Introduction
Numerous wear particles exist in the interface
membranes between bone and artificial joints, which has been
associated with osteolysis in numerous animal models and may induce
the inflammatory reaction of macrophages in vitro (1,2).
Lochner et al (3)
discovered that apoptotic rates were increased in osteoblasts
exposed to pure titanium (Ti) particles, titanium oxide,
polymethylmethacrylate and particulate zirconium oxide. Piao et
al (4) recently reported that
Ti particles induced osteogenic inhibition and bone
destruction.
Hyperoside (Hy), a flavonoid glycoside compound
extracted from plants, is widely found in the fruits and herbs of a
number of different plant families, including Hypericaceae,
Rosaceae, Campanulaceae, Ericaceae and Labiatae (4,5).
Protective effects of Hy against liver damage, depression,
inflammation, thrombus, oxidative stress, apoptosis and cancer have
been documented in previous studies (6–9).
Recently, Zhang et al (10)
reported that Hy inhibited the phosphorylation of transcription
factor p65/nuclear factor (NF)-κB, mitogen activated protein kinase
(MAPK; including p38 MAPKs, MAPK 8, MAPK 1 and MAPK 3) activated
transcription factor 3 protein expression, and additionally
suppressed apoptosis regulator BAX (Bax), cytochrome c, caspase-9
and caspase-3 in the liver tissues of diabetic mice.
Tumor necrosis factor ligand superfamily member 12
(TWEAK) is a transmembrane protein composed of 249 amino acids and
located at 17p13 of the chromosome (11). As a member of the tumor necrosis
factor (TNF) superfamily, TWEAK is a TNF family ligand expressed in
numerous human tissues (12,13).
Previous studies demonstrated that TWEAK exerts a variety of
biological effects, including releasing pro-inflammatory cytokines,
mediating immunoreactions, promoting apoptosis, and regulating the
repair and regeneration of tissues in combination with its receptor
(14,15). TWEAK was able to activate the
classical NF-κB signaling pathway and the non-classical NF-κB and
MAPK pathways (16–18). With high conservation, the MAPK
signaling pathways extensively exist in cells, functioning as a
transmitter of stimulatory signals from the outside to the inside
of the cell to induce a series of biological responses (19). p38 MAPK is a classical MAPK
pathway. In the presence of environmental stimuli or stimulating
factors, including TNF-α, TWEAK, interleukin-1 and
ischemia/reperfusion, extracellular signals of p38 MAPK
specifically bind with receptors to promote apoptosis,
differentiation, migration, infiltration or inflammation (20–22).
Apoptosis and autophagy have dual roles; they exert
protective effects when subjected to short and moderate-intensity
levels of cell stress, and induce cell death when excessive
(23). The present study aimed to
investigate the effects of Hy on the apoptosis and autophagy of
osteoblasts exposed to Ti particles, and examine whether TWEAK and
p38 MAPK are involved in the mechanism.
Materials and methods
Cell culture
MC3T3-E1 cells were purchased from National
Infrastructure of Cell Line Resource (Beijing, China), cultured in
α-minimum essential medium (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing 10% fetal calf serum (Shanghai Macklin
Biochemical Co., Ltd., Shanghai, China), and incubated in a 5%
CO2 incubator at 37°C.
Immunocytochemical staining
Cells were seeded at a density of 3×104
cells/ml on sterilized glass coverslips. Following fixation for 10
min with 1 ml/well Immunol Staining Fix Solution (Beyotime
Institute of Biotechnology, Haimen, China) at room temperature, the
coverslips were soaked in 0.75% H2O2-PBS for
10 min at 4°C to block. The slides were incubated overnight with
primary anti-OPG antibody (1:100; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA; polyclonal goat antibody n-20; cat. no. sc:8468),
diluted in 1% bovine serum albumin (Thermo Fisher Scientific,
Inc.). Subsequent to returning to room temperature and being
washed, the slides were incubated with biotinylated secondary
antibodies [goat anti-rabbit immunoglobulin (Ig)G-B; cat. no.
sc-2040; rabbit anti-goat IgG-B; cat. no. sc-2774; 1:100; Santa
Cruz Biotechnology, Inc.] for 1 h at room temperature. A
streptavidin-biotin-peroxidase complex (ABC kit; Vectastain; Vector
Laboratories, Inc., Burlingame, CA, USA) was subsequently added for
30 min at room temperature, followed by chromogen
3,3′diaminobenzidine tetrahydrochloride hydrate (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany), added with 3% hydrogen peroxide in
PBS for 1 min at room temperature. The slides were counterstained
with Harris' hematoxylin at room temperature for 2 min. The
analysis was conducted using the Axio Imager M1 light microscope
(Zeiss AG, Oberkochen, Germany) with magnification, ×200.
Grouping
Concentrations of 0.1 and 1 mg/ml of Ti (Beijing
Yannuo Xincheng Technology Co., Ltd., Beijing, China) were firstly
used to treat MC3T3-E1 cells for 24 h at room temperature. To
investigate the effects of Hy (Nanjing Daofufu Biotechnology Co.,
Ltd., Jiangsu, China) on cell viability, apoptosis, autophagy and
expression levels of associated genes, the MC3T3-E1 cells were
divided into four groups: Control group; Ti group; Hy-1+Ti group;
and Hy-2+Ti group. In the Hy-1+Ti group and the Hy-2+Ti group,
cells were pretreated with 200 and 400 µg/ml Hy at room
temperature, respectively, for 6 h, and subsequently treated with 1
mg/ml Ti particles for 12 h. In the Ti group, cells were treated
with 1 mg/ml Ti particles for 12 h for the following experiments.
Cells were treated with anisomycin (60 µM for 1 h at room
temperature; MedChemExpress USA, Monmouth Junction, NJ, USA) to
verify the role of the MAPK pathway.
Cell counting kit-8 (CCK-8) assay
Cell viability in each group was detected using a
CCK-8 assay (Beyotime Institute of Biotechnology). Cells were
grouped and seeded in 96-well plates at a density of
3×104 cells/ml, and subsequently incubated at 37°C in a
5% CO2 incubator for 4 h. Following the addition of 10
µl CCK-8 reagent to each well, cells were placed into a 5%
CO2 incubator at 37°C for 2 h. Optical density values of
each group were measured at 450 nm using a spectrophotometer
(Sigma-Aldrich; Merck KGaA).
Flow cytometry (FCM)
Cells in the logarithmic phase were collected and
incubated in 6-well plates at a density of 1×105
cells/well. Cells were digested with EDTA-free trypsin (Beyotime
Institute of Biotechnology), stained with Annexin V-fluorescein
isothiocyanate and propidium iodide (MedChem Express LLC, Monmouth
Junction, NJ, USA), and incubated in a dark place for 15 min at
room temperature. The apoptosis rates of each group were detected
using a flow cytometer (EPICS XL-MCL FCM; Beckman Coulter, Inc.,
Brea, CA, USA) with an excitation wavelength of 488 nm and an
emission wavelength of 530 nm. Data was analyzed using FCS Express
version 3.0 (De Novo Software, Glendale, CA, USA).
Cells were treated for 2 h with the lysosomal
inhibitors E64d and pepstatin A (10 mg/ml; MedChemExpress USA) or
dimethyl sulfoxide (DMSO) following transfection.
Monodansylcadaverine (MDC) powder was purchased from Sigma-Aldrich;
Merck KGaA and dissolved in DMSO at 0.1 mol/l of the stock
concentration. The working concentration was 50 µm/l. The cells
were incubated with the MDC dye for 45 min in the dark at 4°C. The
cells were subsequently washed with PBS three times. The positive
rate of autophagy was determined using a flow cytometer (EPICS
XL-MCL FCM; Beckman Coulter, Inc.). Data was analyzed using FCS
Express version 3.0 (De Novo Software, Glendale, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Expression levels of caspase-3, Bax, apoptosis
regulator Bcl-2 (Bcl-2), cellular tumor antigen p53 (p53), Beclin1
and microtubule-associated protein light chain 3 conversion
(LC3-II/I) mRNA were detected using RT-qPCR. Cells were seeded in
6-well plates at a density of 2×106 cells/well. Total
RNA was extracted with TRIzol® (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
concentration of extracted RNA was read using an ultraviolet
spectrophotometer (Thermo Fisher Scientific, Inc.). cDNA was
synthesized by RT with the Takara PrimeScript RT kit (Takara Bio,
Inc., Otsu, Japan) at 37°C for 60 min, 85°C for 5 min and 4°C for 5
min. Quantification of mRNA was performed using a TaqMan Gene
Expression Assay (Thermo Fisher Scientific, Inc.). PCR was
conducted by activating the DNA polymerase at 95°C for 10 min,
followed by 40 cycles of two-step PCR (95°C for 15 sec and 60°C for
45 sec), a final extension at 75°C for 10 min and holding at 4°C.
