Introduction
Lung cancer ranks as the third most common human
malignancy and the leading cause of cancer-associated mortality
worldwide (1). Lung cancer is
divided into small cell lung cancer (SCLC) and non-small cell lung
cancer (NSCLC) subtypes, based on the pathological characteristics
(2). NSCLC is the main type of
lung cancer, accounting for approximately 85% of lung cancer cases
(3). It may be further classified
into three major histotypes: Adenocarcinoma, squamous cell
carcinoma and large cell carcinoma (4). In recent decades, the incidence of
NSCLC has markedly increased in many countries, including China
(5,6). Despite considerable advancement in
several treatments, patients with NSCLC diagnosed at the advanced
stage have extremely poor prognosis, with a 5-year survival rate of
<5% (7,8). Rapid tumor growth, recurrence and
metastasis are the major factors responsible (9). The poor outcomes of NSCLC highlight
the urgent need to better understand the molecular mechanisms
underlying NSCLC occurrence and development, which may facilitate
the identification of effective therapeutic techniques.
microRNAs (miRNAs/miRs) have emerged as a group of
endogenous, non-coding and short RNA molecules that function as
regulators of gene expression by base pairing with a partially
complementary site in the 3′-untranslated regions (3′-UTRs) of
their target genes, to induce mRNA degradation or repress mRNA
translation (10,11). Approximately one-third to one-half
of all human protein-coding genes are directly or indirectly
modulated by miRNAs (12), which
indicates that miRNAs may be closely associated with a variety of
disorders, including NSCLC (13).
Several studies have reported that numerous miRNAs are dysregulated
in NSCLC. For example, miR-183 (14), miR-215 (15) and miR-615 (16) are downregulated in NSCLC, whereas
miR-9 (17), miR-106b (18) and miR-875 (19) are upregulated. Aberrantly expressed
miRNAs may function as tumor-suppressors or oncogenes in NSCLC
initiation and progression, depending on the characteristics of
their target genes (20). Hence,
miRNAs have potential as targets in NSCLC diagnosis, treatment and
prognosis.
miR-577 has been reported to be abnormally expressed
in several tumor types (21–24).
However, the expression pattern, roles and underlying mechanisms of
miR-577 in NSCLC have not been clarified. In the present study,
miR-577 expression was detected in NSCLC tissues and cell lines and
the effects of miR-577 on the proliferation and invasion of NSCLC
cells were examined in vitro. In addition, the underlying
mechanisms of miR-577 in NSCLC cells were investigated. It was
found that miR-577 was downregulated in NSCLC, and miR-577
inhibited the NSCLC cell proliferation and invasion by directly
targeting homeobox A1 (HOXA1). The present study may provide an
effective target for the therapy of patients with lung cancer.
Materials and methods
Ethical statement and clinical
specimens
The present study was approved by the Ethical
Committee of China-Japan Union Hospital of Jilin University
(approval no. 20140311). All patients enrolled in the research
provided written consent and were informed of the study's purpose.
In total, 35 pairs of NSCLC and adjacent non-tumor tissues were
collected from patients (21 males and 14 females; age range, 42–69)
who received surgical resection at China-Japan Union Hospital of
Jilin University between March 2014 and April 2017. None of the
patients underwent any pre-operative chemotherapy or radiotherapy
treatment. Patients who had been treated with pre-operative
chemotherapy or radiotherapy were excluded from this study. All
tissue specimens were rapidly frozen in liquid nitrogen and stored
at −80°C.
Cell lines
A nontumorigenic bronchial epithelium cell line
(BEAS2B) and four NSCLC cell lines (NCI-H460, SK-MES-1, NCI-H522
and A549) were purchased from the American Type Culture Collection
(Manassas, VA, USA). BEAS2B cells were cultured in LHC9 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.). All NSCLC cell lines were maintained in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS and 1%
penicillin/streptomycin mixture. Cells were cultured in a
humidified atmosphere at 37°C containing 5% CO2.
Transfection
Cells were plated into 6-well culture plates with a
density of 7×105 cells/well 1 day before transfection
and maintained in an incubator at 37°C containing 5%
CO2. miR-577 mimics and miRNA mimics negative control
(miR-NC) were chemically synthesized by Shanghai GenePharma Co.,
Ltd. (Shanghai, China) and transfected into cells at a final
concentration of 100 nM. The miR-577 mimics sequence was
5′-UAGAUAAAAUGUUGGUACCUG-3′ and the miR-NC sequence was
5′-UUCUCCGAACGUGUCACGUTT-3′. HOXA1 overexpression plasmid
pcDNA3.1-HOXA1 (pc-HOXA1) and empty pcDNA3.1 plasmid were provided
by Guangzhou RiboBio Co., Ltd. (Guangzhou, China). Cells were
transfected with miRNA mimics (100 pmol) or plasmid (4 µg) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Following a 6 h incubation at 37°C with 5% CO2, the
culture medium was removed and replaced with fresh DMEM containing
10% FBS.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from tissue specimens or
cells using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) in accordance with the manufacturer's
instructions. The concentration of total RNA was determined with a
NanoDrop 2000/2000c spectrophotometer (NanoDrop Technologies;
Thermo Fisher Scientific, Inc.). The All-in-One™ miRNA qRT-PCR
Detection kit (GeneCopoeia, Inc., Rockville, MD, USA) was used to
detect miR-577 expression, and was carried out according to the
manufacturer's instructions. To analyze HOXA1 mRNA expression,
reverse transcription was conducted using PrimeScript 1st Strand
cDNA Synthesis kit (Takara Biotechnology Co., Ltd., Dalian, China).
The synthesized complementary DNA (cDNA) was then subjected to qPCR
using SYBR Premix Ex Taq™ (Takara Biotechnology Co.,
Ltd.), and qPCR was performed according to the manufacturer's
instructions. The relative expression of miR-577 and HOXA1 was
calculated using the 2−ΔΔCq method (25) and was normalized to U6 snRNA and
GAPDH mRNA, respectively. The primers were designed as follows:
miR-577, 5′-TGCGGTAGATAAAATATTGG-3′ (forward) and
5′-GTGCAGGGTCCGAGGT-3′ (reverse); U6, 5′-CTCGCTTCGGCAGCACA-3′
(forward) and 5′-AACGCTTCACGAATTTGCGT-3′ (reverse); HOXA1,
5′-TCCTGGAATACCCCATACTTAGC-3′ (forward) and
5′-GCACGACTGGAAAGTTGTAATCC-3′ (reverse); and GAPDH,
5′-CTGGGCTACACTGAGCACC-3′ (forward) and 5′-AAGTGGTCGTTGAGGGCAATG-3′
(reverse).
