Introduction
The mechanisms underlying intracranial aneurysm (IA)
remain unclear; however, hemodynamics is considered a crucial
factor in the induction of IA. Li et al (1) demonstrated that alterations in
hemodynamic conditions may lead to vascular endothelial injury and
a decrease in P120 catenin (P120ctn) expression in endothelial
cells (ECs). P120ctn has emerged as a master regulator of cadherin
retention and stability at the cell surface. The stabilizing
effects of P120ctn are associated with its direct interaction with
the cytoplasmic region of cadherin; however, how this interaction
is modulated to control cell adhesion remains unclear. Sakamoto
et al (2) described the
changes in morphology, shape and distribution of ECs after being
subjected to an impinging flow for 24 h. Endothelial injury and
vascular wall inflammation may contribute to the formation of IA,
and the bifurcation of an artery is a common location where IA
occurs (3). The aneurysm-promoting
environment is characterized by a high wall shear stress (WSS) and
a high positive wall shear stress gradient (WSSG) (4).
Adherens junctions (AJs) are specialized areas of
the plasma membrane where bundles of the actin cytoskeleton attach
to the membrane through transmembrane linkers. Vascular endothelial
(VE)-Cadherin is the major cell-cell adhesion molecule in vascular
ECs, and is regarded as a master organizer of the endothelial
phenotype (5) and an active
guardian of vascular integrity (6). VE-Cadherin attaches through its
extracellular domains to cadherin in the neighboring cell
membranes. P120ctn has been identified as one of several cofactors
that interacts with the cadherin tail and modulates cadherin
function (7). P120ctn and
VE-Cadherin form a cadherin-catenin complex (CCC) to stabilize the
AJs between ECs, as well as the EC phenotype.
Inflammation in the vascular endothelium is
considered to be associated with the induction of endothelial
injury and remodeling (8). The
expression of P120ctn has been reported to exert a positive effect
on preventing inflammation in ECs (9). Loss of P120ctn may upregulate the
expression levels of some proinflammatory factors, including Kaiso
and matrix metalloproteinase-2 (MMP-2), thus inducing inflammation
in ECs, and affecting the morphology and functions of ECs (10). Kaiso is a ubiquitously expressed
BTB/POZ domain transcription factor, which was initially identified
as an interaction partner of P120ctn in the cytoplasm (11), and is able to induce inflammation
in vitro and in vivo (12). Further studies are required to
clarify whether inflammation, induced by various hemodynamic
conditions, leads to endothelial injury, and to investigate the
roles of P120ctn, Kaiso and MMP-2 during this process.
At present, to the best of our knowledge, in
vitro studies regarding the effects of impinging flow with
various hemodynamic conditions on AJs between ECs are few. In the
present study, the role of P120ctn in endothelial injury was
investigated in vitro.
Materials and methods
T chamber system and experimental
protocols
A modified T chamber system, which is able to mimic
the bifurcation of an artery with various hemodynamic conditions,
was designed (13). The patent for
this system was registered at the state intellectual property
office of the People's Republic of China (14). The T chamber system consists of a
‘T’ shape chamber with two pressure gauges set at branches, a pump,
a reservoir containing flow media and connecting pipes. Forces
generated by impinging flow were demonstrated as WSS and WSSG. The
formula to calculate average WSS is force per unit area, as
follows: τ = F/A. Where τ is shear stress; F
is the force applied and A is the cross-sectional area of
material with area parallel to the applied force vector (15). The flow media used, methods of
morphological examination and specific protocols of the modified
system were elucidated in our previous study (13). In the present study, ImageJ 1.48
software (National Institutes of Health, Bethesda, MA, USA) was
used to count the number of ECs on the coverslip. The flow rates
selected were 250 and 500 ml/min. ECs that were not subjected to
any impinging flow were identified as the static control group. All
experiments were performed in a humidified incubator at 37°C.
Characterization of the morphology, including shape, size and
orientation, of ECs on the coverslip was achieved by observing
cells under an inverted phase contrast microscope (IX71; Olympus
Corporation, Tokyo, Japan), at ×10 magnification. Digital images
were captured using an inverted microscope (Olympus Corporation)
and IP Lab software (7.1; Olympus Corporation).
Cell culture
Cell cultures were prepared and maintained according
to standard cell culture procedures. Briefly, human umbilical vein
endothelial cells (HUVECs; Lonza Group, Ltd., Basel, Switzerland)
were cultured in endothelial basal medium (RPMI-1640; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 1
µg/ml hydrocortisone, 12 µg/ml bovine brain extract, 50 µg/ml
gentamicin, 50 ng/ml amphotericin-B, 10 ng/ml epidermal growth
factor and 10% fetal calf serum, which were all provided by Gibco
(Thermo Fisher Scientific, Inc.). HUVECs were incubated at 37°C and
were cultured in an incubator containing 5% CO2. Cells
were passaged at regular intervals depending on their growth
characteristics using 25% trypsin (Biochrom GmbH, Berlin, Germany)
as described by Walter et al (16).
Targeted silencing of endogenous
P120ctn with small interfering (si)RNA
Knockdown of P120ctn expression in ECs was performed
using a siRNA oligonucleotide duplex synthesized by GE Healthcare
Dharmacon, Inc. (Lafayette, CO, USA). The siRNA was generated based
on the human P120ctn gene sequence, accession number NM_001331, and
its sequence was as follows: 5′-GAAUGUGAUGGUUUAGUUUU-3′. The
siCONTROL Non-Targeting siRNA (NT-siRNA; GE Healthcare Dharmacon,
Inc.) acted as negative control. Transfection was conducted
according to the protocol described by Zhang et al (17). HUVECs, at 50–60% confluence, were
seeded in 60-mm dishes. Cells were transfected with 200 pM siRNA
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. A
total of 24, 48 and 72 h post-transfection, cells were trypsinized
and plated onto 35-mm dishes to assess knockdown efficiency. The
confirmation of P120ctn knockdown was determined by western blot
analysis.