GAPDH was applied as the internal control to normalize the other
mRNA expression levels. The following primers in the present study
were designed by Sangon Biotech Co., Ltd. (Shanghai, China):
Forward 5′-AGAGCTGGACTGCGGTATTG-3′ and
reverse,≈5′-CCATGACCCGTCCCTTGAAT-3′ for caspase-3 (product: 145
bp); forward, 5′-TGGCGATGAACTGGACAACA-3′ and reverse,
5′-CACGGAAGAAGACCTCTCGG-3′ for Bax (product: 86 bp); forward,
5′-TTCTTTCCCCGGAAGGATGG-3′ and reverse, 5′-AGTATCCCACTCGTAGCCCC-3′
for Bcl-2 (product: 112 bp); forward, 5′-ATGAGCGTTGCTCTGATGGT-3′
and reverse, 5′-GGTGGCTCATACGGTACCAC-3′ for p53 (product: 133 bp);
forward, 5′-AACCCCATGCTGTCCTTTCC-3′ and reverse,
5′-CAACTGTGTGCCACAAGCATC-3′ for Beclin1 (product: 171 bp); forward,
5′-TCTGAGTCAAGAGGAGGGGT-3′ and reverse, 5′-ATCTCTGCCTAATCCACCCG-3′
for LC3-I (product: 113 bp); forward, 5′-TCCCAAGAAACCTTCGGCTT-3′
and reverse, 5′-CCAGGACTTGGTATGCTGGC-3′ for LC3-II (product: 185
bp) and forward, 5′-GGCTCATGACCACAGTCCAT-3′ and reverse,
5′-ACATTGGGGGTAGGAACACG-3′ for GAPDH (product: 202 bp). Each
reaction was run in triplicate. The quantification of gene
expression data was analyzed using the 2−ΔΔCq method
(24).
Western blotting
Cells were incubated in 6-well plates at a density
of 2×106 cells/well, and grouped. Cells were harvested
and washed twice with PBS, and lysed in ice-cold
radioimmunoprecipitation assay buffer (Shanghai Yeasen
Biotechnology Co., Ltd., Shanghai, China) with freshly mixed 0.01%
phenylmethanesulfonyl fluoride as a protease inhibitor (Shanghai
Macklin Biochemical Co., Ltd.), and subsequently incubated for 30
min on ice. Cell lysates were centrifuged at 10,000 × g for 5 min
at 4°C; supernatants containing 20–30 µg protein were collected,
and protein concentration was determined using a bicinchoninic acid
kit (Thermo Fisher Scientific, Inc.). Samples (20 µg/lane) were run
on 10% SDS-PAGE gels and electrophoretically transferred to
nitrocellulose membranes (Thermo Fisher Scientific, Inc.). To block
the non-specific proteins, 5% fat-free milk was incubated with the
membranes for 2 h at room temperature. Membranes were incubated
with the following primary specific antibodies at 4°C for 6 h and
subsequently at room temperature for 4 h: Anti-caspase-3 antibody
(1:500; cat. no. ab13847), anti-Bax antibody (1:1,000; cat. no.
ab32503), anti-Bcl-2 antibody (1:1,000; cat. no. ab692), anti-p53
antibody (1:1,000; cat. no. ab26), anti-Beclin1 antibody (1:1,000;
cat. no. ab62557), anti-LC3B antibody (1:1,000; cat. no. ab48394),
anti-TWEAK antibody (1:1,000; cat. no. ab37170), anti-p38 (phospho
T180+Y182) antibody (1:1,000; cat. no. ab45381), anti-p38 antibody
(1:1,000; cat. no. ab31828), and anti-GAPDH antibody (1:2,000; cat.
no. ab8245; all Abcam). The horseradish peroxidase-conjugated
secondary antibodies were goat anti-mouse IgG heavy and light
chains (H&L; 1:2,000; cat. no. ab6789) and donkey anti-goat IgG
H&L (1:2,000; cat. no. ab6885). The membranes were subsequently
incubated at room temperature for 1 h. GAPDH was used as the
reference protein. Blots were visualized using enhanced
chemiluminescence (Thermo Fisher Scientific, Inc.). The density of
the blots was analyzed using Quantity One software version 2.4
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
The data were analyzed using Prism GraphPad version
6.0 software (GraphPad Software, Inc., La Jolla, CA, USA) and are
presented as the mean ± standard deviation. Comparisons of multiple
treatment groups were conducted using one-way analysis of variance
followed by Tukey's and Bonferroni's post-hoc tests. P<0.05 was
considered to indicate a statistically significant difference.
Results
MC3T3-E1 cells are successfully
identified
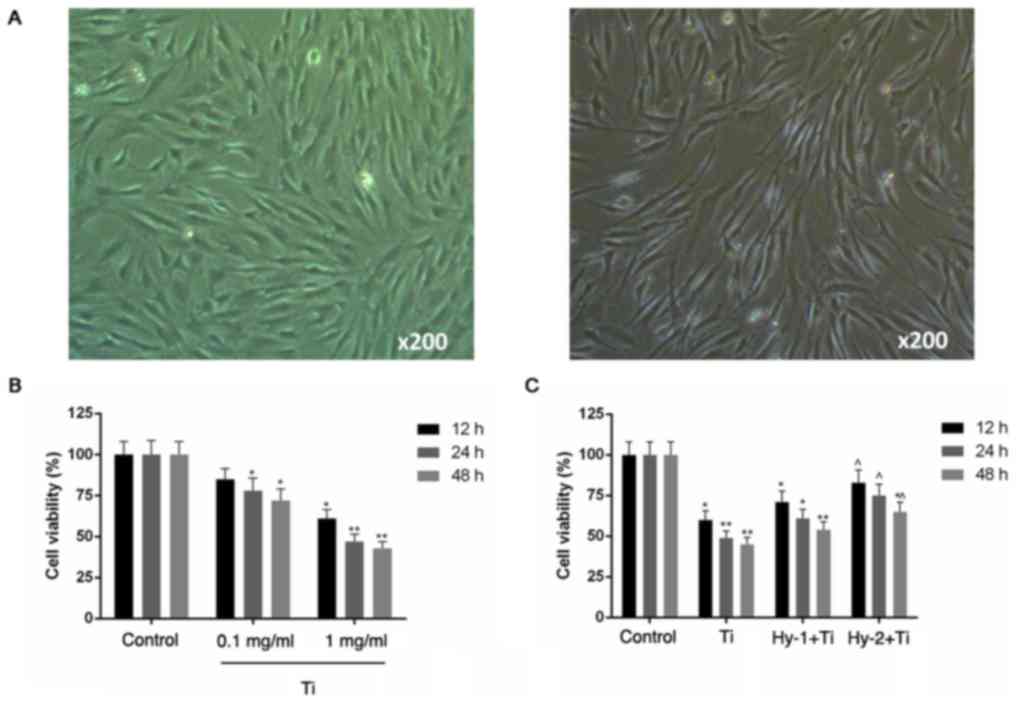
Under the inverted microscope (×200), MC3T3-E1 cells
were successfully observed in serum medium and by
immunocytochemical staining following 24 h of culture (Fig. 1A).
Pretreatment with Hy increases
viability of Ti particle-injured MC3T3-E1 cells
The CCK-8 assay results demonstrated a negative
influence of Ti particles on the viability of MC3T3-E1 cells in a
time- and concentration-dependent manner (P<0.05; Fig. 1B). In groups that were pretreated
with Hy, cells were protected against Ti particle-induced injury
and exhibited increased viability, compared with the Ti group
(P<0.05; Fig. 1C).
