Introduction
Although the growing range of treatment options for
chronic lymphocytic leukemia and for other lymphoproliferative
neoplasms has improved patient survival (1–4),
these diseases remain incurable. In addition, there are patients
who do not respond to the applied therapy. The serious problems
associated with the diagnostic procedure and the design of suitable
treatments seem to be linked to the coexistence in patient
peripheral blood of quiescent and cycling cells population; this
constitutes a special challenge in predicting an effective approach
for treating CLL patients (5,6).
Differences in cell signaling trafficking, as well
as in the expression of factors involved in apoptosis or
microenvironmental factors, might contribute to differences
(between patients) in the cell response to anticancer agents
between patients. In addition, it is well accepted that diversity
in the accumulation of genetic aberrations and epigenetic
modifications could also account for heterogeneity in the clinical
course of CLL (7–9) and the response to therapy (10,11).
Moreover, another factor that could imply in the course of CLL as
well as response to therapy is the expression of miR-155. This
microRNA is associated with the progression of CLL and weak
response to therapy (12). The
presence of several factors important for disease development
reveals the necessity for the use of personalized medicine, by
testing the potential reaction of the patient's cells to anticancer
drugs before treatment, to avoid administration of an ineffective
regimen (8,13–16).
Therefore, it is very important to search for new anticancer agents
with the potential to induce apoptosis in CLL cells (17–20).
Cyclin-dependent kinases (CDK) are fundamental
factors involved in the regulation of the cell-cycle, transcription
and apoptosis. Their frequent deregulation in cancers provides
novel targets for pharmacological intervention in oncology
(21). Various small-molecule CDK
inhibitors have been developed, including CDK4/CDK6-specific
palbociclib and ribociclib, recently FDA-approved for multiple
myeloma and breast cancer, respectively (22,23).
Besides the cell cycle, CDKs play critical roles also in a
non-proliferative CLL and in cell lines where the CDK inhibitor
flavopiridol has been designed as an orphan drug for CLL (24). Flavopiridol however suffers several
side effects, such as significant toxicity including high rates of
major tumor lysis syndrome, cytokine release syndrome and secretory
diarrhea (24). Other CDK
inhibitors are therefore studied as new drugs for CLL, such as
roscovitine, dinaciclib or SNS-032 (25). These compounds target multiple
CDKs, including CDK1, CDK2, CDK5, CDK7 and CDK9, and trigger
cytotoxic effects through interruption of the transcription of key
antiapoptotic genes responsible for sustenance of the leukemia
cell, such as MCL-1 (21,24).
We have recently modified roscovitine to increase
its potency and the optimization yielded new
2-substituted-6-biarylmethylamino-9-cyclopentylpurine derivatives
BP14 and BP30, which display selective and potent inhibition of
CDKs 1, 2, 7 and 9 with low nanomolar IC50 values (26). Both BP14 and BP30 exhibit strong
cytotoxicity in human cancer cell lines that correlate with robust
CDK1 and CDK2 inhibition and caspase activation. BP14 has
demonstrated efficacy against xenografted human liver carcinomas,
effectively repressing tumor growth (27). In addition, BP14 potently inhibited
transcriptional regulator CDK9 and downregulated anti-apoptotic
protein MCL-1 (27,28), key mediator of CLL-cell
survival.
The aim of the current work was to observe the
importance of drug doses for anticancer response in leukemic cells.
For this purpose we have compared the apoptosis induction potential
of new CDK inhibitors as potential drugs for CLL and compare them
with standard treatments. The present study compares the
cytotoxicity (cell viability, apoptosis or necrosis level) of novel
roscovitine derivatives BP14 and BP30 and anticancer drugs used in
hematological clinics for treating CLL (CM, cladribine +
mafosfamide; RCM, rituximab + cladribine + mafosfamide) on controls
and leukemic cells obtained from peripheral blood of CLL patients
untreated with anticancer agents.
Materials and methods
Drugs and treatments
The studied compounds BP14
(N2-(trans-4-aminocyclohexyl)-9-cyclopentyl-N6-[[6-(2-furanyl)-3-piridinyl]
methyl]-9H-purine-2,6-diamine) and BP30
(N2-(trans-4-amino-cyclohexyl)-9-cyclopentyl-N6-[[6-(2-thienyl)-3-pyridinyl]
methyl] −9H-purine-2,6-diamine) were synthesized and characterized
as described in our previous report (26). The identities of the compounds were
confirmed by mass spectrometry, NMR and elemental analysis. The
purity of the compounds was at least 99.5%. 10 mM stock solutions
of the compounds were prepared in DMSO. The final DMSO
concentrations did not exceed 0.1%, a concentration verified not to
affect respective experimental parameters.
Cladribine (Biodrybin) and rituximab were obtained
from the Institute of Biotechnology and Antibiotics (Warsaw,
Poland) and from Roche (Basel, Switzerland), respectively.
Mafosfamide was purchased from Niomech IIT GmbH (Bielefeld,
Germany). These three drugs were used in the following
combinations: CM (cladribine + mafosfamide), RCM (rituximab +
cladribine + mafosfamide). The concentration of the drugs used for
cell incubations were as follows: cladribine 50 ng/ml
(139 nM), mafosfamide 1 µg/ml (2.4 µM), rituximab 20 µg/ml (170
nM). Cells incubated only with medium or with appropriately diluted
DMSO served as a control.
Patients
Blood samples were obtained from five CLL patients:
One female patient and four male (cooperation with the Department
of Hematology, Medical University of Lodz, Poland; Head: Prof. T.
Robak). The median age was 65 years (54–81). Leukocytosis ranged
from 60 to 700×103/µl (Table I).
Samples were collected on EDTA to sterile, disposable tubes. Cell
marker studies were performed to confirm B-cell origin and
monoclonal proliferation. All B lymphocytes were CD5, CD19 and CD23
positive.
 | Table I.Characteristics of patients. |
Table I.
Characteristics of patients.
| Patient number | Sex | Age, years | Leukocytosis
(×103/µl) | Rai stage |
|---|
| 12 | M | 81 | 470 | 4 |
| 14 | M | 57 | 700 | 3 |
| 15 | M | 65 | 60 | 4 |
| 16 | F | 66 | 153 | 4 |
| 17 | M | 54 | 262.3 | 2 |
Ethics statement
The study was approved by the Local Ethics Committee
from the University of Lodz (20/KBBM-UŁ/2015 and
5/KBBMUŁ/2017).
Isolation of mononuclear cells and
cell cultures with anticancer agents
Mononuclear cells were isolated from CLL samples by
centrifugation on a Histopaque 1077 gradient (900 rpm, 19 min,
24°C). The obtained cells were resuspended in RPMI-1640 medium with
10% fetal bovine serum, 2 mM L-glutamine, 100 U/ml penicillin and
100 µg/ml streptomycin. The cell culture density was 3×106/ml. In
the next step, the cells were incubated with drugs/novel anticancer
compounds. After 24 and 48 h of cell incubations with anticancer
agents cell viability was tested.
Flow cytometry analysis
Cell viability, the rate of apoptosis and necrosis
were estimated using a Vybrant Apoptosis Assay #4 kit after 0, 24
and 48 h, as in previous studies (14,15).
