Introduction
Epithelial ovarian cancer (EOC) is the most lethal
malignancy among females, exhibiting highly aggressive behaviors
and a lack of early symptoms, leading to a large number of
mortalities yearly (1). Although
novel technologies have enabled advances in detection and
therapeutic methods, improved understanding about the molecular
events underlying this highly fatal form of tumorigenesis is
required.
MicroRNAs (miRNA/miRs) have been demonstrated to be
associated with the regulation of gene expression. miRNAs are
endogenous RNA molecules that destabilize the transcripts of target
genes or interact with complementary sequences in the
3′-untranslated region (UTR) of the target transcripts to repress
their translation (2). By
inhibiting translation, they lead to the downregulation of gene
expression and increased degradation of RNAs, negatively regulating
gene expression that may be involved in the regulation of critical
cellular processes including the cell cycle, cell differentiation
and apoptosis (3,4). Dysregulation of miRNA expression may
lead to the potentially abnormal regulation of their target mRNAs,
which has been identified to be associated with clinical outcome in
specific cancer types. In ovarian cancer, overexpressed miRNAs
include miR-200a, miR-141, miR-200c and miR-200b; by contrast,
miR-199a, miR-140, miR-145 and miR-125b1 are downregulated. The
abnormal expression of these small molecules may allow them to
serve as oncogenes or tumor suppressor genes, depending on the
targets that they regulate (5).
miR-145 may modulate tumor cell growth, apoptosis
and survival by targeting friend leukemia integration 1
transcription factor (6), insulin
receptor substrate 1 (7), myc
proto-oncogene protein (c-Myc) (8)
and DNA fragmentation factor subunit alpha (DFF45) (9), and affect tumor migration, invasion
and metastasis by targeting mucin 1 (MUC1) (10), p70S6 kinase (11) and 7,8-dihydro-8-oxoguanine
triphosphate (12). Downregulation
of miR-145 has been detected in breast (13) and colon cancer (7,11),
lung adenocarcinoma (12),
hepatocellular carcinoma (14),
bladder cancer (15), pituitary
tumors (16), and ovarian
(11,17,18)
and gallbladder cancer (19).
Previously, the mechanism through which the downregulation of
miR-145 modulates cell growth and invasion in ovarian cancer by
modifying the expression of certain oncogenes has been revealed in
a small number of studies (20,21),
but to the best of our knowledge, no studies have investigated its
regulatory functions in association with the targeting of cyclins
protein and/or transcription factor.
Cyclin D2 (CCND2) belongs to the D-type cyclin
family, the members of which serve as the primary integral
mediators associated with cell cycle progression in the
extracellular signaling environment (22). CCND2 forms a complex with
cyclin-dependent kinase (CDK)4 or CDK6 and functions as a
regulatory subunit of the complex, the activity of which is
required for G1/S transition in the cell cycle (23). Through its ability to shorten the
G1 phase, CCND2 primarily participates in cell cycle progression,
and has therefore been implicated in the induction of cancer
progression (24). E2F
transcription factor 3 (E2F3) is a member of the E2F transcription
factor family involved in regulating cell proliferation (25). As an oncogene, it serves as a
transcriptional activator and regulates the G1/S transition of the
cell cycle (26,27). The regulation of E2F1/2/3 activity
is achieved by formation of a complex with retinoblastoma protein
(Rb), which drives proliferation by promoting the expression of
target genes involved in DNA synthesis (28,29).
In this way, E2F3 may serve an essential role in the development of
various types of cancer. CCND2 and E2F3 are target genes of
miR-145; however, there is a lack of previous data regarding their
association with ovarian cancer.
In the present study, the association between
miR-145 and human ovarian cancer was first assessed, and then
whether miR-145 was associated with CCND2 and E2F3 was examined in
ovarian cancer cell lines, in order to explore the molecular
mechanisms by which miR-145 may mediate cancer cell growth and
invasion. The results may offer mechanistic insight into how
miR-145 mediates the suppression of ovarian cancer.
Materials and methods
Tissue specimens and cell culture
The present study was approved by the Ethics
Committee of the Affiliated Hospital of Nantong University. Tumor
tissues were collected from 20 patients with serous ovarian cancer
and eight patients with clear cell ovarian cancer between January
2015 and September 2016 (age, 43–67; mean age, 53.89; standard
deviation of the mean age, 6.64) in the Affiliated Hospital of
Nantong University. The normal tissues were collected from 20
patients with simple ovarian cysts or adenomyosis undergoing
oophorectomy surgery. Written informed consent was obtained from
all enrolled subjects.
Normal human ovarian surface epithelial cells
(HOSEpiC; ScienCell Research Laboratories, Inc., Carlsbad, CA, USA)
were cultured in RPMI-1640 medium, (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) 293 cells (American Type
Culture Collection, Manassas, VA, USA) were cultured in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc.) medium, whereas ovarian cancer ES-2 and SKOV3 cells (American
Type Culture Collection (Manassas, VA, USA) were cultured in
McCoy's 5a Medium (HyClone; GE Healthcare Life Sciences, Logan, UT,
USA). All media were supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.). The cells were maintained
in a humidified incubator at 37°C with 5% CO2.
RNA extraction and reverse
transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA (including miRNA) was extracted from cells
and tissues using TRIzol® (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. PCR was performed
with SYBR Green PCR Master Mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. For
miRNA detection, 10 ng RNA was reverse transcribed using a miRNA RT
kit (cat. no. 4366596; Thermo Fisher Scientific, Inc.), and the
miR-145 primers used were as follows: Stem-loop primer,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAGGGAT-3′; PCR
forward (F) primer, 5′-GTCCAGTTTTCCCAGGAATCC-3′, PCR reverse (R)
5′-CAGTGCAGGGTCCGAGGTAT-3′. The reaction conditions were as
follows: 95°C for 5 min, followed by 40 cycles of amplification at
95°C for 30 sec, 57°C for 30 sec and 72°C for 30 sec. The primers
for CCND2 were 5′-CAGCCGTCCACTTCAGC-3′ (F) and
5′-TGCCTTTGGGTCTTCC-3′ (R). The primers for E2F3 were
5′-CCCTAAACCCGCTTCC-3′ (F) and 5′-GTTCACAAACGGTCCTTCTA-3′ (R).
Conditions for amplifying mRNAs were as follows: Initial
denaturation of 95°C for 30 sec, followed by 40 cycles of 95°C
(denaturation) for 5 sec and 60°C for 30 sec (annealing and
elongation, two-step PCR). The expression of miR-145 was normalized
to that of U6 small nuclear B non-coding RNA (RNU6B), while the
levels of CCND2 and E2F3 were normalized to those of GAPDH, using
the 2−ΔΔCq method (30). The primers for GAPDH were
5′-AGGTGGTCTCCTCTGACTTCAA-3′ (F) and 5′-TTCGTTGTCATACCAGGAAATG-3′
(R). The RNU6B RT primer (stem-loop primer) was
5′-AACGCTTCACGAATTTGCGT-3′, and the PCR primers were
5′-CTCGCTTCGGCAGCACA-3′ (F) and 5′-AACGCTTCACGAATTTGCGT-3′ (R).
