Introduction
The liver and intestine are connected by the
gut-liver axis (1). The liver is
an endocrine gland that secretes bile acids (BAs) into the
intestine to maintain the stability of the intestinal flora
(2,3). Gut-liver axis dysfunction is
characterized by metabolic disorders of Bas (1). Excessive BAs in the liver induce
hepatocyte death and aggravate inflammatory injury (4–7).
Decreased BAs in the gut, in contrast, lead to intestinal dysbiosis
that impairs intestinal barrier function, inducing bacterial
translocation to allow pathogens, including Bacteroidetes
(Gram-negative bacteria) and their products, lipopolysaccharide
(LPS), into the liver, aggravating hepatic inflammation (8,9).
Previous studies demonstrated the significance of the gut-liver
axis (1,10,11).
Retrospective analyses of a large cohort of clinical samples
demonstrated that patients with inflammatory bowel disease had a
higher incidence of primary sclerosing cholangitis (12,13).
These previous studies suggested that bacterial translocation and
bacteremia in the portal vein aggravated primary sclerosing
cholangitis. Similarly, loss of intestinal epithelial stemness
contributed to bile duct ligation-induced cholestatic liver injury
(14). Furthermore, postnatal
development of intestinal microbiota, including
Proteobacteria, was identified as an important
susceptibility factor for biliary atresia in mice (15).
The inflammasome is a large multiprotein complex
that recognizes diverse microbial-, stress- and danger-associated
signals, and subsequently triggers the maturation of
pro-inflammatory cytokines, including interleukin (IL)-1β and
IL-18, promoting innate immunity (16). IL-1β, activated by the
inflammasome, is involved in liver inflammation (17), whereas, IL-18 has been demonstrated
to be involved in modulating the gut microbiota (18). Previous studies demonstrated that
the intestinal epithelial NACHT, LRR and PYD domains-containing
protein (NLRP)6 inflammasome maintains the intestinal barrier and
the intestinal microbial balance (19,20),
whereas, the NLRP3 inflammasome serves as an intracellular pattern
recognition receptor (PRR) that mediates innate immunity and
aggravates inflammatory liver injury (21–23).
Furthermore, inflammasome inhibitors, including the caspase
inhibitor IDN-6556, have exhibited protective effects in liver
injury (24).
In the gut-liver axis, metabolic disorders of BAs
caused the imbalance of intestinal microflora and hepatic
inflammatory injury (25), and the
inflammasome has been demonstrated to be associated with gut
barrier integrity, microbial composition and liver injury (18,26).
Furthermore, BAs serve as danger signals that may have a direct or
indirect effect on the intracellular inflammasome (21,27–29).
Therefore, understanding the role of the inflammasome in the
gut-liver axis may provide insights into effective treatments for
liver and gut diseases. The present review discusses the roles of
the inflammasome in the gut-liver axis and the associations between
the inflammasome and the intestinal microbiota or the BAs.
Inflammasomes are intracellular PRRs
The inflammasome, whose term was initially proposed
in 2002 (30), is comprised of
multiple proteins present in the cytoplasm and serves as a PRR to
recognize various inflammatory stimulations (Table I), including exogenous pathogens
[pathogen-associated molecular patterns (PAMPs)], and endogenous
signals from damaged or dying cells [damage-associated molecular
patterns (DAMPs)], thereby recruiting and regulating the production
of inflammatory cytokines (16).
In addition, the inflammasome is a key component of innate immunity
and serves a role in the regulation of inflammation under various
injury conditions. In previous studies, it was demonstrated that
the inflammasome is involved in a number of diseases, including
liver fibrosis, primary sclerosing cholangitis, cholestasis and
biliary obstruction (21,23,31,32).
 | Table I.Inflammasome activators. |
Table I.
Inflammasome activators.
| A, Whole
pathogens |
|---|
|
|---|
| Activators | Associated
diseases |
|---|
| Bacteria | Infection |
| fungus | Infection |
| Virus | Infection |
| Parasite | Infection |
|
| B,
PAMPs |
|
|
Activators | Associated
diseases |
|
| LPS | Infection |
| Bacterial
pore-forming toxins | Infection |
| Hemozoin | Malaria |
|
| C, Environmental
insults |
|
|
Activators | Associated
diseases |
|
| Silica | Silicosis |
| Asbestos | Asbestosis |
| Ultraviolet
light | Sunburn |
|
| D,
DAMPs |
|
|
Activators | Associated
diseases |
|
| ATP | Necrosis |
| Glucose | Metabolic
syndrome |
| Uric acid | Gout |
| Amyloid β | Alzheimer's
disease |
| Cytochrome C | Apoptosis |
| ROS | Allergy |
| Heat-shock
protein | Japanese
encephalitis virus infection |
| Defensins | Tuberculosis
infection |
| HMGB1 | Glaucoma |
| Fatty acids | Obesity/Type 2
diabetes |
| Hyaluronic
acid | Airway
hyperresponsiveness |
| Mitochondrial
DNA | Autophagy and
apoptosis |
| Cytoplasmic
DNA | Pyroptosis |
| S100 proteins | Rheumatoid
arthritis/Crohn's disease |
| Albumin | Renal tubular
injury |
The inflammasome is comprised of a sensor protein,
an adaptor protein and caspase-1 (16). The sensor protein family includes
NOD-like receptors (NLRs), including NLRP1, NLRP3, NLR family CARD
domain-containing protein 4, NLRP6 and the PYHIN family protein
interferon-inducible protein AIM2 (33). The NLRP3 inflammasome is the best
characterized inflammasome and consists of NLRP3, the
apoptosis-associated speck-like protein containing a CARD (ASC)
adaptor and pro-caspase-1 (Fig. 1)
(34). Furthermore, NLRP3 contains
C-terminal leucine-rich repeats (LRRs), a central
nucleotide-binding and oligomerization (NACHT) domain, and an
N-terminal pyrin domain (PYD) (35). In the presence of PAMPs and DAMPs,
NLRP3 oligomerizes and combines with ASC to assemble the
inflammasome, recruiting and activating caspase-1, that cleaves the
precursors of inflammatory cytokines, in order to produce and
release the mature forms of IL-1β and IL-18 (16).
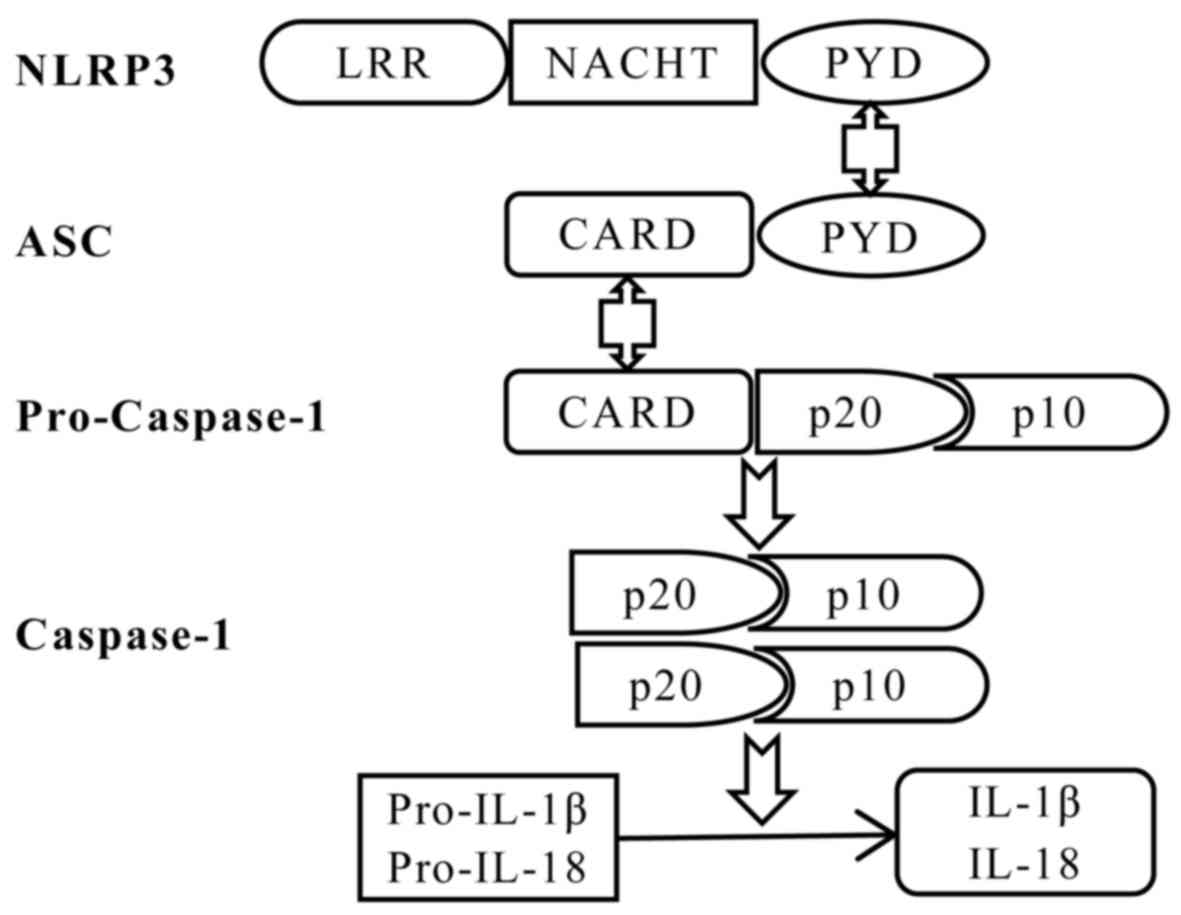 | Figure 1.Schematic diagram of NLRP3
inflammasome assembly. The NLRP3 inflammasome is assembled by
NLRP3, the ASC adaptor and pro-caspase-1. Upon NLRP3 activation,
NLRP3 interacts with ASC via PYDs, and the CARD domain of ASC
recruits the CARD of pro-caspase-1, leading to autocleavage of the
inactive CARD domain from pro-caspase-1. This cleavage allows the
formation of the active caspase-1 p10/p20 tetramer, which cleaves
cytokine precursors to produce and release mature IL-1β and IL-18.