Western blot analysis
ECs were collected and routinely prepared for
western blot analysis and protein concentration determination.
Cells were lysed in radioimmunoprecipitation assay buffer (cat. no.
p0013; Beyotime Institute of Biotechnology, Shanghai, China)
supplemented with 1 mM phenylmethylsulfonyl fluoride
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The cell lysate
was directly obtained for western blot analysis. The samples (50
µg) were loaded at a constant protein concentration and were
separated by 8 or 12.5% SDS-PAGE, after which, they were
electrotransferred to a nitrocellulose membrane. The membrane was
blocked with 5% nonfat milk in Tris-buffered saline with 0.05%
Tween-20 (TBST) at 4°C overnight, and was then incubated overnight
at 4°C with the appropriate primary antibodies [monoclonal: P120ctn
(cat. no. 610133), VE-Cadherin (cat. no. 610252), α-catenin (cat.
no. 610194) and β-catenin (cat. no. 610154), 1:400 dilutions, BD
Transduction Laboratories; BD Biosciences, San Jose, CA, USA;
polyclonal: Kaiso (cat. no. sc-23871) and β-actin cat. no.
(sc-47778), 1:400 dilutions, Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA] diluted in TBST with 1% nonfat dry milk overnight
at 4°C with agitation. After five washes with TBST, the
nitrocellulose membrane was then incubated at 37°C for 2 h with the
appropriate horseradish peroxidase-conjugated secondary antibodies
(cat. no. 58802, 1:2,000, Cell Signaling Technology, Inc., Danvers,
MA. USA). Immune complexes were detected using an enhanced
chemiluminescence kit (GE Healthcare Bio-Sciences, Pittsburgh, PA,
USA) and were semi-quantified by densitometry (ImageJ version 2.0;
National Institutes of Health). β-actin was used as the loading
control.
Statistical analysis
All experiments were performed five times.
Quantitative data are expressed as the means ± standard deviation,
whereas categorical variables are presented as percentages.
Independent samples t-test, Satterthwaite t-test or Wilcoxon
rank-sum test were used to statistically analyze western blot
analyses, and two-way analysis of variance was used to analyze
endothelial cell density. Data were analyzed using SPSS 19.0 (IBM
Corp., Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
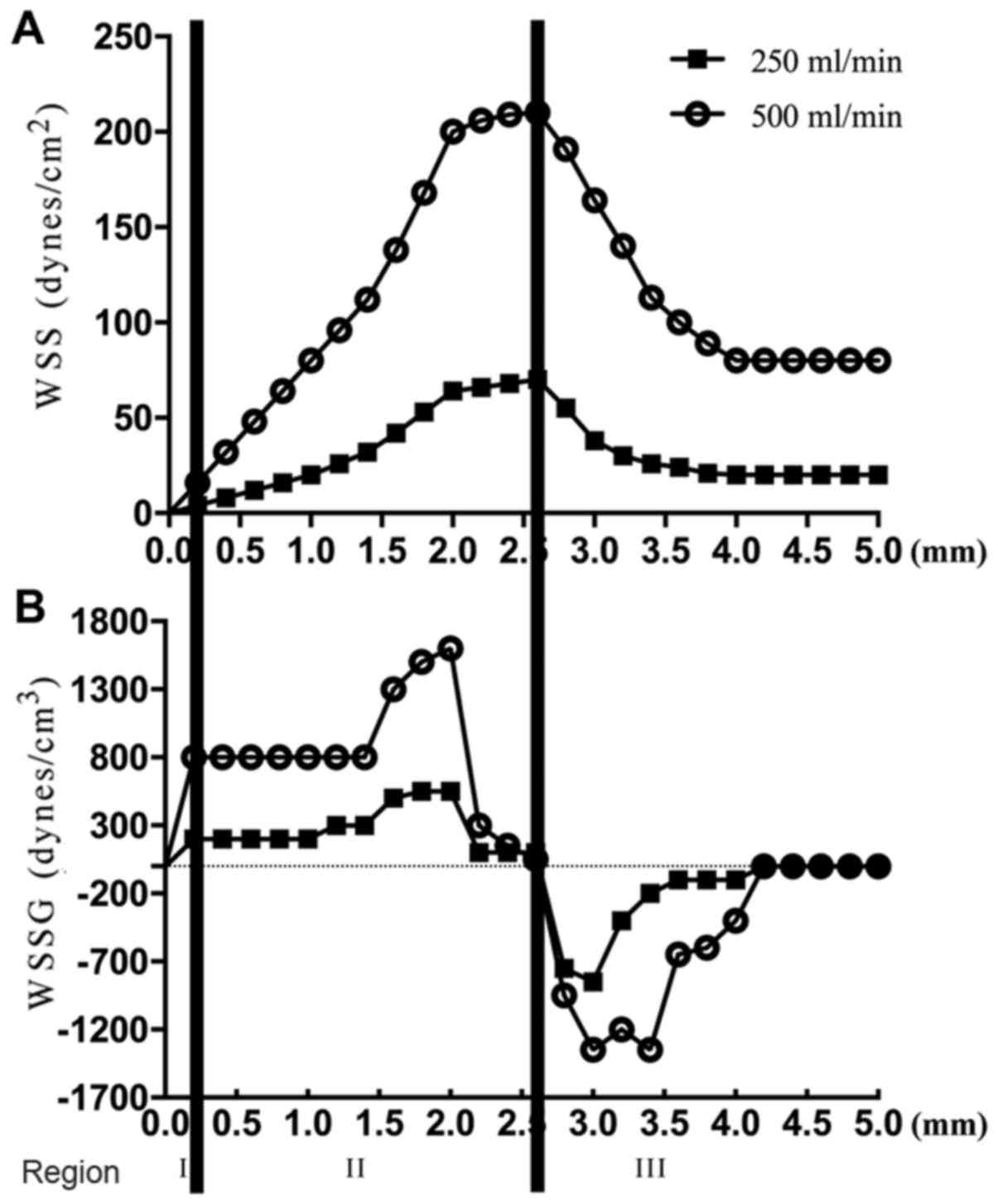
Flow data
According to previous studies (4,13,18,19),
three regions were set to define different conditions of
hemodynamics in the modified T chamber. Region I: A stagnation
point and a low to normal WSS compared with the baseline level in
straight vessels. Region II: A high positive WSSG and high WSS.