Cell Counting Kit-8 (CCK-8) assay
Cells were harvested and plated into 96-well plates
at a density of 3×103 cells/well 24 h after
transfection. Cells were incubated at 37°C with 5% CO2
and proliferation was detected at different time points (0, 24, 48
and 72 h). CCK-8 reagent (10 µl; Dojindo Molecular Technologies,
Inc., Kumamoto, Japan) was added into each well for a further 2 h
at 37°C in a humidified incubator. The optical density of each well
was measured at 450 nm using a microplate reader (Molecular
Devices, LLC, Sunnyvale, CA, USA).
Transwell invasion assay
Transwell inserts (24-well insert; Corning
Incorporated, Corning, NY, USA) containing 8 µm pore size membranes
were employed to determine NSCLC cell invasion capacity. Transwell
inserts were coated with Matrigel® (BD Biosciences, San
Jose, CA, USA) and dried overnight under aseptic conditions.
Transfected cells were harvested 24 h after transfection, suspended
into DMEM without FBS and plated into the upper Transwell inserts
at a density of 5×104 cells/insert. DMEM containing 10%
FBS was used as a chemoattractant in the lower compartment of.
Transwell inserts were then incubated at 37°C with 5%
CO2 for 24 h. The non-invaded cells remaining on the
upper side of the membranes were wiped off with a cotton swab.
Invaded cells were fixed with 4% paraformaldehyde at 37°C for 30
min and stained with 0.1% crystal violet at 37°C for 30 min. Images
of five randomly-selected fields of view for per Transwell insert
were captured under an inverted microscope (×200 magnification;
CKX41; Olympus Corporation, Tokyo, Japan). The invasive ability was
quantified by counting the average number (mean) of invaded cells
in the images.
Bioinformatics predication and
luciferase reporter assay
TargetScan 7.2 (www.targetscan.org) and miRDB 5.0 (www.mirdb.org) were used to search for the potential
targets of miR-577. These indicated that the 3′-UTR of HOXA1
contained the putative miR-577 binding site. The 3′-UTR of HOXA1
containing the wild-type (Wt) or mutant (Mut) miR-577-binding
sequences was generated (Shanghai GenePharma Co., Ltd.). The
chemically synthesized Wt and Mut fragments were inserted into
pMIR-GLOTM Luciferase vector (Promega Corporation, Madison, WI,
USA) and defined as pMIR-Wt-HOXA1-3′-UTR and pMIR-Mut-HOXA1-3′-UTR,
respectively. Cells were plated into 24-well plates at a density of
1.0×105 cells per well. Luciferase reporter plasmids
were introduced into cells in 24-well plates using
Lipofectamine® 2000 and co-transfected with miR-577
mimics or miR-NC. After a 48 h culture, luciferase activity was
measured using a Dual-Luciferase Reporter Assay system (Promega
Corporation) as per the manufacturer's protocol, and was normalized
to Renilla luciferase activity.
Western blot analysis
Total protein was extracted from tissue specimens or
cells using radioimmunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology, Haimen, China). Following protein
extraction, a bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology) was used to detect the concentration of
total protein. Next, equal amounts of protein were subjected to 10%
SDS-PAGE and transferred onto polyvinylidene difluoride membranes
(Beyotime Institute of Biotechnology). Membranes were blocked in 5%
fat-free milk in Tris-buffered saline-0.1% Tween-20 (TBST), the
membranes were incubated overnight at 4°C with primary antibodies
against HOXA1 (cat. no. ab168179; 1:1,000 dilution; Abcam,
Cambridge, UK) or GAPDH (cat. no. ab110305; 1:1,000 dilution;
Abcam). Following extensive washing with TBST, the membranes were
incubated at room temperature for 2 h with horseradish
peroxidase-conjugated goat anti-mouse secondary antibody (cat. no.
ab205719; 1:5,000 dilution; Abcam). An enhanced chemiluminescence
detection kit (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was
used to visualize protein signals. Protein expression was
quantified using Quantity One software version 4.62 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
All data were expressed as the mean ± standard
deviation from at least three independent experiments. Two-tailed
Student's t-test was used to analyze the difference between two
groups. The difference between multiple groups was investigated
using one-way analysis of variance with Student-Newman-Keuls as a
post-hoc test. Spearman's correlation analysis was performed to
explore the relationship between miR-577 and HOXA1 mRNA in NSCLC
tissues. P<0.05 was considered to indicate a statistically
significant difference.
