Introduction
Breast cancer is one of the most common types of
cancer, accounting for 7–10% of systemic malignancies, and is a
serious health threat to women worldwide (1). Surgery and chemotherapy may decrease
the risk of local recurrence or non-localized breast cancer
(2). However, local and regional
treatment outcomes remain unsatisfactory due to the lack of
specific molecular markers and therapeutic targets (3). Therefore, understanding the molecular
mechanisms of breast cancer may improve diagnoses and clinical
outcomes.
Inflammasomes are multiprotein complexes regulating
various inflammatory factors, including interleukin-1β (IL-1β) and
interleukin-18 (IL-18). IL-18 induces programmed cell death protein
1-dependent immunosuppression in cancer (4), and IL-1β is one of the most important
inflammatory mediators eliciting immunosuppressive properties
(5). Therefore, these cytokines
serve important roles in tumorigenesis. NACHT, LRR and PYD
domains-containing protein 3 (NLRP3) is the most widely studied
inflammasome. A previous study demonstrated that activated NLRP3
significantly promoted the protein expression level of IL-18 in the
plasma of patients with lymphoma (6). Furthermore, it was identified that
the activation of caspase-1 may trigger the maturation and
secretion of IL-18 (7).
Additionally, a previous study observed that the NLRP3 inflammasome
components were upregulated in biopsies from head and neck squamous
cell carcinoma tissues, suggesting that the NLRP3 inflammasome may
serve crucial functions in the survival and invasion of human head
and neck squamous cell carcinoma (8). However, the role of NLRP3
inflammasome activation in breast cancer and its underlying
mechanism of action remain unclear.
MicroRNAs (miRNAs) regulate a variety of important
biological processes in cells, including cell proliferation,
differentiation and invasion (9).
Furthermore, dysregulation of miRNAs may contribute to cancer
formation (10). miRNA-107 was
associated with breast cancer progression and negatively regulated
the expression of cyclin dependent kinase 8 inhibiting the
proliferation of breast cancer cells in vitro (11). Additionally, a previous study
demonstrated that miRNA-223-3p (miR-233) was involved in embryo
implantation by suppressing pinopode formation and leukemia
inhibitory factor protein expression (12). Furthermore, miR-233 downregulated
the nuclear factor κ-light-chain-enhancer of activated B cells
signaling to attenuate neutrophilic inflammation (13). These previous studies suggested
that miR-233 may balance the inflammatory response.
The present study suggested that miR-233 suppressed
the growth of breast cancer cells via a mechanism associated with
the inactivation of the NLRP3 inflammasome. The present results
provided novel insight into the molecular functions of miR-233 in
regulating the NLRP3 inflammasome in breast cancer cells.
Materials and methods
Cell culture and RNA transfection
HMEC, MDA-MB231, MCF-7 and SKBR3 cell lines were
purchased from the Type Culture Collection of The Chinese Academy
of Sciences (Bena Culture Collection, Shanghai, China). MDA-MB231
and SKBR3 cell lines were maintained in RPMI-1640 medium
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) containing 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with 100 mg/ml streptomycin and 100 U/ml
penicillin. MCF-7 cell lines and HMEC cells were cultured in
Dulbecco's modified Eagle's medium (Thermo Fisher Scientific, Inc.)
containing 10% FBS, 100 mg/ml streptomycin and 100 U/ml penicillin.
Cells were passaged with PBS (Sigma-Aldrich; Merck KGaA) and 0.02%
EDTA/0.5% trypsin (GE Healthcare, Chicago, IL, USA) every 3 days.
All cells were cultured with 5% CO2 at 37°C until
further experiments were performed.
The short hairpin (sh)-NLRP3, miRNA mimics,
inhibitors and the scrambled negative control oligonucleotides were
purchased from Guangzhou RiboBio Co., Ltd. (Guangzhou, China) and
1,000 ng/µl used for the transfection of MCF-7 cells with
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Transfection was performed with
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. In
total, 2×104 cells/well were seeded into six wells and
each sample was transfected with 0.2 mg RNA. Following
transfection, cells were incubated at 37°C with 5% CO2
for 24 or 48 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
miRNA was extracted from cells using the miRNeasy
FFPE kit (Qiagen China Co., Ltd., Shanghai, China). Primers for
miR-233 (cat. no. HmiRQP0339;
ATATAGCATCTTTCTGTCTCGCCCATCCCGTTGCTCCAATATTCTAACAACAAGTGATTATTGAGCAATGCGCATGTGCGGGATAGACTGATGGCTGC)
were obtained from GeneCopoeia, Inc. (Rockville, MD, USA). NLRP3
primers were forward, 5′-AGACCTCCAAGACCACTAC-3′ and reverse,
5′-ACATAGCAGCGAAGAACTC-3′. Total RNA was extracted from the cells
using RNeasy Mini kit (Qiagen, Hilden, Germany) according to the
manufacturer's protocol. The extracted RNA was reverse transcribed
into cDNA using the ThermoScript™ RT-PCR system at 40°C
for 2 h (Invitrogen; Thermo Fisher Scientific, Inc.). RT-qPCR was
performed using the 7500 Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.) using appropriate primers and the
fluorescent dye SYBR Green (Takara Biotechnology Co., Ltd., Dalian,
China). For quantification of mature miRNA, cDNA was generated
using specific stem-loop universal primers. PCR reaction mixtures
were set up in a total volume of 20 µl. Standard PCR settings (95°C
for 30 sec, 40 cycles of 95°C for 5 sec and 60°C for 34 sec,
followed by a dissociation stage for 15 sec at 95°C, 1 min at 60°C
and 15 sec at 95°C) were used. All samples were run in duplicates.
GAPDH was selected as the normalization control gene. The same
RT-qPCR protocol was used for all the genes and miRNA analyzed.
Results are expressed as fold change with respect to the
experimental control. Quantitation was according to
R=(1+E1)∆Ct1 (Control-Sample)/(1+E2)∆Ct2
(Control-Sample) method (14).
Western blot analysis
Cells and tissues were homogenized in
radioimmunoprecipitation assay buffer (Thermo Fisher Scientific,
Inc.). The protein concentration was determined using a
Bicinchoninic Acid protein assay kit (Thermo Fisher Scientific,
Inc.), and 50 µg protein was loaded per lane with 5% concentrated
SDS gels and 15% separating gel. Following electrophoresis, the gel
was transferred onto a 0.45 µm polyvinylidene fluoride membrane
(Merck KGaA). Following transfer, the membranes were blocked for 2
h with 5% nonfat dry milk at room tempreture. Subsequently, the
membranes were incubated overnight at 4°C with anti-NLRP3 (15101;
1:500), anti-proliferation marker protein Ki67 (9129; 1:1,000;
Ki67; both CST Biological Reagents Co., Ltd., Shanghai, China),
anti-vascular endothelial growth factor (VEGF; ab32152; 1:500
Abcam, Cambridge, UK), anti-PYD and CARD domain-containing protein
(ASC; sc-271054; 1:500; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), anti-IL-1β (ab200478; 1:500), anti-IL-18 (ab71495; 1:500;
both Abcam) and anti-GAPDH (35174.; 1:1,000) primary antibodies,
followed by incubation with secondary antibody conjugated to
horseradish peroxidase (7074; 1:1,000; both CST Biological Reagents
Co., Ltd.) at room tempreture for 2 h. Following washing with TBS,
proteins were visualized using enhanced chemiluminescence reagents
Rabbit IgG (Pierce; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. ImageJ (version number: 1.4.3.67;
National Institutes of Health, Bethesda, MD, USA) was used for
densiometric analysis.
