Introduction
Therapeutic angiogenesis designed to treat limb
ischemic diseases has been extensively studied (1). Two major therapies for therapeutic
angiogenesis include gene therapy, which uses proangiogenic growth
factors to promote angiogenesis, and cell therapy, in which
stem/progenitor cells are transplanted into the ischemic site and
allowed to renew, adhere, migrate and differentiate into
tissue-specific cells together with angiogenesis (2). Endothelial progenitor cells (EPCs),
which may be derived from bone marrow cells or isolated from
peripheral blood (3), can
differentiate into mature endothelial cells and participate in the
formation of new vessels in the ischemic area (4). Thus, EPC transplantation may
ameliorate local ischemia in ischemic diseases. Moreover, the
genetic modification of EPCs, such as the forced expression of
certain angiogenic growth factors, has been shown to increase the
angiogenic response and promote the bioactivity of EPCs (5–7).
Taken together, the use of both gene therapy and cell
transplantation has been considered to be an effective strategy for
angiogenesis in an ischemic region (8).
The platelet-derived growth factor (PDGF) family is
comprised of four genes: PDGF-A, PDGF-B, PDGF-C and PDGF-D
(9,10). PDGFs bind to PDGF receptor α
(PDGFRα) or β (PDGFRβ), and stimulate proliferation, migration and
differentiation into many cell types in both developing and adult
tissues (9,11). Compared with the well-studied roles
of PDGF-A and -B in cardiovascular development and diseases,
PDGF-D, as a newly discovered member of the PDGF family, has been
much less studied. In the field of cancer, PDGF-D has been shown to
promote the cell growth, aggressiveness, angiogenesis and
endothelial-mesenchymal transformation (EMT) of colorectal cancer
(12), and correspondingly, the
inhibition of PDGF-D signaling was found to reduce angiogenesis in
gastric cancer (13). PDGF-D was
also demonstrated to contribute to neointimal hyperplasia in a rat
model of vessel injury (14), to
increase interstitial pressure, to induce macrophage recruitment
and to promote blood vessel maturation (15). However, it remains unknown whether
PDGF-D exerts any impact on the angiogenic activity of EPCs with
regard to proliferation, migration, adhesion and
differentiation.
Given the importance of EPCs in angiogenesis and the
above-mentioned studies, it was hypothesized that PDGF-D may play a
role in the mediation of the biological properties of EPCs,
particularly those required for EPC-assisted angiogenesis, such as
proliferation, migration, adhesion and differentiation. In the
present study, a gain-of-function model was used to investigate the
effects of PDGF-D on the activity of EPCs. The findings of the
present study support the premise that PDGF-D is a potentially
promising target for therapeutic angiogenesis through the
potentiation of the angiogenic activity of EPCs.
Materials and methods
EPCs isolation, culture and
characterization
The isolation of EPCs from rat bone marrow cells was
carried out, as previously detailed (6), with slight modifications. In brief,
female Sprague-Dawley rats (4 weeks of age) were sacrificed by
cervical dislocation. Then, bone marrow mononuclear cells were
separated from the femurs and tibiae using Ficoll density gradient
centrifugation (Amersham Biosciences, Freiburg, Baden-Württemberg,
Germany), and cultured in Endothelial Cell Basal Medium-2 (Lonza
Group, Ltd., Basel, Switzerland) supplemented with EGM-2 MV
SingleQuots (Lonza Group, Ltd.) and 5% fetal bovine serum (FBS).
EPCs at passages 3–8 were used for all studies. For the
immunofluorescence experiments, cells were prepared and analyzed
under a fluorescence microscope (Carl Zeiss, Jena, Thuringia,
Germany), following previously described procedures (6). Briefly, cells were incubated with
primary antibodies against CD133 (Ab2839; Abcam, Cambridge, MA,
USA) at a dilution of 1:150, VEGFR2 (Ab2349; Abcam) at a dilution
of 1:200 and CD34 (ab2839; Abcam) at a dilution of 1:150,
FITC-UEA-lectin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at
a dilution of 1:150 and Dil-AcLDL (Biomedical Technologies, Madrid,
Spain) at a dilution of 1:150, followed by incubation with
anti-Alexa Fluor 647-FITC (Cwbiotech, Beijing, China) secondary
antibody at a dilution of 1:200 as referred to our previous
research (11). Then, cells were
counterstained with DAPI (Roche Diagnostics, Basel, Switzerland)
and imaged with a fluorescence microscope. The experimental
protocol using animals for research was approved by the Ethics
Committee of Chongqing Medical University.
Lentivirus generation
The complementary DNA encoding the full-length
PDGF-D was obtained by PCR in RNA purified from buffalo rat liver
(BRL) cells (ATCC; American Type Culture Collection, Manassas, VA,
USA), and subcloned into the lentiviral-based EGFP vector LV5
(Biosettia, San Diego, CA, USA) via the NotI and
BamHI sites. The sequences of the oligos used for this
subcloning were: 5′-ATAAGAATGCGGCCGCGCCACCATGCACCGGCTCATCTTA-3′
(forward) and 5′-CGGGATCCTTATCGAGGTGGTCTTGAGC-3′ (reverse). The
lentiviruses were generated by transfecting subconfluent 293T cells
with lentiviral expression vectors and packaging plasmids using
calcium phosphate precipitate. The viral supernatants were
collected at 48 h after transfection, centrifuged at 75,000 × g for
90 min, re-suspended and filtered through 0.45 µm filters (EMD
Millipore, Billerica, MA, USA). Infection efficiency was determined
by GFP immunofluorescence. The PDGF-D expressed in EPCs
(PDGF-D-EPCs) was selected by puromycin (Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China) after lentivirus
infection. EPCs that expressed lentiviral-mediated GFP were used as
controls (GFP-EPCs). EPCs without any treatment were defined as
wild-type EPCs (wt-EPCs).