The early apoptotic cells were marked with YO-PRO fluorescent dye,
and late apoptotic and necrotic cells with propidium iodide. For
flow cytometry analysis, 1×106/ml cells were used, centrifuged
(5,000 rpm, 5 min, 24°C), then resuspended in phosphate buffer
saline (PBS) containing fluorescent dyes. Samples were incubated in
the dark for 20 min and then analyzed.
Statistical analysis
Statistical analysis were performed by Kruskal
Wallis test with Dunn's multiple comparison test. The statistical
differences between the control sample (Co.) and samples with the
addition of the anticancer agent was analyzed, respectively.
Statistical analysis were obtained for N=5; *P<0.05,
**P<0.001, ***P<0.0001.
Results
Peripheral mononuclear cells from the group of 6
patients with CLL were exposed to varying concentrations of novel
CDK inhibitors (BP14 and BP30). The representative results for
patients (3 presented as single cases and 2 combined figures with
statistics) were included in current manuscript, showing distinct
patients response to anticancer agents. The effect of CDK
inhibitors on CLL cells were analysed for 6 CLL patients and
compared with standard chemotherapeutic regimens (CM, cladribine +
mafosfamide; RCM, rituximab + cladribine + mafosfamide), as
described in our previous studies with more than 50 patients
(14,15). The CLL cells isolated from
peripheral blood of patients were incubated with anticancer agents
for 24 and 48 h, harvested and then analyzed for viability,
apoptosis and necrosis by flow cytometry. Both studied CDK
inhibitors decreased the viability of the CLL cells in a
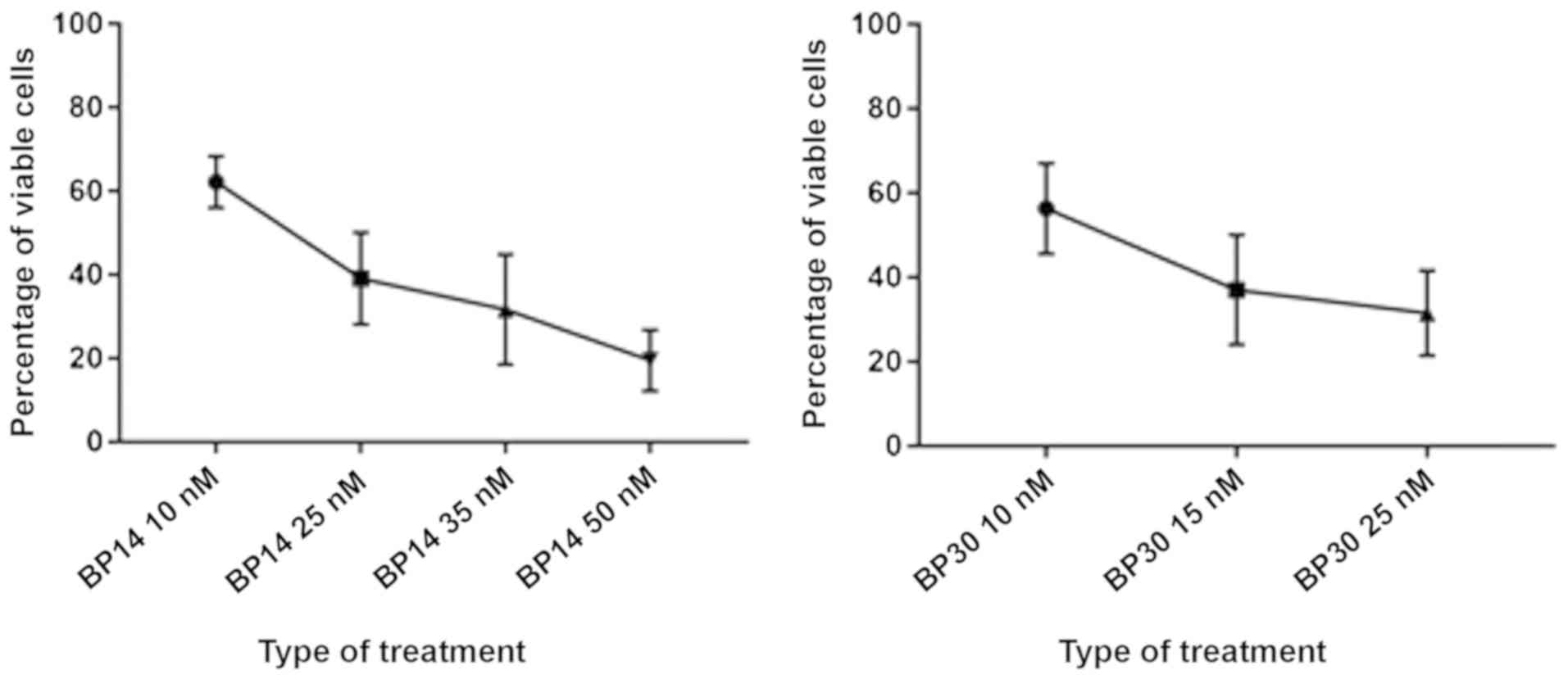
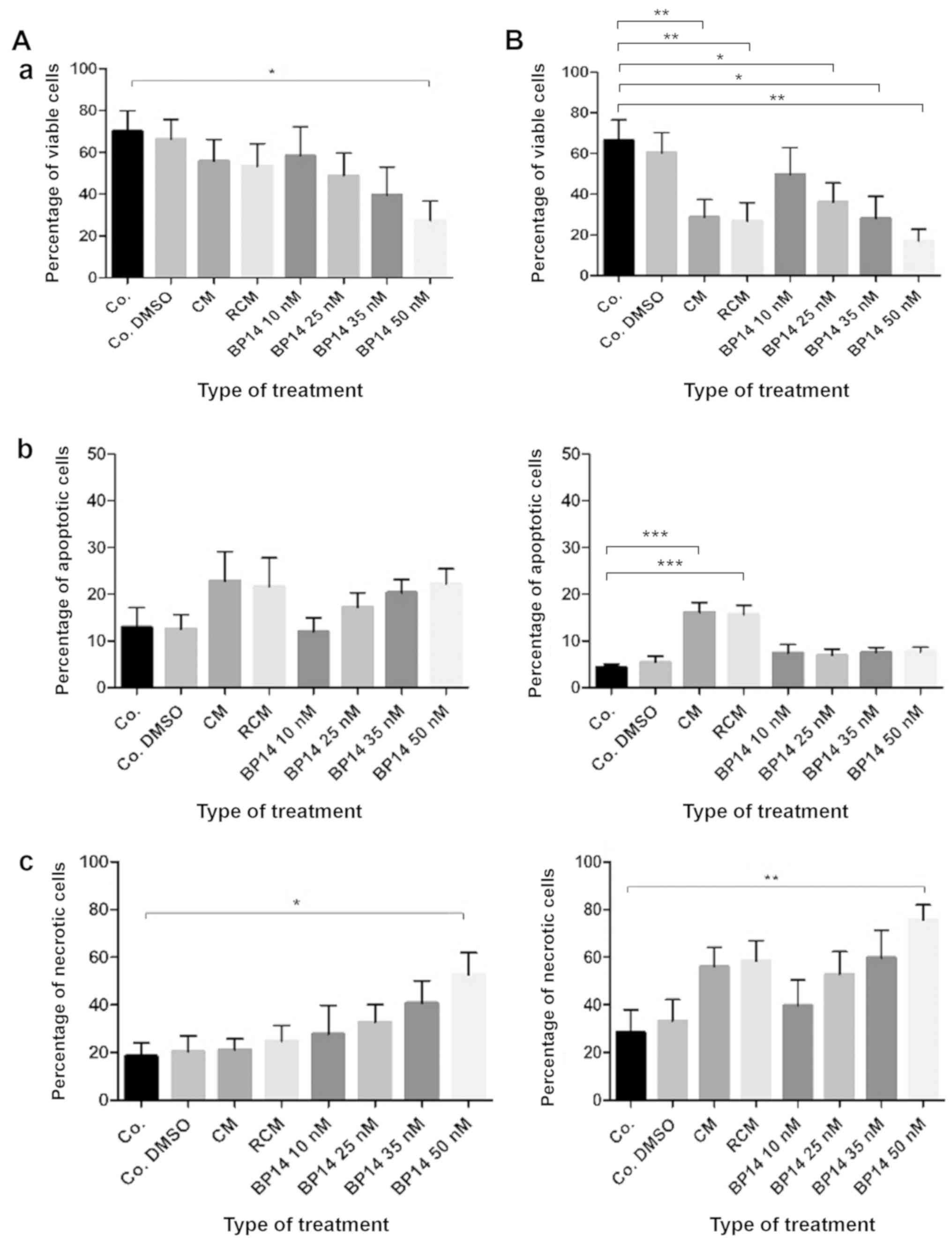
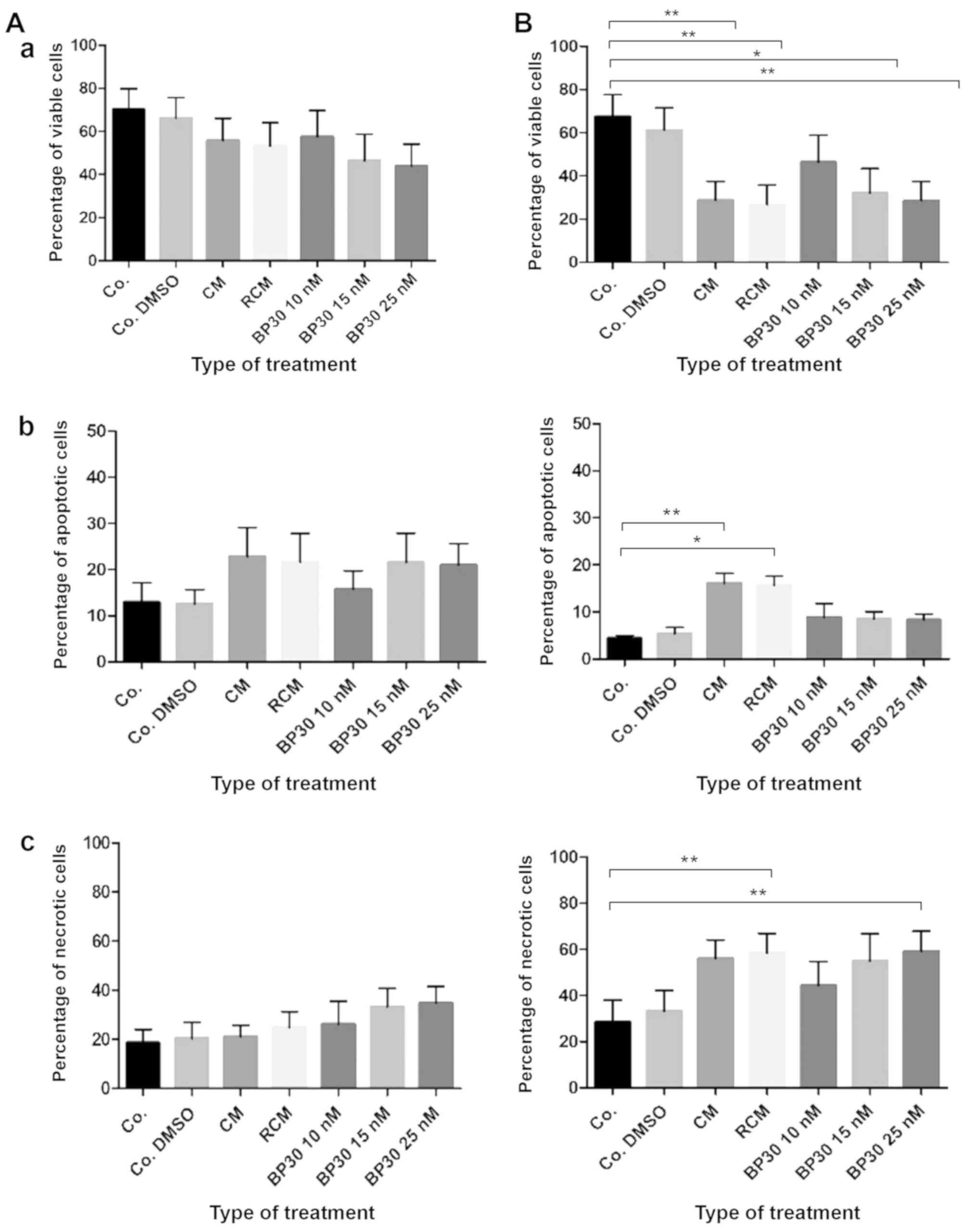
dose-dependent manner (Fig. 1). At
the concentration of 25 nM, the median reduction (IC50) for all
patients in cell viability for both CDK inhibitors was similar,
39.1 and 31.5% for BP14 and BP30, respectively (Fig. 1).