Hoechst staining
Coverslips were soaked in 70% ethanol for 5 min, the
cells were washed three times with 0.9% NaCl and the coverslips
were placed in 6-well plates and incubated at 37°C with 5%
CO2 overnight. Cells were stimulated to undergo
apoptosis by 100 µM H2O2 for 1 h.
Subsequently, the culture solution was discarded and 0.5 ml 4%
paraformaldehyde fixative solution was added for 10 min at 37°C.
The fixative was removed and cells were washed twice with 0.9% NaCl
for 3 min, and the washing solution was discarded. A total of 0.5
ml Hoechst 33258 staining solution was added and cells were stained
for 5 min at 37°C. The slides were sealed with antifluorescence
quenching sealant, and imaged using fluorescence microscopy for 20
fields of view and the analysis were used for Image J version 1.8.0
(National Institutes of Health).
Transwell assay
To analyze cell migration, 2×105 cells
were seeded in six-well plates in triplicate. A Transwell chamber
was placed into the culture plate, with the upper chamber
containing the upper culture medium (DMEM) and the lower chamber
containing the lower culture medium (DMEM). The upper and lower
culture media were separated by a polycarbonate membrane. Following
7 days, cells were plated in the upper chamber and the growth and
migration of cells in the lower culture medium. The cells were
stained with 0.1% crystal violet for 20 min at room temperature and
the unmigrated cells in the upper layer were gently wiped off with
a cotton swab and washed 3 times with PBS and using a light
microscope with magnification, ×10. All experiments were performed
in triplicates and error bars indicate the standard deviation.
Cell proliferation assay
A cell proliferation assay was performed using a
Cell Counting kit-8 (CCK-8). A total of 2×105 cells were
plated into 96-well culture plates. Following 24 h incubation,
CCK-8 solution was added to each well and incubated for 4 h (1
mg/ml; Sigma-Aldrich; Merck KGaA) at 37°C with 5% CO2.
The absorbance was measured at 450 nm. Each experiment was
performed at least three times, each with triplicate samples.
Luciferase reporter assay
Association between miR-223 and NLRP3 was predicted
using the online software Targetscan (http://www.targetscan.org/vert_71). NLRP3 3′
untranslated region (3′UTR) wild-type (WT)
5′-CGCUAUCUUUCUAUUAACUGACC-3′, was cloned downstream of the
Renilla luciferase gene (pLUC-REPORT vector; Promega
Corporation, Madison, WI, USA). A pLUC-REPORT construct containing
a mutant (Mut) NLRP3 3′UTR sequence corresponding to the miR-233-3p
binding site was generated using the following sequence:
5′-CGCUAUCUUUCUAUUUUCUCUCC-3′. For the luciferase reporter assay,
MCF-7 cells were cotransfected using Lipofectamine® 2000
(Thermo Fisher Scientific, Inc.) with a luciferase reporter vector
containing the WT or Mut version of NLRP3 and the miR-233-3p mimics
plasmid and the pEZX-MR04 plasmid containing miR-233-3p precursor
construct or its scrambled control equivalent pEZX-MR04 with 2.5
µg/µl (GeneCopoeia, Inc.). Luciferase activity was measured at 48 h
following transfection using a dual-luciferase assay kit (Promega
Corporation) and normalized to firefly luciferase activity. A total
of three independent experiments were performed in triplicate.
Ethics approval
The animal experiments in the present study were
approved by The Animal Care and Research Committee of Beijing
Tiantan Hospital (Beijing, China). All experiments were performed
in compliance with relevant laws and guidelines. All experiments
were conducted following the institutional guidelines of Beijing
Tiantan Hospital.
Tumor volume and survival curve
The total of 100 female mice were purchased from The
Institute of Zoology, Chinese Academy of Medical Sciences (Bejing,
China). The animals were 8 weeks old and 20–22 g in weight. The
mice were kept in clean rooms at a temperature of ~25°C with a
humidity of 75%, 12-h light/dark cycle and food and water ad
libitum. A total of 3×106 MCF-7 cells transfected
with miR-223 mimics were injected subcutaneously into the shoulder
scapula (n=5) of the nude mice and the tumor volume was assessed by
the formula: Tumor volume (mm3) = maximal length (mm) ×
perpendicular width (mm)2/2 every 5 days. All mice were
sacrificed following measurements. The number of surviving nude
mice was measured and survival curves were calculated.
Immunohistochemistry
Sections of 5 µm in thickness were prepared using
tissue blocks embedded in paraffin and fixed using 4% formaldehyde
overnight at room tempreture, followed by deparaffinization and
hydration using xylene and graded alcohol series. The sections were
treated with a sodium citrate buffer in a microwave for antigen
retrieval and blocked using normal goat serum. Then washed 3 times
with PBS for 5 min each time. Subsequently, sections were stained
using rabbit anti-terminal deoxynucleotidyl transferase (1:100),
anti-Ki67 (1:100) or anti-VEGF (1:100; all Abcam) overnight at 4°C,
and subsequently incubated with a biotinylated goat anti-rabbit
immunoglobulin G secondary antibody for 1 h, followed by staining
with an avidin-biotin peroxidase complex (GeneTex, Inc., Irvine,
CA, USA).
ELISA
Blood samples were collected by enucleation.
Subsequently, samples were kept at room temperature for 2 h and
centrifuged at 2,000 × g for 30 min with 25°C. Subsequently, serum
was transferred into 1.5 ml polypropylene tubes, and stored at
−20°C. Protein expression levels of IL-1β, IL-18 and IL-10 in the
serum were determined using an ELISA kit (Yinggong Corporation,
Shanghai, China) according to the manufacturer's protocol, at 450
nm.
Statistical analysis
SPSS (version 22.0; IBM Corp., Armonk, NY, USA)
software was used for statistical analysis. Data obtained from
experiments using cultured cells are presented as the mean ±
standard deviation. Statistical analysis of normal distribution was
performed on two independent samples. Differential expression of
miRNAs was detected by t-test or one-way analysis of variance
(ANOVA). Survival curves were analyzed using Kaplan-Meier method
and log-rank test. One-way analysis of variance was followed by a
post hoc test for difference value calculation, and Mann-Whitney U
tests were performed to determine statistical differences in
viability between miR-233- and scramble-transfected cells in case
of non-normal distributions. Qualitative data were representative
of more than three independent experiments, with each performed in
triplicate. P<0.05 was considered to indicate a statistically
significant difference.
