Introduction
Scutellaria barbata D. Don (S.
barbata; Ban zhi lian in Chinese) is used as an
immunomodulatory and antitumor agent in traditional Chinese
medicine (1). Extracts of S.
barbata have exhibited growth-inhibitory effects in a number of
types of cancer in vitro and/or in vivo, including
liver cancer (2,3). A recent study revealed that the
immunomodulatory function of S. barbata extracts on Th1 and
Th17 immune responses was involved in its antitumor effect when
treating hepatoma H22-bearing mice (4). The active ingredients in S.
barbata extracts were investigated regarding their different
inhibitory effects on hepatocarcinoma and underlying mechanisms,
and it was found that total flavonoids from S. barbata
impaired the viability (5) and
metastatic capability (6) of
MHCC97H human hepatocarcinoma cells in vitro, which were
associated with regulation of the mitochondrial apoptotic pathway
(5) and matrix metalloproteinases
(6), respectively.
S. barbata polysaccharides (SBPSs) are
another category of main active ingredients extracted from S.
barbata (7). SBPS have been
reported to not only inhibit the proliferation of lung carcinoma
cells in vitro and in a subcutaneous xenograft model in a
dose-dependent manner, but also downregulate phosphorylated
(p)-c-Met and its downstream signaling molecules, including
p-extracellular signal-regulated kinase (p-ERK) and p-protein
kinase B (p-AKT) (8). Yang et
al showed that SBPS potently inhibited cell proliferation and
human epidermal growth factor receptor (HER)2 phosphorylation in
HER2-mutated non-small cell lung cancer in vitro and in
xenograft models (9). In addition,
a previous study revealed that SBPS inhibited the proliferation and
elevated the apoptosis of human colon cancer HT29 cells. SBPS also
inhibited the epithelial-mesenchymal transition of HT29 cells by
upregulating the mRNA expression of E-cadherin and downregulating
that of N-cadherin and vimentin (10). However, there has been limited
investigation of the effects of SBPS on liver cancer so far. In
addition, comprehensive analyses of the molecular mechanisms
underlying the antitumor effect of SBPS using high-throughput omics
technologies are required.
Serum contains an abundance of proteins that are
important indicators of physiological or pathological states.
Therefore, comprehensive determination of the proteome of human
serum with high accuracy and availability has the potential to
identify disease biomarkers, monitor disease development, and
identify the mechanisms underlying disease development (11). Matrix-assisted laser (induced)
desorption ionization (MALDI)-based profiling of serum proteomics
has been approved for routine diagnostics (12). Examining serum proteomics with the
assistance of MALDI time-of-flight mass spectrometry (MALDI-TOF MS)
analysis has identified several potential targets for treating
hepatocellular carcinoma (13,14).
In the present study, an experimental model of mice
bearing H22 hepatic carcinoma was established and treated with
different dosages of SBPS. The antitumor effect of SBPS on hepatic
carcinoma was investigated. In addition, two-dimensional gel
electrophoresis (2-DE) was applied to establish the serum protein
patterns of normal mice, tumor-bearing mice and SBPS-treated
tumor-bearing mice. The differential protein spots were analyzed
using MALDI-TOF MS and the resulting protein sequences were used to
search the National Center for Biotechnology Information (NCBI)
database for validation. Six serum proteins were identified between
the tumor-bearing mice and the tumor-bearing mice treated with
SBPS. This study may provide a novel perspective for treating
hepatocellular carcinoma with S. barbata extracts.
Materials and methods
Establishment of the hepatic carcinoma
mouse model, treatment, and sample collection
Kunming mice (23±1°C,12 h light/dark cycle, with
free access to food and water), weighing 20±2.0 g (males and
females in equal numbers), were provided by Heilongjiang University
of Chinese Medicine (Heilongjiang, China). The murine
hepatocellular carcinoma was purchased from the Cancer Institute
and Hospital (Chinese Academy of Medical Sciences, Beijing, China).
The study was approved by the Ethics Committee of Mudanjiang
Medical University (Mudanjiang, China).
Under sterile conditions, the ascites from mice
bearing H22 hepatoma (established previously) for 6–7 days were
extracted, diluted at a ratio of 1:3 with sterile saline, and
centrifuged at 4°C and 300 × g for 5 min, as previously described
(15). The cell concentration was
adjusted to ~2×l07/ml, with cell viability ≥95%. Mice at
the age of 6–8 weeks were subcutaneously inoculated with 0.2 ml of
cell suspension in the right forelimb axillary region to establish
the solid tumor model.
The mice bearing hepatic carcinoma H22 were randomly
divided into five groups (n=10 in each group): Model (injected with
normal saline), CTX [injected with CTX at 30 mg/(kg·day)], SBPS
high-dose [injected with SBPS at 200 mg/(kg·day)], SBPS
moderate-dose [injected with SBPS at 100 mg/(kg·day)] and SBPS
low-dose groups [injected with SBPS at 50 mg/(kg·day)]. The doses
were determined based on previous literature (16–19).
The injection volume was 10 ml/(kg·day) for mice in all five
groups. From 6 h post-inoculation, saline, CTX, and SBPS were
intraperitoneally injected once a day for 7 days. SBPS was
purchased from Ningbo Dekang Biochem Co., Ltd. (Ningbo, China) and
cyclophosphamide (CTX; cat. no. 12060625) was purchased from Heng
Rui Medicine Co., Ltd. (Jiangsu, China).
The mice were sacrificed 24 h after the final drug
administration by cervical dislocation, and blood samples were
collected from the orbital sinus. The blood samples were maintained
at 4°C for 30 min, and then centrifuged at 3,000 × g and 4°C for
20–30 min to obtain serum. Sera samples from the same group were
pooled together and stored at −80°C.
The tumors were removed and weighed to calculate the
tumor inhibition rate as follows: Inhibition rate = (1 - average
tumor weight of treatment group/average tumor weight of model
group) ×100%.
2-DE and image analysis
The serum samples were preprocessed using the
Calbiochem® ProteoExtract™ Albumin/IgG Removal kit (EMD
Millipore; Merck KGaA, Darmstadt, Germany), and then precipitated
with cold acetone. The precipitated samples were resolved in
protein extraction buffer (200 µl per 10 mg samples) containing 1
mM PMSF, 2 mM EDTA and 10 mM DTT.
The protein concentration was determined using the
2-D Quant kit from Amersham Biosciences; GE Healthcare Life
Sciences, Little Chalfont, UK). Subsequently, 50 or 200 µg of
proteins were loaded in each lane of immobilized pH gradient (IPG)
strips. The first dimension of 2-DE was performed in IPG
isoelectric focusing. The strips were balanced and then transferred
onto sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels
for the second dimension, which was followed by silver staining
(0.25% AgNO3, 0.015% formaldehyde) for 20 min. The 2-DE
gels were scanned using the PowerLook 2100 XL scanner system (Umax
Company, Fremont, CA, USA) and processed using ImageMaster 5.0
software (GE Healthcare Life Sciences).