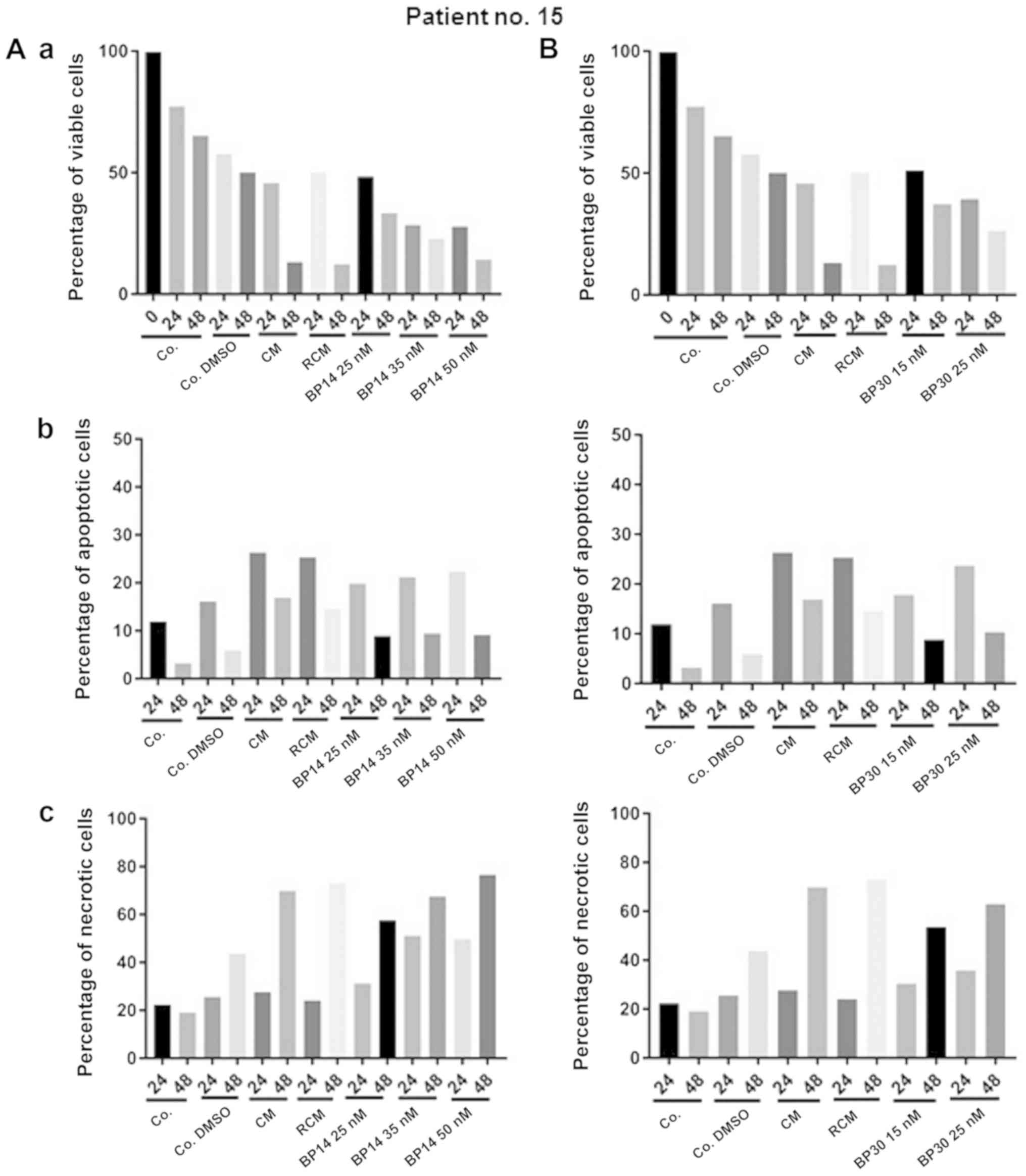
Next we have analysed CLL viability and the level of
necrosis upon treatment with anticancer agents. The cells of
patient no. 15 showed increasing level of necrosis after 24 h
exposition to all used anticancer agents but with distinct extend.
The cells obtained from patient no. 15 were very sensitive toward
all anticancer agents (Figs. 2 and
3). The strong reduction in cell
viability was observed for all used types of treatment, but the
most spectacular effect was noticed when cell were incubated for 48
h with CM and RCM. Moreover, the increase in apoptosis level (24 h
incubation) and the high value of necrosis (48 h) was a consequence
of dynamics in apoptosis realization. The high level of necrosis
after 48 h of cell exposition to anticancer agents could be a
compilation of necrosis as well as advanced apoptosis. Therefore,
for a proper modulation of doses value a special importance should
be given to results obtained at 24 h of cells incubation with
anticancer agents. While for monitoring of apoptosis induction
potential an important time for analysis of anticancer response
efficacy seems to be 48 h of cells exposition to anticancer agents
(Fig. 2).
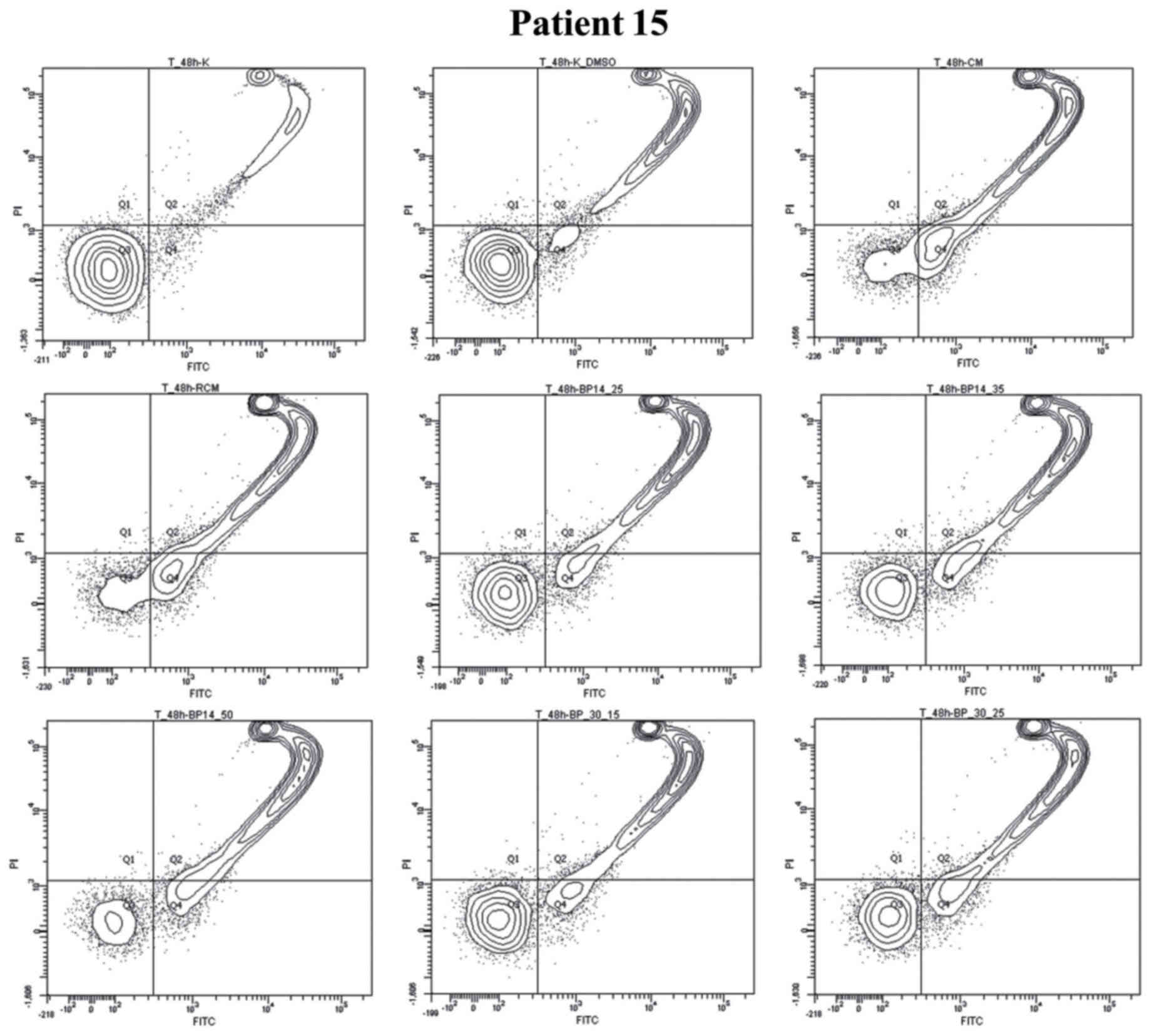 | Figure 3.Graph plots of cell viability (Q3),
apoptotic (Q4), necrotic and late apoptotic cells (Q2), debris
(Q1). CLL cells (Patient no. 15) were incubated for 48 h without
drugs (Co. or Co. DMSO) or with anticancer drugs (CM or RCM) or CDK
inhibitors in several doses BP14.25, BP14.35, BP14.50, and BP