Results
Transfection of sh-NLRP3 affects the
expression of NLRP3 in MCF-7 cells
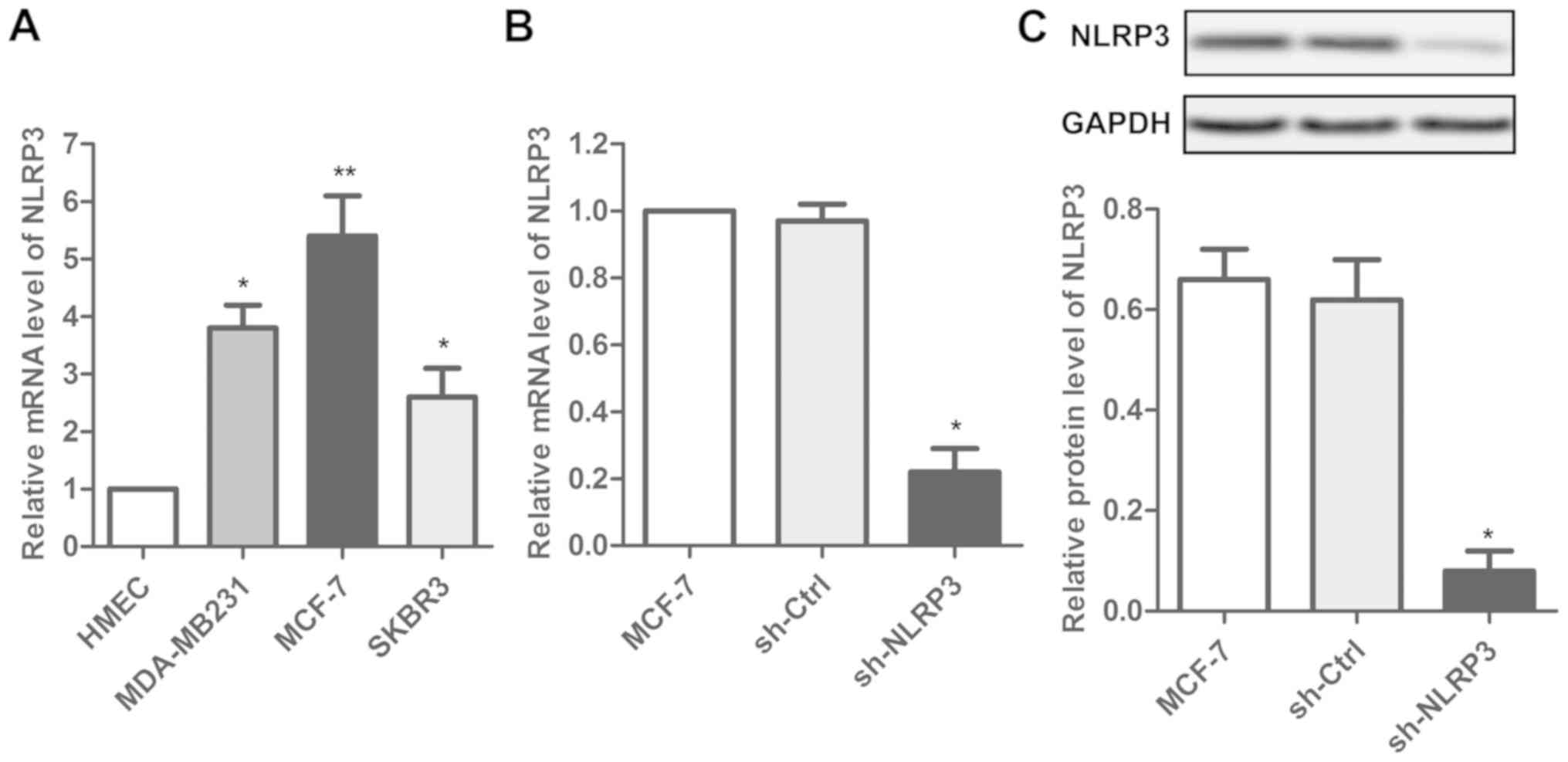
NLRP3 expression levels were measured in three
breast cancer cell lines (MDA-MB231, MCF-7 and SKBR3) and normal
mammary epithelial cells (HMEC). An increase in the NLRP3
expression level was observed in the breast cancer cells compared
with HMEC (Fig. 1A). Among the
three cancer cell lines, the highest expression level of NLRP3 was
measured in MCF-7 cells. Subsequently, the effect of sh-NLRP3 on
MCF-7 cells was investigated. Transfection with sh-NLRP3 decreased
the NLRP3 expression level in MCF-7 cell lines, suggesting that
transfection with sh-NLRP3 affected the expression of NLRP3 in
MCF-7 cell lines (Fig. 1B and
C).
sh-NLRP3 inhibits human breast cancer
MCF-7 cell growth and migration in vitro
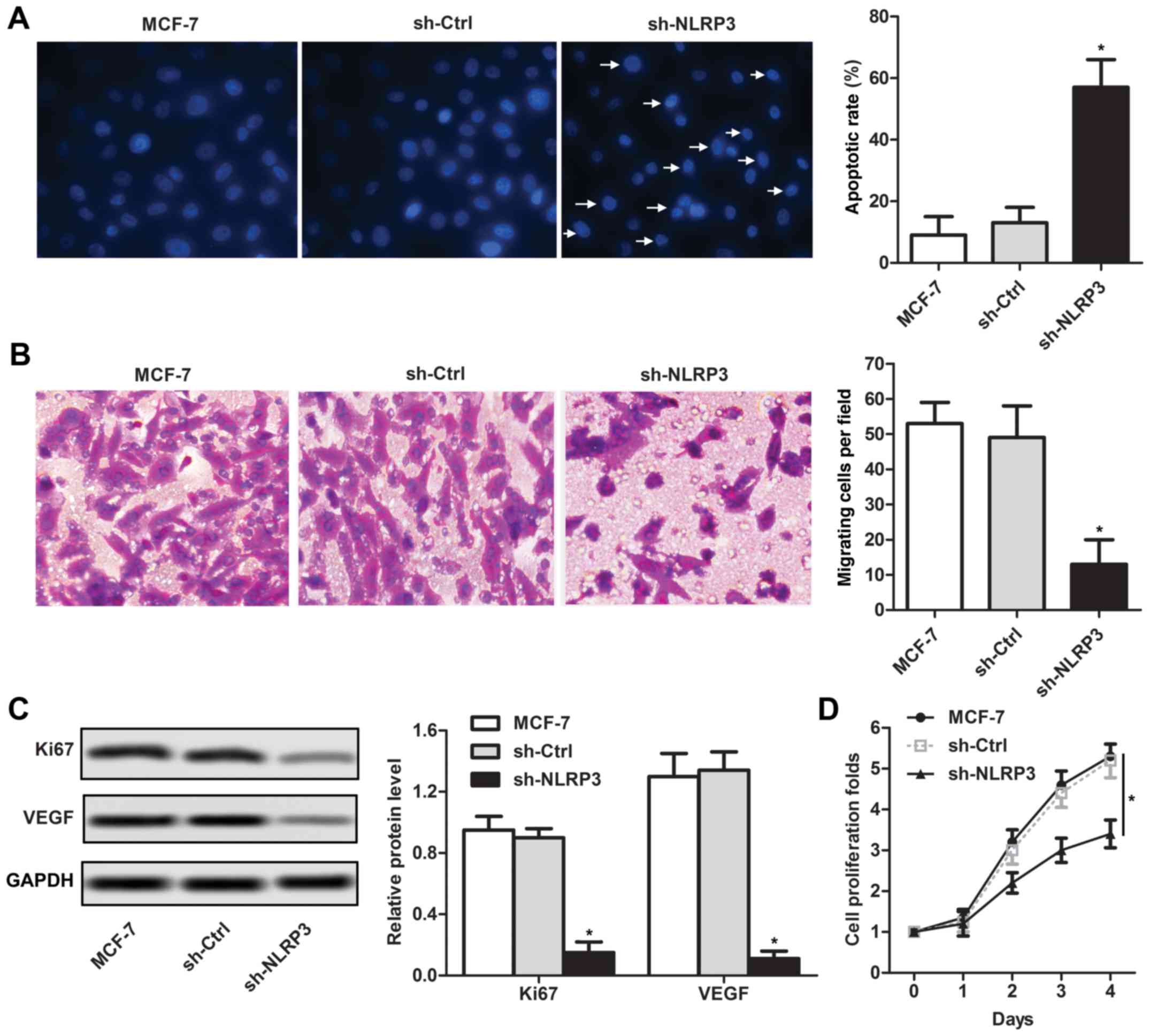
Subsequently, the effects of sh-NLRP3 on cell
function were investigated in breast cancer cells. The percentage
of apoptotic cells following sh-NLRP3 transfection was
significantly higher compared with the MCF-7 control group
(Fig. 2A). The Transwell analysis
suggested that the migratory ability following sh-NLRP3
transfection was significantly lower compared with the MCF-7
control group (Fig. 2B). Decreased
protein expression levels of Ki67 and VEGF were detected in the
sh-NLRP3 group compared with the MCF-7 control group, by western
blotting (Fig. 2C). To further
investigate whether cell proliferation was affected following
transfection with sh-NLRP3, a CCK-8 assay was conducted. sh-NLRP3
transfection significantly decreased the cell proliferation rate in
MCF-7 cell lines at 4 days (Fig.