MALDI-TOF MS
The protein spots were excised from the gel
manually, digested with trypsin and desalinated. The peptide
mixtures were identified via Ultraflex TOF/TOF MS (Bruker
Daltonics, Bremen, Germany) in the reflection mode with a 20-kV
accelerating voltage and 22.4-kV reflecting voltage. A 1-ns pulsed
laser light was produced by a nitrogen laser at 337 nm, with an ion
extraction delay of 0 ns. Subsequently, 100 shots were accumulated
per spectrum, and trypsin autolysis peaks were used as internal
calibrant and adrenocorticotropic hormone as external calibrant to
obtain peptide mass fingerprints (PMFs).
Protein identification
The signal peaks of single isotopes were acquired
from the PMF images using Flexanalysis 3.0 software, and PMFs were
searched against the NCBI database (https://www.ncbi.nlm.nih.gov/protein) using the Mascot
program (Matrix Science, Ltd., London, UK, http://www.matrixscience.com/search_form_select.html).
The search parameters were: Mus musculus; trypsin digestion;
ion species: Monoisotopic and MH+; carbamidomethyl
modification of cysteine as a fixed modification; oxidation of
methionine as a variable modification; and mass error of peptide
fragments: ±0.1%.
Statistical analysis
The data were analyzed using SPSS 13.0 statistical
analysis software (SPSS, Inc., Chicago, IL, USA). The experimental
results are expressed as the mean ± standard deviation. The
differences among groups were compared using one-way analysis of
variance and Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Antitumor effect of SBPS on H22
hepatoma
To evaluate the effect of SBPS on hepatoma growth,
the tumor-bearing mice were injected with SBPS, CTX, or saline
every day for 7 days (~7-10 days after H22 cell inoculation). The
maximum tumor volume was ~10-15 mm3. Compared with the
model group, the tumor weights of the CTX and SBPS high-,
moderate-, and low-dose groups were significantly decreased (all
P<0.01; Table I). Compared with
the CTX group, the low- and high-dose SBPS groups were
significantly different (both P<0.05; Table I); the moderate-dose SBPS group
exhibited comparable antitumor effects (P>0.05; Table I). Therefore, SBPS efficiently
inhibited the growth of hepatoma in mice and the inhibitory rate of
the moderate-dose SBPS was the highest (49.68%; Table I).
 | Table I.Antitumor effect of SBPS on H22
hepatoma. |
Table I.
Antitumor effect of SBPS on H22
hepatoma.
| Group | Dose [mg/(kg ·
day)] | Tumor weight
(g) | Inhibition rate
(%) |
|---|
| Model | – | 1.083±0.236 | – |
| CTX | 30 |
0.390±0.119a | 63.99 |
| High-dose SBPS | 200 |
0.627±0.163a,b | 43.03 |
| Moderate-dose
SBPS | 100 |
0.505±0.185a | 49.68 |
| Low-dose SBPS | 50 |
0.680±0.157a,b | 37.21 |
2-DE mapping of serum proteins in the
normal, tumor and SBPS groups
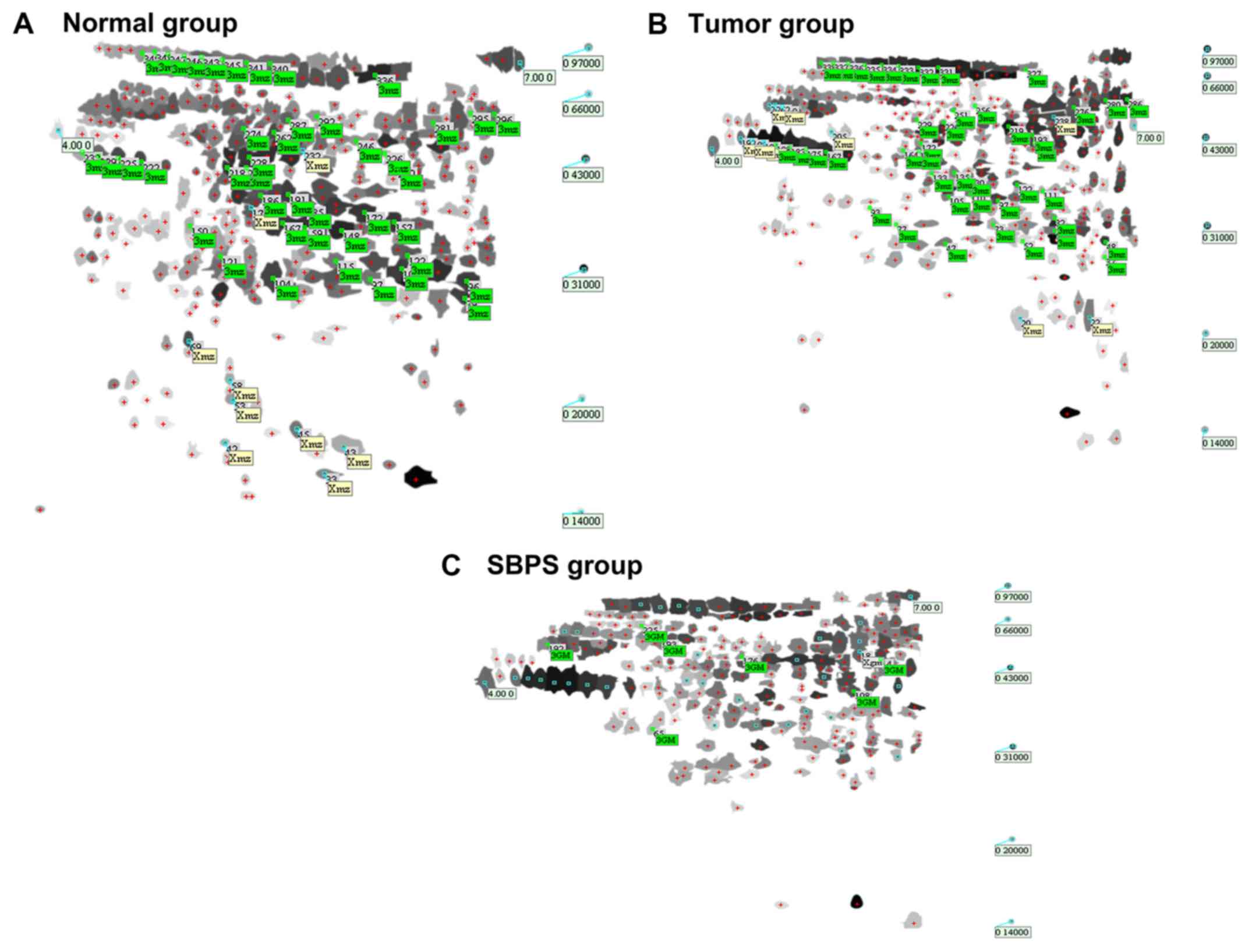
The total numbers of serum protein spots in the
normal, tumor, and SBPS groups were 347±5, 335±3 and 252±7,
respectively. Software analysis showed 62 protein spots with a
change of >3-fold between the normal and tumor groups; 18 spots
had a change of >3-fold between the tumor and SBPS groups
(Fig. 1A-C).
