Introduction
Subjective tinnitus is characterized by the
perception of a sound in the absence of an acoustic source in the
environment. It is a nerve disorder which is accompanied with
hearing loss, cochlear damage (1)
and stress (2–6). Due to demographic changes and to the
increasing use of personal headsets, notably by young people
(7), tinnitus is becoming a
cumulative challenge. It is estimated that ~50 million adults have
experienced tinnitus, whereas 16 million are currently experiencing
it in the United States (8). A
tinnitus animal model that was designed to treat utilized a
high-dose of salicylate and was used to study auditory trauma
associated with tinnitus. Salicylate is well known for its potent
analgesic, antipyretic and anti-inflammatory activity.
Administration of salicylate at specific doses can result in a
unique pattern of auditory dysfunction, characterized by reversible
hearing loss and tinnitus (9).
Consequently, the use of animal models is frequently adopted in
order to establish the condition of tinnitus in various animal
species (10,11). Vascular disturbances in the cochlea
have been demonstrated to result from an inhibition of
cyclooxygenase (COX) by salicylate. COX participates in the
arachidonic acid cascade (12,13).
However, the exact mechanisms of salicylate-induced ototoxicity
remain unclear. Previous studies on salicylate-induced tinnitus
focused on the spontaneous activity of the auditory nerve (14). The cochlea is the major site of
salicylate-induced tinnitus, since the latter can inhibit COX
activity and consequently may result in synapse activation by
cochlear N-methyl-D-aspartate receptors (NMDAR) via the COX
pathway. Based on these results it remains controversial why high
doses of aspirin can cause hearing loss, since it has been proved
that inner hair cell (IHC) ribbon numbers determine the degree of
auditory brain response (ABR) wave restoration (15). Despite the increase in
understanding regarding the function of pre and postsynaptic
regions, the molecular mechanisms underlying the alteration of
ribbon synapses following a lower-dose, long-duration salicylate
treatment are not fully defined. A limited number of studies have
focused on ribbon synapses of IHC. The majority of the laboratory
studies on salicylate ototoxicity involved acute trauma models.
This is due to the fact that a high-dose, short duration salicylate
treatment may readily induce morphological alterations to cochlea
components, which are possibly completely reversible (16–19).
In addition, the majority of behavioral and neural measures of
salicylate ototoxicity rely on sensitivity of the nerve as the
primary indicator of impairment (9). In contrast to the altered spontaneous
electrical activity in the central auditory pathway, a permanent
reduction in compound action potential (CAP) amplitude was observed
following chronic treatment by salicylate (20), which indicates a disorder in ribbon
synapse of IHC. The present study aimed to examine whether an
alteration in γ-amino butyric acid-mediated ribbon synapse
inhibition may result in tinnitus and hearing loss. The specific
aims of the experiment were the following: i) examination of the
effect of salicylate on the hair cells and ribbon synapse of IHC
and ii) examination of the association between the hearing
threshold and the alteration of the ribbon synapse.
Materials and methods
Animal preparation
2-month-old male C57BL/6J mice (18–24 g) were
randomly divided into 2 groups (n=20 each). One group received
saline (control group), whereas the other received salicylate (SA
group; Tianjin Beilian Chemicals Co., Ltd., Tianjin, Chins). A
total of 40 mice were obtained from the SPF Animal Center of Dalian
Medical University (Dalian, China). The animals were housed at
20–23°C, with ~45% humidity and a 12 h light/dark circle. Animals
were handled and treated according to the guidelines established by
the National Institutes of Health and the Institutional Animal Care
and Use Committee of the First Affiliated Hospital of Dalian
Medical University and the protocol of the present study was
approved by the Animal Ethical and Welfare Committee of the Dalian
Medical University.
Salicylate treatments
Previous behavioral evidence of salicylate-induced
tinnitus was tested at a dose of 200 mg/kg, once per day for a
total period of 14 days (21).
These conditions successfully established a tinnitus model in
C57BL/6J mice. Sodium salicylate was dissolved in saline at a
concentration of 50 mg/ml and the animals in the tinnitus group
were administered salicylate solution intraperitoneally (i.p.) at a
dose of 200 mg/kg, once per day, for 14 days.
Assessment of auditory function
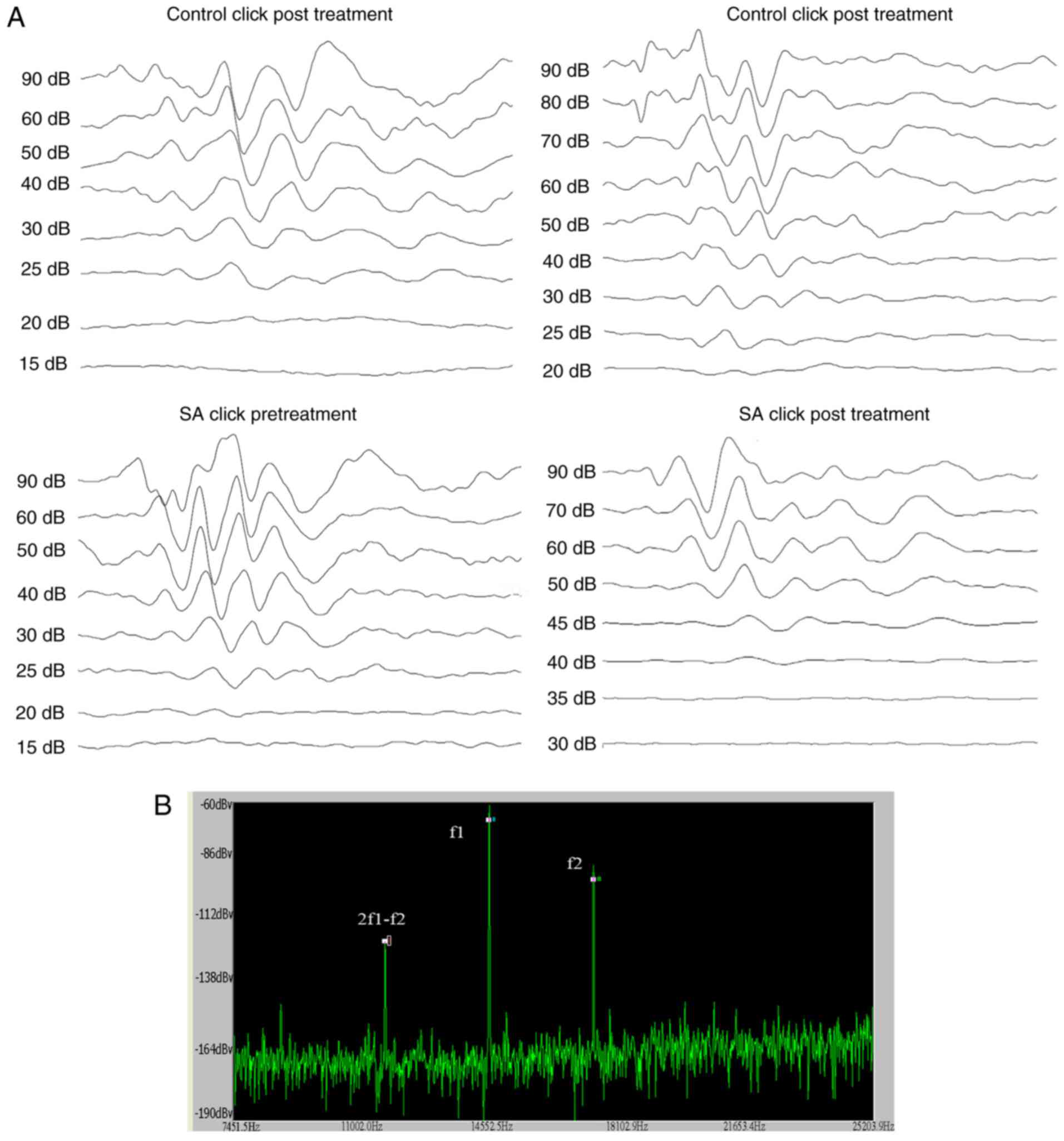
ABR audiograms were conducted at day 14 following
treatment. Mice were tested in a double blinded fashion for ABR
thresholds with equipment from Intelligent Hearing Systems (Miami,
FL, USA). The smart-EP v2.21 was used to generate specific acoustic
stimuli and to amplify measure and display the evoked brainstem