Plasmid construction and
transfection
Overexpression of miR-145 was established using
human miR-145 mimics (5′-GUCCAGUUUUCCCAGGAAUCCCU-3′) and the
corresponding mimic controls (5′-UUCUCCGAACGUGUCACGU-3′), obtained
from Shanghai GenePharma Co., Ltd., (Shanghai, China). The CCND2
overexpression vector primers were
5′-GGGGTACCATGGAGCTGCTGTGCCACGAGGTGG-3′ and
5′-GCTCTAGATCACAGGTCGATATCCCGCACGTCT-3′. The E2F3 overexpression
vector primers were 5′-CGGGATCCAGACTTGGAAACTCCGACTG-3′ and
5′-CGGAATTCTTGGAGGAAGAAGGTAGGAA-3′. The primers were purchased from
Sangon Biotech Co., Ltd. (Shanghai, China). The DNA was extracted
from SKOV3 cells. The PCR reaction conditions were as follows:
Initial denaturation at 95°C for 5 min, followed by 40 cycles of
amplification at 95°C for 30 sec, 55°C for 30 sec and 72°C for 60
sec using Takara LA PCR kit Ver.2.1 (Takara Biotechnology Co.,
Ltd., Dalian, China). The PCR products were inserted into a
pcDNA3.1 plasmid (Shanghai GenePharma Co., Ltd.). The thermocycling
conditions were as follows: 40 cycles of denaturation at 94°C for
30 sec, annealing at 60°C for 15 sec and extension at 72°C for 45
sec using Takara LA PCR kit (Takara Biotechnology Co., Ltd.). Cell
transfection was performed using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) for 48 h according to
the manufacturer's protocol. In total, miRNA at a concentration of
50 nM was transfected with 1 µg expressing plasmids using 1.5 µl
Lipofectamine® 2000 in each well.
3′-UTR luciferase reporter assays
The 3′-UTRs of CCND2 (forward primer,
5′-GGAGTTCTTGGGAATCTTG-3′ and reverse primer,
5′-CCCTTTAGTGGGAGGTAA-3′) and E2F3 (forward primer,
5′-GCCAGTTTACTCCAGGTA-3′ and reverse primer,
5′-AACAATCTAGCCAGGTGA-3′) were amplified by PCR as above-mentioned
from a human SKOV3 cell cDNA library and cloned downstream of the
Renilla luciferase coding sequence, between the Xho I
and Not I sites, of the psiCHECK2 luciferase reporter vector
(Promega Corporation, Madison, WI, USA). The miR-145 target site in
the CCND2 and E2F3 3′UTRs was mutated by altering the 3-nt miR-145
seed match sequence using the QuikChange Site-Directed Mutagenesis
kit (Stratagene; Agilent Technologies, Inc., Santa Clara, CA, USA),
according to manufacturer's protocol. The aforementioned 293 cells
were cotransfected with 100 ng psiCHECK2-CCND2 3′UTR or
psiCHECK2-E2F3 3′UTR, or psiCHECK2-CCND2 3′UTR-Mutant (Mut) or
psiCHECK2-E2F3 3′UTR-Mut luciferase plasmid, and the miR-145 mimics
or miR-145-negative controls (NC) using Lipofectamine®
2000. After 24 h, luciferase activity was measured using the
Dual-Glo Luciferase Reporter Assay System (Promega Corporation)
according to the manufacturer's instructions. Data were normalized
for transfection efficiency by comparison with Renilla
luciferase activity.
Bioinformatics prediction
CCND2 and E2F3 were identified as potential targets
of miR-145 using the bioinformatics software TargetScan (version
7.0; http://www.targetscan.org/cgi-bin/targetscan/vert_71/targetscan.cgi?species=Human&gid=&mir_sc=&mir_c=&mir_nc=&mir_vnc=&mirg=miR-145).
Cell proliferation assay
A total of 24 h after transfection, target cells
were seeded into 96-well plates at a density of 2,000 cells/well
and cultured for 0, 24, 48, 72 and 96 h. Cell proliferation was
detected by Cell Counting Kit-8 (CCK-8) assay according to the
manufacturer's protocol (LakePharma, Inc., San Carlos, CA, USA).
Absorbance values were detected at a wavelength of 450 nm. A total
of 3 wells were measured for cell viability for each group.
Transwell invasion assay
Transwell chambers (EMD Millipore, Billerica, MA,
USA) were used to examine the migration and invasion of cells.
Filters for the invasion assay were precoated at 37°C for 2 h with
Matrigel (BD Biosciences, Franklin Lakes, NJ, USA). A total of
~5×104 cells were added to the upper Transwell chamber
and cultured in serum-free DMEM. The lower compartment of the
Transwell chamber was filled with DMEM containing 10% FBS. Cells
remaining on the lower surface were fixed for 30 min at room
temperature with 4% formaldehyde (Sangon Biotech Co., Ltd.) and
stained with 0.5% crystal violet (Sangon Biotech Co., Ltd.) at room
temperature for 60 min. A total of four randomly-selected
microscope (light microscope; magnification, ×20) fields were
counted for each group.
Cell cycle analysis
The cells were transfected with miR-145 mimics and
miR-145 NC, and CCND2, E2F3 and CCND2 + E2F3 overexpression
plasmids for 48 h, following which the cells were collected and
washed with PBS. Then, the cells were washed with 1% bovine serum
albumin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and fixed
in 95% ice-cold ethanol containing 0.5% Tween-20 for 2 h at −20°C.
Following fixation, cells (1×106) were washed with cold
1% BSA and stained with 10 µg/ml propidium iodide (Sigma-Aldrich;
Merck KGaA) in a solution containing 100 µg/ml RNase in PBS for 30
min at room temperature in the dark. The cells were analyzed using
a flow cytometer (BD FACSCalibur™, BD Biosciences, San Jose, CA,
USA). BD CellQuest Pro software (version, 5.1; BD Biosciences) was
used to analyze the flow cytometry data.
Wound healing assay
Cell migration was assessed by a wound-healing
assay. Cells (2×106) were seeded in 6-well plates and
transfected the following day. At 12 h after transfection, cells
were incubated at 37°C for 24 h, and 95% confluent cells were used
for wound healing assay. Wounds were made using a pipette tip. The
wounded areas were observed and imaged under a light microscope
(200×). The migration distances were imaged at 0 and 24 h after
scratching, and the change in cell migration was determined by
comparing the difference in the wounded area in at least 4 fields.
