Introduction
Colorectal cancer (CRC) ranks as the third most
common cancer in the United States (1) and the fifth greatest cause of
mortality in China in 2015 (2).
Despite marked improvements in therapeutic strategies (3), the prognosis of patients with CRC
remains poor. Thus, novel molecular targets that improve patient
outcomes or serve as standard prognostic factors are warranted.
The human ribonuclease inhibitor (RI) is a 50-kDa
horseshoe-shaped protein that acts as the inhibitor of the
ribonucleolytic activity of RNaseA and angiogenin (ANG) (4–7). The
expression of RI has been investigated in melanoma and bladder
cancer cells (8,9). Based on its ability to inhibit
angiogenesis, RI has been considered as a tumor suppressor.
Previous studies have indicated that exogenous RI prolongs the
survival time of B16 melanoma tumor-bearing mice (10), and RI expression has been
demonstrated to be significantly reduced in human breast cancer
tissues (11). However, to date,
the mechanism underlying RI expression and tumorigenesis remains
unclear. It was previously demonstrated that the overexpression of
RI suppresses proliferation and metastasis in human CRC cells via
the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathway
(12). A recent study has also
suggested that autophagy is activated when Akt is inhibited
(13).
Autophagy, a cellular process that degrades
cytoplasmic components via the lysosomal machinery, is
morphologically characterized by the formation of light chain 3
(LC3)-II autophagic vacuoles (14,15).
In tumorigenesis, autophagy acts as a double-edged sword that may
either promote cell survival or induce cell death (16). However, previous studies have also
indicated that autophagy suppresses the proliferation and
tumorigenicity of human CRC cells (17,18).
The exact effects of RI on autophagy and the functional association
of autophagy with RI remains largely unknown. The present study
investigated the effect of RI on autophagy in CRC cells, and
identified autophagy-associated proteins and pathways to assist in
the identification of biological targets of human CRC.
Materials and methods
Reagents and antibodies
MK-2206 was purchased form Abmole Bioscience, Inc.
(Houston, TX, USA). Bafilomycin A1 (BafiA1) was purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). DMSO and DAPI were
purchased from Beyotime Institute of Biotechnology (Haimen, China).
Anti-RI (cat. no. ab245627), anti-autophagy-related protein (ATG)5
(cat. no. ab228668), anti-ATG7 (cat. no. ab223365), anti-ATG13
(cat. no. ab201467), anti-beclin-1 (BECN1; cat. no. ab62557),
anti-LC3/II (cat. no. ab51520), anti-β-Actin (cat. no. ab119716)
and goat anti-rabbit immunoglobulin G (IgG; Alexa Fluor®
488-conjugated; cat. no. ab150077) antibodies were purchased from
Abcam (Cambridge, UK). Anti-Akt (cat. no. 9272),
anti-phosphorylated (p)-Akt (cat. no. 9271), anti-mTOR (cat. no.
2972), anti-p-mTOR (cat. no. 2971), anti-ULK1 (cat. no. 6888),
anti-p-ULK1 (cat. no. 5869), anti-AMPK (cat. no. 2532S),
anti-p-AMPK (cat. no. 2535), anti-P62/sequestosome 1 (SQSTM1; cat.
no. 38749), goat anti-rabbit IgG horseradish peroxidase
(HRP)-conjugated (cat. no. 7074) and goat anti-mouse IgG
HRP-conjugated (cat. no. 7076) antibodies were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA).
Cell culture
The human CRC cell line HT29 was obtained from The
Cell Bank of Type Culture Collection of Chinese Academy of Sciences
(Shanghai, China) and was cultured in RPMI1640 Medium (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (Beijing Solarbio Science & Technology Co.,
Ltd., Beijing, China) and 100 U/ml penicillin/streptomycin at 37°C
in a humidified atmosphere containing 5% CO2. Cells were
treated with vehicle (0.1% DMSO) or Akt inhibitor MK-2206 (5 µM) at
37°C for 24 h, and the levels of LC3-II were examined by western
blotting. Cells were seeded at a density of 2×105
cells/well into six-well plates and were treated with DMSO (0.1%)
or bafilomycin A1 (1 µM) at 37°C for 72 h.
Overexpression of human RI
HT29 cells were seeded into six-well plates, and the
pcDNA3.1-RI plasmid (4 µg/µl), maintained in the laboratory
(12), was transfected using
Lipofectamine™ 2000 (Thermo Fisher Scientific, Inc.). Approximately
48 h after transfection, 800 µg/ml of geneticin (G418)
(Sigma-Aldrich; Merck KGaA) was added to the medium and incubated
for 2 weeks to select the positive clones that would be employed in
generating a stable transfected cell line, HT29/RI, that
overexpresses RI. Mock cells were transfected with an empty vector
and used as a control (HT29/vector).
Short hairpin RNA (shRNA)-mediated
knockdown assays
All of the plasmids required for shRNA-mediated
knockdown assays were reconstructed and provided by Genepharm, Inc.
(Sunnyvale, CA, USA). The pGPU6/GFP/Neo-shRNA vectors used to knock
down RI, ATG13, BECN1, mTOR and ULK1 were shRI (4 µg/µl), shATG13
(5 µg/µl), shBECN1 (4 µg/µl), shmTOR (4 µg/µl) and shULK1 (4
µg/µl), respectively. The transfection reagent
Lipofectamine® 2000 was used to transfer plasmid DNA
into the HT29 cells. Non-targeting shRNA was used as negative
control (shCTL; 4 µg/µl). Cells were harvested 48 h following
transfection.
Western blotting
The cells were harvested, and total cell lysates
were prepared using a protein extraction kit (Nanjing KeyGen
Biotech Co., Ltd., Nanjing, China) according to the manufacturer's
protocol. Protein concentrations were quantified using a
bicinchoninic acid kit (Beyotime Institute of Biotechnology). Equal
amounts (20 µg per lane) of protein samples were separated using
8–12% SDS-PAGE and transferred onto nitrocellulose membranes. The
membranes were blocked in TBS with 3% nonfat milk for 2 h at 37°C,
followed by incubation with primary antibodies at 4°C overnight.