Region III: A recovery region characterized by a normal WSS and a
negative to zero WSSG (Fig. 1A and
B).
Since normal HUVECs adhered to the coverslips for 3
h (data not shown), the expression levels of proteins and
inflammatory factors in normal HUVECs were evaluated after 3 h.
However, ECs with P120ctn knockdown adhered to the coverslips for
just 1 h under a flow rate of 500 ml/min; therefore, the protocols
were altered and the characteristics of ECs with P120ctn knockdown
were detected every 15 and 30 min, and 1 h.
P120ctn knockdown by siRNA
To elucidate the role of P120ctn in AJs between ECs,
the expression levels of P120ctn were knocked down by siRNA.
P120ctn expression was silenced after 24, 48 and 72 h, as
determined by western blot analysis, whereas the expression levels
of other proteins were normal, including VE-Cadherin, Kaiso,
α-catenin, β-catenin and MMP-2 (Fig.
2A).
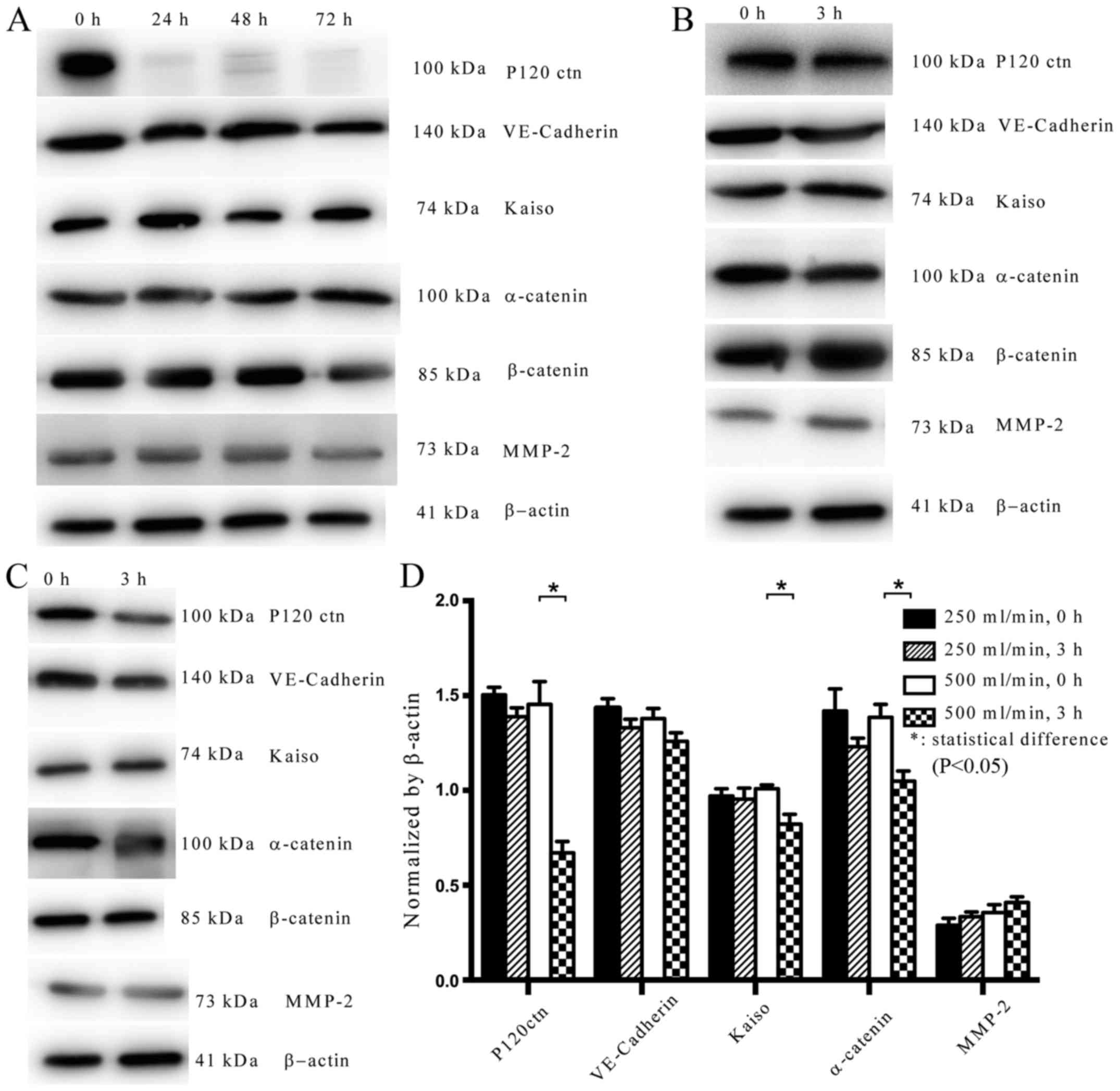 | Figure 2.Expression levels of P120ctn,
VE-Cadherin and other proteins in normal HUVECs. (A) P120ctn was
knocked down and its expression was detected after 24, 48 and 72 h.