Experiments were performed in triplicate and repeated at least 3
times.
Western blot analysis
Cell lysates were prepared using a lysis buffer
(Beyotime Institute of Biotechnology, Haimen, China). The protein
concentration in each sample was assessed using a spectrophotometer
(Nanodrop 1000; Thermo Fisher Scientific, Inc.). A total of 80 µg
protein was loaded in each lane. Proteins were separated by 10%
SDS-PAGE, and transferred to polyvinylidene difluoride membranes
(EMD Millipore). Following blocking using 5% non-fat milk for 2 h
at room temperature, the membranes were incubated with anti-CCND2
monoclonal antibody (cat. no. SC-56305; 1:500; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), anti-E2F3 polyclonal
antibody (cat. no. SC-879; 1:500; Santa Cruz Biotechnology, Inc.),
anti-B cell lymphoma 2 (Bcl-2; cat. no. 4223; 1:1,000; Cell
Signaling Technology, Inc., Danvers, MA, USA),
anti-Bcl-2-associated X protein (Bax; cat. no. 5023; 1:1,000; Cell
Signaling Technology, Inc.) and anti-β-actin (cat. no. 4970;
1:2,000; Cell Signaling Technology, Inc.) overnight at 4°C, which
was followed by incubation with anti-mouse (cat. no. 7076; 1:4,000;
Cell Signaling Technology, Inc.) or anti-rabbit (cat. no. 7074;
1:5,000; Cell Signaling Technology, Inc.) horseradish
peroxidase-conjugated immunoglobulin G at 4°C for 2 h. The blots
were developed using an enhanced chemiluminescent system (Amersham;
GE Healthcare, Chicago, IL, USA), and the density of bands was
detected using ImageJ 1.42q software (National Institutes of
Health, Bethesda, MD, USA).
Statistical analysis
Data were presented as mean ± standard error of the
mean. Statistical analysis was performed using SPSS 11.0 for
Windows (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 7.0 for
Windows (GraphPad Software Inc., La Jolla, CA, USA). The difference
between two groups was analyzed by Student's t-test. One-way
analysis of variance followed by Tukey's post hoc test was used to
compare multiple groups. P<0.05 was considered to indicate a
statistically significant difference. All data are presented as the
mean ± standard error of the mean.
Results
miR-145 is downregulated in human
ovarian cancer tissues and cell lines
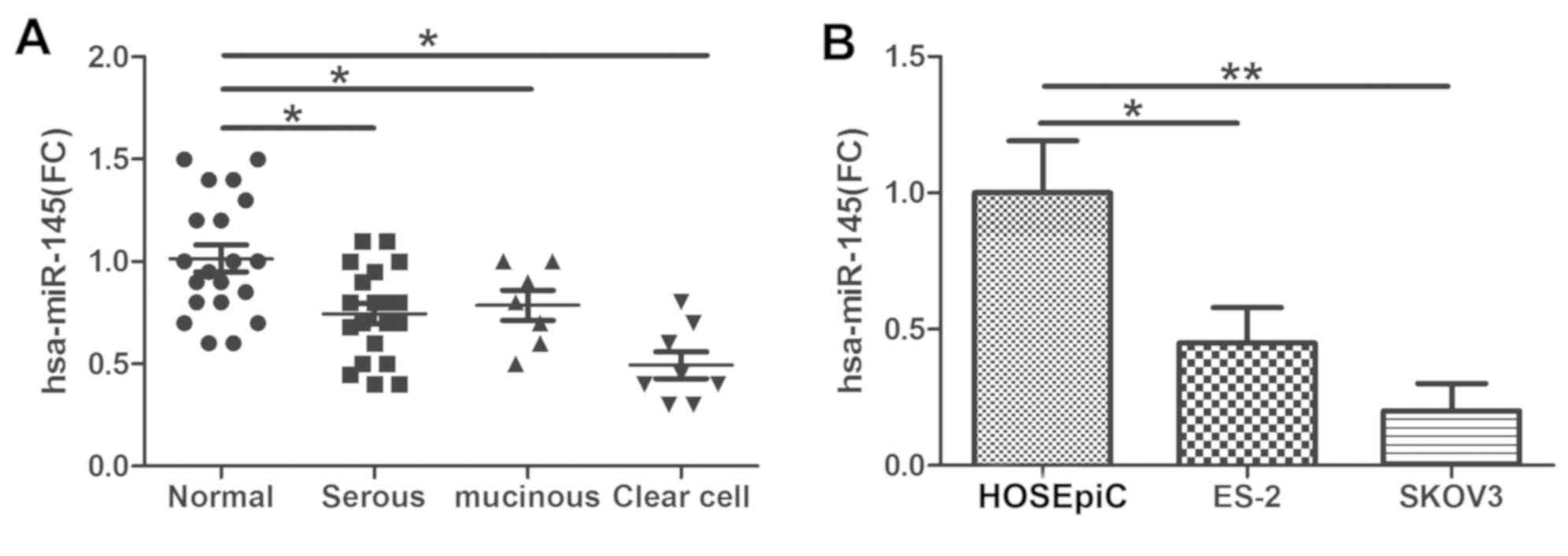
The expression level of miR-145 was examined via
qPCR in tumor tissues obtained from patients with ovarian cancer
(from 20 patients with serous ovarian cancer and 8 with clear cell
ovarian cancer). The data indicated that miR-145 expression was
decreased in serous ovarian cancer and clear cell ovarian cancer
tissues compared with control tissues (Fig. 1A). In concordance with the results
in the cancer tissues, miR-145 was significantly downregulated in
ovarian cancer ES-2 and SKOV3 cell lines compared with HOSEpiC
cells (Fig. 1B).
miR-145 suppresses the proliferation
and invasion of ovarian cancer cells
To investigate the functions of miR-145 in ovarian
cancer cells, miR-145 mimics and mimic NC were transfected into
ES-2 and SKOV3 cells. Then, qPCR was performed to measure the
expression of miR-145. The expression of miR-145 was notably
increased in miR-145 mimic-transfected cells (Fig. 2A). In a CCK-8 assay, the
overexpression of miR-145 significantly inhibited the proliferation
of ovarian cancer cells at 96 h (Fig.
2B). In addition, wound healing (Fig. 2C) and invasion (Fig. 2D) assays also indicated that
miR-145 overexpression markedly suppressed the migratory and
invasive capabilities, which are characteristic metastatic
behaviours, of ovarian cancer cells. Bax and Bcl-2 are members of a
family of cytoplasmic proteins that regulate apoptosis. To
investigate the potential of miR-145 to induce apoptosis, the
levels of pro-apoptotic protein Bax and the anti-apoptotic protein
Bcl-2 were measured in miR-145-transfected or corresponding
control-transfected SKOV3 and ES-2 cells. Compared with those cells
transfected with miR-145 NC, the miR-145-transfected cells
exhibited a decrease in Bcl-2 expression and an increase in Bax
expression. These results suggested that miR-145 promoted the
apoptosis of SKOV3 and ES-2 cells (Fig. 2E).
