Introduction
Influenza A virus (IAV; orthomyxoviridae) infection
in humans affects the upper and lower respiratory tracts, which
often causes acute respiratory diseases ranging from mild to
severe. For example, the symptoms of seasonal influenza virus
infection can include fever, headache and chills, although patients
recover in days (1), whereas lower
respiratory tract infections, including 1918 H1N1 or H51N1, can
contribute to alveolitis and diffuse alveolar damage leading to
mortality (2,3). Clinically available anti-influenza
virus medications include neuraminidase (NA) inhibitors, for
example. oseltamivir and zanamivir, and M2 ion channel blockers,
including amantadine and rimantadine, which have been shown to be
ineffective due to viral genome mutations (4). It was previously reported that amino
acid substitutions in NA (e.g. NA-R292K and NA-Arg292Lys) of H7N9
confer oseltamivir resistance, raising worldwide concerns on
preparedness for an influenza pandemic (5).
Interactions between influenza virus hemagglutinin
and cell surface sialic acid receptors are important for infection
to establish in target cells (6).
During viral replication, structural features, including double
stranded RNA or 5′-triphosphate RNA, are sensed by the host immune
system, leading to elevated pro-inflammatory mediator production
and the recruitment of immune cells to the site of infection
(7). It is well recognized that
the host immune system orchestrates appropriate pro-inflammatory
responses to eliminate invading pathogens and clear infected cells.
However, it is also becoming clear that viral factors and host
immune responses are involved in the pathogenesis of diseases
caused by influenza (8). The
PB1-F2 protein of H5N1(HK/97) and 1918 H1N1, with an amino acid
change at position 66 (N66S), has been found to increase viral
virulence (9). Furthermore,
exacerbated cytokine production and the dysregulated recruitment of
immune cells, including macrophages, following influenza virus
infection contribute to the progression of acute lung injury to
acute respiratory distress syndrome (ARDS) (10,11).
Therefore, data suggests that the most advantageous strategy for
the treatment of influenza diseases combines antiviral compounds
with immunomodulators.
The activation of host signaling pathways is
essential for viral replication and the expression of
pro-inflammatory mediators. Activation of the
phosphoinositide-3-kinase (PI3K)/AKT, nuclear factor (NF)-κB and
mitogen-activated protein kinases (MAPKs), including extracellular
signal-regulated kinase (ERK)1/2 and P38 MAPK signaling cascades
triggered by influenza virus infection, is significant in viral
entry, replication of the viral genome and the nuclear export of
viral ribonucleoproteins (vRNPs), but it also elicits an excessive
pro-inflammatory response and collateral lung damage (12–15).
Therefore, the development of novel compounds that target certain
host signaling pathways may be a promising therapeutic direction
for diseases caused by influenza.
The herb Eucommia ulmoides Oliver (Du-Zhong)
has been used in various clinical situations (16,17);
traditionally, it was used for strengthening muscles and pulmonary
function, reducing blood pressure and preventing miscarriages.
Numerous active components have been identified from Eucommia
ulmoides Oliver, including lignans, polyphenolics, triterpenes
and flavonoids (18). Among the
active components, the main bioactive components, Eucommia
lignans, have protective effects against hypertensive renal injury
(19). However, their effects on
influenza virus infection remain to be fully elucidated. In the
present study, the lignan glycoside
(+)-pinoresinol-O-β-D-glucopyranoside was isolated from Eucommia
ulmoides Oliver and subjected to various assays to characterize
its inhibitory activity, and the underlying mechanisms, against
influenza virus infection.
Materials and methods
General experimental procedures
The nuclear magnetic resonance (NMR) spectra were
obtained using Bruker AVANCE-400 NMR spectrometers (Bruker
Corporation, Billerica, MA, USA). Analytical high-performance
liquid chromatography (HPLC) was performed using the Shimadzu
LC-10A instrument (Shimadzu Corporation, Kyoto, Japan) equipped
with a DAD detector and a reversed-phase C18 column (5-µm, 4.60×250
mm; Shimadzu Corporation). Preparative HPLC was performed on a
Shimadzu LC-8A instrument (Shimadzu Corporation) with a UV SPD-20A
detector using a reversed-phase C18 column (5 µm, 20×250 mm).
Silica gel (200–300 mesh) and silica gel G plates (both from
Qingdao Haiyang Chemical Co., Ltd., Qingdao, China) were used for
thin layer chromatography analysis.
Plant material
Eucommia ulmoides Oliver was collected from
Hubei province (China) and authenticated by Professor Xiping Pan
(Guangzhou Medical University, Guangzhou, China).
Extraction and isolation
The air-dried bark (1.5 kg) of Eucommia
ulmoides Oliver was refluxed with 95% EtOH (v/v, 3×5 l, 1.5 h
each). The combined extracts were concentrated in vacuo to
generate a brown residue (120 g), which was dissolved in
H2O (1.5 l) and subjected to column chromatography (CC)
over Diaion HP20 macroporous adsorptive resins, prior to elution
with MeOH/H2O (0:100-95:5). The 95% EtOH (v/v) eluate
(13.4 g) was subjected to CC on silica gel and eluted with
CH3Cl/MeOH (95:5-0:100) to generate eight fractions (Fr.
1–9). Fr.6 was separated by preparative HPLC and eluted with
MeOH/H2O (2:8-10:0) to obtain one compound (5.6 mg). The
purity of the compound was estimated by HPLC to be >95% and
identified as (+)-pinoresinol-O-β-D-glucopyranoside by NMR
spectroscopy.
Viruses and cell lines
Influenza A/PR/8/34 (H1N1), A/Hongkong/8/68 (H3N2)
and A/Hongkong/Y280/97 (H9N2) were obtained from the American Type
Culture Collection (Manassas, VA, USA). Influenza
A/Guangzhou/GIRD07/09 (H1N1) and B/Lee/1940 (FluB) were isolated
from routine clinical specimens. All viral strains used in the
present study were propagated in Madin-Darby canine kidney (MDCK)
cells. The viral stocks were stored at −80°C and titrated in a 50%
tissue culture infectious dose (TCID50) assay prior to
use.
The MDCK and human alveolar A549 cells were obtained
from the ATCC and maintained at 37°C in a humidified atmosphere of
5% CO2 in DMEM/F12 (1:1) medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.). The A549
cells were transfected with 20 ng poly (I:C) from Sigma-Aldrich;
Merck KGaA (Darmstadt, Germany) using 5 µl Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol.
Cytopathic effect (CPE) inhibition
assay
The MDCK cell monolayers (2×104
cells/well) were grown in 96-well plates and inoculated with 100
TCID50 of serial influenza virus strains at 37°C for 2
h. Subsequently, the inoculum was removed and then incubated with
0–1,000 µg/ml of (+)-pinoresinol-O-β-D-glucopyranoside and the
positive control oseltamivir carboxylate (TLC PharmaChem., Inc.,
Canada) at 37°C, respectively. Following 48 h of incubation, the
influenza virus-infected cells were stained with 0.5% crystal
violet solution and observed under a routine light microscope (DM
3000; Leica Microsystems GmbH, Wetzlar, Germany). The 50%
inhibition concentration (IC50) of the virus-induced CPE
was calculated as previously described (20).
Plaque-reduction assays
The MDCK cell monolayers (5×105
cells/well) were seeded in 6-well plates and incubated overnight at
37°C to ensure adherence. The cells were then inoculated with 40
PFU/well of influenza virus, including influenza A/PR/8/34 (H1N1)
and influenza A/Guangzhou/GIRD07/09 (H1N1), and incubated at 37°C
with constant agitation. Following 2 h of incubation, the inoculum
was removed and replaced with maintenance DMEM (Gibco; Thermo
Fisher Scientific, Inc.) containing 1.5% agarose, 1.5 µg/ml
TPCK-trypsin and the indicated concentration of
(+)-pinoresinol-O-β-D-glucopyranoside. After 3 days, the cells were
fixed in 10% formalin and stained with 1% crystal violet.