Results
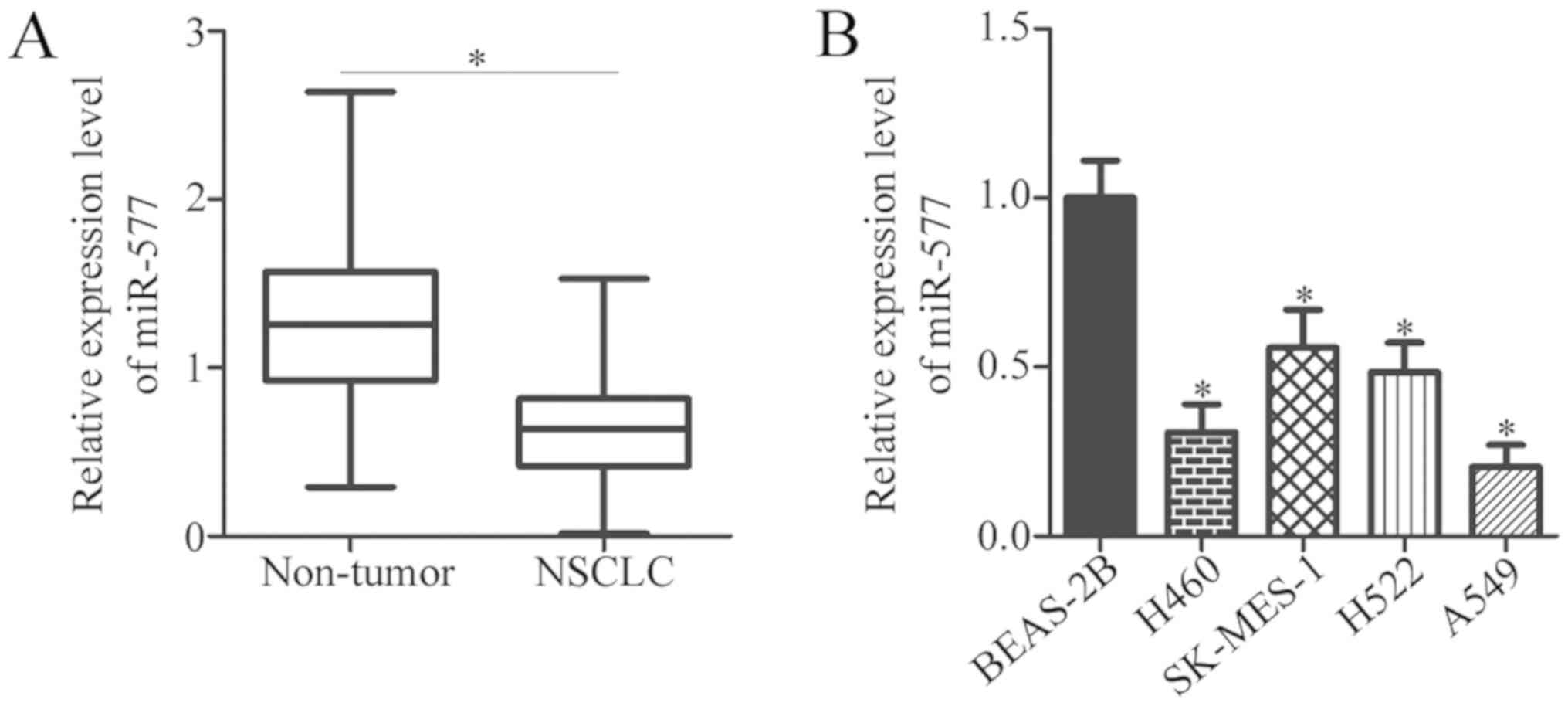
miR-577 is downregulated in NSCLC
tissues and cell lines
To determine the expression pattern of miR-577 in
NSCLC, total RNA was isolated from 35 pairs of NSCLC tissues and
adjacent non-tumor tissues, and RT-qPCR analysis was conducted. The
data indicated that miR-577 expression was downregulated in NSCLC
tissues, compared with non-tumor tissues (P<0.05; Fig. 1A). To confirm this observation, the
expression of miR-577 in NSCLC cell lines was also detected.
Compared with BEAS-2B, all four NSCLC cell lines (H460, SK-MES-1,
H522 and A549) had decreased miR-577 expression, compared with that
in BEAS-2B cells (P<0.05; Fig.
1B). miR-577 expression in H460 and A549 cells was the lowest
among the four NSCLC cell lines; therefore, these two NSCLC cell
lines were selected for subsequent functional experiments.
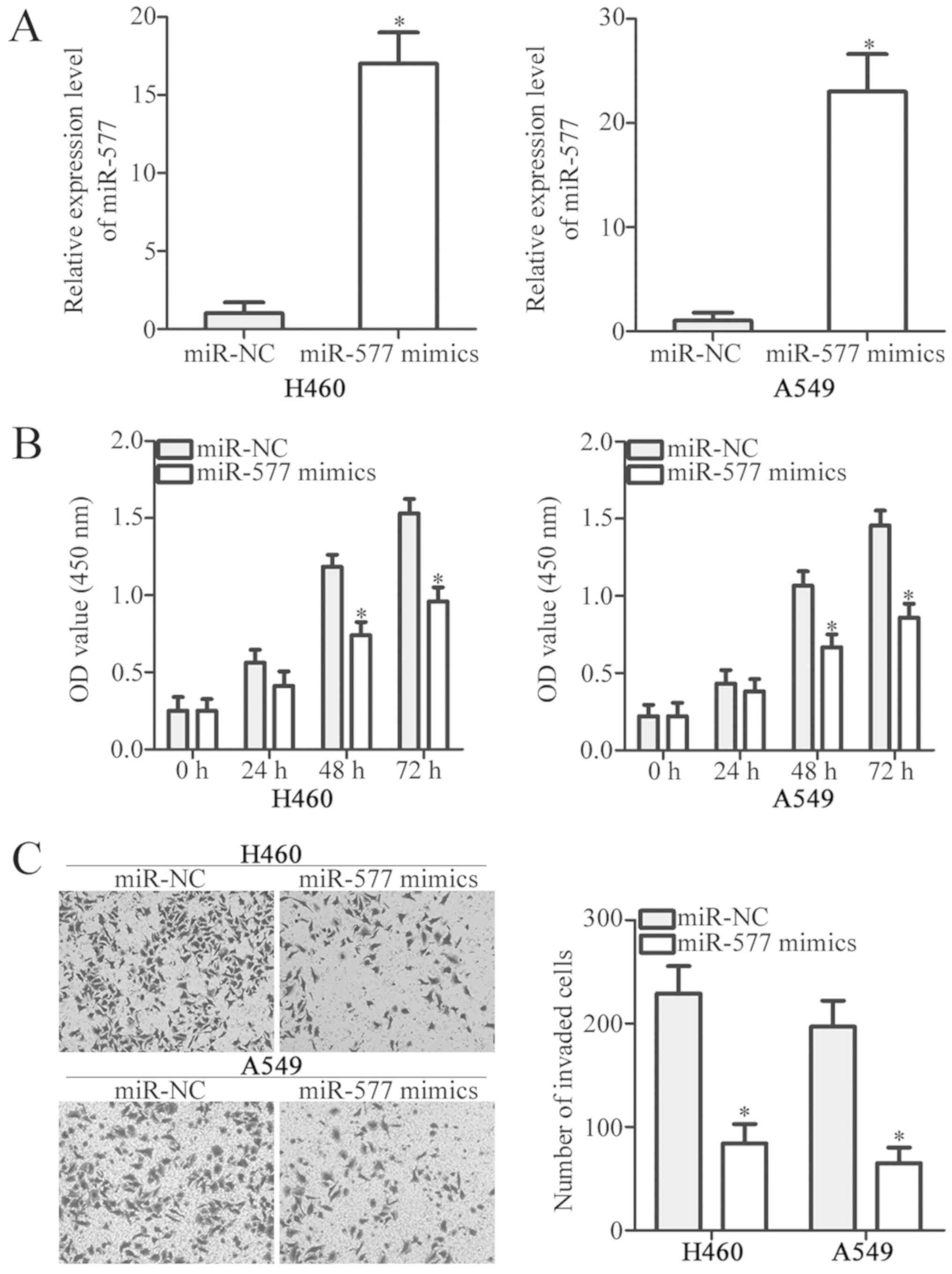
miR-577 restricts proliferation and
invasion of NSCLC cells
To elucidate the functions of miR-577 in NSCLC,
miR-577 mimics were transfected to increase miR-577 expression in
H460 and A549 cells. RT-qPCR results confirmed that miR-577
expression was significantly upregulated in H460 and A549 cells
transfected with miRNA mimics (P<0.05; Fig. 2A). The effect of miR-577
overexpression on NSCLC cell proliferation was determined by CCK-8
assay. Ectopic miR-577 expression evidently decreased the
proliferative ability of H460 and A549 cells, compared with the
miR-NC groups (P<0.05; Fig.