IL, interleukin; CARD, caspase recruitment domain; LRR,
leucine-rich repeat; NACHT, nucleotide-binding and oligomerization
domain; PYD, pyrin domain; NLRP3, NACHT, LRR and PYD
domains-containing protein 3; ASC, apoptotic speck-like protein
containing a CARD. |
In total, two signals lead to the activation of the
NLRP3 inflammasome (Fig. 2). In
the first signal, PAMPs or DAMPs interact with toll-like receptors
(TLRs) on the cell surface, leading to nuclear factor-κB
(NF-κB)-dependent transcription and translation of pro-IL-1β and
pro-IL-18 (36). In addition,
NF-κB activates NLRP3 gene transcription by binding to its
promoter, which is the limiting step for activation of the NLRP3
inflammasome (37). The second
signal involves three concomitant molecular mechanisms. In the
first, extracellular adenosine 5′-triphosphate (ATP) induces P2X
purinoceptor 7-dependent pore formation on the cell membrane and
promotes an intracellular K+ efflux (38), resulting in the translocation of
large-pored pannexin-1 channels into the membrane (39). Subsequently, PAMPs or DAMPs enter
the cell and activate the NLRP3 inflammasome. Furthermore,
phagocytosis of large molecules, including crystalized cholesterol
and uric acid, induces lysosomal disruption, resulting in the
release of its components and activation of the NLRP3 inflammasome
(40). Additionally,
thioredoxin-interacting proteins dissociate from thioredoxin and
bind to the NLRP3 inflammasome to trigger its activation under the
effect of reactive oxygen species (ROS) derived from mitochondria
(41). Notably, the aforementioned
mechanisms occur simultaneously. Furthermore, the second signal
leads to the cleavage and release of pro-IL-1β and pro-IL-18 into
IL-1β and IL-18, respectively (16). Therefore, activation of the NLRP3
inflammasome is associated with the recognition of pathogens
involved in innate immunity, and senses an imbalance in cell
homeostasis, including K+ efflux, Ca2+
signaling, mitochondrial dysfunction and lysosomal rupture.
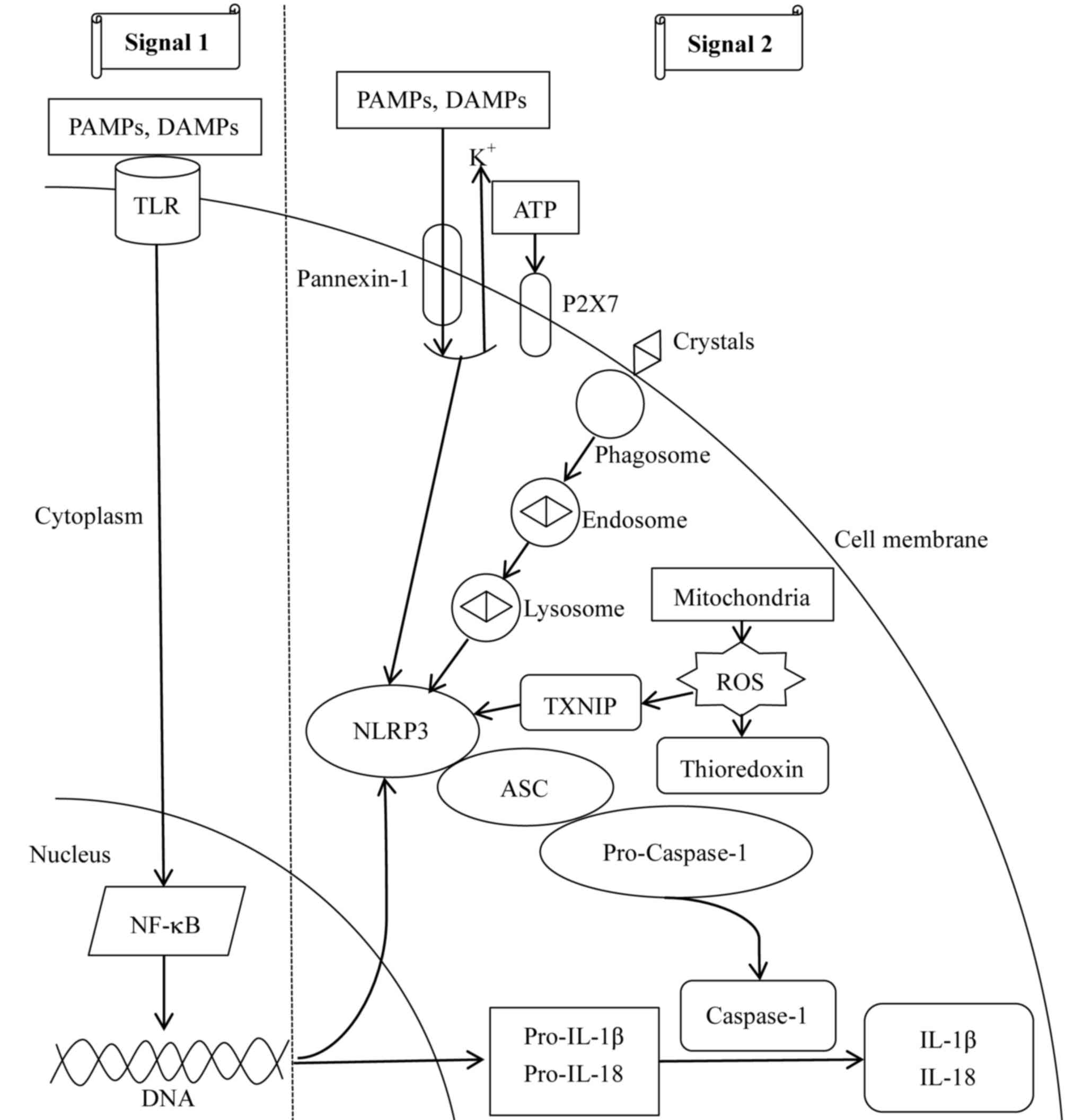 | Figure 2.Mechanisms involved in NLRP3
inflammasome activation. Two signals are required for the
activation of the NLRP3 inflammasome. For signal 1, PAMPs and DAMPs
combine with TLRs on the cell membrane to activate NF-κB-dependent
transcription and translation of NLRP3, pro-IL-1β and pro-IL-18.
For signal 2, three mechanisms have been described. In the first,
ATP interacts with P2X7, leading to intracellular
K+-depletion and opening of a large-pored pannexin-1
channel, through which PAMPs and DAMPs enter the cell and activate
the NLRP3 inflammasome. Furthermore, endocytosis of large
molecules, including crystals, results in lysosomal disruption,
leading to the release of its components and activation of the
NLRP3 inflammasome. Additionally, mitochondria-derived ROS detach
TXNIP from thioredoxin and enable activation of the NLRP3
inflammasome. The second signal results in caspase-1 activation,
and cleavage of pro-IL-1β and pro-IL-18 into mature IL-1β and
IL-18. ASC, apoptosis-associated speck-like protein containing a
CARD; DAMPs, danger-associated molecular patterns; NF-κB, nuclear
factor-κB; P2X7, P2X purinoceptor 7; PAMPs, pathogen-associated
molecular patterns; ROS, reactive oxygen species; TLR, Toll-like
receptor; TXNIP, thioredoxin-interacting protein; IL, interleukin;
NLRP3, NACHT, LRR and PYD domains-containing protein 3; ATP,
adenosine 5′-triphosphate. |
Inflammasome and intestinal homeostasis
Intestinal homeostasis requires an intact intestinal
barrier and a balanced intestinal flora. In the following sections,
different roles of the NLRP6 and NLRP3 inflammasome in the two
components of intestinal homeostasis are discussed.
Intestinal epithelial NLRP6
inflammasome maintains the intestinal barrier and microbial
balance
In humans and mice, NLRP6 is highly expressed in
epithelial cells of the small intestine, colon and goblet cells
(19,42,43),
and is co-expressed with ASC and caspase-1 in the intestinal
epithelium (20). The NLRP6
inflammasome mediates the interaction between the intestinal immune
system and gut microbes, and its activation mechanisms are
summarized in Fig. 3. In infancy,
the colonization of gut microbes, including Proteobacteria,
Firmicutes and Actinobacteria (44), led to the upregulation of the NLRP6
inflammasome and its downstream cytokines (20,45).
Therefore, gut commensal microbes represent one of the first
signals to induce NLRP6 inflammasome-dependent antimicrobial
responses. Furthermore, microbial metabolites regulate the NLRP6
inflammasome metabolism via the second signal;
microbiota-associated metabolite taurine promotes NLRP6 signaling
and IL-18 synthesis, whereas, spermine and histamine inhibit NLRP6
inflammasome activity (20).
Notably, the protozoan Tritrichomonas musculis was described
to induce epithelial IL-18 secretion through ASC inflammasome
activation (46). In addition to
microbial activation mechanisms, the stress-induced
corticotrophin-releasing hormone inhibited the expression of
intestinal NLRP6 inflammasome in rats deprived of water (47), whereas, the nuclear transcription
factor peroxisome proliferator-activated receptor-γ activated the
NLRP6 inflammasome by binding to the NLRP6 promoter (45).
