Introduction
Nasopharyngeal carcinoma (NPC) is a type of head and
neck cancer (1,2). The incidence of NPC is the highest
near the parts of or in the ear, nose and throat malignancies. The
incidence of NPC has obvious regional clusters and certain ethnic
groups are likely to experience a higher incidence of NPC than
others. Incidence is low in most areas of the world, generally
below 1/105 (3).
However, in China NPC is mainly distributed in southern China and
Southeast Asia (4). The onset ages
of NPC are mostly between 40–60 years old, with males having a
higher incidence compared with their female counterparts (5). The etiology of NPC includes viral
infections, genetic factors, environmental and dietary factors as
well as the interaction of various oncogenes and tumor suppressor
genes (6). Almost 70% of NPC
patients are diagnosed at the advanced stages and the five-year
survival rates are lower than 10–40% (7). The main factors contributing to the
failure of treatment are the characteristics of tumor recurrence
and distant metastasis of NPC (8).
Therefore, it is becoming increasingly significant to study how to
reduce distant metastasis so as to improve the outcome of treating
NPC (9).
The epithelial-mesenchymal transition (EMT) process
is prevalent during tumor metastasis. EMT is characterized by
weakened intercellular adhesion and increased metastasis associated
factors, for example, matrix metalloproteinases (MMPs) (10,11).
Tumor cells with EMT frequently manifest invasive phenotypes, which
are associated with difficulty treating the tumor (12,13).
Notably, a couple of studies have focused on how biomarkers
regulate EMT in NPC (14,15). In addition, the aberrant activation
of the Wnt/β-catenin pathway is commonly reported to be involved in
the EMT process of NPC (16).
In recent years, research on NPC in association with
tumor biomarkers has attracted much attention and such studies aid
early diagnosis, treatment efficacy and prognosis of NPC (17–20).
Keratin is a major component of the intermediate filament protein
family and is also a major structural protein of epidermal, and
hair keratinocytes (21). The
expression of keratin is mainly in epithelial tissues (22,23).
Understanding the expression of keratin genes and their normal
function are the basis for maintaining the stability and normal
differentiation of epidermal cells (24). A previous study demonstrated that
keratin may also function to regulate cell growth and apoptosis as
well as protecting cells from impairment and non-mechanical stress
(25). Keratin 6A (KRT6A) is a
family member of keratin proteins and can lead to epidermalization
of squamous epithelium (26,27).
KRT6A is a biomarker that is unique to squamous cells (28), while NPC is an undifferentiated
squamous cell carcinoma (29,30).
However, the correlation of KRT6A and NPC still remains
unclear.
In the present study, the KRT6A silencing cell model
was constructed in order to investigate the association between
KRT6A and EMT in NPC. Taken from the perspective of tumor
metastasis, this study help further understand the formation of
NPC, aiming to improve the diagnostic compliance rate and survival
rate of NPC.
Materials and methods
Cell culture
Human nasopharyngeal epithelial cell line (NP69, as
a control) and NPC cell lines (C666-1, 5-8F and SUNE-1) were
purchased from the American Type Culture Collection (Manassas, MA,
USA), and cultured in 5% CO2 at 37°C. Dulbecco's
modified Eagle's medium (DMEM) was used as basic culture medium.
10% fetal bovine serum (FBS), 100 U/ml streptomycin and 100 µg/ml
penicillin were then added in to the medium. DMEM, FBS,
streptomycin and penicillin were purchased from Biological
Industries (Kibbutz Beit Haemek, Israel). Lithium chloride (LiCl;
20 mM; incubation time 24 h) was purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany) and was used to activate the Wnt
signaling pathway as previously described (31).
Small interfering (si)RNA
transfection
The well-differentiated SUNE-1 cells were evenly
plated in 6-well plates at the initial concentration of
6×104 cells/well. After being cultured for 12 h, the
cells reached 60–70% confluence. The cells were cultured in DMEM
without FBS. A mixture of 50 pmol KRT6A-siRNA (Shanghai GeneChem
Co., Ltd., Shanghai, China), with the sequence of sense
5′-CCAGCAGGAAGAGCUAUA-3′, and antisense 3′-GGUCGUCCUUCUCGAUAUU, and
transfection reagent Lipofectamine® 3000 (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) (2:1), or 50 pmol
scrambled siRNA (as control; Shanghai GeneChem Co., Ltd.), with the
sequence of sense: 5′-UUCUCCGAACGUGUCACGUTT-3′, and antisense:
5′-ACGUGACACGUUCGGAGAA-3′ in mixture with Lipofectamine®
3000 was respectively added to the cells, and cultured for 8 h at
37°C. The transfected cells were accordingly named the siKRT6A
group and the mock group. Cells with non-treatment were named the
Cotl group and treated as the control. The cells were then cultured
in DMEM with FBS for another 48 h before further measurement of
activity.
Cell viability assay
The effect of KRT6A silencing on cell viability of
NPC SUNE-1 cells was measured using Cell Counting Kit-8 (CCK-8;
Beyotime Institute of Biotechnology, Haimen, China) assay. To be
more specific, SUNE-1 cells transfected with KRT6A-siRNA or
scrambled-siRNA, or untreated SUNE-1 cells were respectively seeded
in 96-well plates at an initial density of 2×103
cells/well and incubated at 37°C for 12, 24, and 48 h. Next, 20 µl
CCK-8 reagent was added to each well. Then the plate was incubated
at 37°C for 3 h. Finally, optical density values were read at 450
nm using a microplate reader (BioTek Instruments, Inc., Winooski,
VT, USA). The ratios of cell viabilities to Cotl group at 0 h were
compared.
Cell scratch wound healing assay
The effect of KRT6A silencing on cell metastasis
ability of NPC SUNE-1 cells was measured using wound healing assay.
To be more specific, SUNE-1 cells transfected with KRT6A-siRNA or
scrambled-siRNA, or SUNE-1 cells without transfection were
respectively seeded in 6-well plates at an initial density of
6×104 cells/well. After reaching 80% confluence, a wound
was created using pipette tip scratching. After being cultured for
24 h, the wound closure size images in various fields were captured
and measured by light microscope.
Transwell assay
Cell invasion abilities were measured using
Transwell plates with 8-µm pore filter (Corning, Inc., Corning, NY,
USA) coated with Matrigel (BD Biosciences; Becton, Dickinson and
Company, Franklin Lakes, NJ, USA). Cells (6×104
cells/well) suspended in serum-free medium were seeded in the
upper-chambers, while medium with 20% FBS was added to the
lower-chambers in order to induce cell invasion. After being
cultured at 37°C for 24 h, the cells were stained with crystal
violet (Beyotime Institute of Biotechnology) for 15 min at room
temperature. Finally, cells images were captured and invaded cell
numbers were counted under a light microscope.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The mRNA expression levels of KRT6A in NP69
nasopharyngeal epithelial cells and different NPC cell lines
(C666-1, 5-8F and SUNE-1) were determined. After conducting KRT6A
silencing, levels of KRT6A, E-cadherin, tissue inhibitor of
metalloproteinase-2 (TIMP-2), MMP-2, MMP-9, β-catenin, lymphoid
enhancer binding factor 1 (LEF-1) and T-cell specific factor 4
(TCF-4) were determined in the Cotl, mock and siKRT6A cell groups.
