Introduction
Diabetic nephropathy has become the leading cause of
end-stage renal disease worldwide, developing in ~40% of patients
with type 2 diabetes as glomerular function progressively declines.
The glomerular filtration barrier consists of three main elements:
Capillary endothelium, glomerular basement membrane (GBM) and a
population of specialized cells (podocytes). In type 2 diabetes,
mesangial expansion and GBM thickening are characteristics of renal
dysfunction (1–3). In addition, alterations in podocyte
structure, number and density are present at the onset of diabetic
nephropathy (4,5). In addition, podocyte effacement
appears to contribute to microalbuminuria and to the pathogenesis
of diabetic nephropathy (6–8).
Transient receptor potential (TRP) channels are a
family of nonselective Ca2+-permeable cation channels
that are widely expressed in vertebrate tissues. In particular, TRP
channel 6 (TRPC6) expression and function have been studied in
several tissues (9), including
brain, kidney and heart (10–12).
TRPC6 has been shown to serve an important role in the regulation
of podocyte structure and function (13). Gain-of-function mutations in the
TRPC6 gene resulted in hereditary focal segmental
glomerulosclerosis (14), which is
characterized by proteinuria, progressive renal failure and
glomerular lesions. In addition, TRPC6 overexpression, which is
associated with actin cytoskeleton rearrangement in podocytes, is a
common feature of human proteinuric kidney diseases (10). TRPC6 knockdown by small interfering
RNA (siRNA) attenuated high glucose-induced podocyte apoptosis,
which contributes to the development of diabetic nephropathy
(15). Sonneveld et al
(16) reported that TRPC6
expression in podocytes was regulated by glucose in an Angiotensin
II (AngII)-dependent manner (17).
Taken together, these results suggested that TRPC6 is a potential
therapeutic target for diabetic nephropathy. High glucose levels in
diabetes increases AngII expression in glomeruli, especially in
mesangial cells (18,19). Ang-converting enzyme inhibitors and
AngII receptor blockers (ARBs) have been shown to attenuate
progressive glomerulosclerosis in several disease models (20–22).
The ARB candesartan lowered the peak level of proteinuria by
decreasing the expression of functional molecules in the slit
diaphragm and slowed the progression of diabetic renal disease
(17,23). A recent study demonstrated that
AngII perpetuated podocyte injury via the persistent activation of
Notch1 and Snail signaling (24).
These results demonstrate that AngII participates in mediating the
effects of hyperglycemia in the progression of diabetic nephropathy
(25).
Numerous studies have provided evidence for the
involvement of AngII in the regulation of TRPC6. For example, AngII
increased TPRC6 expression and activated TRPC6 currents in
non-renal cells, including mesenteric artery myocytes and
ventricular myocytes (26,27). Accordingly, currents evoked by
AngII in glomerular podocytes were eliminated by transfection with
siRNA against TRPC6 (28).
Increased TRPC6 expression in the podocyte membrane mediated
apoptosis induced by AngII and albumin overload (29,30).
TRPC6 also participated in the AngII-induced activation of nuclear
factor in activated T cells (NFAT), contributing to the progression
of cardiac hypertrophy (13).
TRPC6-induced activation of the NFAT signaling pathway was
identified as a potential mediator of focal segmental
glomerulosclerosis (31).
Furthermore, TRPC6-mediated Ca2+ influx and activation
of Ca2+-dependent protein phosphatase calcineurin and
its substrate NFAT have been implicated in nephropathy induced by
doxorubicin and AngII (32).
Nevertheless, it remains unclear as to whether AngII mediates
podocyte changes associated with type 2 diabetic nephropathy via
TRPC6.
It was hypothesized that high glucose levels result
in podocyte injury through the activation of a pathway mediated by
AngII, TRPC6 and NFAT. In the present study, it was revealed that
increased AngII expression in glomerular podocytes was accompanied
by enhanced urinary albumin excretion in a rat model of a
high-calorie diet and streptozocin-induced type 2 diabetes. The ARB
valsartan ameliorated diabetic podocyte injury via the
downregulation of TRPC6 and NFAT. The results of the in
vitro studies supported the role of TRPC6 and NFAT in
AngII-induced podocyte injury.
Materials and methods
Animals
Pathogen-free male Wistar rats (n=50; 8-week-old;
200 g) were obtained from the Institute of Drug Control (Qingdao,
China). The rats were housed in individual cages in a
temperature-controlled room with a 12-h light/dark cycle at 50–60%
relative humidity and were given food and water ad libitum.
The rats were allowed to acclimatize for 1 week prior to the
dietary intervention. Protocols for the animal experiments were
approved by the Qingdao University Animal Care and Use Committee
(Shandong, China) and were developed in accordance with guidelines
set by the National Institutes of Health Guide for Care and Use of
Laboratory Animals. The present study was approved by the Ethics
Committee of the Affiliated Hospital of Qingdao University.
The rat model of type 2 diabetes was generated
according to a previously described method (33). The rats were randomly divided into
two groups according to diet: Regular rat chow (n=10; normal
control group) or high-calorie diet (n=40; 10% animal fat, 20% cane
sugar, 2.5% cholesterol, 1% cholate and 66.5% regular chow).
Following 8 weeks, rats fed the high-calorie diet were
intraperitoneally injected with 30 mgxkg−1 streptozocin
to induce type 2 diabetes. One week later, fasting blood glucose
and insulin levels were measured, and the insulin sensitivity index
was calculated as 22.5/[fasting blood glucose (FBG) × insulin
(INS)]. Rats with fasting blood glucose levels >10.0
mmol·l−1 and higher insulin levels (n=40) were further
subdivided into two groups: The ARB treatment group (n=20)
receiving valsartan (40 mg/kgxday given orally; Novartis
International AG, Basel, Switzerland), and the diabetes mellitus
(DM) control group (n=20) receiving normal saline. These treatments
were administered once a day for 12 weeks.
Following the 12-week treatment, the rats were
weighed and urine samples were collected. The rats were then
sacrificed, blood samples were obtained, and the kidneys were
collected and weighed.
Determination of urinary albumin and
creatinine concentrations
To evaluate albumin and creatinine excretion, 24-h
urine samples were collected from the rats every 2 weeks during the
12-week treatment period. Albumin was measured using a
turbidimetric immunoassay kit (Shibayagi Co., Ltd., Shibukawa,
Japan). Creatinine was measured using an automatic biochemistry
analyzer (model no. 7600-020; Hitachi, Ltd., Tokyo, Japan).
Evaluation of metabolic
parameters
Blood samples were obtained from the tail vein once
per week. FBG was determined using a glucometer
(OneTouch™SureStep™; LifeScan, Inc., Milpitas, CA, USA). Serum
insulin levels and glycated hemoglobin (HbA1c) levels were
determined by enzyme-linked immunosorbent assay (cat. nos. YJ-58700
and YJ-0021a, Aquatic Diagnostic Ltd., Stirling, Scotland).
Serum creatinine, urea nitrogen, total cholesterol,
triglyceride, low-density lipoprotein and high-density lipoprotein
levels were determined using an automatic biochemistry analyzer.
Creatinine clearance was calculated using the following formula:
Creatinine clearance=urine creatinine concentration/[serum
creatinine concentration × volume of urine (ml) per min)].