30.15, BP 30.15. BP 30.25. Co., untreated control; Co. DMSO,
control with dimethyl sulfoxide; CM, cladribine combined with
mafosfamide; RCM, CM combined with rituximab; CDK, cyclin-dependent
kinase. |
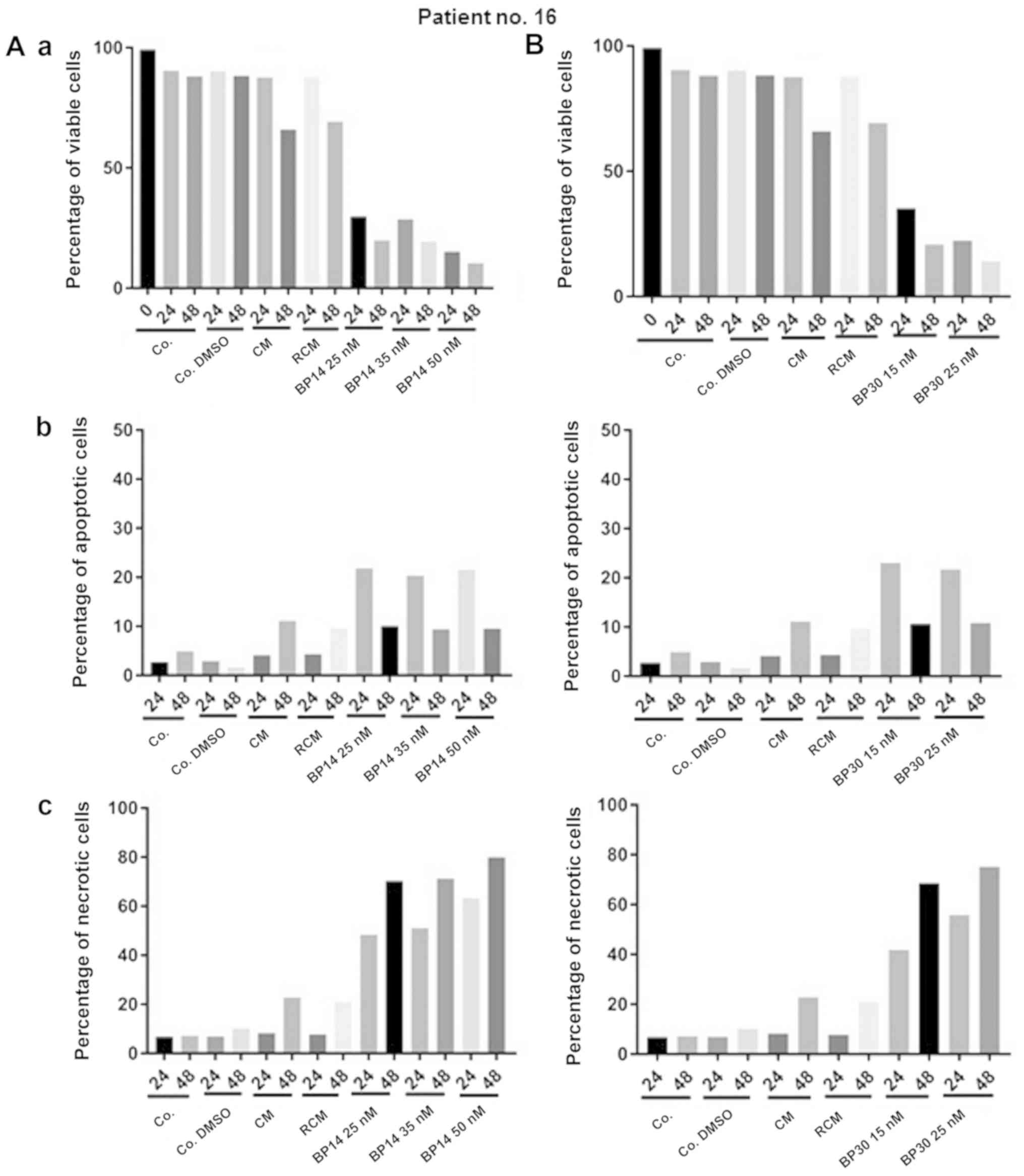
The results obtained for representative patient no.
16 show the potential resistance of patient's cells to drug
combinations used in hematological clinics for CLL treatment. For
this point only BP14/BP30 reflect to be active in apoptosis
induction. Moreover, BP30 and BP14 in all used doses induced very
fast apoptosis. The cells of patient no. 16 (Figs. 4 and 5) demonstrated resistance to CM and RCM,
as well as sensitivity to each analyzed dose of CDK inhibitors
(BP14 and BP30). Interestingly, the level of apoptosis in patients
no. 16's cells for each dose of BP14 and BP30 was relatively
constant, but increasing levels of necrosis/late apoptosis were
observed.
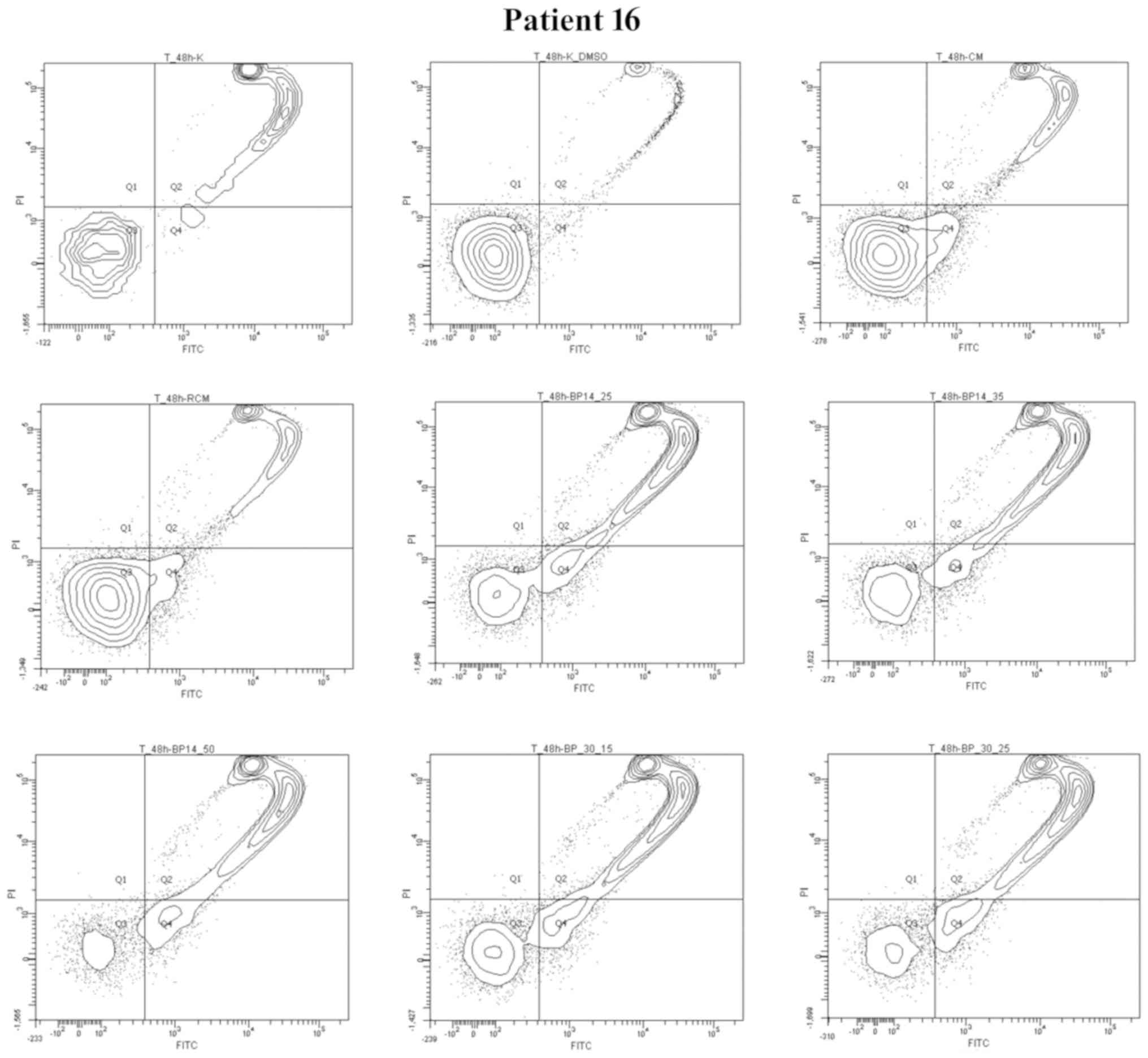 | Figure 5.Graph plots of cell viability (Q3),
apoptotic (Q4), necrotic and late apoptotic cells (Q2), debris
(Q1). CLL cells (Patient no. 16) were incubated for 48 h (Co. or
Co. DMSO) or with anticancer drugs (CM or RCM) or CDK inhibitors in
several doses BP14.25, BP14.35, BP14.50, and BP 30.15, BP 30.15. BP
30.25. Co., untreated control; Co. DMSO, control with dimethyl
sulfoxide; CM, cladribine combined with mafosfamide; RCM, CM
combined with rituximab; CDK, cyclin-dependent kinase. |
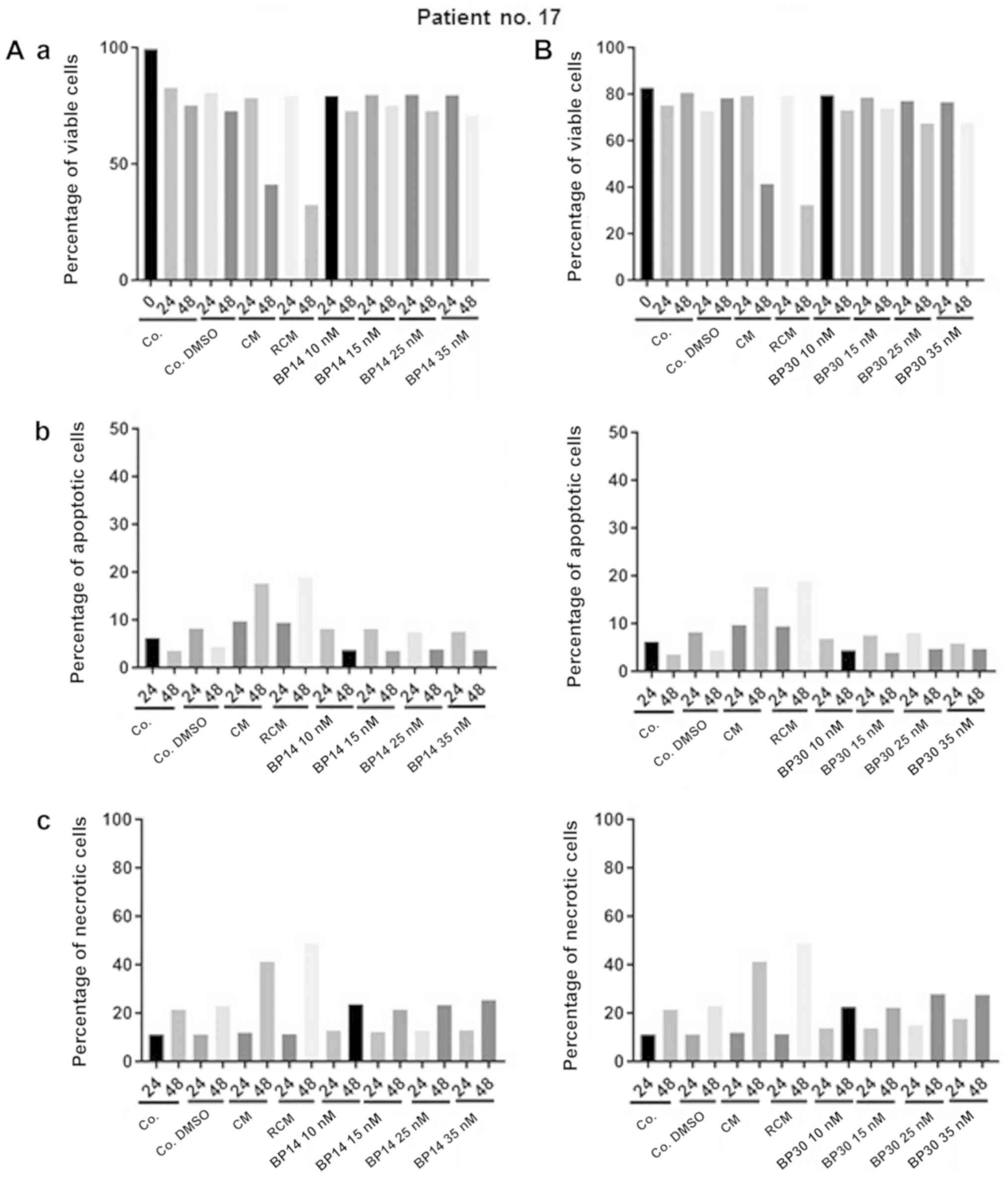
The comparative analysis of patient no. 17 reflects
the opposite reaction to patient no. 16. The cells of patient no.