Pretreatment with Hy decreases
apoptosis in Ti particle-injured MC3T3-E1 cells
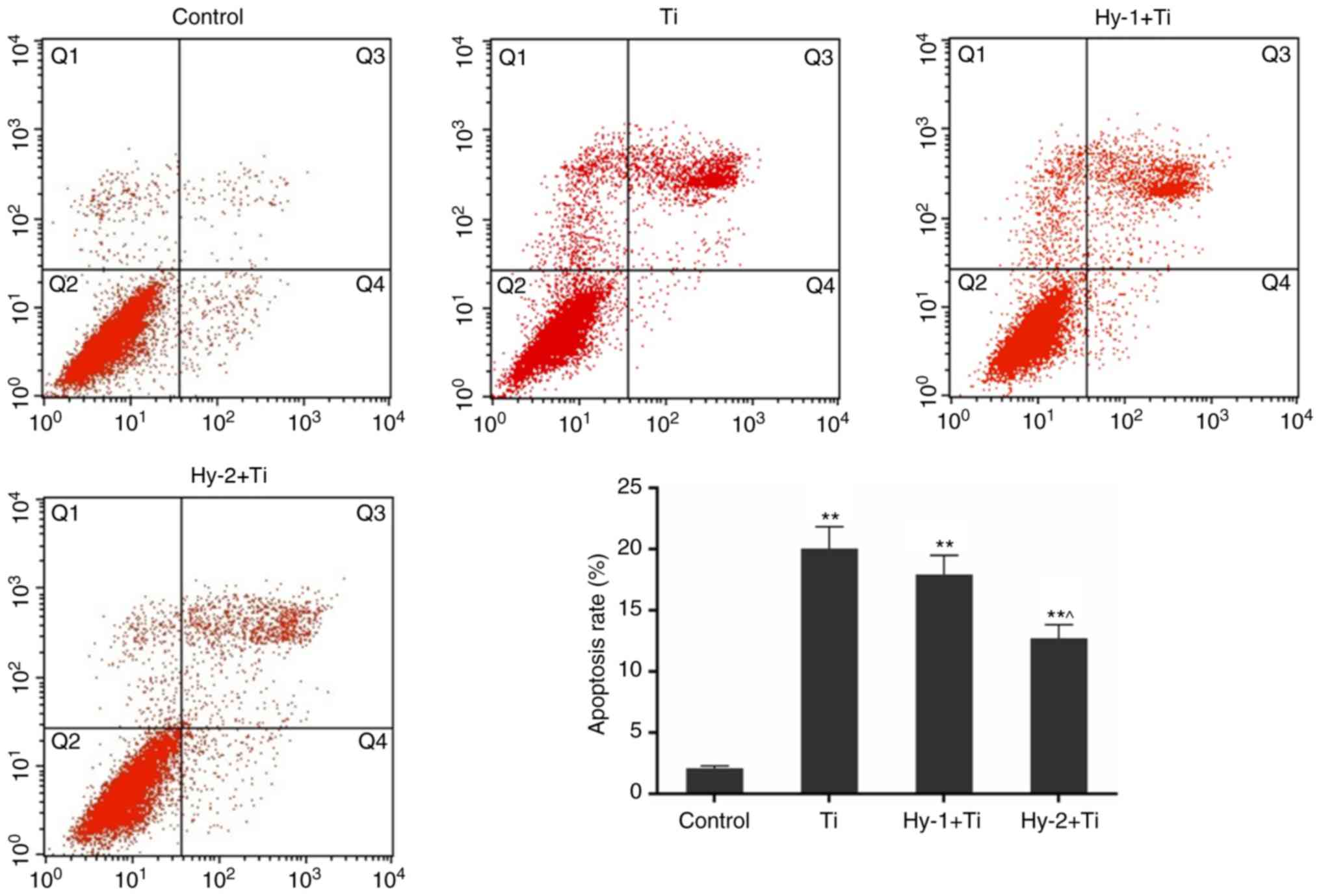
Apoptosis rates in the control, Ti, Hy-1+Ti and
Hy-2+Ti groups were detected by FCM. In response to Ti particles,
the apoptosis rate of MC3T3-E1 cells was significantly increased by
~10 times from 2.08±0.18% in the control group to 20.04±1.79% in
the Ti group (P<0.01). The difference in apoptosis rates between
the Ti and Hy-2+Ti group was significant (P<0.05; Fig. 2).
Hy pretreatment mitigates autophagy of
Ti particle injured MC3T3-E1 cells
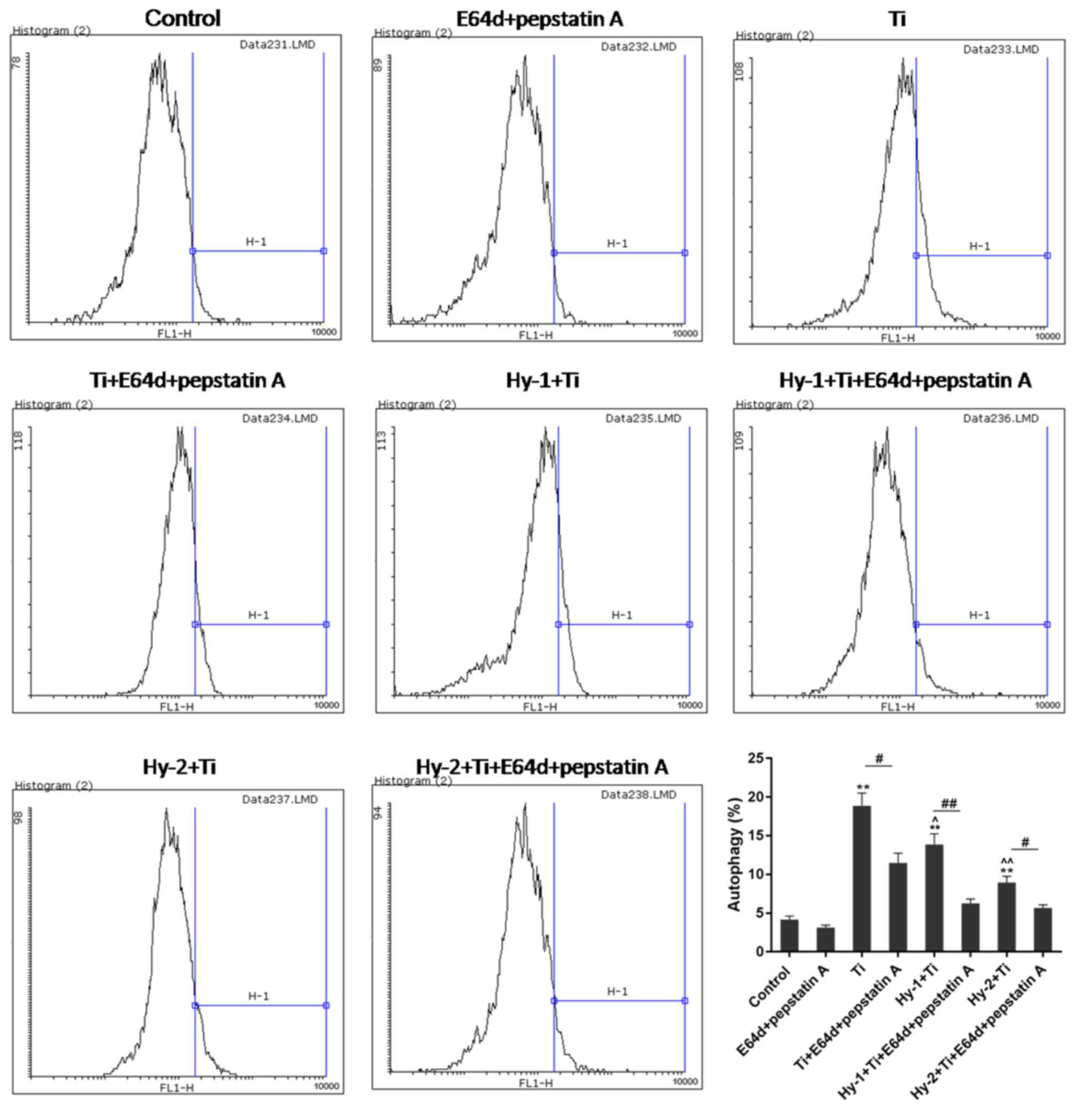
The FCM results demonstrated alterations in
autophagy rates of MC3T3-E1 cells in different conditions. In the
Ti particles treated groups, autophagy was significantly increased,
with an approximately four times increase from normal cells to Ti
treated cells (P<0.01). In the Hy1+Ti and Hy2+Ti groups, Hy
pretreatment mitigated the increase in autophagy in Ti
particle-induced injury. Under the pretreatment condition of high
dose of Hy, the autophagy rate was significantly decreased by half
(P<0.01). With the addition of protease inhibitors (E64d and
pepstatin A; 1:1) in each group, autophagy rates were significantly
blocked (P<0.05; Fig. 3).