Cell viability assay
EPCs (2.0×104 cells/ml) were respectively
transplanted in 96-well plates and co-cultured with PDGF (vehicle,
50 and 100 ng/ml, respectively). At the indicated time points, cell
viability was measured by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assays, according to the manufacturer's protocol. Dimethyl
sulfoxide was added to each well to dissolve the formazan
precipitate. Absorption was measured at a wavelength of 570 nm
using Gloma Multi Detection System (Promega Corporation, Madison,
WI, USA). Data were compiled from six independent experiments, and
each was carried out in duplicate.
Cell adhesion assay
EPCs co-cultured with PDGF (vehicle, 50 and 100
ng/ml, respectively) were respectively seeded on collagen type I
and fibronectin-coated 96-well plates (1×104
cells/well), and incubated for 1 h at 37°C. Non-adherent cells were
removed by washing with PBS. The remaining adherent cells were
measured by MTT assay, according to the manufacturer's protocol.
Data were collected from six independent experiments, and each was
carried out in duplicate.
Transwell assay
In vitro Transwell invasion was assayed on
24-well chambers (Corning Incorporated, Corning, NY, USA),
according to the manufacturer's protocols. In brief, the Matrigel
(BD Biosciences, Franklin Lakes, NJ, USA) pre-coated filter was
solidified for 1 h at 37°C. Then, EPCs co-cultured with PDGF
(vehicle, 50 and 100 ng/ml, respectively, 4.0×104
cells/well) were respectively positioned in the upper chambers, and
allowed to migrate for 72 h. Non-migrated cells were removed by
scraping, while migrated cells were fixed in 20% methanol and
stained with 0.5% crystal violet. These cells were scored from five
randomly selected fields, and images were captured under a light
microscope (Olympus Corporation, Tokyo, Japan) with ×200
magnification. The cell migration rate was presented as a
percentage of the migration in the presence of the vehicle.
Tube formation assay
A pre-coated 24-well plate with Matrigel (BD
Biosciences) was used for the tube formation assay. EPCs
co-cultured with PDGF (vehicle, 50 and 100 ng/ml, respectively;
4.0×104 and 2×105 cells/well, respectively)
were seeded on the Matrigel. After incubation for 72 h at 37°C,
five randomly chosen fields were counted, and images were captured
under a light microscope (Olympus Corporation) with ×400
magnification.
Cell senescence assay
EPCs were co-cultured with PDGF-D (vehicle, 50 and
100 ng/ml, respectively) in a 24-well plate for 24 h. Cell
senescence was measured using a β-galactosidase staining kit
(Beyotime Institute of Biotechnology, Suzhou, Jiangsu, China),
according to the manufacturer's protocol. Data were compiled from
six independent experiments, and each was carried out in
duplicate.
Immunoblotting
Western blot analysis was performed as previously
detailed (11). The following
antibodies (Abs) (Cell Signaling Technology, Inc., Danvers, MA,
USA) were used (all dilutions, 1:1,000): GSK-3β Ab (cat no. 9315),
phospho-GSK-3β Ab (cat no. 9323), STAT3 Ab (cat no. 8232),
phospho-STAT3 Ab (cat no. 9134), mTOR Ab (cat no. 2972),
phospho-mTOR Ab (cat no. 2971), ERK1/2 Ab (cat no. 4696),
phospho-ERK1/2 Ab (cat no. 8544) and PDGF-D Ab (SCBT, Dallas, TX,
USA). The signals were visualized by chemiluminescence (UVP,
Upland, CA, USA) based on the manufacturer's instructions and
grayscale values were calculated by ImageJ version 1.8.0 [National
Institutes of Health (NIH) Bethesda, MD, USA].
Real-time qPCR
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was used to purify the total
RNA from cells. One milligram of total RNA was used for reverse
transcription using a reverse transcription kit (TransGen Biotech,
Beijing, China). Specific products of rat PDGF-D, vascular
endothelial growth factor (VEGF), hepatocyte growth factor (HGF)
and PDGF-B were amplified by qPCR using the following primers:
PDGF-D, 5′-TCCTCGCTTCCCCAACAG-3′ (forward) and
5′-TTCCTCTGACAACGGTGCTG-3′ (reverse); VEGF,
5′-CAAAGCCAGCACATAGGAGAGA-3′ (forward) and
5′-VTATCTTTCTTTGGTCTGCATTCAC-3′ (reverse); HGF,
5′-TTTCCCGTTGTGAAGGAGATAC-3′ (forward) and
5′-TTCAAACTAACCATCCACCCTAC-3′ (reverse); PDGF-B,
5′-TGCTGCTACCTGCGTCTGG-3′ (forward) and 5′-GCTCAGCCCCATCTTCGTC-3′
(reverse); GADPH, 5′-CCCATCTATGAGGGTTACGC-3′ (forward) and
5′-TTTAATGTCACGCACGATTTC-3′ (reverse). The expression levels of
these genes were evaluated by qPCR using a TransStart Green Q-PCR
SuperMix kit (TransGen Biotech) with the following thermocycling
conditions: Denaturation at 95°C for 10 min, followed by 95°C for
30 sec, and 60°C for 20 sec for 40 cycles. The relative expression
of each targeted gene was determined using the 2−∆∆Cq
comparative method (16) and
normalized against that of GAPDH. Each sample was run as triplicate
and repeated in three independent experiments.
ELISA
According to the manufacturer's protocol, an ELISA
kit (cat no. DL-PDGF-D-RA; Dldevelop, Jiangsu, China) was used to
determine the level of PDGF-D present in the extracellular medium.
Following the adsorption of Detection Reagent A, the plates were
incubated with a fixed amount of total protein from the medium.
Thereafter, these plates were incubated with biotinylated Detection
Reagent B and washed, followed by incubation with
streptavidin-horseradish peroxidase. Then, chromogen
tetramethylbenzidine was added, and the readings at an absorbance
of 450 nm were recorded using a microplate reader (SpectraMax M2;
Molecular Devices, LLC, Sunnyvale, CA, USA).