(B) At a flow rate of 250 ml/min, the expression levels of P120ctn,
VE-Cadherin, Kaiso and α-catenin were decreased, whereas MMP-2 was
increased, after 3 h. (C) At a flow rate of 500 ml/min, the
expression levels of P120ctn, VE-Cadherin, Kaiso and α-catenin were
decreased, whereas MMP-2 was increased, after 3 h. (D)
Semi-quantification of protein expression levels. Changes in the
expression of these proteins were more prominent in response to a
flow rate of 500 ml/min. *P<0.05. EC, epithelial cells; HUVECs,
human umbilical vein endothelial cells; MMP, matrix
metalloproteinase; VE, vascular endothelial. |
Expression levels of P120ctn,
VE-Cadherin and other proteins in HUVECs
To investigate the effects of impinging flow with
different hemodynamic conditions on the function and stability of
AJs, the expression levels of specific proteins that constitute AJs
were evaluated by western blot analysis (Fig. 2B-D). In normal HUVECs under a flow
rate of 500 ml/min, the expression levels of P120ctn (P<0.05),
VE-Cadherin, Kaiso (P<0.05) and α-catenin (P<0.05) were
decreased, whereas MMP-2 was increased after 3 h (Fig. 2C and D). In normal HUVECs under a
flow rate of 250 ml/min, the expression levels of P120ctn,
VE-Cadherin, Kaiso and α-catenin were decreased, whereas MMP-2 was
increased after 3 h; however, no significant alterations were
detected (P>0.05; Fig. 2C and
D). More obvious changes in expression were detected in
response to a flow rate of 500 ml/min compared with 250 ml/min
(Fig. 2B-D).
In HUVECs with P120ctn knockdown, the expression
levels of VE-Cadherin, Kaiso and α-catenin were decreased, whereas
MMP-2 expression was increased after 1 h, under both flow rates
(250 and 500 ml/min; P<0.05). Alterations in the expression of
these proteins were more prominent under the rate of 500 ml/min
(Fig. 3A-C). Finally, in HUVECs
infected with the control siRNA, no changes in the expression
levels of P120ctn, Kaiso, α-catenin and MMP-2 were detected after 1
h at both rates (Fig. 3D).
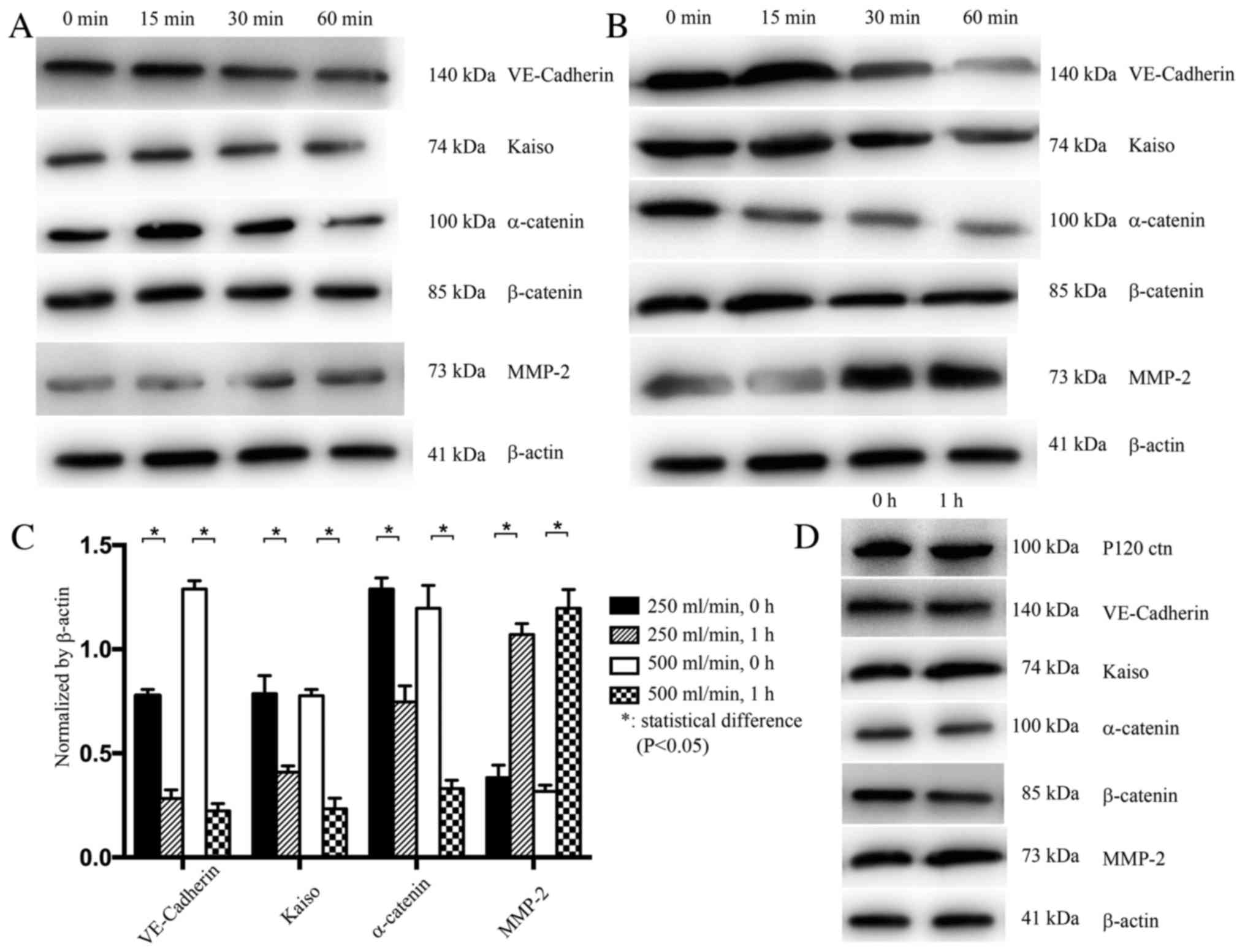 | Figure 3.Expression levels of VE-Cadherin and
other proteins in HUVECs with siRNA-induced P120ctn knockdown, and
in HUVECs transfected with a control siRNA. (A) VE-Cadherin, Kaiso
and α-catenin expression was decreased, whereas MMP-2 expression
was increased, after 1 h under a flow rate of 250 ml/min. (B)
Expression levels of VE-Cadherin, Kaiso and α-catenin were
significantly decreased, whereas MMP-2 expression was increased,
after 1 h under a flow rate of 500 ml/min. (C) Semi-quantification
of the expression levels. Alterations in the expression of these
proteins were more prominent in response to a flow rate of 500
ml/min. (D) In HUVECs transfected with a control siRNA, no
alterations in the expression levels of P120ctn, Kaiso, α-catenin
and MMP-2 were detected after 1 h under a flow rate of 500 ml/min.