responses of mice. Mice were sedated by i.p. injection of sodium
pentobarbitone (100 mg/kg; Sigma-Aldrich Merck KGaA, Darmstadt,
Germany) and ABR testing was conducted in response to click
stimuli. The ABR was recorded by three silver needle electrodes
placed subdermally over the vertex (positive), broadband click and
tone bursts of 4, 8, 16 and 32 kHz was delivered through an insert
earphone (Intelligent Hearing Systems) that was placed directly in
the ear canal. Auditory thresholds which were based on the
visibility and reproducibility of wave III (22) were obtained for each stimulus by
variations in the sound pressure level (SPL) in 5-dB steps. The
variations were conducted in a fluctuating manner to identify the
lowest level at which an ABR pattern could be recognized. ABR
thresholds were determined for each stimulus frequency by
identifying the lowest intensity producing a reproducible ABR
pattern on the computer screen (at least two consistent peaks;
Fig. 1A).
Distortion Product Otoacoustic Emissions (DPOAEs)
were measured using an ER-10B+ (Etymotic Research, Inc., Elk Grove
Village, IL, USA) microphone coupled with two EC1 speakers. Stimuli
of two primary tones f1 and f2 (f2/f1=1.2) were presented with
f1=65 dB, f2=55 dB increments and swept from 8 to 32 kHz in 1/2
octave steps. Stimuli were generated and attenuated digitally (200
kHz sampling). The ear canal sound pressure was preamplified and
digitized. Amplitudes of 2f1-f2 were measured based on the baseline
(Fig. 1B).
Cochlear tissue processing
The mice were decapitated under deep anesthesia
following treatment for 14 days. The cochleae were rapidly removed
from the skull. The round and oval windows and the apex of the
cochlea were dissected, and perfusion was carried out in the
presence of 4% paraformaldehyde at 4°C overnight. Following
fixation, the cochlea shell was decalcified with 10% EDTA for 4–6 h
and subsequently separated from the basal turn under a dissecting
light microscope in 0.01 mmol/l PBS solutions. The parietal gyrus
of the basilar membrane was separated and the vestibular membrane
and covering membranes were removed.
Immunochemistry
The separated basilar membranes were washed three
times in 0.01 M PBS and preincubated for 30 min at room temperature
in blocking solution of 5% normal donkey serum (S30; EMD Millipore,
Billerica, MA, USA) in 0.01 M PBS. The one side of the ears
corresponding to each mouse was incubated with a combination of
goat anti-mouse C-terminal-binding protein 2 (CtBP2; E 16; C
terminal binding protein 2, C, end of combination of protein;
sc-5966; 1:200; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
and rabbit anti-mouse postsynaptic dendrite 95 (PSD95;
postsynaptic) antibodies (ab18258; 1:200; Abcam, Cambridge, MA,
USA), whereas the other side was incubated with a combination of
goat anti-mouse CtBP2 and rabbit anti-mouse GluR2/3 antibodies
(AB1506; 1:100; EMD Millipore) at 4°C overnight. The incubated
samples were washed in 0.01 M PBS three times and incubated in
donkey anti-goat 488 (ab150129; 1:200; Abcam) and donkey
anti-rabbit 568 antibodies (ab150129; 1:200; Abcam) at room
temperature for 60 min. The samples were subsequently, washed three
times. A limited volume (~40 µl) of DAPI (sc-3598; Santa Cruz
Biotechnology, Inc.) was added dropwise in the slide and the
basement membranes were tiled under a dissecting microscope with
the coverslip covering the slide. The samples were viewed directly
with fluorescent microscopy to test the specificity of the primary
antibody, as detailed below.
Laser scanning confocal microscopy
imaging
The laser scanning confocal microscope was an
Olympus FV1000 configuration (Olympus Corporation, Tokyo, Japan)
with 180X oil immersion objective. The excitation wavelengths were
488 and 568 nm for the two antibodies, respectively. Sequence
scanning was conducted in cochlear IHCs with an interval of 0.12
µm. Due to immunohistochemical double-staining that was carried out
at 488 and 568 nm as the emission wavelengths of the secondary
antibodies, the double-labeled fluorescence appeared orange in
color. The sequence scanning initiated in the region of the color
pair fluorescence, whereas it was stopped in the region where the
fluorescein color pairs disappeared. The scanning interval for
sequential scanning was set to 0.15 µm in order to ensure that each
synapse would be marked to the size of mature IHC ribbon synapses
that ranged from 150 to 200 nm (23). Following completion of serial
scanning, the two-dimensional image files were opened successively
using Autodesk 3Ds Max software (2010 release; Autodesk, Inc., San
Rafael, CA, USA). The fluorescein color pairs were marked by
spheres in the first two-dimensional image and the process was
repeated for subsequent images. Finally, the number of spheres was
counted and the synapses were reconstructed in 3D images. The
sequence scanning for the parietal gyrus of 60 basilar membranes
was carried out. A total of one visual field was selected from each
basilar membrane for scanning and 60 files were obtained.