Progeny virus yield reduction
assay
The A549 cells were grown to 90% confluency in
6-well plates and then infected with influenza (MOI=0.1) with or
without the indicated concentration of
(+)-pinoresinol-O-β-D-glucopyranoside. After 24 h, the supernatants
were harvested, and the confluent monolayers of MDCK cells
(2×104 cells/well) in the 96-well plate were inoculated
with 10-fold dilutions of the supernatants at 37°C for 2 h.
Subsequently, the inoculum was removed and replaced with serum-free
DMEM containing 1.5 µg/ml TPCK-trypsin. After 48 h, the viral
plaques were visualized using trypan blue and observed under a
light microscope.
Cell viability assay
The cytotoxic effects induced by
(+)-pinoresinol-O-β-D-glucopyranoside in A549 cells were evaluated
using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assay. In brief, the A549 cells (1×105
cells/well) were seeded into 96-well plates and then incubated with
(+)-pinoresinol-O-β-D-glucopyranoside at different concentrations
(0–1,000 µg/ml) for 48 h. Subsequently, the cells were washed twice
with PBS to remove the drug and incubated with 200 µl MTT solution
(5 mg/ml) for an additional 4 h. The formazan crystals generated in
each well were dissolved with dimethyl sulfoxide (Sigma-Aldrich;
Merck KGaA). The absorbance was determined at 490 nm using a
microplate reader (Synergy HT; BioTek Instruments, Inc., Winooski,
VT, USA).
Western blot analysis
The following primary antibodies (Cell Signaling
Technology, Inc., Danvers, MA, USA) were used for western blot
analysis: NF-κB p65 (cat. no. 8242), phosphorylated
(phosphor)-NF-κB p65 (Ser536) (cat. no. 3033), p38 MAPK
(cat. no. 8690), phospho-p38 MAPK
(Thr180/Tyr182) (cat. no. 4511), AKT (cat.
no. 4691), phospho-AKT (Thr308) (cat. no. 13038), ERK1/2
MAPK (cat. no. 4695), phospho-ERK1/2 MAPK
(Thr202/Tyr204) (cat. no. 9101), c-Jun
N-terminal kinase (JNK) MAPK (cat. no. 9252), phospho-JNK MAPK
(Thr183/Tyr185) (cat. no. 4671),
cyclooxygenase-2 (COX-2; cat. no. 12282) and GAPDH (cat. no. 5174).
The HRP-conjugated secondary antibody (cat. no. BAB1302) was
acquired from Multisciences Biotech Co., Ltd. (Hangzhou,
China).
The cells were rinsed twice with ice-cold PBS and
lysed in RIPA lysis buffer containing 50 mM Tris (pH 7.4), 150 mM
NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 1 mM PMSF
and protease inhibitors (Sigma-Aldrich; Merck KGaA). The
supernatants from each treatment were collected by centrifugation
of the lysates at 13,000 × g for 15 min at 4°C, and then evaluated
to determine the protein concentration using a BCA protein assay
kit (Pierce; Thermo Fisher Scientific, Inc.). Equivalent quantities
of protein (20 µg/lane) were resolved on a 10% polyacrylamide gel
and transferred onto a PVDF membrane (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The membranes were then blocked with 5% non-fat
milk (w/v) in 1X TBS/Tween-20 buffer (0.1%, v/v) for 1 h at room
temperature prior to incubation with the primary and secondary
antibodies. Then, the membranes were incubated overnight at 4°C
with 1:1,000 dilution of primary antibody in 5% BSA (w/v) in
TBS/Tween-20 buffer (0.1% v/v). The HRP-conjugated secondary
antibody was used to detect the primary antibody at a dilution of
1:500 for 1 h at room temperature. The bands were detected using an
enhanced chemiluminescence reaction kit (Amersham; GE Healthcare
Life Sciences, Chalfont, UK). The intensity of the phosphorylated
bands was quantified using ImageJ software version 1.43 (National
Institutes of Health, Bethesda, MD, USA).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
RT-qPCR analysis was performed to determine the
relative mRNA levels of cytokines and chemokines. Briefly, the
influenza A virus-infected cells were treated with the indicated
concentrations of (+)-pinoresinol-O-β-D-glucopyranoside. Total
cellular RNA was extracted using TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc.); the cDNA was then synthesized from 1 µg total
RNA using a PrimeScript™ RT Reagent kit (Takara Bio, Inc., Otsu,
Japan), at 37°C for 15 min followed by 5 sec at 85°C to inactivate
the reaction. The qPCR analysis was performed using a Premix Ex
Taq™ Reagent kit (Takara Bio, Inc.), with initial heating to 95°C
for 30 sec, followed by 40 cycles of denaturation at 95°C for 5 sec
and annealing and elongation at 60°C for 40 sec in an Applied
Biosystems 7500 Real-Time PCR system with the primers and probes
specified in Table I. GAPDH was
used as an internal reference gene. The relative mRNA expression
data were calculated using the 2−ΔΔCq method (21).
 | Table I.Primers and probe sequences for
reverse transcription-quantitative polymerase chain reaction
analysis. |
Table I.
Primers and probe sequences for
reverse transcription-quantitative polymerase chain reaction
analysis.
| Gene | Primer and
probe | Sequence
(5′→3′) |
|---|
| TNF-α | Forward |
AACATCCAACCTTCCCAAACG |
|
| Reverse |
GACCCTAAGCCCCCAATTCTC |
|
| Probe |
CCCCCTCCTTCAGACACCCTCAACC |
| IL-6 | Forward |
CGGGAACGAAAGAGAAGCTCTA |
|
| Reverse |
CGCTTGTGGAGAAGGAGTTCA |
|
| Probe |
TCCCCTCCAGGAGCCCAGCT |
| IL-8 | Forward |
TTGGCAGCCTTCCTGATTTC |
|
| Reverse |
TATGCACTGACATCTAAGTTCTTTAGCA |
|
| Probe |
CCTTGGCAAAACTGCACCTTCACACA |
| MCP-1 | Forward |
CAAGCAGAAGTGGGTTCAGGAT |
|
| Reverse |
AGTGAGTGTTCAAGTCTTCGGAGTT |
|
| Probe |
CATGGACCACCTGGACAAGCAAACC |
| COX-2 | Forward |
GAATCATTCACCAGGCAAATTG |
|
| Reverse |
TTTCTGTACTGCGGGTGGAAC |
|
| Probe |
TTCCTACCACCAGCAACCCTGCCA |
| GAPDH | Forward |
GAAGGTGAAGGTCGGAGTC |
|
| Reverse |
GAAGATGGTGATGGGATTTC |
|
| Probe |
CAAGCTTCCCGTTCTCAGCC |
Pro-inflammatory mediator
measurements
The inhibitory effects of
(+)-pinoresinol-O-β-D-glucopyranoside on the influenza
virus-induced production of pro-inflammatory mediators were
measured via Luminex assays and ELISAs, respectively. Briefly, the
A549 cells in 6-well plates were inoculated with A/PR/8/34 (H1N1)
for 2 h, followed by treatment with different concentrations of
(+)-pinoresinol-O-β-D-glucopyranoside. Following another 24 h of
incubation, the culture supernatants were collected to evaluate the
levels of tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-8,
monocyte chemoattractant protein 1 (MCP-1) and prostaglandin E2
(PGE2) using a Luminex kit (Bio-Rad Laboratories, Inc.) and ELISA
kits (Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocols.
