Introduction
Breast cancer is one of the most common malignant
tumors in women worldwide. Metastasis and invasion are the
predominant causes of mortality in patients (1). A recent study identified that
epithelial-mesenchymal transition (EMT) serves an important role in
metastasis and invasion in breast cancer (2). EMT is a process by which the
epithelial characteristics of a cell are gradually substituted with
a mesenchymal phenotype that is associated with increased invasion
and metastasis. This process is characterized by the degradation of
the extracellular matrix, upregulation of interstitial marker gene
expression, downregulation of epithelial marker gene expression,
and the loss of cell polarity and invasive ability (3). EMT is a dynamic and complex process
that is closely associated with the interaction of various growth
factors, protein molecules, transcription factors and pathways.
Transcription factors that induce EMT include zinc finger protein
SNAI3 (Snail), zinc finger protein SNAI2 (Slug) and twist-related
protein 1 (Twist) (4). These
transcription factors regulate the generation and development of
EMT through various signaling pathways and interactions between
these pathways. During EMT, the expression of the epithelial marker
gene E-cadherin is inhibited by a number of transcription factors
(5).
It is acknowledged that the tumor microenvironment
is critically involved in tumor development (3). The tumor microenvironment is complex
and necessary for the survival of cancer cells. It is predominantly
composed of the extracellular matrix and matrix cells, including
inflammatory, immune, endothelial and mesenchymal stem cells (MSCs)
(6). In recent decades, MSCs in
the tumor microenvironment have been widely researched. MSCs
undergo self-renewal, have multiple differentiation potentials and
are recruited by tumor cells into their microenvironment (7) to stimulate tumor and/or anti-tumor
adjacent cells by releasing endocrine and paracrine signals
(8).
Transforming growth factor (TGF)-β is a cytokine
that mediates complex functions and is widely involved in various
pathological and physiological processes. TGF-β is closely
associated with the occurrence and development of various
conditions, including inflammation, trauma and organ fibrosis
(9). However, its association with
tumor cells remains unclear. It has been observed that the TGF-β
signaling pathway inhibits the proliferation of tumor cells in the
early stages; however, promotes tumor migration in the advanced
stages of cancer (10).
Notably, TGF-β activates mothers against
decapentaplegic homolog (Smad) dependent and Smad independent
signaling pathways, which promotes the different effects of TGF-β
in tumor cells (11–13). Previous studies have demonstrated
that the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)
pathway is activated by TGF-β, which has synergistic/antagonistic
effects on the Smad signaling pathways (14,15).
A previous study additionally demonstrated that adipose-derived
stem cells (ADSCs) induce breast cancer cells to secrete TGF-β,
which consequently results in EMT in breast cancer cells (16).
In the present study, a coculture model of breast
cancer cells and human adipose-derived stem cells (Hu-ADSCs) was
established in vitro to determine how this microenvironment
affected tumor migration and invasion. The underlying signaling
pathways were examined in order to clarify the mechanisms involved
in these phenomena. The data demonstrated that Hu-ADSCs enhanced
the migration and invasion of breast cancer cells, which was
accompanied by decreased E-cadherin expression, in addition to
increased N-cadherin and EMT transcription factor expression.
Notably, it was demonstrated that Hu-ADSCs enhanced EMT in breast
cancer cells by cross interacting with the TGF-β/Smad and PI3K/AKT
signaling pathways.
Materials and methods
Isolation and culture of ADSCs
The present study was conducted in accordance with
the ethical standards in the Declaration of Helsinki (1975) and was
approved by the Institutional Ethics Committee at Shengjing
Hospital of China Medical University (Shenyang, China). All donors
came from the plastic surgery ward between October and December
2017 and were free of major diseases and provided written informed
consent. Adult adipose tissues were obtained by facial or abdominal
liposuction from 7 female donors (aged 19–52), and Hu-ADSCs were
isolated and cultured as previously described (17). Fresh adipose tissues were
collected, washed with sterile PBS, minced into small pieces and
incubated with 0.1% collagenase (type I; Roche Diagnostics GmbH,
Mannheim, Germany) in Dulbecco's modified Eagle's medium (DMEM)/F12
(Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) for 1 h at
37°C. Subsequently, the tissues were added to an equal volume of
DMEM/F12 with 10% fetal bovine serum (FBS; Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China) to neutralize enzyme
digestion. This reaction mixture was centrifuged at 1,200 × g for
10 min at room temperature to remove floating adipose tissues and
the supernatant. The deposited cells were seeded into DMEM/F12
medium with 10% MesenCult™ MSC Stimulatory Supplements
(Stemcell Technologies, Inc., Vancouver, BC, Canada) and the
cultures were maintained at 37°C in a 5% CO2 incubator.
After 48 h, non-adherent cells were removed. When the adherent
cells reached >80% confluency, they were detached using 0.05%
trypsin-EDTA (Beijing Solarbio Science & Technology Co., Ltd.)
and subcultured at a 1:3 ratio under the same culture conditions.
Hu-ADSCs between passages 3–5 were used in all experiments.
Hu-ADSC characterization
Hu-ADSCs were analyzed via flow cytometry with
respect to cellular membrane marker expression using fluorescein
isothiocyanate (FITC)-labeled antibodies (BD Biosciences, Franklin
Lakes, NJ, USA) to endoglin (CD105; undiluted; cat. no. 561443),
5′-nucleotidase (CD73; undiluted; cat. no. 561254), Thy-1 (CD90;
undiluted; cat. no. 555595), CD34 (undiluted; cat. no. 652802),
lymphocyte common antigen (CD45, undiluted; cat. no. 347463) and
human leukocyte antigen-antigen D related (HLA-DR, undiluted; cat.
no. 347364). The negative control stain was FITC-conjugated mouse
immunoglobulin G1-isotype. Hu-ADSCs were suspended in PBS at
concentration of 106/ml. Then, 50 µl cells were
incubated with 20 µl FITC-conjugated CD105, CD73, CD90, CD34, CD45
and HLA-DR for 15 min at 4°C and washed with PBS. Subsequently, the
cells were diluted in 500 µl PBS and analyzed by flow cytometry;
5,000 cell events per sample were acquired on a FACSCalibur flow
cytometer (BD Biosciences). Independent experiments were repeated
three times. The capacity of Hu-ADSCs to differentiate into
adipocytes and osteoblasts was assessed as previously described
(18). Hu-ADSCs were treated with
an Adipogenesis and Osteogenesis Differentiation kit (CTCC
Bioscience, Jiangyin, China). The medium was changed three times
per week. After 4 weeks of differentiation, the Hu-ADSCs were fixed
with 4% formalin for 15 min and stained with 1% Oil Red O and 0.2%
Alizarin Red S, all for 30 min and all at room temperature. Then
the stained cells were observed and photographed under an light
microscope (magnification, ×200).
Cell lines and culture conditions
Breast cancer MCF7 cells were obtained from the Cell
Biology Department of China Medical University (Shenyang, China).
