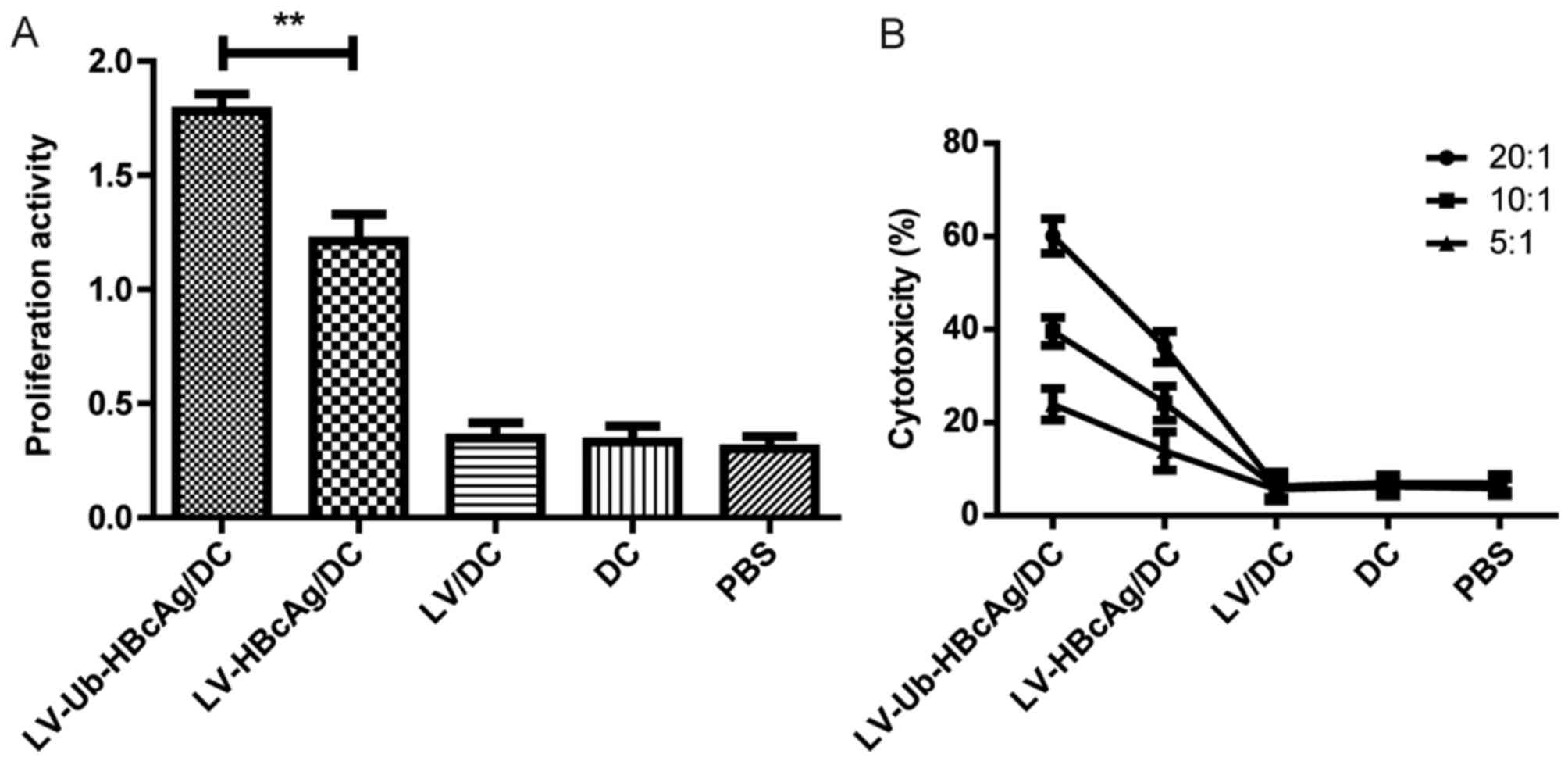
LV-Ub-HBcAg/DC enhances T cell
proliferation and the specific CTL response
The activity of T lymphocyte proliferation from the
immunized animals was examined. T lymphocyte proliferative activity
in the LV-Ub-HBcAg/DC group was higher compared with that in the
other groups (P<0.01; Fig. 3A).
The CTL response was further assessed in the different groups. The
LV-Ub-HBcAg/DC group induced higher percentages of specific
cytolysis at E:T ratios of 20:1, 10:1 and 5:1, respectively,
compared with the other groups (P<0.05; Fig. 3B).