17 (Fig. 6) were sensitive to both
standard CLL treatments (CM and RCM), but displayed resistance to
CDK inhibitors, which was clear in all assays, including cell
viability, apoptosis and necrosis.
Finally, the comparative analysis of the results of
the 24 h (A) and 48 h (B) CLL cell incubations with anticancer
agents for five patients (Table I)
are presented on Fig. 7 (for BP14)
and Fig. 8 (for BP30), with a
statistical analysis performed by the Kruskal-Wallis test. It must
be stated that these combined results are difficult to compare with
those of each single patient. It is important to note that the
results for patients 15, 16 or 17 presented here indicate that the
median results presented in scientific papers usually provide
incorrect recommendations (13–15)
concerning patient resistance/sensitivity to anticancer agents, and
these would not be effective in treating patient no. 16.
Discussion
During CLL development, long-lived B lymphocytes
accumulate in peripheral blood and in other lymphatic organs of
patients with the advanced form of the disease (29). This accumulation occurs due to
suppression of apoptosis and usually happens in the resting form of
the disease (9,29). Since the disease could transform
into an active form, the coexistence of quiescent and cycling cell
populations complicate the diagnostics and prediction of therapy
efficacy (5,6). Therefore, an analysis of the
chemosensitivity profile of patient cells prior to therapy followed
by the use of personalized medicine based on apoptosis induction
would markedly reduce the ineffective treatment (8,13–15).