Marking of IHC ribbon synapses and
counting the number of ribbon synapses
Serial scanning generated two-dimensional images,
which were used to mark IHC ribbon synapses. The image files were
opened successively from top to bottom using 3Ds Max Software. The
images were magnified in a ‘zoomed top view’ to identify IHC ribbon
synapses. The orange fluorescence that indicated the IHC ribbon
synapses appeared in each image and was initially marked by a
sphere. The size of the sphere was adjusted to match the size of
the area of the orange fluorescence. Prior to the analysis of the
orange fluorescence of each image, the previous marked image was
opened for comparison. Fluorescence analysis was omitted to avoid
repetitive labeling of the same synapse provided the orange
fluorescence appeared at the same location of the previous image.
All the images were overlapped and the number of spheres
(indicating IHC ribbon synapses) was counted using the layer
manager of 3Ds Max.
Statistical analysis
The ABR was repeated 3 times, and an average was
calculated as the threshold of the mouse. Immunohistochemistry was
performed in every mouse with both ears. Statistical analyses were
carried out using SPSS 15.0 software (SPSS, Inc., Chicago, IL,
USA). All data are expressed as the mean ± standard error of the
mean. The control and salicylate-treated ABR audiograms
(specifically the SPLs) of the groups were compared, using a
one-way analysis of variance. For multiple comparisons of the
number of IHC ribbon synapses, Tukey's post-hoc multiple comparison
test was used. Multivariate regression analysis was used to analyze
the associations of mismatched synapses and hearing loss. P<0.05
was considered to indicate a statistically significant
difference.
Results
Elevated of the hearing threshold
following i.p. injection of sodium salicylate (tinnitus group) but
not following injection of saline (control group)
Using click stimuli that were spectrally dominated
by frequencies <32 kHz, ABR thresholds of the tinnitus and
control groups were detected prior to the salicylate/sodium
treatment and at the 14th day post-treatment. The ABR thresholds
for the pre and post treatment groups were 24.75±4.13 and
39.0±7.54, respectively for the tinnitus group, and 23.0±2.99 and
22.25±3.02, for the control group (Fig. 2A), suggesting mild hearing loss in
the tinnitus group. Furthermore, the hearing loss was significantly
different in the tinnitus group compared with the control group on
the 14th day post-treatment (P<0.05). Additionally, the
salicylate treatment caused a significant ABR threshold elevation
across all frequencies especially the high frequencies (P<0.05).
On the 14th day post-treatment, statistically significant threshold
alterations were demonstrated at 16 and 32 kHz (Fig. 2B; P<0.01) in the salicylate
group, indicating increasing hearing impairment.
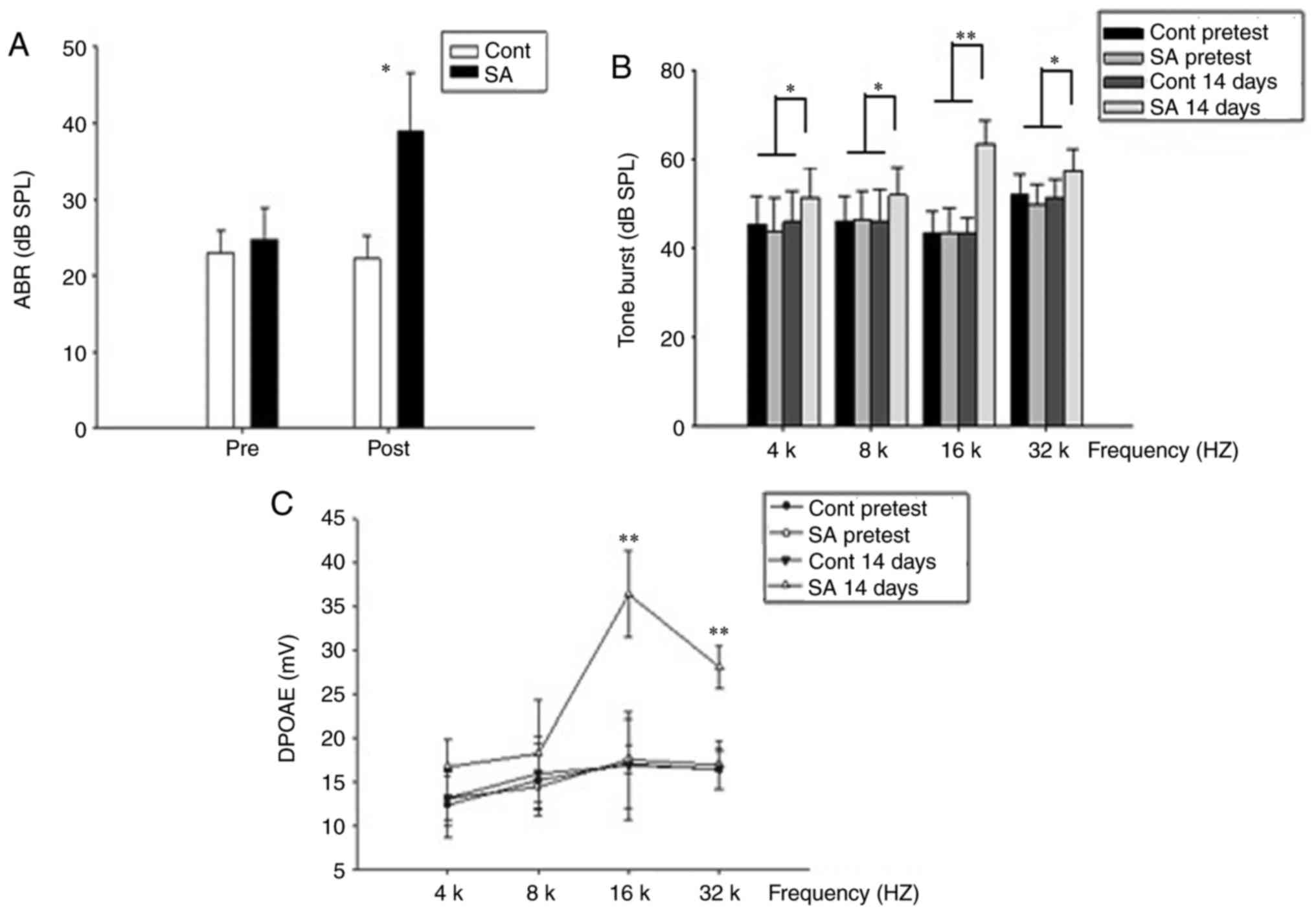 | Figure 2.The hearing threshold is elevated
following intraperitoneal injection of sodium salicylate (SA group)
compared with injection of saline (control group). (A) In the
control group, the mean ABR threshold of mice pretreatment was
23.0±2.99 dB SPL (n=20) and 22.25±3.02 dB SPL post treatment
(n=20), respectively. The mean ABR threshold of SA group mice
pretreatment was 24.75±4.13 dB SPL (n=20) and 39.0±7.54 dB SPL post
treatment (n=20), respectively. ABR thresholds of the SA group were
compared to the control group. The results were presented as the
mean ± standard error of the mean of SPL (dB). *P<0.05; post-SA
group vs. the control group. (B) On the 14th post-treatment day,
thresholds elevation demonstrated a significant improvement at 16
and 32 kHz in SA group compared with those in the control group.