Noninvasive blood pressure
measurement
Blood pressure was measured using a tail cuff system
(model LE5002; Diagnostic Systems Laboratories, Webster, TX, USA)
in conscious rats, while animals rested in a climate-controlled
room (23°C). Systolic blood pressure was measured five times
consecutively.
Glomerular morphological
characteristics
Morphological characteristics of 50-nm renal cortex
sections, including GBM thickness and the condition of podocyte
processes, were examined and photographed using a JEM-1200
transmission electron microscope (JEOL, Ltd., Tokyo, Japan).
Cell culture
A conditionally immortalized mouse podocyte cell
line, MPC5, was donated by The Central Laboratory of Shandong
University (Shandong, China), and was cultured as previously
described (34). The cell density
at 60% were grown on plates in RPMI-1640 medium supplemented with
10% fetal bovine serum (cat. no. 1213G057, Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China), 10–50 U/ml recombinant
mouse γ-interferon (IFN), 100 U/ml penicillin and 100 mg/ml
streptomycin at 33°C under a humidified atmosphere containing 5%
CO2. Then the conditions were changed to 37°C (without
γ-IFN) to induce cell differentiation into mature podocytes and
cells were cultured for 10–14 days. Differentiated podocytes were
then divided into five groups: Normal glucose (NG) group (5.6
mmol/l), high glucose (HG) group (30 mmol/l), high mannitol group
(NG + mannitol 25 mmol/l), the valsartan (VAL) group (HG + the
AngII receptor blocker VAR, 10−5 mmol/l), and HS group
[HG + the TRP channel inhibitor (35), SAR7334, 1 µM) cultured in 37°C for
48 h.
Western blot analysis
Cultured podocytes or homogenized renal tissue were
lysed in cold cell lysis buffer (50 mM Tris, 150 mM NaCl, 10 mM
ethylene diamine tetraacetic acid and 1% Triton X-100) containing
protease and phosphatase inhibitors. BCA protein assay kit (P0012,
Beyotime Institute of Biotechnology, Haimen, China) and microplate
reader (MD-SpectraMax, M5, USA) was used to determine total protein
concentrations according to specification's instruction. The
quantity of protein loaded per lane was 35 g/10 l. The proteins
were separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and subsequently transferred to nitrocellulose
membranes. Membranes were blocked with 3% nonfat dry milk for 2 h
at room temperature. Primary antibodies against TRPC6 (sc-19196,
goat anti-rat; 1:500), NFAT (sc-7296, mouse anti-rat; 1:2,000) and
AngII (sc-20718, rabbit anti-rat; 1:1,000) were obtained from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA) and incubated at 4°C
overnight. Horseradish peroxidase-conjugated anti-immunoglobulin G
served as the secondary antibody (ZB-2301, 1:5,000, Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd.; OriGene
Technologies, Inc., Beijing, China) and incubated at room
temperature for 1 h, and proteins were detected using an enhanced
chemiluminescence kit (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.; OriGene Technologies, Inc.) Image J
(version 1.8.0, National Institutes of Health, Bethesda, MD, USA)
was used for intensity analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from kidney cortex samples
and cultured podocytes using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The RNA was analyzed by
0.6% agarose gel electrophoresis, visualized by ethidium bromide
and was quantified by spectrometry. Complementary cDNA was
synthesized using PrimeScript RT Reagent Kit (cat. no. DRR037A;
Takara Biotechnology Co., Ltd., Dalian, China). RT reactions were
for 45 min at 25°C, 5 min at 85°C and then held at 4°C. RT-qPCR was
carried out using SYBR Premix Ex Taq (Takara Biotechnology Co.,
Ltd.) on an ABI PRISM 7000 HT (cat. no. 11744-100; Applied
Biosystems; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Sequences of primers targeting TRPC6,
AngII, and NFAT2 are shown in Table
I. Reactions were incubated at 95°C for 30 sec, followed by 40
cycles of 95°C for 5 sec, 60°C for 30 sec and 72°C for 40 sec,
followed by melting curve analysis. Relative gene expression was
performed using the comparative Cq method (2−∆∆Cq)
(36) with GAPDH as the internal
control.
 | Table I.Primers used in the in vitro
and in vivo studies. |
Table I.
Primers used in the in vitro
and in vivo studies.
| A, In vitro
studies using mouse podocytes |
|---|
|
|---|
| GenBank accession
no. | Gene | Sequence
(5′-3′) | Length (bp) |
|---|
| NM_001282086.1 | TRPC6 (F) |
TCTCTGGTTTACGGCAGCAGA | 228 |
|
| TRPC6 (R) |
GGAGCTTGGTGCCTTCAAATC |
|
| NM_00164109.1 | NFAT2 (F) |
GGTGCCTTTTGCGAGCAGTA | 185 |
|
| NFAT2 (R) |
TGAGCCCTGTGGTGAGACTTG |
|
| NM_001161731.2 | AngII (F) |
ACTGCGAAAGTATGATGGTGAA | 90 |
|
| AngII (R) |
CCTTGATGTTGTTCTTGGTGTC |
|
| NM_001289726.1 | GAPDH (F) |
CTCATGACCACAGTCCATGC | 201 |
|
| GAPDH (R) |
CACATTGGGGGTAGGAACAC |
|
|
| B, In
vivo studies in rats |
|
| GenBank
accession no. | Gene | Sequence
(5′-3′) | Length
(bp) |
|
| NM_053559.1 | TRPC6 (F) |
TACGGATTGTGGAGGCTATTCT | 98 |
|
| TRPC6 (R) |
AAAGTCATCTTGCTGGAGTTCA |
|
| NM_001244933.1 | NFAT2 (F) |
GAGGGAAGAAGATGGTGTTGTC | 125 |
|
| NFAT2 (R) |
GCACAGGTCTCGGTCAGTTT |
|
| NM_001006992.1 | AngII (F) |
GCAAGCATACAGGAGGGTCTC | 88 |
|
| AngII (R) |
CCATTCTCACAGGCAATAACAA |
|
| NM_001289726.1 | GAPDH (F) |
CTCATGACCACAGTCCATGC | 201 |
|
| GAPDH (R) |
CACATTGGGGGTAGGAACAC |
|
Statistical analysis
Results were expressed as the mean ± standard
deviation of at least three independent experiments. Group
differences were compared by one-way analysis of variance followed
by Student-Newman-Keuls post hoc test. P<0.05 was considered to
indicate a statistically significant difference. Least significant
different t-test was used for pairwise comparison, while Tamhane's
T2 was used for Heteroscedasticity. All analyses were performed
using SPSS software (version 22; IBM Corp., Armonk, IL, USA).
Results
Evaluation of metabolic
parameters
The present study measured the metabolic parameters
of rats in the normal control, DM and ARB treatment groups (n=10
per group). The results revealed that there was a lower insulin
sensitivity index, and increased levels of FBG, HbA1c and lipids in
the DM group than in the normal controls (Table II). However, fasting insulin and
serum creatinine levels did not differ between the groups. In
addition, no significant differences in metabolic parameters were
observed between the DM and ARB treatment groups (Table II).
 | Table II.Biochemical parameters in diabetic
rats following 12-week treatment with the angiotensin II receptor
blocker, valsartan. |
Table II.