The current study compares cytometric analyses of
cell viability, the levels of apoptosis and necrosis in ex vivo CLL
cells isolated from five patients to determine whether the response
has some connection with the type of used anticancer agents. The
special attention was given for observation of the role of
anticancer agent doses on cell response.
Our findings reveal significant differences in the
response to anticancer agents among leukemic patients, as noticed
in previous studies (8,14,15).
Moreover, our results show that the comparison of cell viability,
apoptosis rate, and the level of necrosis underline the importance
of proper anticancer agent dose selection to avoid the occurrence
of a high necrosis level that could transform in vivo into
inflammation. To eliminate potential resistance to therapy, the
therapeutic approach should be chosen before drug administration in
the clinic using personalized therapy tests (14,15).
Such techniques have good value for predicting CLL cell sensitivity
to anticancer drugs. This prediction is of importance as it allows
for the optimal course of therapy to be chosen for the patient,
thus avoiding ineffective anticancer administration and minimizing
the level of necrosis that could influence the activity of the drug
in vivo. In order to expand the range of possible
therapeutic options for leukemia treatment, there is an urgent need
to identify new compounds with anticancer properties.
Recent developments in the field of drug discovery
include the identification of potent CDK inhibitors. Currently, it
is not clear whether highly CDK specific inhibitors, such as
palbociclib, or pan-CDK specific inhibitors, such as flavopiridol
or dinaciclib, are more suitable for cancer therapy. A number of
studies have found them to have promising efficacy when used in
combination with standard anticancer drugs or with other
molecularly targeted agents (21).
The procedure for identifying cancers that can respond to a
particular course of therapy has also been frequently discussed.
CDK inhibitors have also been widely studied in CLL in clinical
settings. For example flavopiridol, SNS032 or dinaciclib have
demonstrated activity against CLL, but have also caused a
significant decrease in cell viability (24,30–33).
These CDK inhibitors downregulate mRNA and protein expression of
the important anti-apoptotic proteins of the BCL2 family such as
MCL1 and BCL-xL and block oncogenic pathways (e.g. STAT3, MAPK,
NF-κB), which have been shown to be essential for CLL cell survival
(25,34).
The present study examines the anti-CLL potential of
two novel CDK inhibitors that have already displayed high
anticancer activity (26). Our
results further support previous findings that CDK inhibitors
induce apoptosis usually much faster than standard treatments used
for CLL patients (24). However, a
high variability noticed in disease development (29) translates on individual patient
sensitivity to anticancer agents. Our recent findings reveal that
the dose of the anticancer drug strongly influences the response to
treatment. While the correct dose of the active drug will induce a
high level of apoptosis and should lead to natural cell elimination
by programmed cell death. The high dose usually induces necrosis,
which could induce inflammation. In addition, our findings
demonstrate that drug dose could play an important role in the
modulation of leukemic cell response to the anticancer agent(s). It
has also been found that 5-HT7 receptors play a role in the
induction of inflammation by release of sirtuin, a nicotinamide
adenine dinucleotide-dependent deacetylase that could influence
gene expression (35), as well as
in the level of serotonin regulation (36). Therefore, it is possible that an
excessive dose of a drug induces a high level of necrosis, and that
could be transferred into inflammation. It might also have an
impact on the diversities in cell signaling. Interestingly, it has
been suggested that inflammation could cause conditions directed to
osteoporosis (37), which can
suggest that factors involved in the development of inflammation
could also disturb other metabolic pathways, leading to the
possible occurrence of a range of other metabolic alterations that
could lead to osteoporosis. Interestingly, the aberrant products
observed in cancer cells involved in the Krebs cycle could promote
cancer progression (38).
The results of our studies confirm the need for
personalized therapy for CLL, as such an individual approach would
greatly avoid the chance of ineffective treatment being used. Our
findings also highlight the importance of the application of the
correct dose for treatment, and demonstrate that the drugs could
induce apoptosis at different rates, which can be also monitored
in vitro by incubating cells with anticancer agents.
Acknowledgements
The authors would like to thank Professor Tadeusz
Robak (Department of Hematology, Medical University of Lodz, Lodz,
Poland) for their help with the personalized therapy for CLL, as
well as Mr. Edward Lowczowski (Medical University of Lodz) for
editorial assistance.
Funding
The present study was supported by the Ministry of
Education, Youth and Sports of the Czech Republic (National Program
of Sustainability I; grant no. LO1204), Czech Science Foundation
(grant no. 15-15264S) and Palacky University in Olomouc (grant no.
IGA_PrF_2018_006). The equipment for cell culture and media was
sponsored by the University of Lodz for MSc degrees.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MK and AS performed the experiments. JZB and PR
determined the clinical material characteristics. MM performed and
organised the blood transfers in the clinic. TG and VK synthesized
the new purine analogs. MR wrote the manuscript, and conceived and
designed the experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Local Ethics
Committee from the University of Lodz (nos. 20/KBBM-UŁ/2015 and
5/KBBMUŁ/2017). The requirement for written, informed consent was
waived.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Besbes S, Mirshahi M, Pocard M and Billard
C: Strategies targeting apoptosis proteins to improve therapy of
chronic lymphocytic leukemia. Blood Rev. 29:345–350. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cramer P, Eichhorst B, Reinhardt HC and
Hallek M: Current strategies to create tailored and risk-adapted
therapies for CLL patients. Best Pract Res Clin Haematol.
29:111–121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Visentin A, Facco M, Frezzato F, Castelli
M, Trimarco V, Martini V, Gattazzo C, Severin F, Chiodin G,
Martines A, et al: Integrated CLL scoring system, a new and simple
index to predict time to treatment and overall survival in patients
with chronic lymphocytic leukemia. Clin Lymphoma Myeloma Leuk.
15:612–620.e1-5. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koffman B and Schorr A: The 21st century
revolution in CLL: Why this matters to patients. Best Pract Res
Clin Haematol. 29:122–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Klein U and Dalla-Favera R: Germinal
centres: Role in B-cell physiology and malignancy. Nat Rev Immunol.
8:22–33. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hallek M: Chronic lymphocytic leukemia:
2013 update on diagnosis, risk stratification and treatment. Am J
Hematol. 88:803–816. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martin-Subero JI, López-Otín C and Campo
E: Genetic and epigenetic basis of chronic lymphocytic leukemia.
Curr Opin Hematol. 20:362–368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rogalińska M, Franiak-Pietryga I, Błoński
JZ, Góralski P, Maciejewski H, Janus A, Robak P, Mirowski M,
Piekarski H, Robak T and Kiliańska ZM: Toward personalized therapy
for chronic lymphocytic leukemia: DSC and cDNA microarray
assessment of two cases. Cancer Biol Ther. 14:6–12. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rodriquez-Vicente AE, Díaz MG and
Hernández-Rivas JM: Chronic lymphocytic leukemia: A clinical and
molecular heterogenous disease. Cancer Genet. 206:49–62. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rogalinska M, Goralski P, Wozniak K,
Bednarek JD, Blonski JZ, Robak T, Piekarski H, Hanausek M, Walaszek
Z and Kilianska ZM: Calorimetric study as a potential test for
choosing treatment of B-cell chronic lymphocytic leukemia. Leuk
Res. 33:308–314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jeyakumar D and O'Brien S: B cell receptor
inhibition as a target for CLL therapy. Best Pract Res Clin
Haematol. 29:2–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ferrajoli A, Shanafelt TD, Ivan C, Shimizu
M, Rabe KG, Nouraee N, Ikuo M, Ghosh AK, Lerner S, Rassenti LZ, et
al: Prognostic value of miR-155 in individuals with monoclonal
B-cell lymphocytosis and patients with B chronic lymphocytic
leukemia. Blood. 122:1891–1899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rogalińska M and Kiliańska ZM:
Personalised therapy versus targeted therapy, differences in
meaning. Glo J Res Anal. 4:5–8. 2015.
|
|
14
|
Rogalińska M, Błoński JZ, Góralski P,
Wawrzyniak E, Hartman M, Rogalska A, Robak P, Koceva-Chyła A,
Piekarski H, Robak T and Kiliańska ZM: Relationship between in
vitro drug sensitivity and clinical response of patients to
treatment in chronic lymphocytic leukemia. Int J Oncol.