LV-Ub-HBcAg/DC decreases the serum
HBsAg and HBV DNA levels and enhances ALT and AST levels
Serum was collected from the immunized mice 2 weeks
following the first and the final immunization. The aim of the
subsequent experiments was to evaluate whether LV-Ub-HBcAg/DC was
able to clear HBV in the mice. The LV-Ub-HBcAg/DC group exhibited
decreased levels of serum HBsAg and HBV DNA titer compared with the
other groups (Fig. 4A and B).
Furthermore, the ALT and AST levels were assessed in the serum 2
weeks following the final immunization of the mice, and the levels
of serum ALT and AST in the LV-Ub-HBcAg/DC group were higher
compared with those in the other groups (Fig. 4C and D).
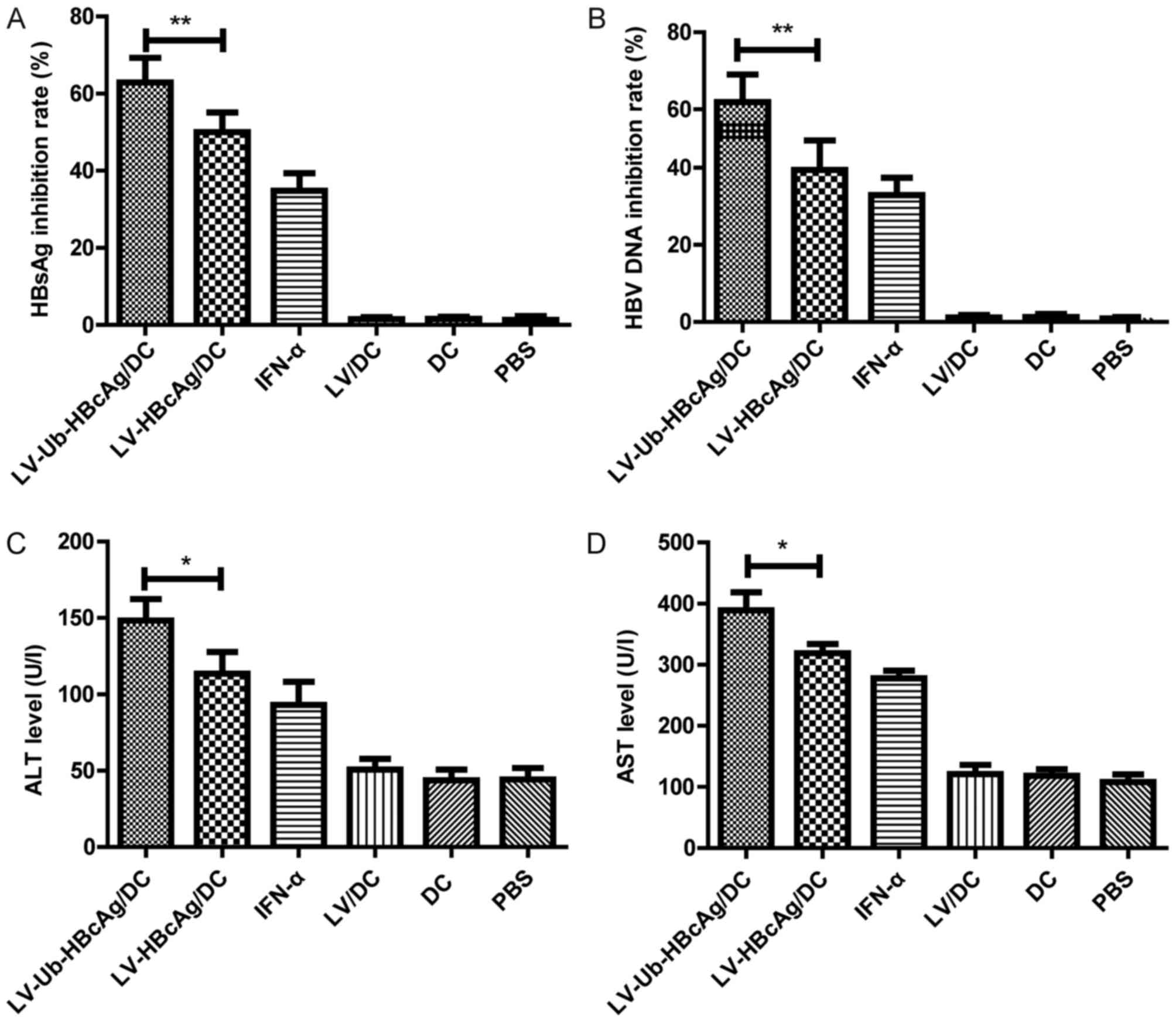 | Figure 4.Suppression of serum levels of HBsAg,
HBV DNA, ALT and AST in immunized HBV transgenic mice. (A) HBsAg
and (B) HBV DNA inhibitory rates in the serum samples from
immunized mice were evaluated. (C) ALT and (D) AST levels in the
mice were detected 2 weeks following the final immunization. The
data are presented as the mean ± standard deviation (n=6).
