Introduction
Nasopharyngeal carcinoma (NPC) is a malignancy of
the head and neck that exhibits a unique geographical distribution
(1,2). NPC usually develops in the
nasopharyngeal mucosa; the majority of NPCs are squamous cell
carcinomas (3). NPC is more common
in males than in females (4);
however, the incidence of nasopharyngeal cancer has increased in
previous decades (5).
Chemotherapy, radiotherapy and surgery are three
main methods for treating NPC (6).
As NPC is more sensitive to chemotherapy, a combined therapy,
including induction, concurrent and adjuvant chemotherapy is
commonly used to treat late-stage NPC (7–9).
Clinical studies reported that combined chemotherapy demonstrated
beneficial short-term effects on NPC (10,11);
however, the incidences of acute recurrence and toxicity following
chemotherapy are high (12,13).
A novel anticancer approach, targeted therapy, has effectively
treated lung cancer, melanoma, leukemia and other tumors (14–17).
Therefore, it is important to investigate the mechanisms underlying
the pathogenesis of NPC to aid the identification of potential
novel targets in the treatment of NPC.
The gene encoding serpin peptidase inhibitor clade C
member 1 (SERPINC1), also known as antithrombin III (ATIII), is
located on chromosome 1q23-25.1 (18). ATIII regulates coagulation via the
inhibition of coagulation factors, and exhibits anti-inflammatory
effects on epithelial cells (19–21).
Studies have previously reported that the occurrence and
development of certain cancers was also closely associated with
SERPINC1; Zietek et al (22,23)
reported increased levels of ATIII in the serum of patients with
kidney cancer and the tumor tissues of patients with bladder
cancer. Other studies observed that SERPINC1-encoded proteins
exhibited inhibitory effects on angiogenesis and suppressed
proliferation (24,25). To the best of our knowledge, the
role of SERPINC1 in the occurrence and development of NPC has not
been investigated.
RNA interference is commonly used to study the role
of genes by targeting the mRNA, and it allows the selective
silencing of one or several genes (26). The present study investigated the
role of SERPINC1 in the proliferation of NPC cells via determining
the expression of the SERPINC1 gene in tissues from patients with
NPC and silencing the gene in NPC cells. The results may provide
novel insight and targets for the treatment of NPC.
Materials and methods
NPC samples
From September 2016 to September 2017, 41 NPC tumor
and adjacent healthy tissues were collected from 19 males and 22
females (aged 49–80 years, with a mean of 58.53±6.36 years) at the
Department of Pathology, Tongde Hospital of Zhejiang Province
(Hangzhou, China). The samples were stored at −80°C prior to
subsequent experimentation. Patients included in the present study
exhibited NPC that was histologically confirmed by biopsy, and no
history of previous head and neck cancer. Patients with incomplete
clinical data were excluded. In addition, NPC patients were staged
according to the criteria of the 8th edition of The Union for
International Cancer Control/American Joint Committee on Cancer
staging system (27). Reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) was
performed to determine the expression levels of SERPINC1 mRNA in
each tissue sample. The patients were divided into low- and
high-expression groups according to the median value of SERPINC1
mRNA expression. A total of 6 cases were randomly selected from the
41 patients the analysis of SERPINC1 protein via western blotting.
All patients provided signed informed consent, and the experiment
was approved by the Ethics Committee of Tongde Hospital of Zhejiang
Province.
Cell culture
The poorly differentiated squamous cell carcinoma
NPC cell line HNE3 was purchased from the American Type Culture
Collection (Manassas, VA, USA). Cells were cultured in RPMI-1640
medium containing 10% fetal bovine serum, 100 U/ml penicillin, 100
µg/ml streptomycin at 37°C in an incubator with 5% CO2. All culture
reagents were purchased from Gibco (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
Transfection of short interfering RNA
(siRNA)
In total, 2×105 cells/well were seeded in 24-well
culture plates, and 0.5 ml medium without antibiotic was added in
each well. Cells at 90% confluence were transfected. siRNA targeted
against SERPINC1 (SERPINC1-siRNA) was purchased from Shanghai
GenePharma Co., Ltd. (Shanghai, China). siRNA (50 nM) transfection
was performed using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) to generate the SERPINC1-siRNA
group. Negative-siRNA (Thermo Fisher Scientific, Inc.) was inserted
into an empty vector (pcDNA3.1; Thermo Fisher Scientific, Inc.),
which served as the empty vector control group; a non-transfected
control group was also established. The sequences of the siRNAs
used in the present study were as follows: SERPINC1-siRNA forward,
AUCACAUUGGAAUACAUGGCC and reverse, CCAUGUAUUCCAAUGUGAUAG;
negative-siRNA forward, CAUGUGGUCUGUCGCAUAAUA and reverse,
CGGUACACCAGACAGCGUAUU. Following transfection for 48 h, cells were
harvested, and the efficiency of transfection was determined via
RT-qPCR and western blot analysis.