Analysis of the differences in the
2-DE map
The clear, large and heavily stained protein spots
were selected as differentially expressed proteins from the three
independent 2-DE images. The normal group vs. model group and the
SBPS group vs. model group were compared to identify the
differentially expressed serum proteins. The spots with
fold-changes of >3 or <0.3 (tumor/normal or SBPS/tumor) were
selected (Tables II and III). Table IV shows the serum proteins which
were differentially expressed between the normal and tumor-bearing
mice but rescued by SBPS treatment. Among them, spots 33, 176, 232
and 257 were expressed in the normal group, absent in the tumor
group, and to a certain extent, restored in the SBPS group; whereas
spots 20, 22, 205 and 238 exclusively appeared in the tumor group
and disappeared upon SBPS treatment. In addition, spots 150, 196,
198, 283 and 310 in the tumor group were lower than those in the
normal group; however, following SBPS treatment, the expression
levels of these proteins were either restored to an extent (spots
150 and 196) or even exceeded the normal level (spots 198, 283 and
310). By contrast, spots 234, 296 and 349 in the tumor group were
higher than those in the normal group; following SBPS treatment,
the expression of these proteins were either restored to an extent
(spot 234) or were below the normal level (spots 296 and 349).
 | Table II.Differentially expressed proteins
between the normal and tumor-bearing mice with a fold-change of
>3 or <0.3. |
Table II.
Differentially expressed proteins
between the normal and tumor-bearing mice with a fold-change of
>3 or <0.3.
| ID | Protein isoelectric
point | MW (Da) | Fold-change (tumor
vs. normal) |
|---|
| 33a | 5.73 | 15,799 | – |
| 42a | 5.09 | 17,393 | – |
| 43a | 5.86 | 17,211 | – |
| 45a | 5.55 | 18,195 | – |
| 53a | 5.13 | 19,925 | – |
| 58a | 5.12 | 21,334 | – |
| 69a | 4.85 | 24,353 | – |
| 176a | 5.25 | 37,256 | – |
| 232a | 5.58 | 45,714 | – |
| 157 | 6.18 | 35,678 | 0.140 |
| 79 | 6.64 | 28,093 | 0.144 |
| 218 | 5.10 | 41,945 | 0.159 |
| 97 | 6.02 | 30,039 | 0.198 |
| 228 | 5.24 | 43,818 | 0.202 |
| 262 | 5.40 | 51,344 | 0.206 |
| 287 | 5.49 | 55,973 | 0.209 |
| 336 | 6.06 | 77,358 | 0.217 |
| 172 | 5.99 | 36,731 | 0.219 |
| 159 | 5.60 | 35,150 | 0.235 |
| 104 | 5.39 | 30,112 | 0.239 |
| 167 | 5.46 | 35,627 | 0.242 |
| 150 | 4.85 | 35,300 | 0.244 |
| 186 | 5.31 | 38,657 | 0.256 |
| 109 | 6.23 | 31,088 | 0.270 |
| 122 | 6.27 | 32,303 | 0.273 |
| 121 | 5.05 | 32,280 | 0.279 |
| 215 | 5.22 | 42,005 | 0.280 |
| 292 | 5.68 | 57,306 | 0.282 |
| 274 | 5.20 | 52,815 | 0.292 |
| 185 | 5.60 | 37,389 | 0.294 |
| 148 | 5.84 | 34,827 | 0.304 |
| 191 | 5.49 | 38,767 | 0.306 |
| 115 | 5.80 | 31,780 | 0.311 |
| 96 | 6.66 | 29,846 | 0.329 |
| 348 | 4.63 | 93,782 | 3.16 |
| 340 | 5.37 | 85,259 | 3.32 |
| 296 | 6.83 | 57,848 | 3.33 |
| 349 | 4.54 | 93,040 | 3.48 |
| 281 | 6.43 | 54,928 | 3.61 |
| 295 | 6.68 | 58,487 | 3.61 |
| 226 | 6.12 | 44,861 | 3.72 |
| 222 | 4.54 | 42,726 | 4.11 |
| 343 | 4.93 | 89,953 | 4.28 |
| 345 | 5.07 | 87,836 | 4.28 |
| 246 | 5.93 | 48,676 | 4.30 |
| 347 | 4.71 | 92,488 | 4.34 |
| 341 | 5.22 | 86,281 | 4.61 |
| 346 | 4.82 | 90,850 | 4.62 |
| 225 | 4.39 | 43,407 | 5.15 |
| 210 | 6.20 | 41,975 | 5.54 |
| 229 | 4.26 | 44,024 | 7.16 |
| 233 | 4.15 | 45,002 | 8.41 |
| 20b | 6.19 | 21,225 | – |
| 22b | 6.68 | 21,243 | – |
| 192b | 4.21 | 42,905 | – |
| 195b | 4.37 | 42,243 | – |
| 197b | 4.28 | 42,557 | – |
| 205b | 4.85 | 44,630 | – |
| 238b | 6.43 | 49,488 | – |
| 262b | 4.45 | 53,826 | – |
| 266b | 4.41 | 53,975 | – |
| 269b | 4.50 | 53,310 | – |
 | Table III.Differentially expressed proteins
between the SBPS-treated and tumor-bearing mice with a fold-change
of >3 or <0.3. |
Table III.
Differentially expressed proteins
between the SBPS-treated and tumor-bearing mice with a fold-change
of >3 or <0.3.
| ID | Protein isoelectric
point | MW (Da) | Fold-change (SPBS
vs. tumor) |
|---|
| 12a | 4.66 | 15,103 | – |
| 16a | 4.64 | 19,473 | – |
| 20a | 6.19 | 21,225 | – |
| 22a | 6.68 | 21,243 | – |
| 100a | 4.56 | 34,106 | – |
| 106a | 4.49 | 34,385 | – |
| 138a | 5.92 | 37,999 | – |
| 205a | 4.85 | 44,630 | – |
| 238a | 6.43 | 49,488 | – |
| 260a | 6.10 | 53,604 | – |
| 213 | 6.69 | 46,770 | 0.271 |
| 266 | 4.41 | 53,975 | 3.490 |
| 268 | 5.20 | 54,874 | 3.690 |
| 93 | 5.12 | 33,111 | 3.740 |
| 305 | 5.07 | 61,268 | 3.760 |
| 144 | 6.52 | 38,538 | 4.190 |
| 239 | 5.76 | 50,451 | 4.510 |
| 18b | 6.64 | 49,338 | – |
 | Table IV.Serum proteins differentially
expressed between the normal and tumor-bearing mice but rescued by
SBPS treatment. |
Table IV.
