Introduction
Insulin resistance, one of the major pathogenic
events in type-2 diabetes mellitus, is characterized by the
inertness of tissues for insulin regulation (1). Insulin resistance impairs glucose
uptake in the peripheral tissue and increases hepatic glucose
output. In 2013, Turner et al (2) reported that diet-induced obesity
substantially increased the risk of insulin resistance. In obese
states, the excessive secretion of free fatty acids (FFA) and
adipokines result in endocrine effects to further lower insulin
sensitivity in muscle and liver (2). Rodents fed with a high-fat diet (HFD)
frequently develop obesity and insulin resistance, providing the
potential to study the development of this disease, and investigate
novel treatments to mitigate the disease-associated complications
(2). Thus far, a number of
plant-derived compounds have demonstrated hypoglycemic properties
and the potential to ameliorate insulin resistance associated with
obesity.
Dioscin, as a plant-derived steroidal saponin, is
abundant in Discorea species (yams) which has traditionally
been used for the treatment of asthma, abscesses, chronic diarrhea
and ulcers (3). Extracts of
Dioscorea batatas were additionally revealed to ameliorate
the insulin resistance of mice fed with a HFD (4). Dioscin has a wide spectrum of
biological activities, including anti-cancer (5) and antiviral activities (6). Meanwhile, it is also used for the
management of hyperglycemia and dyslipidemia due to its
cardiovascular protective activity (7). In addition, the functions in reducing
oxidative stress and inflammatory responses have been exploited to
attenuate ischemic-reperfusion injuries in multiple organs
(8). Previous evidence indicates
that dioscin has potent effects against obesity in mice (9), in which dioscin was able to alleviate
the increased body weight and liver lipid accumulation. Given that
a HFD and obesity are important contributors to insulin resistance,
dioscin is a promising drug for the treatment of insulin resistance
induced by a HFD.
The hyperactivity of stress-associated and
inflammatory pathways is a common characteristic of
insulin-resistant adipose tissues (10). Signaling pathways involved in
oxidative stress and inflammatory responses, including the
phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) signaling
pathway, have been considered as targets to reduce insulin
resistance (11). Gallagher et
al (12) reported that the
suppression of PI3K induced hyperglycemia in a mouse with insulin
resistance and hyperinsulinemia, suggesting that drugs restoring
the activity of PI3K may potentially alleviate insulin resistance.
In fact, the roles of dioscin in modulating the PI3K/Akt pathway
have already been demonstrated in 2013 (13).
Based on this, the present study aimed to
corroborate the effect of dioscin on regulating insulin resistance
induced by a HFD. The ability of dioscin in modulating phenotypic
parameters of the serum, including glucose, triglyceride (TG) and
total cholesterol (TC) levels, was investigated. To ascertain the
regulatory mechanism of dioscin, the involvement of the insulin
receptor substrate 1 (IRS-1)/PI3K/Akt signaling pathway and
peroxisome proliferator-activated receptor γ (PPAR-γ) pathway were
characterized. Furthermore, factors associated with fatty acid
synthesis, including fatty acid synthetase (FAS) and sterol
regulatory element-binding protein 1 (SREBP-1c; a transcription
factor that activates fatty acid synthesis) and the
adipocyte-derived insulin-sensitizing protein adiponectin were
monitored. Considering the prevalence of insulin resistance and the
critical need for an effective drug to ameliorate this disease, the
results of the present study may verify dioscin as an effective
drug to restore the insulin sensitivity of adipose tissues.
Materials and methods
Animals
All animal experiments were performed and ethically
approved by the Animal Care and Use Committee of the The Affiliated
Yantai Yuhuangding Hospital of Qingdao University (Yantai, China).
A total of 50 healthy male C57BL/6J mice (20–23 g) aged 6 weeks
were purchased from Nanjing Junke Biological Engineering Co., Ltd.
(Nanjing, China; http://njjkswgc.china.herostart.com/). Regular food
(10% kcal from fat, 70% kcal from carbohydrates) and high-fat food
(60% kcal from fat, 20% kcal from carbohydrates) were purchased
from Research Diets, Inc. (New Brunswick, NJ, USA). Mice were
housed in a specific pathogen-free room at 22–25°C and 40–50%
humidity under a 12 h light-dark cycle and had ad libitum
access to food and water.
The animals were allowed to adapt for 7 days prior
to being randomized into five groups: A control group (n=10)
receiving regular food and treated with saline through gavage; a
HFD group (n=10) were fed with a HFD and treated with saline
through gavage; and dioscin treatment groups treated with a HFD and
5 mg/kg/day (low), 10 mg/kg/day (medium) or 20 mg/kg/day (high)
dioscin through gavage. Over 12 weeks, body weight and body fat
were recorded. Subsequent to the end of the 12 weeks, the mice were
fasted for 1 day and blood was collected for serum biochemical
analysis. Then mice were then sacrificed in a CO2
chamber. Adipose tissues were harvested, washed with saline, fixed
in 10% formalin at 4°C for 1 h and stored frozen in liquid nitrogen
until use.
Serum biomedical analysis
Total TG, TC and glucose levels in serum were
measured using enzyme-linked immunosorbent assay kits (Shanghai
Kexin Biotechnology, Co., Ltd., Shanghai, China) according to the
manufacturer's protocol. The absorbance was measured using a plate
reader (Molecular Devices, LLC, Sunnyvale, CA, USA) at a wavelength
of 510 nm.
Determination of adiposity,
homeostasis model assessment of insulin resistance (HOMA-IR) and
adipose insulin resistance (Adipo-IR) levels
The following equations were used to determine the
adiposity, HOMA-IR and Adipo-IR levels of the mice:
Adiposity index = (body fat/final body mass) ×
100
HOMA-IR = [insulin level subsequent to fasting
(µIU/ml) × glucose level following fasting (mM)]/22.5
Adipo-IR = insulin level subsequent to fasting
(mmol/l) × non-esterified fatty acids (NEFA) level following
fasting (pmol/l)
Reverse transcription-quantitative
polymerase chain reaction
Total RNA in the adipose tissues was isolated using
a TRIzol kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Synthesis of cDNA was performed using a PrimeScript RT reagent kit
with gDNA Eraser (Takara Biotechnology Co., Ltd., Dalian, China).