Statistical analysis
The statistical significance of differences among
groups was tested using analysis of variance (ANOVA), followed by
Tukey's honestly significant difference (HSD) test after a
normality test. GraphPad Prism v.6.01 software (GraphPad Software,
Inc., La Jolla, CA, USA) was used for analysis. P-values <0.05
and <0.01 were considered to indicate statistically significant
differences.
Results
Characterization of EPCs
The biological properties of EPCs derived from rat
bone marrow cells were first characterized using a previously
described system, in which the cells were cultured in the
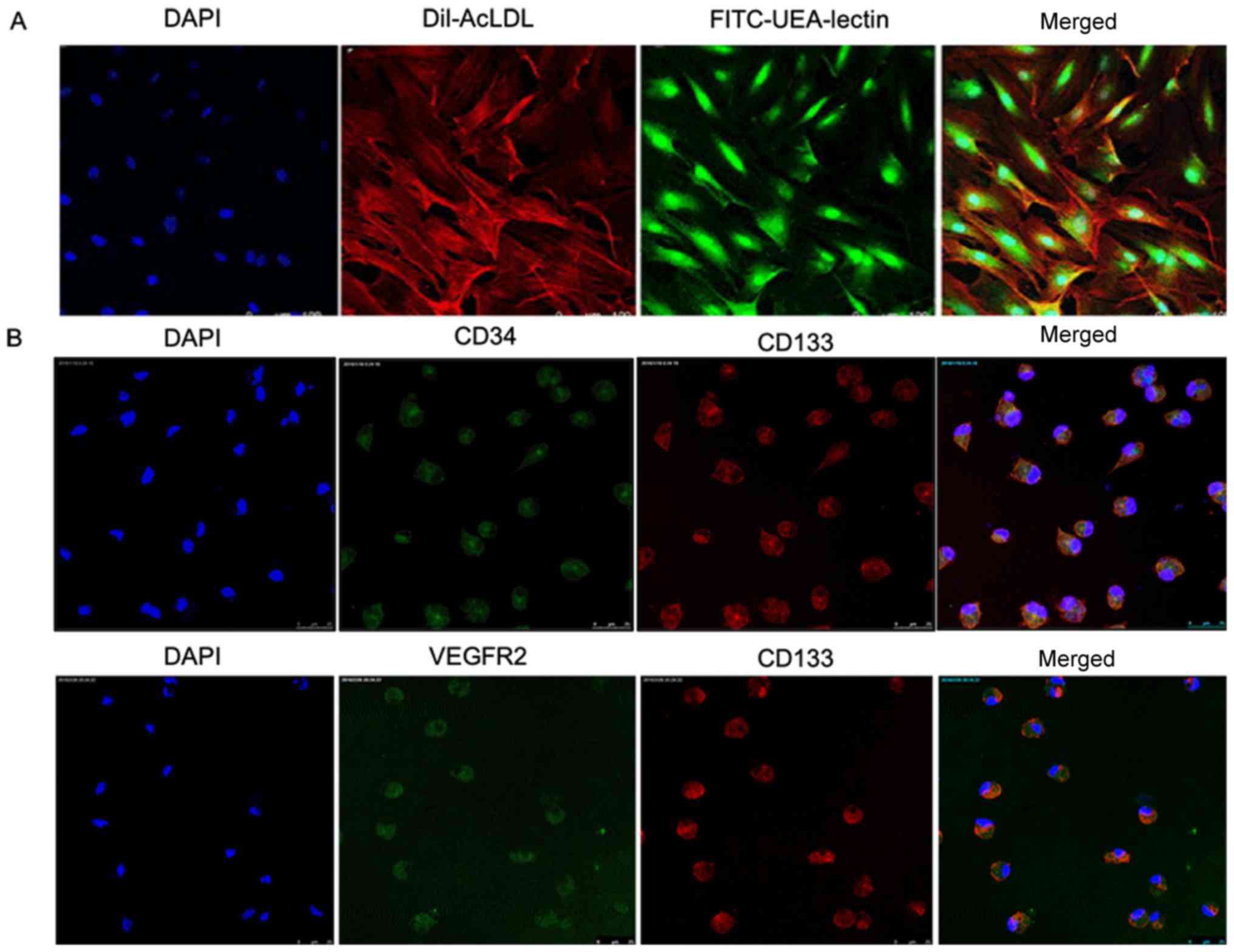
endothelial cell selection medium and isolated (6). The obtained cells had a spindle shape
with an endothelial cell-like morphology at day 7 (Fig. 1A). In addition, 90% of the attached
cells exhibited an uptake of Dil-acLDL and the concurrent binding
to FITC-UEA-lectin (Fig. 1A),
which are the important characteristics of EPCs (17). Furthermore, it was found that these
cells expressed VEGFR2, CD34 and CD133 (Fig. 1B), which are well-known endothelial
markers (18). Taken together,
this demonstrates that the cells derived from the rat bone marrow
cells were EPCs. EPCs were used at passages 3–8 for the subsequent
experiments.