*P<0.05 and **P<0.01. Thresholds at 4 and 8 kHz are slightly
higher in SA group compared with those in the control group. (C) In
the control group, DPOAEs in 4, 8, 16, 32 kHz were 13.17±3.12,
15.95±4.17, 16.89±6.16, 16.5±2.34 at 14th day post treatment. A
dramatic increase of the DPOAE amplitude at 14th day in SA group in
16, 32 kHz was presented, 36.44±4.93, 28.14±2.38, respectively.
**P<0.01 vs. the control group. APR, auditory brainstem
response; SPL, sound pressure level; SA, salicylate; Cont, control;
DPOAE, distortion product otoacoustic emission. |
Contrary to the ABR, an increase in amplitude of
DPOAE was obtained following salicylate treatment. At 16 kHz, the
DPOAE amplitude increased most significantly (Fig. 2C; P<0.01).
Irritation from salicylate does not
affect the characteristics of cochlear hair cells
In the mammalian cochlea, IHC are responsible for
voice encoding, whereas the outer hair cells are responsible for
sound amplification (24). In the
present study, DAPI nuclear staining was used to investigate
whether salicylate exposure could induce morphological alterations
to cochlear hair cells. OHCs and IHCs exhibited normal morphology
as indicated by the nuclei of the hair cells throughout the period
of salicylate exposure (Fig. 3A;
DAPI nuclei staining where indicated). This result suggested that
cochlear hair cells are not affected by salicylate exposure.
Furthermore, the DPOAEs of salicylate group indicated normal
function of OHCs following 14-day salicylate treatment (Fig. 2C).
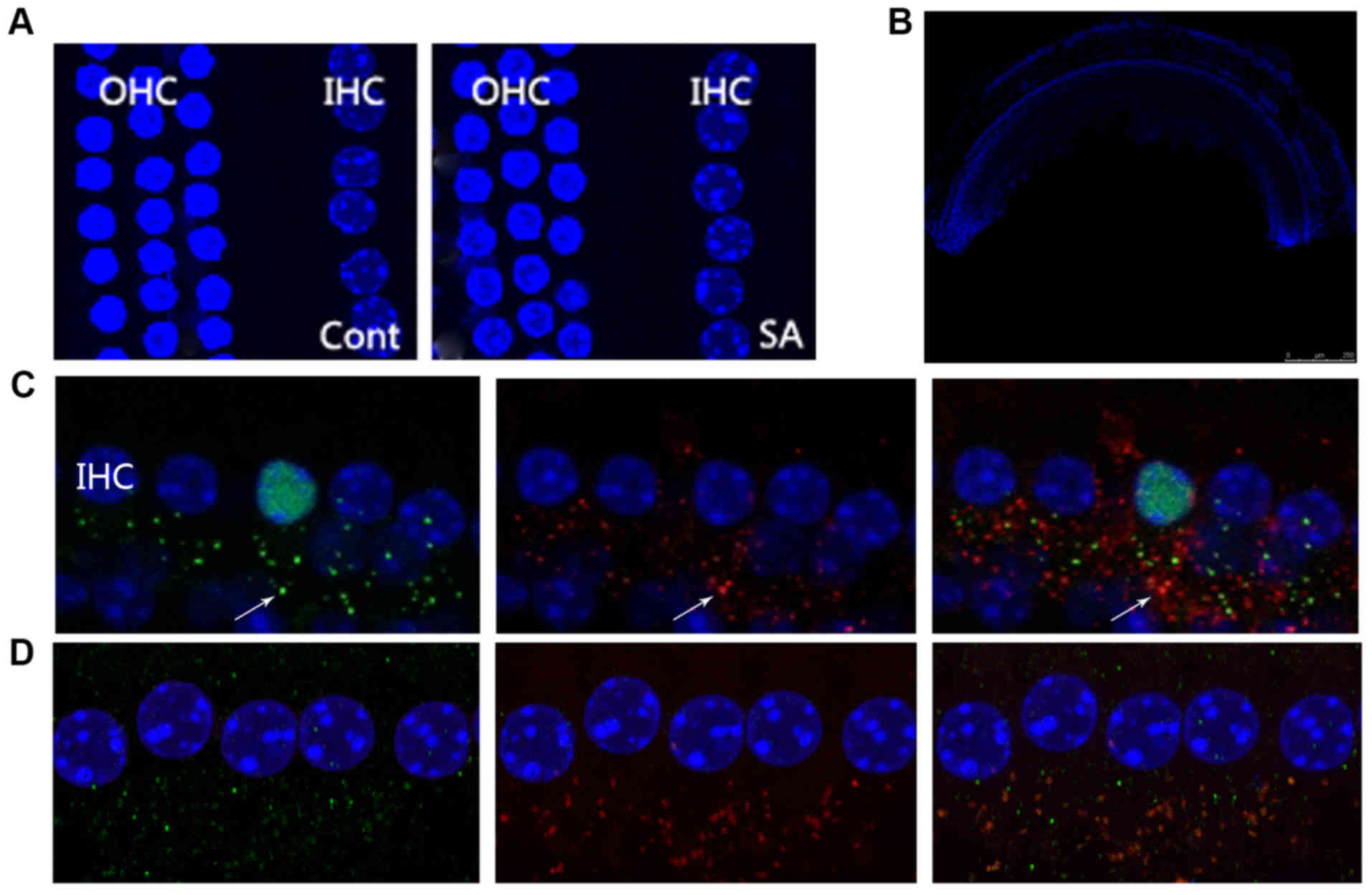 | Figure 3.Irritation from salicylate does not
affect the morphology of cochlear hair cells, although it increases
the number of IHCs at the ribbon synapse. (A) Microscopy images
reveal a normal morphological array in the control and SA groups. A
row of IHCs and three rows of OHCs were included. Blue labeling
indicates DAPI-positive hair cell nuclei. Scale bar=50 µm. (B) The
morphology of the basical turn of basilar membrane. Scale bar=500
µm. (C) CtBP2 of the control group, the presynaptic marker of IHC
ribbon synapses, were labeled by anti-CtBP2 antibody in green
beneath the IHCs as indicated by the white arrow. Postsynaptic
receptors were labeled by a PSD95 antibody in red beneath the IHCs
as indicated by the white arrow. The merged image indicates an
intact ribbon synapse in orange fluorescence beneath the IHCs
depicted by the white arrow. Scale bar=10 µm. (D) CtBP2 of the SA
group at the 14th day following salicylate treatment, including
CtBP2 and PSD95, were observed in increasing numbers. IHCs, inner
hair cells; OHCs, outer hair cells; PSD95, postsynaptic density
protein 95; CtBP2, c-terminal binding protein 2; SA, salicylate;
Cont, control. |
Salicylate induces number alterations
of IHC ribbon synapses
IHC ribbon synapses are excitable synapses that
encode sound signals. In the present study, since the DPOAEs of the
salicylate group increased in high frequencies, the basic gyri of
basic membranes between the two groups were compared (Fig. 3B). The specific presynaptic protein
RIBEYE/CtBP2 was labeled with an anti-CtBP2 antibody that is
located solely in ribbon synapses (25,26).