*P<0.05. EC, epithelial cells; HUVECs, human umbilical vein
endothelial cells; MMP, matrix metalloproteinase; VE, vascular
endothelial. |
Morphology, alignment and distribution
of ECs
The effects of different hemodynamics on the
behavior of ECs were examined by observing the morphology,
alignment and distribution of ECs under impinging flow produced by
the T chamber system. Alterations in the morphology, alignment,
distribution and cell density of ECs were conducted as described in
our previous research (13).
In HUVECs with P120ctn knockdown, under a flow rate
of 250 ml/min, compared with in the static control group (ECs that
were not subjected to any impinging flow), the shape of HUVECs
remained polygonal after 1 h. The number of ECs in region II was
slightly decreased, whereas the number of ECs in region III was
increased. At a flow rate of 500 ml/min, no marked alterations in
the morphology, alignment and distribution of ECs were observed
within 30 min. After 1 h, ECs in region III were overcrowded, and
the number of ECs in region II was decreased. The shape of the
HUVECs was elongated in the direction of the flow. The number of
ECs in region III was increased and ECs in this region were
overcrowded (Fig. 4). To exclude
the possibility that the transfected siRNA itself induced the
aforementioned alterations in ECs, the morphological changes of ECs
transfected with the control siRNA were investigated. No changes in
the morphology, alignment and distribution of such ECs were
observed compared with in the control group after 1 h at a flow
rate of 500 ml/min (Fig. 4).
Furthermore, the density of ECs on the coverslip after 15, 30 and
60 min in response to both flow rates was counted (Table I). To calculate cell density,
experiments were performed five times. At both flow rates, no
significant alterations were detected in cell density after 0, 15,
30 and 60 min (P>0.05).
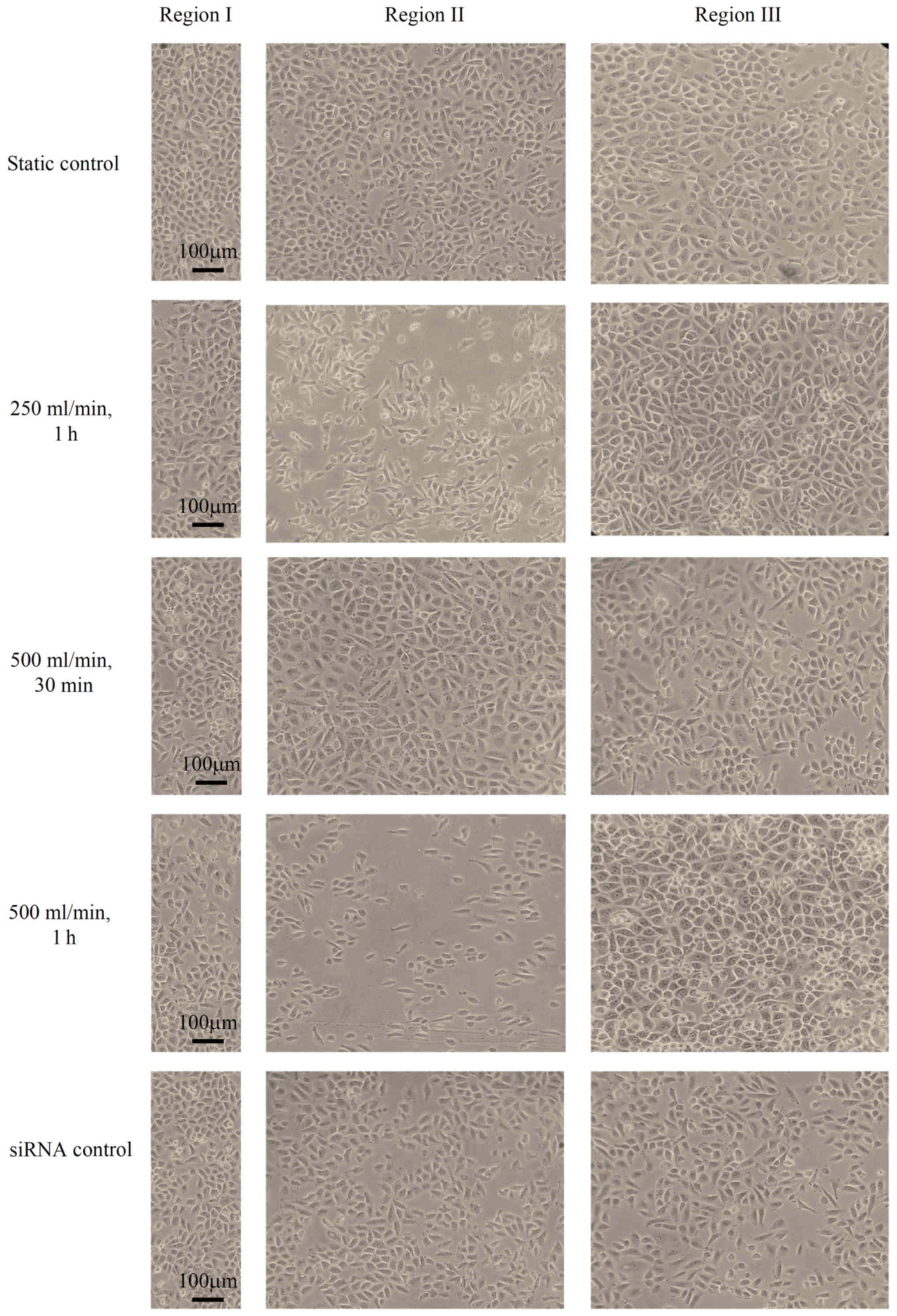 | Figure 4.Morphology, alignment and distribution
of HUVECs with P120ctn knockdown. The static control group
consisted of ECs that were not subjected to impinging flow; no
marked alterations in the morphology, alignment and distribution of
ECs were observed in this groups. When ECs were subjected to
impinging flow at a flow rate of 250 ml/min, the shape of HUVECs
remained polygonal. The number of ECs in region II was slightly
decreased, whereas the number of ECs in region III was increased,
after 1 h. Furthermore, no marked alterations in the morphology,
alignment and distribution of ECs were observed after 30 min under
a flow rate of 500 ml/min. However, after 1 h at 500 ml/min, ECs in
region I were overcrowded, and the number of ECs in region II was
decreased. The shape of HUVECs was elongated in the direction of
the flow. The number of ECs in region III was increased and ECs in
this region were overcrowded. In the siRNA control group, no
alterations in the morphology, alignment and distribution of ECs
were observed after 1 h at 500 ml/min. EC, epithelial cells; HUVEC,
human umbilical vein endothelial cells. |
 | Table I.Endothelial cell density on coverslips
following P120ctn knockdown and impinging flow at 250 and 500
ml/min. |
Table I.
