Introduction
Cardiac hypertrophy, the first phase of
cardiovascular disease, induces heart failure. However, cardiac
hypertrophy is an important compensatory mechanism in response to
physiological or pathological stimuli that involve regulation of
cellular signaling mediators and transcript factors (1–3).
Hypertrophic signals result in increased protein synthesis and
increased cell cycle (4). Cardiac
hypertrophy is characterized by cell enlargement, involving
physiological and pathological hypertrophy (5). Pathological cardiac hypertrophy is
often coupled with interstitial and perivascular fibrosis, in
addition to apoptosis and the release of atrial natriuretic
peptides (ANP) and brain/B-type natriuretic peptides (BNP). With
the initiation of cardiac hypertrophy, concentric hypertrophy is
the primary phenotype that highly resists after-load and is known
as the adaptive phase. As the cardiac damage progresses, cell
length increases, leading to increased hypertrophy (6). In cardiac hypertrophy, nuclear factor
of activated T-cells (NFAT) is considered an important mediator of
several signal-transduction pathways involved in the coordination
of pathological stimulation (7).
The role of reactive oxygen species (ROS) in the induction of
cardiac hypertrophy has been demonstrated (8). However, oxidative stress induces
apoptosis in addition to cardiac hypertrophy (9). Therefore, the development of a model
of cardiac hypertrophy only without apoptosis is essential.
Potassium bromate (KBrO3) has been widely used as a
food additive and is also a by-product of disinfecting drinking
water by ozonization (10). KBrO3
is applied primarily due to its oxidizing properties and may cause
lipid peroxidation and oxidative DNA damage in humans and other
mammals (11). KBrO3 is also known
as a rodent carcinogen (12,13)
and as a renal and/or neuro-toxicant in humans (14).
Free radicals produced by KBrO3 are easily
associated with cardiac injury, as the heart is very sensitive to
ROS-induced damage (15). Thus,
KBrO3 has been classified as one of the cardiac toxins because
lipid peroxidation increases with a significant reduction in
cardiac antioxidant capacity (16). Recently, vanillin has been
demonstrated as an antioxidant as it improves KBrO3-induced cardiac
injury in mice (17). However, the
effect of KBrO3 on cardiac hypertrophy remains undetermined.
In the present study, KBrO3 was applied to the
cardiac cell line H9c2, which is widely used to induce cardiac
hypertrophy (18). The aim was to
develop a novel and simple model of cardiac hypertrophy without
apoptosis in vitro.
Materials and methods
Materials
Potassium bromate (KBrO3), cyclosporine A, and
antioxidant (tiron) were purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). All other reagents were obtained from the
supplier as indicated and were at least of analytical grade.
Cell culture
The H9c2 cells (cat. no. 60096; Bioresource
Collection and Research Center, Hsinchu, Taiwan) were cultured as
previously described (19). In
brief, H9c2 cells were maintained in Dulbecco's modified Eagle's
medium (pH 7.2; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (GE Healthcare Life
Sciences, Little Chalfont, UK). The H9c2 cells were plated at a
density of 6,000 cells/cm2 and were allowed to
proliferate in the growth medium. Following plating, the medium was
replaced on the second day. The next day, cells were incubated with
testing agent(s) as described below.
Experimental protocol
Briefly, H9c2 cells were incubated with KBrO3 to
identify the changes in cell size. Then, inhibitor was used to
pretreat at 37°C for 60 min prior to the addition of KBrO3. Solvent
applied to dissolve the inhibitor was also pretreated in same
manner at same volume and was termed the vehicle-treated group.
Specific inhibitor(s) were applied to investigate the potential
mechanism(s) of KBrO3. Cyclosporine A (CsA) is a powerful
immunosuppressant and has also been used to inhibit calcineurin
(20). Therefore, as previously
reported (21), CsA at an
effective concentration (500 nM) was incubated with H9c2 cells at
37°C for 1 h prior to the treatment with KBrO3. Additionally, tiron
is a water-soluble and cell permeable antioxidant that scavenges
superoxide (22,23). It was also applied as detailed
above for CsA to investigate the role of free radicals in the
effects of KBrO3.
Measurement of cardiac
hypertrophy
H9c2 cells at a density of 7.5×103
cells/ml were arranged on a 24-well plate (Greiner Bio-One, Monroe,
North Carolina, USA). Cells were starved for 4 h in a serum-free
medium prior to treatment with KBrO3 at 37°C for 72 h. Briefly,
following washing twice with cold PBS, the cells were fixed in 4%
paraformaldehyde at room temperature for 15 min and washed with PBS
containing 2% bovine serum albumin (GE Health Care Life Sciences)
and 0.1% Triton X-100. Cells were stained with rhodamine phalloidin
(Invitrogen; Thermo Fisher Scientific, Inc.) for 25 min at room
temperature to identify the actin filaments and with DAPI (Abcam,
Cambridge, UK) to stain the nucleus. An entire field of vision was
characterized using a fluorescence microscope (IX71; Olympus
Corporation, Tokyo, Japan) connected to an imaging system (DP2-BSW;
Olympus Corporation). The cell sizes were magnified ×200 and
analyzed by the imaging system. Cell surface area size was
determined and quantified by imaging to the complete boundary of
the individual cells. The results were subsequently expressed as
the percentage change of the surface area level of the cells, based
on analysis using image J program, version 1.46 (National
Institutes of Health, Bethesda, MD, USA; http://imagej.nih.gov/ij/) as described previously
(24).
Identification of intracellular superoxide levels.
Following the methods described in a previous study (25), H9c2 cells were seeded in 24-well
plates at a density of 7.5×103 cells/ml at 37°C
overnight. Following starvation for 4 h in a serum-free medium, the
cells were treated with 250 µM KBrO3 at 37°C for an additional 72
h. For the detection of the intracellular superoxide levels,
dihydroergotamine (10 µM) from Thermo Fisher Scientific Inc. was
applied to react with intracellular superoxide ions at 37°C for 30
min following treatment with KBrO3. An entire field of vision was
characterized using a fluorescence microscope (IX71, Olympus
Corporation) connected to an imaging system (DP2-BSW, Olympus
Corporation). The results were subsequently expressed as a
percentage of the intracellular superoxide levels in the cells
based on the analysis using image J program, version 1.46 (National
Institutes of Health), as described previously (24).
Determination of intracellular
calcium
Changes in the intracellular calcium concentrations
were also detected using a fluorescent probe, fura-2, as described
previously (26). Fluorescence was
continuously recorded by a fluorescence spectrofluorometer (F-2000;
Hitachi, Ltd., Tokyo, Japan). The values of [Ca2+]i were
determined as described previously (26).