These kits were used according to the manufacturers' protocols. PCR
reaction conditions were as follows: 95°C for 5 min, and 40 cycles
of 95°C for 20 sec, 60°C for 30 sec and 72°C for 30 sec. PCR was
performed using a CFX96 Real Time PCR detection system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) with SYBR green ІІ. Primers
used for RT-qPCR are described in Table I. β-actin was used as an internal
control. Quantification of the mRNA level was performed using the
2−ΔΔCq method (14).
 | Table I.Primer sequences of adipose tissue
genes. |
Table I.
Primer sequences of adipose tissue
genes.
| Genes | Primer sequence
(5′-3′) |
|---|
| Phosphoinositide
3-kinase | Forward:
CGGTTTCTCCCTTCTACTTCCTG |
|
| Reverse:
GCTCTGCCTCAGCCTTTTATTG |
| Peroxisome
proliferator-activated receptor γ | Forward:
GCCCTTTGGTGACTTTATGGAG |
|
| Reverse:
TGTCCCCACATATTCGACACTC |
| Protein kinase B | Forward:
GAAGACCCAAAGACCAAGATGC |
|
| Reverse:
TCTGACAACAAAGCAGGAGGTG |
| Insulin receptor | Forward:
GTTGCCTTCTTGGGACTGATGT |
|
| Reverse:
GGTCTGTTGTGGGTGGTATCCT |
| Insulin receptor
susbtrate-1 | Forward:
CTTCTGTTACACCTCAAGGGGC |
|
| Reverse:
GGTTATGGTTGGGACTTAGGTTCA |
| Fatty acid
synthetase | Forward:
ACCTCATCACTAGGAAGCCACCAG |
|
| Reverse:
GTGGTACTTGGCCTTGGGTTTA |
| Adiponectin | Forward:
CGTTCTCTTCACCTACGACCAGT |
|
| Reverse:
ATTGTTGTCCCCCTTCCCCATAC |
| Sterol regulatory
element-binding protein 1 | Forward:
CCTGGAGCGAGCATTGAACT |
|
| Reverse:
ACTGACAGAGAAGCTGCACGC |
| β-actin | Forward:
ACGGTCAGGTCATCACTATCG |
|
| Reverse:
GGCATAGAGGTCTTTACGGATG |
Hematoxylin and eosin (H&E)
staining
Adipose tissues were harvested from the mice, fixed
with 10% formalin at 4°C for 24 h, and embedded in paraffin at 60°C
for 1 h. The tissue block was sectioned at a thickness of 5 µm. The
tissue was dewaxed twice with xylene for 15 min at 24°C. Following
hydration by passing a series of graded ethanol concentrations (95%
ethanol for 5 min, 90% ethanol for 5 min, 70% ethanol for 2 min and
distilled water for 5 min). Tissues were then stained using
hematoxylin and 0.5% eosin (H&E) (Nanchang Yulu Experimental
Equipment Co., Ltd., Jiangxi, China; http://shop1379475340812.cn.makepolo.com/) at room
temperature for 10 min, followed by dehydration and covering with a
thin glass. The morphology of the adipose tissue was examined under
a light microscope (magnification, ×200; Olympus Corporation,
Tokyo, Japan).
Western blot analysis
Adipose tissue of the mice was sectioned and
homogenized, followed by centrifugation at 5,000 × g for 10 min at
4°C. The supernatant was collected and the protein concentration
was determined using a Coomassie blue protein assay (Thermo Fisher
Scientific, Inc.). Protein lysates (30 µg) were then loaded in 19%
SDS-PAGE, then transferred to polyvinylidene fluoride membranes
(Merck KGaA, Darmstadt, Germany). Non-fat milk dissolved in
tris-buffered saline with Tween-20 (0.1%; TBST) was used to block
the membrane for 1 h at room temperature. The membranes were
incubated overnight at 4°C with the following primary antibodies:
IRS-1 Ser307 (1:800 dilution; cat. no. AI618; Beyotime Institute of
Biotechnology, Shanghai, China), phosphorylated (p-) IRS1 Ser307
(1:800 dilution; cat. no. AI623-1; Beyotime Institute of
Biotechnology), Akt (1:1,000 dilution; cat. no. 9272; Cell
Signaling Technology, Inc., Danvers, MA, USA), p-Akt Ser473
(1:1,000 dilution; cat. no. 4060; Cell Signaling Technology, Inc.),
PI3K (1:1,000 dilution; cat. no. 4249; Cell Signaling Technology,
Inc.) and β-actin (1:50,000 dilution; cat. no. 3700; Cell Signaling
Technology, Inc.). Subsequent to extensive washing (3 times) with
TBST, horseradish peroxidase-conjugated goat anti-rabbit secondary
antibodies (1:1,000; cat. no. ab150084; Abcam, Cambridge, UK)
diluted in phosphate buffered saline were applied to the membrane
followed by incubation for 1 h at room temperature. Subsequent to
washing, enhanced chemiluminescence substrates (cat. no. 34580;
Thermo Fisher Scientific, Inc.) were added to the membrane. Images
of the membranes were analyzed using Quantity One 1-D analysis
software (Bio-Rad Laboratories, Inc.).
Immunohistochemistry
Adipose tissue was fixed using 4% polyformaldehyde
(Beijing Baiao Laibo Technology Co., Ltd., Beijing, China) for 20
min at room temperature and was sliced into 4 µm-thick sections.
Subsequent to dewaxing and hydration, these slices were soaked in
3% H2O2 at room temperature for 10 min,
followed by the repair of antigens by electric stove heating, and
subsequently the following primary antibodies were added: p-IRS1
Ser307 (cat no. ab60946; 1:1,000; Abcam, Cambridge, MA, USA) and
p-Akt Ser473 (cat no. #4060; 1:100; Cell Signaling Technology,
Inc.) and incubated for 30 min at 37°C. The horseradish
peroxidase-conjugated secondary antibody-goat anti rabbit IgG (cat
no. A0208; 1:50; Beyotime Institute of Biotechnology, Haimen,
China) was added and incubated with specific antibody-binding
capacity for 30 min at 37°C. Subsequent to staining with
3,3′-diaminobenzidine followed by counterstaining with hematoxylin
and dehydration for 5 min, and the membranes were then treated with
gradient alcohol for 10 min each then two changes of xylene for 3
min and sealed, all at room temperature. The samples were then
observed under a Olympus BX51 fluorescence upright microscope
(Olympus Corporation, Tokyo, Japan).