The right or left ear was randomly selected and identified using an
PSD95 antibody which binds to postsynaptic dendrites (15,27),
whereas the other side of the ears was identified using an
anti-GluR2/3 antibody, which binds to
α-amino-3-hydroxy-5-methyl-4-isoxa-zole-propionate receptors
(AMPARs) (28,29). A merged image that included
labeling of the RIBEYE/CtBP2 protein and NMDARs and/or AMPARs
indicated an intact synapse (Fig. 3B
and C).
The 3Ds Max Software package can effectively
reconstruct a template model of anatomical targets using
two-dimensional images (30–34).
The authors' previous study demonstrated that 3Ds Max can be used
to calculate the number of IHC ribbon synapses in healthy C57 mice.
Therefore, 3Ds Max is considered appropriate for quantitative
analysis of IHC ribbon synapses when mice are stimulated with
salicylate treatment. Using this method, it was noted that the
number of RIBEYE/CtBP2 and PSD95 was increased to a similar level
(Fig. 4A). The merged image that
included labeling of the RIBEYE/CtBP2 and PSD95 proteins was
17.15±1.39 and 16.7±1.69, respectively in the control and tinnitus
groups prior to treatment and 16.4±1.73 and 21.4±1.70 at the 14th
day of treatment, respectively. However, the decrease of AMPARs was
detected in the tinnitus group (Fig.
4B). The fluorescence value of GluR2/3 was 16.40±1.73 and
16.7±1.69 in the control and tinnitus groups prior to treatment,
and 16.30±1.53 and 3.9±3.95 at the 14th day of treatment,
respectively. Mismatch of CtBP2 and GluR2/3 was associated with
hearing loss following salicylate treatment (Fig. 4C and D).
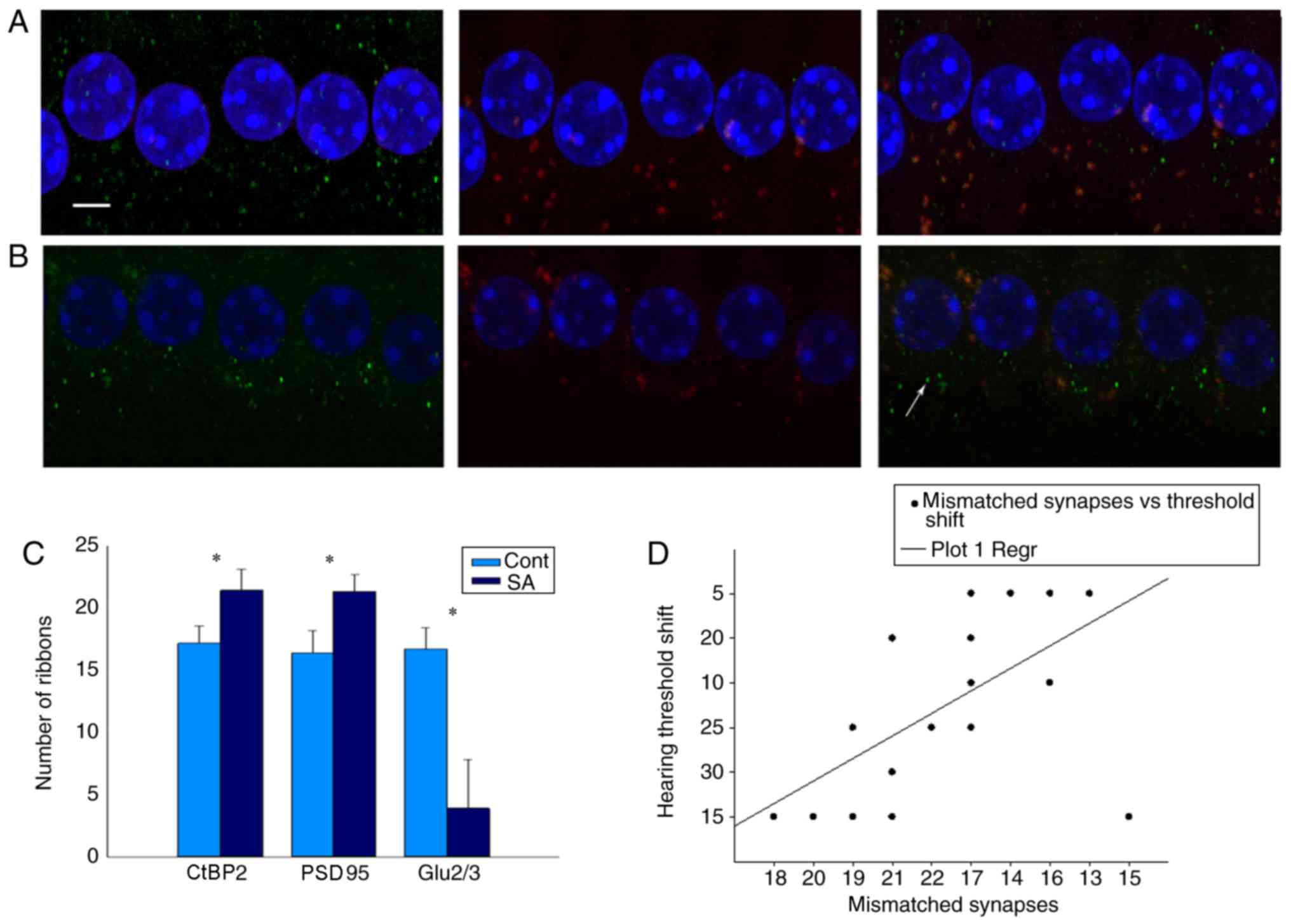 | Figure 4.Salicylate reduces the number of post
synaptic AMPARs, which causes mismatch between pre and post
synapses. (A) CtBP2 of the control group, the presynaptic marker of
IHC ribbon synapses, is labeled by an anti-CtBP2 antibody in green
beneath the IHCs. Postsynaptic receptors are labeled by a GluR2/3
antibody in red beneath the IHCs. Merged image indicates an intact
ribbon synapse in orange fluorescence beneath the IHCs. Scale
bar=10 µm. (B) CtBP2 of the SA group at the 14th day following
salicylate treatment, the presynaptic marker of IHC ribbon synapses
(CtBP2) increased, while the postsynaptic marker (GluR2/3)
decreased. Solitary green marks indicate a mismatched synapse
beneath the IHCs indicated by white arrow. (C) Numbers of pre- and
post-synaptic receptors of the SA group were compared to the
control group at the 14th day following treatment. Results are
presented as the mean ± standard error of the mean of SPL (dB). The
merged image labeling RIBEYE/CtBP2 and PSD95 was 16.4±1.73 and
21.4±1.70 in the control group and tinnitus group at the 14th day
following treatment. However, decrease of AMPARs was detected in
tinnitus group. The number of GluR2/3 was 16.40±1.73, 3.9±3.95, at
the 14th day following treatment, respectively; *P<0.05 vs. the
control. (D) There was a significant correlation between the number
of mismatched synapses and the hearing threshold (r=−0.904,
P<0.01). PSD95, postsynaptic density protein 95; CtBP2,
c-terminal binding protein 2; AMPARs,
α-amino-3-hydroxy-5-methyl-4-isoxa-zole-propionate receptors; IHC,
inner hair cells; Cont, control; SA, salicylate. |
The alteration in the number of ribbon synapses
suggested that IHC ribbon synapses were significantly affected by
exposure to salicylate. Therefore, IHC ribbon synapses were
demonstrated to be susceptible to salicylate exposure. Significant
deterioration of the hearing thresholds can be caused by severe
ribbon loss, despite intact OHC functions (35). This result demonstrated that
tinnitus animals with increased NMDAR and transmitter release of
the tinnitus animals that exhibited higher ABR thresholds.
Discussion
The results of the present study demonstrate that
salicylate improved DPOAE amplitudes during treatment. These
results are consistent with the view that salicylate enhances OHC
function by upregulating prestin expression, which increases OHC
electromotility (20). Although
DPOAEs were enhanced, a reduction in ABR amplitude was observed
unexpectedly. Thresholds recorded 14 days following drug treatments
demonstrated a decrease compared with the control. These results,