2B). Transwell invasion assays were then performed to detect
invasion of H460 and A549 cells transfected with miR-577 mimics or
miR-NC. miR-577 overexpression significantly inhibited H460 and
A549 cell invasion (P<0.05; Fig.
2C). These results indicated that miR-577 may be a tumor
suppressor in NSCLC.
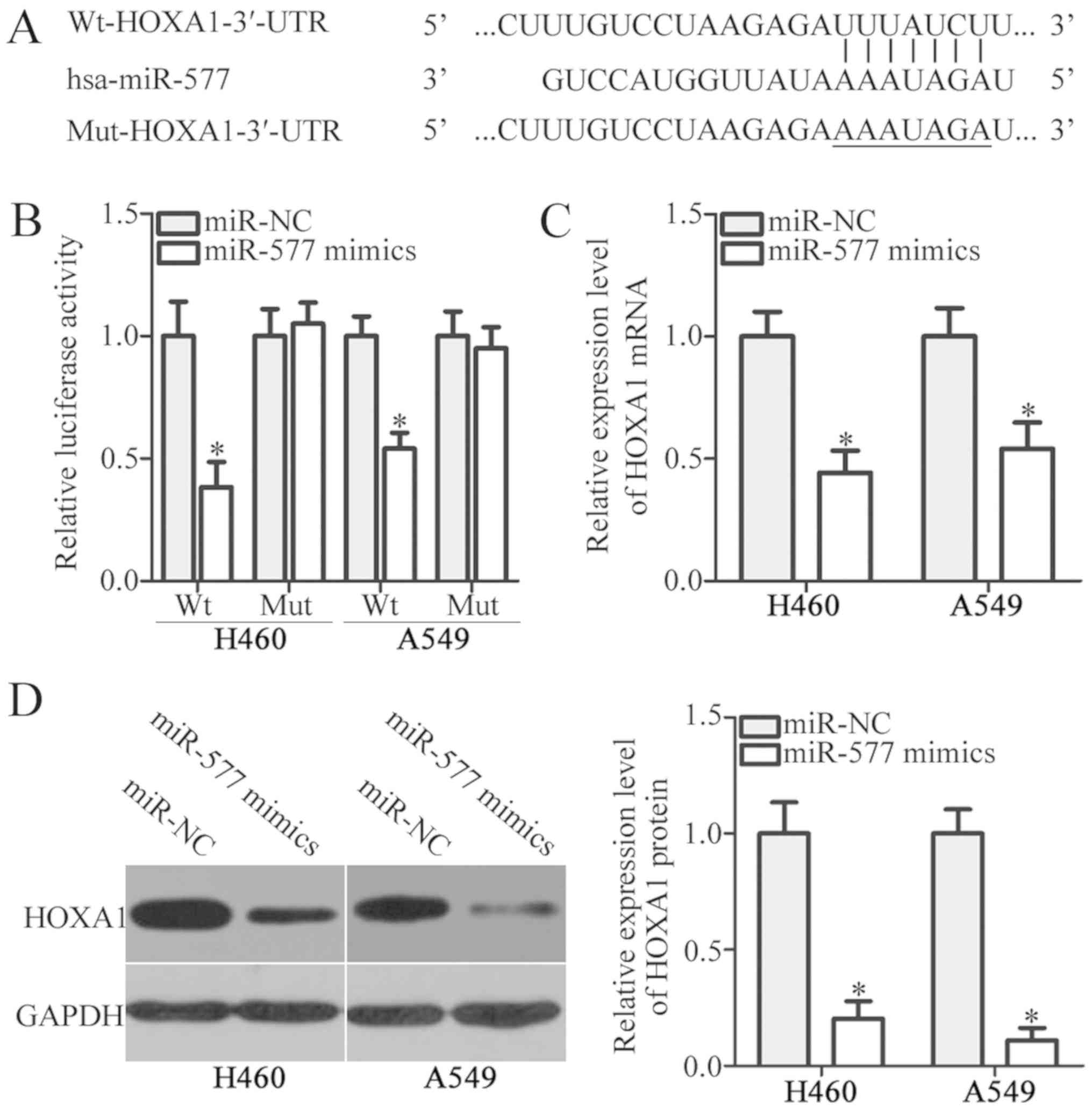
HOXA1 is a direct target gene of
miR-577 in NSCLC cells
To examine the mechanisms by which miR-577 affected
NSCLC cell proliferation and invasion, bioinformatics analysis was
performed to predict the putative targets of miR-577. The miRNA
target prediction algorithms (TargetScan and miRDB) indicated that
the 3′-UTR of HOXA1 contained the putative miR-577-binding site
(Fig. 3A). To determine whether
HOXA1 was a direct target of miR-577, luciferase reporter assays
were performed in H460 and A549 cells following co-transfection
with miR-577 mimics or miR-NC and pMIR-Wt-HOXA1-3′-UTR or
pMIR-Mut-HOXA1-3′-UTR. miR-577 overexpression suppressed the
luciferase activity of pMIR-Wt-HOXA1-3′-UTR in H460 and A549 cells
(P<0.05). There was no decrease in the luciferase activity in
the pMIR-Mut-HOXA1-3′-UTR transfected group (Fig. 3B). Next, the mRNA and protein
expression of HOXA1 in H460 and A549 cells was measured, following
transfection with miR-577 mimics or miR-NC. The results revealed
that miR-577 mimic transfection in H460 and A549 cells
significantly reduced the mRNA (P<0.05; Fig. 3C) and protein (P<0.05; Fig. 3D) expression of HOXA1. Taken
together, these results demonstrated that HOXA1 was a direct target
of miR-577 in NSCLC cells.