Serum proteins differentially
expressed between the normal and tumor-bearing mice but rescued by
SBPS treatment.
|
|
|
| Fold-change |
|
|
|---|
|
|
|
|
|
|
|
|---|
| ID | PI | MW (Da) | Normal | Tumor | SBPS | Expression level
(tumor vs. normal) | Fold-change (SPBS
vs. normal) |
|---|
| 257a | 6.42 | 50,071 | 1,354.16 | – | 318.46 | – | 0.235 |
| 232a | 5.58 | 45,714 | 675.51 | – | 559.23 | – | 0.828 |
| 33a | 5.73 | 15,799 | 623.64 | – | 137.48 | – | 0.220 |
| 176a | 5.25 | 37,256 | 417.18 | – | 567.86 | – | 1.360 |
| 310 | 4.25 | 61,307 | 1,109.01 | 85.52 | 2,060.76 | 0.0771 | 1.860 |
| 218 | 5.10 | 41,945 | 847.48 | 145.76 | 444.41 | 0.172 | 0.524 |
| 150 | 4.85 | 35,300 | 435.68 | 81.82 | 307.92 | 0.188 | 0.707 |
| 283 | 6.13 | 55,710 | 668.29 | 126.31 | 1,830.14 | 0.189 | 2.740 |
| 196 | 6.38 | 39,969 | 1,319.16 | 299.31 | 1,261.29 | 0.227 | 0.956 |
