Introduction
Primary intrahepatic stone (PIS) are rare among
patients in Western countries (1,2). As
reported in the literature, most PIS patients are Asian, and there
is a high incidence of the disease in East Asia (3,4).
Currently, PISs remain a common biliary system disease in Southwest
China (5). Approximately 200–250
surgical procedures for PISs are performed in the Southwest
Hospital, Third Military Medical University (Chongqing, China),
each year.
Relapse and persistent progress of PISs to an
advanced stage may result in biliary cirrhosis or intrahepatic
cholangiocarcinoma (6), two of the
most important causes of mortality due to benign biliary tract
disease. At present, the etiology of PISs has not been fully
elucidated; it is generally believed that chronic inflammation of
the bile duct and bile stasis promote the formation of gallstones
and that other factors such as bacterial infection, malnutrition
and living environment may also be involved (7–9). The
distribution of PIS cases in Eastern and Western countries and in
the northern and southern parts of China differs notably,
reflecting the strong racial and regional characteristics of PIS
(10). Therefore, the possible
role of genetic background in the pathogenesis of PISs is worthy of
further investigation.
The protein encoded by the ATP binding cassette
subfamily B member (ABCB)11 gene is known as the bile salt
export pump (BSEP). Its primary function is to transport bile acid
from the cell cavity to the bile duct and to facilitate cholesterol
dissolution and inhibit its supersaturated crystallization
(11–13). In a previous study of
hepatolithiasis, it was found that the mRNA and protein expression
levels of sodium/taurine co-aligners and cholesterol 7α
hydroxylase, which are associated with bile salt and cholesterol
metabolism, were significantly lower in hepatolithiasis patients
than in normal controls (14).
Therefore, this phenomenon needs to be investigated further.
It has been demonstrated that mutation of the
ABCB11 gene is closely associated with cholestasis,
including benign recurrent intrahepatic cholestasis 2 (15,16),
progressive familial intrahepatic cholestasis 2 (17–19),
and intrahepatic cholestasis of pregnancy (20–22),
and bile duct stones often form in patients with BSEP deletion
syndrome caused by ABCB11 gene mutations (23). In our previous study (5), two missense (rs2287617 and rs2287622)
and one synonymous (rs3815675) mutations were detected in exons 9,
13 and 4, respectively. However, the distribution of allele
frequencies for these three mutations was not significantly
different between patients and controls. Conversely, one missense
mutation (rs118109635) and two synonymous mutations (rs497692 and
rs2287616) were also detected in exons 21, 24 and 9, respectively.
The distribution of these allele frequencies were significantly
different in PIS patients compared with those in the healthy group.
The two mutations (rs118109635 and rs497692) in ABCB11 as well as
decreased expression of its encoded protein BSEP, were closely
associated with the recurrence of cholangitis and the level of
jaundice of the PIS patients (5).
The aim of the present study was to further verify the association
between ABCB11 gene mutations and clinical morbidity in PIS
patients on the basis of an expanded sample size. In addition, the
present study focused on the observation of the effects of
ABCB11 gene mutations on BSEP expression and on its
distribution in the cell membrane. The mRNA and protein expression
levels of the ABCB11 gene in the liver tissues of PIS
patients were determined using quantitative polymerase chain
reaction (PCR) and western blot analysis. To further investigate
the effect of these mutations on the protein's expression and
distribution, a wild-type plasmid containing all open reading
frames of the human ABCB11 gene was constructed, and mutant
plasmids were subsequently constructed by site-directed
mutagenesis. After transfection of 293 cells with the mutant
plasmids, changes in the expression of ABCB11 mRNA and cell
membrane BSEP were detected. Subsequently, based on the polar
characteristics of Madin-Darby canine kidney (MDCK) cells, the
effect of the ABCB11 gene mutation on the distribution of
BSEP at the cellular level was analyzed using immunofluorescence
and laser confocal scanning microscopy.
Materials and methods
Clinical samples
A total of 443 patients (male, 134; female, 309; age
range 17–81 years; male/female, 0.434) with PISs treated in the
Southwest Hospital, Third Military Medical University (Chongqing,
China) from December 2012 to May 2016 were included in the present
study. Furthermore, 560 healthy individuals (male 245; female 315;
age range 16–78 years; male/female, 0.778) were selected from the
physical examination center of the same hospital as the control
group. The inclusion criteria of the experimental group were
confirmed diagnosis of PISs by imaging study; no prior liver
resection, no history of chronic liver disease, no hepatitis A, B,
or C virus or cytomegalovirus infection, no gallstones, and no
tumors. The inclusion criteria of the control group were confirmed
absence of PISs by imaging, no history of chronic liver disease, no
hepatitis A, B, or C virus or cytomegalovirus infection, and no
gallstones. Five milliliters of peripheral blood were collected
from each of the above PIS patients and healthy participants, and
the lymphocytes were immediately separated using human peripheral
lymphocyte separation medium (Tianjin Hao Yang Biological
Manufacturing Co., Ltd., Tianjin, China; http://www.tbdscience.com). The obtained samples were
stored in a −80°C freezer.
Clinical data, including information on sex, age,
height, body weight, indicators in the preoperative liver function
test, preoperative serum jaundice, recurrent cholangitis, and stone
type were collected (Tables I and
II). Stone removal of the PIS
patients was confirmed by abdominal ultrasound, abdominal CT,
cholangiography and magnetic resonance imaging. Postoperatively,
the patients were followed up every three months, and a stone found
at follow-up was considered as a recurrence. The experiments were
approved by the Ethics Committee of the Southwest Hospital of the
Third Military Medical University of China. Written informed
consent was obtained from all participants.
 | Table I.Clinical data of patients with
primary intrahepatic stones. |
Table I.
Clinical data of patients with
primary intrahepatic stones.
| Parameters | Patients, n | Median (range) |
|---|
| Sex,
male/female | 134/309 |
|
| Age, male/female
(years) | 134/309 | 52 (17–81)/49
(17–78) |
| Height, male/female
(cm) | 134/309 | 167 (155–187)/155
(144–176) |
| Weight, male/female
(kg) | 134/309 | 60 (41–93)/51
(36–80) |
| Glutamic-pyruvic
transaminase (IU/l) | 443 | 39.0
(4.9–587.0) |
| Glutamic oxalacetic
transaminase (IU/l) | 443 | 38.0
(10.0–621.0) |
| Glutamyl
transpeptidase (IU/l) | 443 | 165.0
(7.0–1968.0) |
| Alkaline
phosphatase (IU/l) | 443 | 167.0
(31.0–1527.0) |
| Total protein
(g/l) | 443 | 71.2
(36.2–87.2) |
| Albumin (g/l) | 443 | 40.0
(20.4–60.0) |
| Alobulin (g/l) | 443 | 31.2
(14.4–50.0) |
| Total bilirubin
(µmol/l) | 443 | 16.4
(5.4–460.4) |
| Direct bilirubin
(µmol/l) | 443 | 3.8
(0.4–271.9) |
| Indirect bilirubin
(µmol/l) | 443 | 12.1
(3.7–196.6) |
| Total bile acid
(µmol/l) | 443 | 5.1
(0.2–261.6) |
 | Table II.Genotype distribution in groups of
recurrence of cholangitis, preoperative jaundice, types of
gallstone or recurrence of PIS. |
Table II.
