Introduction
Esophageal cancer (EC) is considered to be one of
the most invasive carcinomas and is ranked sixth among the lethal
aggressive carcinomas globally. EC is considered to be a fatal
disease for patients diagnosed during the advanced stage, with an
overall survival rate of 10–20% (1). The incidence and mortality rates
associated with EC continue to rise, displaying great geographical
and sociocultural variation as well as a tendency for familial
aggregation in recent years, as is evident in the central regions
of China (2). The main
histological types of EC include adenocarcinoma and squamous cell
carcinoma based on etiology and pathological features (3). Esophageal squamous cell carcinoma
(ESCC) is the most prevalent histological subclass that is derived
from esophageal epithelial cells (4). Although advancements in surgery,
chemotherapy and radiotherapy have been achieved to treat ESCC
patients, the overall 5-year survival rate still remains <40%
worldwide owing to the high incidence of local invasion and distant
metastasis, particularly in late-stage ESCC patients (5). The prognosis of patients with ESCC
remains poor as the detailed mechanisms of the molecular and
genetic alterations underlying ESCC progression are unknown.
Therefore, investigating novel biomarkers, therapeutic agents and
mechanisms underlying ESCC initiation and progression are pivotal
in improving the early diagnosis and treatment of ESCC.
Long non-coding RNAs (lncRNAs) are a novel
transcript class of RNA molecules, longer than 200 nucleotides,
which lack the capacity to be translated into proteins (6). LncRNAs are aberrantly expressed or
dysregulated in a variety of physiological and pathological
processes via complex mechanisms (3,7).
Accumulating evidence has confirmed that lncRNAs essentially
regulate the expression of their downstream-reacting genes at the
epigenetic, transcriptional and post-transcriptional levels
(8). LncRNAs have been associated
with the genesis, progression and prognosis of various types of
cancer, and increasingly emerge either as tumor suppressors or
promoters that potentially possess oncogenic functions (9). Furthermore, lncRNAs perform their
respective biological functions at the RNA level and also regulate
microRNA (miR) function by competing with target mRNAs (10). Although a decade of research has
contributed to establishing the mechanism underlying the function
of lncRNAs, a large number of lncRNAs with potential roles,
particularly in cancer, have not been discovered or thoroughly
investigated. In the past few years, previous studies have
indicated a close association between the abnormal expression of
lncRNAs and several malignant types of cancer including human EC,
suggesting that lncRNAs could serve important roles in the
initiation of EC, with particular emphasis on ESCC (7,11,12).
The lncRNA, distal-less homeobox 6 antisense 1
(DLX6-AS1), is localized at the 7q21.3 chromosomal region in humans
and is transcribed as a 1,990 bp noncoding RNA. Previous studies
have suggested that DLX6-AS1 may function as a noncoding oncogene,
and that its dysregulation is closely associated with the
progression and poor prognosis of tumors in lung adenocarcinoma
(13), renal cell carcinoma
(14) and hepatocellular carcinoma
(15). These studies implied that
tumorigenesis or progression was associated with the dysregulation
of DLX6-AS1. However, the potential mechanisms underlying the role
of DLX6-AS1 in ESCC have yet to be elucidated and the associated
studies are few in number. In the present study, the expression
profile of DLX6-AS1 was characterized in ESCC tissues and the
adjacent histologically normal tissues. The association between the
expression patterns of DLX6-AS1 and the clinical characteristics of
patients with ESCC were also investigated. Furthermore, the
functional impact of DLX6-AS1 on ESCC cell proliferation, apoptosis
and invasive ability was investigated by performing in vitro
lncRNA knockdown assays. The results revealed the potential roles
of DLX6-AS1 and provided a novel perspective on the progression and
pathogenesis of ESCC.
Materials and methods
Clinical tissue samples
Paired primary ESCC and matched adjacent
non-neoplastic specimens were obtained from 73 eligible patients
enrolled in the present study at the First Affiliated Hospital of
Zhengzhou University (Zhengzhou, China) between April 2015 and July
2016 (Table I). All of the biopsy
samples were obtained from ESCC patients who did not undergo
chemotherapy or radiotherapy and were not suffering from any other
serious diseases. Following surgical resection, the samples were
frozen and stored using liquid nitrogen until RNA extraction. The
histopathology of the tumor tissues was independently reviewed by
at least two pathologists. The Union for International Cancer
Control/American Joint Committee on Cancer classification
guidelines were applied to determine the tumor stage in each
individual (16). The acquired
clinicopathological information for all of the samples is available
in Table I. Written informed
consent was obtained from each patient prior to using the clinical
samples for research purposes. The Ethics Committee of Zhengzhou
University (Henan, China) approved the study protocol.
 | Table I.Association between the
clinicopathological factors and the expression of DLX6-AS1. |
Table I.