Statistical analysis
Data were represented as the mean ± the standard
deviation, and analyzed using SPSS 19.0 (IBM Corp., Armonk, NY,
USA). Comparisons among different groups were based on one-way
analysis of variance analysis. Paired comparisons between different
groups were performed using the Student Newman Keul's method.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of dioscin on the phenotypic
parameters of mice
Subsequent to being fed with high-fat food for 12
weeks, biochemical parameters, including initial body mass, final
body mass, total fat and adiposity index, were significantly
increased compared with the control group (P<0.05; Table II). Compared with the HFD group,
mice in the medium and high dioscin groups were revealed to have
significantly decreased levels of final body weight, and in all
three dioscin groups had significantly decreased levels of total
fat and adiposity index in a dose-dependent manner (P<0.05).
 | Table II.Phenotypic parameters of mice in
different groups. |
Table II.
Phenotypic parameters of mice in
different groups.
|
| Group |
|---|
|
|
|
|---|
| Parameter | Control | High-fat diet | Dioscin (low) | Dioscin (medium) | Dioscin (high) |
|---|
| Initial body mass
(g) | 20.8±0.6 | 21.1±0.4 | 20.7±0.3 | 21.3±0.5 | 20.4±0.7 |
| Final body mass
(g) | 29.2±0.7 | 40.3±1.1a | 38.2±1.2 | 34.9±0.9c | 31.5±0.8c |
| Total fat (g) | 0.77±0.04 |
8.07±0.13b |
6.94±0.15c |
5.38±0.11c |
2.91±0.07d |
| Adiposity index
(%) | 2.51±0.41 |
20.45±0.58b |
17.21±0.43c |
15.08±0.52c |
9.37±0.37c |
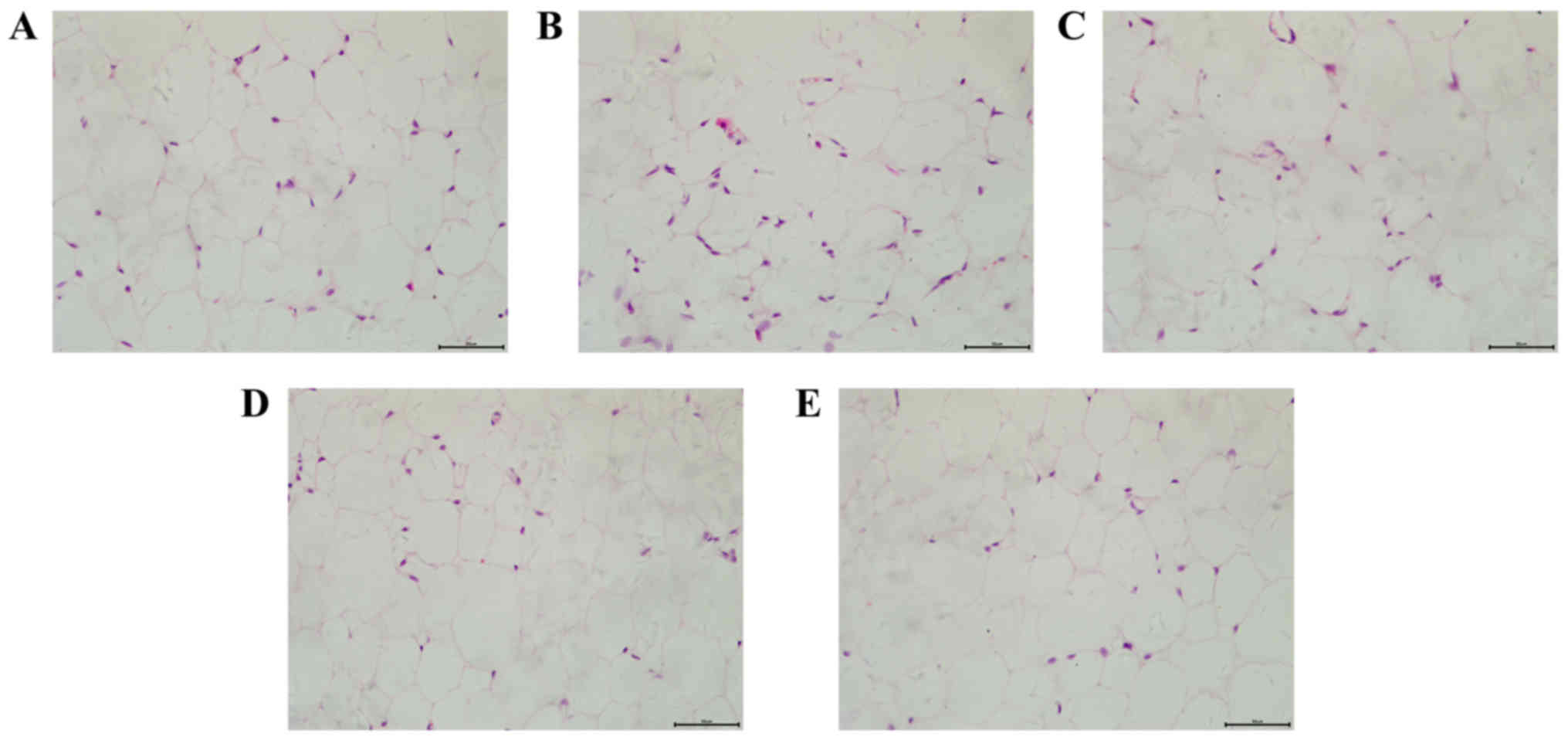
Effects of dioscin on the pathology
changes of adipose cells
H&E staining was applied to detect the
morphology of adipose cells in a number of different groups. As
presented in Fig. 1, mice in the
HFD group demonstrated an increased volume and decreased number of
adipose cells, accompanied with a more varied shape and size of
cells compared with the control group. However, dioscin treatment
attenuated the morphological changes of adipose cells, further
demonstrating the effect of dioscin on restoring the phenotypic
parameters in a HFD model.
Regulation of insulin resistance by
dioscin
Next, the present study examined whether dioscin
reduced insulin resistance by evaluating the changes of biochemical
parameters of the serum obtained from the mice. As presented in
Table III, mice fed with a HFD
demonstrated a significant elevation of blood glucose, insulin, TC,
TG and NEFA levels when compared with the control group
(P<0.05), resulting in significantly higher HOMA-IR and Adipo-IR
values (P<0.01; Table III).