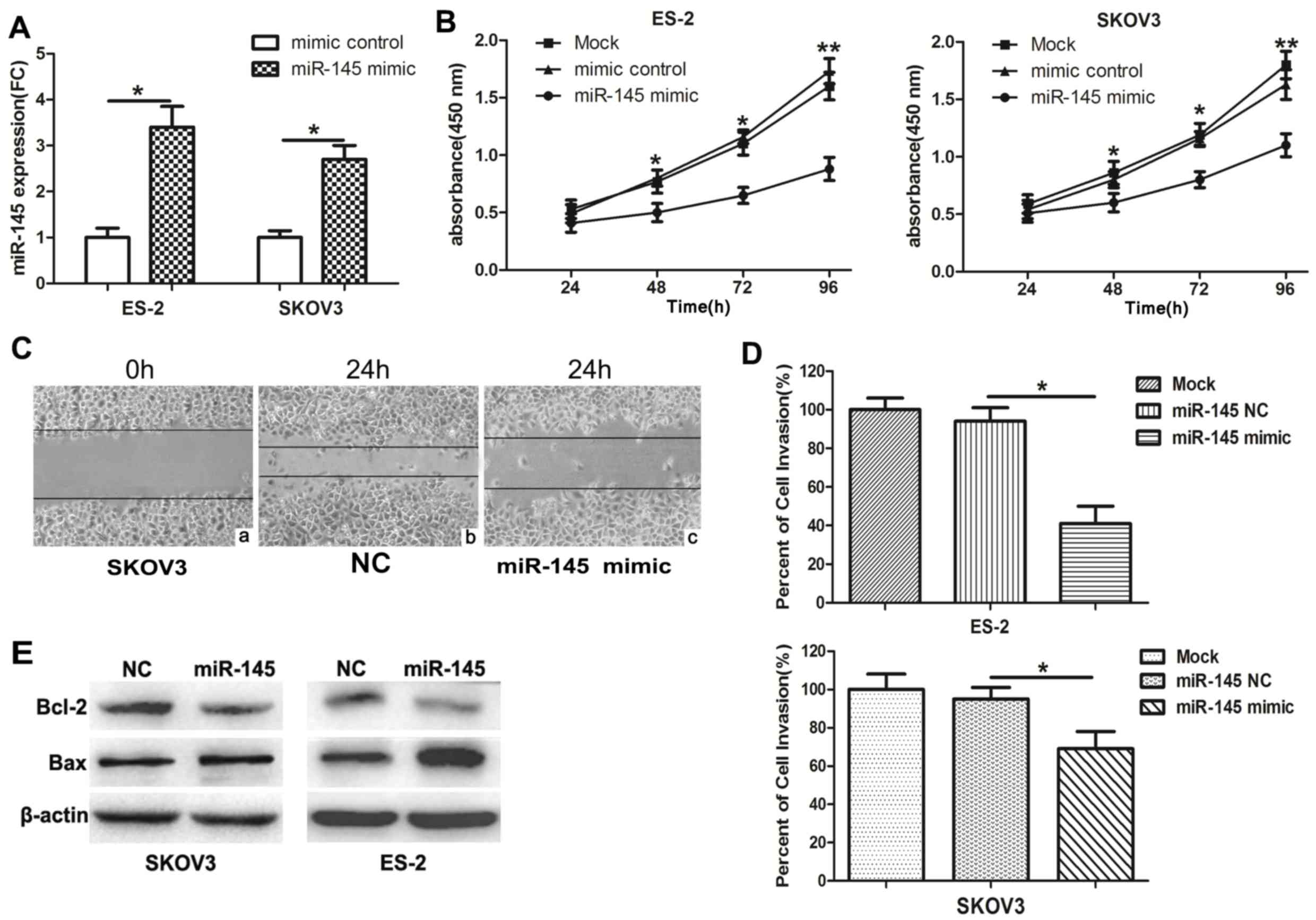 | Figure 2.miR-145 inhibits ovarian cancer cell
proliferation, cell cycle, invasion and migration. (A) SKOV3 and
ES-2 cells were transfected with miR-145 mimics or the control
mimics, and the expression of miR-145 was determined by reverse
transcription quantitative polymerase chain reaction. (B) Cell
viability in cells transfected with miR-145 mimics or NC was
measured by Cell Counting Kit-8 assay at 4 time points (0, 24, 48,
72 and 96 h). UV-visible absorbance was measured at 450 nm.
*P<0.05 and **P<0.01 for mimic control and miR-145 mimic
groups vs. mock group. (C) Wound healing assay was performed at 24
h post-transfection (magnification, 200×). (C-a) SKOV3 cells at 0
h. Following 24 h, the migration ability of SKOV3 cells transfected
with (C-b) miR-NC significantly increased compared with (C-c) cells
transfected with miR-145. The results indicated that overexpression
of miR-145 inhibits cell migration. (D) Invasion assays of ovarian
cancer cells transfected with miR-145 or the control mimics. (E)
The expression levels of Bcl-2 and Bax were detected in SKOV3 and
ES-2 cells using western blot analysis. Experiments were performed
in triplicate. *P<0.05 and **P<0.01. miR, microRNA; NC,
negative control; FC, fold change. |
CCND2 and E2F3 are targets of miR-145
in ovarian cancer cells
CCND2 and E2F3 were identified as potential targets
of miR-145 using the bioinformatics software TargetScan. To
validate this prediction, luciferase candidate genes were
constructed with the 3′-UTR of each target downstream of the
reporter (Fig. 3A). The luciferase
activity assays identified that miR-145 mimics significantly
inhibited luciferase activity when cells were co-transfected with
luciferase plasmid containing the wild type (WT) but not the Mut
3′-UTR of CCND2 or E2F3 (Fig. 3B).
RT-qPCR assays also demonstrated that the relative mRNA expression
levels of CCND2 and E2F2 were downregulated following transfection
with miR-145 in SKOV3 cells (Fig.
3C). Furthermore, CCND2 and E2F3 expression was detected in
ovarian cancer tissues. The results of the western blot analysis
indicated that the protein levels of CCND2 and E2F3 were increased
in ovarian cancer tissues compared with the normal tissues
(Fig. 3D). Additionally, in SKOV3
cells, overexpression of miR-145 significantly suppressed CCND2 and
E2F3 protein levels (Fig. 3E and
F). Taken together, these data verified a direct interaction
between miR-145 and the two target genes CCND2 and E2F3.