Verification of EPCs stably expressing
PDGF-D
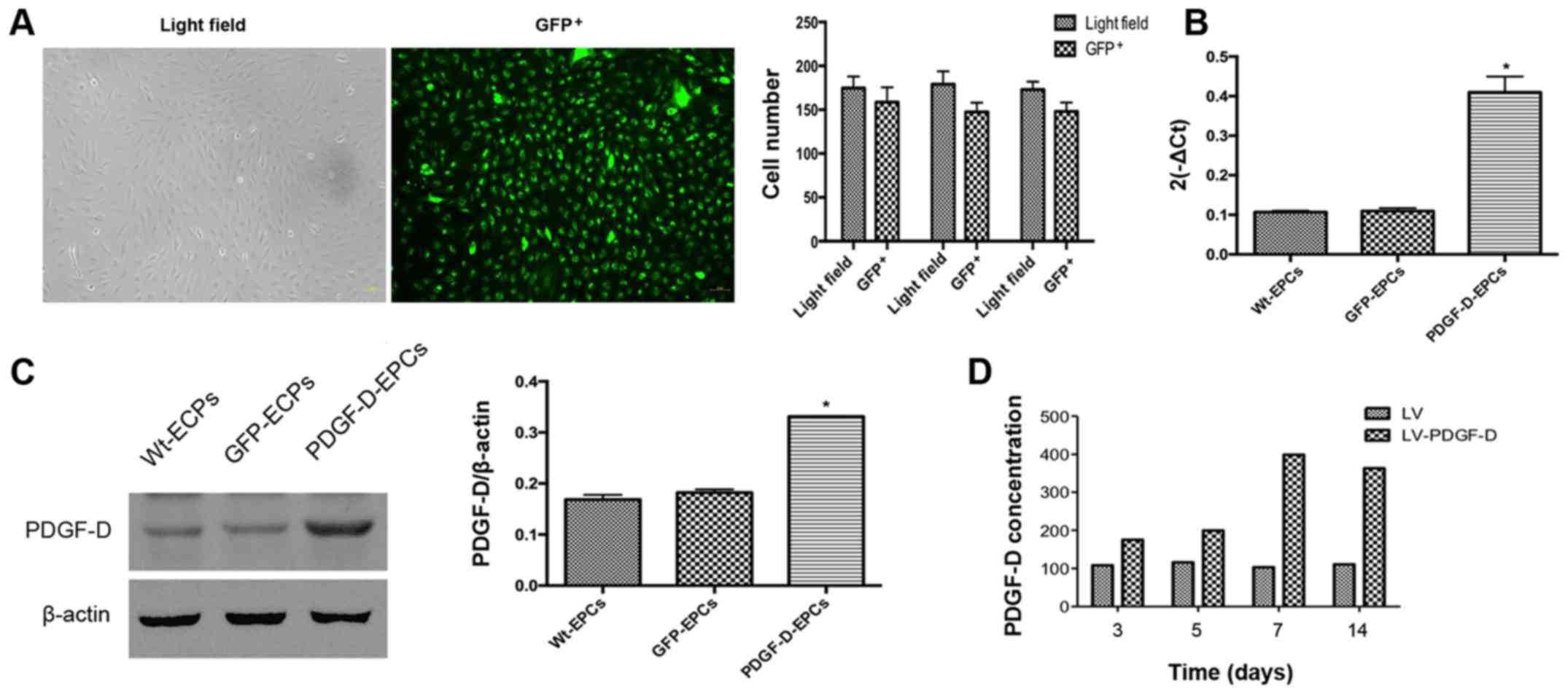
In order to generate EPCs stably expressing GFP as
controls (GFP-EPCs) or PDGF-D (PDGF-D-EPCs), EPCs were infected
with lentiviruses encoding GFP alone or encoding both PDGF-D and
GFP, followed by puromycin selection for one week. Infection
efficiency was first evaluated by observing GFP using a
fluorescence microscope. As shown in Fig. 2A, the infection efficiency of EPCs
by lentiviruses was >90%. Next, the expression levels of PDGF-D
in the stably expressing EPCs after drug selection by RT-qPCR and
western blot analysis were assessed, and it was found that the mRNA
expression in the PDGF-D-EPCs was 0.5-fold more than that in the
GFP-EPCs (Fig. 2B). Consistent
with the RT-qPCR results, western blot analysis also revealed a
significantly elevated PDGF-D expression in PDGF-D-EPCs, when
compared with that in the GFP-EPCs (Fig. 2C). In addition, it was found that
the secretion of PDGF-D in the culture medium of PDGF-D-EPCs was
significantly increased within 14 days of examination, when
compared with the GFP-EPCs (Fig.
2D).
PDGF-D in the microenvironment
promotes EPCs proliferation, migration, adhesion and tube formation
and inhibits senescence
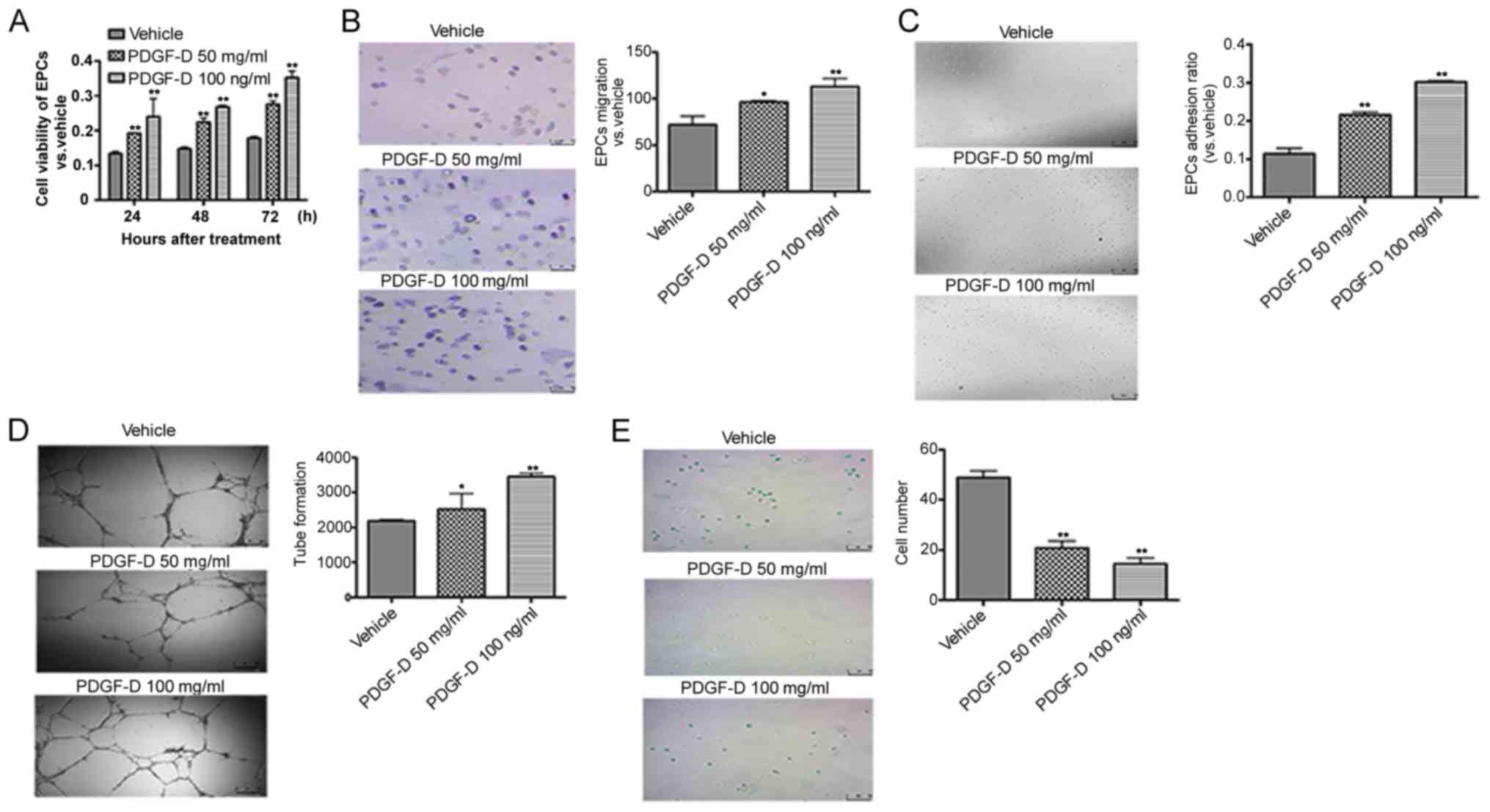
Next, the effects of PDGF-D in the microenvironment
on the biological activities of EPCs in vitro were examined.
MTT assays revealed that PDGF-D treatment significantly promoted
EPCs proliferation, when compared with that noted in the vehicle
(Fig. 3A). Given that the
migration and adhesion of EPCs are required for vasculature
regeneration (19), Transwell
assays were performed to examine the effects of PDGF-D on EPC
migration. The migration ability of EPCs stimulated by PDGF-D (100
ng/ml) was ~2.5-fold higher than the ability of EPCs stimulated by
the vehicle (Fig. 3B). In
addition, PDGF-D significantly enhanced the adhesion of EPCs, when
compared with the vehicle (Fig.
3C). Tube formation in EPCs has been shown to be one of the key
steps to promote angiogenesis (20). EPCs were co-cultured with different
concentrations of PDGF-D (vehicle, 50 and 100 ng/ml, respectively)
on Matrigel, and their capability to form capillary tubes was
evaluated. EPCs co-cultured with PDGF-D (100 ng/ml) had
significantly increased tube formation, when compared with those
co-cultured with the vehicle (Fig.
3D). Furthermore, analysis of senescence was carried out to
investigate the effect of PDGF-D on EPCs senescence, as it was
shown that delayed senescence is helpful for EPCs to migrate and
adhere to ischemic sites and excise angiogenesis (21). It was found that the number of