Association between the
clinicopathological factors and the expression of DLX6-AS1.
|
| Expression of
DLX6-AS1 |
|
|---|
|
|
|
|
|---|
| Characteristics | Low-DLX6-AS1 group
(n) | High-DLX6-AS1 group
(n) | P-value |
|---|
| Sex |
|
| 0.393 |
| Male | 23 | 20 |
|
|
Female | 13 | 17 |
|
| Age, years |
|
| 0.549 |
|
<60 | 21 | 19 |
|
|
≥60 | 15 | 18 |
|
| Tumor location |
|
| 0.693 |
| Upper
1/3 | 9 | 8 |
|
| Middle
1/3 | 14 | 12 |
|
| Lower
1/3 | 13 | 17 |
|
| Histological
grade |
|
| 0.004 |
| Low
differentiation | 8 | 19 |
|
| Middle
differentiation | 10 | 12 |
|
| High
differentiation | 18 | 6 |
|
| Lymph node
metastasis |
|
| 0.003 |
|
Yes | 14 | 27 |
|
| No | 22 | 10 |
|
| TNM stage |
|
| 0.024 |
|
I/II | 25 | 16 |
|
|
III/IV | 11 | 21 |
|
Cell culture
The human ESCC cell lines, EC109 and KYSE30, were
purchased from the Shanghai Institute of Biochemistry and Cell
Biology (Chinese Academy of Sciences, Shanghai, China). The human
immortalized normal esophageal epithelial cell line, Het-1A, was
obtained from American Type Culture Collection (ATCC, Manassas, VA,
USA). All of the cells were maintained in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100
U/ml penicillin and 100 µg/ml streptomycin at 37°C in a humidified
chamber containing 5% CO2.
Cell transfection
Small interfering RNAs (siRNAs) specifically
targeting lncRNA DLX6-AS1 were synthesized by Shanghai GenePharma
Co., Ltd. (Shanghai, China) including a negative control siRNA with
no definite target. EC109 and KYSE30 cells of 5×104
cells/ml were cultured overnight in 6-well plates till 70%
confluency was reached and then transfected with 10 nmol/l lncRNA
DLX6-AS1 siRNA (si-lnc) or the negative control (si-NC) using
Lipofectamine 2000™ (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The cells were collected
following 48 h of transfection to determine the efficiency of the
knockdown via reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) analysis and other functional experiments. The
target sequence for the lncRNA DLX6-AS1 siRNA was as follows:
5′-AAUAAAGAACACUUACACUACUG-3′ (15).
Cell proliferation assay
To analyze the growth of the EC109 and KYSE30 cell
lines, a Cell Counting Kit-8 (CCK-8; Beyotime Institute of
Biotechnology, Haimen, China) assay was performed according to the
manufacturer's protocol. Log-phase cells were harvested and
cultured at a density of 5×103 cells/well in a 96-well
plate, with quintuplicate wells per group. Following transfection
with either lncRNA DLX6-AS1 siRNA or si-NC for 24, 48 or 72 h, the
cells were treated with the CCK-8 solution (10 µl) in the plates
and incubated for 2 h at 37°C. The optical density was measured for
each well at a wavelength of 450 nm using a microplate reader
(Infinite M200; Tecan Group, Ltd., Männedorf, Switzerland) to
estimate cell viability. Growth curves were plotted using the mean
results obtained independently from triplicate experiments.
Apoptosis analysis
The Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) Apoptosis Detection kit (Beyotime
Institute of Biotechnology) was used to quantify the apoptotic rate
of the ESCC cells. Briefly, cells were harvested from each group
via 0.25% trypsinization following a 48 h transfection. The cells
were washed with PBS, resuspended in Annexin-binding buffer and
adjusted to a density of 1×106 cells/ml. They were then
vortexed and analyzed using a flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA) following incubation with a working
solution of FITC-Annexin V and PI at room temperature for 15 min.
The early and late apoptotic cells were counted and distinguished
from the viable and dead cells using FlowJo software (version 7.6,
FlowJo LLC, Ashland, OR, USA). Relative apoptotic ratios were
calculated and compared with those of the control group. Each assay
was performed independently in triplicate.
Invasion assay
The Matrigel-coated Transwell chamber system (8-µm
pore size; Corning Incorporated, Corning, NY, USA) was used to
analyze the invasive capabilities of EC109 and KYSE30 cells as per
the manufacturer's protocol. The cell suspension (200 µl), at a
concentration of 1×105 cells/ml, containing serum-free
RPMI-1640 medium following 48 h of transfection, was loaded in the
upper chamber containing a membrane coated with Matrigel.
Additionally, 500 µl culture medium supplemented with 10% FBS was
added to the lower chamber as the chemoattractant. The system was
then incubated for 48 h at 37°C and 5% CO2. Following
incubation, the noninvasive cells on the surface of the upper
membrane were scraped off using a cotton swab. The cells that
successfully invaded through the filter to the lower regions of the
chambers were fixed with 100% methanol for 15 min at 4°C, stained
with 0.1% crystal violet for 15 min at 37°C and dried for 30 min at
80°C. A total of five randomly selected fields per chamber were
captured using a bright-field microscope (magnification, ×200) to
evaluate the invasive ability of the ESCC cells. Data was obtained
from three independent experiments.
Cell migration assay
EC109 and KYSE30 cell motility was assessed using a
scratch wound healing assay by measuring the movement of cells in
an artificially scraped wound. The cells at concentration of
5×104 cells/ml were seeded onto plastic 6-well plates
until cell confluence reached ~80% following 24 h of transfection.
A 200 µl pipette tip was used to scratch and create wounds in the
confluent monolayer cell cultures, followed by 3 washes with PBS to
remove the debris and floating cells. Images of wound closure were
captured following 0 and 48 h of incubation using a Leica DMIL
inverted microscope to assess the level of migration in each group
of transfected cells. A total of 5 random fields per well were
captured to quantify migration by measuring the distance that the
cells had migrated toward the original wound field using Image Pro
Plus v6.0 software (Media Cybernetics Inc., Rockville, MD, USA).
Each experiment was performed independently in triplicate.