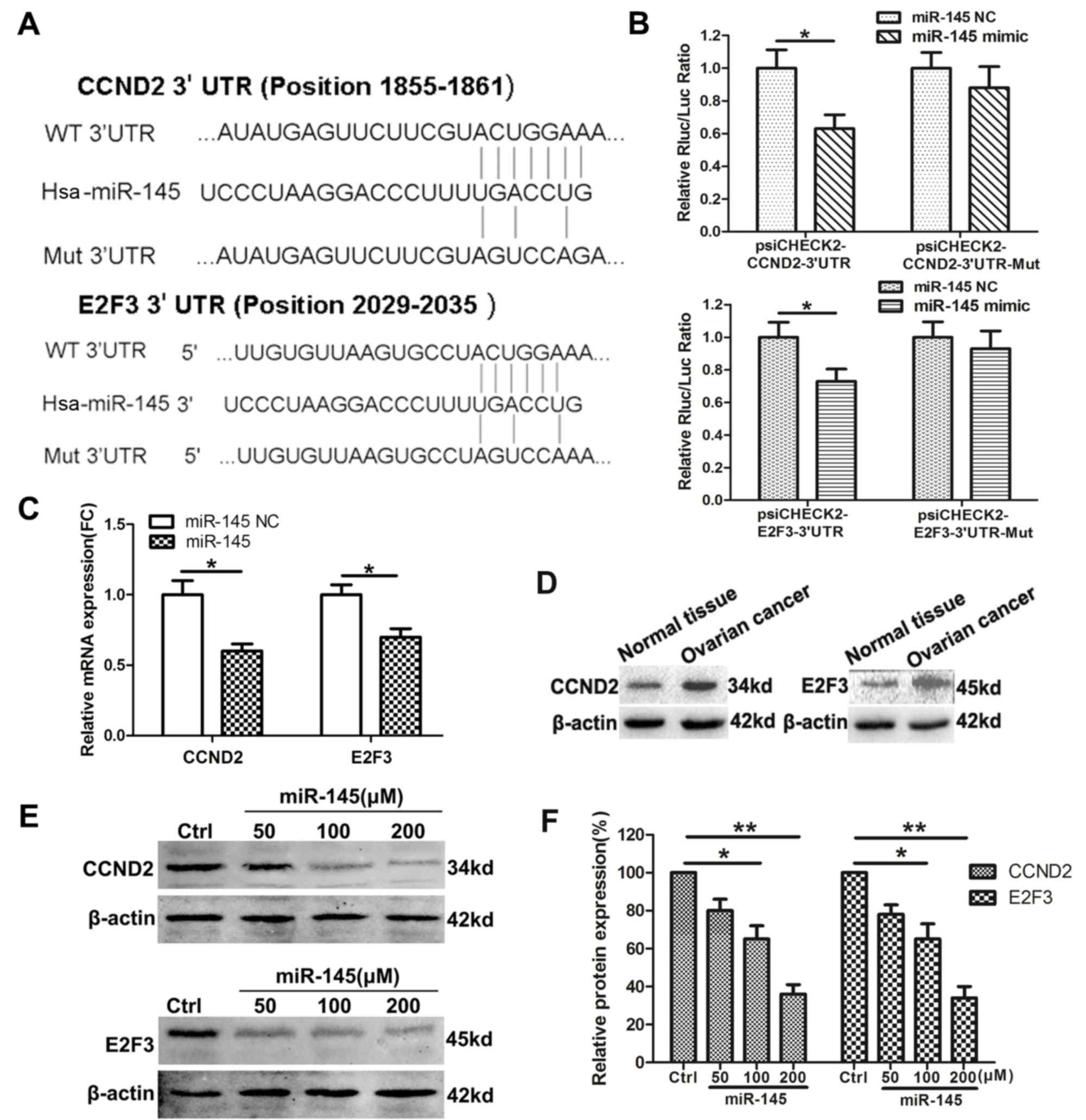 | Figure 3.CCND2 and E2F3 are the targets of
miR-145 in ovarian cancer cells. (A) The schematic construction of
WT and Mut 3′-UTR of CCND2 and E2F3. (B) SKOV3 cells were
co-transfected with WT or Mut 3′-UTR of CCND2 and E2F3, followed by
transfection with the miR-145 or NC mimics. The luciferase
activities were examined 48 h after transfection. (C) SKOV3 cells
were transfected with miR-145 or the control mimics, and CCND2 and
E2F3 mRNA were detected by qPCR. (D) The expression levels of CCND2
and E2F3 were detected in ovarian cancer tissues and normal ovarian
tissues using western blot analysis. (E) The protein level of CCND2
and E2F3 were detected in SKOV3 cells transfected with miR-145 or
the NC mimics. (F) Densitometric evaluation of band intensities.