2D). The present data suggested that sh-NLRP3 inhibited the
growth and migration of MCF-7 cells in vitro.
miR-233 and NLRP3 are inversely
correlated in human breast cancer cells
The physical association between miR-223 and NLRP3
was predicted by online software Targetscan. The expression of
miR-223 was detected using RT-qPCR in MCF-7 cells transfected with
miR-223 mimics or miR-223 inhibitor. The present results
demonstrated that miR-223 was significantly increased in the
miR-223 mimics group and was significantly decreased following
miR-223 inhibitor transfection compared with the MCF-7 control
(Fig. 3A). Subsequently, the
effect of miR-223 mimics alone or in combination with pcDNA-NLRP3
on the expression level of NLRP3 was examined. The present results
suggested that the upregulation of NLRP3 following NLRP3
overexpression was attenuated by cotransfecting miR-223 mimics
(Fig. 3B). Furthermore, the
upregulation of NLRP3 following miR-223 inhibitor transfection was
inhibited by sh-NLRP3 (Fig. 3C).
The present results suggested that miR-223 and NLRP3 are inversely
associated in human breast cancer cells. Additionally, the effects
of miR-223 mimics on the luciferase activity of a reporter plasmid
containing NLRP3 WT 3′UTR or NLRP3 Mut 3′UTR were detected by a
luciferase assay. The present results suggested that miR-223 mimics
decreased the NLRP3 expression level only when cotransfected with
NLRP3 WT and not with NLRP3 Mut (Fig.
3D and E).
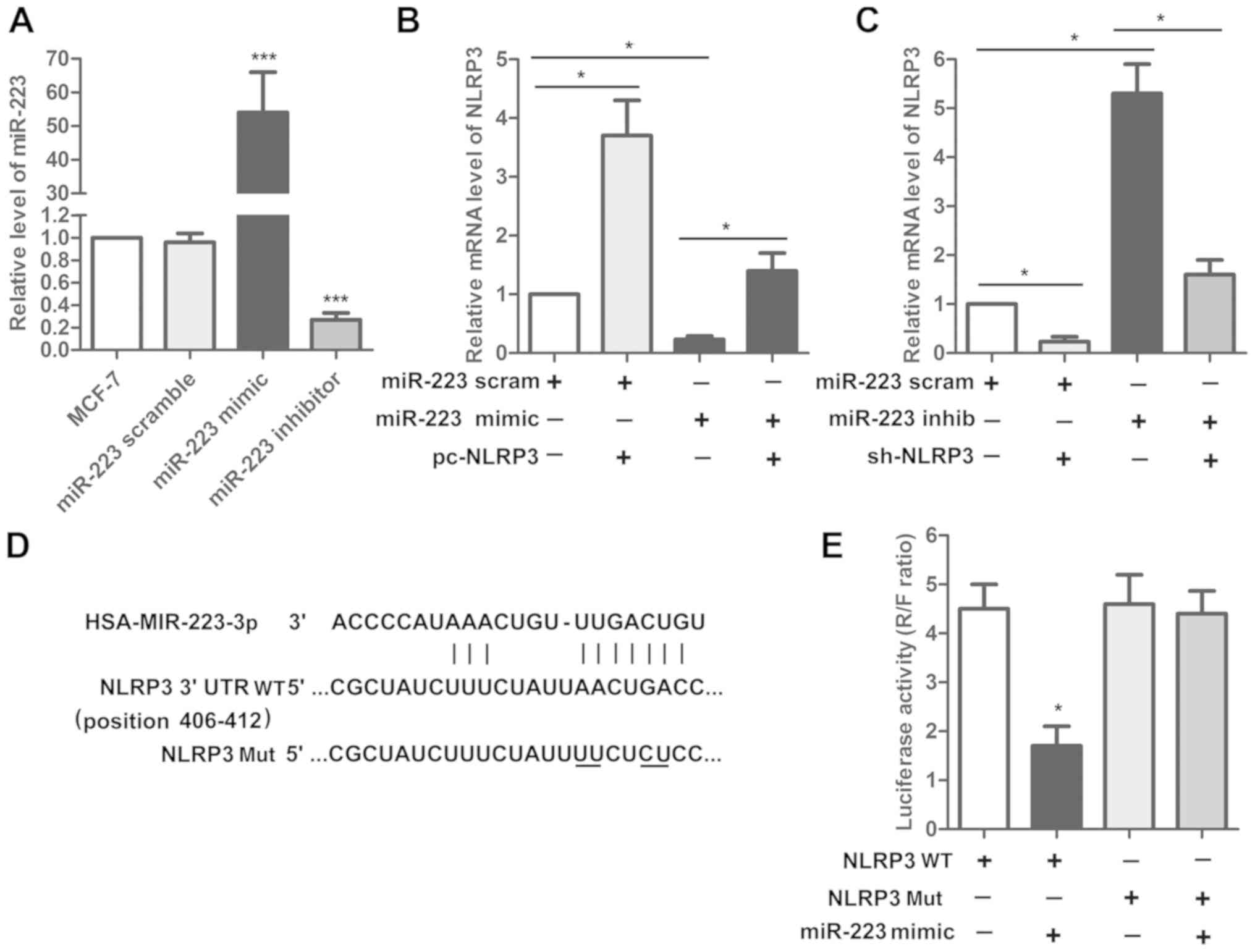 | Figure 3.miR-223 and NLRP3 are inversely
associated in human breast cancer cells. (A) Expression of miR-223
was measured by RT-qPCR. ***P<0.001 vs. MCF-7. (B) Effect of
miR-223 mimics alone and in combination with pcDNA-NLRP3 on NLRP3
expression was measured by RT-qPCR. (C) Effect of miR-223 inhibitor
alone and in combination with sh-NLRP3 on NLRP3 expression was
detected by RT-qPCR. *P<0.05. (D) Association between
miR-223 and NLRP3 was predicted using the online software
Targetscan (http://www.targetscan.org/vert_71). (E) Effect of
miR-223 mimics on NLRP3 WT and NLRP3 Mut was detected by a
luciferase assay. *P<0.05 vs. NLRP3 WT. miR, microRNA; NLRP3,
NACHT, LRR and PYD domains-containing protein 3; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; sh, short
hairpin; WT, wild-type; Mut, mutant; 3′UTR, 3′ untranslated region;
scram, scramble; inhib, inhibitor. |
miR-233 suppresses the growth,
migration and NLRP3 inflammasome activity in human breast cancer
cells by inhibiting the NLRP3 pathway
To further investigate the association between
miR-233 and NLRP3 in human breast cancer cells, NLRP3 was
overexpressed by transfecting cells with a pcDNA vector containing
NLRP3. The present results suggested that NLRP3 promoteed the
growth of breast cancer cells, while miR-233 could inhibit its
proliferation at the tissue level (Fig. 4A). Similarly, at the protein level,
overexpression of NLRP3 and miR-233 scrambled upregulated the
expression of Ki67 and VEGF, indicating the downregulation of NLRP3
by miR-233 (Fig. 4B). NLRP3
overexpression attenuated the inhibitory effect of miR-223 mimics
on cell growth (Fig. 4A-C).