Pretreatment with Hy downregulates the
expression of pro-apoptotic genes in Ti particle-injured MC3T3-E1
cells
Using RT-qPCR and western blotting, alterations in
the expression levels of apoptosis-associated gene products were
detected among groups. It was observed that under treatment with Ti
particles, the mRNA and protein expression levels of pro-apoptotic
genes caspase-3, Bax and p53 were significantly increased, and the
anti-apoptotic gene Bcl-2 was significantly decreased, compared
with the control group (P<0.05 or P<0.01). In the conditions
with Hy pretreatment, the differences were not as pronounced. In
particular, in the high-dose Hy group, the expression levels of
caspase-3, Bax and p53 were significantly decreased; whereas the
expression level of Bcl-2 gene products was significantly
upregulated compared with the Ti group (P<0.05 or P<0.01;
Fig. 4A-E).
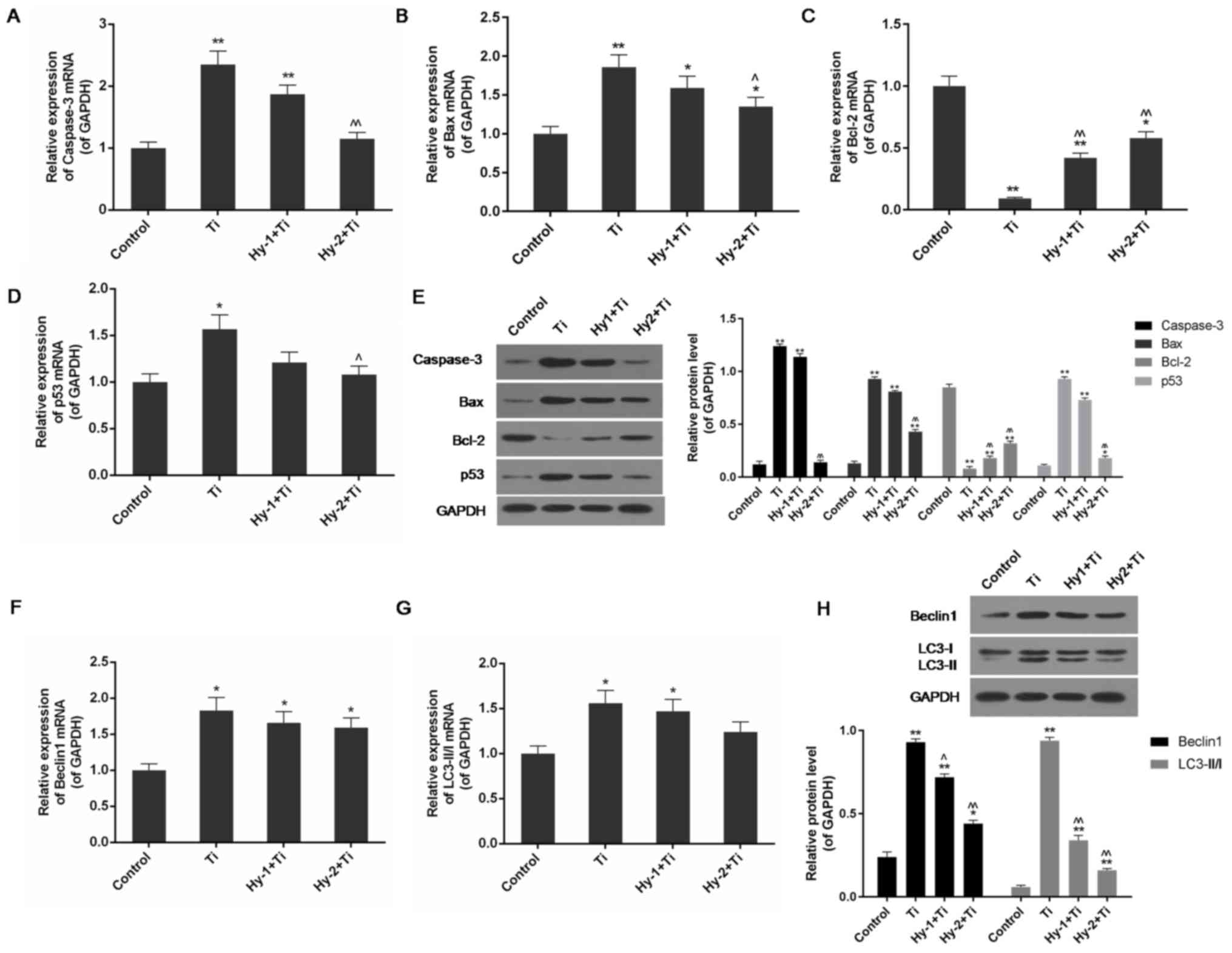 | Figure 4.Expression levels of apoptosis and
autophagy associated genes are detected by reverse
transcription-quantitative polymerase chain reaction and western
blotting in the control group, Ti (1 mg/ml) group, Hy-1 (200
µg/ml)+Ti group and Hy-2 (400 µg/ml)+Ti group. (A) Hy pretreatment
downregulated expression of caspase-3 mRNA in Ti particle injured
cells. (B) Hy pretreatment downregulated expression of Bax mRNA in
Ti particle injured cells. (C) Hy pretreatment upregulated
expression of Bcl-2 mRNA in Ti particle injured cells. (D) Hy
pretreatment downregulated expression of p53 mRNA in Ti particle
injured cells. (E) Hy pretreatment decreased protein expression
levels of caspase-3, Bax and p53; however increased the expression
level of Bcl-2 in Ti particle induced injury. (F) Hy pretreatment
downregulated expression of Beclin1 mRNA in Ti particle injured
cells. (G) Hy pretreatment downregulated the mRNA expression level
of LC3-II/I in Ti particle injured cells. (H) Hy pretreatment
decreased the protein expression level of Beclin1 and the ratio of
LC3-II and LC3-I in Ti particle injured cells. Data are presented
as mean ± standard deviation. n=3. *P<0.05, **P<0.01 vs.
control group; ^P<0.05 and ^^P<0.01 vs.
Ti (1 mg/ml) group. Ti, titanium; Hy, hyperoside; Bax, apoptosis
regulator BAX; Bcl-2, apoptosis regulator Bcl-2; p53, cell tumor
antigen p53; LC-II/I, microtubule-associated protein light chain 3
conversion LC3-II/I. |
Pretreatment with Hy downregulates the
expression of Beclin1 and LC3-II/I in Ti particle-injured MC3T3-E1
cells
RT-qPCR and western blotting demonstrated that the
autophagy-associated genes Beclin1 and LC3-II/I were highly
expressed in Ti particle-injured cells, as there was a significant
increase in the expression levels of their mRNA and proteins
compared with the control cells (P<0.05 or P<0.01). In the Ti
group, the protein expression levels of Beclin1 and LC3-II/I were
approximately four and 15 times increased, respectively, in
comparison with the control group (P<0.01). In Hy-pretreated
cells, the expression levels of these two genes were decreased, and
the effect of pretreatment with Hy on downregulating Beclin1 and
LC3-II/I was demonstrated; a significant decrease in their protein
expression levels compared with the Ti group was observed
(P<0.05 or P<0.01; Fig.
4F-H).