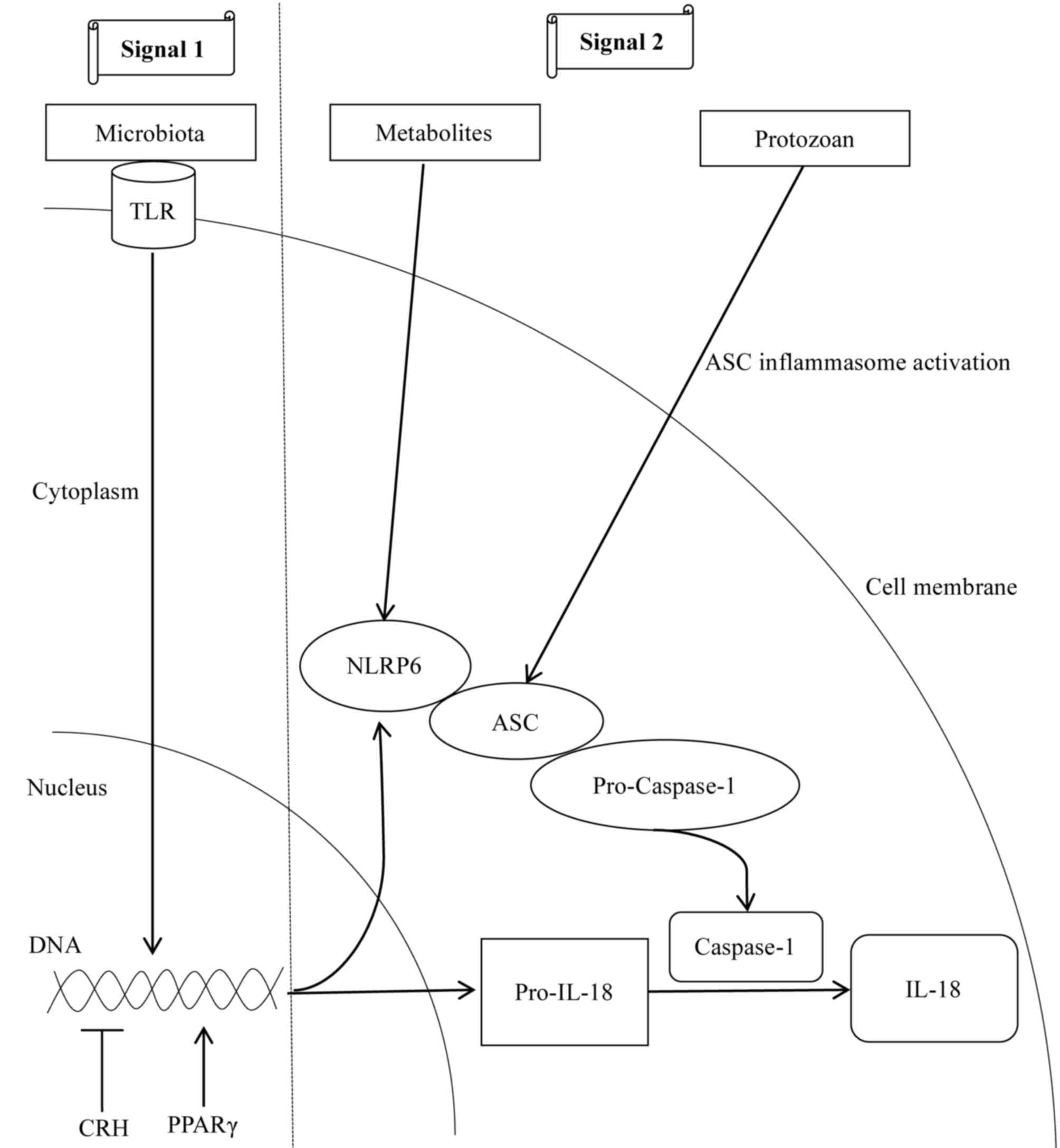 | Figure 3.Mechanisms involved in NLRP6
inflammasome activation. Intestinal microbiota initiate two signals
for the activation of the NLRP6 inflammasome. In the first signal,
the commensal microbiota serve as a TLR ligand and promotes the
transcription of NLRP6 and pro-IL-18. For the second signal,
microbial metabolites, including taurine, promote the multiprotein
complex assembly to activate the NLRP6 inflammasome. In particular,
commensal protozoans promote epithelial IL-18 secretion via
activation of the ASC inflammasome. In addition to the microbial
roles, CRH inhibits the transcription of NLRP6 inflammasome
components, whereas, the nuclear transcription factor PPAR-γ
activates NLRP6 by binding to its promoter region. Arrows indicate
‘promotion’, whereas, the symbol ‘┴’ indicates ‘inhibition’. CRH,
corticotrophin-releasing hormone; PPAR-γ, peroxisome
proliferator-activated receptor-γ; NLRP6, NACHT, LRR and PYD
domains-containing protein 6; IL-18, interleukin 18; ASC,
apoptosis-associated speck-like protein containing a CARD; TLR,
Toll-like receptor. |
Accumulating evidence suggested that the NLRP6
inflammasome regulates host-gut commensal microbiota interaction
via two mechanisms. In the first mechanism, the NLRP6 inflammasome
promotes the secretion of mucus by goblet cells (19). NLRP6−/− mice with
defective goblet cell exocytosis were vulnerable to colonization by
Citrobacter rodentium, and bacteria penetrated in the
epithelial crypts deeper, compared with wild-type mice (19). One possible mechanism for this
phenotype may involve intestinal epithelium autophagy dysfunction.
A recent study demonstrated that sentinel goblet cells secrete
mucus when activated by the NLRP6 inflammasome in a
calcium-dependent manner (48). In
the second mechanism, the NLRP6 inflammasome maintains the balance
of the intestinal flora by promoting intestinal epithelial cells to
synthesize antimicrobial peptides, including angiogenin-4,
intelectin-1 and resistin-like molecule β (20). In this process, gut microbiota
initially activate the NLRP6 inflammasome and induce intestinal
epithelial synthesis of IL-18, which promotes the synthesis of
antimicrobial peptides in an autocrine or in an IL-22-dependent
manner (49). In addition, the
NLRP6 inflammasome is involved in preventing enterovirus infection
by binding to viral RNA, promoting the expression of
interferon-stimulated genes (50).
The microbial metabolites/NLRP6
inflammasome/IL-18/antimicrobial peptide axis serves a critical
role in the regulation of the intestinal inflammatory response
(20). Due to the presence of the
aforementioned metabolic axis, NLRP6−/− mice were unable
to synthesize the protective antimicrobial peptides (42,51),
resulting in intestinal flora disorder, characterized by the
increase of Prevotellaceae species and members of the TM7
phylum, and the decrease of bacteria belonging to the genus
Lactobacillus, phylum Firmicutes (42). Notably, the phenotype of
exacerbated colitis in NLRP6−/− mice may be transferred
to wild-type mice (20). The
reason for this phenomenon involves the transfer of dysbiotic
microbiota from inflammasome-deficient mice to wild-type mice.
Although wild-type mice have an intact NLRP6 inflammasome,
dysbiotic microbiota-produced metabolites, including spermine and
histamine have the ability to inhibit NLRP6 inflammasome-associated
signals and suppress colonic IL-18 expression levels, thereby
reducing antimicrobial peptide synthesis, leading to the
development of dysbiosis and aggravated colitis in wild-type mice.
Nevertheless, antibiotic treatment in addition to exogenous
administration of IL-18 abrogated all the aforementioned effects
(20). Collectively, these data
demonstrated that inflammasome-deficient mice possess a
communicable dysbiotic microbiota that enhances susceptibility to
colitis. Furthermore, NLRP6-deficient mice had increased
susceptibility to colitis-associated colon tumorigenesis, which may
be caused by microbiota-induced chemokine (C-C motif) ligand 5
(CCL5)-driven inflammation. The subsequent inability to resolve
inflammation and repair damaged epithelium, promotes epithelial
cell proliferation, leading to the formation of cancer (52–54).
A previous study suggested that the NLRP6
inflammasome has an indirect effect on the liver by regulating gut
microbiota, and mice lacking the NLRP6 inflammasome presented
dysbiotic microbiota consisting of Porphyromonadaceae and
Prevotellaceae. Furthermore, these intestinal bacteria or
their derived products, including LPS and double-stranded bacterial
DNA, triggered activation of TLR4 and TLR9 in the portal
circulation, and enhanced tumor necrosis factor (TNF)-α expression
in the liver, aggravating the progression of non-alcoholic fatty
hepatitis in mice (18).
Collectively, the NLRP6 inflammasome serves a key role in
maintaining the intestinal flora and preventing metabolic liver
injury.
Intestinal NLRP3 inflammasome is
involved in mucosal defense
The NLRP3 inflammasome has been extensively studied
in myeloid cells; however, the presence of NLRP3 in the gut
epithelium is controversial. Previous studies demonstrated that the
NLRP3 gene is highly expressed in intestinal bone marrow-derived
cells, including macrophages and monocytes (55–57).
Therefore, in the majority of the previous studies, the role of the
NLRP3 inflammasome in intestinal diseases has been investigated by
examining its role in myeloid cells. In colonic epithelial cells,
NLRP3 expression level is low or approximately undetectable
(57,58). However, it was recently observed
that the NLRP3 protein was expressed in human intestinal epithelial
cells (59).
The NLRP3 inflammasome exacerbates intestinal
inflammation. In a rat colitis model, increased expression levels
of the NLRP3 inflammasome were observed (60). Intestinal pathogens activated the
NLRP3 inflammasome and induced IL-β production in monocytes,
aggravating intestinal inflammation (55). In addition, titanium
dioxide-containing nanoparticles exacerbated dextran sodium sulfate
(DSS)-induced colitis by activating the NLRP3 inflammasome in
intestinal epithelial cells and macrophages (59). Furthermore, Escherichia coli
isolated from patients with inflammatory bowel disease activated
the NLRP3 inflammasome, exacerbating the inflammatory response
(61). Conversely, in
NLRP3−/− mice, colonic inflammation in a model of
inflammatory bowel disease was attenuated (62).
The NLRP3 inflammasome exerts a protective effect on
intestinal inflammation by maintaining the intestinal mucosa. Allen
et al (57) demonstrated
that the NLRP3 inflammasome served a protective role in DSS-induced
colitis, and that NLRP3−/− mice exhibited more severe
colitis. These observations may be explained by the following
mechanisms. Hirota et al (63) demonstrated that the expression
levels of IL-1β-induced anti-inflammatory cytokine IL-10 and
transforming growth factor β1 (TGF-β1) were decreased in
NLRP3−/− mice. Additionally, peritoneal macrophages
isolated from NLRP3−/− mice did not exhibit a response
to bacterial muramyldipeptide and neutrophils exhibited decreased
chemotaxis and increased apoptosis. Furthermore,
NLRP3−/− mice exhibited decreased expression levels of
β-defensin and antimicrobial peptides. Conversely, the level of
pathogenic microorganisms, including Enterobacteriaceae and
Mycobacterium was increased. Additionally,
NLRP3−/− mice exhibited decreased expression levels of
IL-18, resulting in the loss of intestinal epithelial integrity,
systemic leukocyte infiltration, and increased chemokine levels,
aggravating DSS-induced colitis (64).