Notably, dioscin treatment significantly lowered HOMA-IR
(P<0.05) and Adipo-IR levels (P<0.01) in a dose-dependent
manner.
 | Table III.Biochemical parameters of plasma. |
Table III.
Biochemical parameters of plasma.
|
| Groups |
|---|
|
|
|
|---|
| Parameters | Control | High-fat diet | Dioscin (low) | Dioscin
(medium) | Dioscin (high) |
|---|
| Plasma glucose
(mg/dl) | 106.77±7.92 |
131.93±9.17a | 124.39±6.53 |
118.94±5.23c |
111.34±7.30c |
| Plasma insulin
(ng/ml) | 0.34±0.07 |
2.41±0.21b | 1.93±0.25 |
1.53±0.17c |
0.90±0.11d |
| Triglyceride
(mg/dl) | 51.69±5.16 |
90.47±7.08b | 79.21±5.93 |
68.38±5.02c |
59.07±6.17d |
| Total cholesterol
(mg/dl) | 68.39±5.33 |
89.04±7.48a | 78.38±6.47 |
72.79±6.09c |
70.33±5.73c |
| Non-esterified
fatty acids (mEq/l) | 0.95±0.11 |
1.45±0.15a | 1.28±0.20 | 1.19±0.18 |
1.04±0.10c |
| Homeostasis model
assessment of insulin resistance index | 2.15±0.24 |
18.79±1.96b | 14.19±1.33 |
10.75±1.06c |
6.03±0.79c |
| Adipose insulin
resistance index | 55.61±6.76 |
601.70±19.05b |
425.37±16.38c |
313.50±15.79d |
161.17±17.23d |
Effects of dioscin on the
IRS-1/PI3K/Akt and PPARγ signaling pathway
To better understand the underlying mechanisms of
dioscin, the present study examined the potential cell signal
pathways involved and revealed that a HFD significantly
downregulated the mRNA levels of PI3K, Akt, insulin receptor (IR)
and IRS-1 (P<0.05; Fig. 2),
when compared with the control group, whilst the high dioscin
treatment group exhibited a significant effective restoration of
the aberrant expression of the aforementioned genes (P<0.05;
Fig. 2). Interestingly, it was
additionally revealed that dioscin may significantly reverse the
decrease in the expression of PPARγ incurred by a HFD treatment
(P<0.05; Fig. 3A) with no
prominent regulation effects on the levels of FAS, adiponectin and
SREBP-1c (Fig. 3B-D).
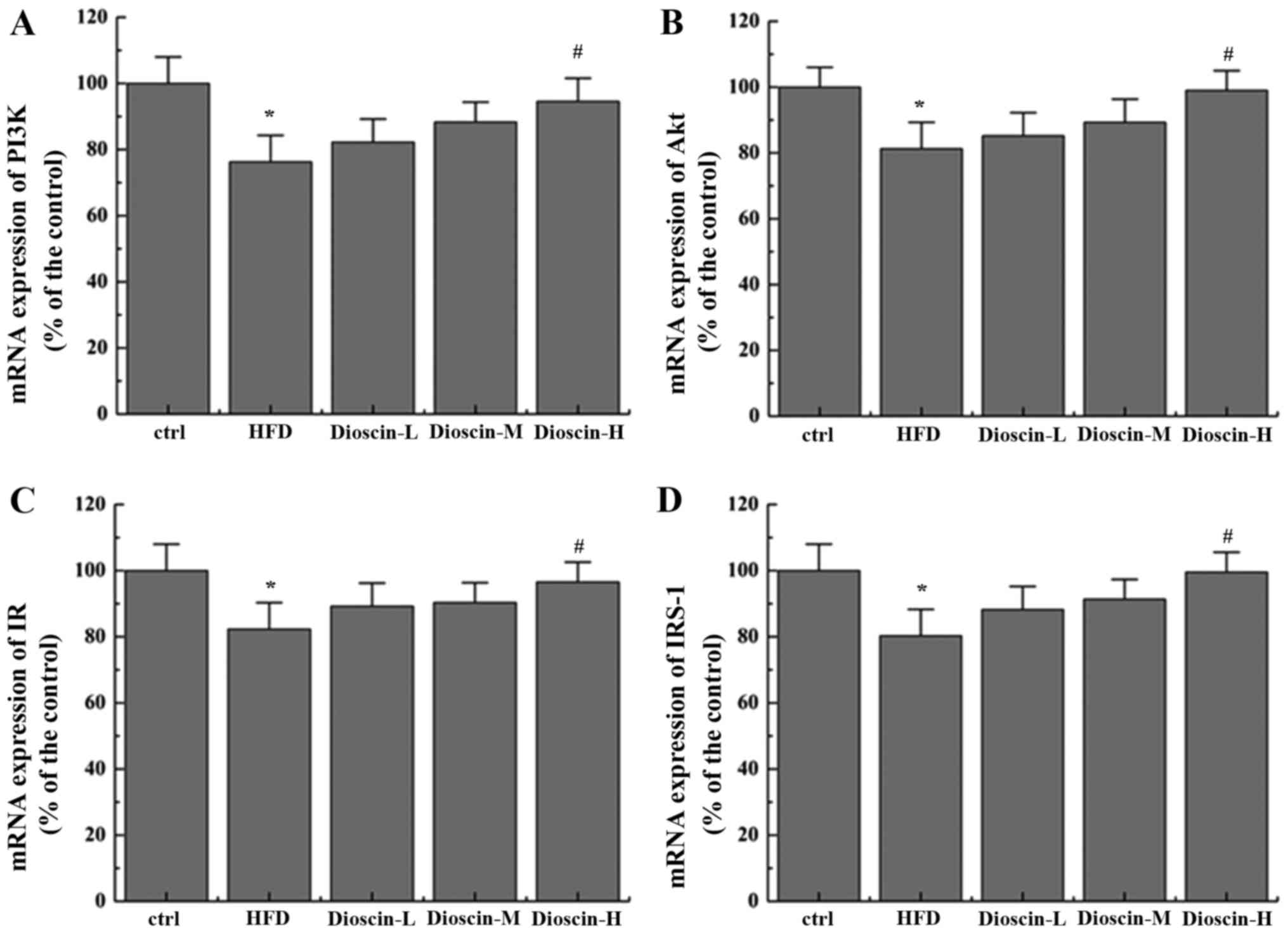 | Figure 2.Effects of dioscin on the expression
of genes in the IRS-1/PI3 K/Akt pathway. The relative mRNA levels
of (A) PI3K, (B) Akt, (C) IR and (D) IRS-1 were presented.