MCF7 cells were cultured in high-glucose DMEM culture medium
(Hyclone; GE Healthcare Life Sciences) with 10% FBS (Beijing
Solarbio Science & Technology) in a humidified 5%
CO2 incubator at 37°C.
Transwell coculture
A non-contact coculture system of Hu-ADSCs and MCF7
cells was established using a Transwell suspension culture chamber
with a polyethylene terephthalate film combined with a 6-pore plate
(0.4 µm pores; Corning, Inc., Corning, NY, USA). Hu-ADSCs were
seeded into the upper chamber at 105 cells/well and MCF7
cells were added to the lower chamber at 5×104
cells/well. Hu-ADSC complete medium (DMEM/F12 medium with 10%
MesenCult™ MSC Stimulatory Supplements) was used in the
coculture system. MCF7 seeded into the 6-pore plate with Hu-ADSC
complete medium served as the control group, to eliminate the
influence of Hu-ADSC complete medium on MCF7 cells. In another two
groups, TGF-β1 neutralizing antibody anti-TGF-β1 (cat. no. ab27969;
Abcam, Cambridge, UK) or PI3K inhibitor LY294002 (cat. no.
HY-10108; MedChemExpress, LLC, Monmouth Junction, NJ, USA) was
added to the upper chamber of the coculture system at a
concentration of 8 µg/ml and 50 µmol/l, respectively. All groups
were maintained at 37°C in a 5% CO2 incubator. After 72
h of culture, MCF7 cells were harvested for the subsequent
experiments.
Cell proliferation assay
The effect of Hu-ADSCs on the proliferation of MCF7
cells was evaluated by a Cell Counting Kit-8 (CCK-8; Nanjing KeyGen
Biotech Co., Ltd., Nanjing, China). Following coculture for 72 h,
the MCF7 cells (1×104 cells/well) were plated in 96-well
plates and incubated for 24, 48 and 72 h. MCF7 cells cultured alone
were grown for 72 h and subsequently added to 96-well plates as a
control. Each group was equipped with four compound pores and 10 µl
CCK-8 solution was added to each well. The absorbance was
determined at the wavelength of 450 nm after incubation for 1
h.
Transwell migration assay
MCF7 cell migration was evaluated using a 24-well
Transwell chamber (8 µm pores; Corning, Inc.). Following coculture
for 72 h, the MCF7 cells (1×105 cells/well) were plated
into the top chamber and high glucose DMEM medium containing 30%
FBS was placed in the bottom chamber. MCF7 cells cultured alone
were used as the control. Cells were incubated for 24 or 48 h.
Cells in the upper chamber were subsequently removed with the aid
of a cotton swab and the remaining cells in the lower chamber were
fixed with 4% formalin for 15 min at room temperature. Cells that
migrated to the lower surface of the membrane were stained with
0.5% crystal violet for 15 min at room temperature and counted with
an inverted phase contrast light microscope (magnification, ×200;
Motic Incorporation, Ltd., Causeway Bay, Hong Kong). In total, five
visual fields were randomly selected for each assay. The mean
number of the migrating cells in these five fields was taken as the
cell migration number of the group. Independent experiments were
repeated three times.
Matrigel invasion assay
MCF7 cell invasion was evaluated in 24-well
Transwell chamber (8 µm pores; Corning, Inc.) coated with 40 µl BD
Matrigel™ Matrix (1:3 dilution; BD Biosciences). The
remaining steps were the same as the Transwell migration assay.
Conditioned medium (CM)
When Hu-ADSCs reached ~80% confluency, the culture
bottle was washed twice with PBS and completely exchanged with
fresh DMEM/F12 serum-free medium. The supernatant was collected
after 24, 48 and 72 h. Subsequently, the supernatant was
centrifuged at 1,200 × g for 10 min at room temperature to remove
cell fragments and filtered with a 0.22-µm filtration membrane to
remove bacteria.
ELISA
TGF-β1 expression in the conditioned medium was
measured using a double antibody sandwich ELISA according to the
manufacturer's protocol (cat. no. DZE10135; Shanghai HoraBio, Inc.,
Shanghai, China). DMEM/F12 serum-free medium without Hu-ADSCs was
placed in the incubator for 24, 48 and 72 h as the control groups.
All specimens were tested in duplicate wells.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Gene expression in cocultured MCF7 cells and MCF7
cells alone was quantified by RT-qPCR. Total RNA was isolated using
an RNAprep pure cell kit (BioTeke Corporation, Beijing, China) and
reverse-transcribed into complementary DNA using a first
complementary DNA Synthesis kit with oligo(dT)15
(BioTeke Corporation; 10 min at 25°C, 50 min at 42°C and 5 min at
95°C). The expressions of the genes of interest were measured by
PCR (Exicycler™ 96; Bioneer Corporation, Daejeon, Korea)
using SYBR Green Master Mix (Beijing Solarbio Science &
Technology). The primers were designed and synthesized by Sangon
Biotech Co., Ltd. (Shanghai, China) and the primer sequences are
listed in Table I. β-actin was
used as an endogenous control to normalize gene expression levels.
qPCR was performed for 10 min at 94°C followed by 40 amplification
cycles (10 sec at 94°C, 20 sec at 60°C and 30 sec at 72°C) to
determine the epithelium or interstitial gene expression. The
2−ΔΔCq method (19) was
used for the analysis of relative gene expression data. Independent
experiments were repeated three times.
 | Table I.Sequences of primers used for reverse
transcription-quantitative polymerase chain reaction analysis |
Table I.
Sequences of primers used for reverse
transcription-quantitative polymerase chain reaction analysis
| Target gene | Primer sequences
(5′→3′) |
|---|
| E-cadherin | F:
AGAACGCATTGCCACATACA |
|
| R:
TAAGCGATGGCGGCATTGTA |
| N-cadherin | F:
GGACCATCACTCGGCTTA |
|
| R:
CACTGGCAAACCTTCACG |
| Snail | F:
GCCCCACAGGACTTTGATGA |
|
| R:
AGTGAGTCTGTCAGCCTTTGTC |
| Slug | F:
AGCGAACTGGACACACATAC |
|
| R:
GCCCCAAAGATGAGGAGTAT |
| Twist | F:
GGAGTCCGCAGTCTTACGA |
|
| R:
CCAGCTTGAGGGTCTGAATC |
| β-actin | F:
CTTAGTTGCGTTACACCCTTTCTTG |
|
| R:
CTGTCACCTTCACCGTTCCAGTTT |
Western blot analysis
To assess the expression of phosphorylated
(p)-Smad2/3, p-AKT, Smad2/3, AKT, Snail, Slug, Twist, E-cadherin
and N-cadherin, total cellular protein was extracted from MCF7
cells from different treatment groups. Protein was isolated from
harvested cells using a Total Protein Extraction kit (Wanleibio
Co., Ltd., Shanghai, China) according to the manufacturer's
protocol. The protein concentration of the samples was determined
with a bicinchoninic acid protein assay kit (Wanleibio Co., Ltd.).