Pretreatment with Hy inhibits the
activation of the TWEAK-p38 pathway in Ti particle-injured MC3T3-E1
cells
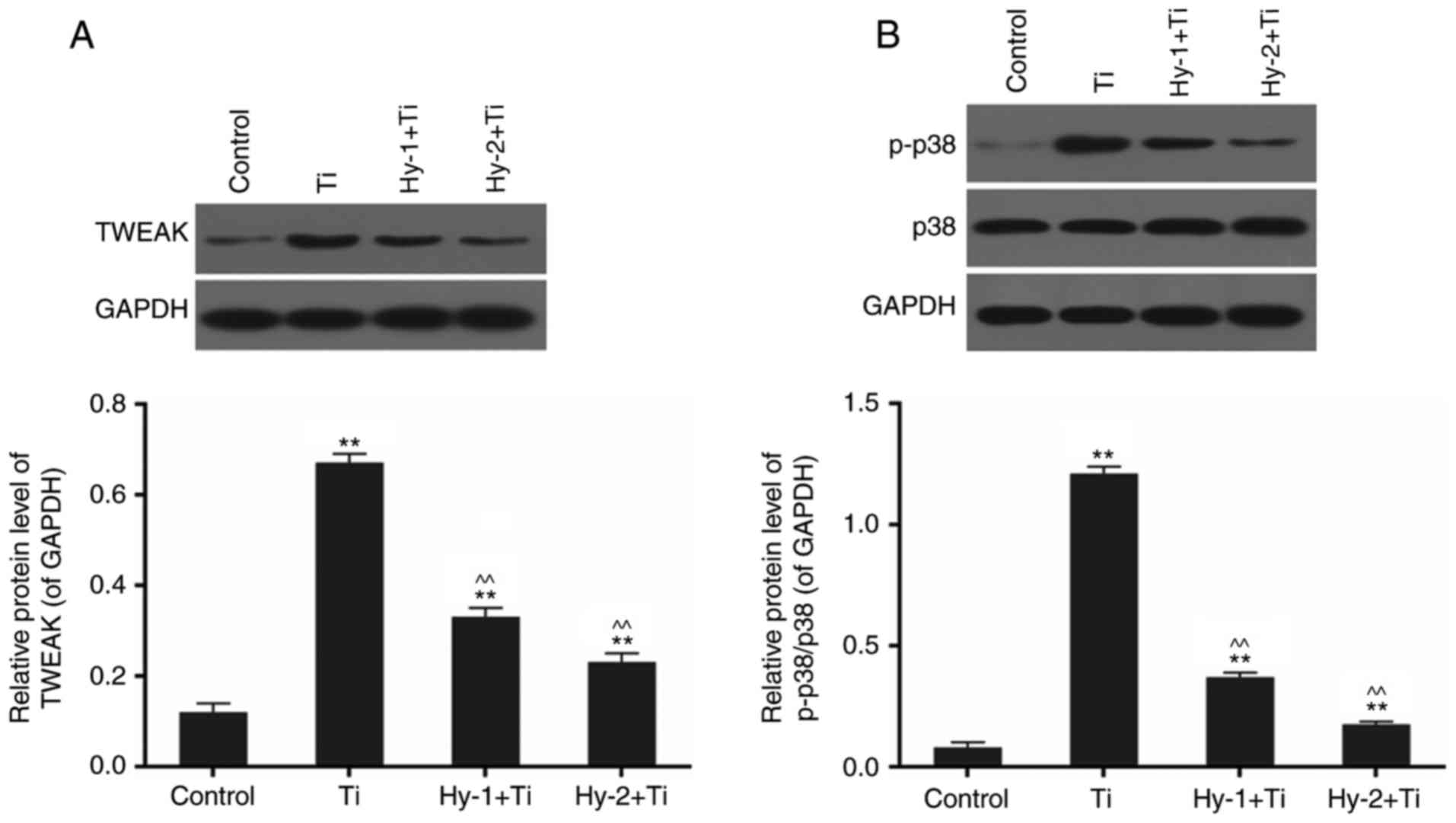
Detection of protein expression levels via western
blotting revealed a significant alteration in the expression of the
TWEAK-p38 pathway in the different conditions. Treatment with Ti
particles significantly upregulated the expression of TWEAK and
activated the phosphorylation of p38 by five and 15 times,
respectively, compared with the control (P<0.01; Fig. 5). In cells subjected to
pretreatment with Hy, the phosphorylation levels of the TWEAK-p38
pathway were decreased. There were significant differences in the
Hy-pretreated groups compared with the Ti group (P<0.01;
Fig. 5).
TWEAK overexpression and p38MAPK
activation elevates the rates of apoptosis and autophagy in Ti
particle-injured MC3T3-E1 cells, even under pretreatment with
Hy
Based on the aforementioned experiments, Ti
particles induced high apoptosis and autophagy rates of MC3T3-E1
cells, and pretreatment with Hy may mitigate the injury with
inhibited levels of TWEAK and p38 MAPK activation (Fig. 5B). To verify that the TWEAK and p38
MAPK signaling pathways are the mechanisms of action of
pretreatment with Hy, TWEAK was overexpressed and the p38 MAPK
pathway was activated. Apoptosis and autophagy rates were
subsequently detected in the Ti, Hy+Ti, TWEAK+Ti, TWEAK+Hy+Ti,
Anisomycin+Ti and Anisomycin+Hy+Ti groups. The results suggested
that TWEAK upregulation and p38 MAPK phosphorylation may increase
the apoptosis and autophagy rates in Ti particle-injured cells,
even with pretreatment with Hy (P<0.05; Fig. 6), which suggested that TWEAK and
p38 MAPK may serve important roles in the protective actions of Hy
in Ti particle-induced injury.
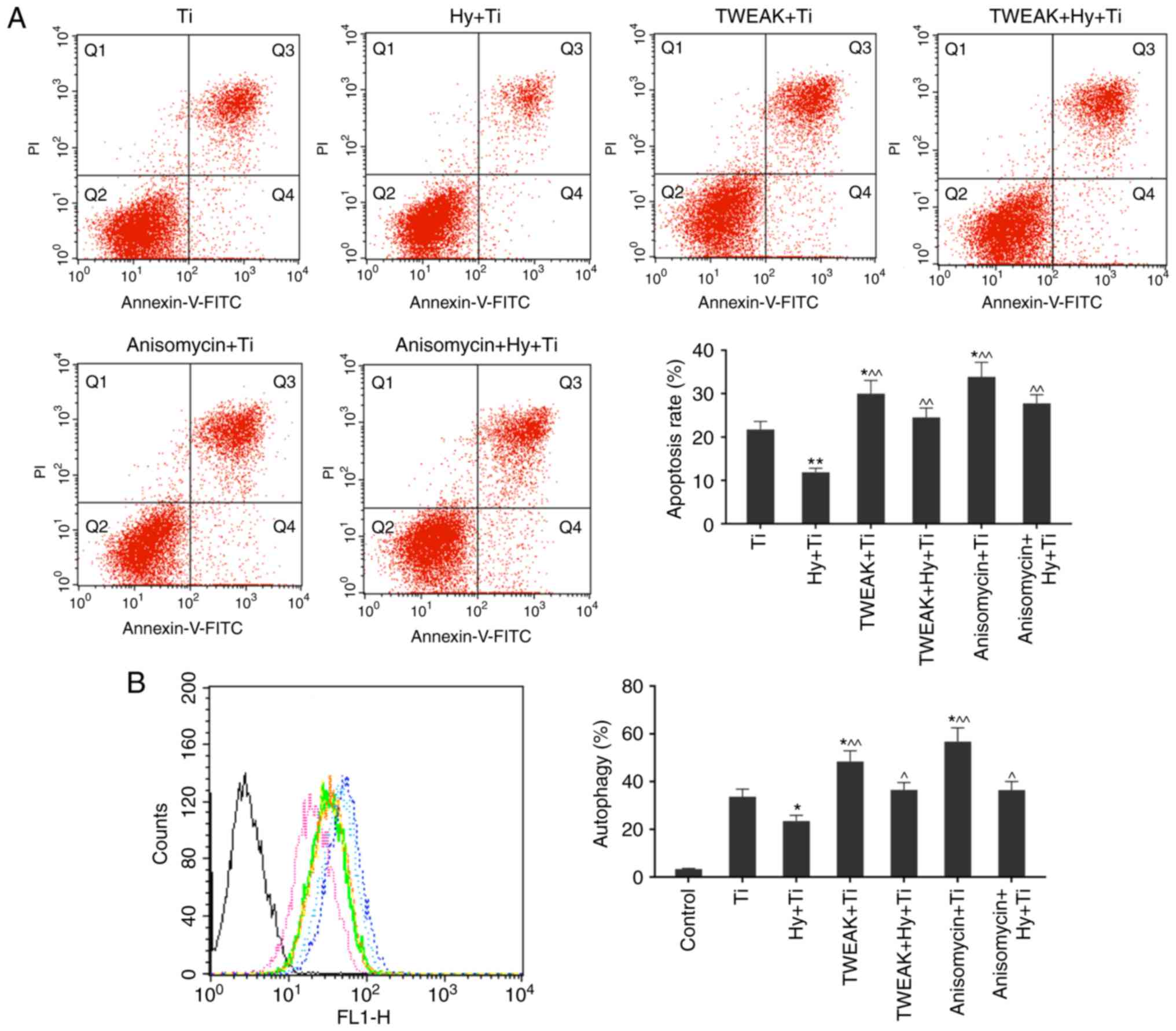 | Figure 6.Apoptosis and autophagy rates in Ti,
Hy (400 µg/ml)+Ti, TWEAK+Ti, TWEAK+Hy+Ti, Anisomycin+Ti and
Anisomycin+Hy+Ti groups are determined using flow cytometry. (A)
TWEAK overexpression and p38 MAPK activation by anisomycin were
able to elevate the apoptosis rate in Ti particle-injured cells,
even under Hy pretreatment. (B) TWEAK overexpression and p38 MAPK
activation by anisomycin may enhance the autophagy rate in Ti
particle-injured cells, even under pretreatment with Hy condition.