Primers used are listed in Table I
and GAPDH was treated as an internal control. The process was
performed as follows: Total RNA was first extracted using the
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and
then reverse transcribed to cDNA using Transcriptor Universal cDNA
Master (Roche Diagnostics, Indianapolis, IN, USA), with the
protocol of 70°C for 5 min and 42°C for 60 min. Then, the PCR
amplification process was conducted using LightCycler®
Multiplex Masters (Roche Diagnostics) with LightCycler®
480II System (Roche Diagnostics) under the conditions as follows:
Pre-denaturation at 95°C for 2 min, 36 cycles (denaturation at 95°C
for 25 sec, annealing at 60°C for 25 sec, extension at 72°C for 30
sec) and a final extension at 72°C for 10 min. The changes were
calculated by the 2−ΔΔCq method (32).
 | Table I.Primer sequences used in the reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used in the reverse
transcription-quantitative polymerase chain reaction.
| Name | Type | Sequence
(5′-3′) |
|---|
| GAPDH | Forward |
CCATCTTCCAGGAGCGAGAT |
|
| Reverse |
TGCTGATGATCTTGAGGCTG |
| KRT6A | Forward |
TCACCGTCAACCAGAGTCTC |
|
| Reverse |
GAACCTTGTTCTGCTGCTCC |
| E-cadherin | Forward |
ACGCATTGCCACATACACTC |
|
| Reverse |
GGTGTTCACATCATCGTCCG |
| TIMP-2 | Forward |
TTCAAAGGGCCTGAGAAGGA |
|
| Reverse |
TCAGGCTCTTCTTCTGGGTG |
| MMP-2 | Forward |
CAGCCCTGCAAGTTTCCATT |
|
| Reverse |
GTTGCCCAGGAAAGTGAAGG |
| MMP-9 | Forward |
GAGACTCTACACCCAGGACG |
|
| Reverse |
GAAAGTGAAGGGGAAGACGC |
| β-Catenin | Forward |
CTTGTGCGTACTGTCCTTCG |
|
| Reverse |
AGTGGGATGGTGGGTGTAAG |
| LEF-1 | Forward |
ACGAGCACTTTTCTCCAGGA |
|
| Reverse |
ATAGCTGGATGAGGGATGCC |
| TCF-4 | Forward |
TCCTCCGATGTCCACTTTCC |
|
| Reverse |
CCTGCTGAGAGAGATGGAGG |
Western blotting
The protein levels of KRT6A were detected in NP69
nasopharyngeal epithelial cells and in the different NPC cell lines
(C666-1, 5-8F and SUNE-1). After conducting KRT6A silencing, levels
of KRT6A, E-cadherin, TIMP-2, MMP-2, MMP-9, β-catenin, LEF-1 and
TCF-4 were measured in Cotl, mock and siKRT6A cell groups. The
cells were lysed on ice using radio immunoprecipitation assay lysis
buffer (Wuhan Boster Biological Technology, Ltd., Wuhan, China) for
30 min and centrifuged at 1×104 × g at 4°C for 20 min.
Next, the proteins in supernatant were quantified using BCA protein
assay kit (Pierce; Thermo Fisher Scientific, Inc.). A total of 20
µg protein was first loaded to each well that contained 12%
SDS-PAGE and then separated and transferred onto polyvinylidene
fluoride membranes (Thermo Fisher Scientific, Inc.). Next, the
membranes were blocked in 5% non-fat dry milk for 1 h at room
temperature and incubated with specific primary antibodies
overnight at 4°C. Finally, the membranes were incubated with
secondary antibodies conjugated with horseradish peroxidase:
Anti-rabbit IgG, HRP-linked Antibody (Cell Signaling Technology,
Inc., Danvers, MA, USA; cat. no. 7074, 1:5,000) for 1 h at room
temperature. GAPDH was treated as a loading control. The proteins
were detected by enhanced chemiluminescence detection reagents
(Pierce; Thermo Fisher Scientific, Inc.) and analyzed by Bio-Rad
ChemiDoc XRS densitometry with Image Lab™ Software version 4.1
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The primary
antibodies used were as follows: Rabbit anti-KRT6A (Abcam,
Cambridge, UK; cat. no. ab18586; 1:200), E-cadherin (Abcam; cat.
no. ab15148; 1:500), TIMP-2 (Abcam; cat. no. ab180630; 1:1,000),
MMP-2 (Abcam; cat. no. ab92536; 1:1,000), MMP-9 (Abcam; cat. no.
ab38898; 1:1,000), β-catenin (Abcam; cat. no. ab16051; 1:5,000),
LEF-1 (Abcam; cat. no. ab22884; 1:500), TCF-4 (Abcam; cat. no.
ab185736; 1:1,000) and GAPDH (Abcam; cat. no. ab9485; 1:2,000).