Endothelial cell density on coverslips
following P120ctn knockdown and impinging flow at 250 and 500
ml/min.
| Cell density | Flow rate
(ml/min) | 0 min | 15 min | 30 min | 60 min | P-value |
|---|
|
105/ml | 250 | 23.9±0.5 | 23.3±1.3 | 22.8±1.4 | 22.6±0.5 | 0.6432 |
|
| 500 | 23.1±0.7 | 22.5±1.4 | 22.3±0.5 | 21.8±1.3 | 0.5856 |
Discussion
As previously reported by Szymanski et al
(4), bovine aortic ECs around the
stagnation point maintain a polygonal shape; however, cell density
is reduced. Conversely, cells in adjacent regions exposed to very
high WSS and WSSG are elongated, aligned parallel to the flow and
cell density is increased. However, this previous study did not
mention speed control and whether flow rate was adjustable.
Furthermore, pressure clamps were used to set the specific pressure
value in the chamber following establishment of the experimental
flow, yet the authors provided no detailed methods to adjust the
velocity of flow and pressure in the chamber.
Different hemodynamic conditions can be established
in an artery bifurcation model using a modified T chamber system;
the results of previous studies have demonstrated that in regions
with high WSS and WSSG, more severe EC damage is induced compared
with in the other regions (1,13). A
possible molecular mechanism associated with AJs was investigated.
The expression levels of P120ctn, VE-Cadherin and Kaiso were
reduced, whereas the expression levels of MMP-2 were increased in
response to impinging flow. When P120ctn was knocked down by siRNA,
the period during which ECs adhered to the coverslip was reduced to
1 h under a flow rate of 500 ml/min, and more obvious damage to ECs
was observed. The expression levels of VE-Cadherin, Kaiso and
α-catenin in HUVECs with P120 knockdown were decreased compared
with in normal HUVECs. These results confirmed the effects of
hemodynamic conditions on the function and morphology of ECs, and
demonstrated a potential molecular mechanism; however, this
requires further investigation. Different hemodynamic conditions,
including various flow velocities and pressure values, can be
achieved in the chamber using the present modified system, and may
be used to build a more convincing simulation of hemodynamic
conditions in humans.
With adjustable velocity and pressure, the modified
T-chamber system used in this study is able to mimic different
hemodynamic conditions of the bifurcation of an artery. In our
previous study (13), it was
demonstrated that the number of normal ECs in region II is
decreased and gaps between cells are enlarged in response to
impinging flow, at a rate of 500 ml/min, for 12 h. In addition, the
number of cells in region III is increased and cells are
accumulated in other regions, whereas the alignment of cells
follows the direction of the impinging flow; these findings are in
accordance with those of Szymanski et al (4). Since no significant differences were
detected in the total density of ECs on the coverslip at different
time points, it may be hypothesized that the ECs lost from region
II moved to region III and accumulated there. The present study
indicated that normal ECs in the region characterized by high WSS
and WSSG may be damaged at a higher flow rate, and some ECs will
move downstream and crowd there. Therefore, in response to
hemodynamic alterations, vessel walls in the bifurcation of an
artery may be damaged in this manner; such remodeling of the
vascular wall may be an essential and potential factor in the
induction of IA, however this needs to be validated.
It has been suggested that damage to AJs between ECs
may be the possible mechanism underlying endothelial injury, of
which, P120ctn may be a key regulator. In response to hemodynamic
alterations, the AJs between ECs may be injured and the vessel wall
may be remodeled. In the present study, after 3 h under a flow rate
of 250 and 500 ml/min, the expression levels of P120ctn,
VE-Cadherin and Kaiso were decreased, whereas MMP-2 expression was
increased. The changes in the expression levels of these proteins
were more prominent in response to a flow rate of 500 ml/min,
compared with a flow rate of 250 ml/min. These results suggested
that it is hemodynamic alterations that affect the expression of
these proteins and damage the AJs between ECs. The results of the
present study were in concordance with those of Noren et al
(20), which indicated that the
expression levels of P120ctn are positively associated with those
of Kaiso.