The controversial role of the NLRP3 inflammasome in
intestinal inflammation may be attributed to one or more of the
following factors. Previous studies on NLRP3 inflammasome function
primarily relied on transgenic animal models (62–64),
in which breeding, environmental factors or different models of
inflammation may affect the course of the disease. Furthermore, the
role of the NLRP3 inflammasome in intestinal inflammation is
predominantly based on the cell types affected. As mentioned above,
in case the NLRP3 inflammasome is activated in intestinal
epithelial cells, the synthesis of defensin is promoted by inducing
IL-18 to maintain the intestinal microbial balance, thereby
exerting a protective effect (64). However, once the intestinal
epithelial barrier is destroyed, micro-organisms and antigens may
access the intestinal lamina propria. At this level, PAMPs may be
recognized by macrophages and by dendritic cells via PRRs.
Subsequently, the NLRP3 inflammasome in myeloid cells is activated,
inducing IL-1β, thereby aggravating inflammation (55).
Similar to NLRP6, the NLRP3 inflammasome may
aggravate liver damage by affecting intestinal flora in the
gut-liver axis. Pierantonelli et al (26) demonstrated that NLRP3−/−
mice fed a Western diet, provided with water containing fructose,
exhibited impaired intestinal antimicrobial peptide synthesis. This
event led to an increased permeability of the intestinal mucosal
barrier, and induced dysbiosis in the form of an increased
Firmicutes/Bacteroidetes ratio and increase in
Proteobacteria, causing bacterial translocation, leading to
the increased expression of the LPS receptor TLR4 and
double-stranded bacterial DNA receptor TLR9 in the liver.
Antibiotic treatment decreased bacterial translocation and
alleviated liver inflammation.
Inflammasome increases liver injury by
inducing inflammation
Apart from the aforementioned roles of the
inflammasomes in the gut, the present review focuses on the roles
of inflammasomes in the liver. The inflammasome is expressed in
liver parenchymal cells and immune cells (65). In hepatocytes, inflammasome
activation induces hepatocyte death via pyroptosis and aggravates
non-alcoholic steatohepatitis (16). In immune cells, however,
gut-derived PAMPs activate the intracellular inflammasome via PRRs,
resulting in increased synthesis of IL-1β and IL-18, with the
former being a crucial pro-inflammatory cytokine in liver
inflammation (17). A previous
study demonstrated that IL-1β promoted the synthesis of monocyte
chemoattractant protein 1 (MCP-1) and TNF-α (66), thereby potentiating TNF-α
cytotoxicity in hepatocytes, and activating hepatic stellate cells
to promote liver fibrosis (67).
In addition, DAMPs that are released from dead hepatocytes may
activate inflammatory responses in immune cells and hepatic
stellate cells (68). Therefore,
the inflammasome serves a key role in the hepatic inflammatory
network. Previous studies investigating the pathogenesis of liver
disease primarily focused on NLRP3 and AIM2 inflammasomes (65,69).
In contrast, the NLRP6 inflammasome does not have a direct effect
on the liver; however, it was demonstrated to indirectly influence
liver disease via the gut-liver axis (18).
Inflammasome regulates the barrier of
biliary epithelium
Similar to the protective roles exerted by the
inflammasome in maintaining the intestinal barrier, the
inflammasome regulates the integrity of the biliary epithelium
barrier. The biliary epithelial barrier serves a significant role
in BA metabolism (70). Once a
barrier, including tight junctions, is disrupted, BAs may leak into
the portal tracts, thereby inducing the recruitment of leukocytes
around the bile duct, activating the pro-inflammatory cytokines
TNF-α, IL-1β and fibrosis factor TGF-β1, leading to periductal
inflammation and fibrosis (70).
Subsequent to fibrosis, bile duct epithelial cells may separate
from the peribiliary plexus, and induce autophagy and cell death in
the bile duct epithelium, leading to obstructive jaundice (71). A recent study has suggested that
the inflammasome is involved in the regulation of biliary
epithelial barrier function. Maroni et al (72) demonstrated that NLRP3 inflammasome
activation affected epithelial barrier function in vitro and
in vivo. In patients with primary sclerosing cholangitis and
mouse models, an increased expression of the NLRP3 inflammasome was
observed, that stimulated IL-18 expression in bile duct epithelial
cells. No significant alterations in IL-1β were identified. In
contrast to the protective role in the intestine, NLRP3
inflammasome activation in wild-type mice decreased the expression
of E-cadherin and Zonulin-1, and increased the permeability of
cholangiocytes. Furthermore, in NLRP3−/− mice, only
minor alterations were observed in the aforementioned cell adhesion
markers and cholangiocyte permeability (72). Collectively, these findings
suggested that the NLRP3 inflammasome is expressed in reactive
cholangiocytes and that activation of the NLRP3 inflammasome
affected epithelial integrity of cholangiocytes by inducing
pro-inflammatory cytokine production.
BA metabolism and its effect on the
inflammasome in the gut-liver axis
In the gut-liver axis, BAs serve a principal role.
BAs are synthesized from cholesterol in hepatocytes by cholesterol
7α-hydroxylase (CYP7A1). Subsequently, primary BAs cholic acid (CA)
and chenodeoxycholic acid (CDCA) conjugate with glycocholic acid
(GCA) and glycochendeoxycholic acid or taurine (taurocholate and
taurodeoxycholic acid) to generate conjugated primary Bas (73). BAs are secreted from the
hepatocytes into bile via the canalicular bile salt export pump
(66,67), subsequently reabsorbed in the
terminal ileum by apical sodium-dependent bile salt transporter,
and returned to the liver via the portal vein (74,75).
Finally, 95% of BAs are taken up by the basolateral transport
systems into hepatocytes. The remaining primary BAs in the
intestine are dehydroxylated to secondary BAs, including
deoxycholic acid (DCA) and lithocholic acid (LCA), by bacteria
(76). Therefore, factors
affecting the metabolism of BAs may lead to gut-liver axis
dysfunction (77).
BAs serve as endocrine signaling molecules by
binding to the nuclear farnesoid-X receptor (FXR) and membrane
Takeda G-protein receptor 5 (TGR5) in the gut-liver axis (78–80).
FXR is highly expressed in enterocytes and hepatocytes, whereas,
TGR5 is not present in parenchymal cells; however, is present in
enteroendocrine cells, Kupffer cells, sinusoidal endothelial cells,
stellate cells and cholangiocytes (81,82).
Among the BAs, CDCA is the strongest FXR agonist (CDCA > DCA
> CA > LCA; in order of decreasing potency) (79), whereas, LCA is the most potent in
activating TGR5 (LCA > DCA > CDCA > CA) (83). Accumulating evidence has suggested
that BAs are involved in a series of pathological processes in the
gut-liver axis by activating FXR. For example, CA and DCA inhibited
bacterial overgrowth and had a positive effect on mucosal injury in
ileum caused by bile duct ligation (84), whereas, mice lacking FXR had
impaired gut barrier integrity (85). BAs modulated gut microbial
composition and mice fed with CA exhibited a marked decrease in the
Bacteroidetes/Firmicutes ratio (2,86).
In addition, FXR is a negative regulator of NF-κB-mediated hepatic
inflammation and inhibited liver fibrosis, promoting regeneration
(87–89). Therefore, FXR may represent a novel
therapeutic target for a broad range of gut and liver diseases.
At physiological levels, BAs are unable to activate
the NLRP3 inflammasome. However, intrahepatic BAs may reach a
critical concentration in cholestasis, causing DCA and CDCA to
function as DAMPs to induce a strong activation of the macrophage
NLRP3 inflammasome in a dose- and time-dependent manner via signal
1 and signal 2 (Fig. 4) (21,28).
The activation of the NLRP3 inflammasome may result in cholestatic
liver injury and liver fibrosis, and its activation mechanisms may
be involved in calcium influx, ROS production, K+ efflux
and intracellular ATP release (21,28).
In addition to the aforementioned direct roles, BAs are able to
indirectly regulate the NLRP3 inflammasome via their receptors
(Fig. 4). Guo et al
(27) demonstrated that specific
BAs, including CA, GCA, CDCA, DCA, ursodeoxycholic acid, LCA and
taurolithocholic acid interacted with the macrophage membrane
receptor TGR5 and induced cyclic adenosine
3′,5′-monophosphate-dependent protein kinase A (PKA) activation.
PKA kinase in turn phosphorylated NLRP3, thereby preventing NLRP3
inflammasome activation, thus exerting anti-inflammatory effects.
In addition, Hao et al (28) identified another mechanism via
which the BA-activated nuclear receptor FXR negatively regulated
the NLRP3 inflammasome. FXR interacted with NLRP3 and caspase-1,
thereby preventing assembly of the NLRP3 inflammasome. However, the
expression of FXR in cholestasis was downregulated in the liver,
leading to the extensive activation of the NLRP3 inflammasome in
macrophages, aggravating cholestatic liver injury. Furthermore, Xie
et al (29) confirmed that
the FXR activator GW4064 is able to inhibit NLRP3 inflammasome
activation, mitigating liver inflammation. The BA receptors FXR and
TGR5 not only inhibited intrahepatic BA synthesis and reduced
cholestatic liver injury (90,91);
however, additionally negatively regulated the NLRP3 inflammasome,
exerting anti-inflammatory effects. Accordingly, using novel
ligands of FXR and TGR5 may represent a potential therapeutic
strategy for the treatment of cholestasis.