Statistical analysis
SPSS software (version 20.0; IBM Corps, Armonk, NY,
USA) was used for data analysis. Three replications were conducted
in each assay. Data were expressed with the mean ± standard
deviation. The data were analyzed by one-way analysis of variance
and Dunnett t's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
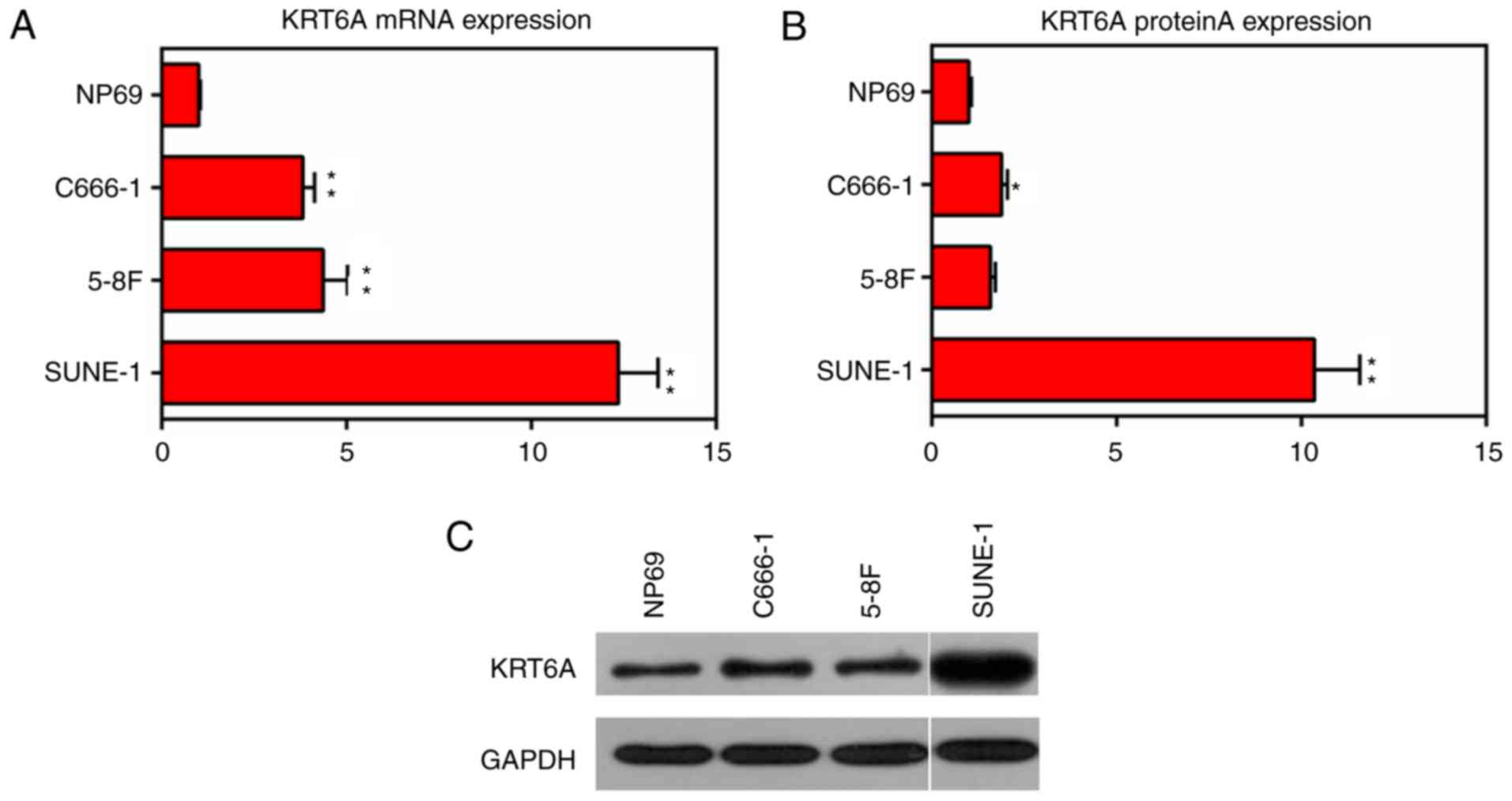
KRT6A is upregulated in NPC cells
The expression levels of KRT6A in the nasopharyngeal
epithelial cell line (NP69, as control) and NPC cell lines (C666-1,
5-8F and SUNE-1) were measured. The results of RT-qPCR and western
blotting demonstrated that the mRNA and protein levels of KRT6A
were significantly upregulated in all detected NPC cells, among
which the value was the highest in the SUNE-1 cells (P<0.01;
Fig. 1). Therefore, SUNE-1 cells
were selected for the following gene modification experiments.
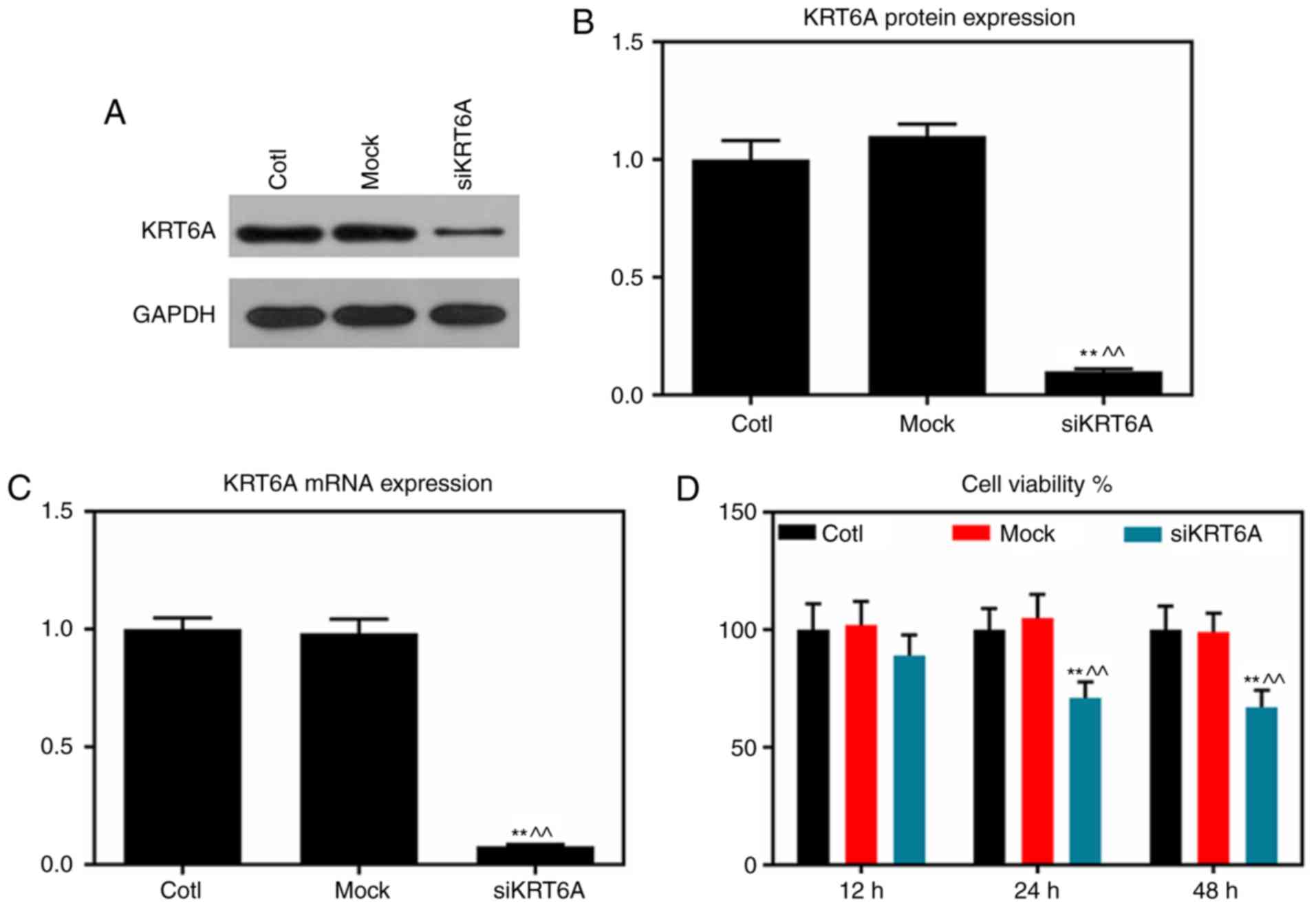
KRT6A silencing inhibits the cell
viability of NPC cells
KRT6A-siRNA was transfected into NPC SUNE-1 cells
using the liposome method. RT-qPCR and western blot assays analysis
demonstrated that the mRNA and protein levels of KRT6A were
significantly decreased in the siKRT6A group, compared with those
of the Cotl and mock groups (P<0.01; Fig. 2A-C). CCK-8 assay results
demonstrated that cell viabilities in the siKRT6A group was
inhibited compared with the two groups, Cotl and mock (P<0.01;
Fig. 2D).