Total protein (40 µg/lane) was resolved by 5–10% SDS-PAGE and
subsequently transferred to a polyvinylidene difluoride membrane.
The membrane was blocked with 5% non-fat milk at room temperature
for 1 h in Tris-buffered saline containing 0.1% Tween-20 and
subsequently incubated with diluted primary antibody (1:500;
p-smad2/3 antibody cat. no. WL02305, p-AKT antibody cat. no.
WLP001, smad2/3 antibody cat. no. WL01520, AKT antibody cat. no.
WL0003, Snail antibody cat. no. WL01863, Slug antibody cat. no.
WL01508, Twist antibody cat. no. WL00997, E-cadherin antibody cat.
no. WL01482, N-cadherin antibody cat. no. WL01047 and β-actin
antibody cat. no. WL01845; all from Wanleibio, Co., Ltd.) at 4°C
overnight. The membrane was subsequently incubated with a secondary
antibody (1:5,000, cat. no. WLA023, Wanleibio Co., Ltd., Shenyang,
China) for 45 min at room temperature. Proteins were visualized
with an enhanced chemiluminescence reagent (Wanleibio Co., Ltd.).
β-actin was used as an internal control to normalize the loading
materials. Finally, the protein bands were quantified using
Gel-Pro-Analyzer version 4.0 (Media Cybernetics, Inc., Rockville,
MD, USA).
Statistical analysis
All experiments were repeated three times. The
results were analyzed by SPSS version 22.0 (IBM Corp., Armonk, NY,
USA). Data are presented as the mean ± standard deviation. Single
comparisons were performed by independent samples t-test and
multiple comparisons were performed by one-way analysis of variance
followed by Tukey's post-hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Isolation and identification of
Hu-ADSCs
The Hu-ADSCs were isolated from human adipose tissue
and adhered to the culture plate 24 h after seeding. After 3 or 4
days of culture, the majority of the cells exhibited
spindle-shaped, homogeneous and whirlpool-like growth (Fig. 1A). After 4 weeks, Hu-ADSCs were
successfully induced to differentiate into adipocytes and
osteoblasts (Fig. 1A). To further
characterize the cell surface markers of Hu-ADSCs, immunophenotypic
analysis was performed by flow cytometry. Hu-ADSCs exhibited