Black, control; green, Ti; pink, Hy+Ti; light blue, TWEAK+Ti;
yellow, TWEAK+Hy+Ti; blue, Anisomycin+Ti; orange, Anisomycin+Hy+Ti.
Data are presented as the mean ± standard deviation. n=3.
*P<0.05, **P<0.01 vs. Ti group; ^P<0.05,
^^P<0.01 vs. Hy+Ti group. Ti, titanium; Hy,
hyperoside; TWEAK, tumor necrosis factor ligand superfamily member
12; p38, mitogen activated protein kinase 11; MAPK, mitogen
activated protein kinase; FITC, fluorescein isothiocyanate; PI,
propidium iodide. |
Discussion
In the present study, a reduction in cell viability
was observed in MC3T3-E1 cells with the addition of Ti particles,
and the inhibitory effect was exerted in a dose-dependent manner.
In the condition with Ti particles, the apoptosis and autophagy
rates of MC3T3-E1 cells were significantly increased. Pretreatment
with Hy, a flavonoid glycoside compound extracted from natural
plants, was demonstrated to mitigate apoptosis and autophagy in
MC3T3-E1 cells in Ti particle-induced injury, revealing its
potential protective effect on osteoblasts.
The stability of bone structure relies on the
dynamic equilibrium of osteolysis and osteogenesis; any factor
which activates osteoclasts or inhibits osteoblasts may directly or
indirectly disturb the balance, resulting in loss of bone mass
(25,26). Numerous previous studies have
demonstrated that wear particles stimulate cells around a
prosthesis to release cytokines, including IL-6, IL-8 and TNF-α, to
affect the viability, proliferation, phenotype and function of
osteoblasts, resulting in periprosthetical osteolysis and
prosthetic loosening (27–30).
Previous studies demonstrated that various apoptotic
macrophages, foreign body giant cells and T lymphocytes, in
addition to highly expressed caspase-3, Bax and p53 were detected
in interface membranes between a prosthesis and the joint (30,31).
It was observed that the regulation of caspase-3 expression and
apoptosis was closely implicated in the local accumulation of wear
particles and osteolysis (31,32).
Excessive autophagy may additionally activate apoptosis or convert
itself to autophagic death, although initial autophagy inhibits
oxidative stress injury to protect the cell (33–36).
Autophagy, which serves a key role in cell stress and environmental
adaptation, is strongly associated with cell damage repair,
replication and proliferation (37). Stimulatory factors, including
glucocorticoid and monosodium urate monohydrate, were demonstrated
to induce autophagy in osteoblasts or osteocytes (37–39).
In the process of cellular apoptosis, caspase-3, a
primary cleavage enzyme and a reliable marker in the mammalian
apoptotic and inflammatory pathways, is activated by regulating
caspase-9 and/or caspase-8 in the mitochondrial pathway and/or the
death receptor-mediated pathway to induce cellular apoptosis
(40–42). The anti-apoptotic gene Bcl-2 and
the pro-apoptotic gene Bax are two key apoptosis-associated genes
(43). When cells are stimulated
via death signals, pro-apoptotic proteins will undergo a
conformational change, transposition from the cytoplasm to
membranes of organelles and reaction with anti-apoptotic proteins,
thus reversing their inhibitory effect on apoptosis and releasing a
series of pro-apoptotic factors, eventually resulting in apoptosis
(44,45). p53, located at chromosome 17q13.1,
is a cancer suppressor gene with a strong association with tumors
(46). p53 is a transcription
factor at the convergence of numerous cellular stress pathways,
including oncogene activation, hypoxia, DNA damage and endoplasmic
reticulum stress, to induce different biological cell responses,
including cell cycle arrest at the G1 or G2
phases, DNA repair, senescence or even apoptosis (47,48).
In the present study, high expression levels of the pro-apoptotic
genescaspase-3, Bax and p53 were observed, with a decreased
expression level of the anti-apoptotic gene Bcl-2 in the Ti model
group and an increased apoptosis rate compared with normal cells.
Pretreatment with Hy markedly downregulated the expression levels
of pro-apoptotic genes and upregulated the expression level of
Bcl-2 in the Ti injury model, and the effect was exerted in a
concentration-dependent manner, which suggested a protective effect
of Hy on Ti particle-induced apoptosis.
Autophagy is an important mechanism for the
self-protection of cells through lysosomes, which assist cells in
maintaining the cellular synthesis, degradation and progression
cycle to promote cell survival (37). However, excessive autophagy induces
apoptosis (35,49). BECN1 and LC3 are two autophagy
regulatory genes involved in the formation of autophagosomes.
Beclin1 is a protein that in humans is encoded by the BECN1 gene,
which regulates autophagy by combining with phosphatidylinositol 3
kinase and regulating ubiquitin-like modifier-activating enzyme
proteins (50,51). The BECN-1 gene is essential for the
maintenance of homeostasis, cell development and differentiation,
tumorigenesis and cellular adaptation (52). LC3, divided into LC3-I and LC3-II,
is able to target to the membrane of autophagosomes. Generally,
LC3-I is regularly expressed in the cytoplasm and once autophagy
occurs, LC3-I combines with phosphatidyl ethanolamine in the
process of modification to form LC3-II (53). The LC3-II content is positively
correlated with the number of autophagic vacuoles (54). Along with alterations in the
autophagy rate under different conditions of Ti particles and Hy,
consistent expression levels of Beclin1 and LC3-II/Iin MC3T3-E1
cells were observed. Upon the addition of Ti particles, the
expression levels of Beclin1 and LC3-II/I were significantly
upregulated to promote autophagy. Upon pretreatment with Hy, these
protein expression levels were decreased.
Numerous immune cells, including
monocyte-macrophages, dendritic cells and activated T cells, are
able to generate soluble forms of cytokines (55–57).
Upon inflammation or tissue injury, the expression of TWEAK may be
significantly upregulated (14,15).