Genotype distribution in groups of
recurrence of cholangitis, preoperative jaundice, types of
gallstone or recurrence of PIS.
|
| Recurrence of
cholangitis | Preoperative
jaundice | Types of
gallstone | Recurrence of
PIS |
|---|
|
|
|
|
|
|
|---|
| Mutation (n) | Yes 309 (%) | No 134 (%) | P-value | Yes 143 (%) | No 300 (%) | P-value | CBS 420 (%) | CS 23 (%) | P-value | Yes 95 (%) | No 348 (%) | P-value |
|---|
| RS3815675T
>C |
|
|
|
|
|
|
|
|
|
|
|
|
| TT
(n=218) | 154 (49.8) | 64 (47.8) | 0.222 | 71 (49.7) | 147 (49.0) | 0.895 | 209 (49.8) | 9 (39.1) | 0.426 | 46 (48.4) | 172 (49.4) | 0.266 |
| TC
(n=179) | 128 (41.4) | 51 (38.1) |
| 56 (39.2) | 123 (41.0) |
| 169 (40.2) | 10 (43.5) |
| 43 (45.3) | 136 (39.1) |
|
| CC
(n=46) | 27 (8.7) | 19 (14.2) |
| 16 (11.1) | 30 (10.0) |
| 42 (10.0) | 4 (17.4) |
| 6 (6.3) | 40 (11.5) |
|
| RS2287616T
>C |
|
|
|
|
|
|
|
|
|
|
|
|
| TT
(n=210) | 148 (47.9) | 62 (46.3) | 0.143 | 68 (47.6) | 142 (47.3) | 0.998 | 201 (47.9) | 9 (39.1) | 0.493 | 48 (50.5) | 162 (46.6) | 0.303 |
| TC
(n=186) | 134 (43.4) | 52 (38.8) |
| 60 (42.0) | 126 (42.0) |
| 176 (41.9) | 10 (43.5) |
| 41 (43.2) | 145 (41.7) |
|
| CC
(n=47) | 27 (8.7) | 20 (14.9) |
| 15 (10.5) | 32 (10.7) |
| 43 (10.2) | 4 (17.4) |
| 6 (6.3) | 41 (11.8) |
|
| RS2287617G
>A |
|
|
|
|
|
|
|
|
|
|
|
|
| GG
(n=440) | 308 (99.7) | 132 (98.5) | 0.168 | 141 (98.6) | 299 (99.7) | 0.510 | 418 (99.5) | 22 (95.7) | 0.369 | 92 (96.8) | 348 (100) | 0.009 |
| GA
(n=3) | 1 (0.3) | 2 (1.5) |
| 2 (1.4) | 1 (0.3) |
| 2 (0.5) | 1 (4.3) |
| 3 (3.2) | 0 (0.0) |
|
| AA
(n=0) | 0 (0.0) | 0 (0.0) |
| 0 (0.0) | 0 (0.0) |
| 0 (0.0) | 0 (0.0) |
| 0 (0.0) | 0 (0.0) |
|
| RS2287622T
>C |
|
|
|
|
|
|
|
|
|
|
|
|
| TT
(n=39) | 25 (8.1) | 14 (10.4) | 0.621 | 15 (10.5) | 24 (8.0) | 0.679 | 38 (9.0) | 1 (4.4) | 0.693 | 12 (12.6) | 27 (7.8) | 0.061 |
| TC
(n=187) | 134 (43.4) | 53 (39.6) |
| 60 (42.0) | 127 (42.3) |
| 176 (41.9) | 11 (47.8) |
| 46 (48.4) | 141 (40.5) |
|
| CC
(n=217) | 150 (48.5) | 67 (50.0) |
| 68 (47.6) | 149 (49.7) |
| 206 (49.0) | 11 (47.8) |
| 37 (38.9) | 180 (51.7) |
|
|
RS118109635C>T |
|
|
|
|
|
|
|
|
|
|
|
|
| CC
(n=423) | 295 (95.5) | 128 (95.5) | 0.980 | 132 (92.3) | 291 (97.0) | 0.026 | 402 (95.7) | 21 (91.3) | 0.634 | 90 (95.5) | 333 (95.7) | 0.692 |
| CT
(n=20) | 14 (4.5) | 6 (4.5) |
| 11 (7.7) | 9 (3.0) |
| 18 (4.3) | 2 (8.7) |
| 5 (5.3) | 15 (4.3) |
|
| TT
(n=0) | 0 (0.0) | 0 (0.0) |
| 0 (0.0) | 0 (0.0) |
| 0 (0.0) | 0 (0.0) |
| 0 (0.0) | 0 (0.0) |
|
| RS497692A
>G |
|
|
|
|
|
|
|
|
|
|
|
|
| AA
(n=55) | 37 (12.0) | 18 (13.4) | 0.828 | 13 (9.1) | 42 (14.0) | 0.011 | 51 (12.1) | 4 (17.4) | 0.599 | 13 (13.7) | 42 (12.1) | 0.560 |
| AG
(n=194) | 138 (44.7) | 56 (41.8) |
| 53 (37.1) | 141 (47.0) |
| 183 (43.6) | 11 (47.8) |
| 45 (47.4) | 149 (42.8) |
|
| GG
(n=194) | 134 (43.4) | 60 (44.8) |
| 77 (53.8) | 117 (39.0) |
| 186 (44.3) | 8 (34.8) |
| 37 (38.9) | 157 (45.1) |
|
Genomic DNA extraction, primer design
and ABCB11 gene exon sequencing
The Blood Genome DNA Extraction kit (Takara Bio,
Inc., Otsu, Japan) was used to extract genomic DNA from the
peripheral blood lymphocytes of patients and controls. In the 28
exon coding regions of the ABCB11 gene, the first exon does
not participate in the coding of the protein, so Primer 6.0 primer
design software (primerdesign.co.uk/home) was used to design 27 pairs
of primers for the PCR amplification of the remaining exons
(Table III). The PCR
amplification was performed with the PrimeSTAR Max DNA Polymerase
kit (Takara Bio, Inc.) and the cycling conditions were as follow:
30 cycles of 98°C for 10 sec, 55°C for 5 sec and 72°C for 30 sec.
The amplified products were then sequenced using the ABI 3730XL
Gene Analyzer (Applied Biosystems; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
 | Table III.Primer pairs for the amplification of
the ABCB11 coding sequence. |
Table III.
Primer pairs for the amplification of
the ABCB11 coding sequence.