*P<0.05, **P<0.01. LV, lentivirus; HBcAg, hepatitis B core
antigen, HBsAg, hepatitis B surface antigen; Ub-HBcAg,
ubiquitinated HBcAg; HBV, hepatitis B virus; ALT, alanine
aminotransferase; AST, aspartate transaminase; DC, dendritic cell;
IFN, interferon; PBS, phosphate-buffered saline. |
LV-Ub-HBcAg/DC increases the
inflammatory reaction and reduces the expression of HBsAg and HBcAg
in liver tissue
The H&E-stained sections of liver tissues were
observed in order to evaluate the histological changes. A higher
level of lymphocyte infiltration was observed in the liver of mice
immunized with LV-Ub-HBcAg/DC compared with the other groups
(Fig. 5A). To further evaluate
whether LV-Ub-HBcAg/DC resulted in viral clearance in transgenic
mice, the therapeutic effects of this agent were detected by
immunohistological analysis in the liver tissues from the immunized
mice. The results suggested that LV-Ub-HBcAg/DC immunization
reduced the expression levels of HBsAg in the cytoplasm and the
expression levels of HBcAg in the nuclei (stained brownish yellow)
compared with those noted in the other groups (Fig. 5B and C).
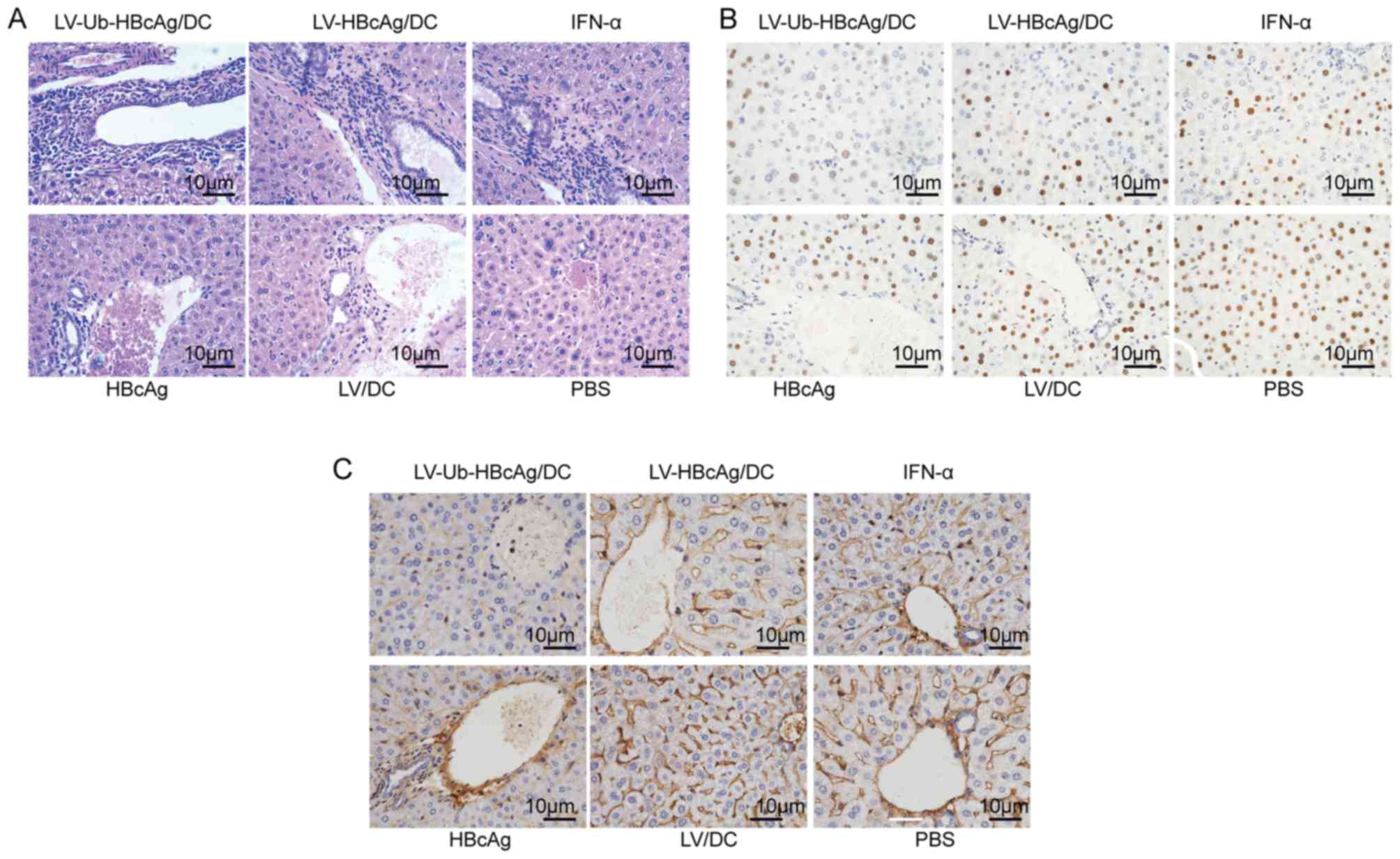 | Figure 5.Histological analysis and
immunohistochemical staining for HBcAg and HBsAg in liver sections