Experiments were performed in triplicate. *P<0.05 and
**P<0.01 vs. control. CCND2, cyclin D2; E2F3, E2F transcription
factor 3; miR, microRNA; WT, wild type, Mut, mutant; UTR,
untranslated region; NC/ctrl, negative control; FC, fold
change. |
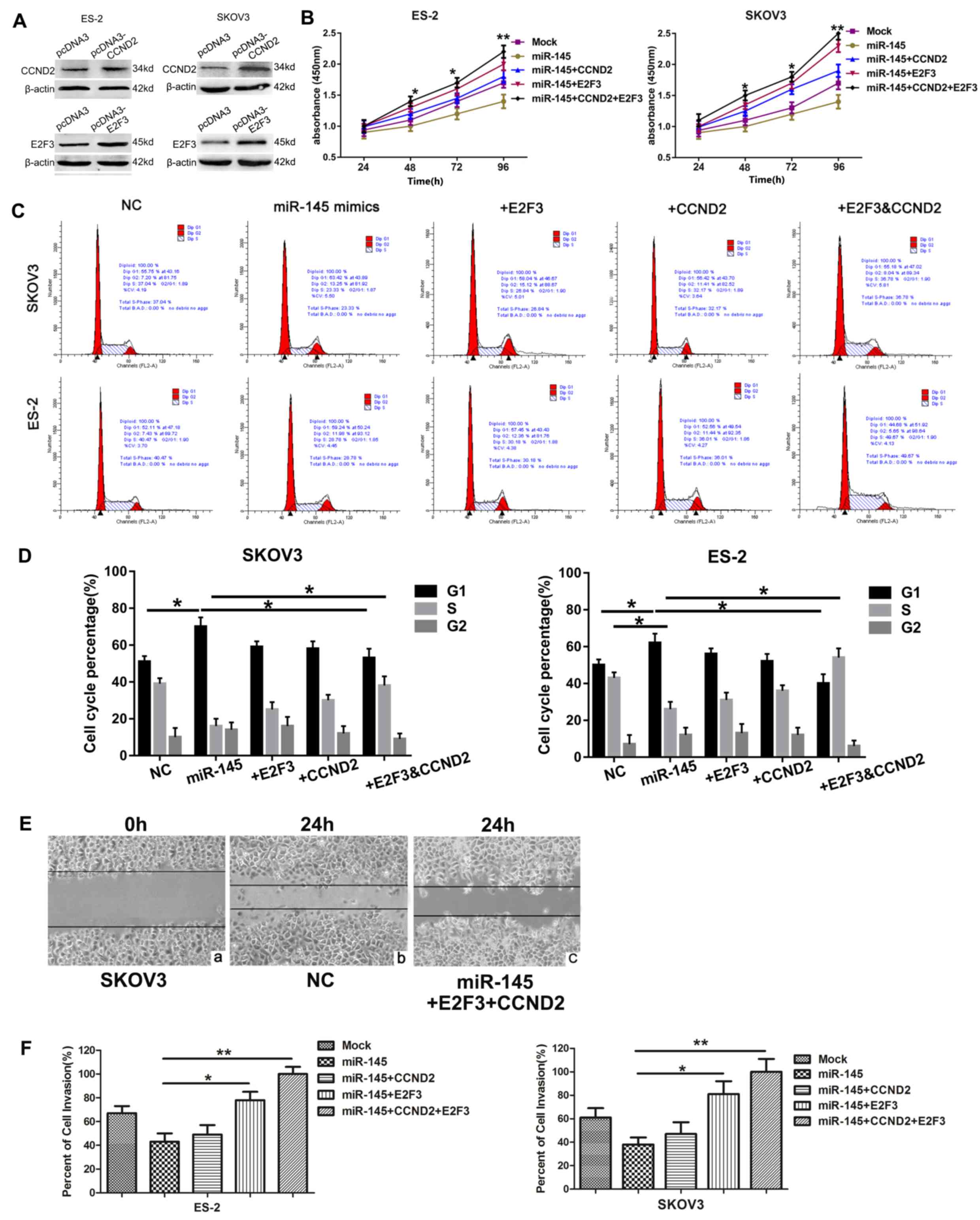
Restoration of CCND2 and E2F3 reverses
the effects of miR-145
CCND2 or E2F3, alone or together, were not
transfected into the ovarian cancer cell line in the absence of
miR-145 to clarify the role of CCND2 and E2F3 in tumorigenesis, as
it has been confirmed in a previous study (31). Whether CCND2 and E2F3 were
associated with the function of miR-145 was additionally
investigated. A total of 2 cell lines were transfected with CCND2
and E2F3 overexpression plasmids, and expression patterns of the
proteins were examined by western blot analysis. The protein
expression levels of CCND2 and E2F3 were significantly increased in
the cells transfected with the target gene overexpression plasmids
(Fig. 4A). Subsequently, the cells
were co-transfected with miR-145 mimics or NC vector and the target
genes overexpression plasmids. The effect of miR-145 and its target
genes on cell proliferation was examined using the CCK-8 assay
(Fig. 4B). It was observed that
cell viability gradually increased over the indicated time points
following transfection with CCND2 or E2F3 overexpressed plasmids.
The effects of miR-145 and its target genes on cell cycle
progression were also determined using flow cytometry, which
revealed the percentages of ES-2 and SKOV3 cells in G1 phase to be
increased following transfection with miR-145, compared with those
of the controls, providing evidence that upregulated miR-145
induced G1 cell cycle arrest in ovarian cancer cells. Similarly,
CCND2 and E2F3 significantly increased the populations of SKOV3 and
ES-2 cells in S phase, and therefore appeared to promote entry into
the S phase, thereby increasing proliferation (Fig. 4C and D). In addition, wound healing
(Fig. 4E) and invasion (Fig. 4F) assays demonstrated that
restoration of CCND2 or E2F3 significantly reversed the tumor
suppressive effects of miR-145. Overall, these data suggest that
miR-145 functions as a tumor suppressor at least in part by
targeting CCND2 and E2F3.
Discussion
Increasing evidence has demonstrated that miRNAs are
significantly involved in the differentiation, proliferation and
apoptosis of various malignancies by interacting with the 3′-UTR of
their target genes. miR-145 is located on chromosome 5 (5q32-33),
an established fragile site in the human genome (32). It targets numerous oncogenes, which
regulate cellular processes including the cell cycle,
proliferation, apoptosis and invasion. Accordingly, decreased
expression of miR-145 has been revealed in a number of types of
cancer. However, limited studies have demonstrated the
downregulation of miR-145, as a tumor suppressor, in human ovarian
cancer, which has been suggested as a potential diagnostic marker
or as a target for sensitizing ovarian cancer cells to chemotherapy
(33,34). The results from the present study
suggested that miR-145 was downregulated in 3 different
pathological types of EOC, and furthermore that its expression was
decreased in the ovarian cancer cell lines SKOV3 and ES-2. miR-145
mimics were subsequently transfected into the cell lines, and the
increased expression of miR-145 was confirmed by qPCR. In the
following experiments, it was identified that the percentage of
cells in G1 phase increased, while cell proliferation, migration
and invasion were suppressed. These results indicate that miR-145
may exert its inhibitory effects on its target oncogenes and
thereby mediate cell cycle regulation in ovarian cancer cells.
At present, a number of oncogenes have been
identified as targets of miR-145. Previous studies have confirmed
that miR-145 may suppress cell proliferation by targeting growth
factor-associated genes including insulin receptor substrate 1,
insulin like growth factor receptor and epidermal growth factor
receptor (EGFR) (14,35,36).
It may also inhibit DFF45, protein phosphatase 3 catalytic subunit
alpha, core-binding factor subunit beta, clathrin interactor 1 and
c-Myc, promoting cell apoptosis and interrupting the cell cycle. In
addition, certain oncogenes that are involved in cell invasion and
metastasis, including neural precursor cell expressed,
developmentally down-regulated 9, fascin actin-bundling protein 1,
MUC1, cell surface associated and sex-determining region box 9, may
also be regulated by miR-145 (10,37–40).
The identification of an increasing number of target genes
indicates that miR-145 may be involved in a complicated regulatory
network. In the present study, it was confirmed that CCND2 and E2F3
were direct targets of miR-145 by a luciferase activity assay.
D-type cyclins (D1, D2 and D3) are considered to be potentially
oncogenic proteins, as they serve critical roles in regulating cell
cycle progression through the G1 phase to S phase. D-type cyclins
bind to and activate CDK4/CDK6, which induces cell cycling
(40,41). The cyclin D-CDK4/CDK6 complex may
inactivate Rb during early G1 phase by progressive
multi-phosphorylation, resulting in the upregulation of
transcription factor E2F. E2F may then target genes that promote
cell cycle progression into the S phase (42), including cyclin E, cyclin A, CDK2
and cell cycle division 25 (41,43).