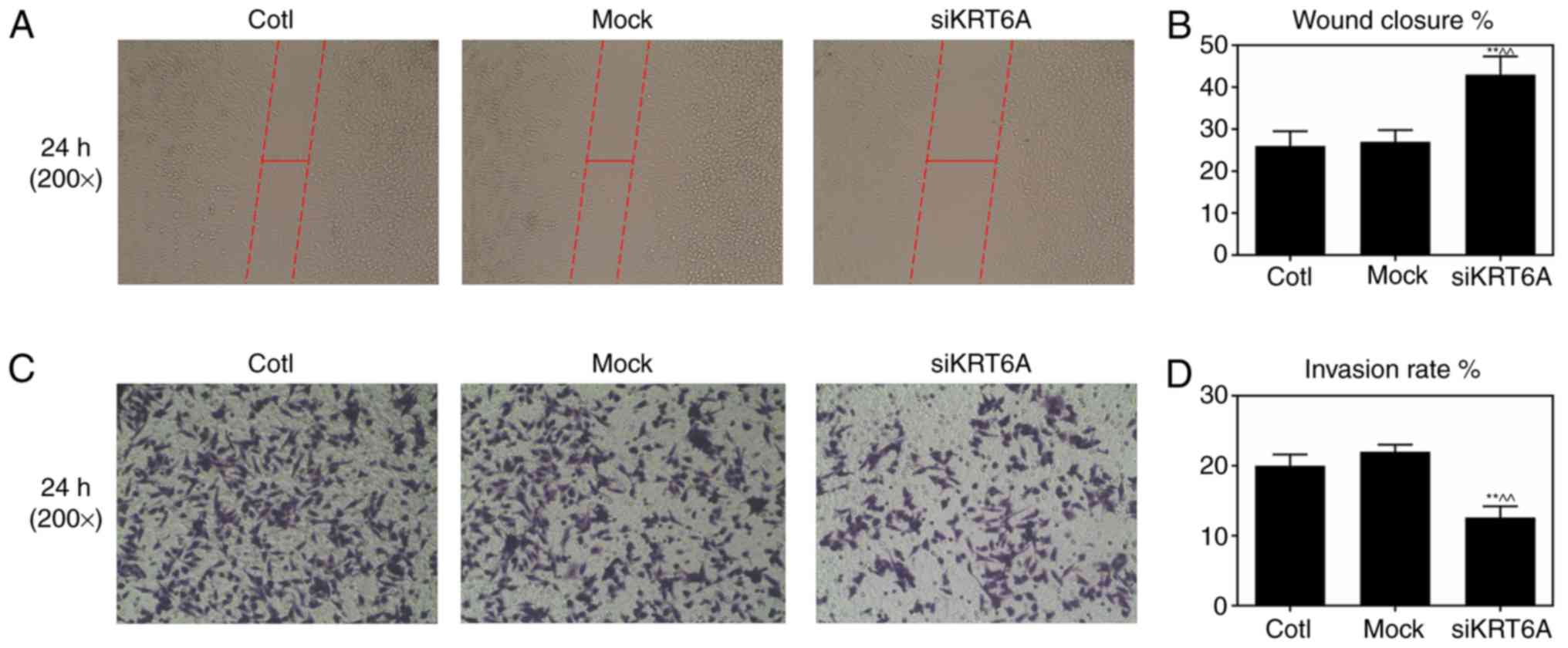
KRT6A silencing inhibits cell
metastasis and invasion of NPC cells
Cell metastasis and invasive abilities of SUNE-1 NPC
cells prior to and following performing KRT6A silencing were
measured by scratch wound healing and Transwell assays,
respectively following 24 h of cell growth. The wound distance
siKRT6A group were significantly increased compared with the Cotl
and mock groups, indicating that KRT6A silencing inhibited cell
metastasis of SUNE-1 NPC cells (P<0.01; Fig. 3A and B). In addition, the invasion
rates of SUNE-1 NPC cells significantly decreased in the siKRT6A
group, suggesting that KRT6A silencing inhibited cell invasion of
SUNE-1 NPC cells (P<0.01; Fig. 3C
and D).
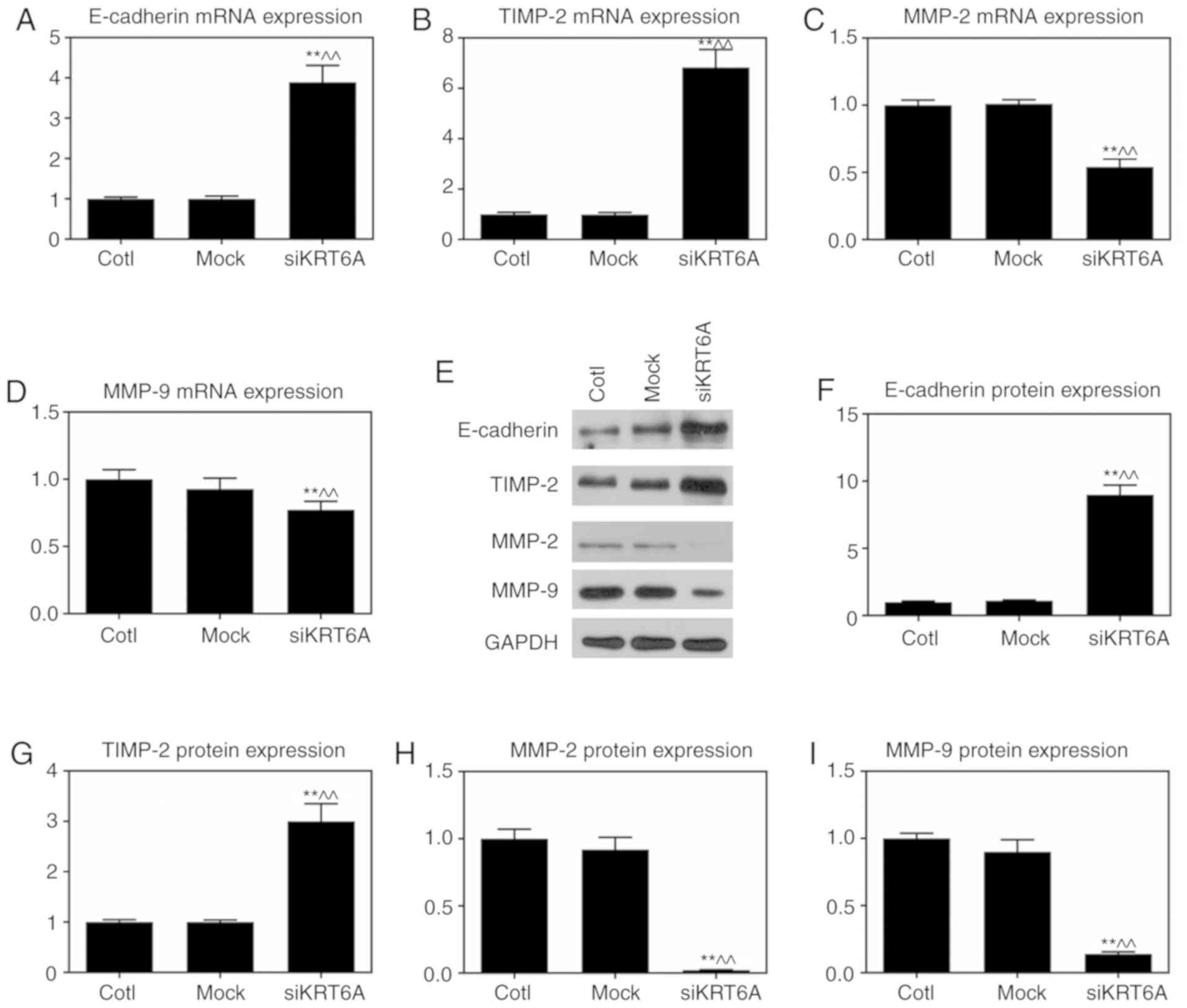
KRT6A silencing attenuates EMT in NPC
cells
EMT is the major mechanism of cell metastasis and
invasion, Therefore, the epithelial marker E-cadherin and matrix
degrading enzyme-associated factors were measured. RT-qPCR
demonstrated though the mRNA and protein expression levels of
E-cadherin and TIMP-2 were significantly promoted in the siKRT6A
group (P<0.01), the mRNA and protein expression levels of MMP-2
and MMP-9 were significantly reduced compared with those in both
the Cotl and mock groups (P<0.01; Fig. 4).