RNA isolation and RT-qPCR
analysis
The RT-qPCR assays were conducted to detect the
expression of DLX6-AS1. TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract total RNA from ESCC tissues
or cells stored at −80°C, according to the manufacturer's protocol.
The cDNAs for subsequent qPCRs were synthesized using the
PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd., Dalian,
China) by reverse transcribing 1 µg of total RNA using random
primers at 42°C for 30 min and 85°C for 5 min. PCR amplification
was conducted using the Applied Biosystems 7500 fast real-time PCR
system (Thermo Fisher Scientific, Inc.) in 20 µl reaction mixtures
containing SYBR Green Real-time PCR Master Mix (Takara
Biotechnology Co., Ltd.) at 95°C for 30 sec of initial
denaturation, followed by 40 cycles of 95°C for 5 sec and 60°C for
30 sec. The Cq value was used to determine the relative expression
levels of DLX6-AS1 and to calculate the fold-change; the expression
levels were internally normalized to that of β-actin using the
2−ΔΔCq method (17).
The primers used for analysis are as follows: lncRNA DLX6-AS1
forward, 5′-AGTTTCTCTCTAGATTGCCTT-3′ and reverse,
5′-ATTGACATGTTAGTGCCCTT-3′; β-actin forward,
5′-TCCCTGGAGAAGAGCTACGA-3′ and reverse, 5′-ACCTGAGGCTTTGGATTCCT-3′.
Each experiment was performed independently in triplicate.
Western blot analysis
EC109 and KYSE30 cells in each group were lysed
using a radioimmunoprecipitation assay buffer (Beyotime Institute
of Biotechnology) containing the protease inhibitor
phenylmethylsulfonyl fluoride (Beyotime Institute of
Biotechnology), as per the manufacturer's protocol for total
protein extraction. The protein concentration was determined via a
bicinchoninic acid assay (Thermo Fisher Scientific, Inc.). Protein
samples (50 µg) were separated using 10% SDS-PAGE and then
transferred to a nitrocellulose membrane (EMD Millipore, Billerica,
MA, USA). Following blocking with 5% non-fat milk for 2 h at 4°C,
the membrane was subsequently incubated overnight at 4°C with
primary antibodies in Tris-buffered saline (TBS). Primary
antibodies against epithelial (E)-cadherin (1:800; ab76055; Abcam,
Cambridge, UK), neural (N)-cadherin (1:800; ab98952; Abcam),
vimentin (1:800; 49636; Cell Signaling Technology, Inc., Danvers,
MA, USA) and β-actin (1:1,000; ab6276; Abcam) were used for western
blot analysis. Following washing with TBS containing 0.1% Tween-20,
the membrane was incubated with secondary antibodies (horseradish
peroxidase-conjugated goat anti-mouse Immunoglobulin G; 1:2,000;
HAF007; R&D Systems, Inc., Minneapolis, MN, USA) for 2 h at
37°C. Normalized to the β-actin protein density, the target protein
band densities were measured using an enhanced chemiluminescence
array (Beyotime Institute of Biotechnology) and densitometry were
detected by Image-Pro Plus software version 6.0 (Media Cybernetics,
Inc., Rockville, MD, USA).
Statistical analysis
Statistical analysis was conducted using SPSS
software (version 21.0; IBM, Corps., Armonk, NY, USA). All of the
data is presented as the mean ± standard deviation. The statistical
differences were evaluated using independent two-tailed Student's
t-tests, one-way analysis of variance and Dunnett's test,
chi-square tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression profile of DLX6-AS1 in
human ESCC tissues and cell lines
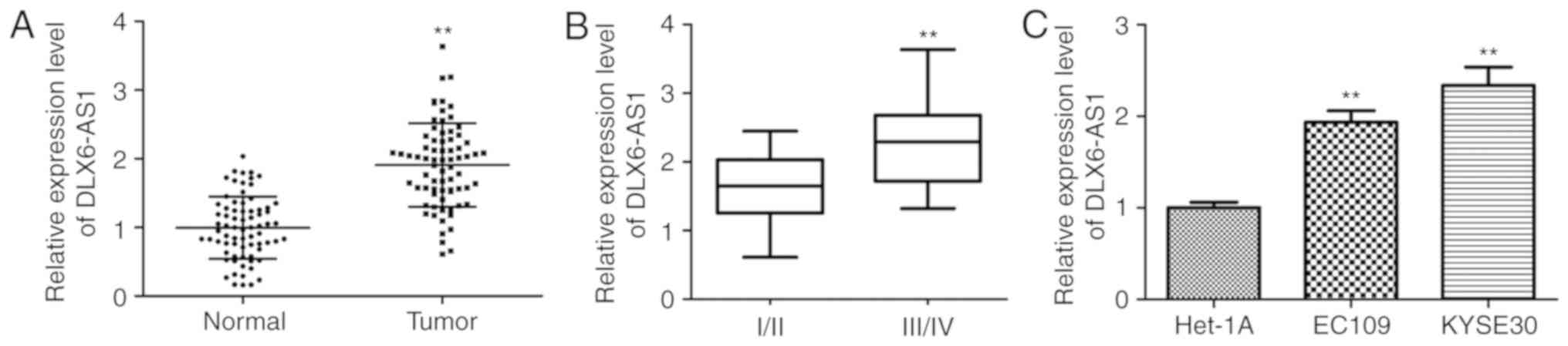
To identify the biological roles of DLX6-AS1 in ESCC
development, RT-qPCR analysis was conducted to assess the
expression of DLX6-AS1 in tumor tissues (n=73) and paired adjacent
normal tissues (n=73). As illustrated in Fig. 1A, DLX6-AS1 expression was
significantly elevated in the ESCC tissue samples when compared
with the corresponding adjacent noncancerous tissue (P<0.01).