The primary antibodies used for western blotting were the
following: Anti-RI (1:2,000), anti-ATG5 (1:500), anti-ATG7
(1:1,000), anti-ATG13(1:1,000), anti-(BECN1) (1:600), anti-LC3/II
(1:3,000), anti-β-Actin (1:1,000) anti-Akt (1:500), anti-p-Akt
(1:1,000), anti-mTOR(1:1,000), anti-p-mTOR (1:1,000), anti-ULK1
(1:500), anti-p-ULK1 (1:500), anti-AMPK (1:500), anti-p-AMPK
(1:500), anti-P62/SQSTM1 (1:1,000). The following secondary
antibodies were incubated for 1 h at 37°C: Goat anti-rabbit
HRP-linked IgG (1:2,000) or goat anti-mouse HRP-conjugated IgG
(1:2,000). Chemiluminescent signals were detected using the
Enhanced Chemiluminescent Plus kit (GE Healthcare, Chicago, IL,
USA) and the signal intensity was measured by densitometric
analysis using the Versa-Doc™ Imaging system (version 4.0; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). β-actin was used as the
internal control to normalize the samples.
Immunofluorescence staining
Cells were grown in six-well plates and fixed with
3.5% formaldehyde in PBS for 10 min at room temperature, washed
twice with PBS, permeabilized with 0.1% Triton X-100 for 10 min,
and blocked with 0.5% bovine serum albumin (Beyotime Institute of
Biotechnology) for 15 min at room temperature. Following two washes
with PBS, the cells were incubated with an LC3-II primary-antibody
(1:500) for 2 h at room temperature, followed by incubation with an
Alexa Fluor® 488-conjugated secondary antibody (1:200)
for 1 h at room temperature. Fluorescent signals were detected
using a fluorescence microscope (Olympus Corporation, Tokyo, Japan;
magnification, ×400). Autophagy was quantified by counting the
number of LC3-II dots per cell (a minimum of 50 cells per
preparation in three independent experiments).
Colony-formation assay
For the clonogenic assay, cells were transfected
with control shRNA (shCTL), RI shRNA (shRI), RI plasmid (RI) or
empty vector (Vector) for 48 h respectively. Cells in different
groups were subsequently seeded with appropriate dilutions into 6
cm dishes, followed by incubation at 37°C for 2 weeks. Colonies
were fixed with 4% paraformaldehyde at 4°C for 30 min, and
subsequently stained with crystal violet (0.5% w/v) at room
temperature for 5 min. The dishes were imaged using a light
microscope (Olympus Corporation; magnification, ×400) and the
numbers of distinct colonies (≥50 cells per colony) were counted
under the microscope (small, <0.3 mm; medium, 0.3–0.6 mm; large,
>0.6 mm). The results were averaged for each treatment
group.
Cell viability assay
The cell viability was determined by Cell Counting
Kit-8 (CCK-8) assay (Beyotime Institute of Technology). Briefly,