Biochemical parameters in diabetic
rats following 12-week treatment with the angiotensin II receptor
blocker, valsartan.
|
Characteristics | NC (n=10) | DM (n=10) | ARB (n=10) |
|---|
| ISI | 0.220±0.024 |
0.05±0.004a |
0.060±0.005a |
| FINS ng/ml | 18.57±1.01 | 22.09±1.75 | 20.16±1.57 |
| FBG mmol/l | 5.56±0.64 |
19.44±0.47a |
18.58±0.58a |
| HbA1C% | 2.84±0.33 |
6.64±0.45a |
6.40±0.72a |
| TG mmol/l | 1.49±0.15 |
4.83±0.69a |
4.42±0.48a |
| TC mmol/l | 0.76±0.24 |
3.99±0.25a |
3.78±0.26a |
| LDL mmol/l | 1.04±0.19 |
7.38±0.44a |
7.07±0.45a |
| HDL mmol/l | 1.03±0.20 |
0.50±0.04a |
0.52±0.04a |
| Scr mmol/l | 48.5±7.09 | 53.7±7.83 | 50±8.84 |
Kidney function parameters
To assess glomerular injury, the present study
measured urinary albumin excretion and found a significant
difference between the DM and normal control groups. However, the
ARB VAL significantly decreased urinary albumin excretion in the
diabetic rats following 8 weeks of treatment (Fig. 1A). ARB treatment also decreased the
elevated kidney weight/body weight ratio observed in diabetic rats
(Fig. 1B) but did not affect
creatinine clearance or systolic blood pressure (Fig. 1C and D).
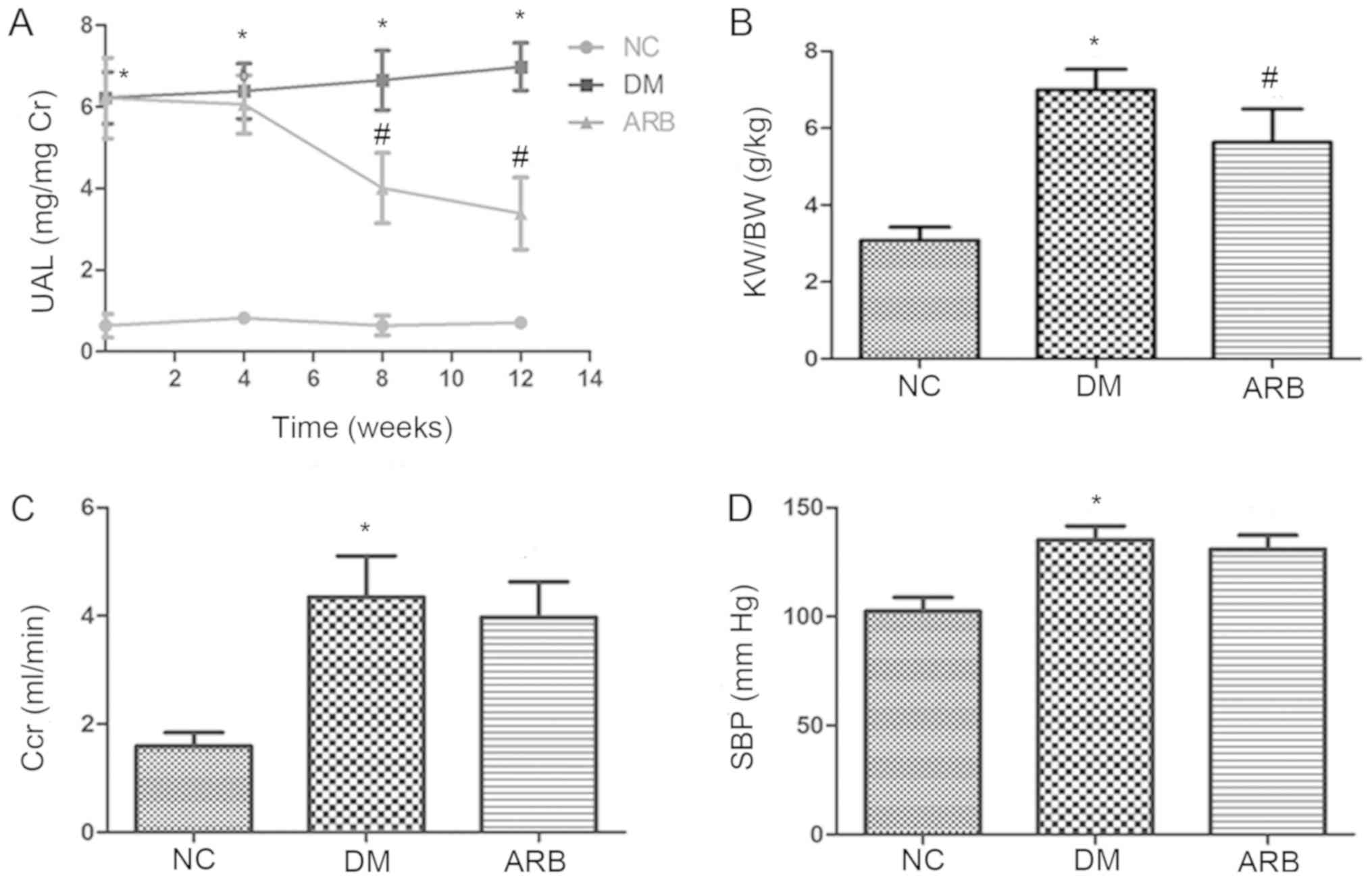 | Figure 1.Effect of angiotensin II receptor
blocker valsartan on functional parameters of diabetic rats. (A)
UAL was elevated in rats with streptozocin-induced DM when compared
with NC; however, albumin excretion was decreased in rats treated
with the ARB, valsartan. (B) Valsartan also decreased the ratio of
KW/BW in diabetic rats, but did not alter the (C) Ccr or (D) SBP.