of HBV transgenic mice. The mice were treated with PBS, LV/DC,
HBcAg, IFN-α, LV-HBcAg/DC or LV-Ub-HBcAg/DC. (A) Liver sections
were stained with hematoxylin and eosin and inflammatory
infiltrates were observed by light microscopy. Immunohistochemical
staining for (B) HBcAg and (C) HBsAg was performed. Representative
images are shown (original magnifications, ×400). LV, lentivirus;
HBcAg, hepatitis B core antigen; HBsAg, hepatitis B surface
antigen; Ub-HBcAg, ubiquitinated HBcAg; DC, dendritic cell; IFN,
interferon; PBS, phosphate-buffered saline. |
LV-Ub-HBcAg/DC enhances the expression
levels of p-P38-MAPK and p-JNK
LV-Ub-HBcAg/DC affected the MAPK/JNK signaling
pathway. To investigate the underlying mechanism, the expression
levels of P38-MAPK, p-P38-MAPK, JNK and p-JNK were analyzed in the
T cells derived from the LV-Ub-HBcAg/DC, LV-HBcAg/DC, LV/DC and PBS
mice groups. The results demonstrated that p-P38-MAPK and p-JNK
were significantly upregulated in the LV-Ub-HBcAg/DC group compared
with the corresponding protein expression in the other groups
(P<0.01; Fig. 6).
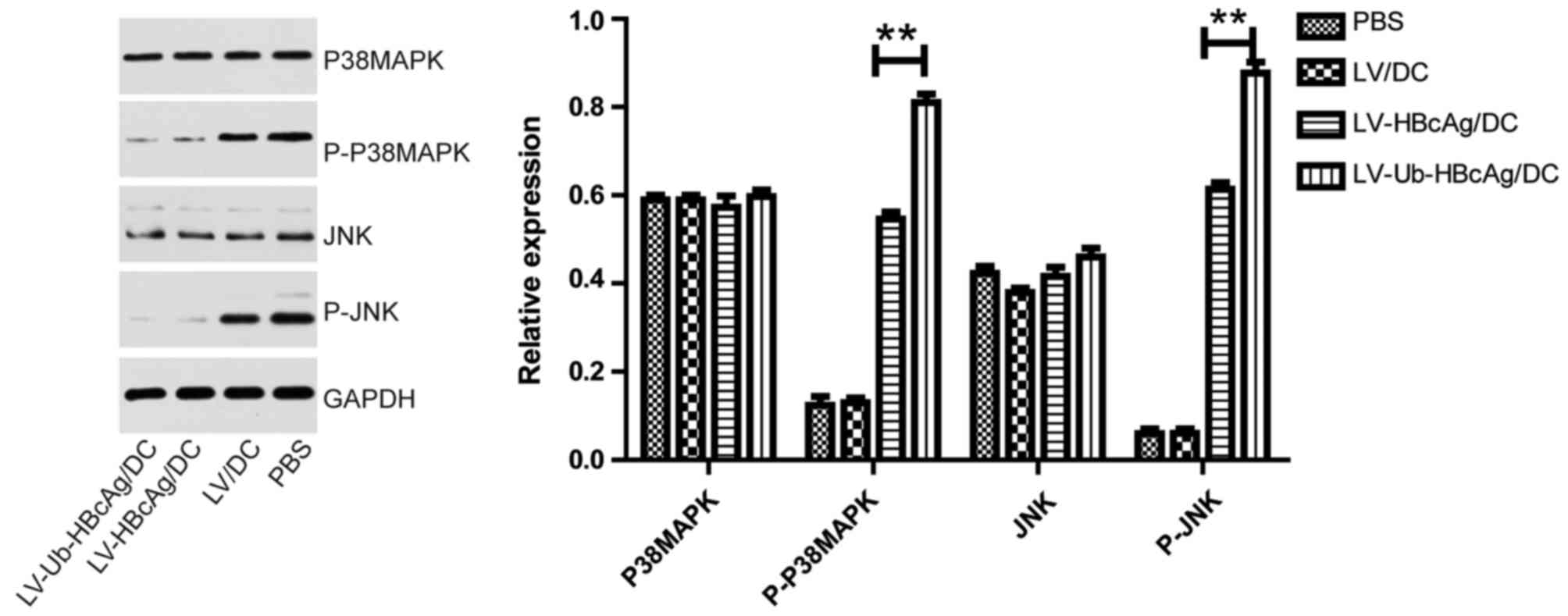 | Figure 6.Expression levels of MAPK/JNK pathway
proteins. The expression levels of p-P38MAPK and p-JNK were
significantly upregulated in mice immunized with LV-Ub-HBcAg/DC.