Statistical analyses
All data were analyzed using SPSS v.18.0 statistical
software (SPSS, Inc., Chicago, IL, USA) and are presented as the
mean ± standard deviation based on at least three independent
experiments. Statistical analyses were performed using one-way
analysis of variance followed by Bonferroni's multiple comparisons
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Structural elucidation of
(+)-pinoresinol-O-β-D-glucopyranoside
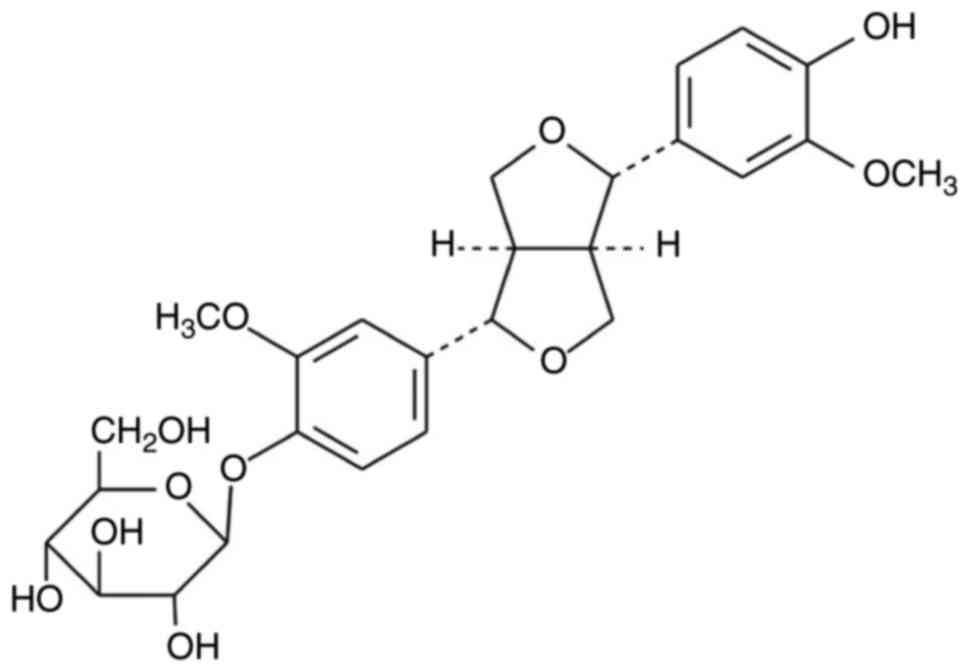
White amorphous powder, 1H-NMR (MeOD,400 MHz):δ7.15
(1H, d, J=8.4 Hz, H-5′), 6.95 (1H, d, J=1.6 Hz, H-2′), 6.92 (1H,
dd, J=8.4, 1.6 Hz, H-6′), 6.82 (1H, br, J=8.4 Hz, H-6), 6.80(1H, d,
J=1.6 Hz, H-2), 6.77 (1H, d, J=8.4 Hz, H-5), 4.88 (1H, d, J=6.0 Hz,
Glc-1), 4.76 (1H, d, J=4.0 Hz, H-7′), 4.71 (1H, d, J=4.0 Hz, H-7),
3.88 (3H, s, 3′-OMe), 3.87 (3H, s, 3-OMe). 13C-NMR (MeOD, 100 MHz):
148.0 (C-3′), 146.17 (C-3), 144.53 (C-4′), 144.36 (C-4), 134.49
(C-1′), 130.78 (C-1), 117.10 (C-6), 116.84 (C-6), 115.02 (C-5′),
113.12 (C-5), 108.64 (C-2′), 108.0 (C-2), 99.87 (Glc-1), 84.54
(C-7), 84.15 (C-7′), 75.24 (Glc-3), 74.87 (Glc-5), 71.94 (Glc-2),
69.76 (C-9′), 69.72 (C-9), 68.36 (Glc-4), 59.5 (Glc-6), 53.8
(3-OMe), 53.45 (3′-OMe), 52.58 (C-8′), 52.39 (C-8). The data were
in accordance with the literature regarding
(+)-pinoresinol-O-β-D-glucopyranoside (Fig. 1) (22).
Anti-influenza effects of
(+)-pinoresinol-O-β-D-glucopyranoside in vitro
The present study initially evaluated the
anti-influenza effects of (+)-pinoresinol-O-β-D-glucopyranoside
using a CPE reduction assay. The MDCK cells were inoculated with
100 TCID50 of influenza viruses and then incubated with
a concentration series of (+)-pinoresinol-O-β-D-glucopyranoside or
oseltamivir carboxylate following removal of the inoculum.
(+)-pinoresinol-O-β-D-glucopyranoside was found to reduce the CPE
induced by two influenza A/H1N1 viral strains (A/PR/8/34 and
A/Guangzhou/GIRD07/09), with IC50 values of
176.24–408.81 µg/ml and SI values of 1.80–4.17 (Table II). However,
(+)-pinoresinol-O-β-D-glucopyranoside did not exhibit antiviral
effects against influenza virus A/Hongkong/8/68 (H3N2),
A/Hongkong/Y280/97 (H9N2) or B/Lee/1940 (FluB) (Table II). The activity against A/PR/8/34
and A/Guangzhou/GIRD07/09 was confirmed using plaque reduction
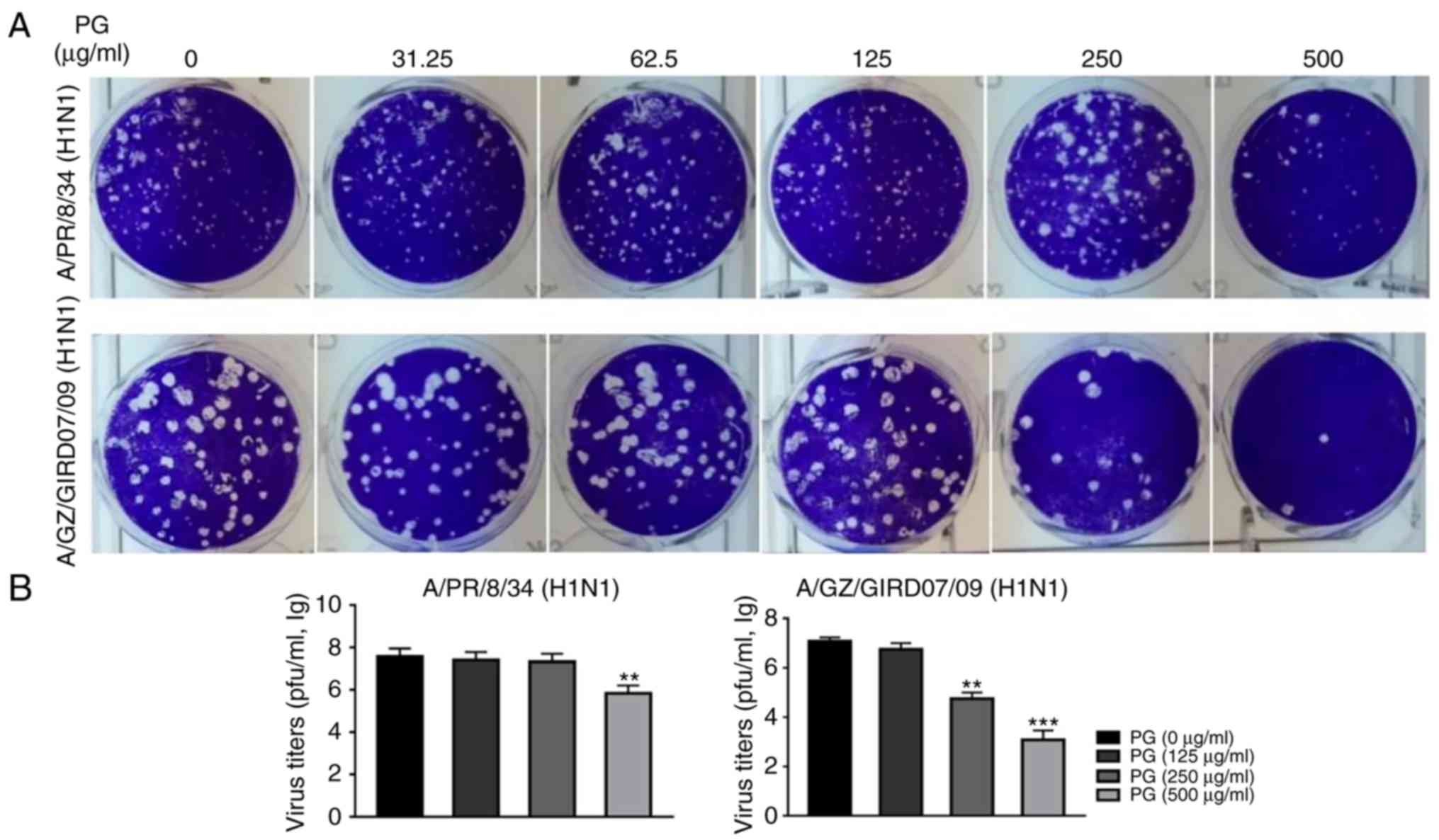
assays and progeny virus yield reduction assays. As shown in
Fig. 2A,
(+)-pinoresinol-O-β-D-glucopyranoside treatment significantly
reduced plaque formation in the A/PR/8/34 and A/Guangzhou/GIRD07/09
(H1N1) virus-infected cells. Furthermore, the progeny virus titers
of the two virus strains were significantly decreased by treatment
with (+)-pinoresinol-O-β-D-glucopyranoside at the concentration of
250 or 500 µg/ml (Fig. 2B).