*P<0.01 vs. NC group; #P<0.01 vs. DM group. UAL,
urinary albumin level; DM, diabetes mellitus; NC, normal control;
ARB, Angiotensin II receptor blocker; KW/BW, kidney weight to body
weight; Ccr, creatinine clearance; SBP, systolic blood
pressure. |
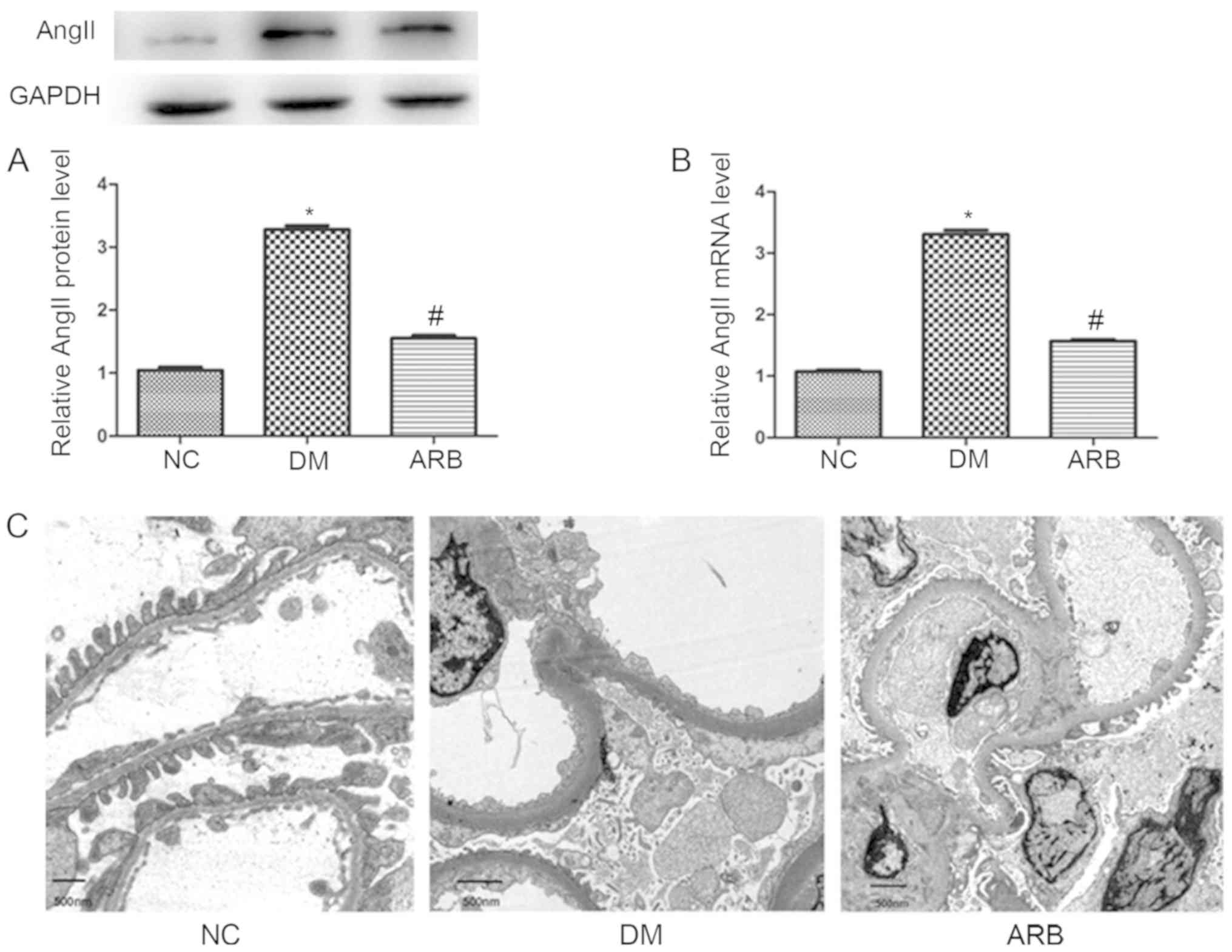
AngII expression in glomerular
podocytes and podocyte lesions in type 2 diabetic nephropathy
To better understand the role of AngII in podocytes,
the present study analyzed AngII expression changes in the
glomerular podocytes of type 2 diabetic rats via western blotting
and qPCR. In addition, the effects of ARB treatment on GBM
thickness and podocyte effacement were examined by electron
microscopy. In the DM group, the mRNA and protein levels of AngII
were significantly higher than those of the normal control group
(Fig. 2A and B); however,
treatment with VAL (40 mg/kgxday for 12 weeks) decreased AngII
expression to levels almost equivalent to those of the normal
controls. In DM rats, diabetic nephropathy was observed, which
manifested as GBM thickening and podocyte process effacement
(Fig. 2C). In addition, impairment
of the glomerular filtration barrier was ameliorated by ARB
treatment (Fig. 2).
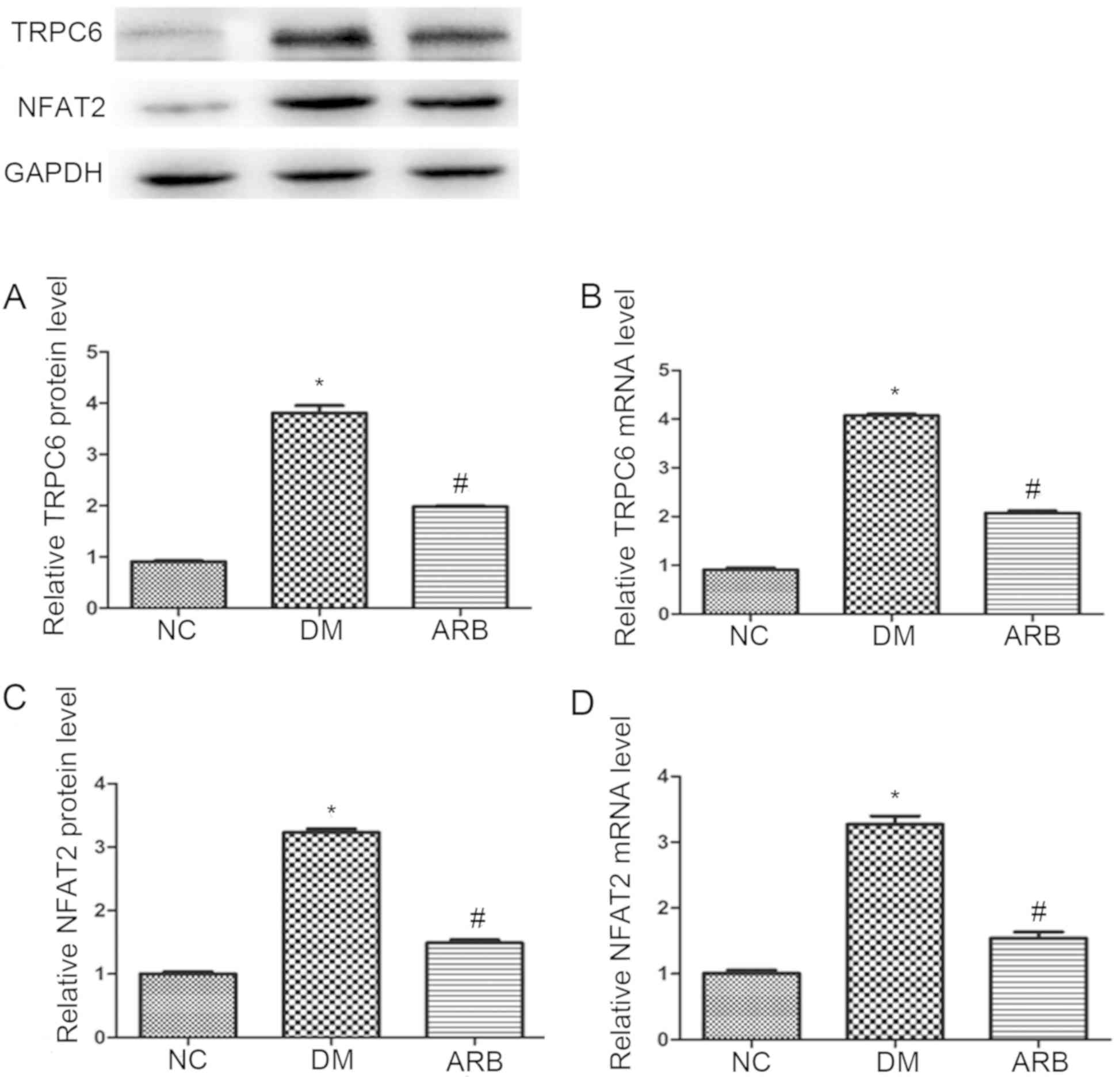
Involvement of TRPC6 and NFAT in
AngII-induced podocyte injury
To determine whether TRPC6 and NFAT were involved in
AngII-induced podocyte injury in type 2 diabetes, the present study
evaluated the expression of TRPC6 and NFAT in the rat model
employed. The results revealed higher mRNA and protein levels of
TRPC6 and NFAT in diabetic rats when compared with the normal
controls (Fig. 3). These changes
were attenuated by ARB treatment (Fig.