| 198 | 6.70 | 40,396 | 306.08 | 116.53 | 1,047.45 | 0.381 | 3.420 |
| 325 | 4.90 | 71,029 | 187.60 | 94.61 | 357.99 | 0.504 | 1.910 |
| 296 | 6.83 | 57,848 | 761.96 | 1,954.87 | 583.35 | 2.57 | 0.766 |
| 349 | 4.54 | 93,040 | 539.96 | 1,444.62 | 187.35 | 2.68 | 0.347 |
| 234 | 6.65 | 46,292 | 263.39 | 1,485.30 | 404.69 | 5.64 | 1.540 |
| 205b | 4.85 | 44,630 | – | 138.75 | – | – | – |
| 20b | 6.19 | 21,225 | – | 696.90 | – | – | – |
| 22b | 6.68 | 21,243 | – | 1,382.00 | – | – | – |
| 238b | 6.43 | 49,488 | – | 2,139.15 | – | – | – |
MALDI-TOF MS analysis
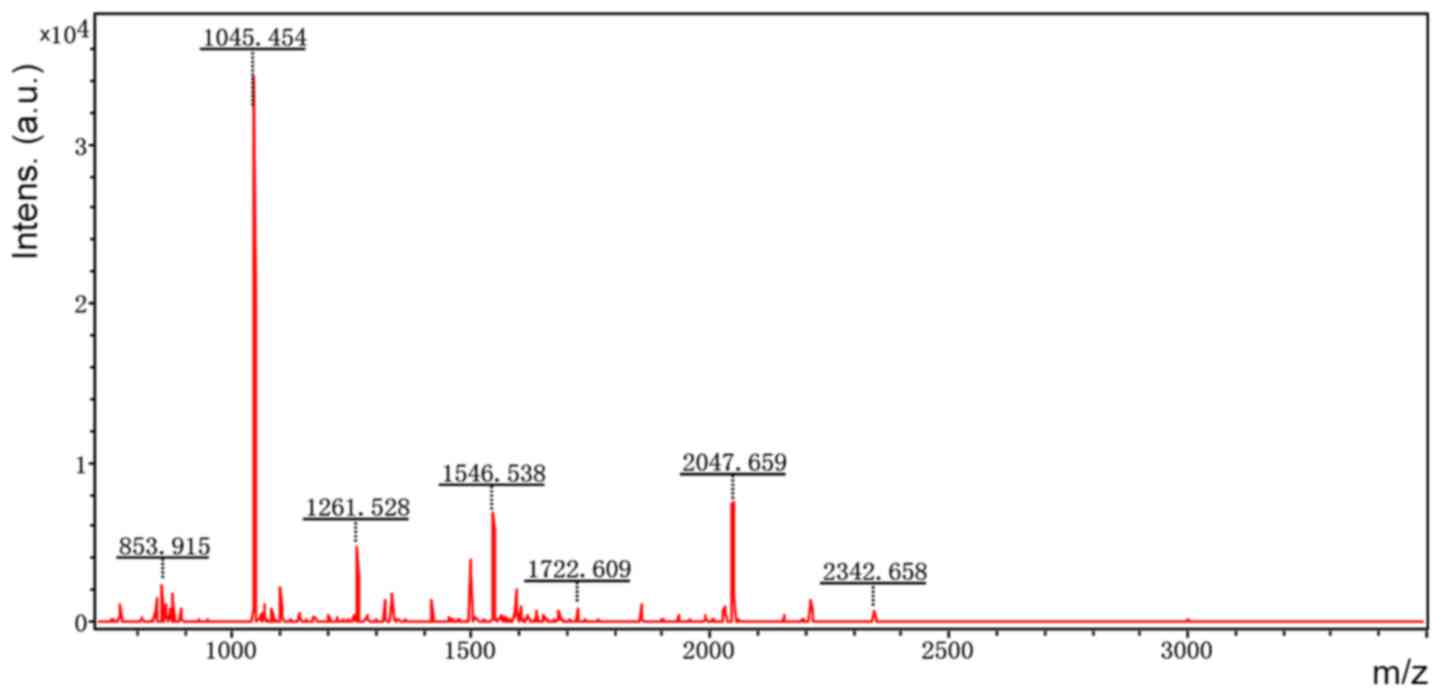
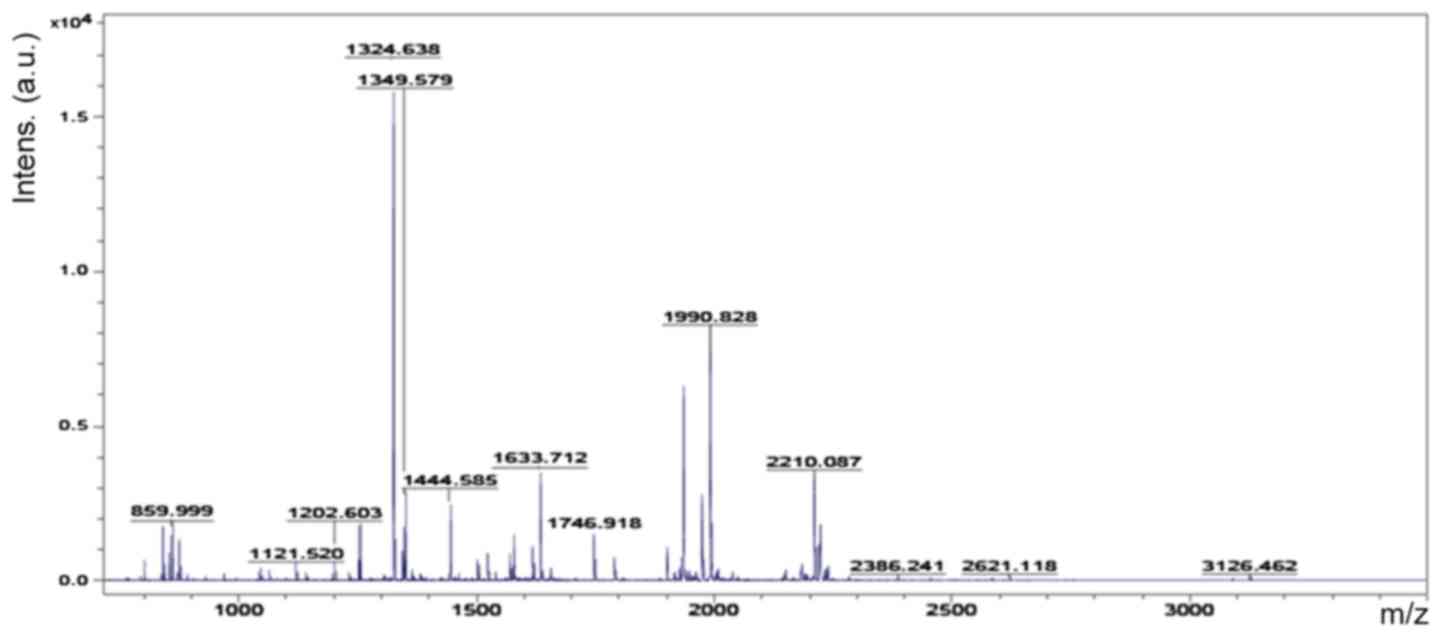
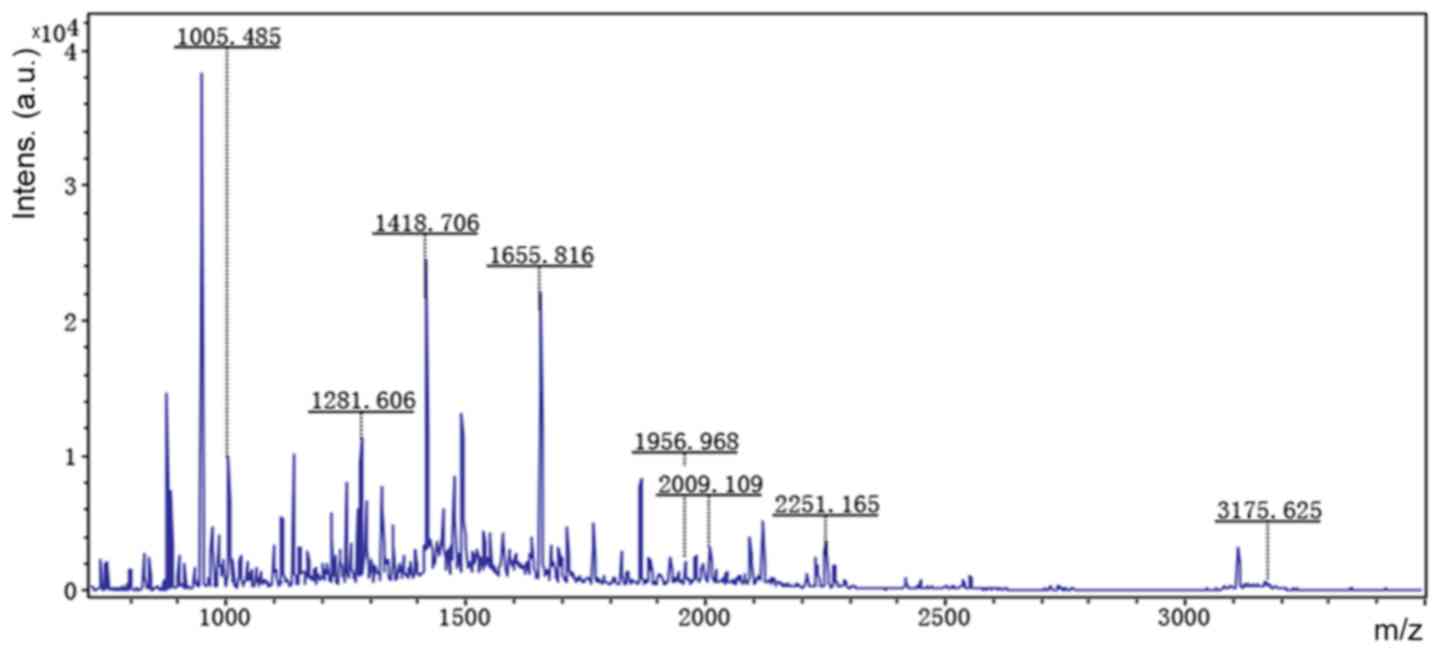
Accordingly, six representative protein spots (150,
196, 234, 296, 232 and 238) were selected for further analysis.
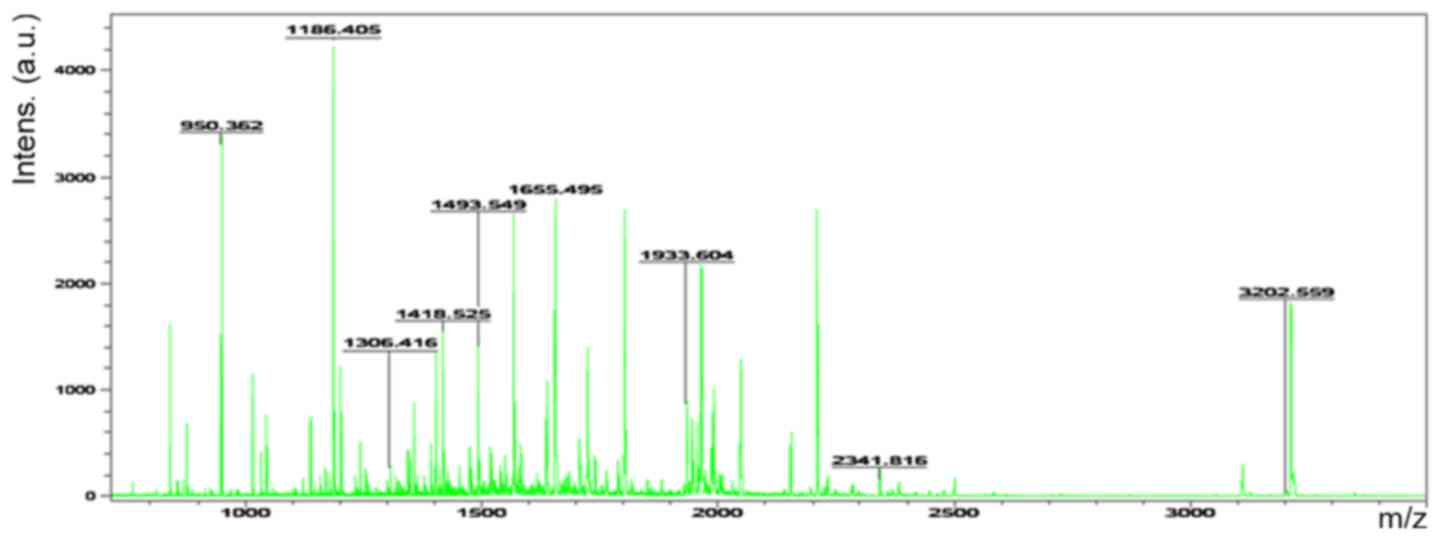