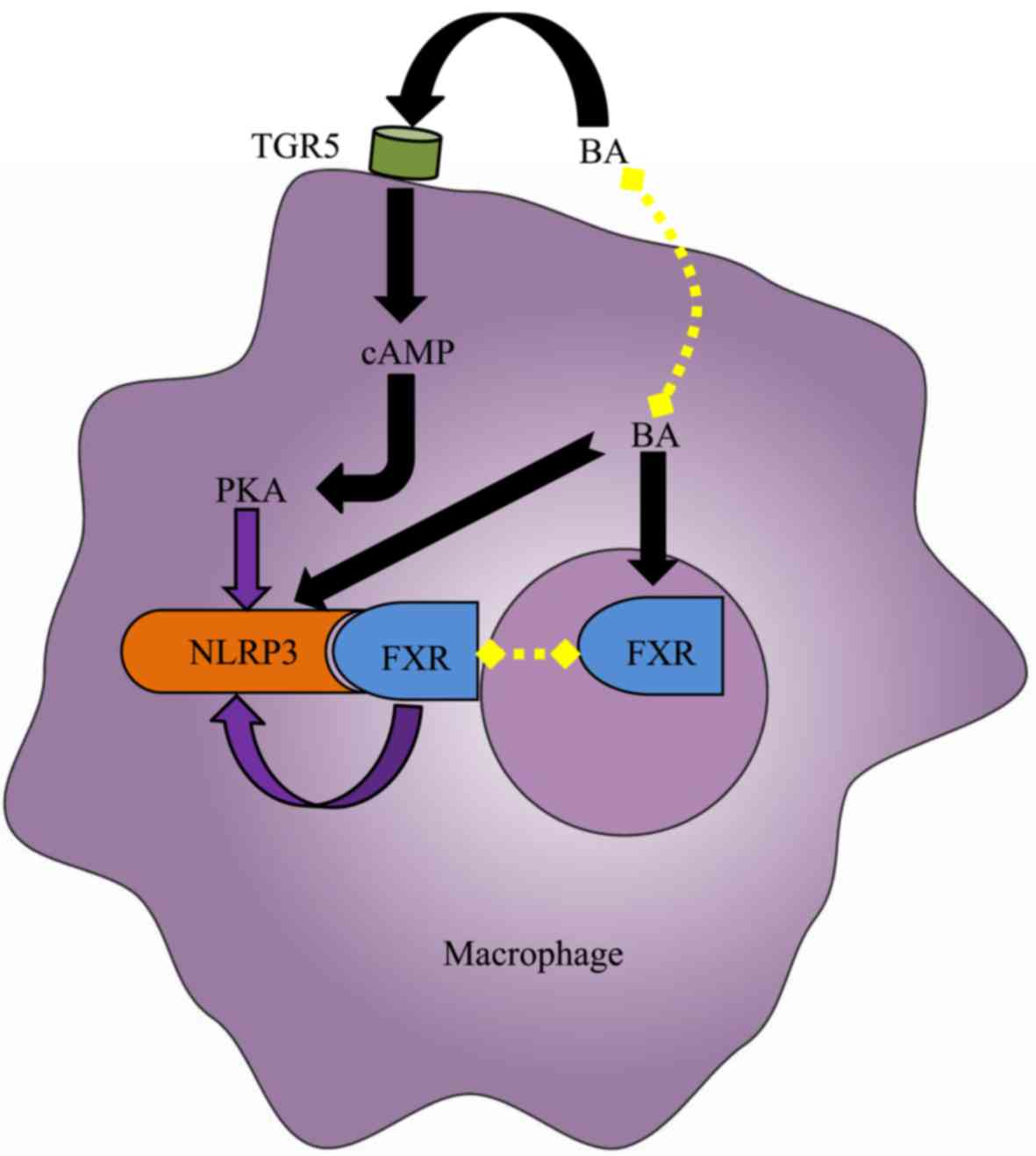 | Figure 4.Effect of BA on the NLRP3
inflammasome in the macrophage. Elevated intracellular BAs,
including deoxycholic acid and chenodeoxycholic acid, directly
activate the NLRP3 inflammasome in macrophages. However, the BA
nuclear receptor FXR interacts with NLRP3 to prevent the assembly
of NLRP3 inflammasome components, thereby repressing its
activation. Furthermore, the BA membrane receptor TGR5 may
negatively regulate NLRP3 inflammasome activation by TGR5-cAMP-PKA
axis-dependent NLRP3 phosphorylation and ubiquitination. However,
due to the limited expression of FXR and TGR5 under cholestasis
conditions, the aforementioned protective mechanisms fail to
counteract the cytotoxic effects of BAs. Black arrows indicate
‘promotion’, whereas, purple arrow indicates ‘inhibition’. BA, bile
acid; cAMP, cylic adenosine monophosphate; FXR, farnesoid-X
receptor; PKA, protein kinase A; TGR5, Takeda G-protein receptor 5;
NLRP3, NACHT, LRR, and PYD domains-containing protein 3. |
Inflammasome connects gut and liver
Similar to BAs, the inflammasome has been
demonstrated to connect the gut and liver. According to De Minicis
et al (8), intestinal NLRP3
inflammasome expression was downregulated in a bile duct ligation
mouse model. Collectively, the roles of the inflammasome in the
gut-liver axis are presented in Fig.
5. In gut-liver dysfunction, decreased BAs in the intestine
lead to dysbiosis characterized by increased Gram-negative
bacteria, whose metabolites, including spermine and histamine may
inhibit the expression of the NLRP6 inflammasome in the intestinal
epithelium. Additionally, dysbiosis leads to a decreased synthesis
of IL-18, intestinal antimicrobial peptides, and mucus secretion by
goblet cells, increasing intestinal permeability. Increased
permeability leads to intestinal flora disruption and bacterial
translocation, and eventually results in gut-derived PAMPs and
DAMPs to enter the liver via the portal vein. In the liver, PAMPs
and DAMPs interact with TLRs to activate the intracellular NLRP3
inflammasome that leads to the synthesis of IL-1β in macrophages or
Kupffer cells (17). It has been
previously reported that IL-1β exacerbates liver injury in the
following ways. IL-1β aggravates inflammation by recruiting
inflammatory cells. Additionally, in combination with TNF-α, IL-1β
induces pyroptosis of hepatocytes. Furthermore, IL-1β activates
hepatic stellate cells and promotes fibrosis (67).
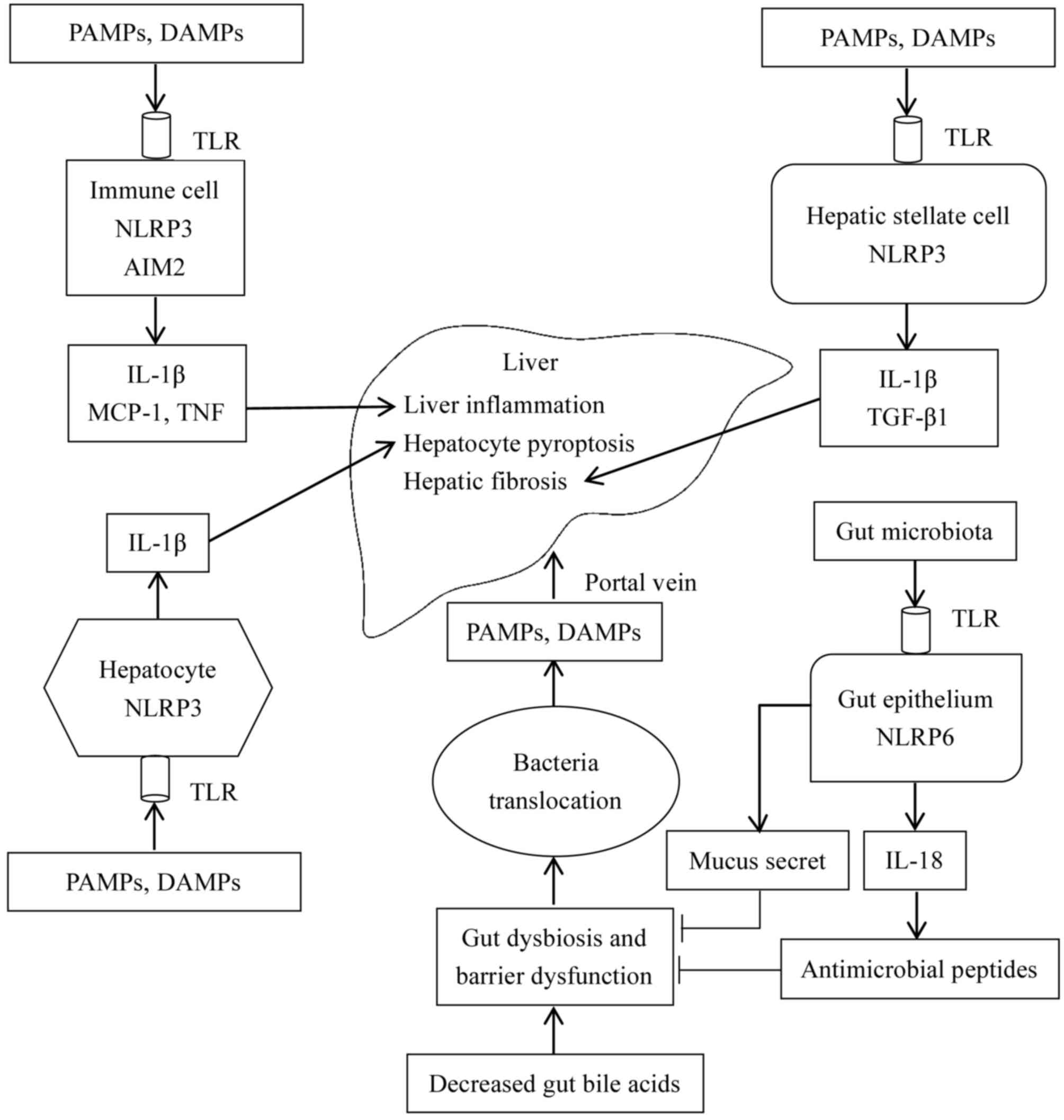 | Figure 5.Roles of the inflammasome in the
gut-liver axis. Decreased intestinal BAs induce intestinal flora
disorders, increase intestinal permeability, and impair intestinal
barrier function. The NLRP6 inflammasome in the intestinal
epithelium physiologically induces IL-18 synthesis, and promotes
the production of antimicrobial peptides and mucus secretion by
goblet cells, which eventually inhibits intestinal barrier
disruption and maintains intestinal microbial balance. However, in
gut-liver dysfunction, protective effects of the NLRP6 inflammasome
may be inhibited, resulting in bacterial translocation, and the
transfer of PAMPs and DAMPs to the liver via the portal vein. In
the liver, accumulative PAMPs and DAMPs act on TLRs on the cell
membrane to activate the intracellular inflammasome. Furthermore,
in immune cells, NLRP3 and AIM2 inflammasome activation induces the
synthesis of IL-1β, which primarily mediates inflammation in the
liver, and increases the expression of MCP-1 and TNF-α. In
hepatocytes, MCP-1 further aggravates hepatocyte steatosis.