3).
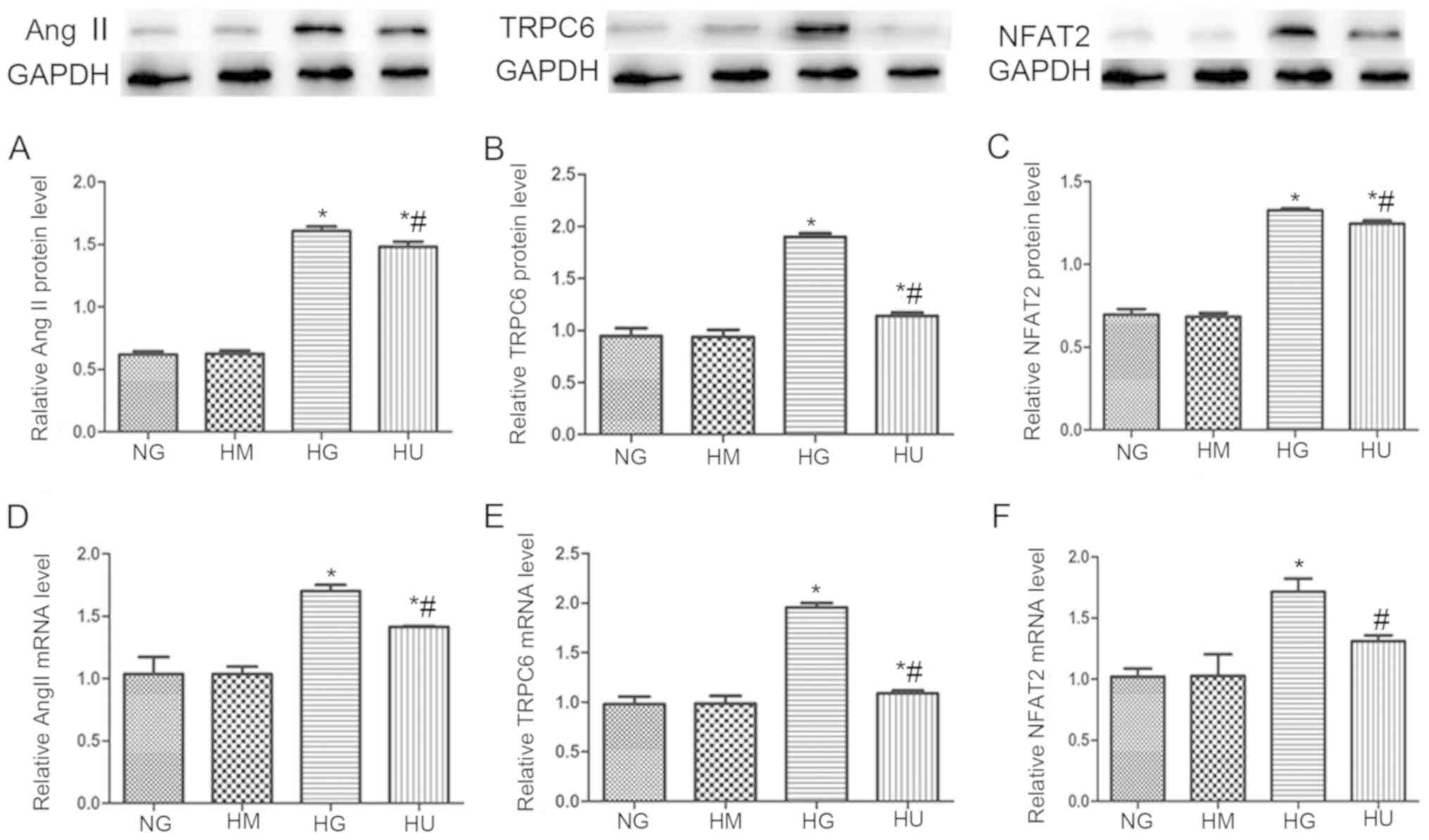
ARB treatment attenuates HG-induced
upregulation of AngII, TRPC6, and NFAT in vitro
As the results of the present study thus far
suggested that the effects of AngII in the pathogenesis of diabetic
nephropathy were mediated by TRPC6/NFAT signaling, the present
study then investigated whether this pathway was activated by HG
levels in cultured podocytes. The protein levels (Fig. 4A-C) and mRNA levels (Fig. 4D-F) of AngII, TRPC6, and NFAT were
markedly higher in cells exposed to HG (30 mmol/l) for 48 h when
compared with cells exposed to NG levels (5.6 mmol/l). This effect
was significantly attenuated by ARB treatment with VAL. Cells
cultured with 5.6 mmol/l glucose and 25 mmol/l mannitol were also
evaluated. The expression of AngII, TRPC6 and NFAT in these cells
was similar to that of the NG control group, thereby ruling out the
osmotic effect of glucose.
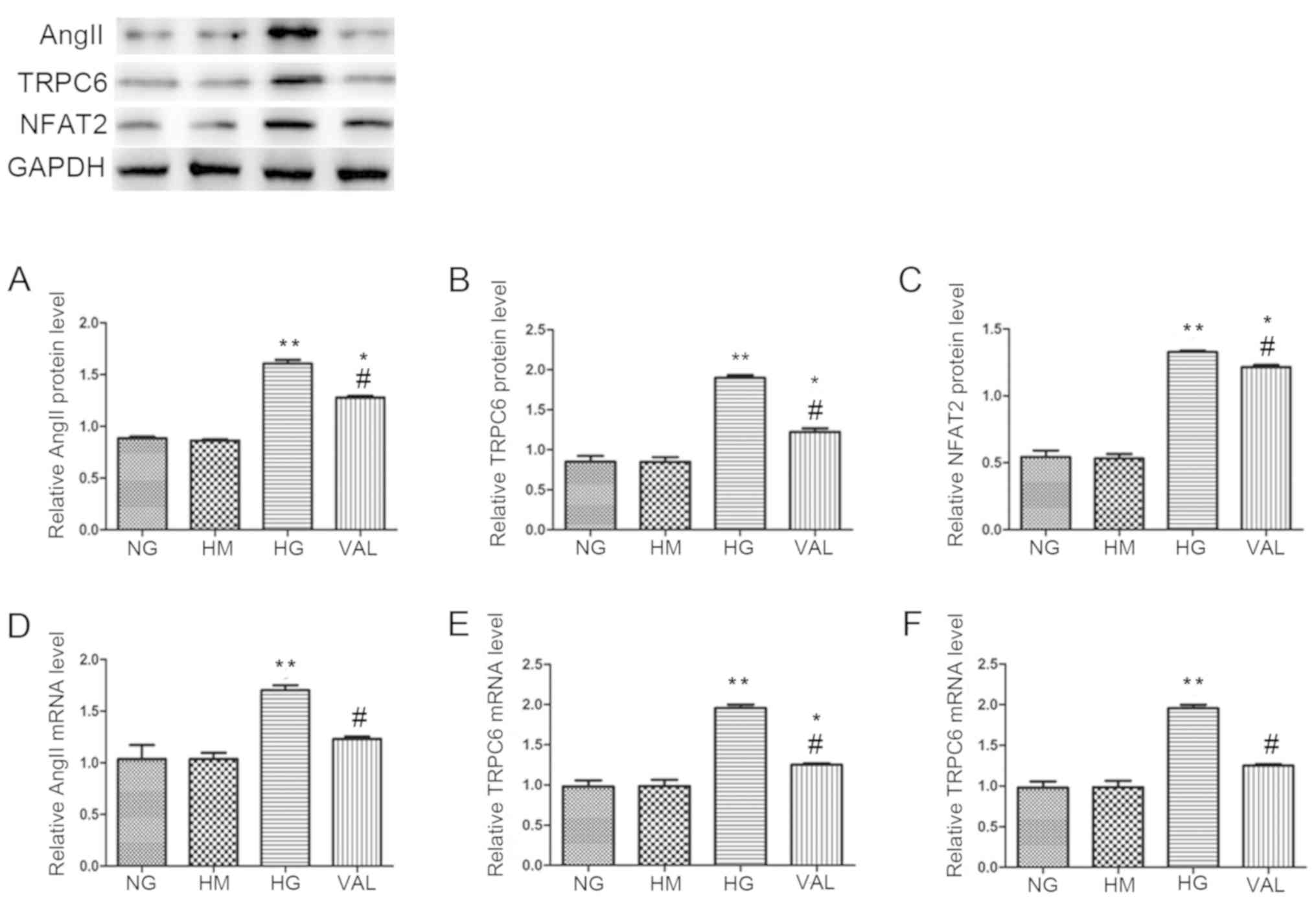 | Figure 4.ARB VAL attenuates the HG-induced
upregulation of AngII, TRPC6 and NFAT in vitro. Expression
of AngII, TRPC6 and NFAT was assessed by (A-C) western blotting and
(D-F) reverse transcription-quantitative polymerase chain reaction
analysis in podocytes cultured with NG (5.6 mmol/l) or HG (30
mmol/l). Podocytes cultured with NG and HM (25 mmol/l) served as a
control for the effects of osmotic pressure. The effect of the ARB
was evaluated in podocytes cultured with HG (30 mmol/l) and treated
with VAL (10.5 mmol/l). *P<0.05 and **P<0.01 vs. NG group;
#P<0.05 vs. HG group. AngII, angiotensin II; ARB,
angiotensin II receptor blocker; NG, normal glucose; HG, high
glucose; HM, high mannitol; VAL, valsartan; TRPC6, transient
receptor potential channel 6; NFAT, nuclear factor of activated
T-cells. |
TRP channel inhibitor attenuates
HG-induced NFAT overexpression in vitro
To further study the role of TRPC6 in this signaling
pathway, the present study evaluated the effect of the TRP channel
inhibitor SAR7334 on TRPC6 and NFAT expression in cultured
podocytes. The results revealed that SAR7334 (the HS group)
significantly attenuated the HG-induced increase of AngII, TRPC6
and NFAT expression, at the protein (Fig. 5A-C) and mRNA levels (Fig. 5D-F).
Discussion
Previous studies have reported significantly
decreased podocyte numbers and density in patients with diabetic
nephropathy, as well as a negative correlation between proteinuria
and podocyte injury (37). These
investigations contributed greatly to our understanding of the role
podocyte injury serves in the glomerular dysfunction of diabetic
nephropathy (38,39). Identifying mechanisms underlying
these podocyte structural and functional changes may provide novel
therapeutic targets for diabetic nephropathy. The present study
revealed that treatment with the ARB VAL had no significant effect
on systolic blood pressure or creatinine clearance at the
administered dose, suggesting that VAL reduced proteinuria by
protecting against podocyte injury.
Podocytes in the kidneys as well as in endothelial
and distal tubular cells express TRPC6 (40–42).
To further explore this phenomenon, the present study measured
TRPC6 expression in cultured podocytes. The results revealed the
increase in AngII and TRPC6 in the kidneys of diabetic rats and
HG-treated podocytes were consistent with those of studies by