Certain studies have demonstrated that CCND2 and E2F3 are
associated with tumorigenesis (31). Chang et al (44) revealed that CCND2 is involved in
stimulating the proliferation, cell cycle progression, migration
and invasion of ovarian cancer cells. In addition, E2F3 is
critically involved in EGFR-mediated proliferation in ovarian
cancer and is clinically relevant in ovarian cancer (45). Therefore, experiments to clarify
the function of CCND2 and E2F3 on cell growth, migration and
invasion were not repeated in the present study. The present study
aimed to clarify whether the participation of CCND2 and E2F3 in
cell growth, migration and invasion was mediated by miR-145.
The results indicated that the protein expression
levels of CCND2 and E2F3 were downregulated by transfection with
miR-145. Conversely, the restoration of CCND2 and E2F3 by
transfection with CCND2 and E2F3 overexpression plasmids reversed
the effects of miR-145. This restoration was observed in cells
transfected with separate CCND2 and E2F3 overexpressed plasmids;
the co-transfection of the 2 genes together additionally augmented
the restoration. Collectively, the data from the present study
confirmed the effect of miR-145 in targeting upstream (CCND2) and
downstream (E2F3) molecules, which appeared to markedly affect the
biological behavior of ovarian cancer cells. It was also observed
that the effect of miR-145 was not only partially reversed, but
abrogated compared with the mock control, and it was hypothesized
that CCND2 and E2F3 may participate in other potential mechanisms,
which led to this result. In summary, the present study
demonstrated that miR-145 expression was decreased in serous
ovarian cancer and clear cell ovarian cancer tissues, and in the
corresponding ovarian cancer cell lines. Overexpression of miR-145
was able to suppress ovarian cancer cell proliferation, migration
and invasion. Additionally, CCND2 and E2F3 were confirmed as
targets of miR-145 in ovarian cancer cells, and restoration of
these 2 genes partly reversed the tumor-suppressive effect of
miR-145. The results of the present study suggested that miR-145
may function as a tumor-inhibiting factor by regulating the
oncogenes CCND2 and E2F3 in ovarian cancer. An in vivo study
will be conducted in future to verify these data. A mouse model of
ovarian cancer has been established (unpublished data), in order to
confirm the effect of miR-145 on the biological behavior of ovarian
cancer by local injection, intraperitoneal administration and
intravenous administration, and to examine the potential
applications of miR-145 in ovarian cancer therapeutics.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Science Plan
Program of Nantong Municipal Health and Family Planning Commission
(grant no. WQ2016073), The Science Plan Program of Nantong (grant
no. MS12017014-5), Jiangsu Provincial Key Medical Talents Program
for Youth (grant no. QNRC2016404) and The Six-One Project of
Jiangsu Provincial Health Committee (grant no. LGY2018036).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
MH and JZ designed the experiments. YQ, MS, JY and
YC performed the experiments. XC and WC analyzed the data. MH and
JZ interpreted the data. YQ wrote the manuscript. All authors read
and approved the manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of the Affiliated Hospital of Nantong University. Written
informed consent was obtained from all enrolled subjects.
Patient consent for publication
All patients within this study provided consent for
the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Novoa-Vargas A: Natural history of ovary
cancer. Ginecol Obstet Mex. 82:613–622. 2014.(In Spanish).
PubMed/NCBI
|
|
2
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Garofalo M and Croce CM: microRNAs: Master
regulators as potential therapeutics in cancer. Annu Rev Pharmacol
Toxicol. 51:25–43. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mendell JT and Olson EN: MicroRNAs in
stress signaling and human disease. Cell. 148:1172–1187. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iorio MV, Visone R, Di Leva G, Donati V,
Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, et
al: MicroRNA signatures in human ovarian cancer. Cancer Res.
67:8699–8707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang J, Guo H, Zhang H, Wang H, Qian G,
Fan X, Hoffman AR, Hu JF and Ge S: Putative tumor suppressor
miR-145 inhibits colon cancer cell growth by targeting oncogene
Friend leukemia virus integration 1 gene. Cancer. 117:86–95. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu Z, Xu T, Wang L, Wang X, Zhong S, Xu C
and Shen Z: MicroRNA-145 directly targets the insulin-like growth
factor receptor I in human bladder cancer cells. FEBS Lett.
588:3180–3185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sachdeva M, Zhu S, Wu F, Wu H, Walia V,
Kumar S, Elble R, Watabe K and Mo YY: p53 represses c-Myc through
induction of the tumor suppressor miR-145. Proc Natl Acad Sci USA.
106:3207–3212. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang J, Guo H, Qian G, Ge S, Ji H, Hu X
and Chen W: MiR-145, a new regulator of the DNA fragmentation
factor-45 (DFF45)-mediated apoptotic network. Mol Cancer.
9:2112010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sachdeva M and Mo YY: MicroRNA-145
suppresses cell invasion and metastasis by directly targeting mucin
1. Cancer Res. 70:378–387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu Q, Liu LZ, Qian X, Chen Q, Jiang Y, Li
D, Lai L and Jiang BH: MiR-145 directly targets p70S6K1 in cancer
cells to inhibit tumor growth and angiogenesis. Nucleic Acids Res.
40:761–774. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cho WC, Chow AS and Au JS: MiR-145
inhibits cell proliferation of human lung adenocarcinoma by
targeting EGFR and NUDT1. RNA Biol. 8:125–131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan M, Liaw CS, Ji SM, Tan HH, Wong CY,
Thike AA, Tan PH, Ho GH and Lee AS: Identification of circulating
microRNA signatures for breast cancer detection. Clin Cancer Res.
19:4477–4487. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gramantieri L, Fornari F, Ferracin M,
Veronese A, Sabbioni S, Calin GA, Grazi GL, Croce CM, Bolondi L and
Negrini M: MicroRNA-221 targets Bmf in hepatocellular carcinoma and
correlates with tumor multifocality. Clin Cancer Res. 15:5073–5081.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ichimi T, Enokida H, Okuno Y, Kunimoto R,
Chiyomaru T, Kawamoto K, Kawahara K, Toki K, Kawakami K, Nishiyama
K, et al: Identification of novel microRNA targets based on
microRNA signatures in bladder cancer. Int J Cancer. 125:345–352.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Amaral FC, Torres N, Saggioro F, Neder L,
Machado HR, Silva WJ Jr, Moreira AC and Castro M: MicroRNAs
differentially expressed in ACTH-secreting pituitary tumors. J Clin
Endocrinol Metab. 94:320–323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang D, Sun Y, Hu L, Zheng H, Ji P, Pecot
CV, Zhao Y, Reynolds S, Cheng H, Rupaimoole R, et al: Integrated
analyses identify a master microRNA regulatory network for the
mesenchymal subtype in serous ovarian cancer. Cancer Cell.