Together, these results suggested that
(+)-pinoresinol-O-β-D-glucopyranoside inhibits influenza A H1N1
viruses.
 | Table II.Anti-influenza virus efficacy of
(+)-pinoresinol-O-β-D-glucopyranoside. |
Table II.
Anti-influenza virus efficacy of
(+)-pinoresinol-O-β-D-glucopyranoside.
|
|
(+)-pinoresinol-O-β-D-glucopyranoside
(µg/ml) | Oseltamivir
(µg/ml) |
|---|
|
|
|
|
|---|
| Virus type and
strain |
TC50 |
IC50 | SIa |
TC50 |
IC50 | SIa |
|---|
| A/PR/8/34
(H1N1) | 736.49±34.51 | 408.81±5.24 | 1.80±0.11 | >1,000 | 0.041±0.01 | >1,000 |
| A/GZ/GIRD07/09
(H1N1) | 736.49±34.51 | 176.24±4.41 | 4.17±0.30 | >1,000 | 0.022±0.01 | >1,000 |
| A/HK/8/68
(H3N2) | 736.49±34.51 | >737 | <1 | >1,000 | 0.098±0.01 | >1,000 |
| A/HK/Y280/97
(H9N2) | 736.49± 4.51 | >737 | <1 | >1,000 | 0.756±0.12 | >200 |
| B/Lee/1940
(FluB) | 736.49±34.51 | >737 | <1 | >1,000 | 11.51±1.19 | >100 |
Effects of
(+)-pinoresinol-O-β-D-glucopyranoside on A549 cell viability
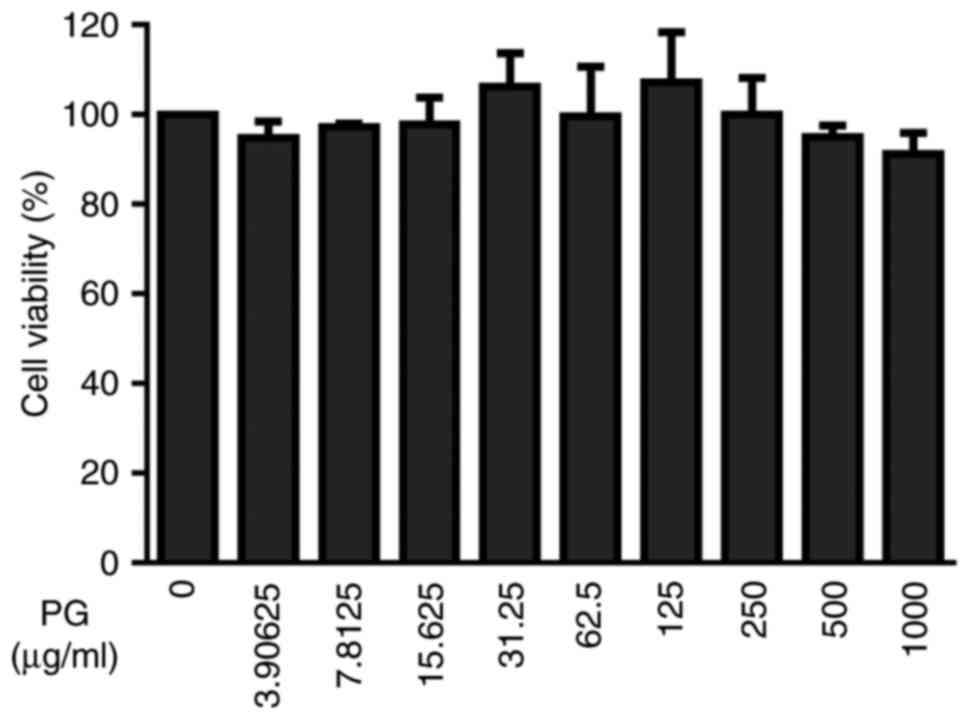
To select appropriate concentrations for further
experiments, the A549 cells were incubated with increasing
concentrations of (+)-pinoresinol-O-β-D-glucopyranoside for 48 h.
Following this, cell viability was assessed with an MTT assay to
evaluate the potential cytotoxicity of lignan
(+)-pinoresinol-O-β-D-glucopyranoside. The results showed that
(+)-pinoresinol-O-β-D-glucopyranoside did not affect the viability
of A549 cells up to a concentration of 1,000 µg/ml (Fig. 3). Therefore, the pharmacological
effects of (+)-pinoresinol-O-β-D-glucopyranoside on viral infection
were investigated using a concentration range of 150–450 µg/ml.
Effect of
(+)-pinoresinol-O-β-D-glucopyranoside on influenza virus-induced
cellular signaling
Studies have revealed that influenza A virus
exploits multiple host cell signaling pathways to facilitate
self-replication (23,24). It has been suggested that the
pharmacological inhibition of cellular signaling may be a potential
strategy for controlling viral infection (25). The results of the study indicated
that (+)-pinoresinol-O-β-D-glucopyranoside possesses antiviral
activity against influenza A H1N1, therefore, whether the anti-H1N1
virus activity was associated with the inhibition of signaling
pathways required for influenza virus infection was determined.