Furthermore, IL-1β sensitizes hepatocytes to TNF-mediated cellular
toxicity and induces pyroptosis of hepatocytes in combination with
TNF-α. In hepatic stellate cells, NLRP3 inflammasome activation
upregulates the expression level of TGF-β1 and promotes hepatic
fibrosis. Arrows indicate ‘promotion’, whereas, the symbol ‘┴’
indicates ‘inhibition’. BA, bile acid; DAMPs, danger-associated
molecular patterns; MCP-1, monocyte chemoattractant protein 1;
PAMPs, pathogen-associated molecular patterns; TLR, Toll-like
receptor; IL, interleukin; NLRP6, NACHT, LRR and PYD
domains-containing protein 6; AIM, interferon-inducible protein
AIM2; TNF, tumor necrosis factor; TGF-β1, transforming growth
factor β1. |
Conclusions and future perspectives
In conclusion, the inflammasome is associated with
the gut-liver axis by affecting the intestinal mucosal barrier and
microbial composition, and by modulating liver inflammation.
However, the majority of the previous studies focused on the role
of the inflammasome in either the gut or liver, and ignored its
complex role in the gut-liver axis. Furthermore, the inflammasome
is assembled by self-oligomerizing scaffold proteins and involves
multiple NLR or non-NLR families, including the NLRP3, NLRP6 and
the AIM2 inflammasome. As hypothesized, various inflammasomes serve
different roles in the same disease. Although the inflammasome may
be the same, its roles in maintaining the integrity of the
intestinal mucosa may differ. In addition, the NLRP3 inflammasome
was downregulated in the gut; however, upregulated in the liver in
the same disease (8). Therefore,
to understand the exact mechanisms of inflammasome in the gut-liver
axis, it is crucial to identify the role of the inflammasome in the
liver and gut, and investigate the interactions among multiple
types of inflammasomes in the same disease.
BAs serve as endocrine signaling molecules in the
pathogenesis of gut-liver axis. The effect of BAs on the NLRP3
inflammasome is controversial. BAs may directly activate the NLRP3
inflammasome. In contrast, BAs may have the opposite effect by
binding to the membrane receptor TGR5 or nuclear receptor FXR. This
discrepancy may be explained by condition-specific effects. At
physiological levels, BAs were described to inhibit the NLRP3
inflammasome in an indirect way, maintaining the cell homeostasis.
However, BAs may exert their cytotoxic effects by directly acting
on the NLRP3 inflammasome. Therefore, BA receptors may represent
the key molecules for the treatment of liver diseases. For example,
FXR agonist obeticholic acid has been approved for the treatment of
primary biliary cholangitis, whereas, TGR5 agonists failed to
improve cholestatic liver injury in a mouse model of sclerosing
cholangitis (92). The underlying
mechanism of the dual role of BAs may be associated with the
different distribution of these two BA receptors in the liver.
Further studies are required to elucidate the interactions among
BAs, BA receptors and the inflammasome.
Previous studies have demonstrated the benefits of
inflammasome-targeting drugs. For instance, the caspase inhibitor
IDN-6556 improved liver injury and liver fibrosis (24). Furthermore, administration of the
IL-1 inhibitor anakinra ameliorated liver inflammation, steatosis,
hepatocellular damage and fibrosis (17). In conclusion, based on the ongoing
mechanistic studies of the inflammasome, novel
inflammasome-targeting drugs may provide novel therapeutics for the
treatment of gut and liver diseases.
Acknowledgements
The authors thank Mr. Jin-Feng Jin for the technical
assistance in figure editing.
Funding
The present study received financial support from
The National Natural Science Foundation of China (grant nos.
81770519 and 81771633), The Science Foundation of Shanghai
(Shanghai, China; grant nos. 16411952200, 16140902300 and
17411960600), Shanghai Hospital Development Center (Shanghai,
China; grant no. SHDC12014106), Shanghai Key Disciplines (Shanghai,
China; grant no. 2017ZZ02022), Shanghai Rising-Star Program
(Shanghai, China; A type; grant no. 15QA1400800) and The Science
Foundation of Shanghai Excellent Youth Scholars (Shanghai, China;
grant no. 2017YQ042).
Availability of data and materials
Not applicable.
Authors' contributions
SZ conceived and designed the theme. JW retrieved
concerned literatures and wrote the article. RD reviewed and edited
the article.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ASC
|
apoptosis-associated speck-like
protein containing a CARD
|
|
ATP
|
adenosine 5′-triphosphate
|
|
BAs
|
bile acids
|
|
CA
|
cholic acid
|
|
CCL5
|
chemokine (C-C motif) ligand 5
|
|
CDCA
|
chenodeoxycholic acid
|
|
CYP7A1
|
cholesterol 7α-hydroxylase
|
|
DAMPs
|
damage-associated molecular
patterns
|
|
DCA
|
deoxycholic acid
|
|
DSS
|
dextran sodium sulfate
|
|
FXR
|
farnesoid-X receptor
|
|
GCA
|
glycocholic acid
|
|
IL
|
interleukin
|
|
LCA
|
lithocholic acid
|
|
LPS
|
lipopolysaccharide
|
|
LRR
|
leucine-rich repeat
|
|
MCP-1
|
monocyte chemoattractant protein 1
|
|
NACHT
|
nucleotide-binding and
oligomerization
|
|
NF-κB
|
nuclear factor-κB
|
|
NLR
|
NOD-like receptor
|
|
NLRP
|
NACHT, LRR and PYD domains-containing
protein
|
|
PAMPs
|
pathogen-associated molecular
patterns
|
|
PKA
|
protein kinase A
|
|
PRR
|
pattern recognition receptor
|
|
PYD
|
pyrin domain
|
|
ROS
|
reactive oxygen species
|
|
TGF-β1
|
transforming growth factor β1
|
|
TGR5
|
Takeda G-protein receptor 5
|
|
TLR
|
Toll-like receptor
|
|
TNF-α
|
tumor necrosis factor α
|
References
|
1
|
Visschers RG, Luyer MD, Schaap FG, Olde
Damink SW and Soeters PB: The gut-liver axis. Curr Opin Clin Nutr
Metab Care. 16:576–581. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Islam KB, Fukiya S, Hagio M, Fujii N,
Ishizuka S, Ooka T, Ogura Y, Hayashi T and Yokota A: Bile acid is a
host factor that regulates the composition of the cecal microbiota
in rats. Gastroenterology. 141:1773–1781. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yokota A, Fukiya S, Islam KB, Ooka T,
Ogura Y, Hayashi T, Hagio M and Ishizuka S: Is bile acid a
determinant of the gut microbiota on a high-fat diet? Gut Microbes.
3:455–459. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cai SY and Boyer JL: Studies on the
mechanisms of bile acid initiated hepatic inflammation in
cholestatic liver injury. Inflamm Cell Signal.
4:e15612017.PubMed/NCBI
|
|
5
|
Cai SY, Ouyang X, Chen Y, Soroka CJ, Wang
J, Mennone A, Wang Y, Mehal WZ, Jain D and Boyer JL: Bile acids
initiate cholestatic liver injury by triggering a
hepatocyte-specific inflammatory response. JCI Insight.
2:e907802017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Perez MJ and Briz O: Bile-acid-induced
cell injury and protection. World J Gastroenterol. 15:1677–1689.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Allen K, Jaeschke H and Copple BL: Bile
acids induce inflammatory genes in hepatocytes: A novel mechanism
of inflammation during obstructive cholestasis. Am J Pathol.
178:175–186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Minicis S, Rychlicki C, Agostinelli L,
Saccomanno S, Candelaresi C, Trozzi L, Mingarelli E, Facinelli B,
Magi G, Palmieri C, et al: Dysbiosis contributes to fibrogenesis in
the course of chronic liver injury in mice. Hepatology.
59:1738–1749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sabino J, Vieira-Silva S, Machiels K,
Joossens M, Falony G, Ballet V, Ferrante M, Van Assche G, Van der
Merwe S, Vermeire S and Raes J: Primary sclerosing cholangitis is
characterised by intestinal dysbiosis independent from IBD. Gut.
65:1681–1689. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wiest R, Albillos A, Trauner M, Bajaj JS
and Jalan R: Intestinal hepatic axis for liver disease. J Hepatol.
67:1084–1103. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tripathi A, Debelius J, Brenner DA, Karin
M, Loomba R, Schnabl B and Knight R: The gut-liver axis and the
intersection with the microbiome. Nat Rev Gastroenterol Hepatol.