| Exon | Forward primer | Reverse primer | Fragment length
(bp) |
|---|
| ABCB11 |
| 2 |
5′-GGCTCTTTCAGGGAGTTATTAACC-3′ |
5′-ACTTGACCAGCTTGTCCTACTT-3′ | 335 |
| 3 |
5′-AGAGACAATATGAGCAGGAAGA-3′ |
5′-CTGCTTTGTGCCTTTGATATGA-3′ | 266 |
| 4 |
5′-TCTGTGAATCGCTAGTGAACCT-3′ |
5′-ACACCCACTGCCATAAATCAAC-3′ | 313 |
| 5 |
5′-ATACGAACTCTGCCACTCAATT-3′ |
5′-GTTAGATACCACTCCAGCTCAG-3′ | 492 |
| 6 |
5′-AATGTAATCTCTGGTGGCTTGA-3′ |
5′-TGTAGTTCTTAGGGCTTCTGAT-3′ | 262 |
| 7 |
5′-CTTAGTTCCCAAGAAGAGGCATT-3′ |
5′-CACACCAAATTGCAGTACCTTG-3′ | 487 |
| 8 |
5′-GAGAGGCTGTTAATGCTATCCA-3′ |
5′-TGTTGCTAACTGTACTCAGGAA-3′ | 410 |
| 9 |
5′-TCTTCCTCCTGTCAATGATGTTAC-3′ |
5′-ATTACTCTGCTTAGCTCCCTCTT-3′ | 412 |
| 10 |
5′-TGCTCTGTGTTTGCGATGATTT-3′ |
5′-TGTTTCCACAGACAGACTCCATA-3′ | 435 |
| 11 |
5′-TCTCTGCGTTAACATGGAAGAC-3′ |
5′-CAAGAGCGAAACTCCATCTCAA-3′ | 418 |
| 12 |
5′-GCAGAGATACGCCAAAGATGTT-3′ |
5′-AAGACACCTCCATTCCCTATTACT-3′ | 337 |
| 13 |
5′-CACAGACACCGAGTATCAACAC-3′ |
5′-CCAGGACAGTCTCAATGTATGC-3′ | 332 |
| 14 |
5′-TTTCTGCCCATTGGTCAAGTAT-3′ |
5′-CTCTTAGTTTCTCCCAGGAATGTA-3′ | 331 |
| 15 |
5′-GATCACTGTCAGAAGCCATCAA-3′ |
5′-TATCAACTACTCCCATCCCTCC-3′ | 336 |
| 16 |
5′-TCTAATGTCTGCACAGCCTATT-3′ |
5′-GTTGGGAGAACAGTGAGTATTGA-3′ | 441 |
| 17 |
5′-TTGCTACTTCTGATGGACTTCTC-3′ |
5′-AGGATTAGGACTACAGAGGACTC-3′ | 437 |
| 18 |
5′-AACTTGGACACCAGTTGATCCT-3′ |
5′-TAGTCTGACTTGAAACACTGCTAG-3′ | 300 |
| 19 |
5′-CCATATCCCATAGACATTTGAGGT-3′ |
5′-ATGAGAAGAAGAAAGCTAGTCCAG-3′ | 335 |
| 20 |
5′-CCACCAGAATGATACATTTCCTAC-3′ |
5′-TGAAGAGGGAGATGTTAGAGAA-3′ | 405 |
| 21 |
5′-GCAATGGGCTGTGTATCTCTTT-3′ |
5′-GTCAGTGTTAGAAGCAGTGGAA-3′ | 444 |
| 22 |
5′-TCTGAGACGGGTTGATTGCTTT-3′ |
5′-GCTTCCTTCAGTCTCTTCGTACTA-3′ | 331 |
| 23 |
5′-CCACTGAAATGTCACGAAAGGA-3′ |
5′-TGGAGACAGAAGAATACACAGAAG-3′ | 524 |
| 24 |
5′-ATGCTTGTTCAGTCCTCTTCTT-3′ |
5′-CCTGTGTCCATGTGTTCTGTT-3′ | 560 |
| 25 |
5′-TGAAGGTATCTCAAGCAGGGATT-3′ |
5′-AAAGTGAGTCTGGCAAAGCAAA-3′ | 397 |
| 26 |
5′-TTAGCCTTGGGATTGTTAGTCTG-3′ |
5′-CACTCTGGTCATTCTACTTCTCC-3′ | 402 |
| 27 |
5′-GAGGAGACCTTGACATGAGTTC-3′ |
5′-GGTTCCACAAAGTATTGCCAAT-3′ | 362 |
| 28 |
5′-GGATTGTTATTCAGGTCGTGTT-3′ |
5′-TTAGCTTGGATTCCGATGTAGG-3′ | 462 |
Extraction of mRNA and reverse
transcription-quantitative PCR (RT-qPCR)
Total mRNA was extracted from the 200 mg freshly
frozen liver tissue of the PIS patients and the control group using
the Eastep Super Total RNA Extraction kit (Promega Corporation,
Madison, WI, USA). PIS patient and control group liver tissue
samples were cryopreserved in liquid nitrogen at the time of
excision to facilitate RNA extraction and RT-qPCR analysis. The
specific experimental procedure was conducted according to the
manufacturer's protocol. DNA removal and reverse transcription were
performed with the PrimeScript™ RT Reagent kit with gDNA
Eraser (Takara Bio, Inc.) at 42°C for 2 min. Finally, the mRNA
expression of the ABCB11 gene in the PIS patients was
detected by RT-qPCR using SYBR Premix Ex Taq II (Takara Bio, Inc.)
and a quantitative fluorescence PCR analyzer.
Total RNA was extracted from 293 cells 48 h after
transfection with wild-type and mutant A856V plasmids using the
Eastep Super Total RNA Extraction kit (Promega Corporation),
followed by DNA removal and reverse transcription using the
PrimeScript™ RT Reagent kit with gDNA Eraser (Takara
Bio, Inc). The mRNA expression was detected by RT-qPCR using SYBR
Premix Ex Taq II (Takara Bio, Inc.) and a quantitative fluorescence
PCR analyzer.
The results of freshly frozen liver tissues and
cells were normalized to the expression of β-actin. The primers for
BSEP were forward primer, 5′-dTTGCCTTTGCCCAGTGCATCAT-3′ and reverse
primer, 5′-dGGTTGTCGGTCCAGCAGTTGAA-3′ (BSEP), and those for β-actin
were forward primer, 5′-dCCTGGCACCCAGCACAAT-3′ and reverse primer,
5′-dGGGCCGGACTCGTCATAC-3′. For the quantitative measurement of
ABCB11 mRNA expression, RT-qPCR was performed using a CFX96
Real-Time system (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
with the following cycling conditions: 95°C for 30 sec, followed by
40 cycles of 95°C for 5 sec and 60°C for 30 sec (24).
Western blot analysis
Membrane proteins were extracted from the liver
tissues of PIS patients and 293 cells transfected with wild-type
and mutant A856V ABCB11, using the Mem-PER™ Plus
Membrane Protein Extraction kit (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The Bicinchoninic Protein
Quantification kit (CWBiotech, Beijing, China) was used for protein
concentration determination. Equal amounts of protein (20 µg) were
separated by 6% SDS-PAGE and transferred to polyvinylidene fluoride
(PVDF) membranes (EMD Millipore, Billerica, MA, USA), which were
blocked and incubated with primary antibodies overnight at 4°C. The
primary antibodies used in this study included the following:
Anti-BSEP (1:500; cat. no. 155421; Abcam, Cambridge, UK) and
anti-Na/K-ATPase (1:1,000; cat. no. 58475; Abcam). The membranes
were washed with Tris-buffered saline/Tween-20 and incubated with a
horseradish peroxidase (HRP)-conjugated secondary antibody for 2 h
at room temperature. The SuperSignal West Femto Substrate Trial kit
(Thermo Fisher Scientific, Inc.) was used for signal detection.
Plasmids and site-directed
mutagenesis
The wild-type plasmid pEZ-M02-BSEP contains all the
open reading frames (GeneCopoeia Inc., Rockville, MD, USA) of the
ABCB11 gene. This plasmid was used as a template to obtain
the A856V mutation by site-directed mutagenesis (25). The ABCB11 coding sequences
of the above plasmids were verified by sequencing.