The data are presented as the mean ± standard deviation (n=6,
**P<0.01). LV, lentivirus; HBcAg, hepatitis B core antigen,
Ub-HBcAg, ubiquitinated HBcAg; DC, dendritic cell; IFN, interferon;
PBS, phosphate-buffered saline; MAPK, mitogen-activated protein
kinase; JNK, c-Jun N-terminal kinase; p-, phosphorylated. |
Discussion
In the present study, DCs transduced with
LV-Ub-HBcAg were used to immunize HBV transgenic mice. Th1-type
(IFN-γ, IL-2 and TNF-α) and Th2-type (IL-4 and IL-10) cytokines
were then assessed as an indicator of the Th1/Th2 immune balance.
The T cell proliferation and specific CTL activities were also
assessed. In addition, to evaluate the specific anti-HBV activity
in vivo, the levels of HBsAg and HBV DNA in the serum were
detected, and the expression levels of HBcAg and HBsAg in liver
tissues were examined. Furthermore, the protein expression levels
of the P38-MAPK/JNK pathway were analyzed by western blot analysis
to examine the mechanism of Th cell differentiation. The results
indicated that LV-Ub-HBcAg/DC induced Th1 cell differentiation and
potent HBV-specific T cell responses in mice. The data also
suggested that the P38-MAPK/JNK pathway may be associated with the
differentiation of Th cells.
The control of HBV replication and the clinical
outcome of HBV infection depend on the immunity of the host. The
resolving infection of HBV results in multiple HBV antigen-specific
CD8+ and CD4+ T cell responses (24,25)
which are associated with viral clearance (26). However, patients with CHB have
impaired and dysfunctional HBV-specific T cell responses (27–29).
The specific T cell responses are mediated by APCs, and DCs are
considered the most potent APCs of the immune system (30).
DCs are important in inducing immunity against viral
infections (31). DC-based
therapeutic vaccines pulsed with HBV antigens can boost
HBV-specific adaptive immunity, including specific CD4+
and CD8+ T cell immune responses (31). LVs are efficient delivery systems
that are used as vaccine vectors to elicit protective T cell immune
responses in infectious diseases and cancer (32). LVs have the ability to transduce
antigens into DCs and generate specific immune responses (32). Accumulated data have shown that
patients with CHB have impaired function of DCs and are at an
immunocompromised state of immune tolerance (33–35).
Our previous study demonstrated that LV-Ub-HBcAg can induce DC
maturation and improve DC function in vitro (16). Our previous studies have shown that
the production of Ub-HBcAg can promote HBcAg degradation into
antigenic peptides by the UPS (16,19).
These peptides are presented by DCs and are readily recognized by
CD8+ T cells, whereas the activation of HBV-specific
CD8+ T cells is critical for HBV control (36,37).
In the present study, it was demonstrated that treatment with DCs
transduced by LV-Ub-HBcAg increased the secretion of Th1-like
cytokines (IFN-γ, IL-2 and TNF-α) and induced HBcAg-specific CTL
activity.
The present study further evaluated whether
LV-Ub-HBcAg/DC immunization can generate antiviral immunity. HBV
transgenic mice are used as a model of chronic HBV infection,
appropriate to assess the efficacy of treatment strategies. The
findings of the present study demonstrated that the inflammatory
reaction in the liver was consistent with specific CTL activity
induced by LV-Ub-HBcAg/DC. LV-Ub-HBcAg/DC reduced serum HBsAg and
HBV DNA levels in the HBV transgenic mice and the expression of
HBsAg and HBcAg in the liver tissue. These data indicated that
LV-Ub-HBcAg/DC induced anti-HBV activity in vivo.