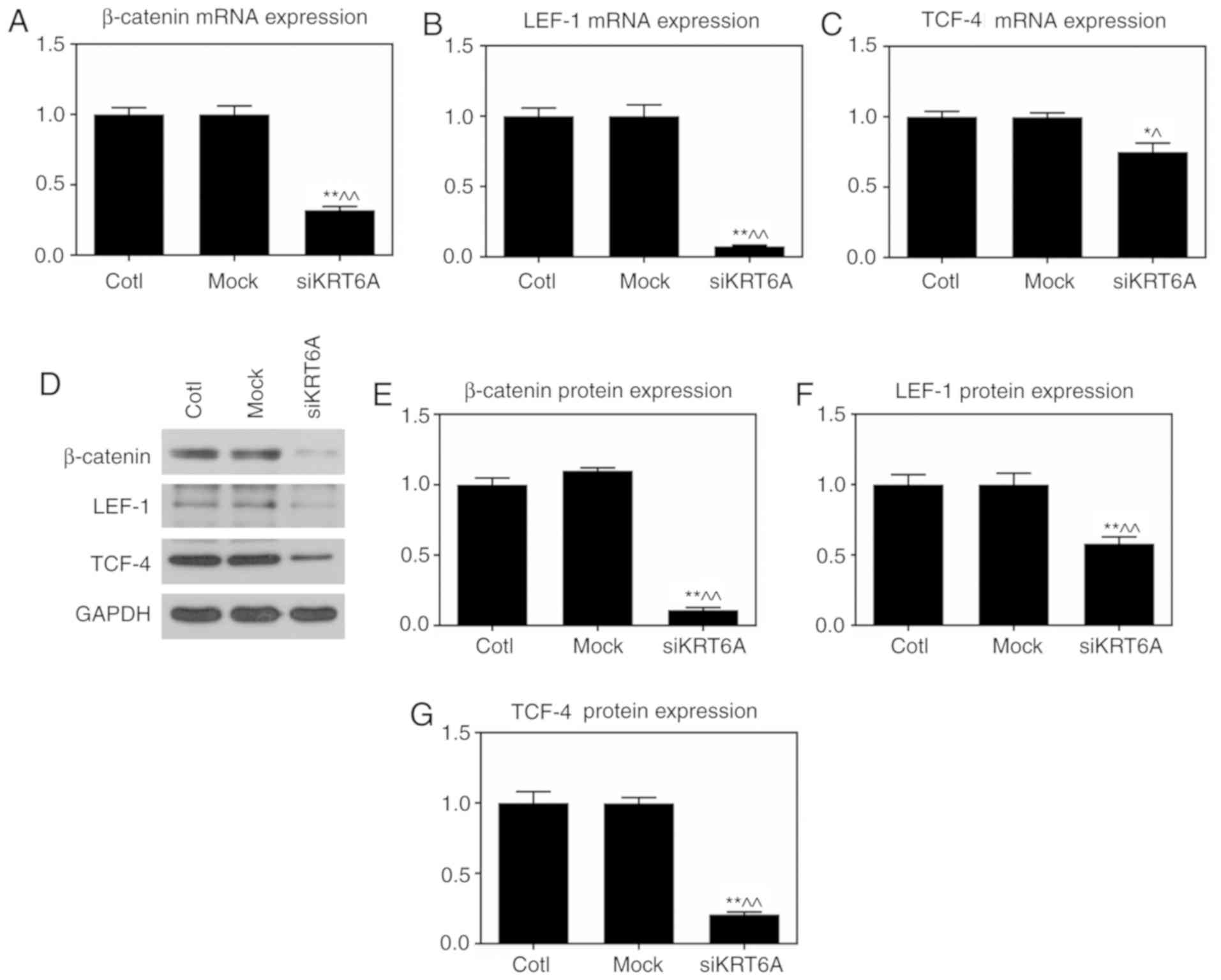
KRT6A silencing inhibits the
Wnt/β-catenin pathway in NPC cells
As the Wnt/β-catenin pathway strongly affects
tumorigenesis, the effect of KRT6A silencing on the Wnt/β-catenin
pathway was investigated using RT-qPCR and western blot assays. The
results demonstrated that both the mRNA and protein levels of
β-catenin, LEF-1 and TCF-4 were significantly reduced in the
siKRT6A group, compared with those in both the Cotl and mock groups
(P<0.01; Fig. 5).