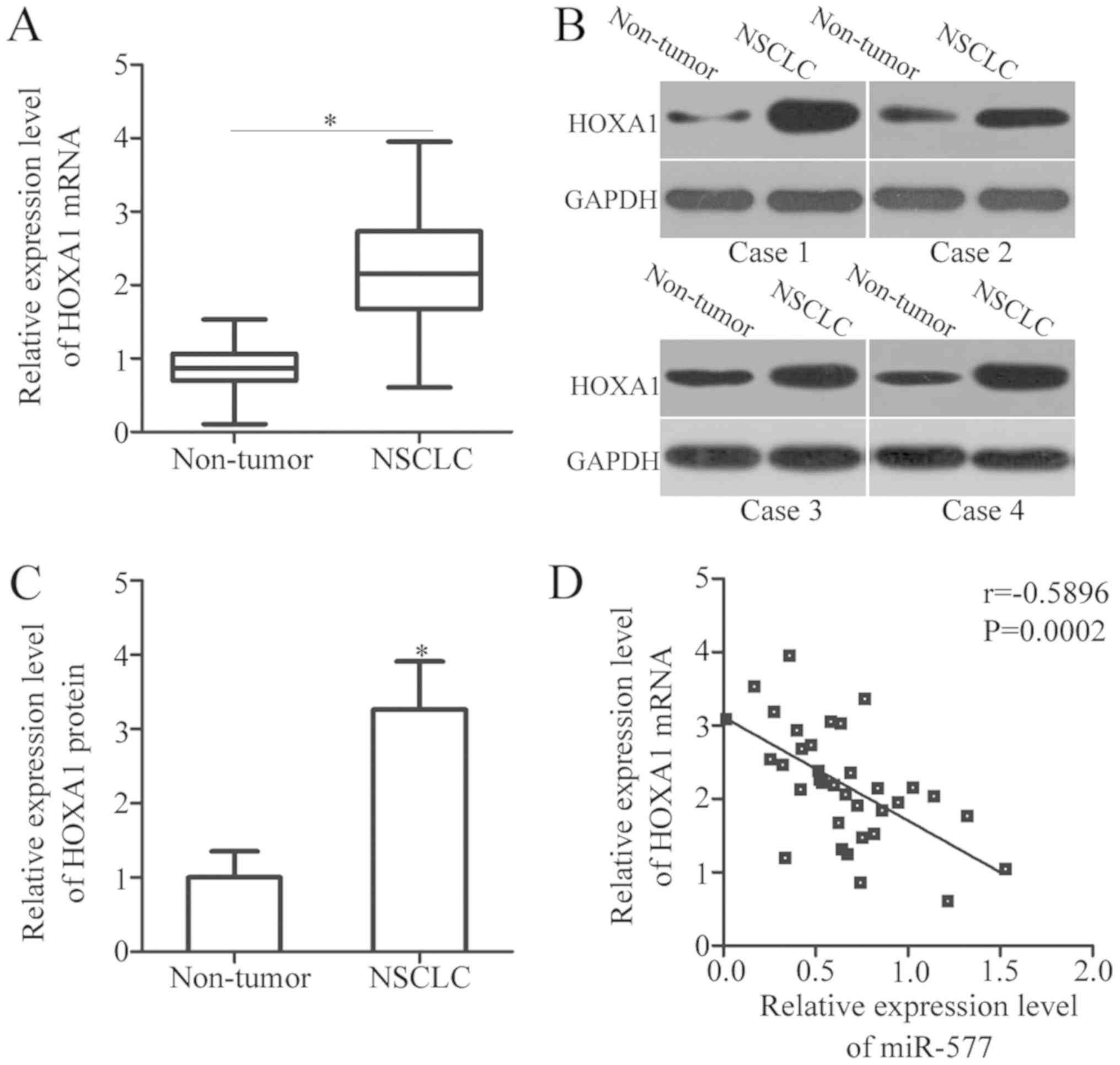
HOXA1 is overexpressed in NSCLC
tissues and inversely correlated with miR-577 level
To further evaluate the relationship between miR-577
and HOXA1 in NSCLC, HOXA1 expression was detected in 35 pairs of
NSCLC tissues and adjacent non-tumor tissues. The mRNA expression
of HOXA1 was notably higher in NSCLC tissues, compared with that in
non-tumor tissues (P<0.05; Fig.
4A). In addition, the protein expression of HOXA1 in several
pairs of NSCLC tissues and adjacent non-tumor tissues was
determined by western blot analysis. The results indicated that
HOXA1 protein expression was upregulated in NSCLC tissues, compared
with the adjacent non-tumor tissues (P<0.05; Fig. 4B and C). Furthermore, an inverse
association between miR-577 and HOXA1 mRNA expression in NSCLC
tissues was observed (r=−0.5896, P=0.0002; Fig. 4D).