senescent EPCs was significantly lower in EPCs treated with 100
ng/ml of PDGF-D than those treated with the vehicle (Fig. 3E). Collectively, these results
indicate that PDGF-D has proangiogenic effects on EPCs.
PDGF-D acts in a PDGFR-dependent and
-independent manner
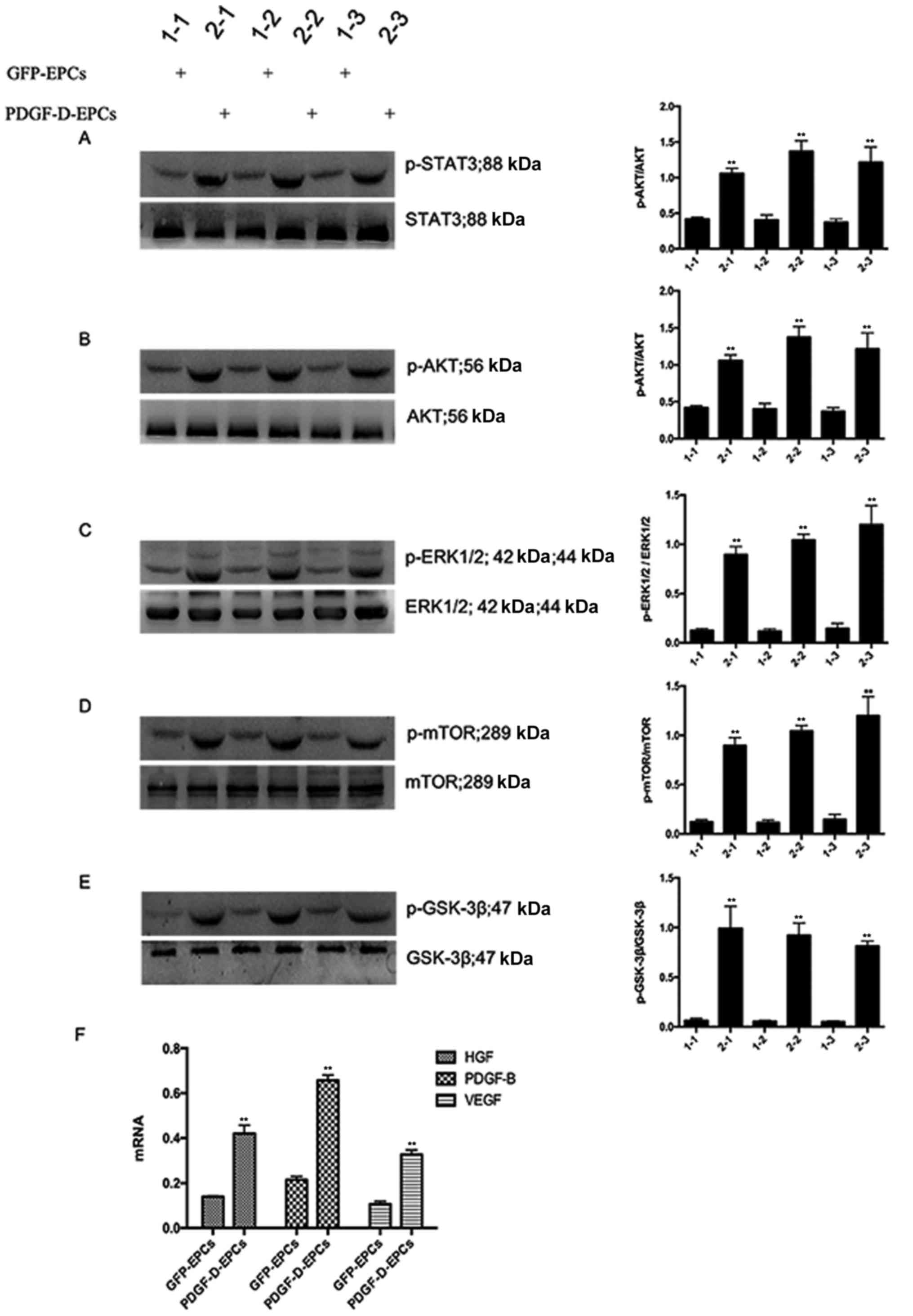
In order to ascertain whether PDGF-D activates PDGFR
and the downstream signaling, the expression of essential kinases
involved in the PDGFR signaling pathway was examined by western
blot analysis. As shown in Fig. 4A and
E, PDGF-D overexpression promoted the phosphorylation of STAT3,
AKT, ERK1/2, mTOR and GSK-3β. Interestingly, PDGF-D altered the
expression and secretion of several PDGFR-independent regulators,
including VEGF, HGF and PDGF-B (Fig.
4F). Collectively, these observations indicate that PDGF-D
mediates endothelial angiogenesis in a PDGFR-dependent and
-independent manner.
Discussion
In the present study, the gain-of-functional
approach was used to examine the impact of PDGF-D on the biological
activities of EPCs obtained from rat bone marrow cells. The major
findings from this study were as follows: i) PDGF-D in the
microenvironment promotes EPC proliferation, migration, adhesion
and tube formation; ii) PDGF-D suppresses EPC senescence; iii)
PDGF-D mediates the biological activities of EPCs through
PDGFR-dependent and -independent mechanisms.
Cell based gene therapy, which has a proangiogenic
effect, appears to be a powerful approach for the treatment of limb
ischemic diseases (5,22,23).
For instance, VEGF-transfected macrophages (5) and reprogrammed human postnatal dermal
fibroblasts with EC transcription factors ER71/ETV2 (23) have been shown to be particularly
effective in thrombus recanalization and resolution. In contrast to
the previous belief that angioblasts are only implicated in
vasculogenesis in pre-natal life, it is presently a general
consensus that EPCs are generated in both pre- and post-natal life
(24). Mesodermal cells produced
during embryogenesis gives rise to hemangioblasts, which
differentiate into EPCs or hematopoietic stem cells (HSCs). The
former exhibits the capacity to differentiate into ECs, and HSCs
generate all blood cell lineages (25), which contributes significantly to
angiogenesis. In the present study, it was found that PDGF-D
substantially enhanced the angiogenic function of EPCs, including
proliferation, adhesion, migration and tube formation. In addition,
angiogenesis is an organized series of events that lead to the
maturation of a mature vascular network, which needs the precise
temporal and spatial regulation of numerous angiogenic factors,