Cell culture and plasmid
transfection
A total of 293 cells and MDCK cells obtained
from the Cell Bank of the Chinese Academy of Sciences were cultured
in Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc.) with 1% penicillin-streptomycin solution
(Beyotime Institute of Biotechnology, Haimen, China) and 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a
5% CO2 humidified atmosphere. A total of
5×105 293 cells were seeded in 6-well plates and
transiently transfected with 2,500 ng DNA and
Lipofectamine® (Invitrogen; Thermo Fisher Scientific,
Inc.) per well after 24 h at 37°C humidified atmosphere containing
5% CO2 when the cell confluence reached 80%. A total of
1×105 MDCK cells were seeded in 24-well plates and
transiently transfected with 500 ng of DNA and
Lipofectamine® (Invitrogen; Thermo Fisher Scientific,
Inc.) per well after 24 h at 37°C humidified atmosphere containing
5% CO2 when the cell confluence reached 70%. The
transfected cells were incubated at 37°C humidified atmosphere
containing 5% CO2, and were harvested 48 h later for
subsequent experiments.
Immunofluorescence and laser confocal
microscopy
The MDCK cells were seeded on slides 24 hour at room
temperature prior to transfection. Forty-eight hours after
transfection, the cells were fixed with 2% paraformaldehyde for 10
min at room temperature and infiltrated with 1% Triton X-100
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 5 min at room
temperature, followed by blocking with 10% goat serum (Gibco;
Thermo Fisher Scientific, Inc.) for 1 h at room temperature.
Following rinsing with PBS, one group of 1×105 cells was
incubated with rabbit anti-BSEP polyclonal antibody (1:100; cat.
no. 155421; Abcam) and chicken anti-Na/K-ATPase monoclonal antibody
(1:200; cat. no. 353; Abcam) overnight at 4°C, then washed with PBS
and incubated with goat anti-rabbit Alexa Fluor 488 (1:200; cat.
no. 150077; Abcam) and goat anti-chicken Alexa Fluor 647 (1:200;
cat. no. 150171; Abcam) at room temperature for 2 h. Another group
of 1×105 cells was incubated with rabbit anti-BSEP
polyclonal antibody (1:100; cat. no. 155421) and mouse
anti-calnexin polyclonal antibody (1:200; cat. no. 112995)
overnight at 4°C, then washed with PBS and incubated with goat
anti-rabbit Alexa Fluor 488 (1:200; cat. no. 150077) and goat
anti-mouse Alexa Fluor 647 (1:200; cat. no. 150115; all Abcam) at
room temperature for 2 h. Images of the cells were obtained using a
Leica CTR 4000 laser scanning confocal microscope.
Statistical analysis
The genotype frequencies at all mutation loci were
directly obtained by counting and the use of the Hardy-Weinberg
equilibrium. The unconditional logistic regression model was used
to evaluate the correlation between the ABCB11 gene
mutations and PISs. The evaluation indicators included odds ratio
(OR) and 95% confidence interval (CI); the odds ratio (OR) was
corrected for sex, age and body mass index. The correlation between
the genotype and the clinical data of the PIS patients was analyzed
using the Chi-square test. All experimental data were analyzed by
SPSS software (PASW Statistics 22, IBM Corp., Armonk, NY, USA) or
Graphpad Prism (v6.0 for Windows; GraphPad Software, Inc., La
Jolla, CA, USA). Data are presented as the mean ± standard
deviation of four independent experiments. Statistical analyses
shown in the figures were performed using paired Student's t-tests
or one-way analysis of variance with least significant difference
post hoc test. P<0.05 was considered to indicate a statistically
significant result.
Results
ABCB11 gene mutations may be
associated with the pathogenesis of PISs
In the present study, peripheral blood lymphocytes
were collected from 443 PIS patients and 560 healthy individuals
for ABCB11 exon sequencing. Six mutations were detected,
including three missense mutations and three synonymous mutations
(Table IV). The missense mutation
rs118109635 and the synonymous mutation rs497692 were detected
within exons 21 and 24, of the ABCB11 gene, respectively.
The gene frequency distributions of the two mutational loci in the
PIS patients and the healthy control group differed significantly
(P=0.025 and P=0.017, respectively). In addition, the missense
mutations rs2287617 and rs2287622 and the synonymous mutations
rs3815675 and rs2287616 were located in exons 9, 13, 4 and 9 of the
ABCB11 gene, respectively. However, these four mutations
showed no significant differences between PIS patients and healthy
controls (P>0.05). These results indicated that there are two
significant ABCB11 gene mutations in the PIS patients
(rs118109635 and rs497692), consistent with previous findings
(5).
 | Table IV.Distribution of six mutations, and
the association between the mutations and PIS. |
Table IV.
Distribution of six mutations, and
the association between the mutations and PIS.
| Mutation | Amino acid
change | Genotype | Controls (%)
n=560 | Cases (%)
n=443 | Adjusted OR (95%
CI) | P-value | P-value for HWE in
control |
|---|
| s3815675 | Synonymous | TT | 280 (50.0) | 218 (49.2) | Ref. | Ref. | 0.308 |
|
|
| TC | 227 (40.5) | 179 (40.4) | 0.98
(0.73–1.30) | 0.877 |
|
|
|
| CC | 53 (9.5) | 46 (10.4) | 1.15
(0.72–1.83) | 0.569 |
|
|
|
| TC+TT | 507 (90.5) | 397 (89.6) | 0.86
(0.55–1.36) | 0.524 |
|
| rs2287616 | Synonymous | TT | 277 (49.4) | 210 (47.4) | Ref. | Ref. | 0.545 |
|
|
| TC | 225 (40.2) | 186 (42.0) | 1.06
(0.79–1.41) | 0.694 |
|
|
|
| CC | 58 (10.4) | 47 (10.6) | 1.08
(0.69–1.72) | 0.715 |
|
|
|
| TC+TT | 502 (89.6) | 396 (89.4) | 0.94
(0.61–1.46) | 0.793 |
|
| rs2287617 | R299k | GG | 555 (99.1) | 440 (99.3) | Ref. | Ref. | 0.943 |
|
|
| GA | 5 (0.9) | 3 (0.7) | 0.65
(0.14–3.06) | 0.582 |
|
|
|
| AA | 0 (0.0) | 0 (0.0) | NA | NA |
|
|
|
| GA+AA | 5 (0.9) | 3 (0.7) | 0.65
(0.14–3.06) | 0.582 |
|
| rs2287622 | V444A | TT | 50 (8.9) | 39 (8.8) | Ref. | Ref. | 0.886 |
|
|
| TC | 237 (42.3) | 187 (42.2) | 0.91
(0.55–1.51) | 0.709 |
|
|
|
| CC | 273 (48.8) | 217 (49.0) | 0.92
(0.56–1.52) | 0.750 |
|
|
|
| TC+TT | 287 (51.2) | 226 (51.0) | 1.00
(0.76–1.31) | 0.992 |
|
| rs118109635 | A865V | CC | 551 (98.4) | 423 (95.5) | Ref. | Ref. | 0.627 |
|
|
| CT | 9 (1.6) | 20 (4.5) | 2.67
(1.13–6.31) | 0.025 |
|
|
|
| TT | 0 (0.0) | 0 (0.0) | NA | NA |
|
|
|
| CT+TT | 9 (1.6) | 20 (4.5) | 2.67
(1.13–6.31) | 0.025 |
|
| rs497692 | Synonymous | AA | 106 (18.9) | 55 (12.4) | Ref. | Ref. | 0.549 |
|
|
| AG | 249 (44.5) | 194 (43.8) | 1.44
(0.96–2.17) | 0.079 |
|
|
|
| GG | 205 (36.6) | 194 (43.8) | 1.79
(1.18–2.70) | 0.006 |
|
|
|
| AG+GG | 454 (81.1) | 388 (87.6) | 1.60
(1.08–2.34) | 0.017 |
|
Correlation of ABCB11 gene mutations
and clinical characteristics of PIS patients
The analysis of the ABCB11 gene mutations and
the relevant clinical data for the PIS patients in the present
study (Table II) showed that the
genotype of the rs118109635 mutation (CT genotype) of the
ABCB11 gene was correlated with preoperative jaundice
(P=0.026) and that the homozygous genotype (GG) and the
heterozygous genotype (GA) caused by mutations at the rs497692
locus in the PIS patients in the present study were associated with
preoperative jaundice (P=0.011). In addition, the mutation at the
rs2287617 locus of the ABCB11 gene was associated with the
recurrence of PISs (P=0.009). Therefore, the rs118109635 and
rs497692 mutations of the ABCB11 gene are closely associated
with the clinical characteristics of PISs.