positive expression of CD73, CD90 and CD105, and negative
expression of CD34, CD45 and HLA-DR (Fig. 1B and C).
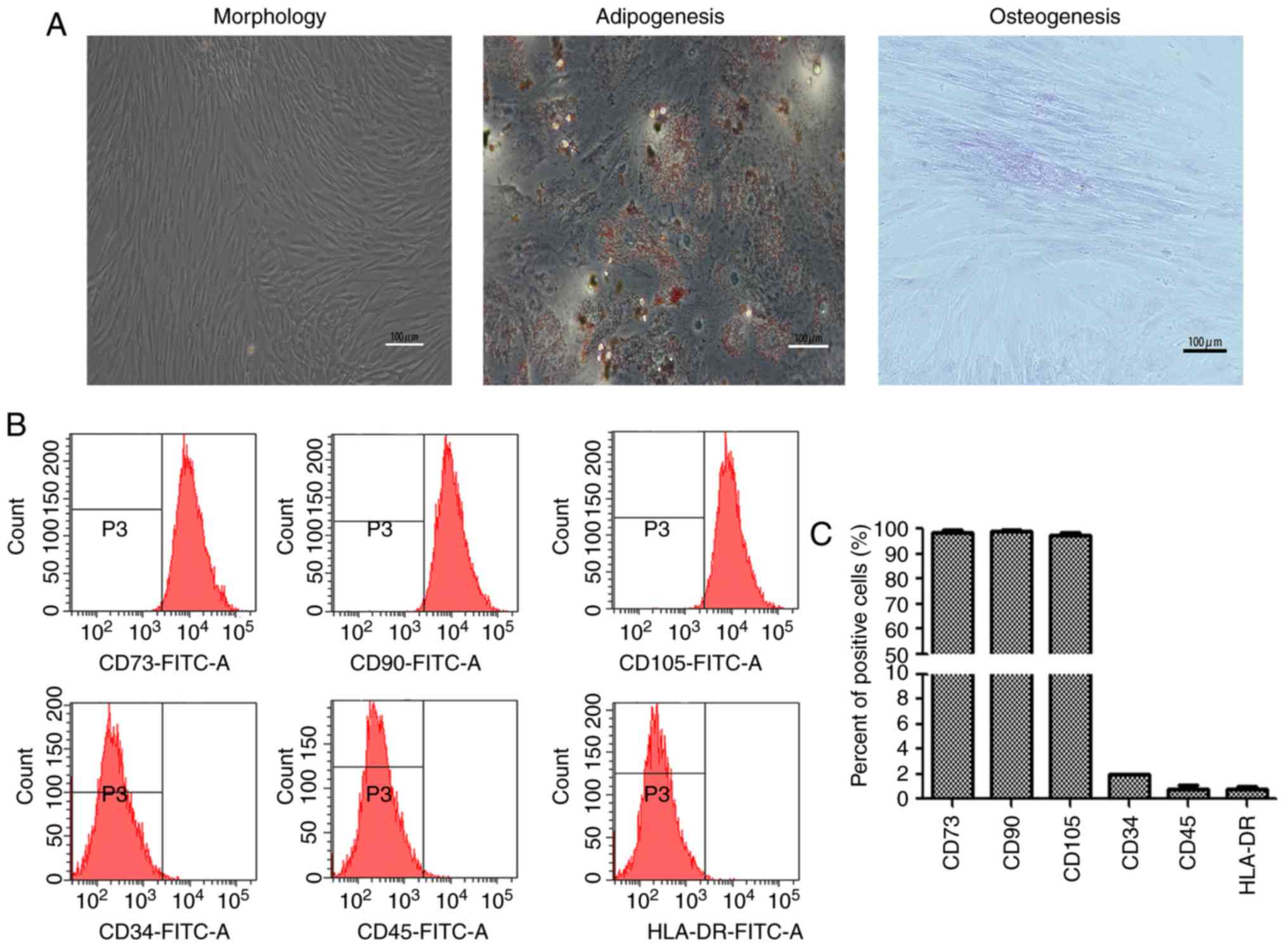 | Figure 1.Isolation and identification of
Hu-ADSCs. (A) Morphological features of isolated Hu-ADSCs at
passage 3. Hu-ADSCs were induced to differentiate into adipocytes
and osteoblasts with red indicating lipid droplets and pink
indicating calcification. Scale bar, 100 µm. (B) Hu-ADSCs examined
by flow cytometry were positive for CD73, CD90 and CD105
expression, and negative for CD34, CD45 and HLA-DR expression. (C)
A total of three independent flow cytometry experiments were
quantified. Hu-ADSCs, human adipose-derived stem cells; CD73,
5′-nucleotidase; CD90, Thy-1; CD105, endoglin; CD45, lymphocyte
common antigen; HLA-DR, human leukocyte antigen-antigen D related;
FITC, fluorescein isothiocyanate. |
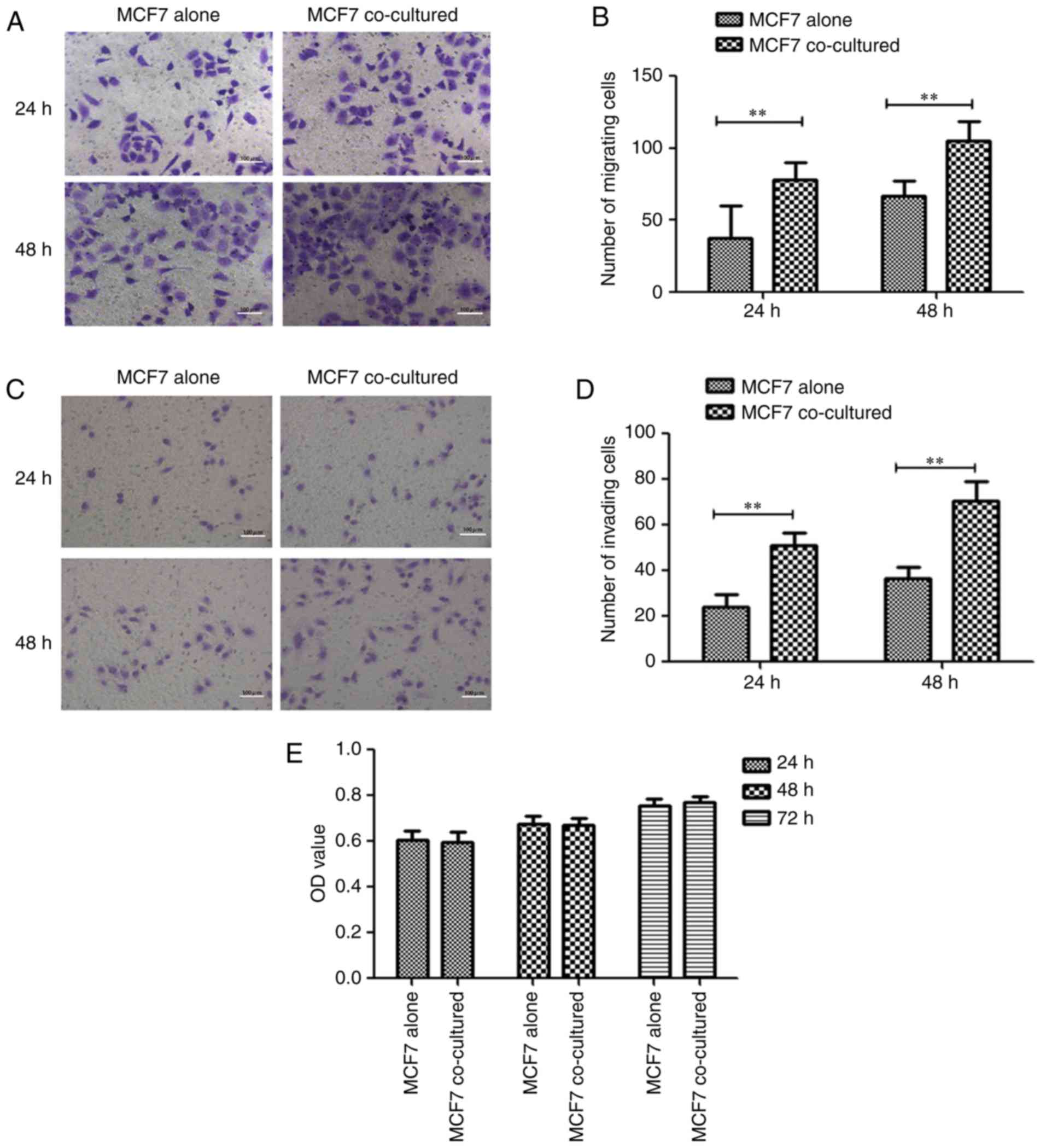
Hu-ADSCs increase MCF7 cell migration
and invasion
The migration and invasion of MCF7 cells cocultured
with Hu-ADSCs were determined by Transwell and Matrigel assays,
respectively, with MCF7 cells cultured alone as the control. In the
Transwell migration assay after 24 and 48 h of culture, the numbers
of migrating MCF7 cells significantly increased in the cocultured
group (78.47±11.37 and 104.93±13.57, respectively) compared with
the control group (37.93±22.00 and 66.53±10.54, respectively; both
P<0.01; Fig. 2A and B). In the
Matrigel invasion assay, the same results as the Transwell
migration assay were observed after 24 and 48 h: The number of
invading MCF7 cells was significantly increased in the cocultured
group (50.93±5.71 and 71.53±8.44, respectively) compared with the
control MCF7 cells (24.27±5.35 and 36.80±4.68, respectively; both
P<0.01; Fig. 2C and D). In
addition, cell proliferation experiments demonstrated that Hu-ADSCs
did not promote MCF7 cell proliferation (Fig. 2E). This suggested that the
ADSC-induced migration and invasion of cocultured MCF7 cells was
not caused by a significant increase in the number of MCF7 cells
(P>0.05); however, rather because the ADSCs specifically
promoted cell migration and invasion.