including VEGF, basic fibroblast growth factor-2 (FGF-2) and PDGF.
Furthermore, it was found in the present study that the
overexpression of PDGF-D increased the release of VEGF, HGF and
PDGF-B into the culture medium. Together, it was postulated that
PDGF-D may promote angiogenesis by enhancing the angiogenic
activity of EPCs and increase the paracrine release of angiogenic
factors.
During the process of angiogenesis and
vasculogenesis, EPCs are activated to reach the proliferative
sites, allowing these cells to enter the circulation (26). Thereafter, EPCs go to their target
tissue, attach to the endothelia of the vessel, and penetrate
through the basement membrane and ECM. After arriving at the
remodeling site, EPCs start their differentiation into or interact
with ECs. Although the exact function of EPCs remain unclear, it
has been widely considered that EPC differentiation goes through
the adhesion to extracellular matrix components, proliferation,
maturation, and the possession of an endothelial phenotype
(27). During this process, PDGF
family members, such as PDGF-A and -B, play important roles
(28). The present study provides
evidence that PDGF-D also potentiates the angiogenic ability of
EPCs, including proliferation, migration and tube formation, and
inhibits their senescence in vitro. Given that decreased
angiogenesis capacity is also an important feature of aging blood
vessels (29,30), mainly manifested as EPCs
senescence, and that the EPCs activity in the circulatory system is
critical for endothelial cell renewal, vascular repair and
neovascularization (31), we
believe that the anti-senescence effect of PDGF-D on EPCs can
reduce vascular aging and enhance their function (32). All these increase the capability of
EPCs to integrate into the major capillary plexus that constitutes
the fundamental vascular network, which is required for
angiogenesis. Thus, the present study suggests that PDGF-D plays a
crucial part in angiogenesis and vasculogenesis by activating
EPCs.
PDGF isoforms play biological roles by binding to
two specific cell surface receptors with different affinities. The
α-receptor binds all three isoforms with high affinity, whereas the
β-receptor (PDGFR-β) binds only to PDGF-B1. PGDFRα and PDGFRβ have
partly different functions, because these are differentially
expressed in different cell types. However, these two PDGF
receptors can regulate different signal transduction pathways in
cells that have both receptor types (33). As mentioned above, in the present
study, it was observed that PDGF-D promoted angiogenesis partially
through the direct potentiation of biological activities of EPCs.