46:1259–1267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rogalińska M, Góralski P, Błoński JZ,
Robak P, Barciszewski J, Koceva-Chyła A, Piekarski H, Robak T and
Kilianska ZM: Personalized therapy tests for the monitoring of
chronic lymphocytic leukemia development. Oncol Let. 13:2079–2084.
2017. View Article : Google Scholar
|
|
16
|
Montserrat E, Bauman T and Delgado J:
Present and future of personalized medicine in CLL. Best Pract Res
Clin Haematol. 29:100–110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Piggin A, Bayly E and Tam CS: Novel agents
versus chemotherapy as frontline treatment of CLL. Leuk Lymph.
58:1320–1324. 2017. View Article : Google Scholar
|
|
18
|
Rogalińska M and Kiliańska ZM: Potential
new agents for chronic lymphocytic leukemia treatment. Anticancer
Agents Med Chem. 10:666–682. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Robak T, Stilgenbauer S and Tedeschi A:
Front-line treatment of CLL in the era of novel agents. Cancer
Treat Rev. 53:70–78. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hallek M: Role and timing of new drugs in
CLL. Hematol Oncol. 35 (Suppl 1):S30–S32. 2017. View Article : Google Scholar
|
|
21
|
Whittaker S, Mallinger A, Workman P and
Clarke PA: Inhibitors of cyclin-dependent kinases as cancer
therapeutics. Pharmacol Ther. 173:83–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Niesvizky R, Badros AZ, Costa LJ, Ely SA,
Singhal SB, Stadtmauer EA, Haideri NA, Yacoub A, Hess G, Lentzsch
S, et al: Phase 1/2 study of cyclin-dependent kinase (CDK)4/6
inhibitor palbociclib (PD-0332991) with bortezomib and
dexamethasone in relapsed/refractory multiple myeloma. Leuk Lymph.
56:3320–3328. 2015. View Article : Google Scholar
|
|
23
|
Edessa D and Sisay M: Recent advances of
cyclin-dependent kinases as potential therapeutic targets in
HR+/HER2-metastatic breast cancer: A focus on ribociclib. Breast
Cancer (Dove Med Press). 9:567–579. 2017.PubMed/NCBI
|
|
24
|
Blachly JS, Byrd JC and Grever M:
Cyclin-dependent kinase inhibitors for the treatment of chronic
lymphocytic leukemia. Semin Oncol. 43:265–273. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Y, Germano S, Clements C, Samuel J,
Shelmani G, Jayne S, Dryer MJ and Macip S: Pro-survival signal
inhibition by CDK inhibitor dinaciclib in Chronic Lymphocytic
Leukaemia. Br J Haematol. 175:641–651. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gucký T, Jorda R, Zatloukal M, Bazgier V,
Berka K, Řezníčková E, Béres T, Strnad M and Kryštof V: A novel
series of highly potent 2,6,9-trisubstituted purine
cyclin-dependent kinase inhibitors. J Med Chem. 56:6234–6247. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Haider C, Grubinger M, Řezníčková E, Weiss
TS, Rotheneder H, Miklos W, Berger W, Jorda R, Zatloukal M, Gucky
T, et al: Novel inhibitors of cyclin-dependent kinases combat
hepatocellular carcinoma without inducing chemoresistance. Mol
Cancer Ther. 12:1947–1957. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Allegri L, Baldan F, Mio C, Puppin C,
Russo D, Kryštof V and Damante G: Effects of BP-14, a novel
cyclin-dependent kinase inhibitor, on anaplastic thyroid cancer
cells. Oncol Rep. 35:2413–2418. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rodriguez D, Bretones G, Arango JR,
Valdespino V, Campo E, Quesada V and López-Otín C: Molecular
pathogenesis of CLL and its evolution. Int J Hematol. 101:219–228.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tong WG, Chen R, Plunkett W, Siegel D,
Sinha R, Harvey RD, Badros AZ, Popplewell L, Coutre S, Fox JA, et
al: Phase I and pharmacologic study of SNS-032, a potent and
selective Cdk2, 7, and 9 inhibitor, in patients with advanced
chronic lymphocytic leukemia and multiple myeloma. J Clin Oncol.
28:3015–3022. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ge Y, Lei W, Ma Y, Wang Y, Wei B, Chen X,
Ru G, He X, Mou X and Wang S: Synergistic antitumor effects of CDK
inhibitor SNS-032 and an oncolytic adenovirus co-expressing TRAIL
and Smac in pancreatic cancer. Mol Med Rep. 15:3521–3528. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Flynn J, Jones J, Johnson AJ, Andritsos L,
Maddocks K, Jaglowski S, Hessler J, Grever MR, Im E, Zhou H, et al:
Dinaciclib is a novel cyclin-dependent kinase inhibitor with
significant clinical activity in relapsed and refractory chronic
lymphocytic leukemia. Leukemia. 29:1524–1529. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Robak P and Robak T: Novel synthetic drugs
currently in clinical development for chronic lymphocytic leukemia.
Expert Opin Investig Drugs. 26:1249–1265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Awan FT, Kay NE, Davis ME, Wu W, Geyer SM,
Leung N, Jelinek DF, Tschumper RC, Secreto CR, Lin TS, et al: Mcl-1
expression predicts progression-free survival in chronic
lymphocytic leukemia patients treated with pentostatin,
cyclophosphamide, and rituximab. Blood. 113:535–537. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kozako T, Suzuki T, Yoshimitsu M, Arima N,
Honda S and Soeda S: Anticancer agents targeted to sirtuins.
Molecules. 19:20295–20313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Albayrak A, Halici Z, Cadirci E, Polat B,
Karakus E, Bayir Y, Unal D, Atasoy M and Dogrul A: Inflammation and
peripheral 5-HT7 receptors: The role of 5-HT7 receptors in
carrageenan induced inflammation in rats. Eur J Pharmacol.
715:270–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Polat B, Halici Z, Cadirci E, Albayrak A,
Karakus E, Bayir Y, Bilen H, Sahin A and Yuksel TN: The effect of
alpha-lipoic acid in ovariectomy and inflammation-mediated
osteoporosis on the skeletal status of rat bone. Eur J Pharmacol.
718:469–474. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rogalinska M: The role of mitochondria in
cancer induction, progression and changes in metabolism. Mini Rev
Med Chem. 16:524–530. 2016. View Article : Google Scholar : PubMed/NCBI
|