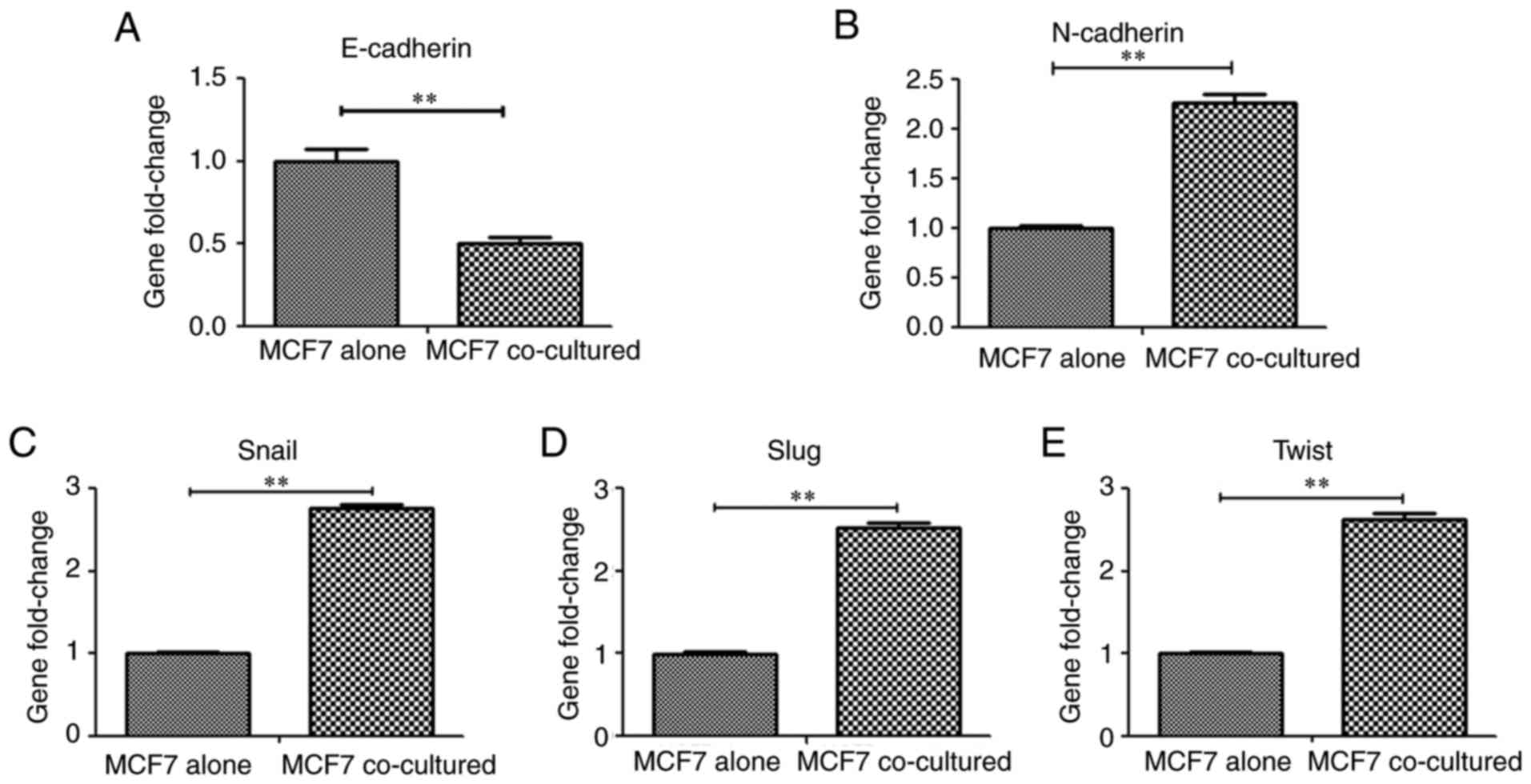
Hu-ADSCs enhance MCF7 cell EMT
E-cadherin is an epithelial marker involved in cell
adhesion/polarity and tissue morphogenesis, and normal epithelial
cells acquire invasive and migratory properties when they express
lower levels of E-cadherin (20).
E-cadherin expression was detected in MCF7 cells cocultured with
Hu-ADSCs by RT-qPCR and MCF7 cells cultured alone served as a
control. Concurrently, alterations in the expression of
interstitial marker gene N-cadherin and EMT-associated
transcription factors, including Snail, Slug and Twist, were
examined in the cocultured MCF7 cells and the control group. The
expression of E-cadherin in the cocultured MCF7 cells was
significantly downregulated compared with the control group
(Fig. 3A; P<0.01), whereas
N-cadherin (Fig. 3B), Snail
(Fig. 3C), Slug (Fig. 3D) and Twist (Fig. 3E) expression was upregulated
compared with the control group (all P<0.01).
Hu-ADSCs promote MCF7 cell EMT by
cross interacting with the TGF-β/Smad and PI3K/AKT pathways
TGF-β is directly dependent on Smad signaling for
tumor inhibition or promotion, and TGF-β may by phosphorylation
activate other signaling pathways that have potential involvement
in tumor cell survival, growth, migration and invasion. (TGF-β
non-Smad pathways) (9). To clarify
the mechanisms of Hu-ADSC action on MCF7 cells, it was determined
whether Hu-ADSCs secreted TGF-β1 by ELISA (Fig. 4A), and the effects of the
TGF-β/Smad and PI3K/AKT pathways on tumor EMT were examined by
western blotting. The results from ELISA demonstrated that the
level of TGF-β1 in CM greater than that in DMEM/F12 serum-free
medium, which was indicated that Hu-ADSCs secreted TGF-β1.
E-cadherin expression in MCF7 cells was significantly decreased
following treatment with Hu-ADSCs, and was significantly increased
in the coculture of MCF7 cells with anti-TGF-β1 or PI3K inhibitor
LY294002 (Fig. 4B and C;
P<0.01). The expressions of N-cadherin (Fig. 4B and D) and EMT-associated
transcription factors had the opposite trend (Fig. 4E-H). Concurrently, p-Smad2/3 (data
not shown) and p-AKT expression was additionally increased in the
cocultured MCF7 cells and this phenomenon was reversed by
anti-TGF-β1 or LY294002 (Fig.
4I-L). These results suggested that the TGF-β/Smad and PI3K/AKT
signaling pathways were involved in the promotion of MCF7 cell EMT
by Hu-ADSCs. As one of the aims of the present study was to confirm
whether TGF-β could activate the PI3K/AKT signaling pathway and
what effect the activated PI3K pathway would have on the EMT of the
cocultured MCF7 cells, the expression of p-AKT after the addition
of anti-TGF-β1 in the co-culture system was tested. Notably, it was
demonstrated that p-AKT was significantly decreased in the
cocultured MCF7 cells treated with anti-TGF-β1 (Fig. 4K and L; P<0.01). This suggested
that TGF-β1 can activated PI3K/AKT signaling pathway and that
Hu-ADSCs promoted MCF7 cell EMT via the TGF-β/Smad and PI3K/AKT
pathways, and that cross talk between the two pathways existed.