cells in each group were plated at a density of 3,000 cells/well in
96-well plates, and incubated at 37°C with 5% CO2 for
12, 24, 48 and 72 h. CCK-8 solution (10 µl) was added to each well,
the optical absorbance was determined at 450 nm following a 3 h
incubation using BioTek™ Epoch™ (BioTek Instruments, Inc.,
Winooski, VT, USA).
Statistical analysis
The data were analyzed using SPSS software 16.0
(SPSS, Inc., Chicago, IL, USA). The results are expressed as the
mean ± standard error of the mean. Each experiment was repeated at
least three times and analyzed using a Students' t-test or one-way
analysis of variance followed by Tukey's test for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
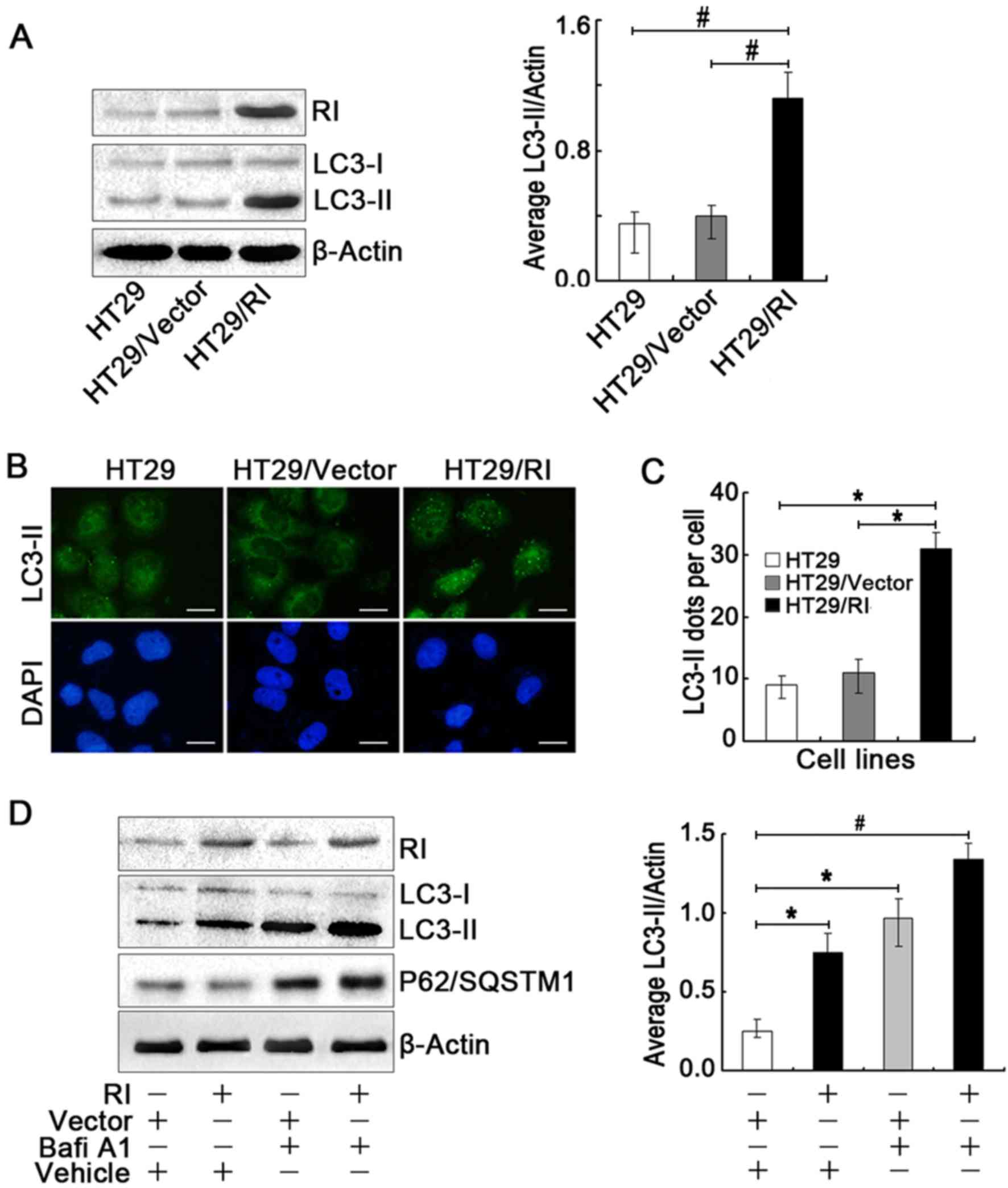
Overexpression of RI induces autophagy
in HT29 CRC cells
Previous studies have demonstrated that Akt
inhibition strongly activates autophagy (19), and that overexpression of RI
suppresses the PI3K/Akt pathway in human CRC cells (12). To study a possible regulatory
effect of RI on autophagy in HT29 cells, these cells were
transfected with pcDNA3.1-RI recombinant vectors, and successfully
transfected cells were selected for further analyses.
Overexpression of RI markedly increased LC3-II levels in the HT29
cells (Fig. 1A), suggesting that
increased RI expression induces autophagy. In addition,
fluorescence microscopy was used to verify the generation of
autophagosomes. An increase in the number of LC3-II puncta was
observed in the HT29/RI cells (Fig.
1B), further substantiating the previous observations. As
visible in Fig. 1B, the majority
of LC3-II puncta were concentrated in the nucleus. The number of
LC3-II puncta/cell significantly increased by >3-fold compared
with the HT29 and HT29/Vector cells controls (Fig. 1C). LC3-II levels were also elevated
during autophagosome-lysosome fusion or autophagic vesicle
degradation. To verify that this LC3-II elevation was induced by
the upregulation of RI, the autophagic flux in the HT29/RI cells
was analyzed in the absence (Vehicle) or presence of BafiA1, an
inhibitor of autophagosome-lysosome fusion and LC3-II degradation.
As indicated in Fig. 1D, BafiA1
increased the levels of LC3-II and of the autophagy-specific
substrate P62/SQSTM1 compared with the control and empty vector
groups, suggesting that LC3-II accumulation in HT29/RI cells was
attributable to the promotion of autophagy but not to autophagic
degradation. Taken together, these results confirmed that the
overexpression of RI triggered autophagy in human CRC cells.
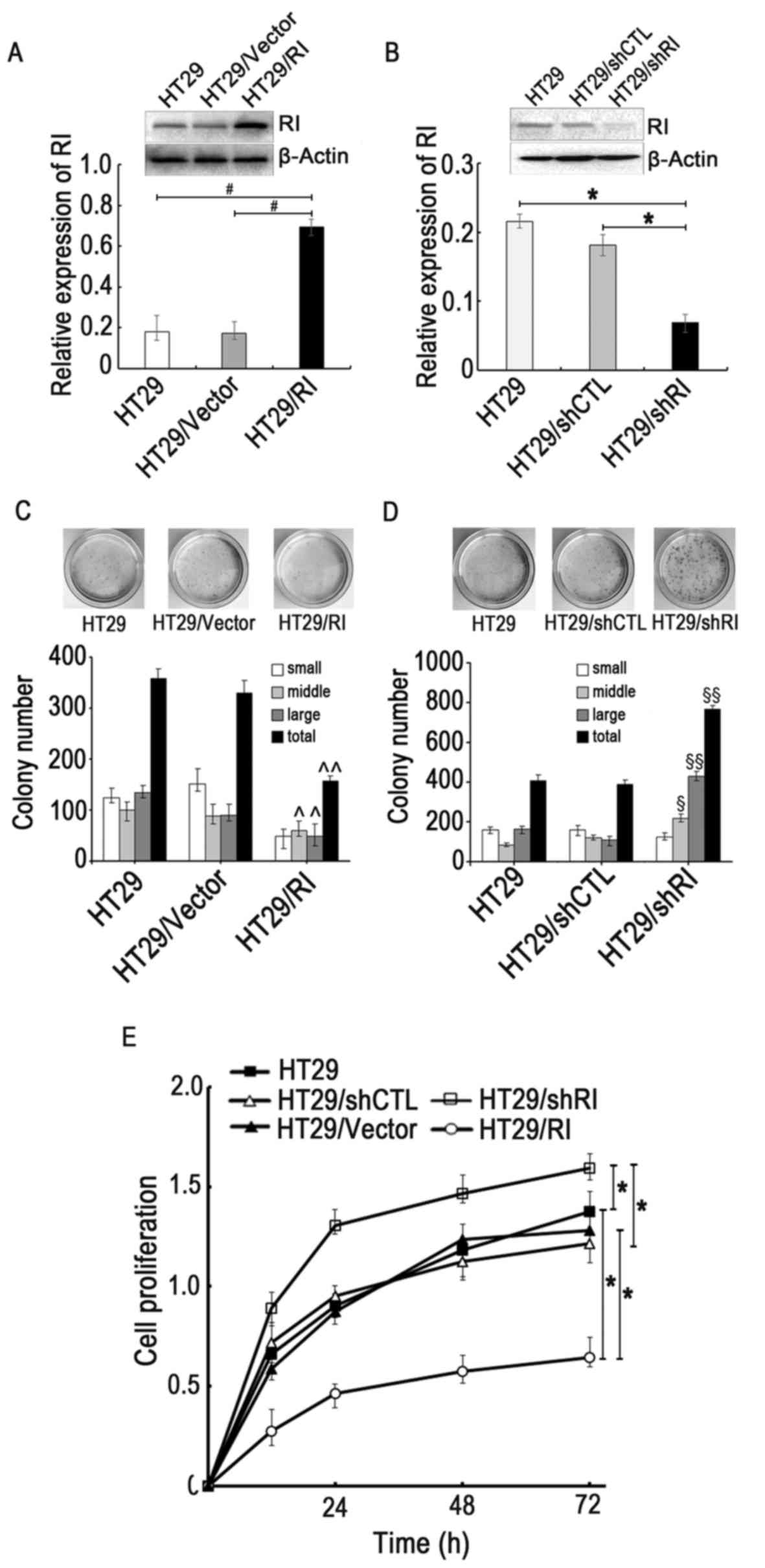