Subsequently, the protein expression levels of ASC, IL-1β and IL-18
were investigated. The present results suggested that the increased
ASC, IL-1β and IL-18 protein expression levels induced by NLRP3
overexpression were decreased by miR-223 mimics (Fig. 4D).
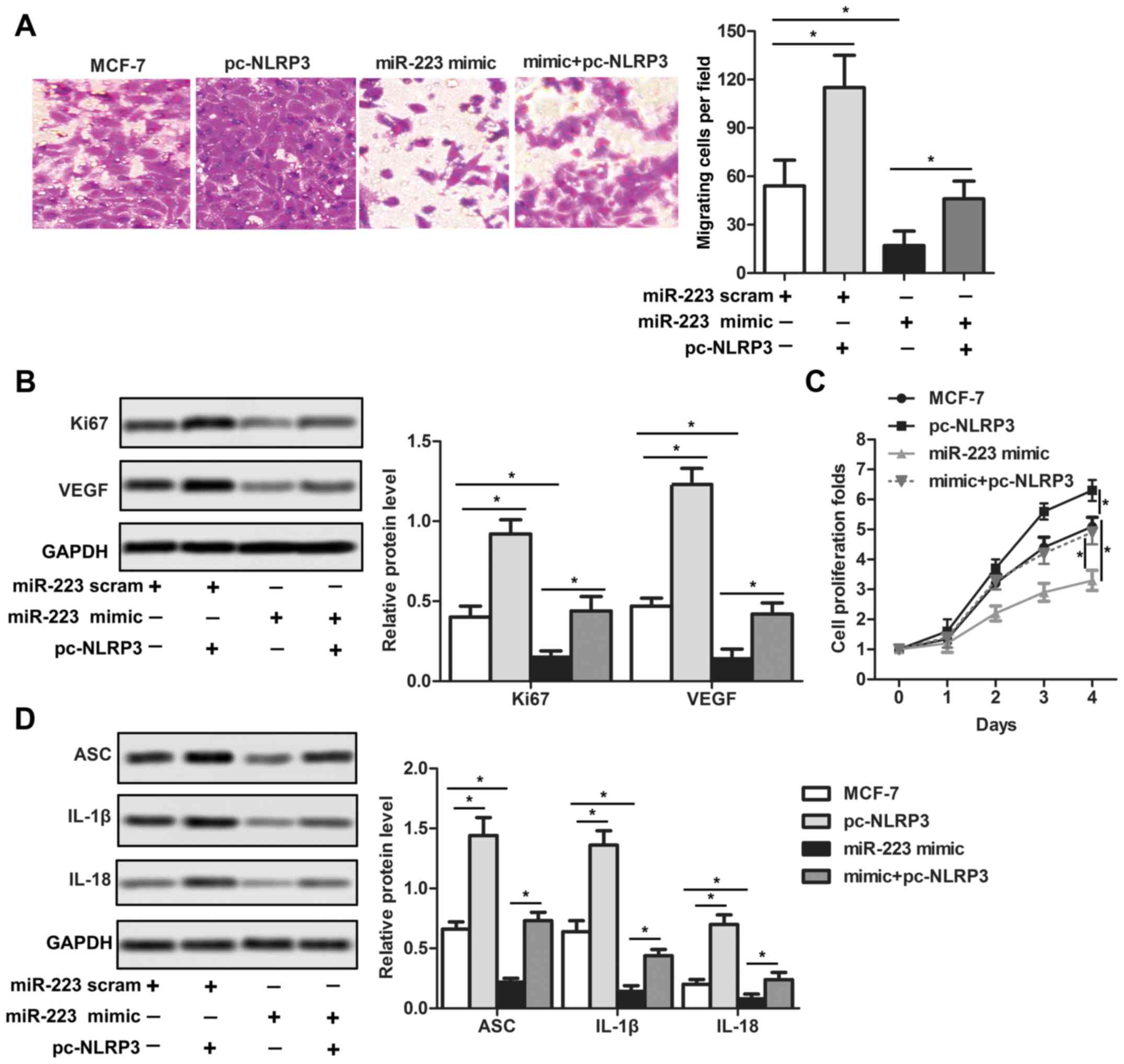 | Figure 4.miR-233 suppresses the growth and
migration of human breast cancer cells by inactivating the NLRP3
pathway. (A) Cell migration was measured by Transwell assays
(magnification, ×400). (B) Ki67 and VEGF protein expression levels
were detected by western blotting. (C) Cell proliferation of breast
cancer cells following transfection with miR-223 mimics and
pc-NLRP3 was measured by Cell Counting kit-8. (D) Protein
expression levels of ASC, IL-1β and IL-18 were detected by western
blotting. *P<0.05. miR, microRNA; NLRP3, NACHT, LRR and PYD
domains-containing protein 3; IL-, interleukin; ASC, PYD and CARD
domain-containing protein; VEGF, vascular endothelial growth
factor; Ki67, proliferation marker protein Ki67; scram,
scramble. |
NLRP3 inflammasome inactivation by
miR-233 decreases the tumor volume in vivo
To investigate the inhibitory effects of miR-233 on
tumor progression, MCF-7 cells transfected with miR-223 mimics were
subcutaneously injected into nude mice. The results suggested that
the tumor volume was significantly lower in the miR-223 mimics
group compared with the MCF-7 control group following 20 and 25
days (Fig. 5A). Furthermore, the
survival rate was increased in the miR-223 mimics group compared
with the negative control groups (Fig.
5B). Subsequently, the number of cells expressing Ki67 and/or
VEGF was analyzed by immunohistochemistry. An increased apoptotic
rate and decreased number of Ki67- and VEGF-positive cells were
detected in the miR-223 mimics group compared with the negative
control group (Fig. 5C).