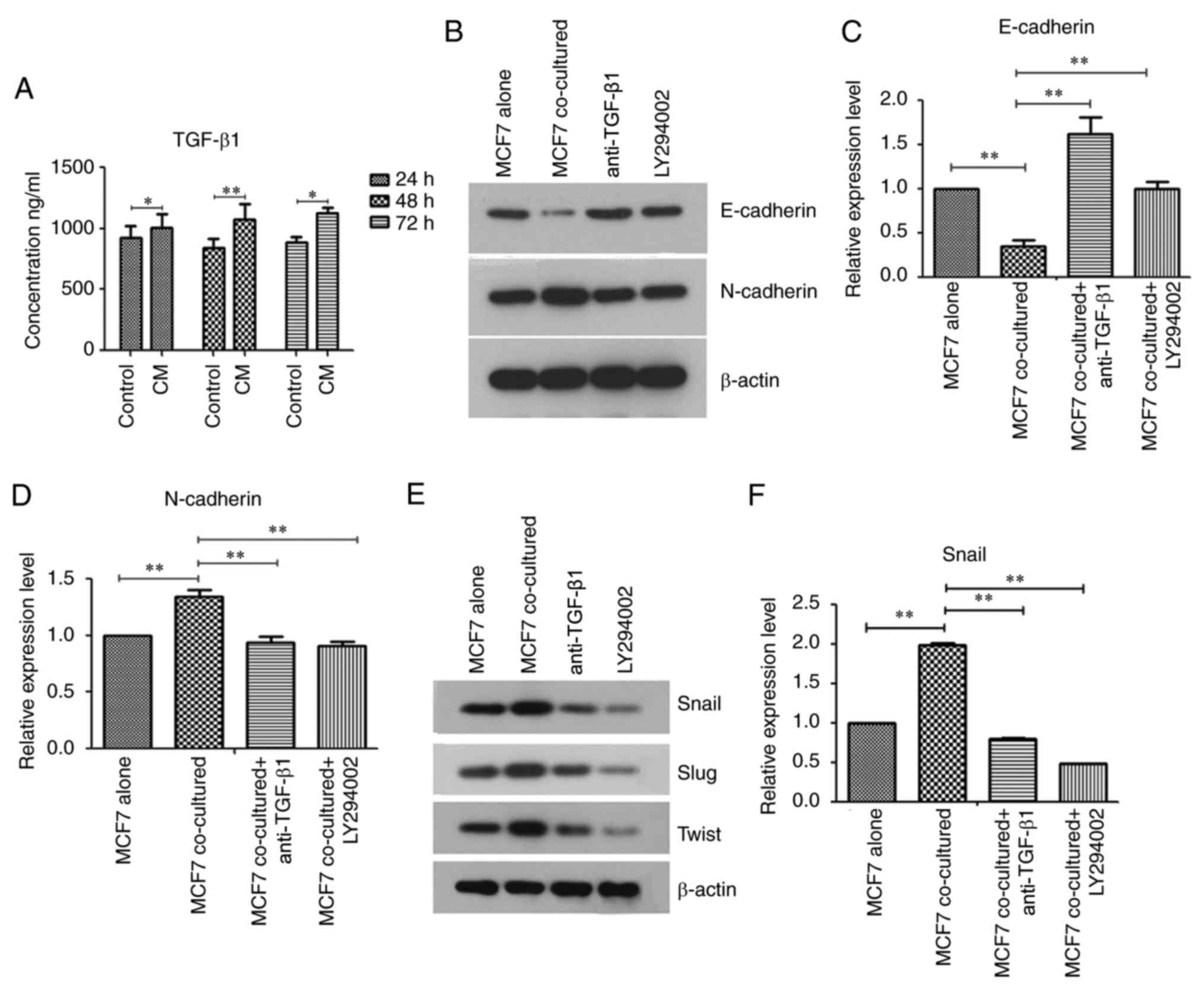 | Figure 4.Human adipose-derived stem cells
promote MCF7 cell epithelial-mesenchymal transition by cross
interacting with the TGF-β Smad and phosphoinositide 3-kinase/AKT
pathways. (A) Secretion of TGF-β1 into CM and DMEM/F12 serum-free
medium was assessed by ELISA. (B) Expression of E-cadherin and
N-cadherin were determined by western blotting. Relative expression
levels of (C) E-cadherin and (D) N-cadherin by densitometric
analysis. (E) Expression of Snail, Slug and Twist were determined
by western blotting. Relative expression levels of (F) Snail by
densitometric analysis. Data are presented as the mean ± standard
deviation of three independent experiments. *P<0.05,
**P<0.01. TGF-β, transforming-β; Snail, zinc finger protein
SNAI3; Slug, zinc finger protein SNAI2; Twist, twist-related
protein-1; p, phosphorylated; Smad, mothers against decapentaplegic
homolog; AKT, protein kinase B; CM, conditioned medium. Relative
expression levels of (G) Slug and (H) Twist by densitometric
analysis. (I) Expression of p-Smad2/3 was determined by western
blotting. Total Smad2/3 was used as a control to confirm equal
protein amounts. (J) Relative expression of p-Smad2/3 was
determined by densitometric analyses. (K) p-AKT expression was
determined by western blotting. Total AKT was used as a control to
confirm equal protein amounts. (L) Relative expression of p-AKT was
determined by densitometric analyses. Data are presented as the
mean ± standard deviation of three independent experiments.
*P<0.05, **P<0.01. Slug, zinc finger protein SNAI2; Twist,
twist-related protein-1; p, phosphorylated; Smad, mothers against
decapentaplegic homolog; AKT, protein kinase B; CM, conditioned
medium. |
Discussion
MSCs have self-renewal and multiple differentiation
functions, and migrate to tumorigenic or inflammatory sites to
exert tumor regulation or immunomodulatory effects by interacting
with tumor or inflammatory cells (8,21).
Based on these particular biological characteristics, MSCs are
considered as candidates for cancer treatment or regenerative
medicine applications (22,23).
However, accumulating evidence has suggested that the role of MSCs
in cancer is controversial. Although they may stimulate the growth,
migration and invasion of tumors by direct or indirect actions on
tumor cells, they may additionally inhibit tumor growth by
inhibiting signal pathways, including Wnt (24,25).
Therefore, the present study aimed to investigate the effects of
MSCs on tumors and to examine the underlying mechanism
involved.
Hu-ADSCs were firstly isolated from adipose tissue.
The morphological characteristics of Hu-ADSCs were assessed using
an appropriate concentration of collagenase for digestion, and the
adherent method was similar to other sources of MSCs, such as bone
marrow and human umbilical cords.
Furthermore, the MSCs stably differentiated into
adipocytes and osteoblasts, and highly expressed CD90, CD73 and
CD105, with low levels of CD34, CD45 and HLA-DR. These results meet
the criteria proposed by the Committee of the International Society
for Cellular Therapy for the biological characteristics of MSCs
(26).
Although it was previously demonstrated that MSCs
are recruited by tumor cells into their microenvironment (27), the interaction between them and
tumor cells remains unclear. By interacting with the tumor
microenvironment, MSCs exert a tumor suppressive effect (28); however, there is accumulating
evidence that MSCs have a tumor promoting effect, including tumors
of the breast, prostate, liver, ovary and pancreas (29–33).
The Hu-ADSC paracrine effect on tumor cells induces an aggressive
phenotype, leading to eventual cell migration (34). Park et al (35) observed that lung cancer cells
promote the differentiation of ADSCs into myofibroblasts and that
the direct coculture of these altered ADSCs increased the EMT of
lung cancer cells. Different from these previous studies, the
present study established a Transwell isolated coculture system and
determined that the paracrine effect of Hu-ADSCs promoted the EMT
of MCF7 cells. A direct coculture method was not adopted, thus
allowing Hu-ADSCs to be distinguished from MCF7 cells and allowing
a more convenient study of the biological characteristics of MCF7
cells. Although the direct co-culture method may be used to
determine the direct contact and paracrine effects of MSCs on tumor
cells, it is not easy to separate the two cell types following
coculture because MSCs lack specific surface markers, which may
additionally be altered following contact with tumor cells
(36). Yan et al (37) identified that ADSCs promote the
proliferation of breast cancer cells. The present study
demonstrated that Hu-ADSCs did not increase the number of MCF7
cells; however, promoted tumor cell EMT, characterized by the
decreased expression of E-cadherin, and increased expressions of
N-cadherin and EMT-associated transcription factors. The
transformation of tumor cell expression from E-cadherin to
N-cadherin increases cancer cell migration and invasion (38).