Reverse-transcription-quantitative
polymerase chain reaction (RT-qPCR)
As previously described (24), Total RNA was extracted from H9c2
cells using TRIzol reagent (Life Technologies; Thermo Fisher
Scientific, Inc.). Total RNA (5 µg) was reverse-transcribed into
cDNA with random hexamer primers (Roche Diagnostics GmbH, Mannheim,
Germany). Analysis was carried out using LightCycler (Roche
Diagnostics GmbH). PCR cycles were under the following conditions:
Pretreatment at 95°C for 10 sec, 96°C for 10 sec, 60°C for 30 sec,
72°C for 1 sec, 40°C for 30 sec (45 cycles). β-actin was used as
the control of the input RNA level. The primers used in RT-qPCR
analysis were designed by Roche (Roche Diagnostics GmbH). The
concentration of each PCR product was calculated relative to a
corresponding standard curve. Analysis of relative gene expression
data using real-time quantitative PCR and the 2−ΔΔCq
method as described previously (27). The relative gene expression was
subsequently indicated as the ratio of the target gene level to
that of β-actin. The primers for BNP, β-Myosin Heavy Chain (β-MHC),
and β-actin were:
BNP forward, 5′-GTCAGTCGCTTGGGCTGT-3′ and reverse,
5′-CCAGAGCTGGGGAAAGAAG-3′;β-MHC forward, 5′-CATCCCCAATGAGACGAAGT-3′
and reverse, 5′-GGGAAGCCCTTCCTACAGAT-3′; β-actin forward,
5′-CTAAGGCCAACCGTGAAAAG-3′ and reverse,
5′-GCCTGGATGGCTACGTACA-3′.
Western blot analysis
Cells were harvested and washed with ice cold
phosphate buffer solution, then homogenized in radio
immunoprecipitation assay buffer which was prepared according to a
previous method (24). Lysates
were mixed and incubated on ice for 10 min. Cell debris was
precipitated at 4°C for 10 min (16,099 × g). Protein concentrations
were measured by bicinchoninic acid protein assay (Thermo Fisher
Scientific, Inc.). Proteins were separated by 10% or 15% SDS-PAGE
and electro-transferred onto a polyvinylidene fluoride membrane.
Nonspecific binding was blocked by incubation in 5% nonfat milk at
room temperature for 2 h. Membranes were subsequently incubated
with the primary antibodies (at 1:1,000 dilution) at 4°C overnight,
washed 3 times with TBST (0.05% Tween-20) and finally incubated
with a HRP-conjugated secondary antibody at room temperature for 1
h. Protein bands were visualized using an chemiluminescence (ECL)
kit (PerkinElmer, Inc., Waltham, MA, USA). The optical densities of
the bands for calcineurin (18 kDa), NFAT3 (100 kDa), Histone H3 (15
kDa), and β-actin (43 kDa) were quantified using a laser
densitometer (CHEM-400; Avegene Life Science, Taipei, Taiwan), as
described in a previous study (28). The target antigens from the protein
extracts were detected using primary antibodies specific for
calcineurin (C0581; Sigma-Aldrich; Merck KGaA), NFAT3 (PA-1-021;
Thermo Fisher Scientific, Inc.), or β-actin (A5441; Sigma-Aldrich;
Merck KGaA) and Histone H3 (SC-8654; Cell Signaling Technology,
Inc., Danvers, MA, USA). Additionally, the apoptotic markers such
as Bcl2 (AB59348; Abcam, Cambridge, MA, USA), AIF (07–208; Merck
KGaA) and Caspase-3 (9662S; Cell Signaling Technology, Inc.) were
also determined.
Nuclear extraction
The extraction of the nuclear fraction was performed
according to a previously described method (28), using a CNMCS Compartmental Protein
Extraction Kit (BioChain Institute, Inc., Hayward, CA, USA).
Briefly, H9c2 cells were collected and ice-cold lysis buffer (2 ml
per 20 million cells) added to them. The cell mixture was passed
through the needle base 50–90 times to disrupt the cell membranes
and to release the nuclei from the cells. The degree of cell
membrane disruption and the release of nuclei were monitored with a
fluorescence microscope. The mixture was then centrifuged at 15,000
× g at 4°C for 20 min. The supernatant, which contained cytoplasmic
proteins, was removed and was saved in a separate tube. The pellet
was resuspended in ice-cold washing buffer (4 ml per 20 million
cells), and the suspension was rotated at 4°C for 5 min, followed
by centrifugation at 15,000 × g at 4°C for 20 min. The supernatant
was removed and ice-cold nuclear extraction buffer (1 ml per 20
million cells) was added to the pellet. Following rotation at 4°C
for 20 min, the suspension was centrifuged at 15,000 × g at 4°C for
20 min. The supernatant, which contained nuclear proteins, was
removed and saved for further use.
Cytotoxicity assay
Cell viability and survival were determined by an
MTT assay (29). Briefly, 5,000
cells were plated in 24-well plates in triplicate and treated with
concentrations between 250 and 5,000 µM of KBrO3 at 37°C for 72 h.
Following treatment, the medium was removed and replaced with 500
µl/well fresh medium, and 50 µl MTT (final concentration 0.5 mg/ml)
was added to each well. The plates were incubated at 37°C for 4 h,
allowing viable cells to reduce the yellow tetrazolium salt into
dark blue formazan crystals. The formazan crystals were dissolved
using a solution of 0.01 M HCl/SDS 10%. Finally, the absorbance in
each individual well was determined at a wavelength of 595 nm, with
the absorbance of the cells counted by a Synergy HT Multi-Mode
Microplate Reader (BioTek Instruments, Inc., Winooski, VT, USA).
Relative cell viabilities were calculated as percentages vs.
control cells: Relative cell viability (%)=(absorbance of treated
cells)/(absorbance of control cells) ×100%.
Staining of live and dead cells
To image the live and dead cells, a LIVE/DEAD
viability assay kit (Molecular Probes; Thermo Fisher Scientific,
Inc.) was used, according to the manufacturer's protocol. H9c2
cells at a density of 5×104 cells/ml were incubated with
two probes, calcein-AM (green color) and ethidium homodimer-1
(EtdD-1, bright red color), for intracellular esterase activity and
plasma membrane integrity, respectively. Subsequently, specimens
were observed under a fluorescence microscope (Olympus IX71;
Olympus Corporation). The live cells are shown in green and the
dead cells in red (magnification, ×4). The images were analyzed
using image J program, version 1.46 (National Institutes of
Health). Each assay performed in triplicate.
Flow cytometry analysis
H9c2 apoptotic cell measurement was performed by
flow cytometry with Annexin V-propidium iodide staining, as
described previously (30), using
a fluorescein isothiocyanate (FITC) Apoptosis Detection Kit (BD
Biosciences, San Diego, CA, USA). The Annexin reagent utilizes
Annexin V to detect phophatiylserine of apoptotic cells and a dead
cell marker as an indicator of membrane stability. H9c2 cells were
seeded in a dish (10 cm) at a density of 1×106
cells/well, and the experimental procedure was performed according
to the manufacturer's protocol. Staining was analyzed by
fluorescence-activated cell sorting on a flow cytometer (NOVO Cyte
3000; ACEA Biosciences, San Diego, CA., USA). Spectral compensation
and data quantification were performed using NovoExpress software
(2017 Version; ACEA Biosciences Inc., San Diego, CA, USA) that
provided a variety of plots and gates for flow cytometry data
analysis.