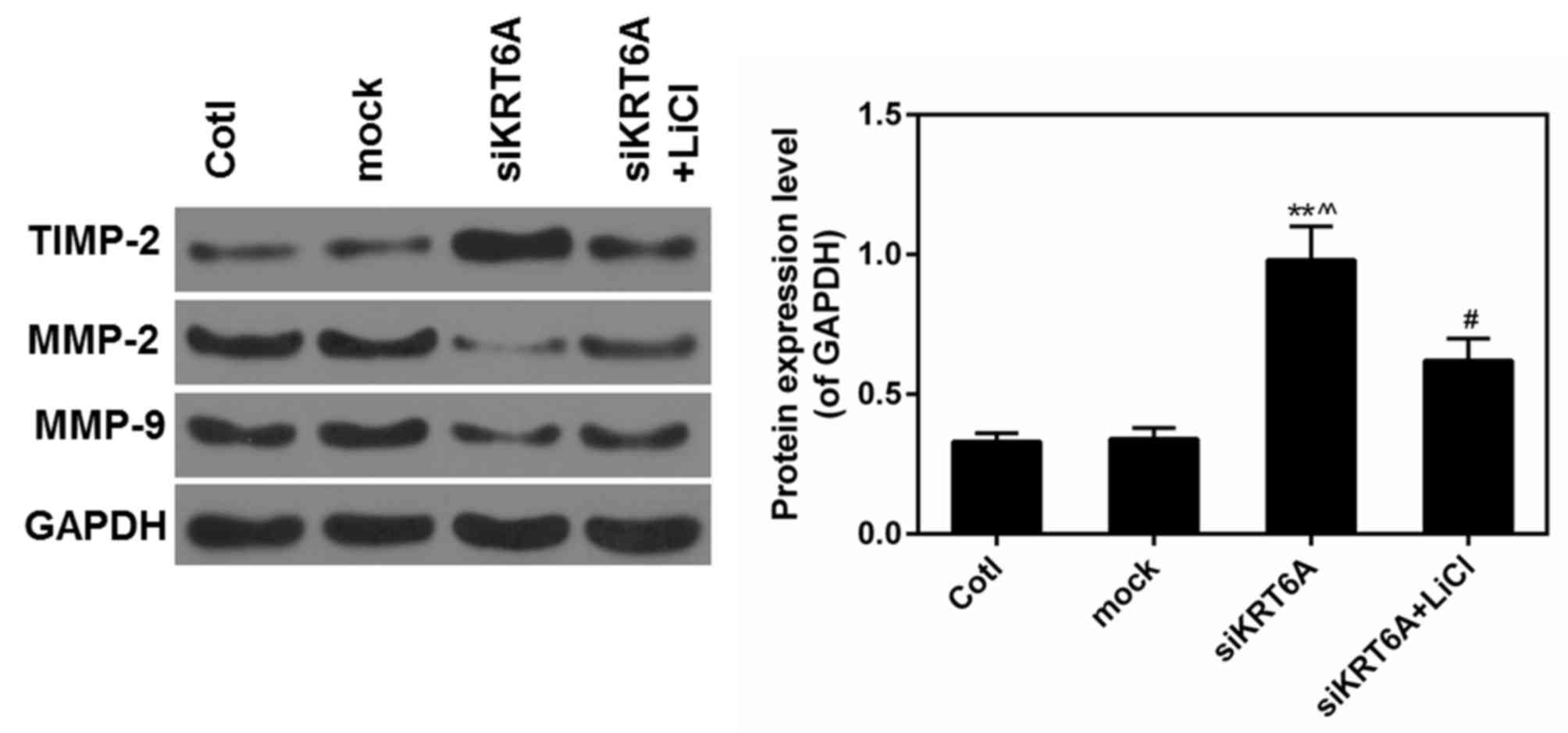
Activation of the Wnt/β-catenin
pathway reverses the effect of si-KRT6A
LiCl was used to activate the Wnt/β-catenin pathway
and determined the association between the Wnt/β-catenin pathway
and si-KRT6A. It was observed that decreased expression of MMP2/9
caused by si-KRT6A was then rescued by LiCl. By contrast, the
expression of TIMP2 was significantly decreased in LiCl+si-KRT6A
group compared with the si-KRT6A group (P<0.05; Fig. 6). This suggested that the effect of
si-KRT6A on NPC cells may be largely dependent on the inactivation
of the Wnt/β-catenin pathway.
Discussion
NPC is widely regarded as an undifferentiated
squamous cell carcinoma that is characterized by distant metastasis
(17,33). KRT6A is a biomarker unique to
squamous cells and the correct expression of keratin genes is the
basis for maintaining the stability, and normal differentiation of
epidermal cells (28). Therefore,
it is interesting to know whether KRT6A affects cell growth and
differentiation as well as distant metastasis in NPC. Therefore,
this study focused on investigating the mechanisms of ectopic KRT6A
in NPC. The expression levels of KRT6A in multiple NPC cell lines,
which include C666-1, 5-8F and SUNE-1 cells were first measured. It
was demonstrated that the expression of KRT6A was high in SUNE-1.
This may be explained by the fact that SUNE-1 is a type of poorly
differentiated carcinoma cell. The poorly differentiated carcinoma
cells have a high malignancy rate and can invade into distant
organs and tissues. Therefore, KRT6A silencing was conducted in
SUNE-1 cells and the effects of KRT6A silencing on cell viability,
metastasis, and invasion of SUNE-1 NPC cells were determined.
As the process of EMT is critical to tumor
metastasis and it has been reported to be involved in the
modification process of numerous biomarkers (34), the expression levels of certain
critical factors were detected in the EMT process. Being
responsible for cell-cell adhesion in epithelial cells, E-cadherin
is one of the most critical hallmarks of EMT (35,36).
Decrease of E-cadherin induces weakened epithelial characteristics
and transition to the mesenchymal phenotype (37). In the present study, upregulated
E-cadherin was tested when KRT6A was silenced in NPC cells. MMPs
are the main proteolytic enzymes in the process of tumor
metastasis, during which MMPs destroy the histological barrier for
cell invasion (38–40). Type IV collagenases, including
MMP-2 and MMP-9, are important in affecting tumor metastasis
(10,41,42).
The present study demonstrated that KRT6A silencing suppressed the
expression of MMP-2 and MMP-9, as well as facilitated the
expression of TIMP-2 in NPC cells.
The Wnt/β-catenin pathway is correlated with the EMT
process in cancer. During the EMT process the stabilization and
nuclear translocation of β-catenin is the critical events in
Wnt/β-catenin pathway (43–46).
LEF-1 and TCF-4, two key members of TCF family, mediate the
regulation of β-catenin-dependent transcription factors (47–50).
To investigate the effect of KRT6A on the canonical β-catenin/TCF
pathway in NPC, alterations of the expression of β-catenin, LEF-1
and TCF-4 were identified when KRT6A was silenced in NPC cells. The
results proved that KRT6A silencing inhibited the expression of
β-catenin, LEF-1 and TCF-4 so as to inactivate β-catenin/TCF
pathway. In addition, the activation of Wnt/β-catenin pathway,
which was caused by LiCl, reversed the effect of KRT6A silencing by
regulating the expression of TIMP2 and MMP2/9. These results
suggested that KRT6A silencing may produce its anti-tumor effect
largely by inhibiting the Wnt/β-catenin pathway.
The present study demonstrated the anti-tumor role
of KRT6A silencing in NPC cells. However, it is equally significant
to further investigate the effect of KRT6A through upregulating its
expression, as well as to confirm the role of KRT6A in animal
experiments.