Additionally, the downstream pathway of NLRP3 was investigated. The
results demonstrated that miR-223 overexpression led to a decrease
in the protein expression levels of ASC, and the concentration of
serum IL-1β and IL-18 decreased; however, the IL-10 protein
expression level increased (Fig. 5D
and E). The association between NLRP3 and miR-223 in breast
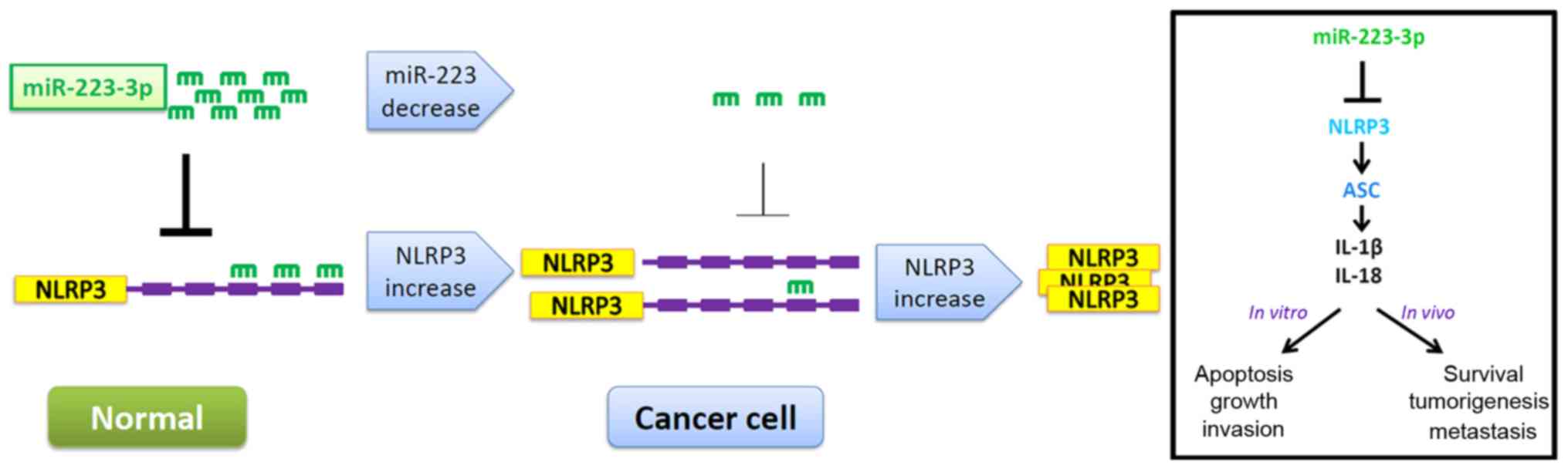
cancer is depicted in Fig. 6.
Collectively, the present data suggested that miR-233 reduced
growth and immunosuppression of breast cancer via inactivation of
the NLRP3 inflammasome in vivo.
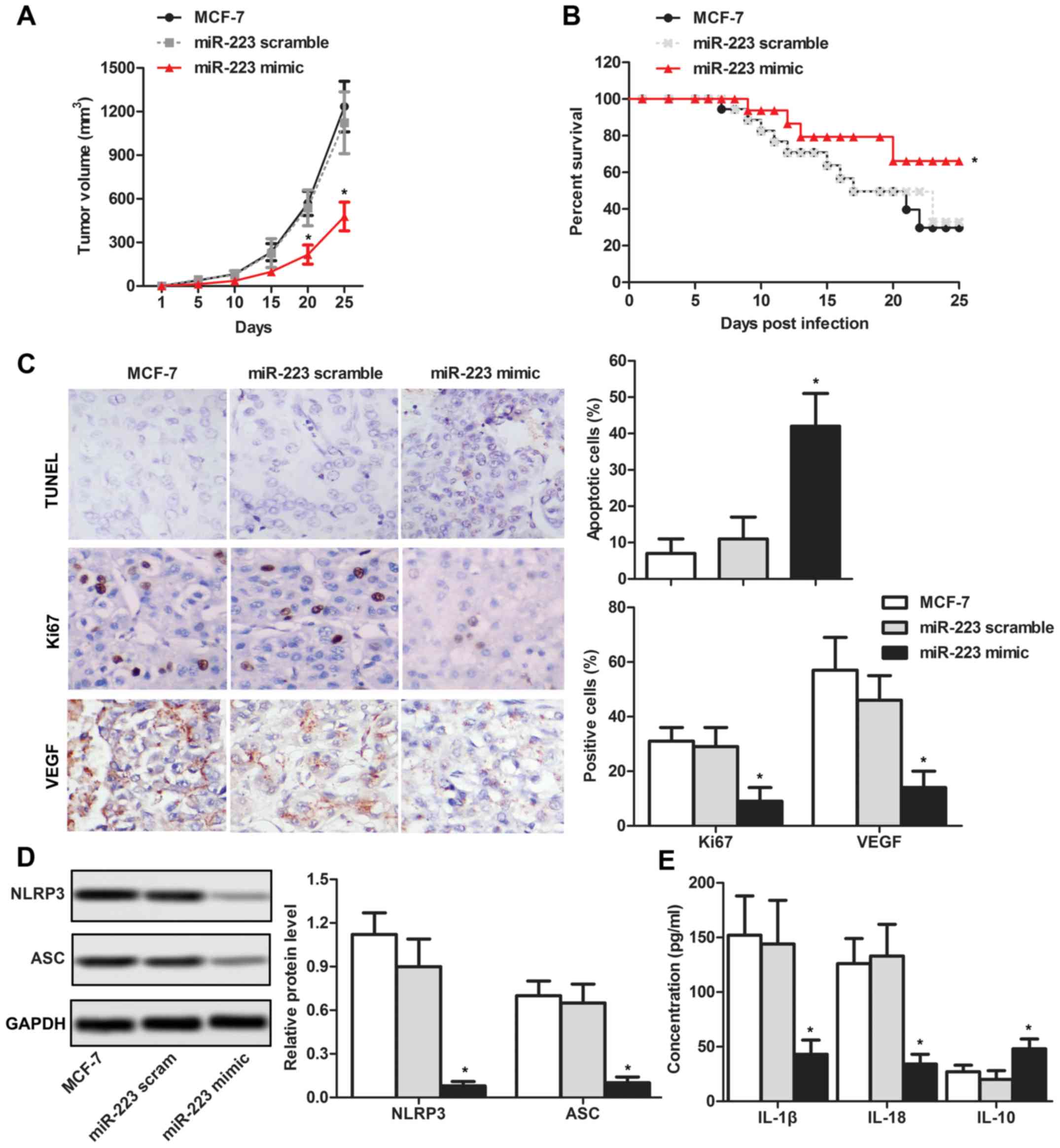 | Figure 5.NLRP3 inflammasome inactivation
driven by miR-233 reduces the development of tumors in vivo.
(A) Tumor volume was measured by subcutaneously injecting MCF-7
cells transfected with miR-223 mimics into nude mice. (B) Survival
curve of nude mice was measured for 25 days. (C) Expression level
of TUNEL, Ki67 and VEGF were detected by immunohistochemistry
(magnification, ×400). (D) Protein expression levels of NLRP3 and
ASC were detected by western blotting. (E) Concentrations of IL-1β,
IL-18 and IL-10 were detected by ELISA. *P<0.05 vs. respective
MCF-7. NLRP3, NACHT, LRR and PYD domains-containing protein 3; IL-,
interleukin; ASC, PYD and CARD domain containing protein; TUNEL,
terminal deoxynucleotidyl transferase dUTP nick end labeling; miR,
microRNA; Ki67, proliferation marker protein Ki67; scram,
scramble. |
Discussion
Activation of the NLRP3 inflammasome has a critical
role in disease development (15–18).
NLRP3 activation was implicated in a number of pathophysiological
processes, including chronic liver disease, lymphoma and cancer
(19,20). Consistent with previous studies
(21), the present results
suggested that NLRP3 expression was higher in the three breast
cancer cell lines tested, compared with HMEC cells. Considering the
increased expression level of NLRP3 in MCF-7 cells compared with
the other two cell lines (MDA-MB231 and SKBR3), it was hypothesized
that the knockdown of NLRP3 may be more effective in MCF-7 cells.