RI regulates HT29 cell survival
A previous study demonstrated that the upregulation
of RI affects the morphology and proliferation of bladder cancer
cells (20). To investigate the
effects of RI on HT29 cell survival, RI expression was upregulated
by introducing an exogenous RI gene using the overexpression
vector pcDNA3.1/RI, or silenced using a specific shRNA
(shRI)-mediated knockdown. As presented in Fig. 2A and B, RI expression levels
significantly increased or decreased in the HT29/RI or HT29/shRI
cells, respectively, compared with the controls, suggesting that
the transfection and RI expression manipulation were
successful.
A colony formation assay was conducted to elucidate
the association between RI expression and HT29 cell metastasis. The
overexpression of RI significantly inhibited CRC cell colony
formation, whereas knocking down RI in HT29 cells increased it,
indicating the inhibitory effect of RI on HT29 cell tumorigenic
ability (Fig. 2C and D). Cell
viability was subsequently assessed using the CCK-8 assay. The
results further provided the evidence that RI expression is
negatively associated with viability in CRC cells (Fig. 2E). These results therefore
supported the observations made with the colony formation assay,
demonstrating that RI overexpression may negatively affect the
viability and tumorigenic abilities of CRC cells.
Autophagy-associated proteins BECN1
and ATG13 are essential for autophagy in response to RI
overexpression in HT29 cells
To determine whether autophagy induced by RI
overexpression contributes to the regulation of specific proteins
of the ATG family, the expression levels of ATG5, ATG7, ATG13 and
BECN1 were assessed by western blotting. Fig. 3A indicates that overexpression of
RI significantly increased the protein levels of BECN1 and ATG13,
but not ATG5 and ATG7. To further validate these observations,
specific shRNA sequences of ATG13 (shATG13) and BECN1 (shBECN1)
were transfected into HT29/RI cells. The results demonstrated that
LC3-II levels significantly decreased due to the knockdown of BECN1
and ATG13 in the HT29/RI cells (Fig.
3B and C). Furthermore, the formation of LC3-II autophagic
vacuoles were observed under fluorescence microscopy. As exhibited
in Fig. 3D, the knockdown of ATG13
or BECN1 significantly attenuated the accumulation of LC3-II in
RI-overexpressing cells. Moreover, the number of LC3-II dots/cell
significantly decreased following silencing of ATG13 or BECN1 in
HT29/RI cells. Taken together, these results indicated that ATG13
and BECN1 may have been responsible for autophagy induced by RI
overexpression in human CRC cells.