Statistical analysis
Data are presented as the mean ± standard error from
the indicated sample size (N) eachperformed in triplicate. One-way
analysis of variance withpost hoc Tukey test was used for multiple
comparisons by SPSS version 16.0 software (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
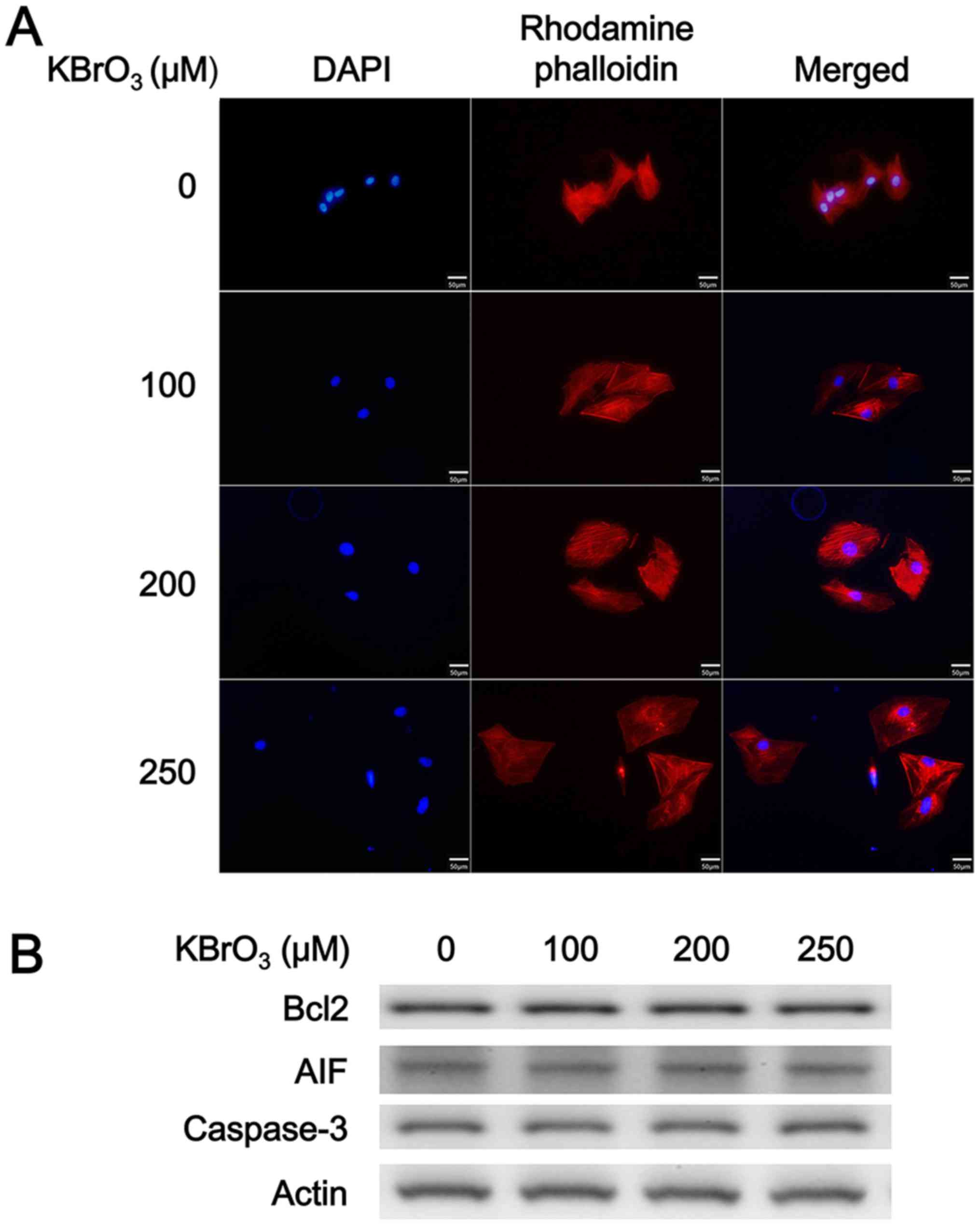
Effect of KBrO3 on H9c2 cell size
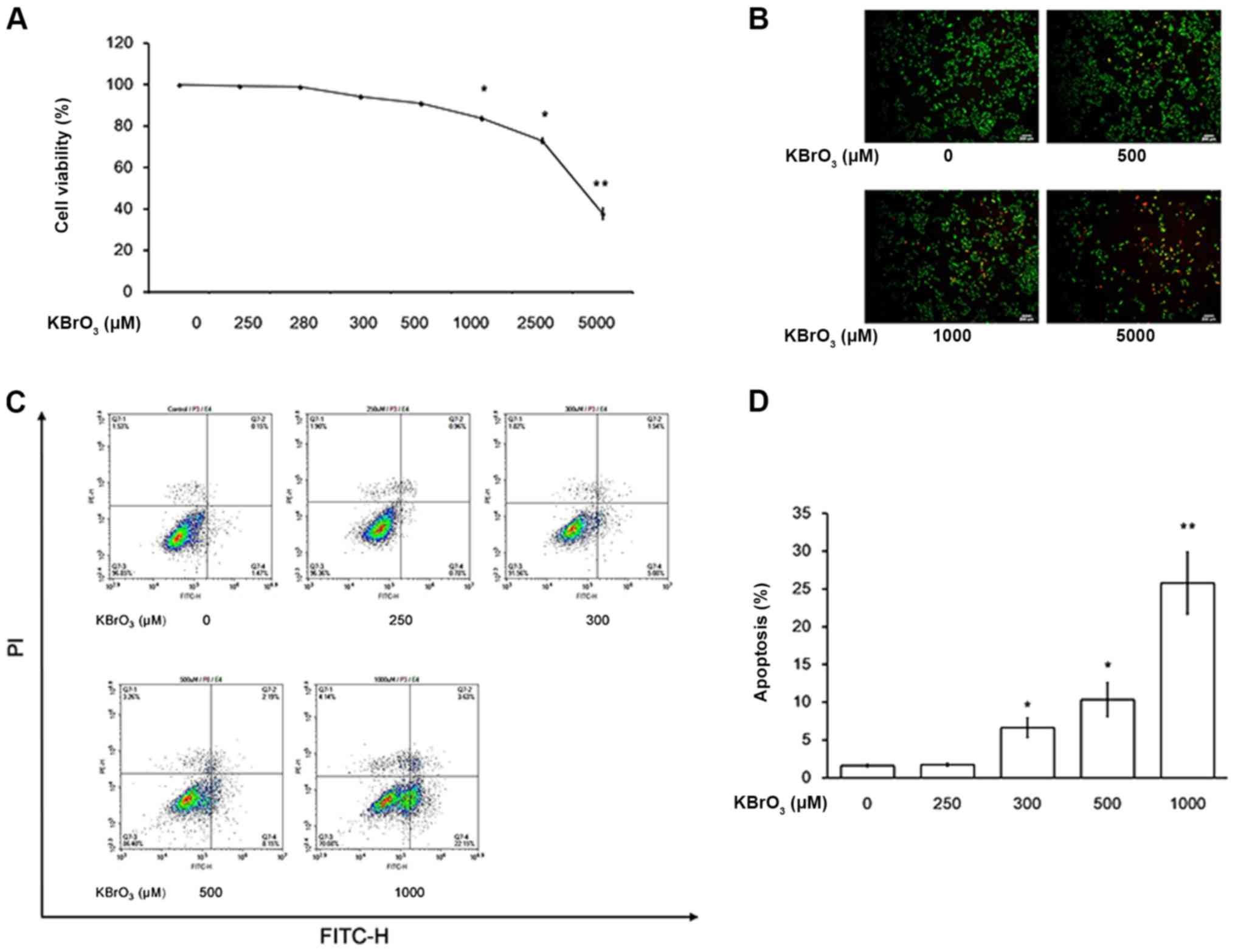
In a preliminary experiment, H9c2 cells were
incubated with KBrO3 (150 µM) for 24, 48, 72 and 84 h. KBrO3
increased the H9c2 cell size gradually, with the maximal response
observed at 72 h following treatment (data not shown). Therefore,
H9c2 cells were incubated with KBrO3 at concentrations of 150 µM
for 72 h. However, different from high glucose, cellular osmolarity
was not influenced by KBrO3 in the preliminary experiments. As
demonstrated in Fig. 1A, KBrO3
increased the H9c2 cell size in a dose-dependent manner as shown in
Table I. Additionally, the mRNA
expression levels of the biomarkers of cardiac hypertrophy, such as
BNP and β-MHC were also increased by KBrO3 in the same manner, as
demonstrated in Table I. However,
KBrO3 did not modify the cell number of H9c2 cells even at the
highest dose (control group: 8.2×104
cells/cm2; KBrO3 250 µM group: 7.8×104
cells/cm2). Notably, apoptosis was not identified in
H9c2 cells incubated with KBrO3 at the effective concentrations as
demonstrated in Fig. 1B and
Table I as the levels of
apoptosis-associated markers remain unchanged.