In conclusion, KRT6A silencing suppressed cell
viability, invasion and metastasis of NPC cells via β-catenin/TCF
pathway. Therefore, KRT6A may be a novel biomarker in the diagnosis
and treatment of NPC. Further research in vivo is required
in order to provide more evidence that illustrates the role of
KRT6A.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CC performed all the experiments. HS contributed to
the conception of the study and wrote the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu TF: Recent developments in diagnosis
and treatment of nasopharyngeal carcinoma. Keio J Med. 40:59–62.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wyatt DE, Brooker DS, Connolly JH and
Coyle PV: Prognostic value of Epstein-Barr virus serology in
patients with nasopharyngeal carcinoma. J Infect. 26:171–175. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Y, Shen C, Lu X and Hu C: The
incidence and prognosis of nasopharyngeal carcinoma patients with
family history. Oncotarget. 8:97323–97330. 2017.PubMed/NCBI
|
|
4
|
Wei KR, Zheng RS, Zhang SW, Liang ZH, Li
ZM and Chen WQ: Nasopharyngeal carcinoma incidence and mortality in
China, 2013. Chin J Cancer. 36:902017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu TX: Advance in diagnosis and management
of local recurrent nasopharyngeal carcinoma. Ai Zheng. 23:230–234.
2004.(In Chinese). PubMed/NCBI
|
|
6
|
Xia L, Zhang W, Zhu L and Yu G: Abnormally
expressed long non-coding RNAs in nasopharyngeal carcinoma: A
meta-analysis. Clin Lab. 64:585–595. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chan AT, Leung TW, Kwan WH, Mok TS, Yeo W,
Lai M and Johnson PJ: Phase II study of Temodal in the treatment of
patients with advanced nasopharyngeal carcinoma. Cancer Chemother
Pharmacol. 42:247–249. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Y, Ou X, Shen C, Xu T, Li W and Hu C:
Patterns of local failures and suggestions for reduction of
clinical target volume for nasopharyngeal carcinoma patients
without cervical lymph node metastasis. Onco Targets Ther.
11:2545–2555. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qi XK, Han HQ, Zhang HJ, Xu M, Li L, Chen
L, Xiang T, Feng QS, Kang T, Qian CN, et al: OVOL2 links stemness
and metastasis via fine-tuning epithelial-mesenchymal transition in
nasopharyngeal carcinoma. Theranostics. 8:2202–2216. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ogasawara N, Kudo T, Sato M, Kawasaki Y,
Yonezawa S, Takahashi S, Miyagi Y, Natori Y and Sugiyama A:
Reduction of membrane protein CRIM1 decreases E-cadherin and
increases claudin-1 and MMPs, enhancing the migration and invasion
of renal carcinoma cells. Biol Pharm Bull. 41:604–611. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tuo Z, Zhang J and Xue W: LncRNA TP73-AS1
predicts the prognosis of bladder cancer patients and functions as
a suppressor for bladder cancer by EMT pathway. Biochem Biophys Res
Commun. 499:875–881. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi B, Wang Y and Yin F:
MALAT1/miR-124/Capn4 axis regulates proliferation, invasion and EMT
in nasopharyngeal carcinoma cells. Cancer Biol Ther. 18:792–800.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li S, Zhang X, Zhang R, Liang Z, Liao W,
Du Z, Gao C, Liu F, Fan Y and Hong H: Hippo pathway contributes to
cisplatin resistant-induced EMT in nasopharyngeal carcinoma cells.
Cell Cycle. 16:1601–1610. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zuo LL, Zhang J, Liu LZ, Zhou Q, Du SJ,
Xin SY, Ning ZP, Yang J, Yu HB, Yue WX, et al: Cadherin 6 is
activated by Epstein-Barr virus LMP1 to mediate EMT and metastasis
as an interplay node of multiple pathways in nasopharyngeal
carcinoma. Oncogenesis. 6:4022017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu SL, Li YJ, Liao K, Shi L, Zhang N, Liu
S, Hu YY, Li SL and Wang Y: 2-Methoxyestradiol inhibits the
proliferation and migration and reduces the radioresistance of
nasopharyngeal carcinoma CNE-2 stem cells via NF-κB/HIF-1 signaling
pathway inactivation and EMT reversal. Oncol Rep. 37:793–802. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu Y, Qi MF, Xu QL, Kong XY, Cai R, Chen
QQ, Tang HY and Jiang W: Candidate tumor suppressor ZNF154
suppresses invasion and metastasis in NPC by inhibiting the EMT via
Wnt/β-catenin signalling. Oncotarget. 8:85749–85758.
2017.PubMed/NCBI
|
|
17
|
Wang G, Zhang L, Zhou Y, Sun Q, Xu H, Cai
F, Xiang P, Chen Z and Jiang H: KAI1/CD82 genetically engineered
endothelial progenitor cells inhibit metastasis of human
nasopharyngeal carcinoma in a mouse model. Med Sci Monit.
24:3146–3152. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aouf S, Laribi A, Gabbouj S, Hassen E,
Bouaouinaa N, Zakhama A and Harizi H: Contribution of Nitric oxide
synthase 3 genetic variants to nasopharyngeal carcinoma risk and
progression in a Tunisian population. Eur Arch Otorhinolaryngol.
13–Feb;2019.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chua HH, Kameyama T, Mayeda A and Yeh TH:
Cancer-specifically re-spliced TSG101 mRNA promotes invasion and
metastasis of nasopharyngeal carcinoma. Int J Mol Sci. 20(pii):
E7732019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Petersson F: EBV-associated
non-keratinizing nasopharyngeal carcinoma with prominent spindled
cell and whorling patterns: A previously unreported histological
variant in a patient presenting with dermatomyositis. Head Neck
Pathol. 13–Feb;2019.(Epub ahead of print). View Article : Google Scholar :
|
|
21
|
Xiao H, Guo Y, Yi J, Xia H, Xu H, Yuan L,
Hu P, Yang Z, He Z, Lu H and Deng H: Identification of a novel
keratin 9 missense mutation in a chinese family with epidermolytic
palmoplantar keratoderma. Cell Physiol Biochem. 46:1919–1929. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jacob JT, Coulombe PA, Kwan R and Omary
MB: Types I and II keratin intermediate filaments. Cold Spring Harb
Perspect Biol. 10(pii): a0182752018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Komori T, Ono M, Hara ES, Ueda J, Nguyen
HTT, Nguyen HT, Yonezawa T, Maeba T, Kimura-Ono A, Takarada T, et
al: Type IV collagen α6 chain is a regulator of keratin 10 in
keratinization of oral mucosal epithelium. Sci Rep. 8:26122018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deo PN and Deshmukh R: Pathophysiology of
keratinization. J Oral Maxillofac Pathol. 22:86–91. 2018.PubMed/NCBI
|
|
25
|
Gu LH and Coulombe PA: Keratin function in
skin epithelia: A broadening palette with surprising shades. Curr
Opin Cell Biol. 19:13–23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chang HH, Dreyfuss JM and Ramoni MF: A
transcriptional network signature characterizes lung cancer
subtypes. Cancer. 117:353–360. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Forrest CE, Casey G, Mordaunt DA, Thompson
EM and Gordon L: Pachyonychia congenita: A spectrum of KRT6a
mutations in australian patients. Pediatr Dermatol. 33:337–342.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cammarata-Scalisi F, Natsuga K, Toyonaga
E, Nishie W, Shimizu H, Avendaño A, Araque D, Da Silva G,
Bellacchio E and Callea M: Early severe pachyonychia congenita
subtype PC-K6a with a novel mutation in the KRT6A gene. J Eur Acad
Dermatol Venereol. 31:e94–e96. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chai AW, Cheung AK, Dai W, Ko JM, Ip JC,
Chan KW, Kwong DL, Ng WT, Lee AW, Ngan RK, et al:
Metastasis-suppressing NID2, an epigenetically-silenced gene, in
the pathogenesis of nasopharyngeal carcinoma and esophageal
squamous cell carcinoma. Oncotarget. 7:78859–78871. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang P, Zhang L, Liu H, Zhao L, Li Y,
Shen JX, Liu Q, Liu MZ and Xi M: Clinicopathologic characteristics
and prognosis of tongue squamous cell carcinoma in patients with
and without a history of radiation for nasopharyngeal carcinoma: A
matched case-control study. Cancer Res Treat. 49:695–705. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Clement-Lacroix P, Ai M, Morvan F,
Roman-Roman S, Vayssière B, Belleville C, Estrera K, Warman ML,
Baron R and Rawadi G: Lrp5-independent activation of Wnt signaling
by lithium chloride increases bone formation and bone mass in mice.