In addition, the expression of Snail, Slug and Twist
in cocultured MCF7 cells were increased in the present study, which
additionally suggested that Hu-ADSCs promoted MCF7 cell EMT. The
expressions of these transcription factors promote tumor cell
transition to a mesenchymal state by inhibiting epithelial cell
marker expression and inducing mesenchymal cell marker expression
(36,39,40).
The results in the present study regarding the
signal pathways involved provide insight for the current
understanding of the effects of Hu-ADSCs on tumor cell EMT. TGF-β
signaling, particularly TGF-β1, is recognized as a signaling
pathway that regulates the EMT process (16,41,42).
TGF-β regulates the expression of EMT transcription factors and
induces EMT through cross talk with other cell factors, including
Wnt, Ras and Notch (43).
Therefore, it was hypothesized that the alterations in MCF7 cells
were associated with TGF-β1 released by Hu-ADSCs. To confirm this
hypothesis, Hu-ADSC TGF-β1 secretion was confirmed by ELISA.
Xu et al (16) identified that Hu-ADSCs regulate EMT
in MCF7 cells by paracrine and induced autocrine TGF-β signaling.
In contrast, the present study identified the signaling pathways
associated with TGF-β. Based on accumulating evidence, EMT is
closely associated with the activation of the TGF-β/Smad and
PI3K/AKT signaling pathways (11,12,14,44).
Therefore, the present study aimed to identify the association
between the activation of these pathways in Hu-ADSCs, and the
effects of Hu-ADSCs on MCF7 cells. The use of anti-TGF-β1 and PI3K
inhibitor LY294002 decreased the EMT of cocultured MCF7 cells. This
suggested that Hu-ADSCs promoted the EMT of MCF7 cells, at least in
part though the TGF-β Smad and non-Smad dependent PI3K/AKT
signaling pathways.
Furthermore, the findings of the present study
suggested that the PI3K/AKT signaling pathway may be activated by
TGF-β1 secreted by Hu-ADSCs during the promotion of breast cancer
EMT, as p-AKT expression decreased following the addition of
anti-TGF-β1 to the coculture system. This phenomenon highlighted
that suppression of the TGF-β/Smad pathway inhibited the PI3K/AKT
pathway, suggesting cross interactions between the two pathways.
The external environment tested differed from the internal
environment, even if it was close to the internal environment.
Further investigations are required to elucidate the underlying
mechanisms. Understanding the association between Hu-ADSCs and
cancer cells may provide targets for breast cancer treatment.
In conclusion, the present study examined the
interaction between Hu-ADSCs and the MCF7 cell line using a
Transwell coculture system to examine the paracrine effects of
Hu-ADSCs in tumor development. This provided evidence that Hu-ADSCs
may stimulate increased MCF7 cell migration and invasion via
alterations in E-cadherin, N-cadherin and EMT-associated
transcription factor expression, which were at least partially
mediated by the activation of the TGF-β Smad and non-Smad dependent
PI3K/AKT pathways.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81270430).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request to any scientist wishing to use them for non-commercial
purposes, without breaching participant confidentiality.
Authors' contributions
SMW and XZ were responsible for designing of the
study and critical review of manuscript. SMW, YW, ZY, QW and HD
performed the experiments. SLW and XL performed statistical
analyses. YW and QW wrote the manuscript. All authors read and
approved the final version of manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the ethical standards in the Declaration of Helsinki (1975) and was
approved by the Institutional Ethics Committee at Shengjing
Hospital of China Medical University (Shenyang, China). All donors
provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EMT
|
epithelial-mesenchymal transition
|
|
FBS
|
fetal bovine serum
|
|
Hu-ADSC
|
human adipose-derived stem cell
|
|
MSC
|
mesenchymal stem cell
|
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zare M, Bastami M, Solali S and Alivand
MR: Aberrant miRNA promoter methylation and EMT-involving miRNAs in
breast cancer metastasis: Diagnosis and therapeutic implications. J
Cell Physiol. 233:3729–3744. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iser IC, Ceschini SM, Onzi GR, Bertoni AP,
Lenz G and Wink MR: Conditioned medium from adipose-derived stem
cells (ADSCs) promotes epithelial-to-mesenchymal-like transition
(EMT-Like) in glioma cells in vitro. Mol Neurobiol. 53:7184–7199.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grzegrzolka J, Biala M, Wojtyra P,
Kobierzycki C, Olbromski M, Gomulkiewicz A, Piotrowska A, Rys J,
Podhorska-Okolow M and Dziegiel P: Expression of EMT Markers SLUG
and TWIST in breast cancer. Anticancer Res. 35:3961–3968.
2015.PubMed/NCBI
|
|
6
|
Bissell MJ and Hines WC: Why don't we get
more cancer? A proposed role of the microenvironment in restraining
cancer progression. Nat Med. 17:320–329. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Uchibori R, Tsukahara T, Mizuguchi H, Saga
Y, Urabe M, Mizukami H, Kume A and Ozawa K: NF-κB activity
regulates mesenchymal stem cell accumulation at tumor sites. Cancer
Res. 73:364–372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Melzer C, Yang Y and Hass R: Interaction
of MSC with tumor cells. Cell Commun Signal. 14:202016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu X, Zheng L, Yuan Q, Zhen G, Crane JL,
Zhou X and Cao X: Transforming growth factor-β in stem cells and
tissue homeostasis. Bone Res. 6:22018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie F, Ling L, van Dam H, Zhou F and Zhang
L: TGF-β signaling in cancer metastasis. Acta Biochim Biophys Sin
(Shanghai). 50:121–132. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smith AL, Iwanaga R, Drasin DJ, Micalizzi
DS, Vartuli RL, Tan AC and Ford HL: The miR-106b-25 cluster targets
Smad7, activates TGF-β signaling, and induces EMT and tumor
initiating cell characteristics downstream of Six1 in human breast
cancer. Oncogene. 31:5162–5171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lamouille S and Derynck R: Cell size and
invasion in TGF-beta-induced epithelial to mesenchymal transition
is regulated by activation of the mTOR pathway. J Cell Biol.