 | Table I.Effects of KBrO3 on cultured cardiac
cells. |
Table I.
Effects of KBrO3 on cultured cardiac
cells.
| Contents | Control | KBrO3 (100 µM) | KBrO3 (200 µM) | KBrO3 (250 µM) |
|---|
| Relative area level
(fold change) | 1.00±0.07 | 1.46±0.06 |
2.07±0.11a |
2.74±0.09b |
| Relative mRNA level
of BNP/β-Actin | 1.00±0.00 | 1.19±0.03 |
1.60±0.05a |
2.09±0.07b |
| Relative mRNA
levels of β-MHC/β-Actin | 1.00±0.00 | 1.13±0.05 | 1.54±0.05a |
1.99±0.01b |
| Ratio of
Bcl2/β-Actin protein | 0.74±0.08 | 0.74±0.09 | 0.73±0.09 | 0.73±0.07 |
| Ratio of
Caspase-3/β-Actin protein | 0.63±0.09 | 0.62±0.08 | 0.61±0.07 | 0.60±0.07 |
| Ratio of
AIF/β-Actin protein | 0.70±0.10 | 0.70±0.09 | 0.67±0.08 | 0.68±0.08 |
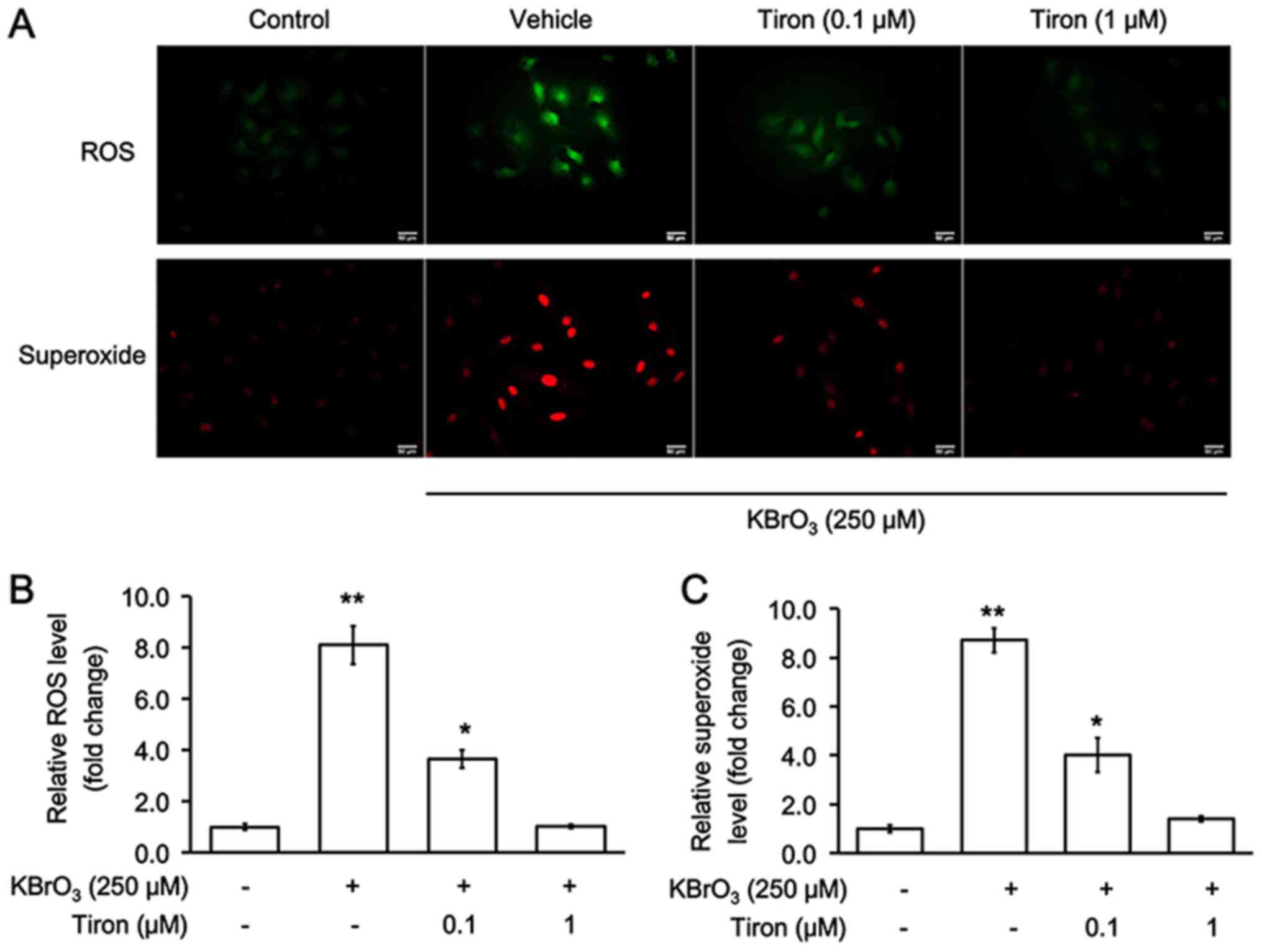
Oxidative stress and KBrO3-induced
cardiac hypertrophy in H9c2 cells
The changes in two oxidative biomarkers, ROS and
superoxide, were investigated following KBrO3-induced cardiac
hypertrophy in H9c2 cells. As demonstrated in Fig. 2, KBrO3 notably increased levels of
both oxidative biomarkers, and these were reversed by tiron at
effective concentrations.