which were unexpected, demonstrated that long term treatment with
salicylate may result in auditory function impairment, but the
target organ was not OHC.
The present study was designed to examine the
mechanisms that are associated with hearing loss and alteration of
ribbon synapses, following induction of tinnitus by salicylate in
mice. The difficulties associated with measuring the activity of
NMDARs at adult synapses, have produced conflicting results about
their expression levels (36).
Several studies reported no detectable current on spiral ganglion
somata and/or calcium alteration in postsynaptic afferent boutons
induced by NMDA application. Cochlear synaptic transmission was
carried out by AMPAR in the absence of NMDARs (34–36).
In contrast to the aforementioned studies, the induction of
tinnitus by COX blockers (salicylate and mefenamate) was mediated
by cochlear NMDARs. In the present study, the data indicated a
discrepancy between PSD95 and Glu2/3 that suggested the presence of
NR1 and NR2B at the postsynaptic density of the IHC synapses. The
results of the present study, directly suggest that NMDARs exist at
this ribbon synapse. This is inconsistent with a previous study
that reported the presence of NMDARs at the IHC synapses by
Patch-clamp recordings (37).
Consequently, it was hypothesized that salicylate may modulate the
IHC synaptic neurotransmission via NMDARs at the IHC synapses. To
the best of our knowledge the results of the present study are
considered novel.
Salicylate increased the levels of arachidonic acid
in the entire part of the cochlea in vivo by enabling the
activation of NMDARs (38).
Therefore, whether the altered number of IHC ribbon synapses is
responsible for hearing loss remains undiscovered. The data
reported in the current study indicated that other cochlear
components, including OHCs and IHCs, were not markedly affected.
This supports the idea that OHCs and IHCs may not be responsible
for the impaired hearing induced by exposure to a low dose of
salicylate. Therefore, it is reasonable to consider that IHC ribbon
synapses may be responsible for the induction of tinnitus and
impaired hearing observed in this study. Salicylate caused an
excitatory effect in the presence of GYKI 53784 that resulted in
complete inhibition of AMPAR (39). This result contradicts the
hypothesis that the activation of IHC NMDA auto receptors as a
unique mechanism of salicylate action. The result that an mGluR2/3
agonist significantly reverses the expression of the dysfunctional
NMDAR in the MK-801 model of schizophrenia indicates a direct
competitive inhibition (39).
These findings were consistent with the data presented in the
present study. Furthermore, it was demonstrated that the number of
IHC ribbon synapses increased on the 14th day following salicylate
exposure, whereas CtBP2 increased in a synchronous manner with
PSD95. However, a dramatic reduction in the number of Glu2/3
occurred at the 14th day.
Researchers demonstrated that salicylate increased
auditory nerve fiber spontaneous activity. During perilymphatic
perfusion of salicylate an increase in the spontaneous spiking rate
was noted that may occur in part due to an activation of the NMDA
auto receptors on the IHCs. This process would in turn increase
glutamate release in the central nervous system, as described in
previous studies (40–43). In addition, the overall
responsiveness of a fiber of the spiral ganglion neuron (SGN) may
be regulated postsynaptically. This mechanism has previously been
demonstrated to exist in the postsynaptic boutons of SGN (44) indicating that the activation of the
NMDAR by salicylate increases the glutamate receptor activity in
the IHC synaptic region. This may provide an explanation of the
protective effect of salicylate on mice against hearing loss caused
by gentamicin ototoxicity (45).
However, the present study is consistent with other studies that
demonstrated a hearing loss following salicylate treatment. The
data suggest that the hearing loss is due to the mismatch between
the presynapses and postsynapses of the AMPR. The mismatch between
pre and post synapses would be expected to be functionally
destructive, due to the lack of synaptic transmission of the
pre-synaptic ribbons (46).