Effects of ABCB11 gene mutations in
PIS patients on the expression of ABCB11 mRNA and its encoded
protein
The expression of ABCB11 mRNA in the liver
tissues of PIS patients with different genotypes was measured by
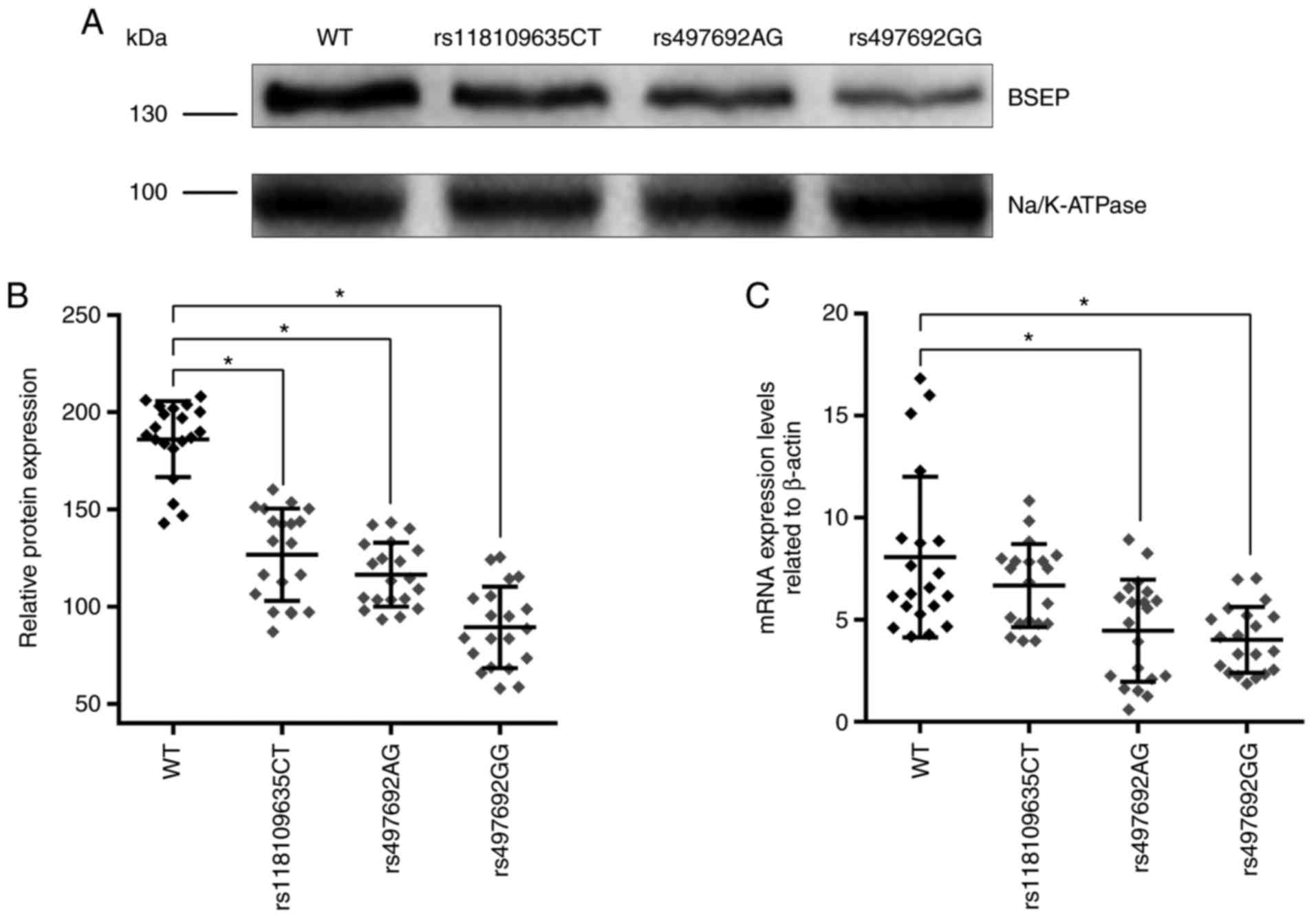
RT-qPCR. The results showed that whereas the mRNA expression levels
of the mutant rs497692 were decreased, the rs118109635 mutation had
no significant impact on ABCB11 mRNA expression levels
(Fig. 1C). The expression of BSEP
in the liver tissue of PIS patients with different genotypes was
detected by western blot analysis; BSEP was detected as a 140–150
kDa band (Fig. 1A). The results
revealed that the expression of BSEP was significantly decreased in
patients with the ABCB11 gene mutations rs118109635 and
rs497692 (Fig. 1A and B). Thus,
the ABCB11 gene mutations (rs118109635 and rs497692) may
affect the transcription and translation of the ABCB11
gene.
Expression of mutated BSEP on the 293
cell membrane
The aim of this experiment was to investigate the
effect of ABCB11 gene mutation on BSEP expression and its
subcellular localization. Therefore, 293 and MDCK cell lines were
used, albeit neither express BSEP protein, as experimental cells,
and investigated the effect of ABCB11 gene mutations on
protein expression and subcellular localization by transfection of
ABCB11 mutant plasmid and wild-type plasmid. Due to the high
expression of BSEP protein in liver cell lines, it may interfere
with the observation of cell transfection experiment results. The
293 and MDCK cells are polarized cells commonly used to study
trafficking of membrane proteins (17,23).
The transfection efficiency in MDCK cells was quite low (25). Thus, to determine the effects of
ABCB11 mutations on BSEP expression, 293 cells were used.