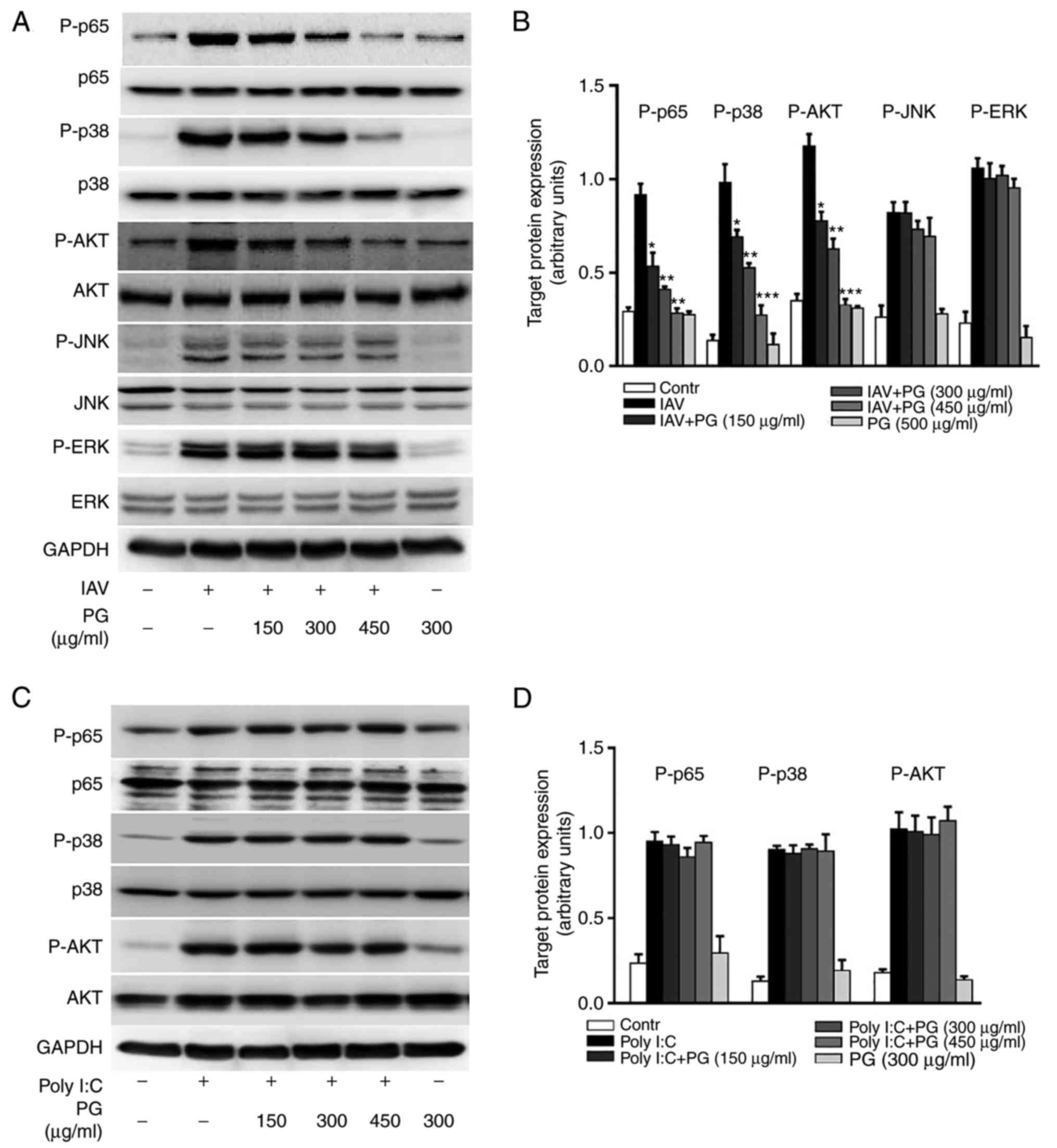
Treatment with (+)-pinoresinol-O-β-D-glucopyranoside significantly
decreased the influenza H1N1-induced activation of multiple
cellular signaling pathways, including the NF-κB, p38, MAPK and AKT
pathways, but not the JNK or ERK MAPK pathways (Fig. 4A and B). As these pathways may also
have been activated by viral products, whether
(+)-pinoresinol-O-β-D-glucopyranoside affected synthetic mimics of
viral RNA poly (I:C)-mediated pathway activation was investigated.
As shown in Fig. 4C and D, it was
found that (+)-pinoresinol-O-β-D-glucopyranoside did not affect the
poly (I:C)-induced activation of NF-κB, p38 MAPK or AKT signaling.
Taken together, these results suggested that
(+)-pinoresinol-O-β-D-glucopyranoside inactivates multiple cellular
signaling pathways triggered by viral infection, therefore exerting
antivirus effects against H1N1.
Effects of
(+)-pinoresinol-O-β-D-glucopyranoside on the influenza
virus-induced expression of pro-inflammatory mediators
The high or low pathogenic influenza virus-induced
hyperinduction of pro-inflammatory mediators was mediated though
specific host cellular pathways, which are considered to affect the
severity of influenza diseases (26,27).
To determine whether (+)-pinoresinol-O-β-D-glucopyranoside can
affect the H1N1 influenza virus-induced expression of
pro-inflammatory mediators though the inhibition of cellular
signaling, the present study assessed the expression of
pro-inflammatory mediators at the mRNA and protein levels via
RT-qPCR and Luminex assays, respectively. As shown in Fig. 5A and B, treatment with
(+)-pinoresinol-O-β-D-glucopyranoside decreased the H1N1 influenza
virus-induced upregulation of cytokine and chemokine expression,
including that of TNF-α, IL-6, IL-8 and MCP-1, in a
concentration-dependent manner. Furthermore, it was found that
treatment with (+)-pinoresinol-O-β-D-glucopyranoside suppressed the
H1N1 virus-induced production of COX-2 (Fig. 5C and D) and that of the derived
PGE2 (Fig. 5E). These results
indicated that (+)-pinoresinol-O-β-D-glucopyranoside decreased the
H1N1 influenza virus-induced expression of pro-inflammatory
mediators via the inhibition of multiple signaling pathways.
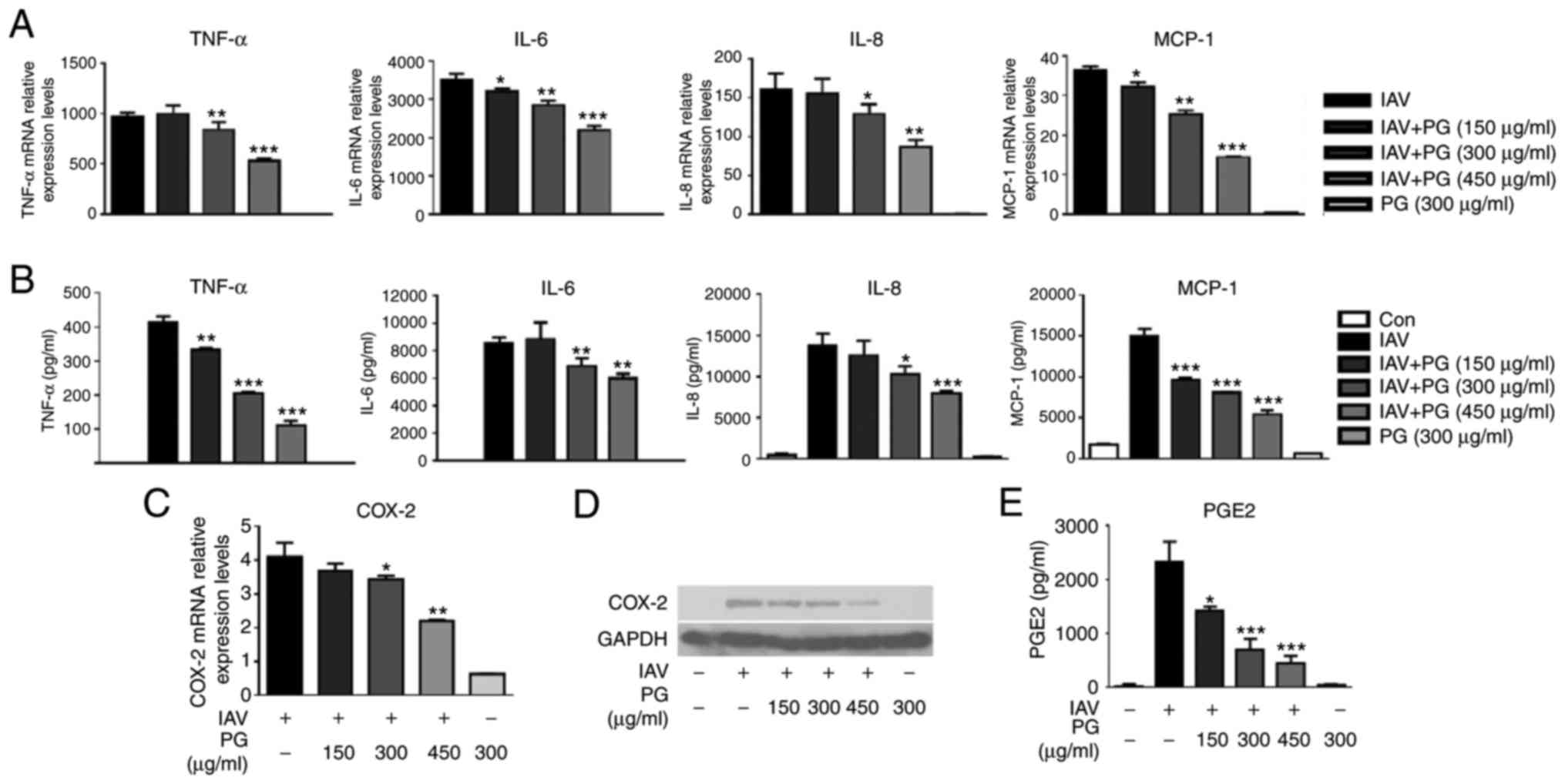 | Figure 5.PG treatment reduces IAV-induced
expression of pro-inflammatory mediators. (A) A549 cells were
either mock-infected or infected with IAV/PR8/34 (H1N1) (MOI=0.1)
in the presence or absence of PG (150–450 µg/ml), and then lysed
with TRIzol reagent for 24 h. Total RNA was isolated and RT-qPCR
analyses were performed to measure the gene expression of TNF-α,
IL-6, IL-8 and MCP-1. (B) Culture supernatants of H1N1
virus-infected A549 cells treated with different concentrations of
PG were harvested for the evaluation of cytokines and chemokines
using a Luminex assay. (C) mRNA and (D) protein levels of COX-2 in
H1N1 virus-infected A549 cells with treated with different
concentrations of PG were analyzed via RT-qPCR and western blot
analyses, respectively. (E) PGE2 levels in the culture supernatants
from virus-infected A549 cells with/without PG treatment were
evaluated using ELISAs. Data are expressed as the mean ± standard
deviation. *P<0.05, **P<0.01 and ***P<0.001, vs. untreated
infected cells. PG, (+)-pinoresinol-O-β-D-glucopyranoside; IAV,
influenza A virus; Con, control; TNF-α, tumor necrosis factor-α;
IL, interleukin; MCP, monocyte chemoattractant protein 1; COX-2,
cyclooxygenase-2; PGE2, prostaglandin E2; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. |
Discussion
In previous decades, the increasing incidence of
antiviral drug-resistant influenza viruses has highlighted the
urgency for novel antiviral drugs. Compounds from Chinese herbal
medicines have gained interest in the development of novel
antiviral medications as they tend to possess multiple activities
and a broad safety window. In the present study, a lignan compound
was isolated from Eucommia ulmoides Oliver and its structure
was subjected to extensive spectroscopic analysis; it was
identified as (+)-pinoresinol-O-β-D-glucopyranoside. Further
investigation showed that the antiviral and anti-inflammatory
effects of (+)-pinoresinol-O-β-D-glucopyranoside against influenza
virus infection likely occur through the inhibition of AKT, NF-κB,
and p38 MAPK signaling.
Our previous study reported on the structure of a
novel lignan glycoside [(+)-pinoresinol
4-O-[6-O-vanilloyl]-β-D-glucopyranoside)] from the latex of
Calotropis gigantean, comprised of
(+)-pinoresinol-O-β-D-glucopyranoside moiety and a vanilloyl group,