In the present study, alterations in the expression
levels of P120ctn, Kaiso and α-catenin were significant under a
flow rate of 500 ml/min for 3 h. The findings suggested that when
hemodynamic alterations occur, P120ctn expression may be lost,
followed by the destruction of AJs, and a decrease in the
expression of VE-Cadherin and α-catenin. Conversely, as the levels
of P120ctn are decreased, the combination of P120ctn and Kaiso may
break down and the inflammatory ability of Kaiso might be
recovered, thus resulting in EC inflammation. The increased
expression of MMP-2 detected in this study might verify this
conclusion; however, its expression was not significantly altered
within 3 h. Further studies are required to clarify the expression
of these proteins (VE-Cadherin) and inflammatory factors (MMP-2,
Kaiso) after 12 h in normal ECs in response to impinging flow, and
to determine whether these changes will be significant.
It is crucial for ECs to maintain the normal
function and morphology of the vascular wall. However, the
stability of ECs can be damaged by various factors, including,
inflammation within the vascular wall and hemodynamic alterations
to the blood flow in vessels. P120ctn is considered an important
component in maintaining the AJs between ECs, alongside α-catenin
and β-catenin (21). P120ctn is
able to form the CCC, which has the ability to maintain normal
functions and signaling pathways between ECs (20).
P120ctn also has the ability to regulate gene
transcription in ECs; for example, P120ctn is able to combine with
Kaiso, an inflammatory factor, and inhibit the combination of Kaiso
and DNA in nuclei (22). In
addition, the inhibitory effects of Kaiso on EC growth, as well as
Kaiso-induced inflammatory damage to the vascular wall, are reduced
by the combination of P120ctn and Kaiso (10). The expression levels of MMP-2 are
increased in vessel walls derived from human IA, and MMP-2 is able
to combine with Kaiso (22). These
findings may suggest that there is an association between P120ctn
and vascular inflammation; however, this requires further
investigation.
When P120ctn expression was knocked down in the
present study, the period during which HUVECs adhered to the
coverslips was reduced to 1 h; in our previous study, normal HUVECs
adhered for 12 h (13). The
expression levels of VE-Cadherin and Kaiso were more prominently
decreased, whereas the expression levels of MMP-2 were more
prominently increased in HUVECs with P120ctn knockdown compared
with in normal HUVECs. A possible mechanism for this may be that
when P120ctn was knocked down, the expression levels of VE-Cadherin
and α-catenin were downregulated, leading to decreased CCC complex
formation, and damaged AJs and ECs.
In response to hemodynamic alterations, P120ctn may
be destroyed first. In the present study, P120ctn was knocked down
in ECs by siRNA. In P120ctn knockdown ECs its expression could not
be detected by western blotting, whereas the expression levels of
other proteins were normal. These findings suggested that
alterations in the expression levels of other proteins may not be
induced by P120ctn knockdown alone. Hemodynamic alterations may be
a key factor in inducing changes in the expression levels of these
proteins; therefore, it was hypothesized that P120ctn may be
destroyed first.
In response to impinging flow and hemodynamic
changes, the stability of the connection between P120ctn knockdown
ECs may be decreased, and more VE-Cadherin broke down or was lost;
therefore, more prominent morphological alterations in ECs were
observed. In addition, the duration that P120ctn knockdown ECs
adhered to the coverslip under 500 ml/min impinging flow was
decreased to 1 h. Furthermore, in response to P120ctn knockdown,
the levels of Kaiso were decreased, whereas those of MMP-2 were
significantly increased. It may be hypothesized that the decreased
expression of Kaiso may induce an increase in MMP-2 expression.
Furthermore, the present study hypothesized that Kaiso may recover
its inflammatory ability in response to P120ctn knockdown, which is
inhibited when Kaiso is combined with P120ctn in the P120ctn-Kasio
complex. Due to the inflammation caused by free Kaiso (23), the expression levels of MMP-2 may
increase, resulting in the induction of a more serious inflammatory
injury to ECs; however, further research is required to verify this
assumption.
VE-Cadherin is also considered a crucial component
of AJs, as is P120ctn. As well as its role in forming the CCC,
VE-Cadherin serves an important role in maintaining the normal
functions and morphology of ECs. It has been reported that as the
levels of VE-Cadherin decrease, the stability of ECs breaks down,
which is considered an initial step in inducing remodeling of the
vascular wall (6). It has also
been revealed that as WSS increases, the levels of VE-Cadherin
decrease in this study. However, the specific mechanism underlying
the effects of P120ctn, VE-Cadherin and other proteins on ECs
remains unclear. In addition, further research is required to
clarify whether VE-Cadherin is broken down or is removed by the
impinging flow, thus resulting in the decreased expression levels
of VE-Cadherin.
The present study was limited by the type of cells
selected; since arterial ECs were not available, HUVECs were
selected. In addition, it is necessary to subject ECs to a longer
period of impinging flow. Other hemodynamic conditions should be
selected in future research to elucidate the association between
AJs and ECs. Although the present study indicated that the
expression levels of P120ctn were dependent on hemodynamics,
further research is required to clarify the effects of P120ctn
overexpression on AJs in response to impinging flow. Finally, more
methods, including confocal microscopy or other histochemical
analyses are required to provide stronger evidence to support the
present conclusions.
In conclusion, the present study revealed that AJs
are crucial for the maintenance of normal EC morphology and
functions in the vascular wall, and P120ctn is an important
regulator of AJs. In addition, it was suggested that a loss in
P120ctn may be induced first by hemodynamic alterations. As
hemodynamic conditions change, losing P120ctn may aggravate AJs
between ECs and induce the inflammation in the vascular wall.
Clinically, in response to hemodynamic alterations, a loss of
P120ctn may induce endothelial injury; therefore, P120ctn may have
a critical role in inducing IA.
Acknowledgements
Not applicable.