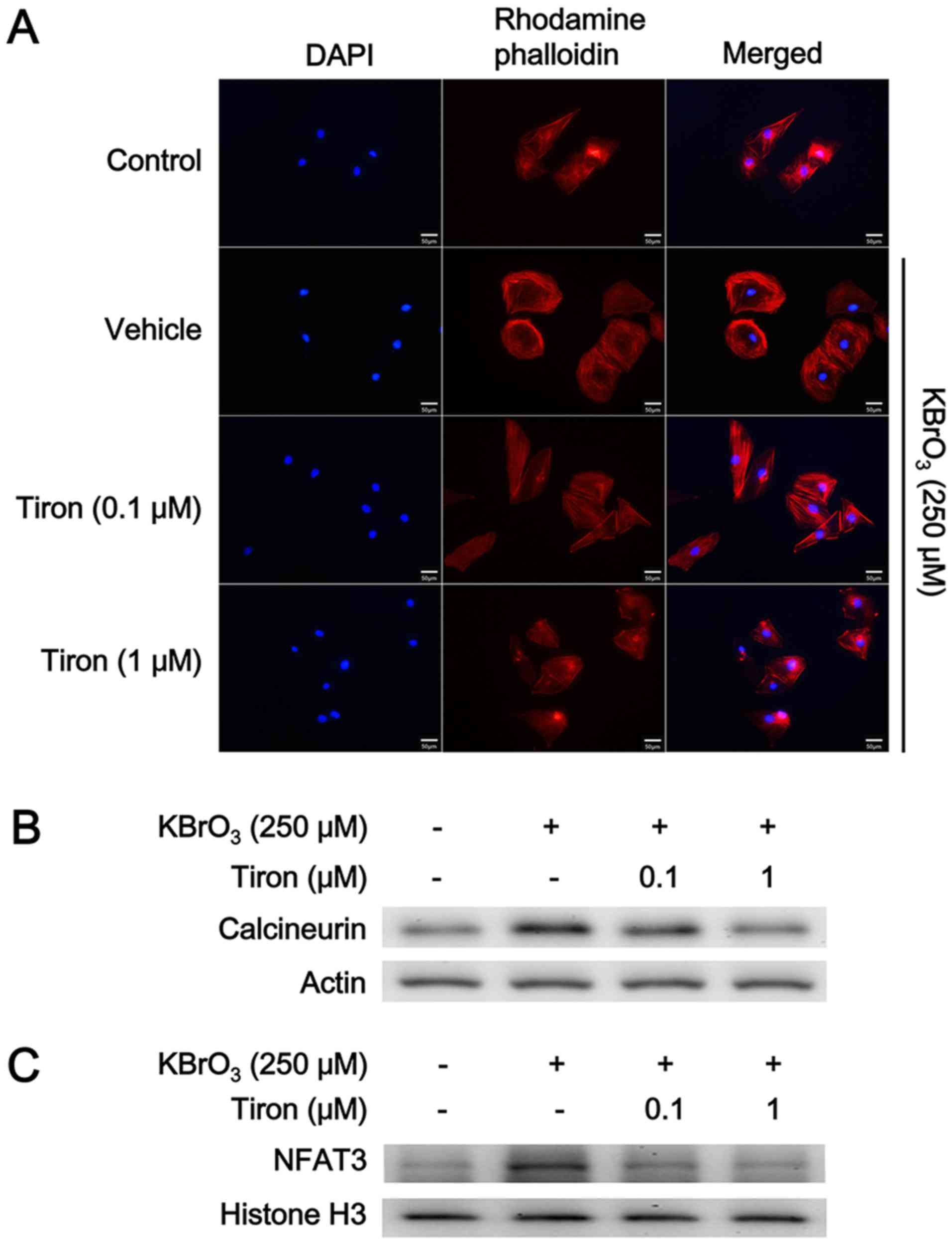
Effect of antioxidant on KBrO3-induced
cardiac hypertrophy in H9c2 cells
H9c2 cells were treated with the antioxidant tiron
to investigate the mediation of oxidative stress following the
KBrO3-induced cardiac hypertrophy. Following pretreatment with
tiron for 30 min, cardiac hypertrophy induced by KBrO3 was reduced
in a dose dependent manner (Fig.
3A). Additionally, the expression levels of the two biomarkers
BNP and β-MHC were also similarly reduced by tiron, as demonstrated
in Table II.
 | Table II.Cardiac hypertrophy induced by KBrO3
is reduced by the antioxidant Tiron. |
Table II.
Cardiac hypertrophy induced by KBrO3
is reduced by the antioxidant Tiron.
| Contents | Control | Vehicle +
KBrO3 (250 µM) | Tiron (0.1 µM) +
KBrO3 (250 µM) | Tiron (1 µM) +
KBrO3 (250 µM) |
|---|
| Relative area level
(fold change) | 1.00±0.08 |
2.56±0.12b |
1.62±0.10a | 1.02±0.09 |
| Relative mRNA level
of BNP/β-Actin | 1.00±0.00 | 1.96±0.04b | 1.48±0.03a | 1.22±0.05 |
| Relative mRNA level
of β-MHC/β-Actin | 1.00±0.00 | 1.91±0.06b | 1.49±0.05a | 1.20±0.04 |
| [Ca2+]nM | 176.30±4.06 | 327.40±11.22b | 257.32±13.28a | 185.69±9.58 |
| Ratio of
calcineurin/β-Actin protein | 0.31±0.04 | 0.81±0.05b | 0.49±0.04a | 0.29±0.03 |
| Ratio of
NFAT3/Histone H3 protein | 0.45±0.02 | 0.87±0.05b | 0.63±0.02a | 0.49±0.04 |
Cellular signaling pathway and
KBrO3-induced cardiac hypertrophy in H9c2 cells
Cardiac hypertrophy is controlled simultaneously by
stimulatory (prohypertrophic) and counter-regulatory
(antihypertrophic) mechanisms via the calcineurin-NFAT signaling
pathway (31). Therefore, the
changes in the signaling pathway were investigated using western
blotting. As demonstrated in Fig.
3, both calcineurin (Fig. 3B)
and NFAT3 expression (Fig. 3C)
levels were elevated by KBrO3 at the concentration sufficient to
induce cardiac hypertrophy in H9c2 cells. However, this action of
KBrO3 was reduced by tiron in a dose dependent manner (Fig. 3B and C; Table II). Moreover, the cellular calcium
levels were measured and it was demonstrated that KBrO3 increased
cellular calcium levels which were then attenuated by tiron, as
demonstrated in Table II.