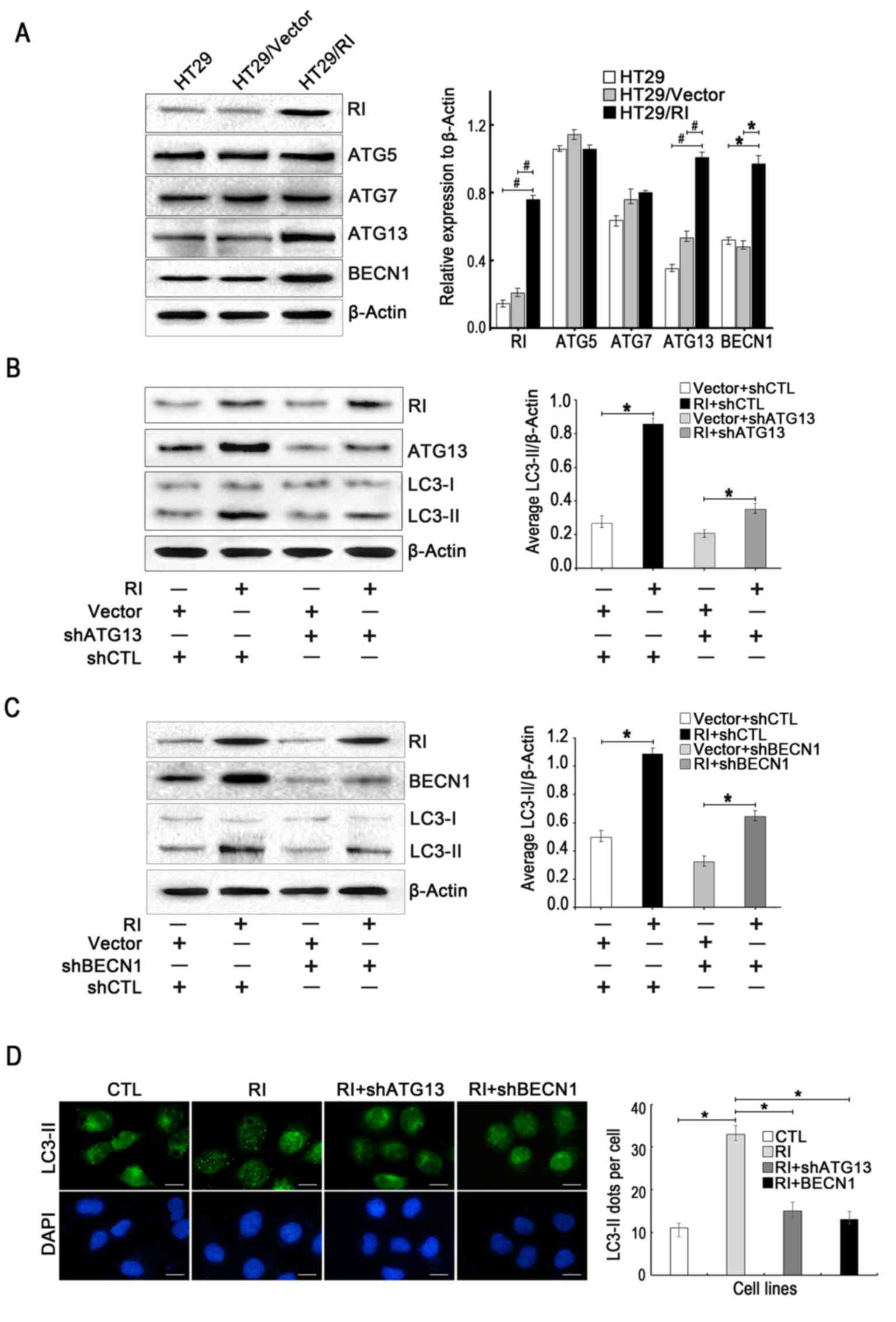 | Figure 3.ATG13 and BECN1 are required for
autophagy in response to RI overexpression in HT29 cells. (A) The
protein levels of ATG5, ATG7, ATG13 and BECN1 (normalized to
β-actin) were analyzed by western blotting. Representative western
blot and densitometric analyses normalized to β-actin demonstrating
the effects of (B) shATG13 and (C) shBECN1 on LC3-II levels
following RI overexpression. (D) Effect of shATG13 and shBECN1 on
LC3-II accumulation induced by RI overexpression compared to the
CTL. The average number of LC3-II dots/cell were counted in more
than five fields with ≥100 cells in each group. All quantitative
data are presented as the mean ± standard error of the mean of at
least three independent experiments. *P<0.05 and
#P<0.01 vs. respective control group. RI,
ribonuclease inhibitor; LC3, light chain 3; ATG, autophagy-related
protein; BECN1, beclin 1; shATG13, short hairpin RNA targeting
ATG13; shBECN1, short hairpin RNA targeting BECN1; Vector, control
vector transfection; shCTL, non-targeting short hairpin RNA used as
a control; CTL, control. |
Akt/mTOR/ULK1 pathway is involved in
the activation of autophagy in CRC cells
To evaluate which pathways may have mediated the
effect of RI on autophagy and cell viability, signal transduction
molecules, including Akt, mTOR, ULK1 and AMPK, were examined in CRC
cells. A previous study demonstrated that the function of RI is
associated with the PI3K/Akt pathway (12). Consistent with these reports, the
present study revealed that Akt and mTOR were significantly
inactivated in HT29/RI cells, whereas ULK1 was activated by
phosphorylation, but there was no effect on the expression levels
of phosphorylated AMPK (p-AMPK), total Akt, total mTOR, total ULK1
and total AMPK (Fig. 4A). These
results suggested that RI overexpression may have induced autophagy
by inhibiting the Akt/mTOR/ULK1 pathway, but not via the activation
of AMPK. To further validate whether Akt, mTOR and its downstream
target ULK1 were involved in RI induced autophagy, the effects of
the Akt inhibitor, MK-2206 (21),
were evaluated by assessing its effects on LC3-II levels. Fig. 4B demonstrates that the inhibition
of activated Akt (p-Akt) resulted in an increase in the amount of
LC3-II. In addition, the downregulation of mTOR (shmTOR) increased
ULK1 phosphorylation and significantly promoted LC3-II accumulation
(Fig. 4C), whereas silencing of
ULK1 markedly reduced LC3-II levels, indicating that mTOR and ULK1,
as the downstream elements of Akt signaling, may also be involved
in the process of RI-induced autophagy in CRC cells (Fig. 4D). Based on these results,
activation of the Akt/mTOR/ULK1 signaling pathway may have been
responsible for RI-induced autophagy in CRC cells.