Plasmids containing wild-type and mutated BSEP cDNA were
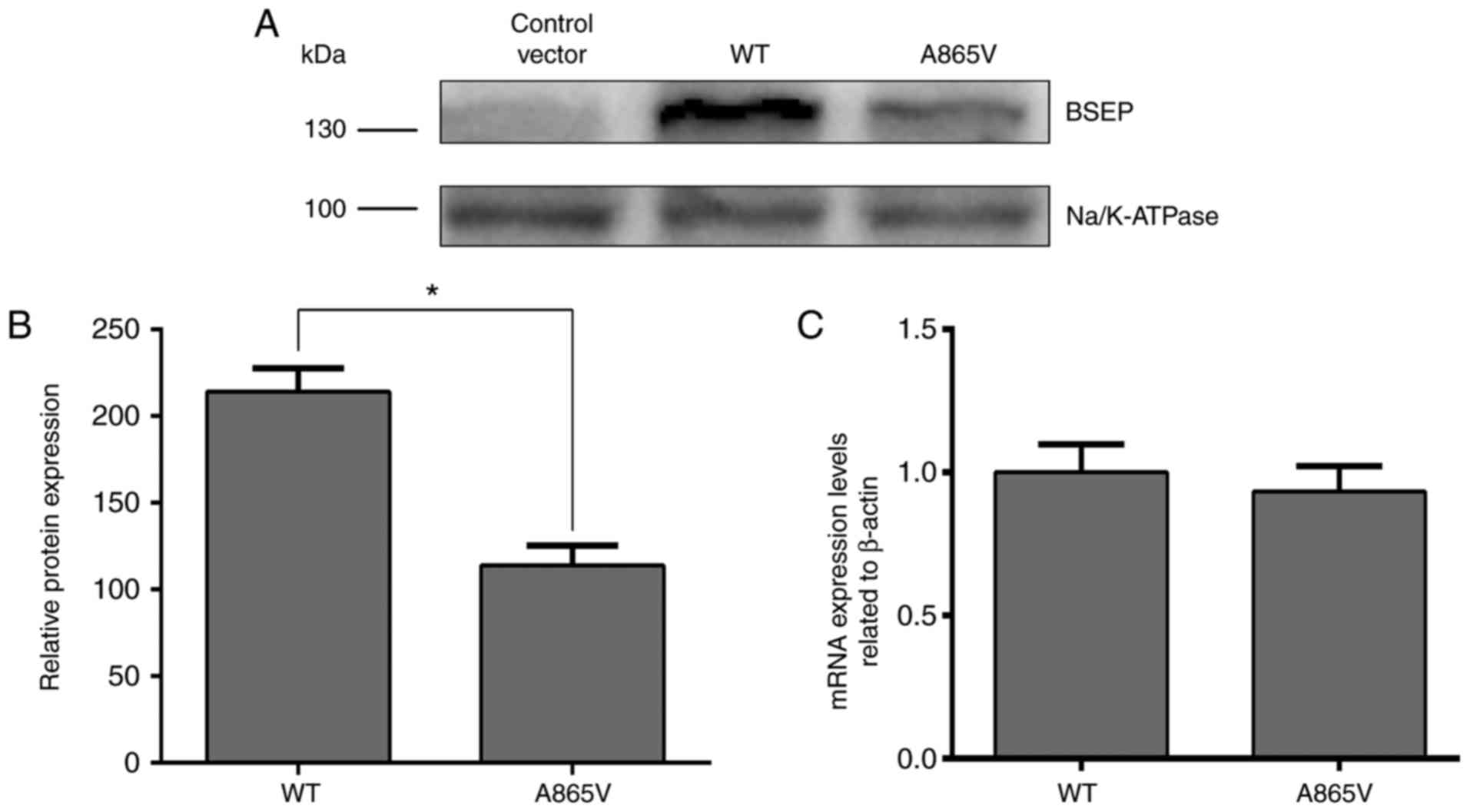
constructed and transfected into 293 cells (25) to determine the effects of the A865V
mutation on BSEP expression. The expression of BSEP in the A865V
mutant measured by western blot analysis was significantly lower
than that in wild-type 293 cells (Fig.
2A and B). Although the A865V mutation had no apparent effect
on BSEP mRNA expression levels (Fig.
2C), the decrease in BSEP protein expression levels may be
associated with the degradation of BSEP due to its retention in the
endoplasmic reticulum as a result of a change in its
three-dimensional structure (17).
Thus, the above results indicated that the A865V mutation
downregulated the translocation of the BSEP protein.
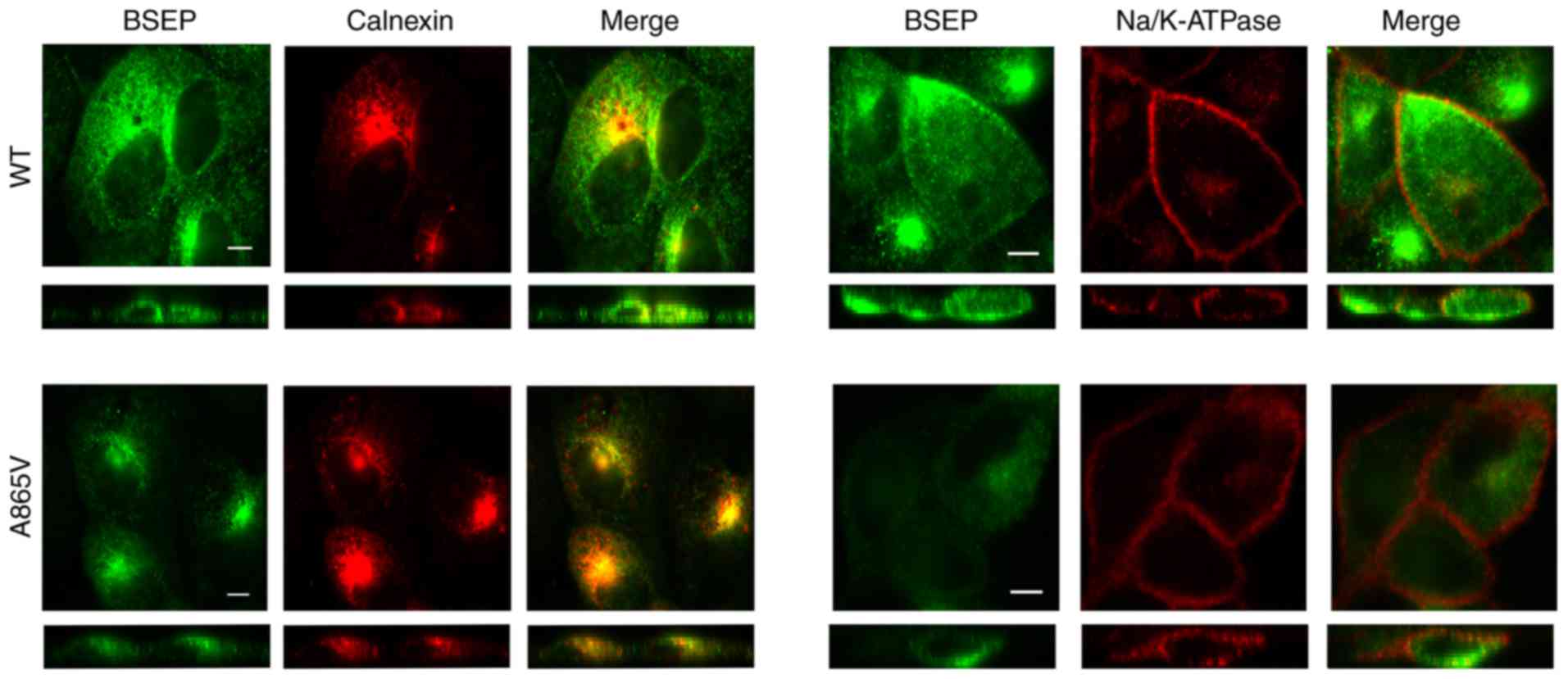
Subcellular localization of wild-type
and mutant BSEP
The cells used in this experiment needed to undergo
multiple antibody incubation and PBS washing steps. The 293 cells
have poor adhesion and can easily be detached from the slide during
multiple incubation and washing (25). Thus, the impact of ABCB11
mutations against BSEP subcellular localization was assessed in
MDCK cells. MDCK cells were transiently transfected with wild-type
and mutant plasmids, and the expressed proteins were labeled and
stained with antibodies to BSEP, calnexin and Na/K-ATPase to
determine the subcellular location of BSEP by immunofluorescence
confocal microscopy. Calnexin and Na/K-ATPase were used as markers
for the endoplasmic reticulum and the cell membrane, respectively.