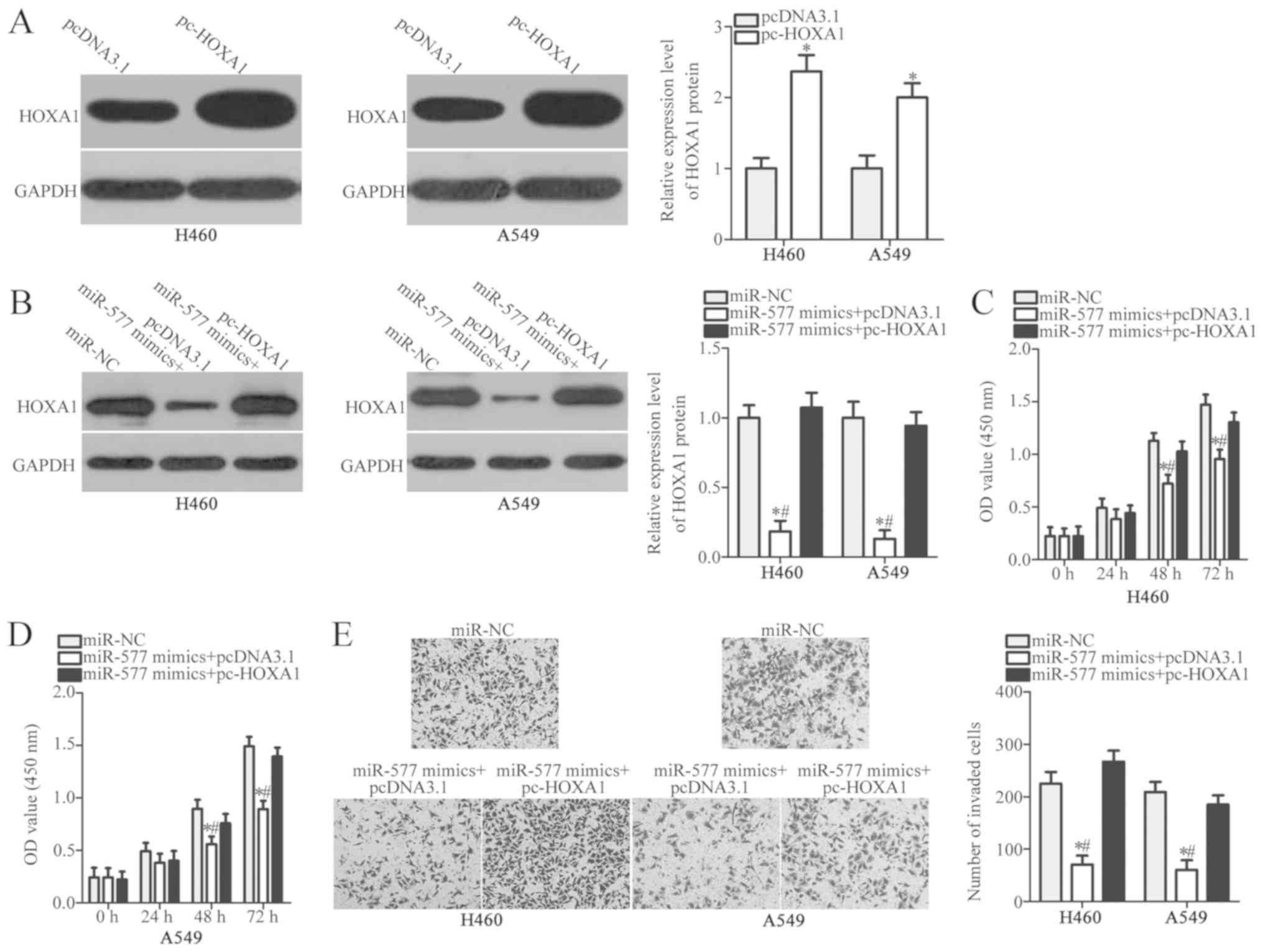
Restored HOXA1 expression prevents the
inhibitory effects of miR-577 overexpression in NSCLC cells
Given that HOXA1 was identified as a direct target
of miR-577, whether HOXA1 was required for the suppressive roles of
miR-577 on NSCLC cells was further clarified. HOXA1 overexpression
plasmid pcDNA3.1-HOXA1 (pc-HOXA1) was used to restore HOXA1
expression in H460 and A549 cells. HOXA1 expression was
significantly increased in pc-HOXA1-transfected H460 and A549
cells, compared with cells transfected with empty pcDNA3.1 plasmid
(P<0.05; Fig. 5A). Next, rescue
experiments were performed by co-transfecting miR-577 mimics and
pc-HOXA1 or pcDNA3.1 into H460 and A549 cells. Following
transfection, the decreased HOXA1 protein level in H460 and A549
cells caused by miR-577 overexpression was recovered by pc-HOXA1
(P<0.05; Fig. 5B). Similarly,
CCK-8 and Transwell invasion assays confirmed that HOXA1
restoration abolished the inhibitory effects of miR-577 mimics on
H460 and A549 cell proliferation (P<0.05; Fig. 5C and D) and invasion (P<0.05;
Fig. 5E). These results suggested
that miR-577 served a tumor suppressive role in NSCLC, at least
partially through targeting HOXA1.
Discussion
An increasing number of studies have indicated the
presence of aberrant miRNA expression in NSCLC (26–28).
miRNA dysregulation is closely associated with NSCLC oncogenesis
and development, by acting as tumor suppressors or oncogenes
(14,16,29).
Therefore, an in-depth understanding of the biological roles of
miRNAs in NSCLC may provide novel therapeutic methods for the
management of patients with this disease. In the present study, it
was demonstrated that miR-577 expression was significantly reduced
in NSCLC tissues and cell lines. The restoration of miR-577
expression significantly decreased the proliferation and invasion
of NSCLC cells. Notably, miR-577 negatively regulated HOXA1
expression by directly binding to its 3′-UTR. Furthermore, HOXA1
expression was upregulated in NSCLC tissues, and the upregulation
of HOXA1 was inversely correlated with miR-577. HOXA1 restoration
prevented the inhibitory effects of miR-577 overexpression on NSCLC
cell proliferation and invasion. These results provided novel
insights into NSCLC development and invasion, and may aid in the
identification of therapeutic strategies.
miR-577 expression has been examined in several
types of human cancer. For example, miR-577 expression is
downregulated in breast cancer, and this downregulation is
significantly correlated with tumor size, stage and lymphatic
metastasis (21). In addition,
miR-577 expression is reduced in hepatocellular carcinoma tissues
and cell lines, and low miR-577 expression is associated with tumor
size and metastasis (22). miR-577
is also downregulated in colorectal cancer (23), papillary thyroid carcinoma
(24), glioblastoma (30) and gastric cancer (31). However, miR-577 expression is
upregulated in esophageal squamous cell carcinoma (32). These findings indicate that the
expression pattern of miR-577 in human cancers is tissue specific.