The significantly increased expression of DLX6-AS1 in advanced
tumor stages (III/IV) compared with early-stage tumors (I/II)
suggested that the overexpression of DLX6-AS1 may be associated
with the development of ESCC (P<0.01; Fig. 1B). Furthermore, the levels of
DLX6-AS1 were validated in the ESCC cell lines (EC109 and KYSE30)
via RT-qPCR normalized to Het-1A. The expression level of DLX6-AS1
was significantly upregulated in the two ESCC cell lines when
compared with the Het-1A cell line (P<0.01; Fig. 1C). It was also demonstrated that
the KYSE30 cells exhibited increased expression of DLX6-AS1 when
compared with EC109 cells. Based on these results, the EC109 and
KYSE30 cell lines were used for the subsequent experiments to
evaluate the effect of DLX6-AS1 in ESCC pathogenesis.
Overexpression of DLX6-AS1 is
associated with the clinicopathological characteristics of ESCC
patients
To further determine whether the expression profile
of DLX6-AS1 was associated with the clinicopathological parameters
of ESCC, using the DLX6-AS1 expression of adjacent noncancerous
tissues as a reference, the 73 patients were divided into DLX6-AS1
low- and high-expression groups, taking the median expression level
of DLX6-AS1 as a cutoff (Table I).
The analysis demonstrated that increased DLX6-AS1 expression was
mainly detected in late-stage ESCC tissues and was significantly
associated with differentiation status (P=0.004), lymph node
metastasis (P=0.003) and advanced Tumor-Node-Metastasis (TNM) stage
(P=0.024). There was insufficient evidence significantly
associating the expression of DLX6-AS1 with other clinical
characteristics including sex, age and tumor location. Significant
associations between the aberrant expression patterns of DLX6-AS1
and the clinicopathological characteristics of ESCC patients
indicated that further functional studies should be conducted in
order to evaluate the role of DLX6-AS1 in the progression of
ESCC.
Knockdown of DLX6-AS1 inhibits the
proliferation of ESCC cells
To further understand the biological roles of
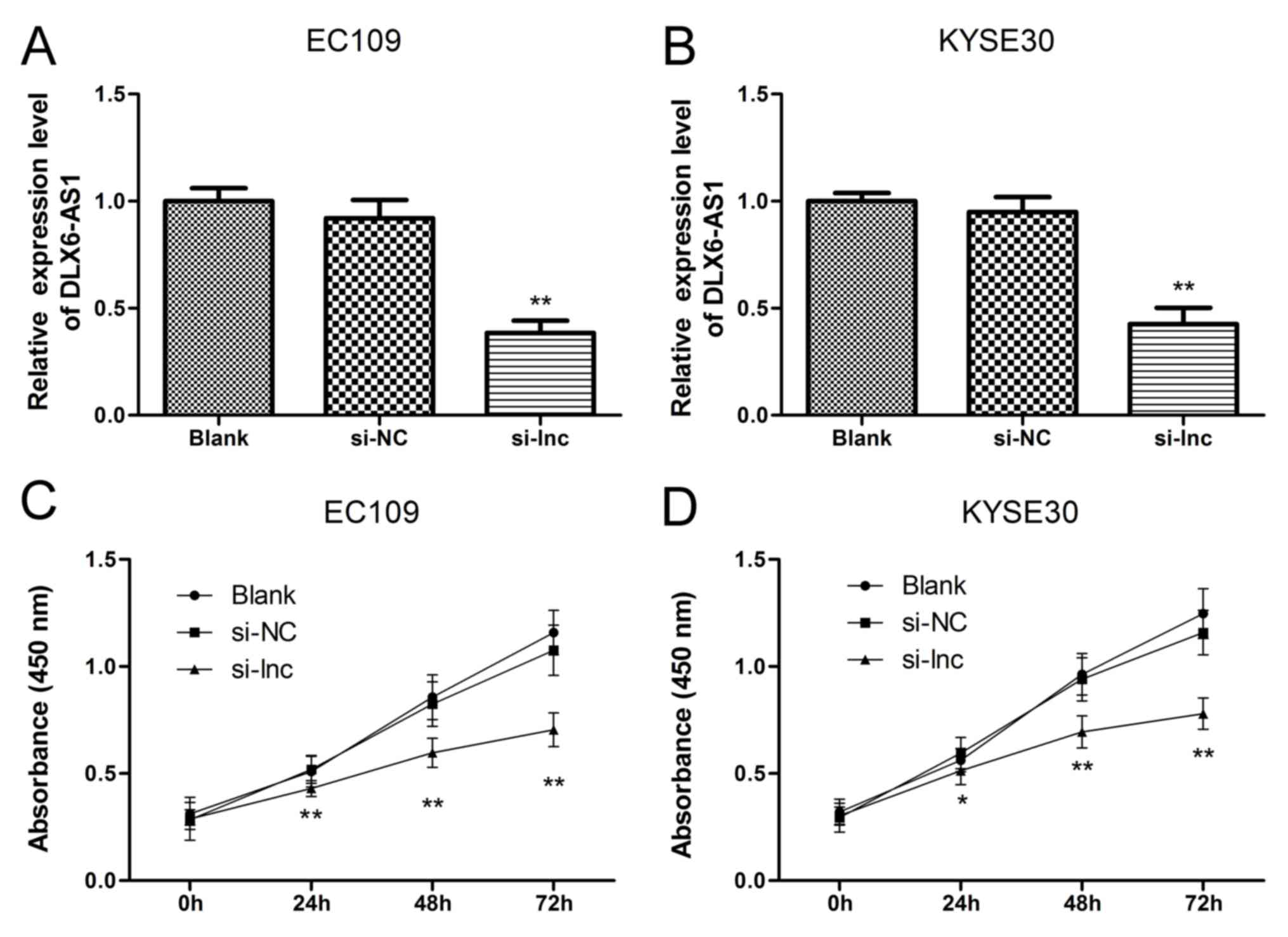
DLX6-AS1 in ESCC cell phenotypes, knockdown assays were performed
by transfecting EC109 and KYSE30 cell lines with the DLX6-AS1 siRNA
as these cell lines contain high levels of DLX6-AS1. The success of
the 48 h siRNA transfection was verified via RT-qPCR in EC109 and
KYSE30 cells. As demonstrated in Fig.