which possessed antiviral activity though the retention of vRNPs in
the nucleus (28). Additionally,
it was found that (+)-pinoresinol-O-β-D-glucopyranoside did not
have any inhibitory effects on influenza A/PR/8/34 (H1N1) virus
with an IC50 value >348.6 µM and SI value <1.0
(28). In the present study, the
antiviral effect of (+)-pinoresinol-O-β-D-glucopyranoside was
re-evaluated, and the compound was found to have antiviral activity
against the influenza A/PR/8/34 (H1N1) virus with an
IC50 value of 408.81±5.24 µg/ml (785.37±10.07 µM)
(Table II), which was higher than
previously reported and for that of (+)-pinoresinol
4-O-[6′-O-vanilloyl]-β-D-glucopyranoside. These results suggested
that (+)-pinoresinol-O-β-D-glucopyranoside was not potent enough to
exert inhibitory effects on the influenza A/PR/8/34 (H1N1) virus at
low doses due to the absence of a vanilloyl moiety. The antiviral
properties of (+)-pinoresinol-O-β-D-glucopyranoside were confirmed
by the result that treatment reduced influenza A/GZ/GIRD07/09
(H1N1) virus-induced CPE in MDCK cells (Table II).
Influenza viruses exploit multiple host cell
signaling cascades to facilitate their replication. An increasing
number of reports have demonstrated that the suppression of
cellular signaling using pharmacological agents can limit the
spread of influenza. The inhibition of NF-κB activity by
acetylsalicylic acid or Bay 11–7082 inhibited influenza virus
propagation via the retention of vRNP in the nucleus, and
effectively reduced viral titers in vitro and in vivo
(13,29). Furthermore, the synthesis of eight
segments of the viral RNA (vRNA) genome was reduced by an NF-κB
inhibitor (30). Similarly, the
inhibition of PI3K/AKT signaling confirmed its importance in viral
processes, including viral uptake, vRNA synthesis and vRNP nuclear
export (14,31). Phosphorylation of the
early-endosomal protein EEA1 and anti-apoptotic factor B-cell
lymphoma 2 by P38 MAPK has been reported to enhance the endocytosis
of virus particles and the nucleocytoplasmic export of viral NP
proteins, and this was eradicated following treatment with a P38
MAPK-specific inhibitor (15,32).
Findings indicated that inhibition of the NF-κB, p38 MAPK, and AKT
signaling pathways by specific inhibitors exerted antiviral
activity. However, certain compounds from Chinese herbal medicines
with NF-κB inhibition activity did not exhibit broad antiviral
activity and the detailed mechanism was not revealed (28,33).
In concordance, although the present found that the virus-induced
NF-κB, p38 MAPK, and AKT signaling pathways were inhibited by
(+)-pinoresinol-O-β-D-glucopyranoside,
(+)-pinoresinol-O-β-D-glucopyranoside did not exert inhibitory
effects on influenza virus A/Hongkong/8/68 (H3N2),
A/Hongkong/Y280/97 (H9N2) or B/Lee/1940 (FluB) (Table II). In the present study, the
reasons why lignan (+)-pinoresinol-O-β-D-glucopyranoside, with its
NF-κB, p38 MAPK, and AKT signaling inhibition properties, did not
show broad antiviral activity were not elucidated. The results
suggested that the inhibition activity of natural compounds from
traditional Chinese medicine on cellular molecules was not potent
enough, compared with specific inhibitors. It is anticipated that
investigations in the future will elucidate the detailed mechanism.
The possible underlying mechanism of
(+)-pinoresinol-O-β-D-glucopyranoside against influenza infection
may involve inactivation of the NF-κB, P38 MAPK and PI3K/AKT
signaling pathways (Fig. 3).
The results of the present study demonstrated that
(+)-pinoresinol-O-β-D-glucopyranoside decreased the expression of
TNF-α, IL-6, IL-8 and MCP-1 (Fig. 5A
and B). During influenza virus infection, the abnormal
activation of host signaling pathways leads to an excessive
inflammatory response, which is considered to result in lung tissue
injury and may progress to ARDS (34). In patients infected with seasonal
influenza viruses, the levels of cytokines, including IL-6, TNF-α
and interferon (IFN)-γ-inducible protein 10 (IP-10), were elevated
on day 1 but had declined rapidly by day 5 (35). By contrast, the persistent
elevation of cytokines in patients infected with avian H7N9 or H5N1
viruses resulted in poor outcomes and even mortality (27,36).
Dysregulation among pro-inflammatory cytokines has served as a
hallmark of influenza disease severity (37). The suppression of NF-κB signaling
has been shown to decrease the influenza virus-mediated expression
of IL-6, IL-8, MCP-1 and RANTES in vitro and in vivo
(13). p50 subunit deficiency in
mice attenuated an array of NF-κB-targeted genes induced by
influenza A (H5N1) (38).