*P<0.05 vs. the ctrl group; #P<0.05 vs. the HFD
group. IRS-1, insulin receptor substrate 1; PI3K, phosphoinositide
3-kinase; IR, insulin receptor; Akt, protein kinase B; HFD,
high-fat diet; ctrl, control; L, low; M, medium; H, high. |
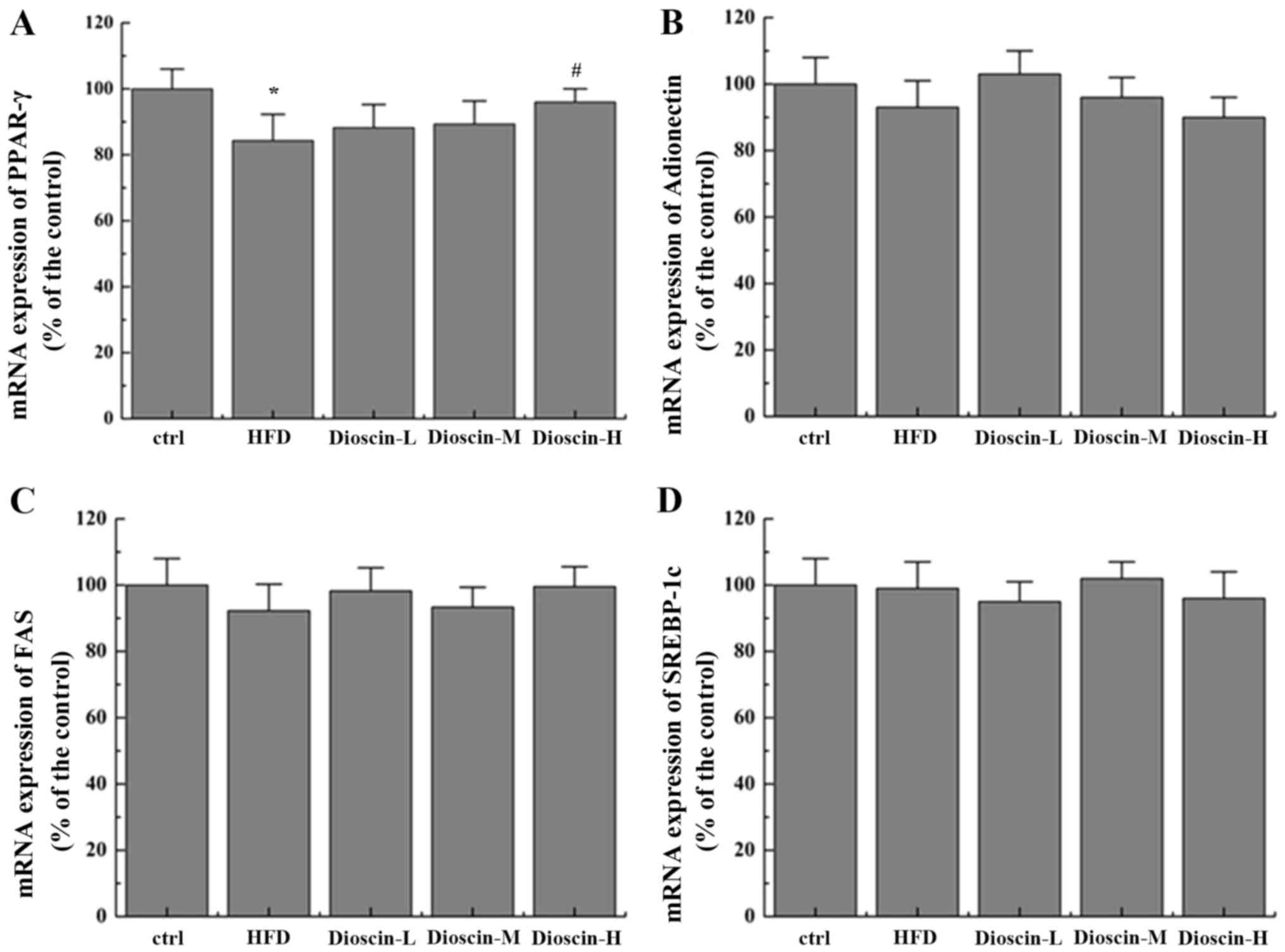 | Figure 3.Effects of dioscin on the expression
of genes in the PPAR-γ pathway. Relative mRNA levels of (A) PPAR-γ,
(B) adiponectin, (C) FAS and (D) SREBP-1c were determined.
*P<0.05 vs. the ctrl group; #P<0.05 vs. the HFD
group. PPAR-γ, Peroxisome proliferator-activated receptor γ; FAS,
fatty acid synthetase; SREBP-1c, sterol regulatory element-binding
protein 1; HFD, high-fat diet; ctrl, control; L, low; M, medium; H,
high. |
Furthermore, p-IRS-1 and IRS-1 levels were examined
by western blot analysis. As presented in Fig. 4A, a HFD significantly upregulated
the p-IRS1/IRS-1 ratio compared with the control group (P<0.01),
and dioscin at doses of 10 and 20 mg/kg/day significantly reduced
this value compared with the HFD group (P<0.05). Meanwhile,
p-Akt/Akt value was significantly decreased in the HFD group in
comparison with the control group (P<0.05; Fig. 4B). In contrast, a high dose of
dioscin significantly increased the p-Akt/Akt value compared with
the HFD group (P<0.05; Fig.
4B).