Hence, miR-577 may be an effective diagnostic biomarker for these
malignant tumors.
miR-577 serves as a tumor suppressor in human cancer
types. For instance, miR-577 overexpression inhibits
epithelial-mesenchymal transition and invasion in breast cancer
cells (21). In hepatocellular
carcinoma, the upregulation of miR-577 suppresses cell
proliferation, promotes cell apoptosis and induces cell cycle
arrest at the G0/G1 phase (22).
In colorectal cancer, miR-577 expression restoration attenuates
cell growth, induces G0/G1 cell cycle arrest in vitro and
inhibits tumor growth in vivo (23). In papillary thyroid carcinoma,
miR-577 expression restricts cell growth, migration and invasion
in vitro (24). In
glioblastoma, ectopic miR-577 overexpression impedes cell viability
and growth (30). In gastric
cancer, miR-577 overexpression represses cell proliferation by
affecting the G1 to S phase transition (31). Nevertheless, miR-577 plays
oncogenic roles in esophageal squamous cell carcinoma and promotes
cell proliferation and colony formation (32). These conflicting pieces of evidence
indicate that the biological roles of miR-577 exhibit evident
tissue specificity and suggest that miR-577 may be a valuable
therapeutic target for treating patients with these cancers.
Various genes have been demonstrated to be the
direct targets of miR-577, including Ras-related protein Rab-25 in
breast cancer (21), β-catenin in
hepatocellular carcinoma (22),
heat shock protein27 in colorectal cancer (23), sphingosine kinase 2 in papillary
thyroid carcinoma (24), E2F
transcription factor 3 in gastric cancer (31) and testis specific 10 in esophageal
squamous cell carcinoma (32). In
the present study, HOXA1, mapped to the short arm of chromosome 7
at band 15.2 (7p15.2), was validated as a direct target gene of
miR-577 in NSCLC cells. It belongs to the homeodomain-containing
transcription factor (HOXA) family and serves crucial roles in
early developmental patterns and organogenesis (33,34).
Previous studies have shown that HOXA1 is markedly upregulated in
NSCLC tissues (35) and has
oncogenic function in the carcinogenesis and progression of NSCLC
(36–38). Herein, it was found that miR-577
directly targeted HOXA1 to inhibit the proliferation and invasion
of NSCLC cells. The present study, together with previous findings,
suggested that the identified miR-577/HOXA1 axis may represent a
promising therapeutic target for patients with NSCLC.
In conclusion, miR-577 expression was decreased in
NSCLC tissues and cell lines. Functional analyses indicated that
miR-577 was able to inhibit the proliferation and invasion of NSCLC
cells. Furthermore, HOXA1 was identified as a direct target gene of
miR-577 in NSCLC, and it was required for the inhibitory effects of
miR-577 on NSCLC cells. These results may help to further
understand the mechanisms underlying the occurrence and development
of NSCLC, and provided evidence for the miR-577/HOXA1 axis as a
potential therapeutic target for the treatment of patients with
this malignancy. However, the sample size of the present study was
small, and the relationship between miR-577 and the
clinicopathological characteristics of NSCLC patients was not
investigated. More samples will be collected to resolve this in
future experiments.
Acknowledgement
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HN designed the research. LM, DN and HN performed
functional experiments. All authors read and approved the final
draft.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of China-Japan Union Hospital of Jilin University
(approval no. 20140311), and was performed in accordance with the
Declaration of Helsinki and the guidelines of the Ethics Committee
of China-Japan Union Hospital of Jilin University. Written informed
consent was provided by all patients for the use of their clinical
tissues.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Laskin JJ and Sandler AB: State of the art
in therapy for non-small cell lung cancer. Cancer Invest.
23:427–442. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ramalingam SS, Owonikoko TK and Khuri FR:
Lung cancer: New biological insights and recent therapeutic
advances. CA Cancer J Clin. 61:91–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Collins LG, Haines C, Perkel R and Enck
RE: Lung cancer: Diagnosis and management. Am Fam Physician.
75:56–63. 2007.PubMed/NCBI
|
|
5
|
Chen W, Zheng R, Zhang S, Zhao P, Zeng H
and Zou X: Report of cancer incidence and mortality in China, 2010.
Ann Transl Med. 2:612014.PubMed/NCBI
|
|
6
|
Kutikhin AG, Yuzhalin AE, Brailovskiy VV,
Zhivotovskiy AS, Magarill YA and Brusina EB: Analysis of cancer
incidence and mortality in the industrial region of South-East
Siberia from 1991 through 2010. Asian Pac J Cancer Prev.
13:5189–5193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bradbury P, Sivajohanathan D, Chan A,
Kulkarni S, Ung Y and Ellis PM: Postoperative adjuvant systemic
therapy in completely resected non-small-cell lung cancer: A
systematic review. Clin Lung Cancer. 18:259–273.e258. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Steeg PS: Metastasis suppressors alter the
signal transduction of cancer cells. Nat Rev Cancer. 3:55–63. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hydbring P and Badalian-Very G: Clinical
applications of microRNAs. F1000Res. 2:1362013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Croce CM and Calin GA: miRNAs, cancer, and
stem cell division. Cell. 122:6–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Castro D, Moreira M, Gouveia AM, Pozza DH
and De Mello RA: MicroRNAs in lung cancer. Oncotarget.