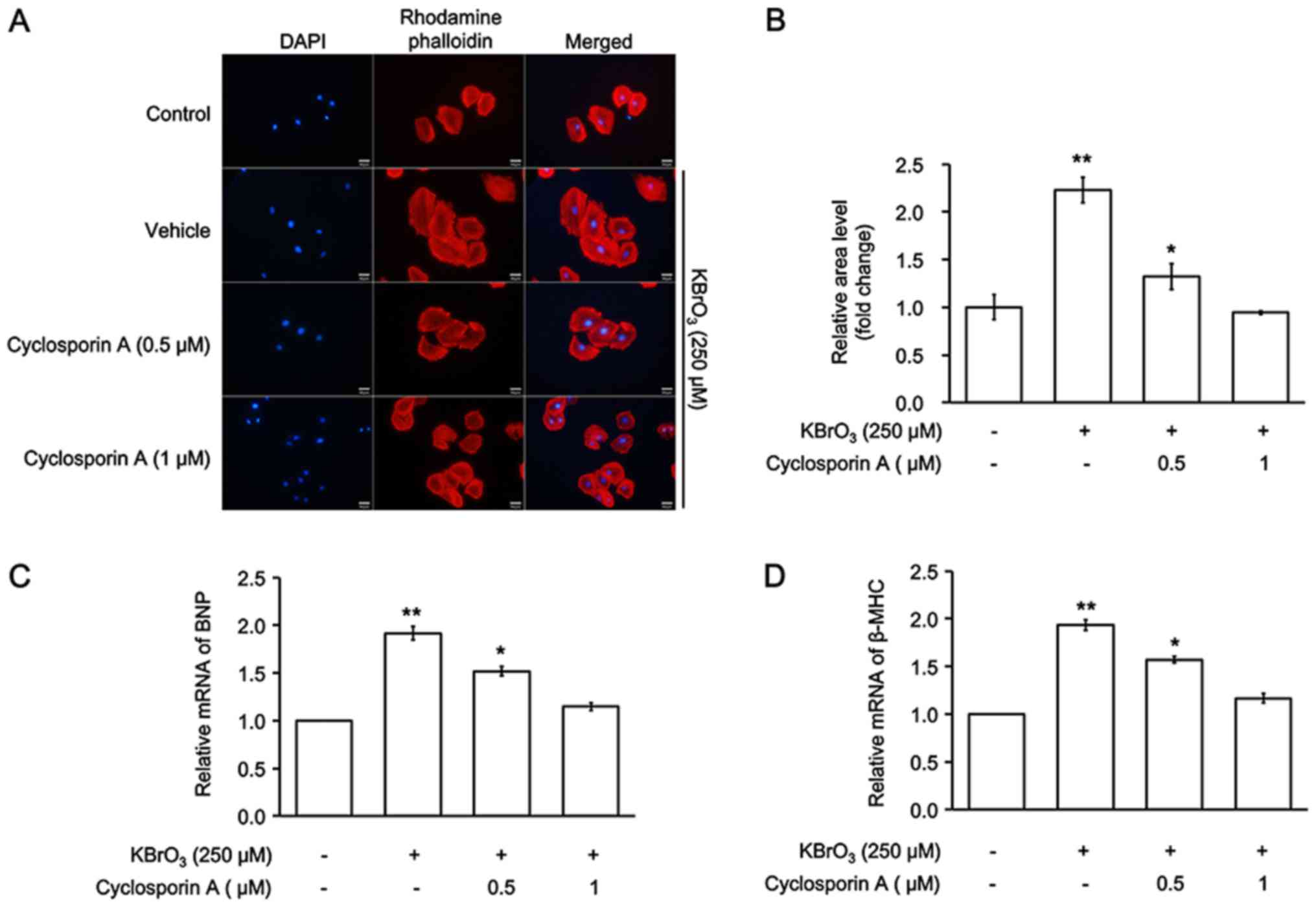
Cyclosporine A inhibits KBrO3-induced
cardiac hypertrophy in H9c2 cells
Cyclosporine A (CsA) is a powerful immunosuppressant
and has also been used to inhibit calcineurin (20). The cell size of H9c2 and mRNA
expression levels of BNP and β-MHC were measured. As illustrated in
Fig. 4, CsA inhibited
KBrO3-induced cardiac hypertrophy in H9c2 cells. Therefore,
mediation of the calcineurin-NFAT signaling pathway in
KBrO3-induced cardiac hypertrophy was identified.
Characterization of KBrO3-induced
apoptosis and damage in H9c2 cells
In a previous study, apoptosis was induced by
H2O2 at concentrations higher than that used
to induce cardiac hypertrophy in cardiac cells (32). Therefore, the possible toxic effect
of KBrO3 on H9c2 cells was investigated. As demonstrated in
Fig. 5A, KBrO3 at high
concentrations may damage H9c2 cells and cell death was observed in
H9c2 cells treated with KBrO3 >1 mM, in a dose-dependent manner.
Epifluorescent staining of cells following treatment with KBrO3
illustrated mostly apoptotic/necrotic cells with orange red
fluorescent nuclei (Fig. 5B) while
cells with green fluorescent nuclei were considered as an indicator
of live cells, indicating that cells underwent apoptosis at high
doses of KBrO3. Additionally, Annexin V-FITC staining was used. As
demonstrated in Fig. 5C and D,
apoptosis induced by KBrO3 at high doses was clearly identified
using the flow cytometry.
Discussion
In the present study, KBrO3 at concentrations
<250 µM induced cardiac hypertrophy without apoptosis in a
cultured cell line. Additionally, it was demonstrated that free
radicals and/or oxidative stress were mediated by the effect of
KBrO3, which is fully consistent with previous reports that KBrO3
induces damage via oxidative stress (10–12).
Cardiac injury induced by KBrO3 has also been observed in rats
(15,16), and histological evidence
demonstrates that KBrO3 induces cardiac apoptosis and/or necrosis
in rodents (33). However, cardiac
hypertrophy induced by KBrO3 has not been previously examined.
The direct effect of KBrO3 on cardiac cells resulted
in an increase in the size of H9c2 cells, which was demonstrated
using visual identification and a parallel increase in biomarkers
of cardiac hypertrophy. Increased expression levels of ANP or BNP
in plasma levels are widely used as reliable indicators of cardiac
hypertrophy in the clinic (34).
In the present study, the mRNA expression level of BNP in H9c2
cells was increased by KBrO3 in a dose-dependent manner. This was
in addition to another indicator β-MHC, which has been described
previously (35). Similar to
natriuretic peptides, β-MHC or α-skeletal actin has also been used
as a biomarker of pathological hypertrophy (36). In the present study, KBrO3, similar
to its role in increasing the expression of BNP, also increased the
expression of β-MHC. Furthermore, KBrO3 increased the size of H9c2
cells (hypertrophy) but did not increase cell numbers
(hyperplasia). Therefore, KBrO3 induced cardiac hypertrophy in H9c2
cells.
ROS produced by KBrO3 are proposed as the primary
mediators in tissue damage (11,16).
Thus, the changes in ROS and superoxide levels were investigated.
Similar to hyperglycemia-induced cardiac damage (24), KBrO3 increased ROS and superoxide
levels in H9c2 cells. Additionally, the effect of tiron, which is a
water-soluble and cell permeable antioxidant that scavenges
superoxide (22,23), reduced the effect of KBrO3 in a
dose-dependent manner. Tiron alleviates cardiac damage through a
marked reduction in oxidative stress (37,38).
In the present study, tiron inhibited the effects of KBrO3
including the induction of cardiac hypertrophy and formation of ROS
in H9c2 cells.
H2O2 induces cardiac
hypertrophy only at concentrations <50 µM in cardiomyocytes
isolated from rats (32).
Furthermore, KBrO3 induced disorder at concentrations <250 µM in
the cultured H9c2 cell line. Similar to the effects of
H2O2, KBrO3 induced apoptosis and/or necrosis
in H9c2 cells at high concentrations (>300 µM). Therefore, the
effective concentration of KBrO3 is limited within this range for
the induction of cardiac hypertrophy in H9c2 cells.
The role of the calcineurin signaling pathway was
also investigated in KBrO3-induced cardiac hypertrophy. Calcineurin
may dephosphorylate NFAT3, leading to nuclear translocation
(31). Subsequently, the nuclear
NFAT3 participates in the promotion of hypertrophic gene expression
including that of BNP and β-MHC to induce cardiac hypertrophy
(6,7). Additionally, ROS increases cellular
calcium (39). In the present
study, it was demonstrated that KBrO3 significantly increased the
calcium level, which may promote the calcineurin and NFAT3
signaling pathway in H9c2 cells. This conclusion is also supported
by the evidence from cyclosporine A, which at a concentration
effective to inhibit calcineurin, attenuated the KBrO3-induced
cardiac hypertrophy. Notably, these effects of KBrO3 were also
reduced by tiron in a dose-dependent manner. Therefore, KBrO3
likely increases hypertrophic signaling via elevation of ROS in
H9c2 cells, but this needs to investigate in the future.