Phosphorylation activates MAPKs, and the signal transmission of
MAPK pathways is fulfilled by the continuous phosphorylation of
downstream substrates (58). p38
MAPK is a classical MAPK pathway. The cascade reaction of p38MAPK
includes four kinases, p21-activated kinase, mixed lineage kinase,
MKK3/6/4 and p38MAPK, which constitute a continuous reaction chain
of protein kinases (20–22). With further research on MAPK, an
increasing number of studies reported that the p38 MAPK signal
pathway served an important role in inflammatory osteolysis,
including osteoarthritis and chronic infectious arthritis (20–22).
Previously, a number of studies suggested that the p38 MAPK pathway
serves a role in the pathological process of inflammatory
osteolysis induced by particles; however, how the proteins are
expressed in the interface membrane of prosthetic loosening
following artificial joint replacement remains controversial
(59,60). Whether TWEAK is involved in
periprosthetical osteolysis is unclear (61,62).
In the present study, the protein expression level of TWEAK was
significantly increased in the Ti model cells in comparison with
the control group. Furthermore, the increasing ratio of p-p38 and
p38, suggesting a higher phosphorylation level of p38 MAPK, was
detected. Pretreatment with Hy of MC3T3-E1 cells in Ti-induced
injury was observed to mitigate the alterations in the activation
of p-p38 and TWEAK. In cells that had undergone pretreatment with
Hy, the expression of TWEAK was downregulated, and the p-p38/p38
expression level was decreased in the Hy-1+Ti and Hy-2+Ti
group.
It was demonstrated that pretreatment with Hy may be
able to improve cell viability and proliferation, and decrease
apoptosis and autophagy to protect MC3T3-E1 cells from Ti
particle-induced damage. The TWEAK and p38 pathways may be
activated to contribute to the repair processes. Hy protected
osteoblasts against Ti particle-induced damage by regulating the
TWEAK-p38 pathway. The present results suggested that Hy has the
potential to function as a protective agent for osteoblasts;
however, further studies on the underlying mechanism are
required.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
X-FZ conceived and designed the study. QZ performed
the experiments, analyzed the data and was the primary contributor
in writing the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Huai'an Second People's Hospital (Huai'an, China) and
the Affiliated Huai'an Hospital of Xuzhou Medical University
(Huai'an, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Amirhosseini M, Andersson G, Aspenberg P
and Fahlgren A: Mechanical instability and titanium particles
induce similar transcriptomic changes in a rat model for
periprosthetic osteolysis and aseptic loosening. Bone Rep. 7:17–25.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haynes DR, Crotti TN and Zreiqat H:
Regulation of osteoclast activity in peri-implant tissues.
Biomaterials. 25:4877–4885. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lochner K, Fritsche A, Jonitz A, Hansmann
D, Mueller P, Mueller-Hilke B and Bader R: The potential role of
human osteoblasts for periprosthetic osteolysis following exposure
to wear particles. Int J Mol Med. 28:1055–1063. 2011.PubMed/NCBI
|
|
4
|
Piao MJ, Kang KA, Zhang R, Ko DO, Wang ZH,
You HJ, Kim HS, Kim JS, Kang SS and Hyun JW: Hyperoside prevents
oxidative damage induced by hydrogen peroxide in lung fibroblast
cells via an antioxidant effect. Biochim Biophys Acta.
1780:1448–1457. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang XN, Li JM, Yang Q, Feng B, Liu SB,
Xu ZH, Guo YY and Zhao MG: Anti-apoptotic effects of hyperoside via
inhibition of NR2B-containing NMDA receptors. Pharmacol Rep.
62:949–955. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choi JH, Kim DW, Yun N, Choi JS, Islam MN,
Kim YS and Lee SM: Protective effects of hyperoside against carbon
tetrachloride-induced liver damage in mice. J Nat Prod.
74:1055–1060. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Haas JS, Stolz ED, Betti AH, Stein AC,
Schripsema J, Poser GL and Rates SM: The anti-immobility effect of
hyperoside on the forced swimming test in rats is mediated by the
D2-like receptors activation. Planta Med. 77:334–339. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ku SK, Kwak S, Kwon OJ and Bae JS:
Hyperoside inhibits high-glucose-induced vascular inflammation in
vitro and in vivo. Inflammation. 37:1389–1400. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li W, Liu M, Xu YF, Feng Y, Che JP, Wang
GC and Zheng JH: Combination of quercetin and hyperoside has
anticancer effects on renal cancer cells through inhibition of
oncogenic microRNA-27a. Oncol Rep. 31:117–124. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Wang M, Dong H, Yu X and Zhang J:
Anti-hypoglycemic and hepatocyte-protective effects of hyperoside
from Zanthoxylum bungeanum leaves in mice with
high-carbohydrate/high-fat diet and alloxan-induced diabetes. Int J
Mol Med. 41:77–86. 2018.PubMed/NCBI
|
|
11
|
Liu Q, Xiao S and Xia Y: TWEAK/Fn14
activation participates in skin inflammation. Mediators Inflamm.
2017:67468702017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tallroth K, Eskola A, Santavirta S,
Konttinen YT and Lindholm TS: Aggressive granulomatous lesions
after hip arthroplasty. J Bone Joint Surg Br. 71:571–575. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harris WH: Osteolysis and particle disease
in hip replacement. A review. Acta Orthop Scand. 65:113–123. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Santavirta S, Takagi M, Gomez-Barrena E,
Nevalainen J, Lassus J, Salo J and Konttinen YT: Studies of host
response to orthopedic implants and biomaterials. J Long Term Eff
Med Implants. 9:67–76. 1999.PubMed/NCBI
|
|
15
|
Knowles HJ and Athanasou NA: Acute hypoxia
and osteoclast activity: A balance between enhanced resorption and
increased apoptosis. J Pathol. 218:256–264. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schmalzried TP, Jasty M and Harris WH:
Periprosthetic bone loss in total hip arthroplasty. Polyethylene
wear debris and the concept of the effective joint space. J Bone
Joint Surg Am. 74:849–863. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wataha JC: Predicting clinical biological
responses to dental materials. Dent Mater. 28:23–40. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cooper HJ, Ranawat AS, Potter HG, Foo LF,
Koob TW and Ranawat CS: Early reactive synovitis and osteolysis
after total hip arthroplasty. Clin Orthop Relat Res. 468:3278–3285.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matzinger P: Tolerance, danger, and the
extended family. Annu Rev Immunol. 12:991–1045. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Konttinen Y, Imai S and Suda A:
Neuropeptides and the puzzle of bone remodeling. State of the art.
Acta Orthop Scand. 67:632–639. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
King KY and Goodell MA: Inflammatory
modulation of HSCs: Viewing the HSC as a foundation for the immune
response. Nat Rev Immunol. 11:685–692. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Murray PJ and Wynn TA: Protective and
pathogenic functions of macrophage subsets. Nat Rev Immunol.
11:723–737. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee YH, Cheng FY, Chiu HW, Tsai JC, Fang
CY, Chen CW and Wang YJ: Cytotoxicity, oxidative stress, apoptosis
and the autophagic effects of silver nanoparticles in mouse
embryonic fibroblasts. Biomaterials. 35:4706–4715. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Buckley CD: Why does chronic inflammation
persist: An unexpected role for fibroblasts. Immunol Lett.
138:12–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nathan C and Ding A: Nonresolving
inflammation. Cell. 140:871–882. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vermes C, Chandrasekaran R, Jacobs JJ,
Galante JO, Roebuck KA and Glant TT: The effects of particulate
wear debris, cytokines, and growth factors on the functions of
MG-63 osteoblasts. J Bone Joint Surg Am. 83-A:201–211. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fritz EA, Glant TT, Vermes C, Jacobs JJ
and Roebuck KA: Titanium particles induce the immediate early
stress responsive chemokines IL-8 and MCP-1 in osteoblasts. J
Orthop Res. 20:490–498. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kusano K, Miyaura C, Inada M, Tamura T,
Ito A, Nagase H, Kamoi K and Suda T: Regulation of matrix
metalloproteinases (MMP-2, −3, −9, and −13) by interleukin-1 and
interleukin-6 in mouse calvaria: Association of MMP induction with
bone resorption. Endocrinology. 139:1338–1345. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takei H, Pioletti DP, Kwon SY and Sung KL:
Combined effect of titanium particles and TNF-alpha on the
production of IL-6 by osteoblast-like cells. J Biomed Mater Res.