8:81679–81685. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang CL, Zheng XL, Ye K, Ge H, Sun YN, Lu
YF and Fan QX: MicroRNA-183 acts as a tumor suppressor in human
non-small cell lung cancer by down-regulating MTA1. Cell Physiol
Biochem. 46:93–106. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yao Y, Shen H, Zhou Y, Yang Z and Hu T:
MicroRNA-215 suppresses the proliferation, migration and invasion
of non-small cell lung carcinoma cells through the downregulation
of matrix metalloproteinase-16 expression. Exp Ther Med.
15:3239–3246. 2018.PubMed/NCBI
|
|
16
|
Liu J, Jia Y, Jia L, Li T, Yang L and
Zhang G: MicroRNA-615-3p inhibits the tumor growth and metastasis
of NSCLC via inhibiting IGF2. Oncol Res. Mar 21–2018.(Epub ahead of
print). doi: 10.3727/096504018X15215019227688.
|
|
17
|
Li G, Wu F, Yang H, Deng X and Yuan Y:
MiR-9-5p promotes cell growth and metastasis in non-small cell lung
cancer through the repression of TGFBR2. Biomed Pharmacother.
96:1170–1178. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei K, Pan C, Yao G, Liu B, Ma T, Xia Y,
Jiang W, Chen L and Chen Y: MiR-106b-5p promotes proliferation and
inhibits apoptosis by regulating BTG3 in non-small cell lung
cancer. Cell Physiol Biochem. 44:1545–1558. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Lu Y, Ding H, Gu T, Gong C, Sun J,
Zhang Z, Zhao Y and Ma C: The miR-875-5p inhibits SATB2 to promote
the invasion of lung cancer cells. Gene. 644:13–19. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li S, Gao M, Li Z, Song L, Gao X, Han J,
Wang F, Chen Y, Li W, Yang J and Han X: Role of microRNAs in
metastasis of non-small cell lung cancer. Front Biosci (Landmark
Ed). 21:998–1005. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yin C, Mou Q, Pan X, Zhang G, Li H and Sun
Y: MiR-577 suppresses epithelial-mesenchymal transition and
metastasis of breast cancer by targeting Rab25. Thorac Cancer.
9:472–479. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang LY, Li B, Jiang HH, Zhuang LW and Liu
Y: Inhibition effect of miR-577 on hepatocellular carcinoma cell
growth via targeting beta-catenin. Asian Pac J Trop Med. 8:923–929.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang H, Ju H, Zhang L, Lu H and Jie K:
microRNA-577 suppresses tumor growth and enhances chemosensitivity
in colorectal cancer. J Biochem Mol Toxicol. 31:2017. View Article : Google Scholar :
|
|
24
|
Xue KC, Hu DD, Zhao L, Li N and Shen HY:
MiR-577 inhibits papillary thyroid carcinoma cell proliferation,
migration and invasion by targeting SphK2. Eur Rev Med Pharmacol
Sci. 21:3794–3800. 2017.PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhan B, Lu D, Luo P and Wang B: Prognostic
value of expression of MicroRNAs in non-small cell lung cancer: A
systematic review and meta-analysis. Clin Lab. 62:2203–2211. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matikas A, Syrigos KN and Agelaki S:
Circulating biomarkers in non-small-cell lung cancer: Current
status and future challenges. Clin Lung Cancer. 17:507–516. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ansari J, Shackelford RE and El-Ost H:
Epigenetics in non-small cell lung cancer: From basics to
therapeutics. Transl Lung Cancer Res. 5:155–171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ding X, Zhong T, Jiang L, Huang J, Xia Y
and Hu R: miR-25 enhances cell migration and invasion in
non-small-cell lung cancer cells via ERK signaling pathway by
inhibiting KLF4. Mol Med Rep. 17:7005–7016. 2018.PubMed/NCBI
|
|
30
|
Zhang W, Shen C, Li C, Yang G, Liu H, Chen
X, Zhu D, Zou H, Zhen Y, Zhang D and Zhao S: miR-577 inhibits
glioblastoma tumor growth via the Wnt signaling pathway. Mol
Carcinog. 55:575–585. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu Z, Zhang W and Deng F: MicroRNA-577
inhibits gastric cancer growth by targeting E2F transcription
factor 3. Oncol Lett. 10:1447–1452. 2015.PubMed/NCBI
|
|
32
|
Yuan X, He J, Sun F and Gu J: Effects and
interactions of MiR-577 and TSGA10 in regulating esophageal
squamous cell carcinoma. Int J Clin Exp Pathol. 6:2651–2667.
2013.PubMed/NCBI
|
|
33
|
Shah N and Sukumar S: The Hox genes and
their roles in oncogenesis. Nat Rev Cancer. 10:361–371. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Grier DG, Thompson A, Kwasniewska A,
McGonigle GJ, Halliday HL and Lappin TR: The pathophysiology of HOX
genes and their role in cancer. J Pathol. 205:154–171. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kusakabe M, Kutomi T, Watanabe K, Emoto N,
Aki N, Kage H, Hamano E, Kitagawa H, Nagase T, Sano A, et al:
Identification of G0S2 as a gene frequently methylated in squamous
lung cancer by combination of in silico and experimental
approaches. Int J Cancer. 126:1895–1902. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Y, Li XJ, He RQ, Wang X, Zhang TT,
Qin Y, Zhang R, Deng Y, Wang HL, Luo DZ and Chen G: Upregulation of
HOXA1 promotes tumorigenesis and development of nonsmall cell lung
cancer: A comprehensive investigation based on reverse
transcription-quantitative polymerase chain reaction and
bioinformatics analysis. Int J Oncol. 53:73–86. 2018.PubMed/NCBI
|
|
37
|
Zhan M, Qu Q, Wang G, Liu YZ, Tan SL, Lou
XY, Yu J and Zhou HH: Let-7c inhibits NSCLC cell proliferation by
targeting HOXA1. Asian Pac J Cancer Prev. 14:387–392. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tian X, Ma J, Wang T, Tian J, Zhang Y, Mao
L, Xu H and Wang S: Long non-coding RNA HOXA transcript antisense
RNA myeloid-specific 1-HOXA1 axis downregulates the
immunosuppressive activity of myeloid-derived suppressor cells in
lung cancer. Front Immunol. 9:4732018. View Article : Google Scholar : PubMed/NCBI
|