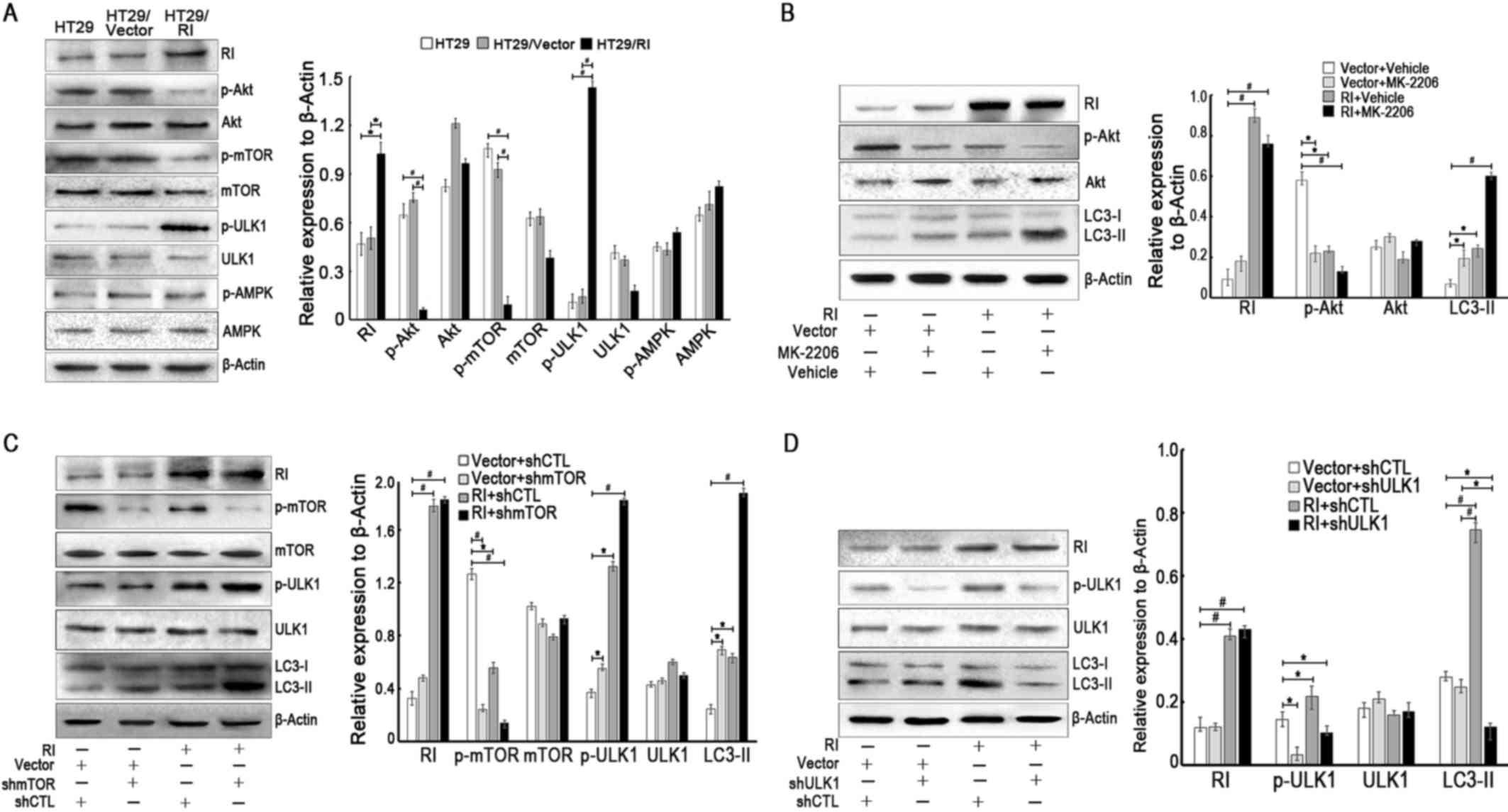 | Figure 4.Akt/mTOR/ULK1 signaling pathway is
responsible for RI-induced autophagy in colorectal cancer cells.
(A) The protein levels of RI, p-Akt, Akt, p-mTOR, mTOR, p-ULK1,
ULK1, p-AMPK and AMPK (normalized to β-actin) were analyzed by
western blotting. (B) HT-29/Vector or HT-29/RI cells were treated
with vehicle (0.1% DMSO) or Akt inhibitor MK-2206 (5 µM) at 37°C
for 24 h, and the levels of LC3-II were examined by western
blotting. Representative figures demonstrating the effect of
inhibition of p-Akt on LC3-II accumulation compared with the
control groups. Representative western blotting images indicating
the effect of (C) shmTOR and (D) shULK1 on LC3-II levels. All
quantitative data are presented as the mean ± standard error of the
mean of at least three independent experiments. *P<0.05 and
#P<0.01. RI, ribonuclease inhibitor; Akt, protein
kinase B; mTOR, mechanistic target of rapamycin; ULK1, Unc-51 like
autophagy activating kinase; AMPK, adenosine
monophosphate-activated protein kinase; p-, phosphorylated; LC3,
light chain 3; shmTOR, short hairpin RNA targeting mTOR; shULK1,
short hairpin RNA targeting ULK1. |
Discussion
RI is a cytoplasmic acidic protein that is involved
in multiple biological processes, including inhibition of RNase A
and ANG activity. Previous studies have demonstrated that RI
regulates stress-induced subcellular localization of angiogenin to
control growth and survival of HeLa cells (22), and that upregulation of RI inhibits
the growth and metastasis in melanoma, breast cancer and bladder
cancer cells (8,20,23).
However, the effect of RI on the growth of colorectal cancer cells
and its underlying mechanisms, were not fully understood. In the
present study, RI protein expression level was significantly
decreased in HT29 cells compared with other cell lines, including
HCT116, CW-2 and LoVo as assessed by western blotting (data not
presented); therefore, HT29 cells were cultured and transfected,
and served as the primary model to study the potential effects of
RI on autophagy in CRC cells. Furthermore, the present study
demonstrated that RI overexpression induces autophagy in human CRC
cells, possibly via the Akt/mTOR/ULK1 signaling pathway.
Autophagy is a critical event which maintains tissue
homeostasis under basal conditions (24). Although the precise role of
autophagy in cancer cells remains unclear, previous studies have
demonstrated that autophagy appears to be a double-edged sword that
may be beneficial or detrimental to cancer development (25). Earlier investigations have reported
that RI serves a noteworthy role in ANG-induced angiogenesis
(26,27), depleting nutrients that may be
utilized by cells. Alternatively, autophagy provides energy by
degrading intracellular macromolecules, proteins and organelles,
which may be beneficial to the growth of tumor cells in a low
vascular environment (25,28). Therefore, defining the
context-specific role of autophagy in CRC cells and the association
between RI and autophagy may guide autophagy-based therapeutic
interventions. In the present study, overexpression of RI was
observed to induce autophagy in human CRC cells.