Since inhibitory synapse has been demonstrated
before (47,48), a great deal was known about the
molecular control of excitatory synapse formation. It was
speculated that functional circuits depend on the proper balance of
synaptic excitation and inhibition to process sensory information
and to perform motor and cognitive tasks. Excitatory-inhibitory
imbalances and synaptic dysfunction lead to neurological and
psychiatric disorders (47,48).
The latest discovery of Zugaib et al (49) demonstrated
endocannabinoid-dependent depolarization-induced suppression of
excitation in glycinergic neurons were enhanced as mice were
exposed to prolonged high doses of salicylate. The results pointed
to an increased inhibition of the dorsal cochlear nucleus (DCN)
inhibitory cartwheel neuron during depolarizations, which is
potentially significant for DCN hyperactivity and tinnitus
generation. Therefore, whether the enhanced NMDARs in the present
study were inhibitory synapses as demonstrated with DCN remains
unclear. Further molecular tests are required to verify it.
The current study demonstrates the previously
mentioned hypothesis and offers a comprehensive analysis regarding
the explanation of the exact etiology of the hearing loss. The
alteration in the number of AMPR as opposed to the number of
ribbons appears to be the primary reason for hearing loss.
In conclusion, the present study suggests that a
different degree of damage of the first synapse occurs within the
auditory pathway and the IHC synapse. The data revealed that
exposure to a low-dose of salicylate over a long-duration could
induce quantitative alterations in the number of the pre and
postsynaptic ribbons of IHCs, while it did not affect the number of
the cochlear hair cells. IHC ribbon synapses were the primary
targets of salicylate and may be responsible for tinnitus and
hearing loss. The current study provides direct evidence of a novel
pharmacological profile of salicylate that induces an increase in
arachidonic acid levels. This increase enabled the activation of
NMDAR at the IHC ribbons. The present study hypothesized that the
increase in the ribbon synapses that is caused by excessive
activation of NMDAR may induce abnormal spontaneous activity of
auditory nerve fibers, which could lead to the induction of
tinnitus. The present study further indicated a competitive
inhibition between NMDAR and AMPAR that induced a mismatch between
ribbons and AMPAR that in turn caused hearing loss. Although, the
mechanism of the modulation of the NMDA/AMPAR ratio in IHC cells
remains unknown, the present study provides a novel molecular
mechanism that accounts for the formation of tinnitus at the
periphery of the auditory system.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81503372)
and Education fund item of Liaoning province (grant no.
LQ2017015).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
LS was responsible for the conception and design of
the study. ML conducted and supervised the experiments. WC and HW
conducted the experiments and wrote the main manuscript text. YC
and XM prepared all the figures. YL performed statistical analysis.
XR collected the data. All authors reviewed the manuscript.
Ethics approval and consent to
participate
The present study is supported by the Education
Foundation of Liaoning province and the protocol was approved by
the Animal Ethical and Welfare Committee of the Dalian Medical
University (Dalian, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fresca TM, Salvi RF and Archela RS:
Dimensões do espaço paranaense. Editora UEL, Londrina. 2002.
|
|
2
|
Moller AR: Pathophysiology of tinnitus.
Otolaryngol Clin North Am. 36:249–266, v-vi. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jastreboff PJ: Tinnitus retraining
therapy. Prog Brain Res. 166:415–423. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zenner HP, Pfister M and Birbaumer N:
Tinnitus sensitization: Sensory and psychophysiological aspects of
a new pathway of acquired centralization of chronic tinnitus. Otol
Neurotol. 27:1054–1063. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Puel JL and Guitton MJ: Salicylate-induced
tinnitus: molecular mechanisms and modulation by anxiety. Prog
Brain Res. 166:141–146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leaver AM, Renier L, Chevillet MA, Morgan
S, Kim HJ and Rauschecker JP: Dysregulation of limbic and auditory
networks in tinnitus. Neuron. 69:33–43. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Langguth B, Salvi R and Elgoyhen AB:
Emerging pharmacotherapy of tinnitus. Expert Opin Emerg Drugs.
14:687–702. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shargorodsky J, Curhan GC and Farwell WR:
Prevalence and characteristics of tinnitus among US adults. Am J
Med. 123:711–718. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cazals Y: Auditory sensori-neural
alterations induced by salicylate. Prog Neurobiol. 62:583–631.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jastreboff PJ, Brennan JF, Coleman JK and
Sasaki CT: Phantom auditory sensation in rats: An animal model for
tinnitus. Behav Neurosci. 102:811–822. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jastreboff PJ, Brennan JF and Sasaki CT:
An animal model for tinnitus. Laryngoscope. 98:280–286. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Escoubet B, Amsallem P, Ferrary E and Tran
Ba Huy P: Prostaglandin synthesis by the cochlea of the guinea pig.
Influence of aspirin, gentamicin and acoustic stimulation.
Prostaglandins. 29:589–599. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jung TT, Miller SK, Rozehnal S, Woo HY,
Park YM and Baer W: Effect of round window membrane application of
salicylate and indomethacin on hearing and levels of arachidonic
acid metabolites in perilymph. Acta Otolaryngol Suppl. 493:81–87.
1992.PubMed/NCBI
|
|
14
|
Martin WH, Schwegler JW, Scheibelhoffer J
and Ronis ML: Salicylate-induced changes in cat auditory nerve
activity. Laryngoscope. 103:600–604. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singer W, Zuccotti A, Jaumann M, Lee SC,
Panford-Walsh R, Xiong H, Zimmermann U, Franz C, Geisler HS,
Köpschall I, et al: Noise-induced inner hair cell ribbon loss
disturbs central arc mobilization: A novel molecular paradigm for
understanding tinnitus. Mol Neurobiol. 47:261–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bernstein JM and Weiss AD: Further
observations on salicylate ototoxicity. J Laryngol Otol.
81:915–925. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Didier A, Miller JM and Nuttall AL: The
vascular component of sodium salicylate ototoxicity in the guinea
pig. Hear Res. 69:199–206. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McFadden D, Plattsmier HS and Pasanen EG:
Aspirin-induced hearing loss as a model of sensorineural hearing
loss. Hear Res. 16:251–260. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McFadden D, Plattsmier HS and Pasanen EG:
Temporary hearing loss induced by combinations of intense sounds
and nonsteroidal anti-inflammatory drugs. Am J Otolaryngol.