Proc Natl Acad Sci USA. 102:17406–17411. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang P, Shen N, Liu D, Ning X, Wu D and
Huang X: TRIM24 siRNA induced cell apoptosis and reduced cell
viability in human nasopharyngeal carcinoma cells. Mol Med Rep.
18:369–376. 2018.PubMed/NCBI
|
|
34
|
Setyaningsih WAW, Arfian N, Suryadi E,
Romi MM, Tranggono U and Sari DCR: Hyperuricemia induces Wnt5a/Ror2
gene expression, epithelial-mesenchymal transition, and kidney
tubular injury in mice. Iran J Med Sci. 43:164–173. 2018.PubMed/NCBI
|
|
35
|
Yang F, Wei K, Qin Z, Liu W, Shao C, Wang
C, Ma L, Xie M, Shu Y and Shen H: MiR-598 suppresses invasion and
migration by negative regulation of derlin-1 and
epithelial-mesenchymal transition in non-small cell lung cancer.
Cell Physiol Biochem. 47:245–256. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zheng P, Li H, Xu P, Wang X, Shi Z, Han Q
and Li Z: High lncRNA HULC expression is associated with poor
prognosis and promotes tumor progression by regulating
epithelial-mesenchymal transition in prostate cancer. Arch Med Sci.
14:679–686. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Y, Zhao L, Wang L, Yang X, Zhou A
and Wang J: Placental growth factor promotes epithelial-mesenchymal
transition-like changes in ARPE-19 cells under hypoxia. Mol Vis.
24:340–352. 2018.PubMed/NCBI
|
|
38
|
Chiu YJ, Hour MJ, Jin YA, Lu CC, Tsai FJ,
Chen TL, Ma H, Juan YN and Yang JS: Disruption of IGF-1R signaling
by a novel quinazoline derivative, HMJ-30, inhibits invasiveness
and reverses epithelial-mesenchymal transition in osteosarcoma U-2
OS cells. Int J Oncol. 16–Mar;2018.(Epub ahead of print).
View Article : Google Scholar
|
|
39
|
Ciaramella V, Della Corte CM, Di Mauro C,
Tomassi S, Di Maro S, Troiani T, Martinelli E, Bianco R, Cosconati
S, Pierantoni R, et al: Antitumor efficacy of Kisspeptin in human
malignant mesothelioma cells. Oncotarget. 9:19273–19282. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fu X, Zhu Y, Zheng B, Zou Y, Wang C, Wu P,
Wang J, Chen H, Du P, Liang B and Fang L: KIFC1, a novel potential
prognostic factor and therapeutic target in hepatocellular
carcinoma. Int J Oncol. 52:1912–1922. 2018.PubMed/NCBI
|
|
41
|
Jiang Y, Jiao Y, Liu Y, Zhang M, Wang Z,
Li Y, Li T, Zhao X and Wang D: Sinomenine hydrochloride inhibits
the metastasis of human glioblastoma cells by suppressing the
expression of matrix metalloproteinase-2/-9 and reversing the
endogenous and exogenous epithelial-mesenchymal transition. Int J
Mol Sci. 19(pii): E8442018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kur-Piotrowska A, Bukowska J, Kopcewicz
MM, Dietrich M, Nynca J, Slowinska M and Gawronska-Kozak B: Foxn1
expression in keratinocytes is stimulated by hypoxia: Further
evidence of its role in skin wound healing. Sci Rep. 8:54252018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bush BM, Brock AT, Deng JA, Nelson RA and
Sumter TF: The Wnt/β-catenin/T-cell factor 4 pathway up-regulates
high-mobility group A1 expression in colon cancer. Cell Biochem
Funct. 31:228–236. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Narasipura SD, Henderson LJ, Fu SW, Chen
L, Kashanchi F and Al-Harthi L: Role of β-catenin and TCF/LEF
family members in transcriptional activity of HIV in astrocytes. J
Virol. 86:1911–1921. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yu XW, Xu Q, Xu Y, Gong YH and Yuan Y:
Expression of the E-cadherin/β-catenin/tcf-4 pathway in gastric
diseases with relation to Helicobacter pylori infection: Clinical
and pathological implications. Asian Pac J Cancer Prev. 15:215–220.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang J, Huang K, Shi Z, Zou J, Wang Y,
Jia Z, Zhang A, Han L, Yue X, Liu N, et al: High β-catenin/Tcf-4
activity confers glioma progression via direct regulation of AKT2
gene expression. Neuro Oncol. 13:600–609. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Qian L, Zhang W, Zhang P, Lei B, Wang X,
Wang M, Bai J and He A: The anti-apoptosis effect of MLAA-34 in
leukemia and the β-catenin/T cell factor 4 protein pathway. Am J
Transl Res. 7:2270–2278. 2015.PubMed/NCBI
|
|
48
|
Sun J, Jia Z, Li B, Zhang A, Wang G, Pu P,
Chen Z, Wang Z and Yang W: MiR-19 regulates the proliferation and
invasion of glioma by RUNX3 via β-catenin/Tcf-4 signaling.
Oncotarget. 8:110785–110796. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang K, Wang X, Zou J, Zhang A, Wan Y, Pu
P, Song Z, Qian C, Chen Y, Yang S and Wang Y: miR-92b controls
glioma proliferation and invasion through regulating
Wnt/beta-catenin signaling via Nemo-like kinase. Neuro Oncol.
15:578–588. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xie J, Xiang DB, Wang H, Zhao C, Chen J,
Xiong F, Li TY and Wang XL: Inhibition of Tcf-4 induces apoptosis
and enhances chemosensitivity of colon cancer cells. PLoS One.
7:e456172012. View Article : Google Scholar : PubMed/NCBI
|