15:397–411. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
O'Toole A, Alakkari A, Keegan D, Doherty
G, Mulcahy H and O'Donoghue D: Primary sclerosing cholangitis and
disease distribution in inflammatory bowel disease. Clin
Gastroenterol Hepatol. 10:439–441. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weismüller TJ, Trivedi PJ, Bergquist A,
Imam M, Lenzen H, Ponsioen CY, Holm K, Gotthardt D, Färkkilä MA,
Marschall HU, et al: Patient age, sex, and inflammatory bowel
disease phenotype associate with course of primary sclerosing
cholangitis. Gastroenterology. 152:1975–1984, e1978. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu R, Li X, Huang Z, Zhao D, Ganesh BS,
Lai G, Pandak WM, Hylemon PB, Bajaj JS, Sanyal AJ and Zhou H: C/EBP
homologous protein-induced loss of intestinal epithelial stemness
contributes to bile duct ligation-induced cholestatic liver injury
in mice. Hepatology. 67:1441–1457. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jee J, Mourya R, Shivakumar P, Fei L,
Wagner M and Bezerra JA: Cxcr2 signaling and the microbiome
suppress inflammation, bile duct injury, and the phenotype of
experimental biliary atresia. PLoS One. 12:e01820892017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schroder K and Tschopp J: The
inflammasomes. Cell. 140:821–832. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Petrasek J, Bala S, Csak T, Lippai D,
Kodys K, Menashy V, Barrieau M, Min SY, Kurt-Jones EA and Szabo G:
IL-1 receptor antagonist ameliorates inflammasome-dependent
alcoholic steatohepatitis in mice. J Clin Invest. 122:3476–3489.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Henao-Mejia J, Elinav E, Jin C, Hao L,
Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ,
et al: Inflammasome-mediated dysbiosis regulates progression of
NAFLD and obesity. Nature. 482:179–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wlodarska M, Thaiss CA, Nowarski R,
Henao-Mejia J, Zhang JP, Brown EM, Frankel G, Levy M, Katz MN,
Philbrick WM, et al: NLRP6 inflammasome orchestrates the colonic
host-microbial interface by regulating goblet cell mucus secretion.
Cell. 156:1045–1059. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Levy M, Thaiss CA, Zeevi D, Dohnalová L,
Zilberman-Schapira G, Mahdi JA, David E, Savidor A, Korem T, Herzig
Y, et al: Microbiota-modulated metabolites shape the intestinal
microenvironment by regulating NLRP6 inflammasome signaling. Cell.
163:1428–1443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gong Z, Zhou J, Zhao S, Tian C, Wang P, Xu
C, Chen Y, Cai W and Wu J: Chenodeoxycholic acid activates NLRP3
inflammasome and contributes to cholestatic liver fibrosis.
Oncotarget. 7:83951–83963. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han J, Bae J, Choi CY, Choi SP, Kang HS,
Jo EK, Park J, Lee YS, Moon HS, Park CG, et al: Autophagy induced
by AXL receptor tyrosine kinase alleviates acute liver injury via
inhibition of NLRP3 inflammasome activation in mice. Autophagy.
12:2326–2343. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wree A, McGeough MD, Inzaugarat ME, Eguchi
A, Schuster S, Johnson CD, Peña CA, Geisler LJ, Papouchado BG,
Hoffman HM and Feldstein AE: NLRP3 inflammasome driven liver injury
and fibrosis. Roles of IL-17 and TNF. Hepatology. 2017.
|
|
24
|
Barreyro FJ, Holod S, Finocchietto PV,
Camino AM, Aquino JB, Avagnina A, Carreras MC, Poderoso JJ and
Gores GJ: The pan-caspase inhibitor Emricasan (IDN-6556) decreases
liver injury and fibrosis in a murine model of non-alcoholic
steatohepatitis. Liver Int. 35:953–966. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alaish SM, Smith AD, Timmons J, Greenspon
J, Eyvazzadeh D, Murphy E, Shea-Donahue T, Cirimotich S, Mongodin
E, Zhao A, et al: Gut microbiota, tight junction protein
expression, intestinal resistance, bacterial translocation and
mortality following cholestasis depend on the genetic background of
the host. Gut Microbes. 4:292–305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pierantonelli I, Rychlicki C, Agostinelli
L, Giordano DM, Gaggini M, Fraumene C, Saponaro C, Manghina V,
Sartini L, Mingarelli E, et al: Lack of NLRP3-inflammasome leads to
gut-liver axis derangement, gut dysbiosis and a worsened phenotype
in a mouse model of NAFLD. Sci Rep. 7:122002017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo C, Xie S, Chi Z, Zhang J, Liu Y, Zhang
L, Zheng M, Zhang X, Xia D, Ke Y, et al: Bile acids control
inflammation and metabolic disorder through inhibition of NLRP3
inflammasome. Immunity. 45:802–816. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hao H, Cao L, Jiang C, Che Y, Zhang S,
Takahashi S, Wang G and Gonzalez FJ: Farnesoid X receptor
regulation of the NLRP3 inflammasome underlies
cholestasis-associated sepsis. Cell Metab. 25:856–867, e855. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xie S, Guo C, Chi Z, Huang B, Wu Y, Wang D
and Xia D: A rapid administration of GW4064 inhibits the NLRP3
inflammasome activation independent of farnesoid X receptor
agonism. FEBS Lett. 591:2836–2847. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Martinon F, Burns K and Tschopp J: The
inflammasome: A molecular platform triggering activation of
inflammatory caspases and processing of proIL-beta. Mol Cell.
10:417–426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Giebeler A, Brandenburg LO, Kaldenbach M,
Erschfeld S, Wasmuth H, Wruck C, Trautwein C and Streetz KL: Lack
of hepatic c-Met and gp130 expression is associated with an
impaired antibacterial response and higher lethality after bile
duct ligation. Lab Invest. 92:1726–1737. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Matsushita H, Miyake Y, Takaki A, Yasunaka
T, Koike K, Ikeda F, Shiraha H, Nouso K and Yamamoto K: TLR4, TLR9,
and NLRP3 in biliary epithelial cells of primary sclerosing
cholangitis: Relationship with clinical characteristics. J
Gastroenterol Hepatol. 30:600–608. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Szabo G and Petrasek J: Inflammasome
activation and function in liver disease. Nat Rev Gastroenterol
Hepatol. 12:387–400. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gross O, Thomas CJ, Guarda G and Tschopp
J: The inflammasome: An integrated view. Immunol Rev. 243:136–151.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ting JP, Lovering RC, Alnemri ES, Bertin
J, Boss JM, Davis BK, Flavell RA, Girardin SE, Godzik A, Harton JA,
et al: The NLR gene family: A standard nomenclature. Immunity.
28:285–287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bauernfeind FG, Horvath G, Stutz A,
Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks
BG, Fitzgerald KA, et al: Cutting edge: NF-kappaB activating
pattern recognition and cytokine receptors license NLRP3
inflammasome activation by regulating NLRP3 expression. J Immunol.
183:787–791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Boaru SG, Borkham-Kamphorst E, Van de Leur
E, Lehnen E, Liedtke C and Weiskirchen R: NLRP3 inflammasome
expression is driven by NF-κB in cultured hepatocytes. Biochem
Biophys Res Commun. 458:700–706. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kahlenberg JM and Dubyak GR: Mechanisms of
caspase-1 activation by P2X7 receptor-mediated K+
release. Am J Physiol Cell Physiol. 286:C1100–C1108. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kanneganti TD, Lamkanfi M, Kim YG, Chen G,
Park JH, Franchi L, Vandenabeele P and Núñez G: Pannexin-1-mediated
recognition of bacterial molecules activates the cryopyrin
inflammasome independent of Toll-like receptor signaling. Immunity.
26:433–443. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hornung V, Bauernfeind F, Halle A, Samstad
EO, Kono H, Rock KL, Fitzgerald KA and Latz E: Silica crystals and
aluminum salts activate the NALP3 inflammasome through phagosomal
destabilization. Nat Immunol. 9:847–856. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou R, Tardivel A, Thorens B, Choi I and
Tschopp J: Thioredoxin-interacting protein links oxidative stress
to inflammasome activation. Nat Immunol. 11:136–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Elinav E, Strowig T, Kau AL, Henao-Mejia
J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon
JI and Flavell RA: NLRP6 inflammasome regulates colonic microbial
ecology and risk for colitis. Cell. 145:745–757. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gremel G, Wanders A, Cedernaes J,
Fagerberg L, Hallström B, Edlund K, Sjöstedt E, Uhlén M and Pontén
F: The human gastrointestinal tract-specific transcriptome and
proteome as defined by RNA sequencing and antibody-based profiling.
J Gastroenterol. 50:46–57. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Del Chierico F, Vernocchi P, Petrucca A,
Paci P, Fuentes S, Praticò G, Capuani G, Masotti A, Reddel S, Russo
A, et al: Phylogenetic and metabolic tracking of gut microbiota
during perinatal development. PLoS One. 10:e01373472015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kempster SL, Belteki G, Forhead AJ, Fowden
AL, Catalano RD, Lam BY, McFarlane I, Charnock-Jones DS and Smith
GC: Developmental control of the Nlrp6 inflammasome and a
substrate, IL-18, in mammalian intestine. Am J Physiol Gastrointest
Liver Physiol. 300:G253–G263. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chudnovskiy A, Mortha A, Kana V, Kennard
A, Ramirez JD, Rahman A, Remark R, Mogno I, Ng R, Gnjatic S, et al:
Host-Protozoan interactions protect from mucosal infections through
activation of the inflammasome. Cell. 167:444–456, e414. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sun Y, Zhang M, Chen CC, Gillilland M III,
Sun X, El-Zaatari M, Huffnagle GB, Young VB, Zhang J, Hong SC, et
al: Stress-induced corticotropin-releasing hormone-mediated NLRP6
inflammasome inhibition and transmissible enteritis in mice.
Gastroenterology. 144:1478–1487, e1471-1487.e1-e8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Birchenough GM, Nyström EE, Johansson ME
and Hansson GC: A sentinel goblet cell guards the colonic crypt by
triggering Nlrp6-dependent Muc2 secretion. Science. 352:1535–1542.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huber S, Gagliani N, Zenewicz LA, Huber
FJ, Bosurgi L, Hu B, Hedl M, Zhang W, O'Connor W Jr, Murphy AJ, et
al: IL-22BP is regulated by the inflammasome and modulates
tumorigenesis in the intestine. Nature. 491:259–263. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang P, Zhu S, Yang L, Cui S, Pan W,
Jackson R, Zheng Y, Rongvaux A, Sun Q, Yang G, et al: Nlrp6
regulates intestinal antiviral innate immunity. Science.
350:826–830. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Elinav E, Thaiss CA and Flavell RA:
Analysis of microbiota alterations in inflammasome-deficient mice.