5:235–241. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen GD, Kermany MH, D'Elia A, Ralli M,
Tanaka C, Bielefeld EC, Ding D, Henderson D and Salvi R: Too much
of a good thing: Long-term treatment with salicylate strengthens
outer hair cell function but impairs auditory neural activity. Hear
Res. 265:63–69. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu N, Zhu ML, Johnson B, Liu YP, Jones RO
and Zhao HB: Prestin up-regulation in chronic salicylate (aspirin)
administration: An implication of functional dependence of prestin
expression. Cell Mol Life Sci. 65:2407–2418. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bourre JM, Durand G, Erre JP and Aran JM:
Changes in auditory brainstem responses in alpha-linolenic acid
deficiency as a function of age in rats. Audiology. 38:13–18. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moser T, Neef A and Khimich D: Mechanisms
underlying the temporal precision of sound coding at the inner hair
cell ribbon synapse. J Physiol. 576:55–62. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Starr A, Picton TW, Sininger Y, Hood LJ
and Berlin CI: Auditory neuropathy. Brain. 119:741–753. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sudhof TC: The synaptic vesicle cycle.
Annu Rev Neurosci. 27:509–547. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roux I, Safieddine S, Nouvian R, Grati M,
Simmler MC, Bahloul A, Perfettini I, Le Gall M, Rostaing P, Hamard
G, et al: Otoferlin, defective in a human deafness form, is
essential for exocytosis at the auditory ribbon synapse. Cell.
127:277–289. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maier W, Bednorz M, Meister S, Roebroek A,
Weggen S, Schmitt U and Pietrzik CU: LRP1 is critical for the
surface distribution and internalization of the NR2B NMDA receptor
subtype. Mol Neurodegener. 8:252013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Q and Green SH: Functional role of
neurotrophin-3 in synapse regeneration by spiral ganglion neurons
on inner hair cells after excitotoxic trauma in vitro. J Neurosci.
31:7938–7949. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Biou V, Bhattacharyya S and Malenka RC:
Endocytosis and recycling of AMPA receptors lacking GluR2/3. Proc
Natl Acad Sci USA. 105:1038–1043. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma B, Wang L, von Wasielewski R,
Lindenmaier W and Dittmar KE: Serial sectioning and
three-dimensional reconstruction of mouse Peyer's patch. Micron.
39:967–975. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Filippi S, Motyl B and Bandera C: Analysis
of existing methods for 3D modelling of femurs starting from two
orthogonal images and development of a script for a commercial
software package. Comput Methods Programs Biomed. 89:76–82. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gruska M, Medalia O, Baumeister W and Leis
A: Electron tomography of vitreous sections from cultured mammalian
cells. J Struct Biol. 161:384–392. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Punge A, Rizzoli SO, Jahn R, Wildanger JD,
Meyer L, Schönle A, Kastrup L and Hell SW: 3D reconstruction of
high-resolution STED microscope images. Microsc Res Tech.
71:644–650. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nakagawa T, Komune S, Uemura T and Akaike
N: Excitatory amino acid response in isolated spiral ganglion cells
of guinea pig cochlea. J Neurophysiol. 65:715–723. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ruel J, Chen C, Pujol R, Bobbin RP and
Puel JL: AMPA-preferring glutamate receptors in cochlear physiology
of adult guinea-pig. J Physiol. 518:667–680. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Glowatzki E and Fuchs PA: Transmitter
release at the hair cell ribbon synapse. Nat Neurosci. 5:147–154.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ruel J, Chabbert C, Nouvian R, Bendris R,
Eybalin M, Leger CL, Bourien J, Mersel M and Puel JL: Salicylate
enables cochlear arachidonic-acid-sensitive NMDA receptor
responses. J Neurosci. 28:7313–7323. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Khimich D, Nouvian R, Pujol R, Tom Dieck
S, Egner A, Gundelfinger ED and Moser T: Hair cell synaptic ribbons
are essential for synchronous auditory signalling. Nature.
434:889–894. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xi D, Li YC, Snyder MA, Gao RY, Adelman
AE, Zhang W, Shumsky JS and Gao WJ: Group II metabotropic glutamate
receptor agonist ameliorates MK801-induced dysfunction of NMDA
receptors via the Akt/GSK-3β pathway in adult rat prefrontal
cortex. Neuropsychopharmacology. 36:1260–1274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Berretta N and Jones RS: Tonic
facilitation of glutamate release by presynaptic
N-methyl-D-aspartate autoreceptors in the entorhinal cortex.
Neuroscience. 75:339–344. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Breukel AI, Besselsen E, Lopes da Silva FH
and Ghijsen WE: A presynaptic N-methyl-D-aspartate autoreceptor in
rat hippocampus modulating amino acid release from a cytoplasmic
pool. Eur J Neurosci. 10:106–114. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Woodhall G, Evans DI, Cunningham MO and
Jones RS: NR2B-containing NMDA autoreceptors at synapses on
entorhinal cortical neurons. J Neurophysiol. 86:1644–1651. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Luccini E, Musante V, Neri E, Raiteri M
and Pittaluga A: N-methyl-D-aspartate autoreceptors respond to low
and high agonist concentrations by facilitating, respectively,
exocytosis and carrier-mediated release of glutamate in rat
hippocampus. J Neurosci Res. 85:3657–3665. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen Z, Kujawa SG and Sewell WF: Auditory
sensitivity regulation via rapid changes in expression of surface
AMPA receptors. Nat Neurosci. 10:1238–1240. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mazurek B, Lou X, Olze H, Haupt H and
Szczepek AJ: In vitro protection of auditory hair cells by
salicylate from the gentamicin-induced but not neomycin-induced
cell loss. Neurosci Lett. 506:107–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shi L, Liu L, He T, Guo X, Yu Z, Yin S and
Wang J: Ribbon synapse plasticity in the cochleae of Guinea pigs
after noise-induced silent damage. PLoS One. 8:e815662013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chubykin AA, Atasoy D, Etherton MR, Brose
N, Kavalali ET, Gibson JR and Südhof TC: Activity-dependent
validation of excitatory versus inhibitory synapses by neuroligin-1
versus neuroligin-2. Neuron. 54:919–931. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mishra A, Traut MH, Becker L, Klopstock T,
Stein V and Klein R: Genetic evidence for the adhesion protein
IgSF9/Dasm1 to regulate inhibitory synapse development independent
of its intracellular domain. J Neurosci. 34:4187–4199. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zugaib J and Leao RM: Enhancement of
endocannabinoid-dependent depolarization-induced suppression of
excitation in glycinergic neurons by prolonged exposure to high
doses of salicylate. Neuroscience. 376:72–79. 2018. View Article : Google Scholar : PubMed/NCBI
|