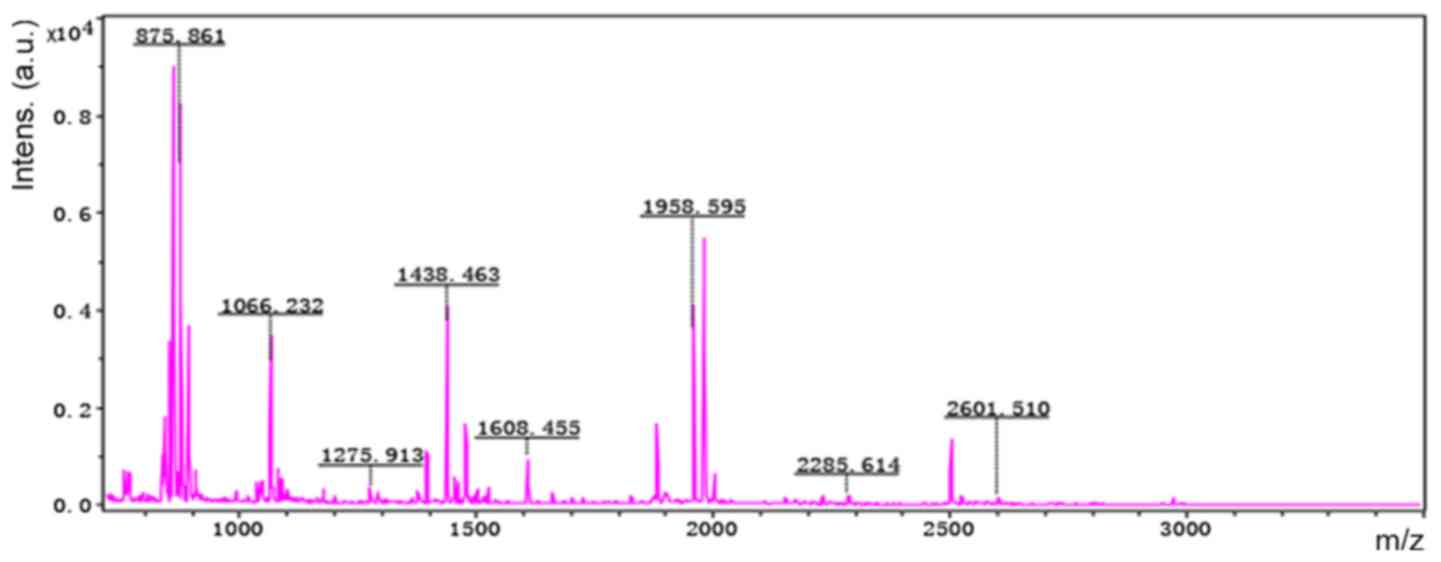
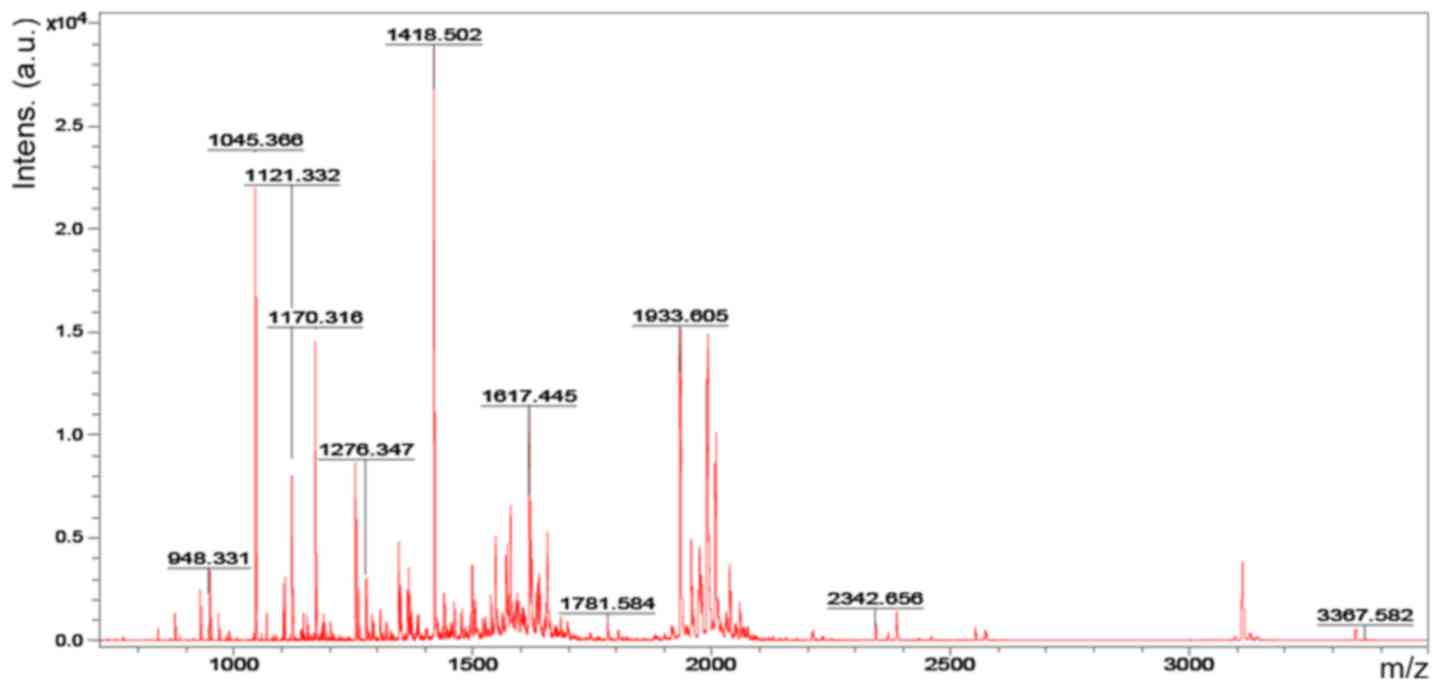
Through MALDI-TOF MS analysis, the respective PMFs of spots 150
(Fig. 2), 196 (Fig. 3), 234 (Fig. 4), 296 (Fig. 5), 232 (Fig. 6) and 238 (Fig. 7) were obtained. The six protein
spots were identified in the NCBI database (Table V). Taken together, the results
showed that pseudouridine synthase 1 (Pus1p; spot 234) and chain A
of the signal recognition particle Alu RNA-binding heterodimer
(Srp9/14; spot 296) were increased in the serum of H22
hepatoma-bearing mice, and both were reduced by SBPS treatment. In
addition, mitochondrial ribosomal protein L24 (MRPL24; spot 232)
was absent from the serum of H22 hepatoma-bearing mice, and this
was restored approximately to the normal level by SBPS
treatment.
 | Table V.Mass spectrometry results of
differentially expressed protein spots. |
Table V.