Specific autophagy-associated genes are involved in
this process. Studies have indicated that more than 40 ATG proteins
are involved in autophagosome formation (29). Previous studies have also reported
that autophagy may be induced through ATG5, BECN1 or ATG7-dependent
or -independent signaling pathways (30), whereas ATG13, one of the components
of the ULK1 kinase complex, is required for the initiation of
autophagosome formation (31). The
present study assessed the expression of ATG5, ATG7, BECN1 and
ATG13 in response to RI overexpression, and revealed that only
BECN1 and ATG13 may be required for CRC cell autophagy, suggesting
that the ULK1 complex may be involved in this process.
Autophagy involves three stages, including
initiation, elongation and maturation (32). Various signaling pathways have been
implicated in the regulation of autophagy (33–35).
mTOR inhibits autophagy by forming a protein complex associated
with ULK1 (also termed the ‘preinitiation’ complex), which is the
central molecule that is involved in autophagy regulation (36,37).
This complex may be negatively and positively regulated by a
variety of intracellular signals. The PI3K pathway is also known to
be involved in autophagy, as the inhibition of PI3K/Akt and mTOR
were demonstrated to upregulate autophagy in breast cancer cells
(38,39). AMPK, which serves a role in the
regulation of cellular lipid and protein metabolism, also induces
autophagy by activating ULK1 or by inactivating mTOR (36). In addition, mTOR may suppress ULK1
under certain conditions (40,41).
As previously reported, the inhibition of Akt may induce autophagy,
and may therefore exert anti-tumor activity (12,42).
Furthermore, the present study revealed that autophagy induced by
RI is dependent on the mTOR/ULK1 pathway, but not on the activation
of AMPK.
Taken together, the results of the present study
indicated that the upregulation of RI promoted the expression of
autophagy-associated genes, including BECN1 and ATG13, and
prevented phosphorylation of Akt and mTOR, which in turn may have
alleviated the inhibition of ULK1, ultimately leading to autophagy
in CRC cells. Therefore, the upregulation of RI may constitute a
novel strategy for the treatment of human CRC.
Acknowledgements
Not applicable.
Funding
This study was supported by a grant from the
National Science Foundation of China (grant no. 31400687).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YiT and FR performed the experiments and wrote the
manuscript. XC, YK, YX and YuT performed the experiments and
performed statistical analysis. JF designed the study and
critically reviewed the manuscript. All authors read and approved
the final version of manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ciombor KK, Wu C and Goldberg RM: Recent
therapeutic advances in the treatment of colorectal cancer. Annu
Rev Med. 66:83–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kobe B and Deisenhofer J: Crystal
structure of porcine ribonuclease inhibitor, a protein with
leucine-rich repeats. Nature. 366:751–756. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McEwan PA, Scott PG, Bishop PN and Bella
J: Structural correlations in the family of small leucine-rich
repeat proteins and proteoglycans. J Struct Biol. 155:294–305.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Datta D, Samanta A, Dasgupta S and Pathak
T: 3′-Oxo-, amino-, thio- and sulfone-acetic acid modified
thymidines: Effect of increased acidity on ribonuclease A
inhibition. Bioorg Med Chem. 21:4634–4645. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dickson KA, Haigis MC and Raines RT:
Ribonuclease inhibitor: Structure and function. Prog Nucleic Acid
Res Mol Biol. 80:349–374. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pan X, Xiong D, Yao X, Xin Y, Zhang L and
Chen J: Up-regulating ribonuclease inhibitor inhibited
epithelial-to-mesenchymal transition and metastasis in murine
melanoma cells. Int J Biochem Cell Biol. 44:998–1008. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yao X, Li D, Xiong DM, Li L, Jiang R and
Chen JX: A novel role of ribonuclease inhibitor in regulation of
epithelial-to-mesenchymal transition and ILK signaling pathway in
bladder cancer cells. Cell Tissue Res. 353:409–423. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen JX, Gao Y, Liu JW, Tian YX, Zhao J
and Cui XY: Antitumor effects of human ribonuclease inhibitor gene
transfected on B16 melanoma cells. Int J Biochem Cell Biol.
37:1219–1231. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tian YX, Wang DM and Cui XY: Analysis of
gene expression of ribonuclease inhibitor in human breast cancer
tissue. Ai Zheng. 23:269–272. 2004.(In Chinese). PubMed/NCBI
|
|
12
|
Tang Y, Liu P, Tian Y, Xu Y, Ren F, Cui X
and Fan J: Overexpression of ribonuclease inhibitor defines good
prognosis and suppresses proliferation and metastasis in human
colorectal cancer cells via PI3K/AKT pathway. Clin Transl Oncol.
17:306–313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Degtyarev M, De Mazière A, Orr C, Lin J,
Lee BB, Tien JY, Prior WW, van Dijk S, Wu H, Gray DC, et al: Akt
inhibition promotes autophagy and sensitizes PTEN-null tumors to
lysosomotropic agents. J Cell Biol. 183:101–116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kabeya Y, Mizushima N, Ueno T, Yamamoto A,
Kirisako T, Noda T, Kominami E, Ohsumi Y and Yoshimori T: LC3, a
mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
White E and DiPaola RS: The double-edged
sword of autophagy modulation in cancer. Clin Cancer Res.