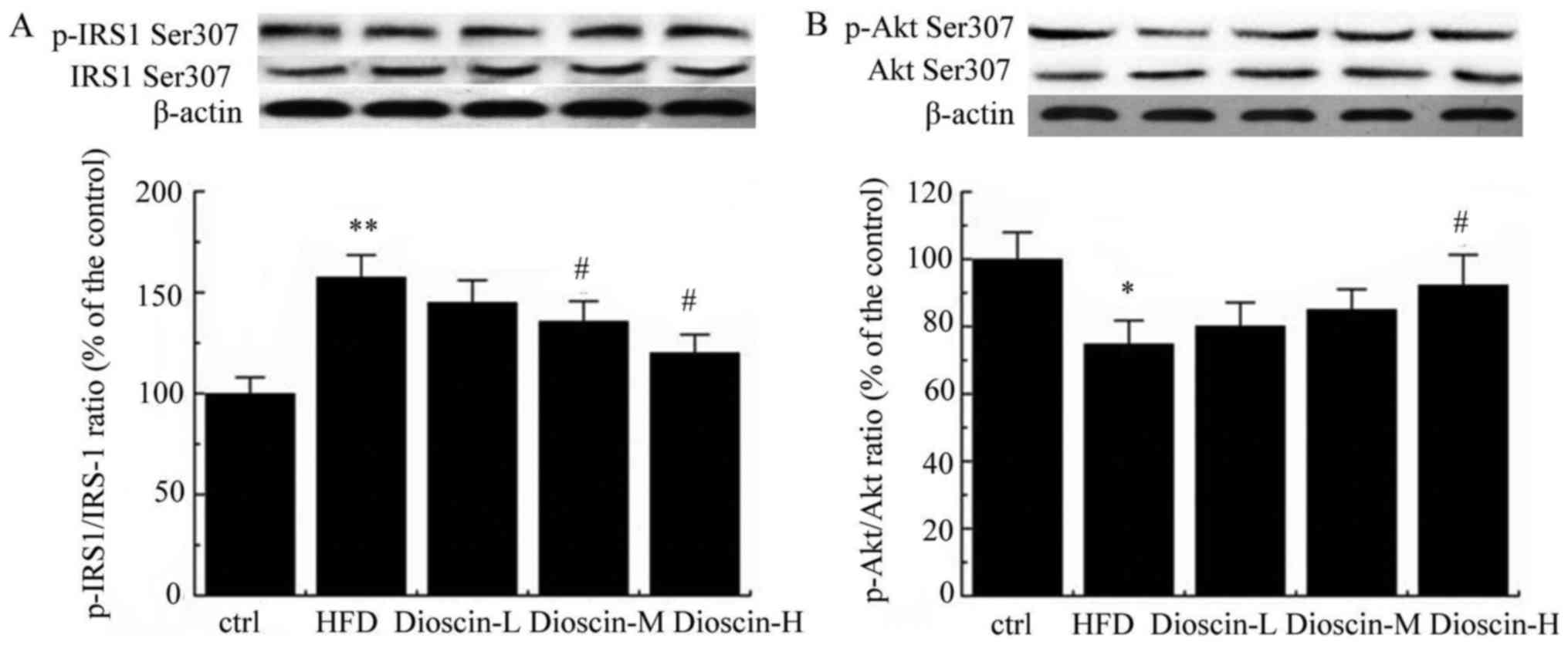 | Figure 4.Western blot analysis of p-IRS-1/IRS-1
and p-Akt/Akt. Protein expression of (A) p-IRS1, IRS-1, (B) p-Akt
and Akt measured using western blot analysis. *P<0.05 and
**P<0.01 vs. the ctrl group; #P<0.05 vs. the HFD
group. P-, phosphorylated; IRS-1, insulin receptor substrate-1;
Akt, protein kinase B; ctrl, control; HFD, high-fat diet; L, low;
M, medium; H, high. |
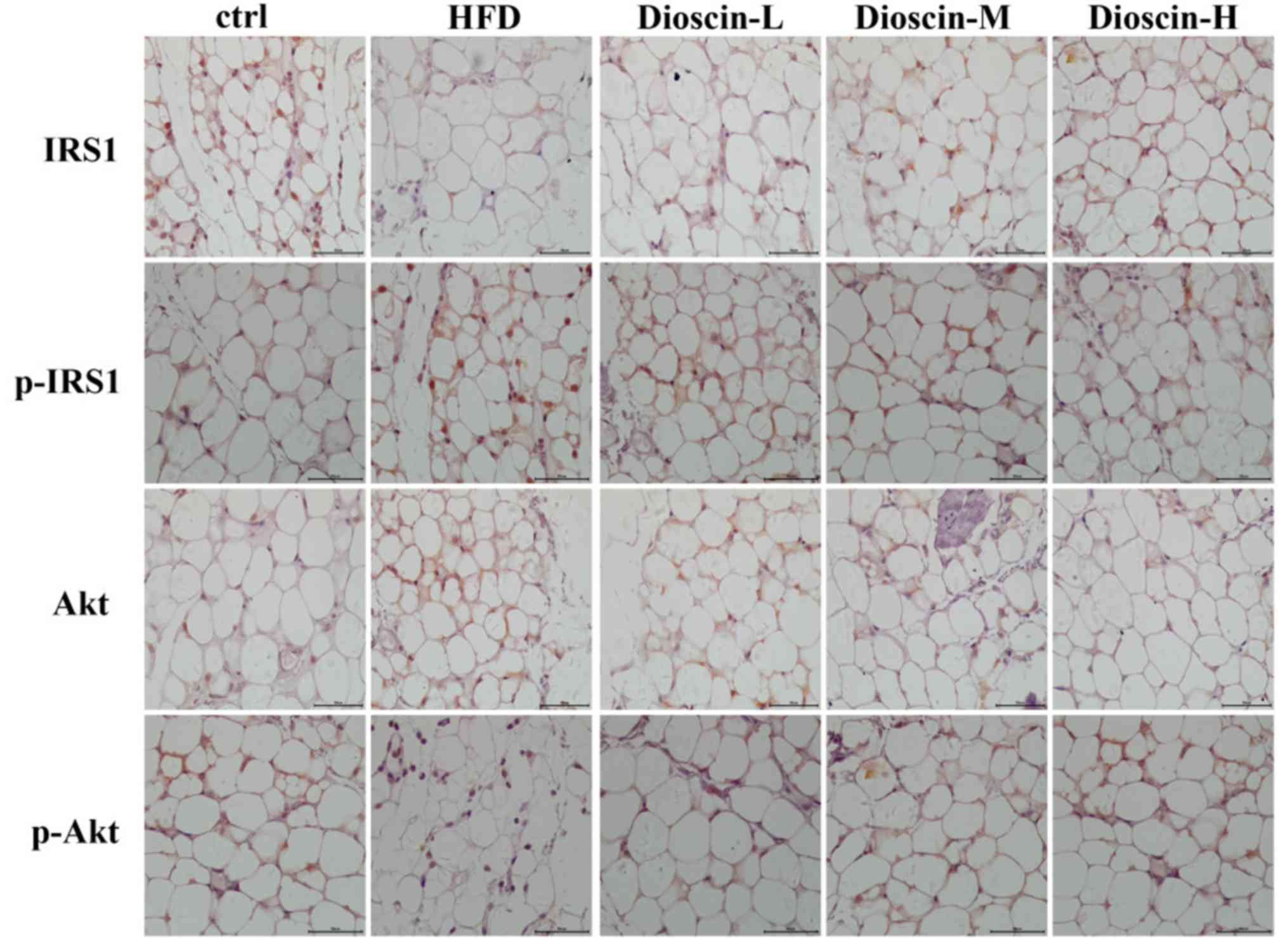
Immunohistochemical analysis
The results of the immunohistochemical analysis were
consistent with the results of the western blot analysis in
Figure 5. Immunohistochemical
staining revealed that the levels of p-IRS1 and p-Akt proteins in
the adipose tissue of the model group were substantially up- and
downregulated, respectively, compared with the control group.