A number of studies have demonstrated the presence
of predominant Th1 responses and increased CTL activity in patients
with CHB who respond positively to antiviral therapy (37); therefore, the activation of Thl
responses may be critical for the successful treatment of HBV
(37,38). The findings of the present study
highlighted that immunization with DCS that were modified by
LV-Ub-HBcAg effectively promoted the secretion of IFN-γ, IL-2 and
TNF-α (Th1-like). Preferential priming of anti-HBcAg Th1 immunity
was evident. Several signaling pathways are required for Th1 cell
differentiation. Certain studies have established that the P38 and
JNK MAPK pathways are selectively induced during the activation of
Th1 effector cells and are required for Th1 immune responses
(39,40). MAPK pathways have an important
regulatory role in the proliferation and migration of T cells
(41,42). In the present study, LV-Ub-HBcAg/DC
increased the production of IFN-γ, which activated MAPK signaling.
The protein expression of the P38MAPK/JNK pathway was analyzed by
western blot analysis. The results revealed that the expression
levels of p-P38MAPK and p-JNK were significantly upregulated in the
LV-Ub-HBcAg/DC-immunized mice group compared with those in the
other groups. It was noted that the difference in the production of
IL-4 and IL-10 (Th2-like) between these groups was not
statistically significant. Cell metabolism and the cellular
environment may be artificially altered during the processes of
cell stimulation or isolation, whereas the levels of the cytokines
in these cells measured using ELISA may not reflect the apparent
biological levels in vivo (43). Further investigations may use more
effective detection methods of these biomarkers. The present study
did not conclude that Th2-like cytokines were associated with the
P38MAPK/JNK pathway. The results indicated that the P38MAPK/JNK
pathway may be associated with the differentiation of Th cells,
which requires additional confirmation using specific inhibitors of
the signaling pathway examined.
Despite the emergence of novel antiviral drugs for
the treatment of patients with CHB, sustained off-treatment
responses have rarely been achieved. In addition, the maintenance
of antiviral therapy with currently available antiviral drugs in
patients with CHB is associated with the long-term risk of viral
resistance and drug toxicity (44,45).
The data obtained in the present study indicated that immunization
with DCs modified with LV-Ub-HBcAg may be a promising candidate for
the treatment of CHB. In conclusion, immunization with DCs modified
with LV-Ub-HBcAg induced predominant Th1 responses and antiviral
immunity in HBV transgenic mice. The activation of the P38-MAPK/JNK
signaling pathway may be involved in this induction. These results
support the conclusion that vaccination with LV-Ub-HBcAg/DC may be
a potential therapeutic strategy for HBV clearance.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81270502
and 81470853) and the Science and Technology Commission of
Zhenjiang Municipality (grant no. SH2016040).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
GZ and ZT conceived and designed the study. SD, XC
and YY performed the experiments. SD wrote the paper. GZ, ZT, XC
and YY reviewed and edited the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was performed according to the
guidelines established by the Laboratory Animal Ethics Committees
of Shanghai Jiao Tong University (Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Perz JF, Armstrong GL, Farrington LA,
Hutin YJ and Bell BP: The contributions of hepatitis B virus and
hepatitis C virus infections to cirrhosis and primary liver cancer
worldwide. J Hepatol. 45:529–538. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schweitzer A, Horn J, Mikolajczyk RT,
Krause G and Ott JJ: Estimations of worldwide prevalence of chronic
hepatitis B virus infection: A systematic review of data published
between 1965 and 2013. Lancet. 386:1546–1555. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lok AS: Chronic hepatitis B. N Engl J Med.
346:1682–1683. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chisari FV, Isogawa M and Wieland SF:
Pathogenesis of hepatitis B virus infection. Pathol Biol (Paris).
58:258–266. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bertoletti A and Ferrari C: Innate and
adaptive immune responses in chronic hepatitis B virus infections:
Towards restoration of immune control of viral infection. Postgrad
Med J. 89:294–304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bertoletti A and Gehring AJ: The immune
response during hepatitis B virus infection. J Gen Virol.
87:1439–1449. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Loggi E, Gamal N, Bihl F, Bernardi M and
Andreone P: Adaptive response in hepatitis B virus infection. J
Viral Hepat. 21:305–313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Asabe S, Wieland SF, Chattopadhyay PK,
Roederer M, Engle RE, Purcell RH and Chisari FV: The size of the
viral inoculum contributes to the outcome of hepatitis B virus
infection. J Virol. 83:9652–9662. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Steinman RM and Hemmi H: Dendritic cells:
Translating innate to adaptive immunity. Curr Top Microbiol
Immunol. 311:17–58. 2006.PubMed/NCBI
|
|
10
|
Santodonato L, D'Agostino G, Nisini R,
Mariotti S, Monque DM, Spada M, Lattanzi L, Perrone MP, Andreotti
M, Belardelli F and Ferrantini M: Monocyte-derived dendritic cells
generated after a short-term culture with IFN-alpha and
granulocyte-macrophage colony-stimulating factor stimulate a potent
Epstein-Barr virus-specific CD8+ T cell response. J Immunol.