2A and B, the expression levels of DLX6-AS1 did not differ
significantly between the non-transfected group (Blank) and the
nonsense siRNA transfected group (NC), suggesting that the nonsense
siRNA had no effect on the expression levels of DLX6-AS1 in the
ESCC cells. The expression profile of DLX6-AS1 was significantly
reduced in the EC109 and KYSE30 cells following transfection with
the lncRNA DLX6-AS1 siRNA (si-lnc group) compared with the Blank
and NC groups (P<0.01). Next, a CCK-8 assay was performed to
further determine the cell viabilities of the EC109 and KYSE30
cells. As demonstrated in Fig. 2C and
D, the corresponding EC109 and KYSE30 cell proliferation curves
indicated that the cell viabilities of the si-lnc group were
significantly inhibited at 24, 48 and 72 h when compared with the
Blank and NC groups (P<0.05). Furthermore, no significant
differences in cell growth were detected between the Blank and NC
groups. The results of the present study suggested that suppressing
DLX6-AS1 expression impeded the growth and survival capacity of
EC109 and KYSE30 cells in vitro and that DLX6-AS1 promoted
ESCC cell proliferation.
Silencing DLX6-AS1 promotes the
apoptosis of EC109 and KYSE30 cells
To investigate the potential mechanisms underlying
the inhibition of cell proliferation induced by DLX6-AS1 knockdown
in ESCC cells, flow cytometry assays were performed to examine the
apoptosis process in EC109 and KYSE30 cells transfected with lncRNA
DLX6-AS1 siRNA for 48 h. As illustrated in Fig. 3, the percentage of apoptotic EC109
and KYSE30 cells significantly increased following DLX6-AS1
knockdown compared with the Blank and NC groups (P<0.01).
Consistent with cell proliferation assays, these flow cytometry
results demonstrated that silencing DLX6-AS1 using siRNA induced
apoptosis in EC109 and KYSE30 cells.
Downregulation of DLX6-AS1 inhibits
invasion and motility of EC109 and KYSE30 cells
Transwell and wound healing assays were conducted to
investigate the roles of DLX6-AS1 in the invasion and motility of
the ESCC cell lines. The Transwell assay results demonstrated that
the number of cells penetrating the Transwell membrane containing
Matrigel did not differ significantly between the Blank and NC
groups. However, significantly fewer invasive cells were observed
in the si-lnc group when compared with the Blank and NC groups
(P<0.01; Fig. 4A). This was
further confirmed in the wound healing assays, which demonstrated
that the wound closure was significantly inhibited in the EC109 and
KYSE30 cells transfected with lncRNA DLX6-AS1 siRNA when compared
with the Blank and NC groups (P<0.01; Fig. 4B). Collectively, the results
indicated that the invasive capabilities and motilities of EC109
and KYSE30 cells were significantly suppressed by the lncRNA
DLX6-AS1 siRNA treatment when compared with the Blank and NC groups
(P<0.01; Fig. 4). Therefore,
DLX6-AS1 is involved in mechanisms that promote ESCC cell invasion
and motility.
Effect of DLX6-AS1 expression on the
epithelial-mesenchymal transition (EMT) of ESCC cells
To investigate the mechanisms underlying the
knockdown of DLX6-AS1 that inhibits the invasion and migration of
ESCC cells, the expression of EMT markers was analyzed as a result
of their involvement in cancer metastatic potential and
progression. The present study sought to determine if the abnormal
expression of DLX6-AS1 was associated with epithelial and
mesenchymal features of the ESCC cell phenotype. The expression of
the EMT-associated markers was measured in the EC109 and KYSE30
cells via western blotting assays following transfection with
DLX6-AS1 siRNA. As demonstrated in Fig. 5, DLX6-AS1 knockdown in EC109 and
KYSE30 cells resulted in significant overexpression of E-cadherin
(epithelial marker), and significantly reduced the expressions of
N-cadherin and vimentin (mesenchymal markers) when compared with
the expression levels of these proteins in the Blank and NC groups
(P<0.01). Therefore, the results implied that DLX6-AS1
suppression markedly inhibited ESCC cell metastasis by affecting
the EMT process.