Results demonstrated that wild-type BSEP was distributed inside the
cells and in the cell membrane, whereas the A856V mutant BSEP was
mainly distributed inside the cells and showed significantly
reduced distribution in the cell membrane (Fig. 3). Thus, the A856V mutation affected
the subcellular localization of BSEP.
Discussion
The present study demonstrated that two mutations in
the ABCB11 gene, rs118109635 (C>T) and rs497692 (A>G),
are associated with the pathogenesis of PISs, and these mutations
are closely associated with the occurrence of preoperative jaundice
in patients with PISs. Compared with the wild-type protein, the
expression of BSEP in the liver tissues of patients with mutations
(rs118109635 and rs497692) was significantly decreased, and the
decrease in protein expression levels was evident is the rs497692
homozygous genotype. Although the ABCB11 mRNA expression
levels of the mutant rs497692 heterozygote and homozygous genotypes
were both decreased, the rs118109635 mutation had no significant
effect on mRNA expression. The in vitro experiments showed
that the A865V mutation significantly decreased BSEP protein
expression levels in the cell membrane, indicating that the A865V
mutation may affect the subcellular localization of BSEP.
According to existing reports, the incidence of PISs
is high in East Asia, and its incidence in Southern China is
significantly higher than in Northern China; thus, it is believed
that the incidence of PISs displays very strong regional and ethnic
differences (1). The identified
mutations in the ABCB11 gene lead to defects in BSEP protein
expression that can cause changes in bile salt content (26), and it has been shown that mutations
in this gene are associated with the pathogenesis of cholestasis
(27–29). Therefore, the role of ABCB11
gene mutations in the pathogenesis of PISs was investigated. In the
present study, ABCB11 gene exon sequencing was performed on
a large sample of 443 cases of PIS patients and 560 healthy
individuals. Two mutations associated with the pathogenesis of
PISs: The missense mutation rs118109635 and the synonymous mutation
rs497692. This result is consistent with the findings reported in a
previous study (5). Additionally,
analysis of the relevant clinical data showed that the
ABCB11 gene mutations rs118109635 and rs497692 were
correlated with the occurrence of preoperative jaundice in PIS
patients. Therefore, the role of ABCB11 gene mutations in
the pathogenesis of PISs is worthy of further investigation.
To further clarify the association between mutations
rs118109635 and rs497692 and the pathogenesis of PISs, the mRNA
expression levels of ABCB11 in the liver tissue of the PIS
patients was measured. The results showed that the missense
mutation rs118109635 had no significant impact on ABCB11
mRNA expression, but western blot analysis showed that BSEP
expression levels were significantly reduced in patients with this
mutation. Western blot analysis and confocal immunofluorescence
microscopy experiments performed in vitro confirmed that
mutation A865V (rs118109635) significantly decreased BSEP
expression levels at the subcellular levels, with no significant
change in the ABCB11 mRNA expression levels. Thus, the
rs118109635 mutation downregulated the expression of the
transmembrane transporter BSEP in the cell membrane. Previous
studies of ABCB11 gene mutations confirmed that
ABCB11 gene missense mutations change the amino acid
sequence of the BSEP protein and alter its three-dimensional
structure (30). Most misfolded
proteins are degraded by proteases in the endoplasmic reticulum,
ultimately leading to reduced protein expression levels of BSEP in
the cell membranes (17).
Therefore, the missense mutation rs118109635 may be involved in the
pathogenesis of PISs due to its effect on the expression of BSEP;
this hypothesis awaits further confirmation.
Furthermore, to determine the role of mutation
rs497692 in the pathogenesis of PISs, PIS clinical specimens were
analyzed by RT-qPCR. The results showed that the expression mRNA
expression levels of rs497692 was decreased and that the expression
levels of mutant BSEP associated with the heterozygote and
homozygous genotypes were significantly decreased compared to those
of the wild-type gene and protein. The relevant studies
demonstrated that mutation rs497692 of the ABCB11 gene is a
synonymous mutation that can change gene splicing by exon skipping,
thus affecting the expression of mature mRNA and ultimately leading
to a decrease in the cellular level of the encoded protein
(30). Thus, mutation rs497692 may
serve an important role in the pathogenesis of PISs by altering the
transcription level of the ABCB11 gene.
A previous study of BSEP transport function showed
no change in the bile transport activity of individual BSEP
molecules in the cell membrane; instead, the decrease in BSEP
transport function observed in the mutant is due to decreased
molecular distribution of BSEP on the membrane, resulting in
overall reduced bile transport by BSEP (17). Therefore, the rs118109635 and
rs497692 mutations decreased BSEP expression hepatocyte apical and
bile duct membrane by different mechanisms, leading to reduced bile
transport capacity in PIS patients, changes in bile composition,
and stone formation. In addition, in vitro experiments have
shown that administration of 4-butyl benzene increases the membrane
expression of BSEP protein transcribed from the mutated
ABCB11 gene in the cell membrane (31). These results may be beneficial in
the treatment of PIS patients with reduced BSEP expression caused
by the identified ABCB11 gene mutation.
The present study has limitations. The regulatory
mechanism of ABCB11 gene mutation on mRNA and BSEP protein
expression levels need to be studied further, and at the same time,
ABCB11 mutated rat models need to be used in order to
further define the biological function of these mutations, and
determine the specific role of ABCB11 gene mutations in
primary intrahepatic stone.
In conclusion, the present study confirmed that the
ABCB11 gene in patients with PISs displayed two
disease-associated mutations, rs118109635 and rs497692. The
rs497692 and rs118109635 mutations affected transcription and
translation of the ABCB11 gene and downregulated BSEP
expression in the cell membrane, possibly affecting the transport
of specific substrates by BSEP and eventually causing changes in
bile composition that serve an important role in the pathogenesis
of PISs. Therefore, mutations rsB8109635 and rs497692 of the
ABCB11 gene may be risk factors for PISs that can provide a
reliable basis for improvement of the clinical treatment of PIS
patients in the future.
Acknowledgements
The authors would like to thank The Institute of
Hepatobiliary Surgery, Southwest Hospital, Third Military Medical
University (Army Medical University), Chongqing, China, for
providing laboratory facilities.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81270535)
and Southwest Hospital (grant no. SWH2016JCYB-10).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
YH, LG and SP designed the study. LG and YH
conducted the experiments and drafted the manuscript. LG, SP, JC,
JB and PJ were involved in the statistical analysis. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The experiments were approved by the Ethics
Committee of the Southwest Hospital of the Third Military Medical
University of China. Written informed consent was obtained from all
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BSEP
|
bile salt export pump
|
|
BRIC2
|
benign recurrent intrahepatic
cholestasis type 2
|
|
CBS
|
calcium bilirubinate stones
|
|
CI
|
confidence interval
|
|
CS
|
cholesterol stones
|
|
293
|
human embryonic kidney 293
|
|
HWE
|
Hardy-Weinberg Equilibrium
|
|
ICP
|
intrahepatic cholestasis of
pregnancy
|
|
MDCK
|
Madin-Darby canine kidney
|
|
PFIC2
|
progressive familial intrahepatic
cholestasis type 2
|
|
OR
|
odds ratio
|
|
PIS
|
primary intrahepatic stone
|
|
SNP
|
single nucleotide polymorphisms
|
References
|
1
|
Clemente G, De Rose AM, Murri R, Ardito F,
Nuzzo G and Giuliante F: Liver resection for primary intrahepatic
stones: Focus on postoperative infectious complications. World J
Surg. 40:433–439. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li SQ, Hua YP, Shen SL, Hu WJ, Peng BG and
Liang LJ: Segmental bile duct-targeted liver resection for
right-sided intrahepatic stones. Medicine (Baltimore).