The mechanism(s) for oxidative stress-induced
cardiac hypertrophy remains unclear. Many parameters have been
examined (40), including
osteopontin (41). However, most
of these reports have investigated hyperglycemia-induced cardiac
damage, particularly apoptosis (40). Hyperglycemia induces cardiac injury
primarily through ROS generation (38). However, the inflammatory parameters
are also involved in diabetic cardiomyopathy, particularly in the
in vivo models (42).
Therefore, the difference in cardiac hypertrophy between
hyperglycemia and KBrO3 should be clarified in the future.
In cardiac research, oxidative stress or ROS-induced
cardiac hypertrophy is widely induced by H2O2
in H9c2 cells (43). Additionally,
4-hydroxy-2-nonenal (44) or
lactosylceramide (45) has also
been applied to induce cardiac hypertrophy via ROS in a way similar
to that of KBrO3. Therefore, KBrO3 could be used as an inducer of
cardiac hypertrophy in cells. In rats, KBrO3 at a dose of 1.2 g/kg
for 4 weeks produces damages without mortality (46), and in Sprague-Dawley rats, KBrO3 at
a dose of 20 mg/kg for 4 weeks induces cardiac injury (47). Generally, KBrO3 at a dose of 20–30
mg/kg damages cardiac tissues in rats (16). The histological evidence also
demonstrates that KBrO3 at 20 mg/kg twice weekly for 4 weeks
induces cardiac fibrosis in rats (33). In a clinic, nine cases of
accidental KBrO3 poisoning in a bakery demonstrated hematemesis and
renal failure as the possible adverse outcomes (48). Although the cardiac injury seems
not so critical in the human response to KBrO3, further
investigations on human cardiac cells are required. Moreover,
characterization of the dose of KBrO3 effective to induce cardiac
hypertrophy in animals is also essential in the future. The role of
signaling pathways need to be investigated using small interfering
or short hairpin RNA techniques, in order to understand the damage
resulting from KBrO3 in cardiomyocytes. Additionally, the effects
of potassium or bromate on cardiac hypertrophy remain unclear.
Further investigations are required to evaluate the
pharmacokinetics and/or pharmacodynamics of KBrO3.
In conclusion, in the present study, KBrO3 at
concentrations <250 µM induced cardiac hypertrophy in H9c2 cells
without apoptosis mainly through free radicals to increase cellular
calcium levels and so activate the calcineurin and NFAT3 signaling
pathway. Therefore, KBrO3 may be applied to develop a novel cell
model of cardiac hypertrophy for future research.
Acknowledgements
Authors would like to thank Miss Yang-Lien Yen and
Mr. Yi-Zhi Chen for their assistance in the experiments.
Funding
The present study was partly supported by a grant
(grant no. CMFHT10503) from Chi-Mei Medical Center, Yong Kang,
Tainan City, Taiwan.
Availability of data and materials
The datasets used and analyzed during the present
study are available from corresponding author on reasonable
request.
Author's contributions
SK, KC and JC designed experiments. YL, YC and WL
performed the experiments and analyzed data. SK, YL and KC drafted
the manuscript. All authors discussed, revised, and approved the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they do not have any
competing interests.
Glossary
Abbreviations
Abbreviations:
|
BNP
|
brain/B-type natriuretic peptides
|
|
NFAT
|
nuclear factor of activated
T-cells
|
|
ROS
|
reactive oxygen species
|
References
|
1
|
Frey N, Katus HA, Olson EN and Hill JA:
Hypertrophy of the heart: A new therapeutic target? Circulation.
109:1580–1589. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Glenn DJ, Rahmutula D, Nishimoto M, Liang
F and Gardner DG: Atrial natriuretic peptide suppresses endothelin
gene expression and proliferation in cardiac fibroblasts through a
GATA4-dependent mechanism. Cardiovasc Res. 84:209–217. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eom GH and Kook H: Role of histone
deacetylase 2 and its posttranslational modifications in cardiac
hypertrophy. BMB Rep. 48:131–138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Glickman MH and Ciechanover A: The
ubiquitin-proteasome proteolytic pathway: Destruction for the sake
of construction. Physiol Rev. 82:373–428. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
D'Ascenzi F, Pelliccia A, Corrado D,
Cameli M, Curci V, Alvino F, Natali BM, Focardi M, Bonifazi M and
Mondillo S: Right ventricular remodelling induced by exercise
training in competitive athletes. Eur Heart J Cardiovasc Imaging.
17:301–307. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bernardo BC, Weeks KL, Pretorius L and
McMullen JR: Molecular distinction between physiological and
pathological cardiac hypertrophy: Experimental findings and
therapeutic strategies. Pharmacol Ther. 128:191–227. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heineke J and Molkentin JD: Regulation of
cardiac hypertrophy by intracellular signalling pathways. Nat Rev
Mol Cell Biol. 7:589–600. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sawyer DB, Siwik DA, Xiao L, Pimentel DR,
Singh K and Colucci WS: Role of oxidative stress in myocardial
hypertrophy and failure. J Mol Cell Cardiol. 34:379–388. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fiorillo C, Nediani C, Ponziani V,
Giannini L, Celli A, Nassi N, Formigli L, Perna AM and Nassi P:
Cardiac volume overload rapidly induces oxidative stress-mediated
myocyte apoptosis and hypertrophy. Biochim Biophys Acta.
1741:173–182. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Parsons JL and Chipman JK: The role of
glutathione in DNA damage by potassium bromate in vitro.
Mutagenesis. 15:311–316. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Watanabe S, Togashi S and Fukui T:
Contribution of nitric oxide to potassium bromate-induced elevation
of methaemoglobin concentration in mouse blood. Biol Pharm Bull.
25:1315–1319. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
DeAngelo AB, George MH, Kilburn SR, Moore
TM and Wolf DC: Carcinogenicity of potassium bromate administered
in the drinking water to male B6C3F1 mice and F344/N rats. Toxicol
Pathol. 26:587–594. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kujawska M, Ignatowicz E, Ewertowska M,
Adamska T, Markowski J and Jodynis-Liebert J: Attenuation of
KBrO3-induced renal and hepatic toxicity by cloudy apple juice in
rat. Phytother Res. 27:1214–1219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ishidate M Jr, Sofuni T, Yoshikawa K,
Hayashi M, Nohmi T, Sawada M and Matsuoka A: Primary mutagenicity
screening of food additives currently used in Japan. Food Chem
Toxicol. 22:623–636. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Priscilla DH and Prince PS:
Cardioprotective effect of gallic acid on cardiac troponin-T,
cardiac marker enzymes, lipid peroxidation products and
antioxidants in experimentally induced myocardial infarction in
Wistar rats. Chem Biol Interact. 179:118–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oseni OA, Ogunmoyole T and Idowu KA: Lipid
profile and cardio-protective effects of aqueous extract of moringa
oleifera (lam) leaf on bromate-induced cardiotoxicity on Wistar
albino rats. Eur J Adv Res Biol Life Sci. 3:52–66. 2015.
|
|
17
|
Saad HB, Boudawara O, Hakim A and Amara
IB: Preventive effect of vanillin on lipid peroxides
andantioxidants in potassium bromate-inducedcardiotoxicity in adult
mice: Biochemical andhistopathological evidences. J Pharmacognosy
Phytochemistry. 6:1379–1383. 2017.