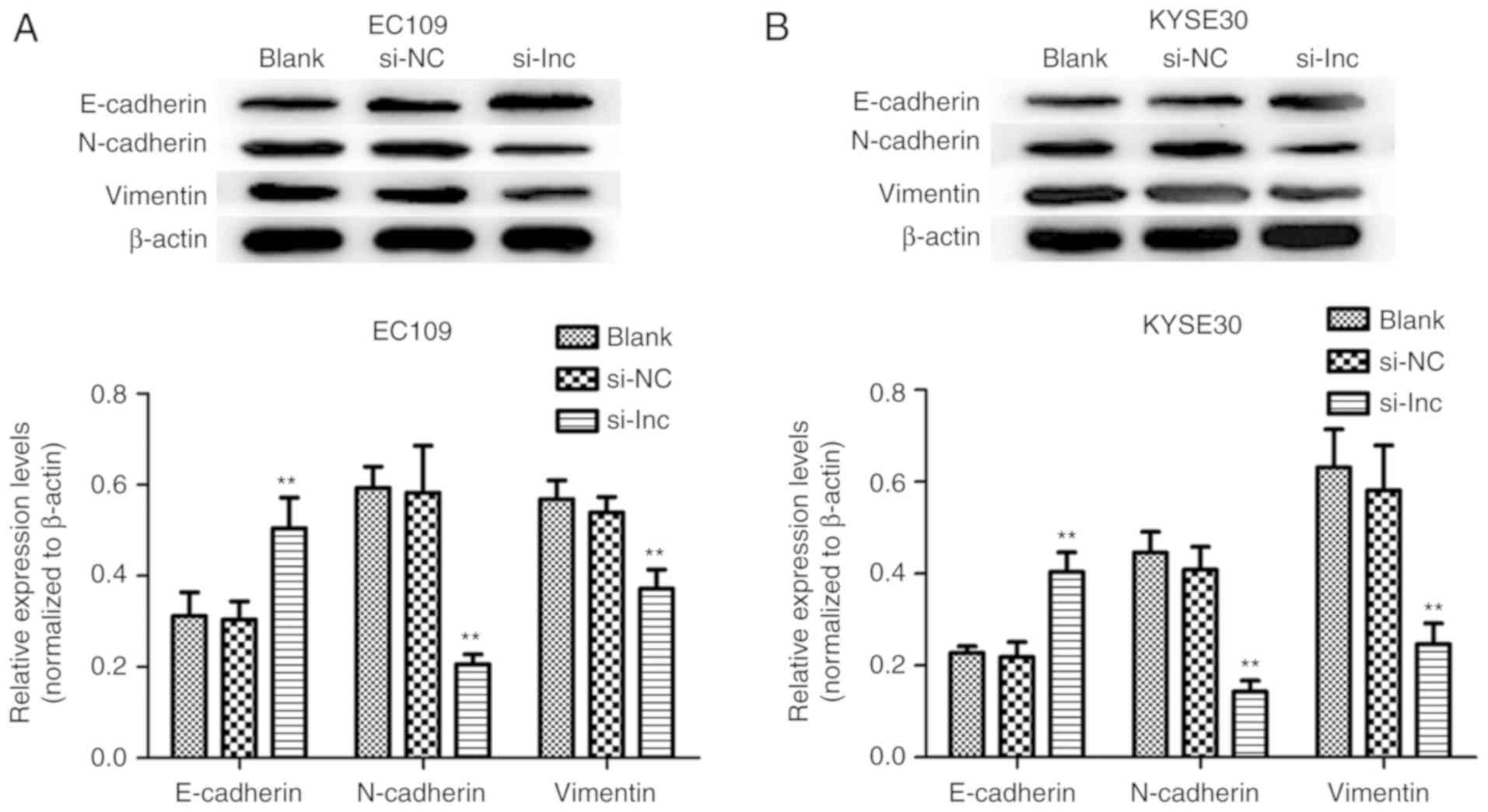 | Figure 5.Effect of the expression of DLX6-AS1
on the regulation of EMT in ESCC. The expression levels EMT markers
(E-cadherin, N-cadherin, and Vimentin) in (A) EC109 and (B) KYSE30
cells transfected with si-lnc, si-NC or Blank at 48 h were analyzed
by western blotting. Knockdown of DLX6-AS1 significantly enhanced
the expression of E-cadherin, and suppressed the expression of
N-cadherin and Vimentin in EC109 and KYSE30 cells in the si-lnc
groups. **P<0.01 compared with the si-NC groups. β-Actin was
used as a loading control. Data are presented as the mean ±
standard deviation. si-NC, nonsense small interfering RNA negative
control; si-lnc, DLX6-AS1 siRNA; DLX6-AS1, distal-less homeobox 6
antisense RNA 1; E, epithelial; N, neural; EMT,
epithelial-mesenchymal transition; Blank, non-transfected. |
Discussion
Genome-wide analysis has demonstrated that
non-protein coding RNAs occupy >90% of the human genome's
transcriptional output in genome-wide analysis (18); in addition, the detailed molecular
mechanisms for the majority of lncRNAs have yet to be elucidated.
Accumulating evidence has illustrated that lncRNAs serve essential
roles during gene regulatory processes and are involved in a number
of biological functions, including cell proliferation and
differentiation in normal and transformed cells (19,20).
Emerging evidence has further demonstrated that dysregulation of
lncRNAs is involved in epigenetic regulatory mechanisms underlying
physiological and pathological processes; therefore, lncRNAs serve
as novel regulators in progressive and uncontrolled tumor growth,
EMT processes and metastasis (12,21,22).