23:186–199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chung YW, Bae HS, Song JY, Lee JK, Lee NW,
Kim T and Lee KW: Detection of microRNA as novel biomarkers of
epithelial ovarian cancer from the serum of ovarian cancer
patients. Int J Gynecol Cancer. 23:673–679. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Letelier P, García P, Leal P, Álvarez H,
Ili C, López J, Castillo J, Brebi P and Roa JC: miR-1 and miR-145
act as tumor suppressor microRNAs in gallbladder cancer. Int J Clin
Exp Pathol. 7:1849–1867. 2014.PubMed/NCBI
|
|
20
|
Wu H, Xiao Z, Wang K, Liu W and Hao Q:
MiR-145 is downregulated in human ovarian cancer and modulates cell
growth and invasion by targeting p70S6K1 and MUC1. Biochem Biophys
Res Commun. 441:693–700. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang W, Wang Q, Yu M, Wu N and Wang H:
MicroRNA-145 function as a cell growth repressor by directly
targeting c-Myc in human ovarian cancer. Technol Cancer Res Treat.
13:161–168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Q, Sakamoto K and Wagner KU: D-type
Cyclins are important downstream effectors of cytokine signaling
that regulate the proliferation of normal and neoplastic mammary
epithelial cells. Mol Cell Endocrinol. 382:583–592. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kato JY and Sherr CJ: Inhibition of
granulocyte differentiation by G1 cyclins D2 and D3 but not D1.
Proc Natl Acad Sci USA. 90:11513–11517. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song H, Hogdall E, Ramus SJ, Dicioccio RA,
Hogdall C, Quaye L, McGuire V, Whittemore AS, Shah M, Greenberg D,
et al: Effects of common germ-line genetic variation in cell cycle
genes on ovarian cancer survival. Clin Cancer Res. 14:1090–1095.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rady B, Chen Y, Vaca P, Wang Q, Wang Y,
Salmon P and Oberholzer J: Overexpression of E2F3 promotes
proliferation of functional human β cells without induction of
apoptosis. Cell Cycle. 12:2691–2702. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bracken AP, Ciro M, Cocito A and Helin K:
E2F target genes: Unraveling the biology. Trends Biochem Sci.
29:409–417. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Johnson DG, Schwarz JK, Cress WD and
Nevins JR: Expression of transcription factor E2F1 induces
quiescent cells to enter S phase. Nature. 365:349–352. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nevins JR: E2F: A link between the Rb
tumor suppressor protein and viral oncoproteins. Science.
258:424–429. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Blais A and Dynlacht BD: Hitting their
targets: An emerging picture of E2F and cell cycle control. Curr
Opin Genet Dev. 14:527–532. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu H, Dougherty U, Robinson V, Mustafi R,
Pekow J, Kupfer S, Li YC, Hart J, Goss K, Fichera A, et al: EGFR
signals downregulate tumor suppressors miR-143 and miR-145 in
Western diet-promoted murine colon cancer: Role of G1 regulators.
Mol Cancer Res. 9:960–975. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Le Beau MM, Lemons RS, Espinosa R III,
Larson RA, Arai N and Rowley JD: Interleukin-4 and interleukin-5
map to human chromosome 5 in a region encoding growth factors and
receptors and are deleted in myeloid leukemias with a del(5q).
Blood. 73:647–650. 1989.PubMed/NCBI
|
|
33
|
Zhu X, Li Y, Xie C, Yin X, Liu Y, Cao Y,
Fang Y, Lin X, Xu Y, Xu W, et al: miR-145 sensitizes ovarian cancer
cells to paclitaxel by targeting Sp1 and Cdk6. Int J Cancer.
135:1286–1296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gadducci A, Sergiampietri C, Lanfredini N
and Guiggi I: Micro-RNAs and ovarian cancer: The state of art and
perspectives of clinical research. Gynecol Endocrinol. 30:266–271.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shi B, Sepp-Lorenzino L, Prisco M, Linsley
P, DeAngelis T and Baserga R: Micro RNA 145 targets the insulin
receptor substrate-1 and inhibits the growth of colon cancer cells.
J Biol Chem. 282:32582–32590. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
La Rocca G, Badin M, Shi B, Xu SQ,
Deangelis T, Sepp-Lorenzinoi L and Baserga R: Mechanism of growth
inhibition by MicroRNA 145: The role of the IGF-I receptor
signaling pathway. J Cell Physiol. 220:485–491. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fuse M, Nohata N, Kojima S, Sakamoto S,
Chiyomaru T, Kawakami K, Enokida H, Nakagawa M, Naya Y, Ichikawa T
and Seki N: Restoration of miR-145 expression suppresses cell
proliferation, migration and invasion in prostate cancer by
targeting FSCN1. Int J Oncol. 38:1093–1101. 2011.PubMed/NCBI
|
|
38
|
Speranza MC, Frattini V, Pisati F, Kapetis
D, Porrati P, Eoli M, Pellegatta S and Finocchiaro G: NEDD9, a
novel target of miR-145, increases the invasiveness of
glioblastoma. Oncotarget. 3:723–734. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu CC, Tsai LL, Wang ML, Yu CH, Lo WL,
Chang YC, Chiou GY, Chou MY and Chiou SH: miR145 targets the
SOX9/ADAM17 axis to inhibit tumor-initiating cells and
IL-6-mediated paracrine effects in head and neck cancer. Cancer
Res. 73:3425–3440. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sherr CJ: G1 phase progression: Cycling on
cue. Cell. 79:551–555. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Weinberg RA: The retinoblastoma protein
and cell cycle control. Cell. 81:323–330. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Trimarchi JM and Lees JA: Sibling rivalry
in the E2F family. Nat Rev Mol Cell Biol. 3:11–20. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
He Y, Franco OE, Jiang M, Williams K, Love
HD, Coleman IM, Nelson PS and Hayward SW: Tissue-specific
consequences of cyclin D1 overexpression in prostate cancer
progression. Cancer Res. 67:8188–8197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chang L, Guo R, Yuan Z, Shi H and Zhang D:
LncRNA HOTAIR regulates CCND1 and CCND2 expression by sponging
miR-206 in ovarian cancer. Cell Physiol Biochem. 49:1289–1303.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Reimer D, Hubalek M, Riedle S, Skvortsov
S, Erdel M, Concin N, Fiegl H, Müller-Holzner E, Marth C, Illmensee
K, et al: E2F3a is critically involved in epidermal growth factor
receptor-directed proliferation in ovarian cancer. Cancer Res.
70:4613–4623. 2010. View Article : Google Scholar : PubMed/NCBI
|