|
|
18
|
Khatua TN, Borkar RM, Mohammed SA, Dinda
AK, Srinivas R and Banerjee SK: Novel sulfur metabolites of garlic
attenuate cardiac hypertrophy and remodeling through induction of
Na+/K+-ATPase Expression. Front Pharmacol. 8:182017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen ZC, Yu BC, Chen LJ, Cheng KC, Lin HJ
and Cheng JT: Characterization of the mechanisms of the increase in
PPARδ expression induced by digoxin in the heart using the H9c2
cell line. Br J Pharmacol. 163:390–398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu W, Zou Y, Shiojima I, Kudoh S, Aikawa
R, Hayashi D, Mizukami M, Toko H, Shibasaki F, Yazaki Y, et al:
Ca2+/calmodulin-dependent kinase II and calcineurin play critical
roles in endothelin-1-induced cardiomyocyte hypertrophy. J Biol
Chem. 275:15239–15245. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Asadi F, Razmi A, Dehpour AR and Shafiei
M: Tropisetron inhibits high glucose-induced calcineurin/NFAT
hypertrophic pathway in H9c2 myocardial cells. J Pharm Pharmacol.
68:485–493. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han YH and Park WH: Tiron, a ROS
scavenger, protects human lung cancer Calu-6 cells against
antimycin A-induced cell death. Oncol Rep. 21:253–261.
2009.PubMed/NCBI
|
|
23
|
Oyewole AO and Birch-Machin MA:
Mitochondria-targeted antioxidants. FASEB J. 29:4766–4771. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lo SH, Hsu CT, Niu HS, Niu CS, Cheng JT
and Chen ZC: Ginsenoside Rh2 improves cardiac fibrosis via
PPARdelta-STAT3 signaling in type 1-like diabetic rats. Int J Mol
Sci. 18(pii): E13642017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li CJ, Lin L, Li H and Yu DM: Cardiac
fibrosis and dysfunction in experimental diabetic cardiomyopathy
are ameliorated by alpha-lipoic acid. Cardiovasc Diabetol.
11:732012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lo SH, Cheng KC, Li YX, Chang CH, Cheng JT
and Lee KS: Development of betulinic acid as an agonist of TGR5
receptor using a new in vitro assay. Drug Des Devel Ther.
10:2669–2676. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang CM, Hsu CT, Niu HS, Chang CH, Cheng
JT and Shieh JM: Lung damage induced by hyperglycemia in diabetic
rats: The role of signal transducer and activator of transcription
3 (STAT3). J Diabetes Complications. 30:1426–1433. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Scudiero DA, Shoemaker RH, Paull KD, Monks
A, Tierney S, Nofziger TH, Currens MJ, Seniff D and Boyd MR:
Evaluation of a soluble tetrazolium/formazan assay for cell growth
and drug sensitivity in culture using human and other tumor cell
lines. Cancer Res. 48:4827–4833. 1988.PubMed/NCBI
|
|
30
|
Calastretti A, Gatti G, Quaresmini C and
Bevilacqua A: Down-modulation of Bcl-2 sensitizes PTEN-mutated
prostate cancer cells to starvation and taxanes. Prostate.
74:1411–1422. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fiedler B and Wollert KC: Interference of
antihypertrophic molecules and signaling pathways with the
Ca2+-calcineurin-NFAT cascade in cardiac myocytes. Cardiovasc Res.
63:450–457. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kwon SH, Pimentel DR, Remondino A, Sawyer
DB and Colucci WS: H(2)O(2) regulates cardiac myocyte phenotype via
concentration-dependent activation of distinct kinase pathways. J
Mol Cell Cardiol. 35:615–621. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
El-Deeb MEE and Abd-El-Hafez AAA: Can
vitamin C affect the KBrO3 induced oxidative stress on
left ventricular myocardium of adult male albino rats? A
histological and immunohistochemical study. J Microsc Ultrastruct.
3:120–136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sagnella GA: Measurement and significance
of circulating natriuretic peptides in cardiovascular disease. Clin
Sci (Lond). 95:519–529. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu N, Chai R, Liu B, Zhang Z, Zhang S,
Zhang J, Liao Y, Cai J, Xia X, Li A, et al: Ubiquitin-specific
protease 14 regulates cardiac hypertrophy progression by increasing
GSK-3β phosphorylation. Biochem Biophys Res Commun. 478:1236–1241.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Waspe LE, Ordahl CP and Simpson PC: The
cardiac beta-myosin heavy chain isogene is induced selectively in
alpha 1-adrenergic receptor-stimulated hypertrophy of cultured rat
heart myocytes. J Clin Invest. 85:1206–1214. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Younce CW, Wang K and Kolattukudy PE:
Hyperglycaemia-induced cardiomyocyte death is mediated via MCP-1
production and induction of a novel zinc-finger protein MCPIP.
Cardiovasc Res. 87:665–674. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zuo L, Youtz DJ and Wold LE: Particulate
matter exposure exacerbates high glucose-induced cardiomyocyte
dysfunction through ROS generation. PLoS One. 6:e231162011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Goldhaber JI: Free radicals enhance
Na+/Ca2+ exchange in ventricular myocytes. Am J Physiol.
271:H823–H833. 1996.PubMed/NCBI
|
|
40
|
Huynh K, Kiriazis H, Du XJ, Love JE, Gray
SP, Jandeleit-Dahm KA, McMullen JR and Ritchie RH: Targeting the
upregulation of reactive oxygen species subsequent to hyperglycemia
prevents type 1 diabetic cardiomyopathy in mice. Free Radic Biol
Med. 60:307–317. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jiang P, Zhang D, Qiu H, Yi X, Zhang Y,
Cao Y, Zhao B, Xia Z and Wang C: Tiron ameliorates high
glucose-induced cardiac myocyte apoptosis by PKCδ-dependent
inhibition of osteopontin. Clin Exp Pharmacol Physiol. 44:760–770.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Khan S, Zhang D, Zhang Y, Li M and Wang C:
Wogonin attenuates diabetic cardiomyopathy through its
anti-inflammatory and anti-oxidative properties. Mol Cell
Endocrinol. 428:101–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schröder E and Eaton P: Hydrogen peroxide
as an endogenous mediator and exogenous tool in cardiovascular
research: Issues and considerations. Curr Opin Pharmacol.
8:153–159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Park JH, Lee JH and Park JW: Attenuated
SAG expression exacerbates 4-hydroxy-2-nonenal-induced apoptosis
and hypertrophy of H9c2 cardiomyocytes. Free Radic Res. 49:962–972.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mishra S and Chatterjee S:
Lactosylceramide promotes hypertrophy through ROS generation and
activation of ERK1/2 in cardiomyocytes. Glycobiology. 24:518–531.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Abdel Gadir EH, Abdel Gadir WS and Adam
SEI: Effects of various levels of dietary potassium bromate on
wistar rats. J Pharmacol Toxicol. 2:672–676. 2007. View Article : Google Scholar
|
|
47
|
Khan MR, Haroon J, Ali Khan R, Bokhari J,
Shabbir M and Rashid U: Prevention of KBrO3-induced
cardiotoxicity by Sonchus asper in rat. J Med Plants Res.
5:2514–2520. 2011.
|
|
48
|
Kumar S and Pankaj P: Accidental potassium
bromate poisoning in nine adults. J Indian Acad Forensic Med.
34:364–366. 2012.
|