On the other hand, previous research suggests that angiogenic
signaling induced by PDGF-D is PDGFRβ-independent (34). PDGFR could bind to scaffold
molecules, which act as bridges to other signaling molecules. For
example, the p85 subunit of phosphatidylinositol 3-kinase
(PI3-kinase) physically interacts with the p110 catalytic subunit
(35) and Grb2 complexes with the
nucleotide exchange factor Sos1 (36), which in turn stimulates Ras and its
downstream signaling Erk1/2 MAP-kinase (37). Similarly, PDGF activation has been
shown to indirectly activate several kinase signaling, including
the Rho, Cdc42 and MAP-kinase pathways (38). Thus, it was speculated that PDGF-D
may also have a paracrine function. Indeed, it was found that the
overexpression of PDGF-D not only potentiates the phosphorylation
of STAT3, AKT, ERK1/2, GSK-3β and mTOR, but also increases the
paracrine release of angiogenic factors, including VEGF, HGF and
PDGF-B. Thus, the investigators consider that PDGF-D promotes the
angiogenic capacity of EPCs through both PDGF signaling-dependent
and -independent pathways, while the exact molecular basis
underpinning the latter, that is, the activation of
PDGF-independent pathways, remains to be elucidated.
Although the enhancement of angiogenic capability of
EPCs by PDGF-D in vitro was observed, it was noted that the
in vivo situation may be different. For example, new vessel
formation in an adult environment requires the process of
neovascularization, which includes not only EPCs formation in the
primitive vascular network, but also ECs activation, in order to
generate a vascular sprout, and all of which are lacking in an
in vitro environment (39).
Hence, this warrants further in vivo investigation to
corroborate our in vitro observations.
In summary, it was demonstrated in the present study
that the newly identified PDGF family member, PDGF-D, boosts the
in vitro anigogenic activity of EPCs, such as proliferation,
adhesion, migration and tube formation. This also provides evidence
that PDGF may exert its positive impact on angiogenesis through
both PDGF signaling-dependent and -independent pathways, while
detailed relevant networks remain to be deciphered. These findings
support the premise that PDGF-D may be potentially used as a
therapeutic target for the treatment of ischemic disease.
References
|
1
|
Annex BH: Therapeutic angiogenesis for
critical limb ischaemia. Nat Rev Cardiol. 10:387–396. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Malo-Michèle M, Bugnon C and Fellmann D:
Cytoimmunological study of the corticotrophic and
corticomelanotrophic cells in the adenohypophysis of Boops salpa L.
(marine teleost) in different experimental conditions (variation of
salinity and of background color). C R Acad Sci Hebd Seances Acad
Sci D. 283:643–646. 1976.(In French). PubMed/NCBI
|
|
3
|
Rehman J, Li J, Orschell CM and March KL:
Peripheral blood ‘endothelial progenitor cells’ are derived from
monocyte/macrophages and secrete angiogenic growth factors.
Circulation. 107:1164–1169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leu S, Day YJ, Sun CK and Yip HK:
tPA-MMP-9 axis plays a pivotal role in mobilization of endothelial
progenitor cells from bone marrow to circulation and ischemic
region for angiogenesis. Stem Cells Int. 2016:54175652016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Modarai B, Humphries J, Burnand KG,
Gossage JA, Waltham M, Wadoodi A, Kanaganayagam GS, Afuwape A,
Paleolog E and Smith A: Adenovirus-mediated VEGF gene therapy
enhances venous thrombus recanalization and resolution.
Arterioscler Thromb Vasc Biol. 28:1753–1759. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ye Y, Li X, Zhang Y, Shen Z and Yang J:
Androgen modulates functions of endothelial progenitor cells
through activated Egr1 signaling. Stem Cells Int. 2016:1–16. 2016.
View Article : Google Scholar
|
|
7
|
Liang J, Huang W, Cai W, Wang L, Guo L,
Paul C, Yu XY and Wang Y: Inhibition of microRNA-495 enhances
therapeutic angiogenesis of human induced pluripotent stem cells.
Stem Cells. 35:337–350. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shimamura M, Nakagami H, Koriyama H and
Morishita R: Gene therapy and cell-based therapies for therapeutic
angiogenesis in peripheral artery disease. Biomed Res Int.
2013:1862152013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li X and Eriksson U: Novel PDGF family
members: PDGF-C and PDGF-D. Cytokine Growth Factor Rev. 14:91–98.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bergsten E, Uutela M, Li X, Pietras K,
Ostman A, Heldin CH, Alitalo K and Eriksson U: PDGF-D is a
specific, protease-activated ligand for the PDGF beta-receptor. Nat
Cell Biol. 3:512–516. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang B, Gong JP, Sun JM, Luo WJ, Chen YK,
Liu ZJ, Li F and Fu J: Construction of a plasmid for expression of
rat platelet-derived growth factor C and its effects on
proliferation, migration and adhesion of endothelial progenitor
cells. Plasmid. 69:195–201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen J, Yuan W, Wu L, Tang Q, Xia Q, Ji J,
Liu Z, Ma Z, Zhou Z, Cheng Y and Shu X: PDGF-D promotes cell
growth, aggressiveness, angiogenesis and EMT transformation of
colorectal cancer by activation of Notch1/Twist1 pathway.