P38-mediated signaling is also involved in the initiation of
pro-inflammatory cytokine synthesis. Treatment with a p38 MAPK
inhibitor (SB203580) reduced the H5N1 virus-mediated expression of
cytokines and chemokines, including TNF-α, IP-10, MCP-1 and RANTES
(39). The cytokine levels,
including those of IP-10 and MCP-1, in patients with severe
influenza A virus infection were positively correlated with the
expression of P38 MAPK in CD4+ lymphocytes (40). During viral replication, the viral
products, including viral RNA sensed by host pattern recognition
receptors can also activate cellular signaling and initiate the
expression of pro-inflammatory cytokines. In examining whether that
the anti-inflammatory effects of
(+)-pinoresinol-O-β-D-glucopyranoside is due to its antiviral
property or the inhibition of cellular signaling triggered by viral
products, the present study found that treatment with
(+)-pinoresinol-O-β-D-glucopyranoside did not affect the poly
(I:C)-mediated activation of NF-κB, p38 kinase or AKT signaling
(Fig. 4B). These results suggested
that the anti-inflammatory effects of
(+)-pinoresinol-O-β-D-glucopyranoside were a result of its
antiviral effects. Therefore, it was hypothesized that the
inhibitory effects of (+)-pinoresinol-O-β-D-glucopyranoside on
infection-activated NF-κB and p38 kinase led to a decrease in the
influenza virus-induced expression of pro-inflammatory
cytokines.
Previous studies have reported that NF-κB and p38
kinase signaling are required for the expression of COX-2, which is
involved in the pathogenesis of pneumococcal pneumonia and
influenza H5N1 viral disease (41–43).
From the data presented in the present study, the inhibitory
effects on NF-κB and p38 kinase signaling by
(+)-pinoresinol-O-β-D-glucopyranoside treatment were correlated
with the decreased expression of COX-2 and PGE2 (Fig. 5C-E). Previous studies have
demonstrated that COX-2 deficiency or inhibition significantly
reduced virus-induced inflammation and changes in body temperature,
and protected against life-threatening influenza challenge
(44,45). Notably, the delayed combination of
antiviral agents with COX-2 inhibitor treatment significantly
prolonged the survival of mice infected with H5N1 (46). Additionally, Coulombe et al
revealed that PGE2 impaired the type I IFN-mediated antiviral
response (47). Therefore, it
appears that suppression of the expression of COX-2 and PGE2 by
(+)-pinoresinol-O-β-D-glucopyranoside is beneficial to the host
during influenza infection.
In conclusion, the present study found that
(+)-pinoresinol-O-β-D-glucopyranoside from Eucommia ulmoides
Oliver exerts antiviral and anti-inflammatory effects through
NF-κB, P38 MAPK and AKT signaling pathway inhibition in influenza
virus-infected cells. Therefore, it was hypothesized that the
product possesses multiple biological activities and low toxicity,
and that it may be a promising anti-influenza candidate drug.
Acknowledgements
Not applicable.
Funding
The present study was financially supported by the
Ministry of Science and Technology of China (grant no.
2015DFM30010), the Secondary Development Projects of Guangdong
Famous and Excellent Traditional Chinese Patent Medicines (grant
no. 20174005), the First Affiliated Hospital of Guangzhou Medical
University (grant no. LJ2016) and the International S&T
Cooperation Program of Guangdong Province (grant no.
2013B051000085).
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding authors on reasonable
request.
Author contributions
ZY, XP and ZJ conceived and designed the
experiments; JL, XL, BZ, XC, PX and HJ performed the experiments;
JL and XL analyzed the data; JL, XL and BZ wrote the manuscript.
ZY, XP and ZJ contributed to revisions of the manuscript. All
authors read and approved the final manuscript.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hayden FG and Gwaltney JMJ: Viral
infections. In textbook of respiratory medicine. Murray JF and
Nadel JA: WB. Saunders Co.; Philadelphia: pp. 977–1035. 1994
|
|
2
|
Peiris JS, Yu WC, Leung CW, Cheung CY, Ng
WF, Nicholls JM, Ng TK, Chan KH, Lai ST, Lim WL, et al:
Re-emergence of fatal human influenza A subtype H5N1 disease.
Lancet. 363:617–619. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kash JC, Tumpey TM, Proll SC, Carter V,
Perwitasari O, Thomas MJ, Basler CF, Palese P, Taubenberger JK,
García-Sastre A, et al: Genomic analysis of increased host immune
and cell death responses induced by 1918 influenza virus. Nature.
443:578–581. 2006.PubMed/NCBI
|
|
4
|
Hayden FG and de Jong MD: Emerging
influenza antiviral resistance threats. J Infect Dis. 203:6–10.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hai R, Schmolke M, Leyva-Grado VH,
Thangavel RR, Margine I, Jaffe EL, Krammer F, Solórzano A,
García-Sastre A, Palese P and Bouvier NM: Influenza A(H7N9) virus
gains neuraminidase inhibitor resistance without loss of in vivo
virulence or transmissibility. Nat Commun. 4:28542013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gamblin SJ and Skehel JJ: Influenza
hemagglutinin and neuraminidase membrane glycoproteins. J Biol
Chem. 285:28403–28409. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thompson AJ and Locarnini SA: Toll-like
receptors, RIG-I-like RNA helicases and the antiviral innate immune
response. Immunol Cell Biol. 85:435–445. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fukuyama S and Kawaoka Y: The pathogenesis
of influenza virus infections: The contributions of virus and host
factors. Curr Opin Immunol. 23:481–486. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Conenello GM, Zamarin D, Perrone LA,
Tumpey T and Palese P: A single mutation in the PB1-F2 of H5N1
(HK/97) and 1918 influenza A viruses contributes to increased
virulence. PLoS Pathog. 3:1414–1421. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Högner K, Wolff T, Pleschka S, Plog S,
Gruber AD, Kalinke U, Walmrath HD, Bodner J, Gattenlöhner S,
Lewe-Schlosser P, et al: Macrophage-expressed IFN-β contributes to
apoptotic alveolar epithelial cell injury in severe influenza virus
pneumonia. PLoS Pathog. 9:e10031882013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hagau N, Slavcovici A, Gonganau DN, Oltean
S, Dirzu DS, Brezoszki ES, Maxim M, Ciuce C, Mlesnite M, Gavrus RL,
et al: Clinical aspects and cytokine response in severe H1N1
influenza A virus infection. Crit Care. 14:R2032010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ludwig S and Planz O: Influenza viruses
and the NF-kappaB signaling pathway-towards a novel concept of
antiviral therapy. Biol Chem. 389:1307–1312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pinto R, Herold S, Cakarova L, Hoegner K,
Lohmeyer J, Planz O and Pleschka S: Inhibition of influenza
virus-induced NF-kappaB and Raf/MEK/ERK activation can reduce both
virus titers and cytokine expression simultaneously in vitro and in
vivo. Antiviral Res. 92:45–56. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ehrhardt C, Marjuki H, Wolff T, Nürnberg
B, Planz O, Pleschka S and Ludwig S: Bivalent role of the
phosphatidylinositol-3-kinase (PI3K) during influenza virus
infection and host cell defence. Cell Microbiol. 8:1336–1348. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marchant D, Singhera GK, Utokaparch S,
Hackett TL, Boyd JH, Luo Z, Si X, Dorscheid DR, McManus BM and
Hegele RG: Toll-like receptor 4-mediated activation of p38
mitogen-activated protein kinase is a determinant of respiratory
virus entry and tropism. J Virol. 84:11359–11373. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu Z, Tang M, Li Y, Liu F, Li X and Dai R:
Antioxidant properties of Du-zhong (Eucommia ulmoides Oliv.)
extracts and their effects on color stability and lipid oxidation
of raw pork patties. J Agric Food Chem. 58:7289–7296. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee MK, Cho SY, Kim DJ, Jang JY, Shin KH,
Park SA, Park EM, Lee JS, Choi MS, Lee JS, et al: Du-zhong
(Eucommia ulmoides Oliv.) cortex water extract alters heme
biosynthesis and erythrocyte antioxidant defense system in
lead-administered rats. J Med Food. 8:86–92. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hussain T, Tan B, Liu G, Oladele OA, Rahu
N, Tossou MC and Yin Y: Health-promoting properties of eucommia
ulmoides: A review. Evid Based Complement Alternat Med.