Durvasula and Shankland (43) and
Yoo et al (44) who
reported that exposure to HG levels increased AngII and its
receptor AngII receptor type 1 in podocytes. Other studies have
demonstrated that increased renin secretion and prorenin receptor
activity contributed to the activation of the local angiotensin
system in a HG environment, driving podocyte injury and loss. In an
attempt to further elucidate the mechanisms underlying
AngII-induced podocyte injury in diabetic nephropathy, the present
study investigated the role of TRPC6 and its substrate NFAT. NFAT
is a transcription factor that is predominantly regulated by
activation and the subsequent translocation from the cytoplasm to
the nucleus. NFAT activation/regulation is measured by total NFAT2
protein/mRNA expression levels (45,46).
The results demonstrated that the ARB VAL attenuated the HG-induced
increase of TRPC6 and NFAT in podocytes in vivo and in
vitro. In addition, changes in albumin excretion and glomerular
morphology were ameliorated by ARB treatment. In particular, VAL
reversed foot process loss and injury, resulting in a near-normal
appearance. This provided further evidence of the role of TRPC6 in
AngII-induced podocyte dysfunction. Recent studies have reported
that TRPC6 was closely associated with HG-induced apoptosis in
cultured podocytes through reactive oxygen species production and
the RhoA/Rho-associated protein kinase signaling pathway (15,47).
In addition, Li et al (48)
described the involvement of the canonical Wnt signaling pathway in
diabetic podocyte injury caused by TRPC6 upregulation. However, Liu
et al (15) and Yang et
al (47) did not confirm their
results in vivo. In the present study, a rat model of a
high-calorie diet and streptozocin-induced type 2 diabetes was
employed to study the role of TRPC6 in podocyte lesions associated
with diabetic nephropathy.
Several lines of evidence have suggested that there
is a close association between AngII and TRPC6 in non-renal and
glomerular cells. For example, TRPC6 has been implicated in
AngII-induced vasoconstrictor responses in mesenteric artery
myocytes (27). Furthermore, the
ARB losartan reversed the effect of AngII on glomerular mesangial
cell proliferation by decreasing TRPC6 expression (49). Other cell culture and animal
studies demonstrated that AngII had a deleterious effect on
podocytes via the upregulation of TRPC6 (32), and that this process required the
generation of reactive oxygen species (28). The present results support our
hypothesis that TRPC6 is a key mediator of AngII-induced podocyte
injury in the progression of type 2 diabetic nephropathy.
To further evaluate the downstream signaling pathway
of AngII in podocytes, the present evaluated the effect of high
glucose conditions on the expression of NFAT, a substrate for
calcineurin. Other studies have reported that HG levels activated
NFAT in vascular smooth muscle and pancreatic β-cells (50,51).
Furthermore, NFAT activation was involved in podocyte injury and
glomerulosclerosis, and NFAT mediated HG-induced glomerular
podocyte apoptosis through the upregulation of B-cell lymphoma
2-associated X protein (Bax) (31,52,53).
Zhang et al (54) recently
demonstrated that an NFAT inhibitor exerted renoprotective effects
in diabetic db/db rats by attenuating HG-induced podocyte
apoptosis.