Funding
The present study received grants from the National
Natural Science Foundation of China (NSFC; grant. nos. 81360188 and
81860225) and from the Natural Science Foundation of Jiangxi
province (grant. no. 20161BAB205238).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
The study was designed by JLZ and MHL. The
experiments were performed and data were collected by JLZ, JWJ and
ZPX, and the data were analyzed by JLZ, ZPX and NZY. The manuscript
was written by JLZ and ZPX, and was revised and approved by all
authors. The manuscript was finally proofread and approved by
MHL.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AJs
|
adherens junctions
|
|
CCC
|
cadherin-catenin complex
|
|
ECs
|
endothelial cells
|
|
HUVECs
|
human umbilical vein endothelial
cells
|
|
IA
|
intracranial aneurysm
|
|
MMP-2
|
matrix metalloproteinase-2
|
|
P120ctn
|
P120 catenin
|
|
WSS
|
wall shear stress
|
|
WSSG
|
wall shear stress gradient
|
References
|
1
|
Li MH, Li PG, Huang QL and Ling J:
Endothelial injury preceding intracranial aneurysm formation in
rabbits. West Indian Med J. 63:167–171. 2014.PubMed/NCBI
|
|
2
|
Sakamoto N, Segawa K, Kanzaki M, Ohashi T
and Sato M: Role of p120-catenin in the morphological changes of
endothelial cells exposed to fluid shear stress. Biochem Biophys
Res Commun. 398:426–432. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huo Y, Wischgoll T and Kassab GS: Flow
patterns in three-dimensional porcine epicardial coronary arterial
tree. Am J Physiol Heart Circ Physiol. 293:H2959–H2970. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Szymanski MP, Metaxa E, Meng H and Kolega
J: Endothelial cell layer subjected to impinging flow mimicking the
apex of an arterial bifurcation. Ann Biomed Eng. 36:1681–1689.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takeichi M: Morphogenetic roles of classic
cadherins. Curr Opin Cell Biol. 7:619–627. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giannotta M, Trani M and Dejana E:
VE-cadherin and endothelial adherens junctions: Active guardians of
vascular integrity. Dev Cell. 26:441–454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lagendijk AK and Hogan BM: VE-cadherin in
vascular development: A coordinator of cell signaling and tissue
morphogenesis. Curr Top Dev Biol. 112:325–352. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Madahian S, Navab KD, Pourtabatabaei N,
Seyedali S, Safar S, Vazirian S and Hough G: Inflammation, high
density lipoprotein and endothelium. Curr Med Chem. 21:2902–2909.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Perez-Moreno M, Song W, Pasolli HA,
Williams SE and Fuchs E: Loss of p120 catenin and links to mitotic
alterations, inflammation, and skin cancer. Proc Natl Acad Sci USA.
105:15399–15404. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
O'Donnell JJ III, Zhuge Y, Holian O, Cheng
F, Thomas LL, Forsyth CB and Lum H: Loss of p120 catenin
upregulates transcription of pro-inflammatory adhesion molecules in
human endothelial cells. Microvasc Res. 82:105–112. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Daniel JM and Reynolds AB: The catenin
p120(ctn) interacts with Kaiso, a novel BTB/POZ domain zinc finger
transcription factor. Mol Cell Biol. 19:3614–3623. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pierre CC, Longo J, Mavor M, Milosavljevic
SB, Chaudhary R, Gilbreath E, Yates C and Daniel JM: Kaiso
overexpression promotes intestinal inflammation and potentiates
intestinal tumorigenesis in Apc(Min/+) mice. Biochim Biophys Acta.
1852:1846–1855. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao JL, Jia L, Wang XB, Zhang LL, Rong
WL, Jiang JW and Li MH: Effects of adjustable impinging flow on the
vascular endothelial cell layer in a modified T chamber. Int J Clin
Exp Med. 10:62017.
|
|
14
|
Li MH, Zhang LL, Zhao JL, Wang XB and Rong
WL: A fluid mechanics experimental device. Patent number: ZL 2014 2
0709792.5. Filed November 24, 2014; isssued, July 8, 2015.
|
|
15
|
Sharma PK: Mechanics of materials. Technol
Health Care. 18:49–61. 2010.PubMed/NCBI
|
|
16
|
Walter C, Pabst A, Ziebart T, Klein M and
Al-Nawas B: Bisphosphonates affect migration ability and cell
viability of HUVEC, fibroblasts and osteoblasts in vitro. Oral Dis.
17:194–199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang J, O'Donnell JJ III, Holian O,
Vincent PA, Kim KS and Lum H: P120 catenin represses
transcriptional activity through Kaiso in endothelial cells.
Microvasc Res. 80:233–239. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meng H, Swartz DD, Wang Z, Hoi Y, Kolega
J, Metaxa EM, Szymanski MP, Yamamoto J, Sauvageau E and Levy EI: A
model system for mapping vascular responses to complex hemodynamics
at arterial bifurcations in vivo. Neurosurgery. 59:1094–1101. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
DePaola N, Gimbrone MA Jr, Davies PF and
Dewey CF Jr: Vascular endothelium responds to fluid shear stress
gradients. Arterioscler Thromb. 12:1254–1257. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Noren NK, Liu BP, Burridge K and Kreft B:
p120 catenin regulates the actin cytoskeleton via Rho family
GTPases. J Cell Biol. 150:567–580. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reynolds AB, Roesel DJ, Kanner SB and
Parsons JT: Transformation-specific tyrosine phosphorylation of a
novel cellular protein in chicken cells expressing oncogenic
variants of the avian cellular src gene. Mol Cell Biol. 9:629–638.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang YL, Malik AB, Sun Y, Hu S, Reynolds
AB, Minshall RD and Hu G: Innate immune function of the adherens
junction protein p120-catenin in endothelial response to endotoxin.
J Immunol. 186:3180–3187. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ogden SR, Wroblewski LE, Weydig C,
Romero-Gallo J, O'Brien DP, Israel DA, Krishna US, Fingleton B,
Reynolds AB, Wessler S, et al: p120 and Kaiso regulate
Helicobacter pylori-induced expression of matrix
metalloproteinase-7. Mol Biol Cell. 19:4110–4121. 2008. View Article : Google Scholar : PubMed/NCBI
|