Therefore, the MCF-7 cell line was used for subsequent in
vitro and in vivo experiments. Following NLRP3
knockdown, decreased proliferation rates and increased apoptotic
rates of MCF-7 cells were detected, suggesting that NLRP3 may
contribute to the progression of breast cancer. However, the
present study presents certain limitations, due to the fact that
MDA-MB231, MCF-7 and SKBR3 are three different subtypes of breast
cancer cell lines. MCF-7 is estrogen receptor (ER)- and
progesterone receptor (PR)-positive (22,23),
and SKBR3 is human epidermal growth factor receptor 2
(HER2)-positive, whereas, MDA-MB231 is ER-, PR- and HER2-negative
(24,25). Considering the limitations of the
present study, further studies are required to investigate the role
of the miR223-NLRP3 axis in MDA-MB231 and SKBR3 cells.
According to the present results, NLRP3 knockdown
induced cell apoptosis in MCF-7 cells and decreased cell migration.
In order to investigate whether the inhibitory effect of
shRNA-NLRP3 on the migratory ability was not due to increased
apoptosis, the expression level of VEGF, a biomarker of cell
migration, was tested. A significant downregulation of VEGF was
detected following NLRP3 knockdown, suggesting an inhibitory effect
of shRNA-NLRP3 on cell migration. In order to further investigate
this phenomenon, the effects of NLRP3 knockdown on
epithelial-mesenchymal transition (testing cellular morphology,
E-cadherin and N-cadherin) of breast cancer cells require
investigation in the future. A previous study demonstrated that
miR-233 regulated cell growth and angiogenic properties in cancer
cells (26). In the present study,
miR-233 reduced proliferation via a mechanism that may be
associated with the inactivation of the NLRP3 inflammasome and its
downstream proteins ASC, IL-1β and IL-18. Previous studies have
suggested that the NLRP3 inflammasome was able to activate
caspase-1, which induced the maturation and secretion of IL-1β and
IL-18 in cancer (7,27). A previous study demonstrated that
secreted IL-10 was associated with the downregulation of
nigericin-induced NLRP3 inflammasome activation in cultured and
primary human immune cells (28).
Additionally, IL-10 and miR-233 were identified as regulators of
NLRP3 in Helicobacter pylori-infected cells (29). In the present study, the protein
expression level of IL-10 was increased following NRLP3
downregulation driven by the miR-223 mimics group. The results were
in agreement with previous studies that demonstrated an increased
immune response following NLRP3 inhibition (30,31).
Additionally, the present study identified a decrease in the
expression level of ASC, IL-1β and IL-18 in the miR-223 mimics
group. Inflammatory cytokines, ASC, IL-1β and IL-18, are aberrantly
expressed in inflammatory-associated diseases and have oncogenic
effects in specific types of cancer, including gastric cancer.
Deswaerte et al (32)
suggested a significant positive association between an elevated
mature IL-18 protein expression level and ASC mRNA expression
levels in cancer. These factors may represent novel therapeutic
targets in cancer, and the long-term objective of breast cancer
research is to identify novel targets for clinical diagnosis and
the treatment of breast cancer. In the present study, the
expression level of NLRP3 increased following miR-233 inhibition in
MCF-7 cells. The present finding suggested that miR-233 may affect
the progression of breast cancer cells by inactivating the NLRP3
inflammasome.
Huang et al (33) suggested that miR-223 may increase
proliferation, promote invasion, and inhibit apoptosis of lung
cancer A549 cells via activation of the nuclear factor
κ-light-chain-enhancer of activated B cells signaling pathway.
However, Guo et al (34)
identified that miR-233 inhibited bladder carcinoma invasiveness
via nuclear receptor coactivator 1. These previous studies
demonstrated the various effect of miR-233 on tumor development. In
order to investigate the mechanism of miR-233 function in breast
cancer progression, in vivo experiments were performed in
the present study. The present results suggested that miR-233
inhibited the growth of tumors and increased the survival rate of
experimental mice. Overexpression of miR-233 suppressed tumor
growth, suggesting an inhibitory role for miR-233 in breast cancer
progression. Consistent with the present in vitro results,
overexpression of miR-233 significantly decreased the expression
level of the NLRP3 inflammasome in vivo. Although a previous
study demonstrated that miR-233 regulated ovarian cancer cell
proliferation and invasion by targeting sex-determining region
Y-box 11 expression (35), the
function of miR-233 and the association between miR-233 and NLRP3
in breast cancer was not examined in previous studies, to the best
of the authors' knowledge. These present results suggested that
NLRP3 inflammasome inactivation driven by miR-233 reduced the
growth of breast cancer in vivo.
Collectively, the present study demonstrated that
miR-233 may suppress breast cancer cell growth via a mechanism
associated with the inactivation of the NLRP3 inflammasome in
vitro and in vivo. The miR-233/NLRP3-mediated cancer
pathway was identified as a molecular mechanism regulating the
growth and immunosuppression of breast cancer, and may represent a
therapeutic target for the treatment of breast cancer.
Acknowledgements
The authors would like to thank Dr Yuwei Zang, Dr
Feng Wang and the members of The Linyi Central Hospital (Linyi,
China) and Beijing Tiantan Hospital, for providing technical
support to the present study.
Funding
The present study was funded by The Youth Foundation
of Beijing Tiantan Hospital, Capital Medical University, Beijing,
China (grant no. 2015-YQN-09).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ analyzed and interpreted the principal data
regarding the cell transfection and in vitro experiments. HL
and YZ were involved in the in vivo experiments and
statistical analysis. FW was responsible for the design of the
study and drafting the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The animal experiments in the present study were
approved by The Animal Care and Research Committee of Beijing
Tiantan Hospital. All experiments were performed in compliance with
relevant laws and guidelines. All experiments were conducted
following the institutional guidelines of Beijing Tiantan
Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sun Q, Liu T, Yuan Y, Guo Z, Xie G, Du S,
Lin X, Xu Z, Liu M, Wang W, et al: MiR-200c inhibits autophagy and
enhances radiosensitivity in breast cancer cells by targeting
UBQLN1. Int J Cancer. 136:1003–1012. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Darby S, McGale P, Correa C, Taylor C,
Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, et
al: Effect of radiotherapy after breast-conserving surgery on
10-year recurrence and 15-year breast cancer death: Meta-analysis
of individual patient data for 10,801 women in 17 randomised
trials. Lancet. 378:1707–1716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deng L, Lei Q, Wang Y, Wang Z, Xie G,
Zhong X, Wang Y, Chen N, Qiu Y, Pu T, et al: Downregulation of
miR-221-3p and upregulation of its target gene PARP1 are prognostic
biomarkers for triple negative breast cancer patients and
associated with poor prognosis. Oncotarget. 8:108712–108725. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Terme M, Ullrich E, Aymeric L, Meinhardt
K, Desbois M, Delahaye N, Viaud S, Ryffel B, Yagita H, Kaplanski G,
et al: IL-18 induces PD-1-dependent immunosuppression in cancer.
Cancer Res. 71:5393–5399. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fan H, Zhao G, Liu L, Liu F, Gong W, Liu
X, Yang L, Wang J and Hou Y: Pre-treatment with IL-1β enhances the
efficacy of MSC transplantation in DSS-induced colitis. Cell Mol
Immunol. 9:473–481. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao X, Zhang C, Hua M, Wang R, Zhong C,
Yu J, Han F, He N, Zhao Y, Liu G, et al: NLRP3 inflammasome
activation plays a carcinogenic role through effector cytokine
IL-18 in lymphoma. Oncotarget. 8:108571–108583. 2017.PubMed/NCBI
|
|
7
|
Martinon F, Burns K and Tschopp J: The
inflammasome: A molecular platform triggering activation of
inflammatory caspases and processing of proIL-beta. Mol Cell.