170:5195–5202. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fonteneau JF, Larsson M, Somersan S,
Sanders C, Münz C, Kwok WW, Bhardwaj N and Jotereau F: Generation
of high quantities of viral and tumor-specific human CD4+ and CD8+
T-cell clones using peptide pulsed mature dendritic cells. J
Immunol Methods. 258:111–126. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boudreau JE, Bonehill A, Thielemans K and
Wan Y: Engineering dendritic cells to enhance cancer immunotherapy.
Mol Ther. 19:841–853. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Akbar SM, Yoshida O, Chen S, Cesar AJ, Abe
M, Matsuura B, Hiasa Y and Onji M: Immune modulator and antiviral
potential of dendritic cells pulsed with both hepatitis B surface
antigen and core antigen for treating chronic HBV infection.
Antivir Ther. 15:887–895. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Akbar SM, Chen S, Al-Mahtab M, Abe M,
Hiasa Y and Onji M: Strong and multi-antigen specific immunity by
hepatitis B core antigen (HBcAg)-based vaccines in a murine model
of chronic hepatitis B: HBcAg is a candidate for a therapeutic
vaccine against hepatitis B virus. Antiviral Res. 96:59–64. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao G and Luo H: The ubiquitin-proteasome
pathway in viral infections. Can J Physiol Pharmacol. 84:5–14.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen JH, Yu YS, Chen XH, Liu HH, Zang GQ
and Tang ZH: Enhancement of CTLs induced by DCs loaded with
ubiquitinated hepatitis B virus core antigen. World J
Gastroenterol. 18:1319–1327. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dai S, Zhuo M, Song L, Chen X, Yu Y, Zang
G and Tang Z: Lentiviral vector encoding ubiquitinated hepatitis B
core antigen induces potent cellular immune responses and
therapeutic immunity in HBV transgenic mice. Immunobiology.
221:813–821. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen A, Wang L, Zhang J, Zou L, Jia Z,
Zhou W, Wan Y and Wu Y: H-2 Kd-restricted hepatitis B virus-derived
epitope whose specific CD8+ T lymphocytes can produce gamma
interferon without cytotoxicity. J Virol. 79:5568–5576. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dai S, Zhuo M, Song L, Chen X, Yu Y, Tang
Z and Zang G: Dendritic cell-based vaccination with lentiviral
vectors encoding ubiquitinated hepatitis B core antigen enhances
hepatitis B virus-specific immune responses in vivo. Acta Biochim
Biophys Sin (Shanghai). 47:870–879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen X, Tang Y, Zhang Y, Zhuo M, Tang Z,
Yu Y and Zang G: Tapasin modification on the intracellular epitope
HBcAg18-27 enhances HBV-specific CTL immune response and inhibits
hepatitis B virus replication in vivo. Lab Invest. 94:478–490.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen X, Liu H, Tang Z, Yu Y and Zang G:
The modification of Tapasin enhances cytotoxic T lymphocyte
activity of intracellularly delivered CTL epitopes via cytoplasmic
transduction peptide. Acta Biochim Biophys Sin (Shanghai).
45:203–212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang Y, Chen Z, Jia H, Wu W, Zhong S and
Zhou C: Induction of Tc1 response and enhanced cytotoxic T
lymphocyte activity in mice by dendritic cells transduced with
adenovirus expressing HBsAg. Clin Immunol. 119:280–290. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bertoletti A, Ferrari C, Fiaccadori F,
Penna A, Margolskee R, Schlicht HJ, Fowler P, Guilhot S and Chisari
FV: HLA class I-restricted human cytotoxic T cells recognize
endogenously synthesized hepatitis B virus nucleocapsid antigen.
Proc Natl Acad Sci USA. 88:10445–10449. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rehermann B, Fowler P, Sidney J, Person J,
Redeker A, Brown M, Moss B, Sette A and Chisari FV: The cytotoxic T
lymphocyte response to multiple hepatitis B virus polymerase
epitopes during and after acute viral hepatitis. J Exp Med.
181:1047–1058. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bertoletti A and Ferrari C: Innate and
adaptive immune responses in chronic hepatitis B virus infections:
Towards restoration of immune control of viral infection. Gut.