52:382–387. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Landgraeber S, von Knoch M, Löer F, Wegner
A, Tsokos M, Hussmann B and Totsch M: Extrinsic and intrinsic
pathways of apoptosis in aseptic loosening after total hip
replacement. Biomaterials. 29:3444–3450. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huk OL, Zukor DJ, Ralston W, Lisbona A and
Petit A: Apoptosis in interface membranes of aseptically loose
total hip arthroplasty. J Mater Sci Mater Med. 12:653–658. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cho DH, Jo YK, Hwang JJ, Lee YM, Roh SA
and Kim JC: Caspase-mediated cleavage of ATG6/Beclin-1 links
apoptosis to autophagy in HeLa cells. Cancer Lett. 274:95–100.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yoo BH, Wu X, Derouet M, Haniff M,
Eskelinen EL and Rosen K: Hypoxia-induced downregulation of
autophagy mediator Beclin 1 reduces the susceptibility of malignant
intestinal epithelial cells to hypoxia-dependent apoptosis.
Autophagy. 5:1166–1179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
She C, Zhu LQ, Zhen YF, Wang XD and Dong
QR: Activation of AMPK protects against hydrogen peroxide-induced
osteoblast apoptosis through autophagy induction and NADPH
maintenance: New implications for osteonecrosis treatment? Cell
Signal. 26:1–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lai EH, Hong CY, Kok SH, Hou KL, Chao LH,
Lin LD, Chen MH, Wu PH and Lin SK: Simvastatin alleviates the
progression of periapical lesions by modulating autophagy and
apoptosis in osteoblasts. J Endod. 38:757–763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Allaeys I, Marceau F and Poubelle PE:
NLRP3 promotes autophagy of urate crystals phagocytized by human
osteoblasts. Arthritis Res Ther. 15:R1762013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xia X, Kar R, Gluhak-Heinrich J, Yao W,
Lane NE, Bonewald LF, Biswas SK, Lo WK and Jiang JX:
Glucocorticoid-induced autophagy in osteocytes. J Bone Miner Res.
25:2479–2488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jia J, Yao W, Guan M, Dai W, Shahnazari M,
Kar R, Bonewald L, Jiang JX and Lane NE: Glucocorticoid dose
determines osteocyte cell fate. FASEB J. 25:3366–3376. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Colell A, Ricci JE, Tait S, Milasta S,
Maurer U, Bouchier-Hayes L, Fitzgerald P, Guio-Carrion A,
Waterhouse NJ, Li CW, et al: GAPDH and autophagy preserve survival
after apoptotic cytochrome c release in the absence of caspase
activation. Cell. 129:983–997. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang C, Yu H, Shen Y, Ni X, Shen S and
Das UN: Polyunsaturated fatty acids trigger apoptosis of colon
cancer cells through a mitochondrial pathway. Arch Med Sci.
11:1081–1094. 2015.PubMed/NCBI
|
|
42
|
Kertmen H, Gurer B, Yilmaz ER, Kanat MA,
Arikok AT, Ergüder BI, Hasturk AE, Ergil J and Sekerci Z:
Antioxidant and antiapoptotic effects of darbepoetin-α against
traumatic brain injury in rats. Arch Med Sci. 11:1119–1128.
2015.PubMed/NCBI
|
|
43
|
Schendel SL and Reed JC: Measuring pore
formation by Bcl-2 family proteins. Methods Enzymol. 322:274–282.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shimizu S, Narita M and Tsujimoto Y: Bcl-2
family proteins regulate the release of apoptogenic cytochrome c by
the mitochondrial channel VDAC. Nature. 399:483–487. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kubicka-Sierszen A and Grzegorczyk JŁ: The
influence of infectious factors on dendritic cell apoptosis. Arch
Med Sci. 11:1044–1051. 2015.PubMed/NCBI
|
|
46
|
Lauwers GY, Wahl SJ, Melamed J and
Rojas-Corona RR: p53 expression in precancerous gastric lesions: An
immunohistochemical study of PAb 1801 monoclonal antibody on
adenomatous and hyperplastic gastric polyps. Am J Gastroenterol.
88:1916–1919. 1993.PubMed/NCBI
|
|
47
|
Yue Y, Yang Y, Shi L and Wang Z:
Suppression of human hepatocellular cancer cell proliferation by
Brucea javanica oil-loaded liposomes via induction of apoptosis.
Arch Med Sci. 11:856–862. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Olivares-Illana V and Fahraeus R: p53
isoforms gain functions. Oncogene. 29:5113–5119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen Y, McMillan-Ward E, Kong J, Israels
SJ and Gibson SB: Oxidative stress induces autophagic cell death
independent of apoptosis in transformed and cancer cells. Cell
Death Differ. 15:171–182. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tassa A, Roux MP, Attaix D and Bechet DM:
Class III phosphoinositide 3-kinase--Beclin1 complex mediates the
amino acid-dependent regulation of autophagy in C2C12 myotubes.
Biochem J. 376:577–586. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liang XH, Kleeman LK, Jiang HH, Gordon G,
Goldman JE, Berry G, Herman B and Levine B: Protection against
fatal Sindbis virus encephalitis by beclin, a novel
Bcl-2-interacting protein. J Virol. 72:8586–8596. 1998.PubMed/NCBI
|
|
52
|
Arsov I, Li X, Matthews G, Coradin J,
Hartmann B, Simon AK, Sealfon SC and Yue Z: BAC-mediated transgenic
expression of fluorescent autophagic protein Beclin 1 reveals a
role for Beclin 1 in lymphocyte development. Cell Death Differ.
15:1385–1395. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li R, Ma M, Li L, Zhao L, Zhang T, Gao X,
Zhang D, Zhu Y, Peng Q, Luo X and Wang M: The protective effect of
autophagy on DNA damage in mouse spermatocyte-derived cells exposed
to 1800 MHz radiofrequency electromagnetic fields. Cell Physiol
Biochem. 48:29–41. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mizushima N: Methods for monitoring
autophagy. Int J Biochem Cell Biol. 36:2491–2502. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Konttinen YT, Zhao D, Beklen A, Ma G,
Takagi M, Kivelä-Rajamäki M, Ashammakhi N and Santavirta S: The
microenvironment around total hip replacement prostheses. Clin
Orthop Relat Res. 28–38. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ren PG, Irani A, Huang Z, Ma T, Biswal S
and Goodman SB: Continuous infusion of UHMWPE particles induces
increased bone macrophages and osteolysis. Clin Orthop Relat Res.
469:113–122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gallo J, Raska M, Mrázek F and Petrek M:
Bone remodeling, particle disease and individual susceptibility to
periprosthetic osteolysis. Physiol Res. 57:339–349. 2008.PubMed/NCBI
|
|
58
|
Gross TS, King KA, Rabaia NA, Pathare P
and Srinivasan S: Upregulation of osteopontin by osteocytes
deprived of mechanical loading or oxygen. J Bone Miner Res.
20:250–256. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chen D, Guo Y, Mao X and Zhang X:
Inhibition of p38 mitogen-activated protein kinase down-regulates
the inflammatory osteolysis response to titanium particles in a
murine osteolysis model. Inflammation. 35:1798–1806. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Iwata Y, Wada T, Furuichi K, Sakai N,
Matsushima K, Yokoyama H and Kobayashi K: p38 Mitogen-activated
protein kinase contributes to autoimmune renal injury in MRL-Fas
lpr mice. J Am Soc Nephrol. 14:57–67. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang L, Bao D, Li P, Lu Z, Pang L, Chen
Z, Guo H, Gao Z and Jin Q: Particle-induced SIRT1 downregulation
promotes osteoclastogenesis and osteolysis through ER stress
regulation. Biomed Pharmacother. 104:300–306. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Feng W, Li J, Liao S, Ma S, Li F, Zhong C,
Li G, Wei Y, Huang H, Wei Q, et al: Gö6983 attenuates titanium
particle-induced osteolysis and RANKL mediated osteoclastogenesis
through the suppression of NFκB/JNK/p38 pathways. Biochem Biophys
Res Commun. 503:62–70. 2018. View Article : Google Scholar : PubMed/NCBI
|