Mass spectrometry results of
differentially expressed protein spots.
| ID | Protein name | gi | MS score | Peptides matched
(n) | Predicted MW
(Da) | Protein isoelectric
point | Sequence coverage
(%) |
|---|
| 150 | Unnamed | gi|26352025 | 85 | 24 | 40,885 | 9.22 | 52 |
| 196 | LOC101056021 | gi|407261771 | 87 | 8 | 10,789 | 11.87 | 89 |
| 234 | Pseudouridine
synthase 1, isoform CRA_b | gi|148688057 | 82 | 21 | 51,127 | 9.10 | 44 |
| 296 | Chain A, signal
recognition particle Alu RNA binding heterodimer, Srp9/14 | gi|157829621 | 73 | 21 | 26,560 | 9.64 | 70 |
| 232 | Mitochondrial
ribosomal protein L24, isoform CRA_b | gi|148683388 | 66 | 11 | 27,036 | 9.49 | 49 |
| 238 | mCG16645, isoform
CRA_b | gi|148703223 | 77 | 27 | 51,801 | 5.47 | 43 |
Discussion
In the present study, a hepatoma mouse model was
established to evaluate whether SBPS had an antitumor effect on H22
hepatoma in vivo and to determine how SBPS treatment
affected the serum proteome of tumor-bearing mice. The study found
that SBPS at different dosages inhibited hepatoma growth.
Differentially expressed proteins were identified on comparing the
serum proteome of the healthy control mice, tumor-bearing mice and
SBPS-treated tumor-bearing mice. Accordingly, several abnormally
expressed serum proteins in the tumor-bearing mice were rescued by
SBPS administration, indicating the antitumor effect of SBPS. In
the present study, the results showed that the inhibitory effects
of SBPS on the tumors were not dose-dependent. The tumor inhibition
rate was lower in the high-dose group than that in the
moderate-dose group. It may be that excessive doses of drugs
increase the burden on the liver and kidney, and may have a
negative impact on the anticancer mechanisms in the body, such as
immune function. Even if the dose-limiting toxicity has been
examined in a previous study, additional toxicity testing of SBPS
is required in order to determine the exact mechanisms of
action.
Other than the direct inhibition of cancer cell
proliferation, SBPS also showed antioxidant effects in vitro
and anti-angiogenic and immune-regulation effects in vivo,
which may systematically regulate the metabolism in patients with
cancer or tumor-bearing animals. Therefore, proteomic analysis is
suitable for the comprehensive evaluation of serum proteins, which
can reflect the multifaceted effects of SBPS on hepatoma mice. SBPS
inhibits tumor growth via different molecular pathways in various
types of cancers, indicating the cancer type-dependent effects of
SBPS. Seven differentially expressed proteins were identified
between healthy control mice, tumor-bearing mice and SBPS-treated
tumor-bearing mice were in the literature, including three
functional proteins relevant to solid tumors, three proteins with
unknown functions and an unnamed protein.
PUS enzymes catalyze the site-specific isomerization
of uridine to generate pseudouridine (PU) (20). Elevated PU excretion has been
detected in different diseases, particularly in the urine of
patients with advanced cancer (21,22).
Previously, the positivity of urinary and serum PU in patients with
hepatoma was reported to be 71.3% and 70.0%, respectively, and PU
levels were reduced to normal levels following tumor resection
(23). The present study showed
that the serum level of Pus1p was low in normal mice, increased in
tumor-bearing mice, but restored to a low level in SBPS-treated
tumor-bearing mice. This may partially explain why PU is abnormally
increased in patients with hepatoma, as Pus1p catalyzes the
generation of PU. Serum Pus1p may be of clinical significance in
the diagnosis and monitoring of primary liver cancer. However, the
way in which SBPS inhibits the expression or secretion of Pus1p
requires investigation in the future.
The heterodimeric protein complex SRP9/SRP14, as a
component of the SRP, binds to 7SL RNA or cytoplasmic Alu RNA to
form a complex known as Alu RNP (24). In these two forms, the SRP9/14
dimer mainly delays peptide elongation and possibly inhibits the
initiation of protein synthesis (25). Reports have indicated the
involvement of SRP9/14 in cancer development. For example, Rho
et al combined 2-D PAGE and tandem mass spectrometry to
identify five differentially expressed proteins between normal
colon and colon cancer tissues. SRP9 was one of the upregulated
proteins in cancer tissues, which was also confirmed by western
blot analysis and immunohistochemistry, suggesting the upregulation
of SRP9 is a candidate biomarker of colon cancer (26). An early study revealed that SRP9
was one of the upregulated genes in Chinese patients with
hepatocellular carcinoma (27).
Consistently, the expression level of SRP9 was found to be
increased in tumor tissues. Regulation of the expression of SRP9/14
in liver cancer remains to be elucidated; however, alterations in
the expression levels of SRP9/14 indicate that SBPS regulates
specific protein synthesis within the tumor or in the whole body of
tumor-bearing mice.
MRPL24 is one of the proteins encoded by nuclear
genes (28). It facilitates the
specific requirements of protein synthesis in mitochondria
(29). In the present study,
MRPL24 was expressed in healthy control mice but absent in
tumor-bearing mice, suggesting that this protein was important in
the process of tumorigenesis. This suggests that MRPL24 may be a
novel target for the diagnosis of liver cancer.
In conclusion, the antitumor effect of SBPS was
confirmed in the hepatoma mouse model and seven differentially
expressed serum proteins were identified using proteomic analysis
in mice with/without SBPS treatment. These differentially expressed
proteins supported the antitumor mechanism of SBPS. They may
provide valuable clues for the pathogenesis, diagnosis and
treatment of liver cancer.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National
Natural Science Foundation Emergency Management Project (grant no.
81441113), the Study on the Effect of PAHs on the Leydig Cells of
Offspring Rats in Pregnancy and its Mechanism (grant no.
2015/01-2015/12), the Research Project of the Health and Family
Planning Commission of Heilongjiang Province (grant no. 2017–582)
and the Regulative Effect of MicroRNA on Gene Expression of k-ras
in Colorectal Cancer (grant no. 2017/1-2018/12).
Availability of data and materials
The dataset supporting the results of this study are
included within the article.
Authors' contributions
GS and LL conceived and supervised the study; GS and
LL designed experiments; LL, XX and LW performed experiments; HZ
completed the animal experiments and blood sample collection; ZH
developed new software and performed simulation experiments; BZ and
YC analyzed data; LL, XX and LW wrote the manuscript; GS and LL
made manuscript revisions. All authors reviewed the results and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Mudanjiang Medical University (Mudanjiang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SBPS
|
Scutellaria barbata
polysaccharides
|
|
MALDI
|
matrix-assisted laser (induced)
desorption ionization
|
|
NCBI
|
National Center for Biotechnology
Information
|
|
IPG
|
immobilized pH gradient
|
|
PMF
|
peptide mass fingerprints
|
|
PUS
|
pseudouridine synthases
|
|
PU
|
pseudouridine
|
|
SRP9
|
signal recognition particle 9
|
|
MRPL24
|
mitochondrial ribosomal protein
L24
|
References
|
1
|
Yu J, Lei J, Yu H, Cai X and Zou G:
Chemical composition and antimicrobial activity of the essential
oil of Scutellaria barbata. Phytochemistry. 65:881–884.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dai ZJ, Wang XJ, Li ZF, Ji ZZ, Ren HT,
Tang W, Liu XX, Kang HF, Guan HT and Song LQ: Scutellaria barbate
extract induces apoptosis of hepatoma H22 cells via the
mitochondrial pathway involving caspase-3. World J Gastroenterol.