94:e11582015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Catena M, Aldrighetti L, Finazzi R, Arzu
G, Arru M, Pulitanò C and Ferla G: Treatment of non-endemic
hepatolithiasis in a Western country. The role of hepatic
resection. Ann R Coll Surg Engl. 88:383–389. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakayama F, Soloway RD, Nakama T, Miyazaki
K, Ichimiya H, Sheen PC, Ker CG, Ong GB, Choi TK, Boey J, et al:
Hepatolithiasis in East Asia. Retrospective study. Dig Dis Sci.
31:21–26. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pan S, Li X, Jiang P, Jiang Y, Shuai L, He
Y and Li Z: Variations of ABCB4 and ABCB11 genes are associated
with primary intrahepatic stones. Mol Med Rep. 11:434–446. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meng ZW, Han SH, Zhu JH, Zhou LY and Chen
YL: Risk factors for cholangiocarcinoma after initial hepatectomy
for intrahepatic stones. World J Surg. 41:835–843. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cetta FM: Bile infection documented as
initial event in the pathogenesis of brown pigment biliary stones.
Hepatology. 6:482–489. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sakpal SV, Babel N and Chamberlain RS:
Surgical management of hepatolithiasis. HPB (Oxford). 11:194–202.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tazuma S: Gallstone disease: Epidemiology,
pathogenesis, and classification of biliary stones (common bile
duct and intrahepatic). Best Pract Res Clin Gastroenterol.
20:1075–1083. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meier Y, Pauli-Magnus C, Zanger UM, Klein
K, Schaeffeler E, Nussler AK, Nussler N, Eichelbaum M, Meier PJ and
Stieger B: Interindividual variability of canalicular
ATP-binding-cassette (ABC)-transporter expression in human liver.
Hepatology. 44:62–74. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kubitz R, Dröge C, Kluge S, Stindt J and
Häussinger D: Genetic variations of bile salt transporters. Drug
Discov Today Technol. 12:e55–e67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kubitz R, Dröge C, Stindt J, Weissenberger
K and Häussinger D: The bile salt export pump (BSEP) in health and
disease. Clin Res Hepatol Gastroenterol. 36:536–553. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen R, Wang J, Tang S, Zhang Y, Lv X, Wu
S, Yang Z, Xia Y, Chen D and Zhan S: Role of polymorphic bile salt
export pump (BSEP, ABCB11) transporters in anti-tuberculosis
drug-induced liver injury in a Chinese cohort. Sci Rep.
6:277502016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chai J, He Y, Cai SY, Jiang Z, Wang H, Li
Q, Chen L, Peng Z, He X, Wu X, et al: Elevated hepatic multidrug
resistance-associated protein 3/ATP-binding cassette subfamily C 3
expression in human obstructive cholestasis is mediated through
tumor necrosis factor alpha and c-Jun NH2-terminal
kinase/stress-activated protein kinase-signaling pathway.
Hepatology. 55:1485–1494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Beausejour Y, Alvarez F, Beaulieu M and
Bilodeau M: Description of two new ABCB11 mutations responsible for
type 2 benign recurrent intrahepatic cholestasis in a
French-Canadian family. Can J Gastroenterol. 25:311–314. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hayashi H, Naoi S, Hirose Y, Matsuzaka Y,
Tanikawa K, Igarashi K, Nagasaka H, Kage M, Inui A and Kusuhara H:
Successful treatment with 4-phenylbutyrate in a patient with benign
recurrent intrahepatic cholestasis type 2 refractory to biliary
drainage and bilirubin absorption. Hepatol Res. 46:192–200. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hayashi H, Takada T, Suzuki H, Akita H and
Sugiyama Y: Two common PFIC2 mutations are associated with the
impaired membrane trafficking of BSEP/ABCB11. Hepatology.
41:916–924. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Waisbourd-Zinman O, Surrey LF, Schwartz
AE, Russo PA and Wen J: A Rare BSEP mutation associated with a mild
form of progressive familial intrahepatic cholestasis type 2. Ann
Hepatol. 16:465–468. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Keitel V, Burdelski M, Warskulat U,
Kühlkamp T, Keppler D, Häussinger D and Kubitz R: Expression and
localization of hepatobiliary transport proteins in progressive
familial intrahepatic cholestasis. Hepatology. 41:1160–1172. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Anzivino C, Odoardi MR, Meschiari E,
Baldelli E, Facchinetti F, Neri I, Ruggiero G, Zampino R,
Bertolotti M, Loria P and Carulli L: ABCB4 and ABCB11 mutations in
intrahepatic cholestasis of pregnancy in an Italian population. Dig
Liver Dis. 45:226–232. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Keitel V, Vogt C, Häussinger D and Kubitz
R: Combined mutations of canalicular transporter proteins cause
severe intrahepatic cholestasis of pregnancy. Gastroenterology.
131:624–629. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lim TY, Coltart I, Foskett P, Thompson R,
Strautnieks S, Penna L, Williamson C, Miquel R and Heneghan MA:
Donor transmitted mutation of the ABCB11 gene and ensuing
intrahepatic cholestasis of pregnancy in a liver transplant
recipient. Liver Transpl. 23:1229–1232. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lam P, Pearson CL, Soroka CJ, Xu S,
Mennone A and Boyer JL: Levels of plasma membrane expression in
progressive and benign mutations of the bile salt export pump
(Bsep/Abcb11) correlate with severity of cholestatic diseases. Am J
Physiol Cell Physiol. 293:C1709–C1716. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Z, Chen K, Jiang P, Zhang X, Li X and
Li Z: CD44v/CD44s expression patterns are associated with the
survival of pancreatic carcinoma patients. Diagn Pathol. 9:792014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gordo-Gilart R, Andueza S, Hierro L,
Martínez-Fernández P, D'Agostino D, Jara P and Alvarez L:
Functional analysis of ABCB4 mutations relates clinical outcomes of
progressive familial intrahepatic cholestasis type 3 to the degree
of MDR3 floppase activity. Gut. 64:147–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang R, Chen HL, Liu L, Sheps JA, Phillips
MJ and Ling V: Compensatory role of P-glycoproteins in knockout
mice lacking the bile salt export pump. Hepatology. 50:948–956.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu G, He P, Liu Z, Chen Q, Zheng B and
Zhang Q: Diagnosis of ABCB11 gene mutations in children with
intrahepatic cholestasis using high resolution melting analysis and
direct sequencing. Mol Med Rep. 10:1264–1274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park JS, Ko JS, Seo JK, Moon JS and Park
SS: Clinical and ABCB11 profiles in Korean infants with progressive
familial intrahepatic cholestasis. World J Gastroenterol.
22:4901–4907. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tai Y, Xie Y and Tang CW: Compound
heterozygous mutations of ABCB11 responsible for benign recurrent
intrahepatic cholestasis. J Dig Dis. 16:299–302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Byrne JA, Strautnieks SS, Ihrke G, Pagani
F, Knisely AS, Linton KJ, Mieli-Vergani G and Thompson RJ: Missense
mutations and single nucleotide polymorphisms in ABCB11 impair bile
salt export pump processing and function or disrupt pre-messenger
RNA splicing. Hepatology. 49:553–567. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hayashi H and Sugiyama Y: 4-phenylbutyrate
enhances the cell surface expression and the transport capacity of
wild-type and mutated bile salt export pumps. Hepatology.
45:1506–1516. 2007. View Article : Google Scholar : PubMed/NCBI
|