Methods Mol Biol. 1040:185–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen GY, Liu M, Wang F, Bertin J and Núñez
G: A functional role for Nlrp6 in intestinal inflammation and
tumorigenesis. J Immunol. 186:7187–7194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Normand S, Delanoye-Crespin A, Bressenot
A, Huot L, Grandjean T, Peyrin-Biroulet L, Lemoine Y, Hot D and
Chamaillard M: Nod-like receptor pyrin domain-containing protein 6
(NLRP6) controls epithelial self-renewal and colorectal
carcinogenesis upon injury. Proc Natl Acad Sci USA. 108:9601–9606.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hu B, Elinav E, Huber S, Strowig T, Hao L,
Hafemann A, Jin C, Wunderlich C, Wunderlich T, Eisenbarth SC and
Flavell RA: Microbiota-induced activation of epithelial IL-6
signaling links inflammasome-driven inflammation with transmissible
cancer. Proc Natl Acad Sci USA. 110:9862–9867. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Seo SU, Kamada N, Muñoz-Planillo R, Kim
YG, Kim D, Koizumi Y, Hasegawa M, Himpsl SD, Browne HP, Lawley TD,
et al: Distinct commensals induce interleukin-1β via NLRP3
inflammasome in inflammatory monocytes to promote intestinal
inflammation in response to injury. Immunity. 42:744–755. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Filardy AA, He J, Bennink J, Yewdell J and
Kelsall BL: Posttranscriptional control of NLRP3 inflammasome
activation in colonic macrophages. Mucosal Immunol. 9:850–858.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Allen IC, TeKippe EM, Woodford RM, Uronis
JM, Holl EK, Rogers AB, Herfarth HH, Jobin C and Ting JP: The NLRP3
inflammasome functions as a negative regulator of tumorigenesis
during colitis-associated cancer. J Exp Med. 207:1045–1056. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hu B, Elinav E, Huber S, Booth CJ, Strowig
T, Jin C, Eisenbarth SC and Flavell RA: Inflammation-induced
tumorigenesis in the colon is regulated by caspase-1 and NLRC4.
Proc Natl Acad Sci USA. 107:21635–21640. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ruiz PA, Morón B, Becker HM, Lang S,
Atrott K, Spalinger MR, Scharl M, Wojtal KA, Fischbeck-Terhalle A,
Frey-Wagner I, et al: Titanium dioxide nanoparticles exacerbate
DSS-induced colitis: Role of the NLRP3 inflammasome. Gut.
66:1216–1224. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zherebiatiev A and Kamyshnyi A: Expression
levels of proinflammatory cytokines and NLRP3 inflammasome in an
experimental model of Oxazolone-induced colitis. Iran J Allergy
Asthma Immunol. 15:39–45. 2016.PubMed/NCBI
|
|
61
|
De la Fuente M, Franchi L, Araya D,
Díaz-Jiménez D, Olivares M, Álvarez-Lobos M, Golenbock D, González
MJ, López-Kostner F, Quera R, et al: Escherichia coli isolates from
inflammatory bowel diseases patients survive in macrophages and
activate NLRP3 inflammasome. Int J Med Microbiol. 304:384–392.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bauer C, Duewell P, Lehr HA, Endres S and
Schnurr M: Protective and aggravating effects of Nlrp3 inflammasome
activation in IBD models: Influence of genetic and environmental
factors. Dig Dis. 30 Suppl 1:S82–S90. 2012. View Article : Google Scholar
|
|
63
|
Hirota SA, Ng J, Lueng A, Khajah M, Parhar
K, Li Y, Lam V, Potentier MS, Ng K, Bawa M, et al: NLRP3
inflammasome plays a key role in the regulation of intestinal
homeostasis. Inflamm Bowel Dis. 17:1359–1372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zaki MH, Boyd KL, Vogel P, Kastan MB,
Lamkanfi M and Kanneganti TD: The NLRP3 inflammasome protects
against loss of epithelial integrity and mortality during
experimental colitis. Immunity. 32:379–391. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Szabo G and Csak T: Inflammasomes in liver
diseases. J Hepatol. 57:642–654. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Mandrekar P, Ambade A, Lim A, Szabo G and
Catalano D: An essential role for monocyte chemoattractant
protein-1 in alcoholic liver injury: Regulation of proinflammatory
cytokines and hepatic steatosis in mice. Hepatology. 54:2185–2197.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Miura K, Kodama Y, Inokuchi S, Schnabl B,
Aoyama T, Ohnishi H, Olefsky JM, Brenner DA and Seki E: Toll-like
receptor 9 promotes steatohepatitis by induction of
interleukin-1beta in mice. Gastroenterology. 139:323–334.e327.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kubes P and Mehal WZ: Sterile inflammation
in the liver. Gastroenterology. 143:1158–1172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Csak T, Pillai A, Ganz M, Lippai D,
Petrasek J, Park JK, Kodys K, Dolganiuc A, Kurt-Jones EA and Szabo
G: Both bone marrow-derived and non-bone marrow-derived cells
contribute to AIM2 and NLRP3 inflammasome activation in a
MyD88-dependent manner in dietary steatohepatitis. Liver Int.
34:1402–1413. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Rao RK and Samak G: Bile duct epithelial
tight junctions and barrier function. Tissue Barriers.
1:e257182013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Fickert P, Fuchsbichler A, Wagner M,
Zollner G, Kaser A, Tilg H, Krause R, Lammert F, Langner C,
Zatloukal K, et al: Regurgitation of bile acids from leaky bile
ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice.
Gastroenterology. 127:261–274. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Maroni L, Agostinelli L, Saccomanno S,
Pinto C, Giordano DM, Rychlicki C, De Minicis S, Trozzi L, Banales
JM, Melum E, et al: Nlrp3 activation induces Il-18 synthesis and
affects the epithelial barrier function in reactive cholangiocytes.
Am J Pathol. 187:366–376. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ikegami T and Honda A: Reciprocal
interactions between bile acids and gut microbiota in human liver
diseases. Hepatol Res. 48:15–27. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hofmann AF: The enterohepatic circulation
of bile acids in mammals: Form and functions. Front Biosci
(Landmark Ed). 14:2584–2598. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
75
|
Dawson PA and Karpen SJ: Intestinal
transport and metabolism of bile acids. J Lipid Res. 56:1085–1099.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ridlon JM, Kang DJ and Hylemon PB: Bile
salt biotransformations by human intestinal bacteria. J Lipid Res.
47:241–259. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Halilbasic E, Claudel T and Trauner M:
Bile acid transporters and regulatory nuclear receptors in the
liver and beyond. J Hepatol. 58:155–168. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Makishima M, Okamoto AY, Repa JJ, Tu H,
Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ and Shan B:
Identification of a nuclear receptor for bile acids. Science.
284:1362–1365. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Parks DJ, Blanchard SG, Bledsoe RK,
Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki
AM, Moore DD and Lehmann JM: Bile acids: Natural ligands for an
orphan nuclear receptor. Science. 284:1365–1368. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Potthoff MJ, Potts A, He T, Duarte JA,
Taussig R, Mangelsdorf DJ, Kliewer SA and Burgess SC: Colesevelam
suppresses hepatic glycogenolysis by TGR5-mediated induction of
GLP-1 action in DIO mice. Am J Physiol Gastrointest Liver Physiol.
304:G371–G380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Schaap FG, Trauner M and Jansen PL: Bile
acid receptors as targets for drug development. Nat Rev
Gastroenterol Hepatol. 11:55–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Reich M, Klindt C, Deutschmann K, Spomer
L, Häussinger D and Keitel V: Role of the G protein-coupled bile
acid receptor TGR5 in liver damage. Dig Dis. 35:235–240. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Kawamata Y, Fujii R, Hosoya M, Harada M,
Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, et al:
A G protein-coupled receptor responsive to bile acids. J Biol Chem.
278:9435–9440. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ding JW, Andersson R, Soltesz V, Willén R
and Bengmark S: The role of bile and bile acids in bacterial
translocation in obstructive jaundice in rats. Eur Surg Res.
25:11–19. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Inagaki T, Moschetta A, Lee YK, Peng L,
Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ, et al:
Regulation of antibacterial defense in the small intestine by the
nuclear bile acid receptor. Proc Natl Acad Sci USA. 103:3920–3925.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wahlström A, Sayin SI, Marschall HU and
Bäckhed F: Intestinal crosstalk between bile acids and microbiota
and its impact on host metabolism. Cell Metab. 24:41–50. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wang YD, Chen WD, Wang M, Yu D, Forman BM
and Huang W: Farnesoid X receptor antagonizes nuclear factor kappaB
in hepatic inflammatory response. Hepatology. 48:1632–1643. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wagner M, Zollner G and Trauner M: Nuclear
receptors in liver disease. Hepatology. 53:1023–1034. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhu C, Fuchs CD, Halilbasic E and Trauner
M: Bile acids in regulation of inflammation and immunity: Friend or
foe? Clin Exp Rheumatol. 34 (4 Suppl 98):S25–S31. 2016.PubMed/NCBI
|
|
90
|
Inagaki T, Choi M, Moschetta A, Peng L,
Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA,
et al: Fibroblast growth factor 15 functions as an enterohepatic
signal to regulate bile acid homeostasis. Cell Metab. 2:217–225.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Péan N, Doignon I, Garcin I, Besnard A,
Julien B, Liu B, Branchereau S, Spraul A, Guettier C, Humbert L, et
al: The receptor TGR5 protects the liver from bile acid overload
during liver regeneration in mice. Hepatology. 58:1451–1460. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Baghdasaryan A, Claudel T, Gumhold J,
Silbert D, Adorini L, Roda A, Vecchiotti S, Gonzalez FJ, Schoonjans
K, Strazzabosco M, et al: Dual farnesoid X receptor/TGR5 agonist
INT-767 reduces liver injury in the Mdr2-/- (Abcb4-/-) mouse
cholangiopathy model by promoting biliary HCO3 output.
Hepatology. 54:1303–1312. 2011. View Article : Google Scholar : PubMed/NCBI
|



