10:417–426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bae JY, Lee SW, Shin YH, Lee JH, Jahng JW
and Park K: P2X7 receptor and NLRP3 inflammasome activation in head
and neck cancer. Oncotarget. 8:48972–48982. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li XY, Luo QF, Wei CK, Li DF, Li J and
Fang L: MiRNA-107 inhibits proliferation and migration by targeting
CDK8 in breast cancer. Int J Clin Exp Med. 7:32–40. 2014.PubMed/NCBI
|
|
12
|
Dong X, Sui C, Huang K, Wang L, Hu D,
Xiong T, Wang R and Zhang H: MicroRNA-223-3p suppresses leukemia
inhibitory factor expression and pinopodes formation during embryo
implantation in mice. Am J Transl Res. 8:1155–1163. 2016.PubMed/NCBI
|
|
13
|
Zhou W, Pal AS, Hsu AY, Gurol T, Zhu X,
Wirbisky-Hershberger SE, Freeman JL, Kasinski AL and Deng Q:
MicroRNA-223 suppresses the canonical NF-κB pathway in basal
keratinocytes to dampen neutrophilic inflammation. Cell Rep.
22:1810–1823. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wong ML and Medrano JF: Real-time PCR for
mRNA quantitation. Biotechniques. 39:75–85. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baldwin AG, Tapia VS, Swanton T, White CS,
Beswick JA, Brough D and Freeman S: Design, synthesis and
evaluation of novel oxazaborine inhibitors of the NLRP3
inflammasome. ChemMedChem. 13:312–320. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Johnson JL, Ramadass M, Haimovich A,
McGeough MD, Zhang J, Hoffman HM and Catz SD: Increased neutrophil
secretion induced by NLRP3 mutation links the inflammasome to
azurophilic granule exocytosis. Front Cell Infect Microbiol.
7:5072017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi X, Qiu S, Zhuang W, Yuan N, Wang C,
Zhang S, Sun T, Guo W, Gao F, Yang S and Qiao Y:
NLRP3-inflammasomes are triggered by age-related hearing loss in
the inner ear of mice. Am J Transl Res. 9:5611–5618.
2017.PubMed/NCBI
|
|
18
|
Ismael S, Nasoohi S and Ishrat T: MCC950,
the selective inhibitor of nucleotide oligomerization domain-like
receptor protein-3 inflammasome, protects mice against traumatic
brain injury. J Neurotrauma. 35:1294–1303. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu X, Dong L, Lin X and Li J: Relevance of
the NLRP3 inflammasome in the pathogenesis of chronic liver
disease. Front Immunol. 8:17282017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao Y, Guo Q, Zhao K, Zhou Y, Li W, Pan
C, Qiang L, Li Z and Lu N: Small molecule GL-V9 protects against
colitis-associated colorectal cancer by limiting NLRP3 inflammasome
through autophagy. Oncoimmunology. 7:e13756402017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu Q, Zhao F, Guo F, Wang C and Fu Z:
Polymeric nanoparticles induce NLRP3 inflammasome activation and
promote breast cancer metastasis. Micromol Biosci. 17–2017.
View Article : Google Scholar
|
|
22
|
Haque I, Ghosh A, Acup S, Banerjee S, Dhar
K, Ray A, Sarkar S, Kambhampati S and Banerjee SK: Leptin-induced
ER-α-positive breast cancer cell viability and migration is
mediated by suppressing CCN5-signaling via activating
JAK/AKT/STAT-pathway. BMC Cancer. 18:992018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Knutson TP, Daniel AR, Fan D, Silverstein
KA, Covington KR, Fuqua SA and Lange CA: Phosphorylated and
sumoylation-deficient progesterone receptors drive proliferative
gene signatures during breast cancer progression. Breast Cancer
Res. 14:R952012. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Okarvi SM and Aljammaz I: Preparation and
in vitro and in vivo characterization of the tumor-specific
antigen-derived peptide as a potential candidate for targeting
human epidermal growth factor receptor 2-positive breast
carcinomas. Anticancer Res. 38:2823–2830. 2018.PubMed/NCBI
|
|
25
|
Salkho NM, Paul V, Kawak P, Vitor RF,
Martins AM, Al Sayah M and Husseini GA: Ultrasonically controlled
estrone-modified liposomes for estrogen-positive breast cancer
therapy. Artif Cells Nanomed Biotechnol. 12:1–11. 2018. View Article : Google Scholar
|
|
26
|
Yang F, Xu Y, Liu C, Ma C, Zou S, Xu X,
Jia J and Liu Z: NF-κB/miR-223-3p/ARID1A axis is involved in
Helicobacter pylori CagA-induced gastric carcinogenesis and
progression. Cell Death Dis. 9:122018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hornung V and Latz E: Intracellular DNA
recognition. Nat Rev Immunol. 10:123–130. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Quan JH, Huang R, Wang Z, Huang S, Choi
IW, Zhou Y, Lee YH and Chu JQ: P2X7 receptor mediates
NLRP3-dependent IL-1β secretion and parasite proliferation in
Toxoplasma gondii-infected human small intestinal epithelial cells.
Parasites Vectors. 11:12018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pachathundikandi SK and Backert S:
Helicobacter pylori controls NLRP3 expression by regulating
hsa-miR-223-3p and IL-10 in cultured and primary human immune
cells. Innate Immun. 24:11–23. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Daley D, Mani VR, Mohan N, Akkad N,
Pandian GSDB, Savadkar S, Lee KB, Torres-Hernandez A, Aykut B,
Diskin B, et al: NLRP3 signaling drives macrophage-induced adaptive
immune suppression in pancreatic carcinoma. J Exp Med. 6:1711–1724.
2017. View Article : Google Scholar
|
|
31
|
van der Heijden T, Kritikou E, Venema W,
van Duijn J, van Santbrink PJ, Slütter B, Foks AC, Bot I and Kuiper
J: NLRP3 inflammasome inhibition by MCC950 reduces atherosclerotic
lesion development in apolipoprotein E-deficient mice-brief report.
Arterioscler Thromb Vasc Biol. 37:1457–1461. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deswaerte V, Nguyen PM, West A, Browning
AF, Yu L, Ruwanpura SM, Balic J, Livis T, Girard C, Preaudet A, et
al: Inflammasome adaptor ASC suppresses apoptosis of gastric cancer
cells by an IL18-mediated inflammation-independent mechanism.
Cancer Res. 78:1293–1307. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang L, Li F, Deng P and Hu C:
MicroRNA-223 promotes tumor progression in lung cancer A549 cells
via activation of the NF-κB signaling pathway. Oncol Res.
24:405–413. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guo J, Cao R, Yu X, Xiao Z and Chen Z:
MicroRNA-223-3p inhibits human bladder cancer cell migration and
invasion. Tumour Biol. 39:10104283176916782017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fang G, Liu J, Wang Q, Huang X, Yang R,
Pang Y and Yang M: MicroRNA-223-3p regulates ovarian cancer cell
proliferation and invasion by targeting SOX11 expression. Int J Mol
Sci. 18:E12082017. View Article : Google Scholar : PubMed/NCBI
|