61:1754–1764. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Das A, Hoare M, Davies N, Lopes AR, Dunn
C, Kennedy PT, Alexander G, Finney H, Lawson A, Plunkett FJ, et al:
Functional skewing of the global CD8 T cell population in chronic
hepatitis B virus infection. J Exp Med. 205:2111–2124. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lopes AR, Kellam P, Das A, Dunn C, Kwan A,
Turner J, Peppa D, Gilson RJ, Gehring A, Bertoletti A and Maini MK:
Bim-mediated deletion of antigen-specific CD8 T cells in patients
unable to control HBV infection. J Clin Invest. 118:1835–1845.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maini MK and Schurich A: The molecular
basis of the failed immune response in chronic HBV: Therapeutic
implications. J Hepatol. 52:616–619. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu L and KewalRamani VN: Dendritic-cell
interactions with HIV: Infection and viral dissemination. Nat Rev
Immunol. 6:859–868. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Farag MM, Hoyler B, Encke J, Stremmel W
and Weigand K: Dendritic cells can effectively be pulsed by HBVsvp
and induce specific immune reactions in mice. Vaccine. 29:200–206.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu B, Tai A and Wang P: Immunization
delivered by lentiviral vectors for cancer and infectious diseases.
Immunol Rev. 239:45–61. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tavakoli S, Mederacke I, Herzog-Hauff S,
Glebe D, Grün S, Strand D, Urban S, Gehring A, Galle PR and Böcher
WO: Peripheral blood dendritic cells are phenotypically and
functionally intact in chronic hepatitis B virus (HBV) infection.
Clin Exp Immunol. 151:61–70. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Akbar SM, Horiike N and Onji M: Immune
therapy including dendritic cell based therapy in chronic hepatitis
B virus infection. World J Gastroenterol. 12:2876–2883. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Op den Brouw ML, Binda RS, van Roosmalen
MH, Protzer U, Janssen HL, van der Molen RG and Woltman AM:
Hepatitis B virus surface antigen impairs myeloid dendritic cell
function: A possible immune escape mechanism of hepatitis B virus.
Immunology. 126:280–289. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Phillips S, Chokshi S, Riva A, Evans A,
Williams R and Naoumov NV: CD8 (+) T cell control of hepatitis B
virus replication: Direct comparison between cytolytic and
noncytolytic functions. J Immunol. 184:287–295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tsai SL, Sheen IS, Chien RN, Chu CM, Huang
HC, Chuang YL, Lee TH, Liao SK, Lin CL, Kuo GC and Liaw YF:
Activation of Th1 immunity is a common immune mechanism for the
successful treatment of hepatitis B and C: Tetramer assay and
therapeutic implications. J Biomed Sci. 10:120–135. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Boni C, Bertoletti A, Penna A, Cavalli A,
Pilli M, Urbani S, Scognamiglio P, Boehme R, Panebianco R,
Fiaccadori F and Ferrari C: Lamivudine treatment can restore T cell
responsiveness in chronic hepatitis B. J Clin Invest. 102:968–975.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rincon M, Conze D, Weiss L, Diehl NL,
Fortner KA, Yang D, Flavell RA, Enslen H, Whitmarsh A and Davis RJ:
Conference highlight: do T cells care about the mitogen-activated
protein kinase signalling pathways? Immunol Cell Biol. 78:166–175.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang DD, Conze D, Whitmarsh AJ, Barrett T,
Davis RJ, Rincón M and Flavell RA: Differentiation of CD4+ T cells
to Th1 cells requires MAP kinase JNK2. Immunity. 9:575–585. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Plotnikov A, Zehorai E, Procaccia S and
Seger R: The MAPK cascades: Signaling components, nuclear roles and
mechanisms of nuclear translocation. Biochim Biophys Acta.
1813:1619–1633. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang X, Hao J, Metzger DL, Ao Z, Chen L,
Ou D, Verchere CB, Mui A and Warnock GL: B7-H4 treatment of T cells
inhibits ERK, JNK, p38, and AKT activation. PLoS One. 7:e282322012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Afkarian M, Sedy JR, Yang J, Jacobson NG,
Cereb N, Yang SY, Murphy TL and Murphy KM: T-bet is a STAT1-induced
regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol.
3:549–557. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wiegand J, van Bömmel F and Berg T:
Management of chronic hepatitis B: Status and challenges beyond
treatment guidelines. Semin Liver Dis. 30:361–377. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fung J, Lai CL, Yuen J, Cheng C, Wu R,
Wong DK, Seto WK, Hung IF and Yuen MF: Randomized trial of
lamivudine versus entecavir in entecavir-treated patients with
undetectable hepatitis B virus DNA: Outcome at 2 Years. Hepatology.
53:1148–1153. 2011. View Article : Google Scholar : PubMed/NCBI
|