Subsequent to treated using dioscin, the protein expression of
p-IRS1 decreased, and the expression of p-Akt increased compared
with the HFD group. The higher the concentration of dioscin, the
more substantial the phosphorylation of IRS1 and Akt appeared to
be.
Discussion
The results of the present study were in agreement
with a previous report that dioscin potently reduced obesity in
mice, and may be explained by the fact that dioscin elevates the
energy expenditure in obese mice (9). HFD impairs fatty acid synthesis and
metabolism in the liver, which increases TG levels and various
other molecules in TG synthesis. The present study revealed that
dioscin is effective in attenuating the increased body weight and
body fat induced by HFD. Upon dioscin treatment, a reduction of
plasma glucose, TG and plasma insulin levels was also observed,
suggesting the amelioration of liver injuries caused by HFD and
ameliorated insulin resistance, as reflected by the decrease in
HOMA-IR and Adipo-IR indexes, in addition to the increase in
adipose cell volume and decrease in adipose cell number, which
corroborated the potential use of dioscin in the clinical
management of insulin resistance.
In the present study, it was revealed that dioscin
regulated PI3K/Akt signaling in HFD mice. A HFD was able to
significantly downregulate the level of PI3K and Akt compared with
the control group (P<0.05), while dioscin demonstrated a
dose-dependent effect on the increased expression of PI3K, Akt, IR
and IRS-1, particularly in the 20 mg/kg/day group. The inhibition
of IRS-1 and Akt signaling was mediated through the phosphorylation
(Ser307) of the proteins. The aforementioned result is preceded by
results that indicate that insulin resistance is activated by the
inhibition of Akt and IRS-1 substrate phosphorylation (15,16).
IRS-1 is one of the key targets of the insulin receptor tyrosine
kinase, which is indispensable for the activation of downstream
metabolism. Insulin specifically binds to the α subunit of insulin
receptor in the cell surface and activates the β subunit inhibited
by the α subunit, resulting in phosphorylation under the action of
tyrosine kinase, activation of IRS and the further cascade reaction
of downstream activation signals, including the PI3K/Akt and
mitogen-activated protein kinase signaling pathway (17). The ability of dioscin to reverse
the blockage of IRS-1-associated signaling improved the sensitivity
of adipose tissue to insulin. This result is consistent with
evidence that numerous saponins are also effective in ameliorating
insulin resistance by modulating the PI3K/Akt signaling pathway
(11,18,19).
The PI3K/Akt and PPAR-γ pathways are the two putative pathways that
are suppressed in diseases associated with excessive oxidative
stress and inflammation (15,18–20).
The restoration of the activity of those two pathways, and
consequent amelioration of oxidative and inflammatory responses,
may potentially account for the effects of dioscin on mitigating
insulin resistance. In 2013, Gao et al (20) reported that Ginsenoside Re
may reduce insulin resistance through the activation of the PPAR-γ
pathway and inhibition of tumor necrosis factor-α (TNF-α)
production. In 2018, Naowaboot et al (21) reported that the administration of a
water extract of V cinerea was able to increase insulin
sensitivity in HFD-induced obese mice by modulating the PI3K/Akt
and AMPK pathways in the liver, skeletal muscle and adipose tissue.
Additionally, Cai et al (22) reported that Sanggua Drink
extract may alleviate insulin resistance in treating type 2
diabetes mellitus rats induced by a HFD through the PI3K/Akt
signaling pathway. Furthermore, Leng et al (23) reported that
1,1,2-Trichloro-1,2,2-trifluoroethane may regulate macrophage
polarization with beneficial effects on adipose tissue
inflammation, and thereby facilitate insulin IRS-1/PI3K signaling,
resulting in the improvement of insulin sensitivity in HFD-fed
mice. Further biochemical analysis on factors associated with
oxidative stress, including levels of malondialdehyde and
glutathione, in addition to inflammatory responses including
interleukin-1 and TNF-α, is necessary to confirm this assumption.
It is also worth noting that dioscin did not demonstrate a
significant impact on the expression of FAS, adiponectin and
SREBP-1c, which are factors associated with insulin-sensitization
or fatty acid synthesis (24),
suggesting that dioscin did not ameliorate insulin resistance in
adipose issue through those mechanisms.
In summary, the present study demonstrated that
dioscin is a promising drug for the treatment of insulin
resistance. In addition, reduced body weight and serum FFA levels
determined dioscin as an excellent candidate in the treatment of
other obesity-induced disorders. For example, a HFD and obesity are
two risk factors of cardiovascular disease, as high fatty acid
levels promote endothelial dysfunction, ultimately resulting in
atherosclerosis and coronary artery diseases (25). The effects of dioscin on reducing
body weight and serum FFA may benefit the prevention and treatment
of cardiovascular diseases. Consequently, it was hypothesized that
dioscin is beneficial in regulating the abnormal metabolism of
obese mice, restoring the activity of the IRS-1/PI3K/Akt pathway
and PPAR-γ pathway, in order to reverse the insulin resistance
induced by a HFD.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HL, LY and CZ conceived designed and performed the
experiments. HL, LY and CZ analyzed the data and prepared the
manuscript. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Affiliated Yantai Yuhuangding Hospital of Qingdao
University (Yantai, China), and all participants provided written
informed consent.