178:437–451. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schrantz N, Bourgeade MF, Mouhamad S, Leca
G, Sharma S and Vazquez A: p38-mediated regulation of an
Fas-associated death domain protein-independent pathway leading to
caspase-8 activation during TGFbeta-induced apoptosis in human
Burkitt lymphoma B cells BL41. Mol Biol Cell. 12:3139–3151. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lamouille S and Derynck R: Emergence of
the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin
axis in transforming growth factor-β-induced epithelial-mesenchymal
transition. Cells Tissues Organs. 193:8–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song K, Wang H, Krebs TL and Danielpour D:
Novel roles of Akt and mTOR in suppressing TGF-beta/ALK5-mediated
Smad3 activation. EMBO J. 25:58–69. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu Q, Wang L, Li H, Han Q, Li J, Qu X,
Huang S and Zhao RC: Mesenchymal stem cells play a potential role
in regulating the establishment and maintenance of
epithelial-mesenchymal transition in MCF7 human breast cancer cells
by paracrine and induced autocrine TGF-β. Int J Oncol. 41:959–968.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Z, Li H, Zhang D, Liu X, Zhao F, Pang
X and Wang Q: Effect of advanced glycosylation end products on
apoptosis in human adipose tissue-derived stem cells in vitro. Cell
Biosci. 5:32015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Chu Y, Yue B, Ma X, Zhang G, Xiang
H, Liu Y, Wang T, Wu X and Chen B: Adipose-derived mesenchymal stem
cells promote osteosarcoma proliferation and metastasis by
activating the STAT3 pathway. Oncotarget. 8:23803–23816.
2017.PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shamir ER and Ewald AJ: Adhesion in
mammary development: Novel roles for E-cadherin in individual and
collective cell migration. Curr Top Dev Biol. 112:353–382. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Davies LC, Heldring N, Kadri N and Le
Blanc K: Mesenchymal stromal cell secretion of programmed death-1
ligands regulates T cell mediated immunosuppression. Stem Cells.
35:766–776. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakamizo A, Marini F, Amano T, Khan A,
Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, Dembinski J, et
al: Human bone marrow-derived mesenchymal stem cells in the
treatment of gliomas. Cancer Res. 65:3307–3318. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kariminekoo S, Movassaghpour A, Rahimzadeh
A, Talebi M, Shamsasenjan K and Akbarzadeh A: Implications of
mesenchymal stem cells in regenerative medicine. Artif Cells
Nanomed Biotechnol. 44:749–757. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hong IS, Lee HY and Kang KS: Mesenchymal
stem cells and cancer: Friends or enemies? Mutat Res. 768:98–106.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yagi H and Kitagawa Y: The role of
mesenchymal stem cells in cancer development. Front Genet.
4:2612013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bajetto A, Pattarozzi A, Corsaro A,
Barbieri F, Daga A, Bosio A, Gatti M, Pisaturo V, Sirito R and
Florio T: Different effects of human umbilical cord mesenchymal
stem cells on glioblastoma stem cells by direct cell interaction or
via released soluble factors. Front Cell Neurosci. 11:3122017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takahara K, Ii M, Inamoto T, Komura K,
Ibuki N, Minami K, Uehara H, Hirano H, Nomi H, Kiyama S, et al:
Adipose-derived stromal cells inhibit prostate cancer cell
proliferation inducing apoptosis. Biochem Biophys Res Commun.
446:1102–1107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kabashima-Niibe A, Higuchi H, Takaishi H,
Masugi Y, Matsuzaki Y, Mabuchi Y, Funakoshi S, Adachi M, Hamamoto
Y, Kawachi S, et al: Mesenchymal stem cells regulate
epithelial-mesenchymal transition and tumor progression of
pancreatic cancer cells. Cancer Sci. 104:157–164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Prantl L, Muehlberg F, Navone NM, Song YH,
Vykoukal J, Logothetis CJ and Alt EU: Adipose tissue-derived stem
cells promote prostate tumor growth. Prostate. 70:1709–1715. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li X, Luo Q, Sun J and Song G: Conditioned
medium from mesenchymal stem cells enhances the migration of
hepatoma cells through CXCR4 up-regulation and F-actin remodeling.
Biotechnol Lett. 37:511–521. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Freese KE, Kokai L, Edwards RP, Philips
BJ, Sheikh MA, Kelley J, Comerci J, Marra KG, Rubin JP and Linkov
F: adipose-derived stems cells and their role in human cancer
development, growth, progression, and metastasis: A systematic
review. Cancer Res. 75:1161–1168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nieman KM, Romero IL, Van Houten B and
Lengyel E: Adipose tissue and adipocytes support tumorigenesis and
metastasis. Biochim Biophys Acta. 1831:1533–1541. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schweizer R, Tsuji W, Gorantla VS, Marra
KG, Rubin JP and Plock JA: The role of adipose-derived stem cells
in breast cancer progression and metastasis. Stem Cells Int.
2015:1209492015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park YM, Yoo SH and Kim SH:
Adipose-derived stem cells induced EMT-like changes in H358 lung
cancer cells. Anticancer Res. 33:4421–4430. 2013.PubMed/NCBI
|
|
36
|
Chou YS and Yang MH:
Epithelial-mesenchymal transition-related factors in solid tumor
and hematological malignancy. J Chin Med Assoc. 78:438–445. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yan XL, Fu CJ, Chen L, Qin JH, Zeng Q,
Yuan HF, Nan X, Chen HX, Zhou JN, Lin YL, et al: Mesenchymal stem
cells from primary breast cancer tissue promote cancer
proliferation and enhance mammosphere formation partially via
EGF/EGFR/Akt pathway. Breast Cancer Res Treat. 132:153–164. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wheelock MJ, Shintani Y, Maeda M, Fukumoto
Y and Johnson KR: Cadherin switching. J Cell Sci. 121:727–735.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brzozowa M, Michalski M, Wyrobiec G,
Piecuch A, Dittfeld A, Harabin-Slowinska M, Boron D and Wojnicz R:
The role of Snail1 transcription factor in colorectal cancer
progression and metastasis. Contemp Oncol (Pozn). 19:265–270.
2015.PubMed/NCBI
|
|
40
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Trivanovic D, Jaukovic A, Krstic J,
Nikolić S, Okić Djordjević I, Kukolj T, Obradović H, Mojsilović S,
Ilić V, Santibanez JF and Bugarski D: Inflammatory cytokines prime
adipose tissue mesenchymal stem cells to enhance malignancy of
MCF-7 breast cancer cells via transforming growth factor-β1. IUBMB
Life. 68:190–200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
McAndrews KM, McGrail DJ, Ravikumar N and
Dawson MR: Mesenchymal stem cells induce directional migration of
invasive breast cancer cells through TGF-β. Sci Rep. 5:169412015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Diaz VM, Vinas-Castells R and Garcia de
Herreros A: Regulation of the protein stability of EMT
transcription factors. Cell Adh Migr. 8:418–428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang YE: Non-Smad pathways in TGF-beta
signaling. Cell Res. 19:128–139. 2009. View Article : Google Scholar : PubMed/NCBI
|