Oncotarget. 8:9961–9973. 2017.PubMed/NCBI
|
|
13
|
Zhao L, Zhang C, Liao G and Long J:
RNAi-mediated inhibition of PDGF-D leads to decreased cell growth,
invasion and angiogenesis in the SGC-7901 gastric cancer xenograft
model. Cancer Biol Ther. 9:42–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen J, Han Y, Lin C, Zhen Y, Song X, Teng
S, Chen C, Chen Y, Zhang Y and Hui R: PDGF-D contributes to
neointimal hyperplasia in rat model of vessel injury. Biochem
Biophys Res Commun. 329:976–983. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Uutela M, Wirzenius M, Paavonen K,
Rajantie I, He Y, Karpanen T, Lohela M, Wiig H, Salven P, Pajusola
K, et al: PDGF-D induces macrophage recruitment, increased
interstitial pressure and blood vessel maturation during
angiogenesis. Blood. 104:3198–3204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nie Z, Xu L, Li C, Tian T, Xie P, Chen X
and Li B: Association of endothelial progenitor cells and peptic
ulcer treatment in patients with type 2 diabetes mellitus. Exp Ther
Med. 11:1581–1586. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoder MC, Mead LE, Prater D, Krier TR,
Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT and Ingram DA:
Redefining endothelial progenitor cells via clonal analysis and
hematopoietic stem/progenitor cell principals. Blood.
109:1801–1809. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun YY, Bai WW, Wang B, Lu XT, Xing YF,
Cheng W, Liu XQ and Zhao YX: Period 2 is essential to maintain
early endothelial progenitor cell function in vitro and
angiogenesis after myocardial infarction in mice. J Cell Mol Med.
18:907–918. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gu Y, Ampofo E, Menger MD and Laschke MW:
miR-191 suppresses angiogenesis by activation of NF-κB signaling.
FASEB J. 31:3321–3333. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van Balkom BW, de Jong OG, Smits M,
Brummelman J, den Ouden K, de Bree PM, van Eijndhoven MA, Pegtel
DM, Stoorvogel W, Würdinger T, et al: Endothelial cells require
miR-214 to secrete exosomes that suppress senescence and induce
angiogenesis in human and mouse endothelial cells. Blood.
121:3997–4006. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lebas B, Galley J, Renaud-Gabardos E,
Pujol F, Lenfant F, Garmy-Susini B, Chaufour X and Prats AC:
Therapeutic benefits and adverse effects of combined proangiogenic
gene therapy in mouse critical leg ischemia. Ann Vasc Surg.
40:252–261. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee S, Park C, Han JW, Kim JY, Cho K, Kim
EJ, Kim S, Lee SJ, Oh SY, Tanaka Y, et al: Direct reprogramming of
human dermal fibroblasts into endothelial cells using ER71/ETV2.
Circ Res. 120:848–861. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Asahara T and Kawamoto A: Endothelial
progenitor cells for postnatal vasculogenesis. Am J Physiol Cell
Physiol. 287:C572–C579. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
De Val S and Black BL: Transcriptional
control of endothelial cell development. Dev Cell. 16:180–195.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Velazquez OC: Angiogenesis and
vasculogenesis: inducing the growth of new blood vessels and wound
healing by stimulation of bone marrow-derived progenitor cell
mobilization and homing. J Vasc Surg. 45 Suppl A:A39–A47. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Caiado F and Dias S: Endothelial
progenitor cells and integrins: Adhesive needs. Fibrogenesis Tissue
Repair. 5:42012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cao R, Bråkenhielm E, Li X, Pietras K,
Widenfalk J, Ostman A, Eriksson U and Cao Y: Angiogenesis
stimulated by PDGF-CC, a novel member in the PDGF family, involves
activation of PDGFR-alphaalpha and -alphabeta receptors. FASEB J.
16:1575–1583. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lakatta EG and Levy D: Arterial and
cardiac aging: Major shareholders in cardiovascular disease
enterprises: Part I: Aging arteries: A ‘set up’ for vascular
disease. Circulation. 107:139–146. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Novella S, Heras M, Hermenegildo C and
Dantas AP: Effects of estrogen on vascular inflammation: A matter
of timing. Arterioscler Thromb Vasc Biol. 32:2035–2042. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Williamson K, Stringer SE and Alexander
MY: Endothelial progenitor cells enter the aging arena. Front
Physiol. 3:302012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Volaklis KA, Tokmakidis SP and Halle M:
Acute and chronic effects of exercise on circulating endothelial
progenitor cells in healthy and diseased patients. Clin Res
Cardiol. 102:249–257. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ekman S, Kallin A, Engström U, Heldin CH
and Rönnstrand L: SHP-2 is involved in heterodimer specific loss of
phosphorylation of Tyr771 in the PDGF beta-receptor. Oncogene.
21:1870–1875. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Muhl L, Folestad EB, Gladh H, Wang Y,
Moessinger C, Jakobsson L and Eriksson U: Neuropilin 1 binds PDGF-D
and is a co-receptor in PDGF-D-PDGFRβ signaling. J Cell Sci.
130:1365–1378. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu J, Zhang Y, McIlroy J, Rordorf-Nikolic
T, Orr GA and Backer JM: Regulation of the p85/p110
phosphatidylinositol 3′-kinase: Stabilization and inhibition of the
p110alpha catalytic subunit by the p85 regulatory subunit. Mol Cell
Biol. 18:1379–1387. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Buday L, Egan SE, Rodriguez Viciana P,
Cantrell DA and Downward J: A complex of Grb2 adaptor protein, Sos
exchange factor and a 36-kDa membrane-bound tyrosine phosphoprotein
is implicated in ras activation in T cells. J Biol Chem.
269:9019–9023. 1994.PubMed/NCBI
|
|
37
|
Rao GN: Hydrogen peroxide induces complex
formation of SHC-Grb2-SOS with receptor tyrosine kinase and
activates Ras and extracellular signal-regulated protein kinases
group of mitogen-activated protein kinases. Oncogene. 13:713–719.
1996.PubMed/NCBI
|
|
38
|
Demoulin JB and Essaghir A: PDGF receptor
signaling networks in normal and cancer cells. Cytokine Growth
Factor Rev. 25:273–283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hasan J, Shnyder SD, Bibby M, Double JA,
Bicknel R and Jayson GC: Quantitative angiogenesis assays in vivo-a
review. Angiogenesis. 7:1–16. 2004. View Article : Google Scholar : PubMed/NCBI
|