Patient consent for publication
All participants provided written informed
consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Samuel VT and Shulman GI: Mechanisms for
insulin resistance: Common threads and missing links. Cell.
148:852–871. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Turner N, Kowalski GM, Leslie SJ, Risis S,
Yang C, Lee-Young RS, Babb JR, Meikle PJ, Lancaster GI, Henstridge
DC, et al: Distinct patterns of tissue-specific lipid accumulation
during the induction of insulin resistance in mice by high-fat
feeding. Diabetologia. 56:1638–1648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tao X, Yin L, Xu L and Peng J: Dioscin: A
diverse acting natural compound with therapeutic potential in
metabolic diseases, cancer, inflammation and infections. Pharmacol
Res. (In press).
|
|
4
|
Kim S, Jwa H, Yanagawa Y and Park T:
Extract from dioscorea batatas ameliorates insulin resistance in
mice fed a high-fat diet. J Med Food. 15:527–534. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aumsuwan P, Khan SI, Khan IA, Ali Z, Avula
B, Walker LA, Shariat-Madar Z, Helferich WG, Katzenellenbogen BS
and Dasmahapatra AK: The anticancer potential of steroidal saponin,
dioscin, isolated from wild yam (Dioscorea villosa) root extract in
invasive human breast cancer cell line MDA-MB-231 in vitro. Arch
Biochem Biophys. 591:98–110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu C, Wang Y, Wu C, Pei R, Song J, Chen S
and Chen X: Dioscin's antiviral effect in vitro. Virus Res.
172:9–14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo CH, Li X and Kang Y: The protective
effect of dioscin-containing serum on hydrogen peroxide injured
cardiomyocytes of neonate rats. Chin J Hosp Pharm. 13:1027–1031.
2012.(In Chinese).
|
|
8
|
Tao X, Wan X, Xu Y, Xu L, Qi Y, Yin L, Han
X, Lin Y and Peng J: Dioscin attenuates hepatic
ischemia-reperfusion injury in rats through inhibition of
oxidative-nitrative stress, inflammation and apoptosis.
Transplantation. 98:604–611. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu M, Xu L, Yin LH, Qi Y, Xu Y, Han X,
Zhao Y, Sun H, Yao J, Lin Y, et al: Corrigendum: Potent effects of
dioscin against obesity in mice. Sci Rep. 5:121832015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kwon H and Pessin JE: Adipokines mediate
inflammation and insulin resistance. Front Endocrinol (Lausanne).
4:712013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu X, Wang S, Xu J, Wang DB, Chen Y and
Yang GZ: Triterpenoid saponins from Stauntonia chinensis ameliorate
insulin resistance via the AMP-activated protein kinase and
IR/IRS-1/PI3K/Akt pathways in insulin-resistant HepG2 cells. Int J
Mol Sci. 15:10446–10458. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gallagher EJ, Fierz Y, Vijayakumar A,
Haddad N, Yakar S and LeRoith D: Inhibiting PI3K reduces mammary
tumor growth and induces hyperglycemia in a mouse model of insulin
resistance and hyperinsulinemia. Oncogene. 31:3213–3222. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsieh MJ, Tsai TL, Hsieh YS, Wang CJ and
Chiou HL: Dioscin-induced autophagy mitigates cell apoptosis
through modulation of PI3K/Akt and ERK and JNK signaling pathways
in human lung cancer cell lines. Arch Toxicol. 87:1927–1937. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Plomgaard P, Bouzakri K, Krogh-Madsen R,
Mittendorfer B, Zierath JR and Pedersen BK: Tumor necrosis
factor-alpha induces skeletal muscle insulin resistance in healthy
human subjects via inhibition of Akt substrate 160 phosphorylation.
Diabetes. 54:2939–2945. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Copps KD and White MF: Regulation of
insulin sensitivity by serine/threonine phosphorylation of insulin
receptor substrate proteins IRS1 and IRS2. Diabetologia.
55:2565–2582. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo S: Insulin signaling, resistance, and
the metabolic syndrome: Insights from mouse models into disease
mechanisms. J Endocrinol. 220:T1–T23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu X, Wang M, Bei W, Han Z and Guo J: The
Chinese herbal medicine FTZ attenuates insulin resistance via IRS1
and PI3K in vitro and in rats with metabolic syndrome. J Transl
Med. 12:472014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Hai J, Cao M, Zhang Y, Pei S,
Wang J and Zhang Q: Silibinin ameliorates steatosis and insulin
resistance during non-alcoholic fatty liver disease development
partly through targeting IRS-1/PI3K/Akt pathway. Int J
Immunopharmacol. 17:714–720. 2013. View Article : Google Scholar
|
|
20
|
Gao Y, Yang MF, Su YP, Jiang HM, You XJ,
Yang YJ and Zhang HL: Ginsenoside Re reduces insulin resistance
through activation of PPAR-γ pathway and inhibition of TNF-α
production. J Ethnopharmacol. 147:509–516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Naowaboot J, Wannasiri S and Pannangpetch
P: Vernonia cinerea water extract improves insulin resistance in
high-fat diet-induced obese mice. Nutr Res. 56:51–60. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cai Y, Wang Y, Zhi F, Xing QC and Chen YZ:
The effect of sanggua drink extract on insulin resistance through
the PI3K/AKT signaling pathway. Evid Based Complement Alternat Med.
2018:94079452018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leng J, Chen MH, Zhou ZH, Lu YW, Wen XD
and Yang J: Triterpenoids-enriched extract from the aerial parts of
salvia miltiorrhiza regulates macrophage polarization and
ameliorates insulin resistance in high-fat fed mice. Phytother Res.
31:100–107. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ranganathan G, Unal R, Pokrovskaya I,
Yao-Borengasser A, Phanavanh B, Lecka-Czernik B, Rasouli N and Kern
PA: The lipogenic enzymes DGAT1, FAS, and LPL in adipose tissue:
Effects of obesity, insulin resistance, and TZD treatment. J Lipid
Res. 47:2444–2450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu K, Zhao W, Gao X, Huang F, Kou J and
Liu B: Diosgenin ameliorates palmitate-induced endothelial
dysfunction and insulin resistance via blocking IKKβ and IRS-1
pathways. Atherosclerosis. 223:350–358. 2012. View Article : Google Scholar : PubMed/NCBI
|