It is essential to investigate tumor-associated lncRNAs in order to
recognize their complex intermolecular interactions in
carcinogenesis, specifically esophageal cancer. Rapid growth and
high incidence of regional and distant metastasis of aggressive
tumors contributes to the poor prognosis of ESCC patients, despite
rapid advances in the diagnosis and treatment of the disease
(23). A clear understanding of
the critical mechanisms involved in the genesis and development of
ESCC is required in order to improve the survival rates of ESCC
patients. Certain investigators have demonstrated that the abnormal
expression of certain lncRNAs, including lncRNAs HOX transcript
antisense RNA (19), POU class 3
homeobox 3 (24) and
SPRY4-intronic transcript 1 (11),
is associated with the progression of ESCC. Therefore, focusing on
specific lncRNAs, which function clinically as tumor promoters or
suppressors during the progression of ESCC with biomarker
applications, can help to improve the diagnosis and treatment of
ESCC. The potential role of DLX6-AS1 in the clinical prognostic
prediction of ESCC focuses attention on its underlying mechanisms
in ESCC.
Recent studies have illustrated that DLX6-AS1 can
act as a positive prognostic marker for the diagnosis of various
types of cancer, including lung adenocarcinoma, hepatocellular
carcinoma and renal cell carcinoma (13–15).
The overexpression of DLX6-AS1 contributes to the metastasis and
invasion of lung adenocarcinoma cells and is positively associated
with the carcinogenesis, advanced histological differentiation and
TNM stage of lung adenocarcinoma (13). Abundance of DLX6-AS1 also promoted
the growth, invasion and migration of hepatocellular carcinoma
cells in in vivo and in vitro assays and effectively
predicted the poor prognosis of hepatocellular carcinoma patients
(15). Its expression is also
elevated in renal cell carcinoma samples and associated with the
progression and metastasis of renal cell carcinoma, indicating that
DLX6-AS1 be an oncogenic agent in renal cell carcinoma (14). However, the underlying mechanism
and role of DLX6-AS1 in ESCC remains uninvestigated. Based on these
associated studies, preliminary functional experiments were
performed to investigate the expression levels and working
mechanisms of DLX6-AS1 in ESCC.
The results of the present study confirmed that the
expression of DLX6-AS1 significantly increased in the majority of
ESCC tissues and cell lines when compared with the adjacent normal
tissue. Aberrant DLX6-AS1 expression was also positively associated
with the clinical prognostic factors of ESCC. To further understand
the roles of DLX6-AS1 in ESCC metastasis and progression,
loss-of-function assays were conducted to suppress the expression
of DLX6-AS1 in EC109 and KYSE30 cells with the help of specifically
designed siRNA. Knockdown of DLX6-AS1 resulted in the inhibition of
the proliferative, migratory and invasive capabilities of the ESCC
cell lines, and induced apoptosis in vitro in the two ESCC
cell lines compared with the control. Previous studies have
demonstrated that cancer cells become motile and invasive via the
EMT process by losing the epithelial phenotype and acquiring
mesenchymal properties, which is associated with invasion and
metastasis in many types of cancer, including ESCC (25,26).
EMT, in particular, has been associated with metastasis and
mortality in ESCC patients, and is regulated by several lncRNAs
(11,22,27).
However, DLX6-AS1′s function in EMT has not been demonstrated until
now. In the present study, the abnormal expression of DLX6-AS1
regulated the expression levels of EMT-induced markers (E-cadherin,
N-cadherin, and Vimentin proteins), suggesting that targeted
silencing of DLX6-AS1 impacts the invasive and metastatic phenotype
of ESCC cells, partly by limiting the EMT process. Therefore, based
on the results of previous studies and the results of the present
study it was hypothesized that DLX6-AS1 probably functions as an
oncogene while modulating ESCC pathogenesis.
The results of the present study confirmed the
association between DLX6-AS1 expression, clinical characteristics
of patients, and the proliferation and invasion of ESCC. Previous
studies have stated that DLX6-AS1 expression promotes tumorigenesis
and progression in hepatocellular carcinoma by regulating the
miR-203a/matrix metalloproteinase-2 pathway and renal cell
carcinoma by modulating miR-26a/phosphatase and tensin homolog axis
(12,13). The present study was limited to
fully elucidating the exact molecular mechanisms underlying
DLX6-AS1-mediated gene regulation. Due to the diversity and
complexity of the regulatory mechanisms of DLX6-AS1 in ESCC,
further experiments are warranted to elucidate which signaling
pathways are involved in the regulatory mechanism of DLX6-AS1 to
expand the understanding of the effect of the high expression of
DLX6-AS1 on the malignancy of ESCC in vitro and in
vivo.
Overall, the present study demonstrated a higher
prevalence of DLX6-AS1 overexpression in ESCC tissues when compared
with the paired noncancerous tissues, suggesting that it could
facilitate ESCC development and progression. Furthermore, the study
also demonstrated that knockdown of DLX6-AS1 inhibited the growth
and invasive abilities of the ESCC cell lines by promoting
apoptosis and disrupting the EMT processes in vitro.
Therefore, these results provide insight into the role of DLX6-AS1
as a potential therapeutic target in ESCC treatments. The
application of DLX6-AS1 as a potential diagnostic and therapeutic
target for ESCC progression and the determination of its precise
mechanism of action should be further investigated in future
studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Support Plan of Science and Technology Innovation Team in
Universities of Henan Province (grant no. 18IRTSTHN029).
Availability of data and materials
The datasets generated during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
SZ and MW conceived the study and participated in
its design and coordination. YL performed the experiments and
contributed to data collection. YY, XL and WY analyzed the data and
drafted the manuscript. MZ assisted in designing experiments. YBL
and KY provided technical expertise in conducting experiments. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from each
patient prior to using the clinical samples for research purposes.