2016:52029082016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li L, Yan J, Hu K, Gu J, Wang JJ, Deng XL,
Li H, Jing X, Li ZY, Ye QF, et al: Protective effects of Eucommia
lignans against hypertensive renal injury by inhibiting expression
of aldose reductase. J Ethnopharmacol. 139:454–461. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reed LJ and Muench H: A simple method of
estimating fifty percent endpoints. American J Epidemiol.
27:493–497. 1938. View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2ΔΔCT method.
|
|
22
|
Sugiyama M and Kikuchi M: Studies on the
constituents of osmanthus species. VII. Structures of lignan
glycosides from the leaves of osmanthus asiaticus NAKAI. Chem Pharm
Bull. 396:483–485. 1991. View Article : Google Scholar
|
|
23
|
Marjuki H, Gornitzky A, Marathe BM,
Ilyushina NA, Aldridge JR, Desai G, Webby RJ and Webster RG:
Influenza A virus-induced early activation of ERK and PI3K mediates
V-ATPase-dependent intracellular pH change required for fusion.
Cell Microbiol. 13:587–601. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marjuki H, Alam MI, Ehrhardt C, Wagner R,
Planz O, Klenk HD, Ludwig S and Pleschka S: Membrane accumulation
of influenza A virus hemagglutinin triggers nuclear export of the
viral genome via protein kinase Calpha-mediated activation of ERK
signaling. J Biol Chem. 281:16707–16715. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ludwig S: Targeting cell signalling
pathways to fight the flu: Towards a paradigm change in
anti-influenza therapy. J Antimicrob Chemother. 64:1–4. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hayden FG, Fritz R, Lobo MC, Alvord W,
Strober W and Straus SE: Local and systemic cytokine responses
during experimental human influenza A virus infection. Relation to
symptom formation and host defense. J Clin Invest. 101:643–649.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de Jong MD, Simmons CP, Thanh TT, Hien VM,
Smith GJ, Chau TN, Hoang DM, Chau NV, Khanh TH, Dong VC, et al:
Fatal outcome of human influenza A (H5N1) is associated with high
viral load and hypercytokinemia. Nat Med. 12:1203–1207. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Parhira S, Yang ZF, Zhu GY, Chen QL, Zhou
BX, Wang YT, Liu L, Bai LP and Jiang ZH: In vitro anti-influenza
virus activities of a new lignan glycoside from the latex of
Calotropis gigantea. PLoS One. 9:e1045442014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mazur I, Wurzer WJ, Ehrhardt C, Pleschka
S, Puthavathana P, Silberzahn T, Wolff T, Planz O and Ludwig S:
Acetylsalicylic acid (ASA) blocks influenza virus propagation via
its NF-kappaB-inhibiting activity. Cell Microbiol. 9:1683–1694.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kumar N, Xin ZT and Liang Y, Ly H and
Liang Y: NF-kappaB signaling differentially regulates influenza
virus RNA synthesis. J Virol. 82:9880–9889. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shin YK, Liu Q, Tikoo SK, Babiuk LA and
Zhou Y: Effect of the phosphatidylinositol 3-kinase/Akt pathway on
influenza A virus propagation. J Gen Virol. 88:942–950. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nencioni L, De Chiara G, Sgarbanti R,
Amatore D, Aquilano K, Marcocci ME, Serafino A, Torcia M, Cozzolino
F, Ciriolo MR, et al: Bcl-2 expression and p38MAPK activity in
cells infected with influenza A virus: Impact on virally induced
apoptosis and viral replication. J Biol Chem. 284:16004–16015.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guan W, Li J, Chen Q, Jiang Z, Zhang R,
Wang X, Yang Z and Pan X: Pterodontic acid isolated from laggera
pterodonta inhibits viral replication and inflammation induced by
influenza A virus. Molecules. 22:E17382017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bauer TT, Ewig S, Rodloff AC and Müller
EE: Acute respiratory distress syndrome and pneumonia: A
comprehensive review of clinical data. Clin Infect Dis. 43:748–756.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bian JR, Nie W, Zang YS, Fang Z, Xiu QY
and Xu XX: Clinical aspects and cytokine response in adults with
seasonal influenza infection. Int J Clin Exp Med. 7:5593–5602.
2014.PubMed/NCBI
|
|
36
|
Yang ZF, Mok CK, Liu XQ, Li XB, He JF,
Guan WD, Xu YH, Pan WQ, Chen LY, Lin YP, et al: Clinical,
virological and immunological features from patients infected with
re-emergent avian-origin human H7N9 influenza disease of varying
severity in Guangdong province. PLoS One. 10:e01178462015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ramos I and Fernandez-Sesma A: Modulating
the innate immune response to influenza A virus: Potential
therapeutic use of anti-inflammatory drugs. Front Immunol.
6:3612015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Droebner K, Reiling SJ and Planz O: Role
of hypercytokinemia in NF-kappaB p50-deficient mice after H5N1
influenza A virus infection. J Virol. 82:11461–11466. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hui KP, Lee SM, Cheung CY, Ng IH, Poon LL,
Guan Y, Ip NY, Lau AS and Peiris JS: Induction of proinflammatory
cytokines in primary human macrophages by influenza A virus (H5N1)
is selectively regulated by IFN regulatory factor 3 and p38 MAPK. J
Immunol. 182:1088–1098. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee N, Wong CK, Chan PK, Lun SW, Lui G,
Wong B, Hui DS, Lam CW, Cockram CS, Choi KW, et al:
Hypercytokinemia and hyperactivation of phospho-p38
mitogen-activated protein kinase in severe human influenza A virus
infection. Clin Infect Dis. 45:723–731. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
N'Guessan PD, Hippenstiel S, Etouem MO,
Zahlten J, Beermann W, Lindner D, Opitz B, Witzenrath M, Rosseau S,
Suttorp N, et al: Streptococcus pneumoniae induced p38 MAPK- and
NF-kappaB-dependent COX-2 expression in human lung epithelium. Am J
Physiol Lung Cell Mol Physiol. 290:L1131–L1138. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Singer CA, Baker KJ, McCaffrey A, AuCoin
DP, Dechert MA and Gerthoffer WT: p38 MAPK and NF-kappaB mediate
COX-2 expression in human airway myocytes. Am J Physiol Lung Cell
Mol Physiol. 285:L1087–L1098. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee SM, Cheung CY, Nicholls JM, Hui KP,
Leung CY, Uiprasertkul M, Tipoe GL, Lau YL, Poon LL, Ip NY, et al:
Hyperinduction of cyclooxygenase-2-mediated proinflammatory
cascade: A mechanism for the pathogenesis of avian influenza H5N1
infection. J Infect Dis. 198:525–535. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Carey MA, Bradbury JA, Seubert JM,
Langenbach R, Zeldin DC and Germolec DR: Contrasting effects of
cyclooxygenase-1 (COX-1) and COX-2 deficiency on the host response
to influenza A viral infection. J Immunol. 175:6878–6884. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Carey MA, Bradbury JA, Rebolloso YD,
Graves JP, Zeldin DC and Germolec DR: Pharmacologic inhibition of
COX-1 and COX-2 in influenza A viral infection in mice. PLoS One.
5:e116102010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zheng BJ, Chan KW, Lin YP, Zhao GY, Chan
C, Zhang HJ, Chen HL, Wong SS, Lau SK, Woo PC, et al: Delayed
antiviral plus immunomodulator treatment still reduces mortality in
mice infected by high inoculum of influenza A/H5N1 virus. Proc Natl
Acad Sci USA. 105:8091–8096. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Coulombe F, Jaworska J, Verway M, Tzelepis
F, Massoud A, Gillard J, Wong G, Kobinger G, Xing Z, Couture C, et
al: Targeted prostaglandin E2 inhibition enhances antiviral
immunity through induction of type I interferon and apoptosis in
macrophages. Immunity. 40:554–568. 2014. View Article : Google Scholar : PubMed/NCBI
|