The present study revealed that HG-induced NFAT
levels were attenuated by treatment with the TRPC6 channel
inhibitor SAR7334 in podocytes, suggesting that hyperglycemia
activated TRPC6 channels via increased AngII expression. The
augmented Ca2+ influx caused the activation of NFAT,
mediating podocyte apoptosis by increasing Bax expression, leading
to glomerular filtration barrier dysfunction.
In conclusion, the results of the present study
suggest that the AngII/TRPC6/NFAT axis may mediate podocyte injury
in the early progression of type 2 diabetic nephropathy. In
addition, TRPC6 may represent a novel therapeutic target for
diabetic nephropathy.
Acknowledgements
The authors would like to thank Professor Liqiu Liu
(Department of Nephrology, Affiliated Hospital of Qingdao
University, Qingdao, China) for providing the conditionally
immortalized mouse podocyte cell line used in the present study, as
well as Professor Donghui Zhen (Department of Pathology, Qilu
Hospital of Shandong University, Qingdao, China)for assisting with
electron microscopy.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RM designed and coordinated the present study, and
performed the experiments. RM, YX, HZ, DZ, DY, LS and YL analyzed
the data. RM, LS and YL wrote the paper.
Ethics approval and consent to
participate
Protocols for the animal experiments were approved
by the Qingdao University Animal Care and Use Committee (Qingdao,
China) and were developed in accordance with guidelines set by the
National Institutes of Health Guide for Care and Use of Laboratory
Animals. The present study was approved by the Ethics Committee of
the Affiliated Hospital of Qingdao University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fioretto P, Mauer M, Brocco E, Velussi M,
Frigato F, Muollo B, Sambataro M, Abaterusso C, Baggio B, Crepaldi
G and Nosadini R: Patterns of renal injury in NIDDM patients with
microalbuminuria. Diabetologia. 39:1569–1576. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Osterby R, Gall MA, Schmitz A, Nielsen FS,
Nyberg G and Parving HH: Glomerular structure and function in
proteinuric type 2 (non-insulin-dependent) diabetic patients.
Diabetologia. 36:1064–1070. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
White KE and Bilous RW: Type 2 diabetic
patients with nephropathy show structural-functional relationships
that are similar to type 1 disease. J Am Soc Nephrol. 11:1667–1673.
2000.PubMed/NCBI
|
|
4
|
Dalla Vestra M, Masiero A, Roiter AM,
Saller A, Crepaldi G and Fioretto P: Is podocyte injury relevant in
diabetic nephropathy? Studies in patients with type 2 diabetes.
Diabetes. 52:1031–1035. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
White KE and Bilous RW; Diabiopsies Study
Group, : Structural alterations to the podocyte are related to
proteinuria in type 2 diabetic patients. Nephrol Dial Transplantat.
19:1437–1440. 2004. View Article : Google Scholar
|
|
6
|
Meyer TW, Bennett PH and Nelson RG:
Podocyte number predicts long-term urinary albumin excretion in
Pima Indians with Type II diabetes and microalbuminuria.
Diabetologia. 42:1341–1344. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakamura T, Ushiyama C, Osada S, Hara M,
Shimada N and Koide H: Pioglitazone reduces urinary podocyte
excretion in type 2 diabetes patients with microalbuminuria.
Metabolism. 50:1193–1196. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pagtalunan ME, Miller PL, Jumping-Eagle S,
Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L and Meyer TW:
Podocyte loss and progressive glomerular injury in type II
diabetes. J Clin Invest. 99:342–348. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Estacion M, Sinkins WG, Jones SW,
Applegate MA and Schilling WP: Human TRPC6 expressed in HEK 293
cells forms non-selective cation channels with limited Ca2+
permeability. J Physiol. 572:359–377. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Möller CC, Wei C, Altintas MM, Li J, Greka
A, Ohse T, Pippin JW, Rastaldi MP, Wawersik S, Schiavi S, et al:
Induction of TRPC6 channel in acquired forms of proteinuric kidney
disease. J Am Soc Nephrol. 18:29–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Onohara N, Nishida M, Inoue R, Kobayashi
H, Sumimoto H, Sato Y, Mori Y, Nagao T and Kurose H: TRPC3 and
TRPC6 are essential for angiotensin II-induced cardiac hypertrophy.
EMBO J. 25:5305–5316. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tai Y, Feng S, Ge R, Du W, Zhang X, He Z
and Wang Y: TRPC6 channels promote dendritic growth via the
CaMKIV-CREB pathway. J Cell Sci. 121:2301–2307. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reiser J, Polu KR, Möller CC, Kenlan P,
Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C,
et al: TRPC6 is a glomerular slit diaphragm-associated channel
required for normal renal function. Nat Genet. 37:739–744. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Winn MP, Conlon PJ, Lynn KL, Farrington
MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S,
Burchette JL, et al: A mutation in the TRPC6 cation channel causes
familial focal segmental glomerulosclerosis. Science.
308:1801–1804. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu BC, Song X, Lu XY, Li DT, Eaton DC,
Shen BZ, Li XQ and Ma HP: High glucose induces podocyte apoptosis
by stimulating TRPC6 via elevation of reactive oxygen species.
Biochim Biophys Acta 1833. 1434–1442. 2013.
|
|
16
|
Sonneveld R, van der Vlag J, Baltissen MP,
Verkaart SA, Wetzels JF, Berden JH, Hoenderop JG and Nijenhuis T:
Glucose specifically regulates TRPC6 expression in the podocyte in
an AngII-dependent manner. Am J Pathol. 184:1715–1726. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brenner BM, Cooper ME, de Zeeuw D, Keane
WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z and
Shahinfar S; RENAAL Study Investigators, : Effects of losartan on
renal and cardiovascular outcomes in patients with type 2 diabetes
and nephropathy. N Engl J Med. 345:861–869. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singh R, Singh AK and Leehey DJ: A novel
mechanism for angiotensin II formation in streptozotocin-diabetic
rat glomeruli. Am J Physiol Renal Physiol. 288:F1183–F1190. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vidotti DB, Casarini DE, Cristovam PC,
Leite CA, Schor N and Boim MA: High glucose concentration
stimulates intracellular renin activity and angiotensin II
generation in rat mesangial cells. Am J Physiol Renal Physiol.
286:F1039–F1045. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bansal SB, Sethi SK, Jha P, Duggal R and
Kher V: Remission of post-transplant focal segmental
glomerulosclerosis with angiotensin receptor blockers. Indian J
Nephrol. 27:154–156. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hamaguchi A, Kim S, Wanibuchi H and Iwao
H: Angiotensin II and calcium blockers prevent glomerular
phenotypic changes in remnant kidney model. J Am Soc Nephrol.
7:687–693. 1996.PubMed/NCBI
|
|
22
|
Hayashi K, Sasamura H, Ishiguro K,
Sakamaki Y, Azegami T and Itoh H: Regression of glomerulosclerosis
in response to transient treatment with angiotensin II blockers is
attenuated by blockade of matrix metalloproteinase-2. Kidney Int.
78:69–78. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suzuki K, Han GD, Miyauchi N, Hashimoto T,
Nakatsue T, Fujioka Y, Koike H, Shimizu F and Kawachi H:
Angiotensin II type 1 and type 2 receptors play opposite roles in
regulating the barrier function of kidney glomerular capillary
wall. Am J Pathol. 170:1841–1853. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gagliardini E, Perico N, Rizzo P, Buelli
S, Longaretti L, Perico L, Tomasoni S, Zoja C, Macconi D, Morigi M,
et al: Angiotensin II contributes to diabetic renal dysfunction in
rodents and humans via Notch1/Snail pathway. Am J Pathol.