The Ethics Committee of Zhengzhou University approved the study
protocol (Henan, China).
Patient consent for publication
All patients agreed the publication of the
research.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sugihara H, Ishimoto T, Miyake K, Izumi D,
Baba Y, Yoshida N, Watanabe M and Baba H: Noncoding RNA expression
aberration is associated with cancer progression and is a potential
biomarker in esophageal squamous cell carcinoma. Int J Mol Sci.
16:27824–27834. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin CY and Xu HM: Novel perspectives of
long non-coding RNAs in esophageal carcinoma. Carcinogenesis.
36:1255–1262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Holmes RS and Vaughan TL: Epidemiology and
pathogenesis of esophageal cancer. Semin Radiat Oncol. 17:2–9.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sjoquist KM, Burmeister BH, Smithers BM,
Zalcberg JR, Simes RJ, Barbour A and Gebski V; Australasian
Gastro-Intestinal Trials Group, : Survival after neoadjuvant
chemotherapy or chemoradiotherapy for resectable oesophageal
carcinoma: An updated meta-analysis. Lancet Oncol. 12:681–692.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen WJ, Zhang F, Zhao X and Xu J: LncRNAs
and esophageal squamous cell carcinoma-implications for
pathogenesis and drug development. J Cancer. 7:1258–1264. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsai MC, Manor O, Wan Y, Mosammaparast N,
Wang JK, Lan F, Shi Y, Segal E and Chang HY: Long noncoding RNA as
modular scaffold of histone modification complexes. Science.
329:689–693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gutschner T and Diederichs S: The
hallmarks of cancer: A long non-coding RNA point of view. RNA Biol.
9:703–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang C, Yuan N, Wu L, Wang X, Dai J, Song
P, Li F, Xu C and Zhao X: An integrated analysis for long noncoding
RNAs and microRNAs with the mediated competing endogenous RNA
network in papillary renal cell carcinoma. Onco Targets Ther.
10:4037–4050. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cui F, Wu D, He X, Wang W, Xi J and Wang
M: Long noncoding RNA SPRY4-IT1 promotes esophageal squamous cell
carcinoma cell proliferation, invasion, and epithelial-mesenchymal
transition. Tumour Biol. 37:10871–10876. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao GL, Pan CF, Xu H, Wei K, Liu B, Zhai R
and Chen YJ: Long noncoding RNA RP11-766N7.4 functions as a tumor
suppressor by regulating epithelial-mesenchymal transition in
esophageal squamous cell carcinoma. Biomed Pharmacother.
88:778–785. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Li P, Zhao W, Yang R, Chen S, Bai Y,
Dun S, Chen X, Du Y, Wang Y, et al: Expression of long non-coding
RNA DLX6-AS1 in lung adenocarcinoma. Cancer Cell Int. 15:482015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zeng X, Hu Z, Ke X, Tang H, Wu B, Wei X
and Liu Z: Long noncoding RNA DLX6-AS1 promotes renal cell
carcinoma progression via miR-26a/PTEN axis. Cell Cycle.
16:2212–2219. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang L, He X, Jin T, Gang L and Jin Z:
Long non-coding RNA DLX6-AS1 aggravates hepatocellular carcinoma
carcinogenesis by modulating miR-203a/MMP-2 pathway. Biomed
Pharmacother. 96:884–891. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rinn JL and Chang HY: Genome regulation by
long noncoding RNAs. Annu Rev Biochem. 81:145–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ge XS, Ma HJ, Zheng XH, Ruan HL, Liao XY,
Xue WQ, Chen YB, Zhang Y and Jia WH: HOTAIR, a prognostic factor in
esophageal squamous cell carcinoma, inhibits WIF-1 expression and
activates Wnt pathway. Cancer Sci. 104:1675–1682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu W, Zhuang P, Song W, Duan S, Xu Q,
Peng M and Zhou J: Knockdown of lncRNA HNF1A-AS1 inhibits oncogenic
phenotypes in colorectal carcinoma. Mol Med Rep. 16:4694–4700.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hauptman N and Glavac D: Long non-coding
RNA in cancer. Int J Mol Sci. 14:4655–4669. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen X, Han H, Li Y, Zhang Q, Mo K and
Chen S: Upregulation of long noncoding RNA HOTTIP promotes
metastasis of esophageal squamous cell carcinoma via induction of
EMT. Oncotarget. 7:84480–84485. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deng HY, Wang YC, Ni PZ, Lin YD and Chen
LQ: Long noncoding RNAs are novel potential prognostic biomarkers
for esophageal squamous cell carcinoma: An overview. J Thorac Dis.
8:E653–E659. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tong YS, Wang XW, Zhou XL, Liu ZH, Yang
TX, Shi WH, Xie HW, Lv J, Wu QQ and Cao XF: Identification of the
long non-coding RNA POU3F3 in plasma as a novel biomarker for
diagnosis of esophageal squamous cell carcinoma. Mol Cancer.
14:32015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng X, Hu H and Li S: High expression of
lncRNA PVT1 promotes invasion by inducing epithelial-to-mesenchymal
transition in esophageal cancer. Oncol Lett. 12:2357–2362. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang C, Cao L, Qiu L, Dai X, Ma L, Zhou
Y, Li H, Gao M, Li W, Zhang Q, et al: Upregulation of H19 promotes
invasion and induces epithelial-to-mesenchymal transition in
esophageal cancer. Oncol Lett. 10:291–296. 2015. View Article : Google Scholar : PubMed/NCBI
|