14:7321–7328. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dai ZJ, Wu WY, Kang HF, Ma XB, Zhang SQ,
Min WL, Kang WF, Ma XB, Zhang SQ, Min WL, et al: Protective effects
of Scutellaria barbata against rat liver tumorigenesis.
Asian Pac J Cancer Prev. 14:261–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kan X, Zhang W, You R, Niu Y, Guo J and
Xue J: Scutellaria barbata D. Don extract inhibits the tumor
growth through down-regulating of Treg cells and manipulating
Th1/Th17 immune response in hepatoma H22-bearing mice. BMC
Complement Altern Med. 17:412017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao J, Lu WF, Dai ZJ, Lin S, Zhao Y, Li S,
Zhao NN, Wang XJ, Kang HF, Ma XB and Zhang WG: Induction of
apoptosis by total flavonoids from Scutellaria barbata D.
Don in human hepatocarcinoma MHCC97-H cells via the mitochondrial
pathway. Tumour Biol. 35:2549–2559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dai ZJ, Wang BF, Lu WF, Wang ZD, Ma XB,
Min WL, Kang HF, Wang XJ and Wu WY: Total flavonoids of
Scutellaria barbata inhibit invasion of hepatocarcinoma via
MMP/TIMP in vitro. Molecules. 18:934–950. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ye CL and Huang Q: Extraction of
polysaccharides from herbal Scutellaria barbata D. Don
(Ban-Zhi-Lian) and their antioxidant activity. Carbohydr Polym.
89:1131–1137. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang X, Yang Y, Tang S, Tang H, Yang G, Xu
Q and Wu J: Anti-tumor effect of polysaccharides from
Scutellaria barbata D. Don on the 95-D xenograft model via
inhibition of the C-met pathway. J Pharmacol Sci. 125:255–263.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang J, Yang G, Hou G, Liu Q, Hu W, Zhao
PU and He YI: Scutellaria barbata D. Don polysaccharides
inhibit the growth of Calu-3 ×enograft tumors via suppression of
the HER2 pathway and angiogenesis. Oncol Lett. 9:2721–2725. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun P, Sun D and Wang X: Effects of
Scutellaria barbata polysaccharide on the proliferation,
apoptosis and EMT of human colon cancer HT29 cells. Carbohydr
Polym. 167:90–96. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang AH, Sun H, Yan GL, Han Y and Wang
XJ: Serum proteomics in biomedical research: A systematic review.
Appl Biochem Biotechnol. 170:774–786. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ng EW, Wong MY and Poon TC: Advances in
MALDI mass spectrometry in clinical diagnostic applications. Top
Curr Chem. 336:139–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao XL, Li H, Yu XL, Liang P, Dong BW, Fan
J, Li M and Liu FY: Predicting early intrahepatic recurrence of
hepatocellular carcinoma after microwave ablation using SELDI-TOF
proteomic signature. PLoS One. 8:e824482013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shen S, Peng H, Wang Y, Xu M, Lin M, Xie
X, Peng B and Kuang M: Screening for immune-potentiating antigens
from hepatocellular carcinoma patients after radiofrequency
ablation by serum proteomic analysis. BMC Cancer. 18:1172018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li H, Gu L, Zhong Y, Chen Y, Zhang L,
Zhang AR, Sobol RW, Chen T and Li J: Administration of
polysaccharide from Panax notoginseng prolonged the survival of H22
tumor-bearing mice. Onco Targets Ther. 9:3433–3441. 2016.PubMed/NCBI
|
|
16
|
Song G, Wang G and Liu H: Experimental
study of Scutellaria barbata polysaccharide on synergistic
and attenuation effects of on cyclophosphamide. Tradit Chinese Med
Inf. 27:107–109. 2010.(In Chinese).
|
|
17
|
Song G, Yu Y and Wang X: Experiments on
antitumor activity of Scutellaria barbata polysaccharides
and its immunological mechanisms. World Sci Technol Mod Trad
Chinese Med. 13:641–643. 2011.(In Chinese).
|
|
18
|
Yu Y, Zhou Q and Yun K: Effects of
Scutellaria barbata polysaccharide combined with adjuvant
chemotherapy drugs on serum TNF-α and VEGF expression in mice.
Tradit Chinese Med Inf. 27:29–31. 2010.(In Chinese).
|
|
19
|
Xu X, Wu L, Li L, et al: Effects of
Scutellaria barbata polysaccharide on tissue protein in H22
tumor bearing mice. J Chinese Gerontol. 36:4709–4710. 2016.(In
Chinese).
|
|
20
|
Hamma T and Ferre-D'Amare AR:
Pseudouridine synthases. Chem Biol. 13:1125–1135. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Waalkes TP, Gehrke CW, Zumwalt RW, Chang
SY, Lakings DB, Tormey DC, Ahmann DL and Moertel CG: The urinary
excretion of nucleosides of ribonucleic acid by patients with
advanced cancer. Cancer. 36:390–398. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rasmuson T and Bjork GR: Urinary excretion
of pseudouridine and prognosis of patients with malignant lymphoma.
Acta Oncol. 34:61–67. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tu Z, Xu S and Wu M: Clinical value of
urinary and serum pseudouridine in diagnosis and monitoring of
primary liver cancer. Chin Med J (Engl). 108:204–208.
1995.PubMed/NCBI
|
|
24
|
Chang DY, Hsu K and Maraia RJ: Monomeric
scAlu and nascent dimeric Alu RNAs induced by adenovirus are
assembled into SRP9/14-containing RNPs in HeLa cells. Nucleic Acids
Res. 24:4165–4170. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Berger A, Ivanova E, Gareau C, Scherrer A,
Mazroui R and Strub K: Direct binding of the Alu binding protein
dimer SRP9/14 to 40S ribosomal subunits promotes stress granule
formation and is regulated by Alu RNA. Nucleic Acids Res.
42:11203–11217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rho JH, Qin S, Wang JY and Roehrl MH:
Proteomic expression analysis of surgical human colorectal cancer
tissues: UP-regulation of PSB7, PRDX1, and SRP9 and hypoxic
adaptation in cancer. J Proteome Res. 7:2959–2972. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Y, Zhu X, Zhu J, Liao S, Tang Q, Liu
K, Guan X, Zhang J and Feng Z: Identification of differential
expression of genes in hepatocellular carcinoma by suppression
subtractive hybridization combined cDNA microarray. Oncol Rep.
18:943–951. 2007.PubMed/NCBI
|
|
28
|
Sylvester JE, Fischel-Ghodsian N, Mougey
EB and O'Brien TW: Mitochondrial ribosomal proteins: Candidate
genes for mitochondrial disease. Genet Med. 6:73–80. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Amunts A, Brown A, Toots J, Scheres SHW
and Ramakrishnan V: Ribosome. The structure of the human
mitochondrial ribosome. Science. 348:95–98. 2015. View Article : Google Scholar : PubMed/NCBI
|