183:119–130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leehey DJ, Singh AK, Alavi N and Singh R:
Role of angiotensin II in diabetic nephropathy. Kidney Int. (Suppl
77):S93–S98. 2000. View Article : Google Scholar
|
|
26
|
Kuwahara K, Wang Y, McAnally J, Richardson
JA, Bassel-Duby R, Hill JA and Olson EN: TRPC6 fulfills a
calcineurin signaling circuit during pathologic cardiac remodeling.
J Clin Invest. 116:3114–3126. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saleh SN, Albert AP, Peppiatt CM and Large
WA: Angiotensin II activates two cation conductances with distinct
TRPC1 and TRPC6 channel properties in rabbit mesenteric artery
myocytes. J Physiol. 577:479–495. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Anderson M, Roshanravan H, Khine J and
Dryer SE: Angiotensin II activation of TRPC6 channels in rat
podocytes requires generation of reactive oxygen species. J Cell
Physiol. 229:434–442. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen S, He FF, Wang H, Fang Z, Shao N,
Tian XJ, Liu JS, Zhu ZH, Wang YM, Wang S, et al: Calcium entry via
TRPC6 mediates albumin overload-induced endoplasmic reticulum
stress and apoptosis in podocytes. Cell Calcium. 50:523–529. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang H, Ding J, Fan Q and Liu S: TRPC6
up-regulation in Ang II-induced podocyte apoptosis might result
from ERK activation and NF-kappaB translocation. Exp Biol Med
(Maywood). 234:1029–1036. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schlöndorff J, Del Camino D, Carrasquillo
R, Lacey V and Pollak MR: TRPC6 mutations associated with focal
segmental glomerulosclerosis cause constitutive activation of
NFAT-dependent transcription. Am J Physiol Cell Physiol.
296:C558–C569. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nijenhuis T, Sloan AJ, Hoenderop JG,
Flesche J, van Goor H, Kistler AD, Bakker M, Bindels RJ, de Boer
RA, Möller CC, et al: Angiotensin II contributes to podocyte injury
by increasing TRPC6 expression via an NFAT-mediated positive
feedback signaling pathway. Am J Pathol. 179:1719–1732. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Danda RS, Habiba NM, Rincon-Choles H,
Bhandari BK, Barnes JL, Abboud HE and Pergola PE: Kidney
involvement in a nongenetic rat model of type 2 diabetes. Kidney
Int. 68:2562–2571. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Graham S, Ding M, Sours-Brothers S, Yorio
T, Ma JX and Ma R: Downregulation of TRPC6 protein expression by
high glucose, a possible mechanism for the impaired Ca2+ signaling
in glomerular mesangial cells in diabetes. Am J Physiol Renal
Physiol. 293:F1381–F1390. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ilatovskaya DV, Palygin O, Levchenko V,
Endres BT and Staruschenko A: The role of angiotensin II in
glomerular volume dynamics and podocyte calcium handling. Sci Rep.
7:2992017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Su J, Li SJ, Chen ZH, Zeng CH, Zhou H, Li
LS and Liu ZH: Evaluation of podocyte lesion in patients with
diabetic nephropathy: Wilms' tumor-1 protein used as a podocyte
marker. Diabetes Res Clin Pract. 87:167–175. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Marshall SM: The podocyte: A potential
therapeutic target in diabetic nephropathy? Curr Pharm Des.
13:2713–2720. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wolf G, Chen S and Ziyadeh FN: From the
periphery of the glomerular capillary wall toward the center of
disease: Podocyte injury comes of age in diabetic nephropathy.
Diabetes. 54:1626–1634. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Menè P, Punzo G and Pirozzi N: TRP
channels as therapeutic targets in kidney disease and hypertension.
Curr Top Med Chem. 13:386–397. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Thilo F, Liu Y, Loddenkemper C, Schuelein
R, Schmidt A, Yan Z, Zhu Z, Zakrzewicz A, Gollasch M and Tepel M:
VEGF regulates TRPC6 channels in podocytes. Nephrol Dial
Transplant. 27:921–929. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Weber EW, Han F, Tauseef M, Birnbaumer L,
Mehta D and Muller WA: TRPC6 is the endothelial calcium channel
that regulates leukocyte transendothelial migration during the
inflammatory response. J Exp Med. 212:1883–1899. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Durvasula RV and Shankland SJ: Activation
of a local renin angiotensin system in podocytes by glucose. Am J
Physiol Renal Physiol. 294:F830–F839. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yoo TH, Li JJ, Kim JJ, Jung DS, Kwak SJ,
Ryu DR, Choi HY, Kim JS, Kim HJ, Han SH, et al: Activation of the
renin-angiotensin system within podocytes in diabetes. Kidney Int.
71:1019–1027. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gabriel CH, Gross F, Karl M, Stephanowitz
H, Hennig AF, Weber M, Gryzik S, Bachmann I, Hecklau K, Wienands J,
et al: Identification of novel nuclear factor of activated T cell
(NFAT)-associated proteins in T cells. J Biol Chem.
291:24172–24187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pachulec E, Neitzke-Montinelli V and Viola
JP: NFAT2 regulates generation of innate-like CD8+ T
lymphocytes and CD8+ T lymphocytes responses. Front
Immunol. 7:4112016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang H, Zhao B, Liao C, Zhang R, Meng K,
Xu J and Jiao J: High glucose-induced apoptosis in cultured
podocytes involves TRPC6-dependent calcium entry via the RhoA/ROCK
pathway. Biochem Biophys Res Commun. 434:394–400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li Z, Xu J, Xu P, Liu S and Yang Z:
Wnt/β-catenin signalling pathway mediates high glucose induced cell
injury through activation of TRPC6 in podocytes. Cell Prolif.
46:76–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Qiu G and Ji Z: AngII-induced glomerular
mesangial cell proliferation inhibited by losartan via changes in
intracellular calcium ion concentration. Clin Exp Med. 14:169–176.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lawrence MC, Bhatt HS and Easom RA: NFAT
regulates insulin gene promoter activity in response to synergistic
pathways induced by glucose and glucagon-like peptide-1. Diabetes.
51:691–698. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nilsson J, Nilsson LM, Chen YW, Molkentin
JD, Erlinge D and Gomez MF: High glucose activates nuclear factor
of activated T cells in native vascular smooth muscle. Arterioscler
Thromb Vasc Biol. 26:794–800. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li R, Zhang L, Shi W, Zhang B, Liang X,
Liu S and Wang W: NFAT2 mediates high glucose-induced glomerular
podocyte apoptosis through increased Bax expression. Exp Cell Res.
319:992–1000. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang L, Chang JH, Paik SY, Tang Y, Eisner
W and Spurney RF: Calcineurin (CN) activation promotes apoptosis of
glomerular podocytes both in vitro and in vivo. Mol Endocrinol.
25:1376–1386. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang L, Li R, Shi W, Liang X, Liu S, Ye
Z, Yu C, Chen Y, Zhang B, Wang W, et al: NFAT2 inhibitor
ameliorates diabetic nephropathy and podocyte injury in db/db mice.
Br J Pharmacol. 170:426–439. 2013. View Article : Google Scholar : PubMed/NCBI
|