15:5308–5316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ellington AA, Berhow M and Singletary KW:
Induction of macroautophagy in human colon cancer cells by soybean
B-group triterpenoid saponins. Carcinogenesis. 26:159–167. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liang C, Feng P, Ku B, Dotan I, Canaani D,
Oh BH and Jung JU: Autophagic and tumour suppressor activity of a
novel Beclin1-binding protein UVRAG. Nat Cell Biol. 8:688–699.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng Y, Ren X, Zhang Y, Patel R, Sharma
A, Wu H, Robertson GP, Yan L, Rubin E and Yang JM: eEF-2 kinase
dictates cross-talk between autophagy and apoptosis induced by Akt
Inhibition, thereby modulating cytotoxicity of novel Akt inhibitor
MK-2206. Cancer Res. 71:2654–2663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhuang X, Lv M, Zhong Z, Zhang L, Jiang R
and Chen J: Interplay between intergrin-linked kinase and
ribonuclease inhibitor affects growth and metastasis of bladder
cancer through signaling ILK pathways. J Exp Clin Cancer Res.
35:1302016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ji S, Lin W, Wang L, Ni Z, Jin F, Zha X
and Fei G: Combined targeting of mTOR and Akt using rapamycin and
MK-2206 in the treatment of tuberous sclerosis complex. J Cancer.
8:555–562. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pizzo E, Sarcinelli C, Sheng J, Fusco S,
Formiggini F, Netti P, Yu W, D'Alessio G and Hu GF:
Ribonuclease/angiogenin inhibitor 1 regulates stress-induced
subcellular localization of angiogenin to control growth and
survival. J Cell Sci. 126:4308–4319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou JH, Tang XY, Zhao R, Wang H and Xia
J: Effects of ribonuclease inhibitor on apoptosis and invasion of
human breast cancer MDA-MB-231 cells. Xi Bao Yu Fen Zi Mian Yi Xue
Za Zhi. 28:260–264. 2012.(In Chinese). PubMed/NCBI
|
|
24
|
Han K, Kim J and Choi M: Autophagy
mediates phase transitions from cell death to life. Heliyon.
1:e000272015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carroll RG and Martin SJ: Autophagy in
multiple myeloma: What makes you stronger can also kill you. Cancer
Cell. 23:425–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dickson KA, Kang DK, Kwon YS, Kim JC,
Leland PA, Kim BM, Chang SI and Raines RT: Ribonuclease inhibitor
regulates neovascularization by human angiogenin. Biochemistry.
48:3804–3806. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peng Y, Li L, Huang M, Duan C, Zhang L and
Chen J: Angiogenin interacts with ribonuclease inhibitor regulating
PI3K/AKT/mTOR signaling pathway in bladder cancer cells. Cell
Signal. 26:2782–2792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu JL, Chang KC, Lo CC, Chu PY and Liu
CH: Expression of autophagy-related protein Beclin-1 in malignant
canine mammary tumors. BMC Vet Res. 9:752013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Young PG, Passalacqua MJ, Chappell K,
Llinas RJ and Bartel B: A facile forward-genetic screen for
Arabidopsis autophagy mutants reveals twenty-one loss-of-function
mutations disrupting six ATG genes. Autophagy. 1–19. 2019.
View Article : Google Scholar
|
|
30
|
Nishida Y, Arakawa S, Fujitani K,
Yamaguchi H, Mizuta T, Kanaseki T, Komatsu M, Otsu K, Tsujimoto Y
and Shimizu S: Discovery of Atg5/Atg7-independent alternative
macroautophagy. Nature. 461:654–658. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qi S, Kim DJ, Stjepanovic G and Hurley JH:
Structure of the human Atg13-Atg101 HORMA heterodimer: An
interaction hub within the ULK1 complex. Structure. 23:1848–1857.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Levine B: Cell biology: Autophagy and
cancer. Nature. 446:745–747. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu M, Zhao G, Zhang D, An W, Lai H, Li X,
Cao S and Lin X: Active fraction of clove induces apoptosis via
PI3K/Akt/mTOR-mediated autophagy in human colorectal cancer HCT-116
cells. Int J Oncol. 53:1363–1373. 2018.PubMed/NCBI
|
|
34
|
Yang L, Liu Y, Wang M, Qian Y, Dai X, Zhu
Y, Chen J, Guo S and Hisamitsu T: Celastrus orbiculatus extract
triggers apoptosis and autophagy via PI3K/Akt/mTOR inhibition in
human colorectal cancer cells. Oncol Lett. 12:3771–3778. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fan XJ, Wang Y, Wang L and Zhu M:
Salidroside induces apoptosis and autophagy in human colorectal
cancer cells through inhibition of PI3K/Akt/mTOR pathway. Oncol
Rep. 36:3559–3567. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Egan DF, Shackelford DB, Mihaylova MM,
Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor
R, et al: Phosphorylation of ULK1 (hATG1) by AMP-activated protein
kinase connects energy sensing to mitophagy. Science. 331:456–461.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lin Y, Kuang W, Wu B, Xie C, Liu C and Tu
Z: IL-12 induces autophagy in human breast cancer cells through
AMPK and the PI3K/Akt pathway. Mol Med Rep. 16:4113–4118. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou X, Yue GG, Chan AM, Tsui SK, Fung KP,
Sun H, Pu J and Lau CB: Eriocalyxin B, a novel autophagy inducer,
exerts anti-tumor activity through the suppression of
Akt/mTOR/p70S6K signaling pathway in breast cancer. Biochem
Pharmacol. 142:58–70. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mizushima N and Komatsu M: Autophagy:
Renovation of cells and tissues. Cell. 147:728–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Green DR and Levine B: To be or not to be?
How selective autophagy and cell death govern cell fate. Cell.
157:65–75. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fujiwara K, Iwado E, Mills GB, Sawaya R,
Kondo S and Kondo Y: Akt inhibitor shows anticancer and
radiosensitizing effects in malignant glioma cells by inducing
autophagy. Int J Oncol. 31:753–760. 2007.PubMed/NCBI
|