Cell viability
A Cell Counting Kit-8 (CCK-8) assay was used to
determine cell viability. Transfected cells at a density of 3×103
cells/well were inoculated in a 96-well plate and incubated at 37°C
with 5% CO2 for 12, 24 and 48 h following transfection. CCK-8
reagent (10 ml; Dojindo Molecular Technologies, Inc., Kumamoto,
Japan) was then added to each well and cultured together at 37°C
with 5% CO2 for 4 h. The absorbance of each well at 450 nm was
measured using a microplate reader (ELx800; BioTek Instruments
Inc., Winooski, VT, USA), and cell viability was calculated
according to the standard curve.
Flow cytometry for cell apoptosis and
proliferation
Flow cytometry was used to evaluate cell apoptosis.
Cells (1×106) were washed with PBS at 4°C and resuspended to a
concentration of 4×105 cells/ml. Phycoerythrin-Annexin V Apoptosis
Detection kit (5 µl; BD Pharmingen; BD Biosciences, San Jose, CA,
USA) was added to cell culture (200 µl), and then 10 µl
7-aminoactinomycin D (20 µg/ml; BD Biosciences) was added. The
samples were incubated at room temperature in the dark for 10 min.
The BD FACSCanto flow cytometer (BD Biosciences) was used to
analyze apoptosis at 488 nm, analysis of data was performed using
the FSC Express software (version 3; De Novo Software, Glendale,
CA, USA). Living cells are presented in the lower left quadrant,
necrotic cells in the upper left quadrant, advanced apoptotic cells
in the upper right quadrant, and early apoptotic cells in the lower
right quadrant. The proliferation of HNE3 cells was also analyzed
via flow cytometry. Click-iT® Plus EdU Pacific Blue flow
cytometry kit (Thermo Fisher Scientific, Inc.) was applied to
detected the cell proliferation.
RT-qPCR
RT-qPCR was performed to investigate the expression
levels of SERPINC1, B-cell lymphoma-2 (Bcl-2)-associated X protein
(Bax), Bcl-2, survivin, cyclin D1 and p53 mRNA; GADPH was used as a
reference gene. RNA was extracted from NPC tissues and cells using
TRIzol® (Thermo Fisher Scientific, Inc.) at 0°C for 5
min, isolated with CHCl3 (Aladdin Shanghai Biochemical Technology
Co., Ltd., Shanghai, China) and then dissolved in diethyl
pyrocarbonate-treated water (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). RNA concentration was determined using a NanoDrop One
Microvolume UV–Vis Spectrophotometer (NanoDrop Technologies; Thermo
Fisher Scientific, Inc., Wilmington, DE, USA). RT was performed on
RNA samples using the PrimeScript first Strand cDNA synthesis kit
(Takara Bio, Inc., Otsu, Japan) to synthesize cDNA. The RT reaction
was performed at 37°C for 15 min, followed by reverse transcriptase
inactivation at 85°C for 15 sec. RT-qPCR reactions were performed
using an ABI 7500 real-time PCR system (Thermo Fisher Scientific,
Inc.), using the SYBR Prellix Ex Taq™ Real-Time PCR Kit (Takara
Bio, Inc.). qPCR was performed by activating the DNA polymerase at
95°C for 5 min, followed by 40 cycles of two-step PCR (at 95°C for
10 sec and at 60°C for 30 sec) and a final extension at 75°C for 10
min prior to holding at 4°C. DNase- and RNase-free water were used
as negative control templates. All primers used were purchased from
Genewiz, Inc. (Suzhou, China) and are presented in Table I. The 2-ΔΔCq method (28) was applied to analyze relative
expression levels of target genes normalized to GADPH. Each
experiment was performed in triplicate.
 | Table I.Sequences of the primers used for
reverse transcription-quantitative polymerase chain reaction. |
Table I.
Sequences of the primers used for
reverse transcription-quantitative polymerase chain reaction.
| Primer name | Sequence
(5′-3′) | Product size
(bp) |
|---|
| SERPINC1,
forward |
GCCTGAAGGTAGCAGCTTGT |
|
| SERPINC1,
reverse |
CCCACACTCCCTCACTCTTC | 313 |
| Bax, forward |
TCCACCAAGAAGCTGAGCGAG |
|
| Bax, reverse |
TTCTTTGAGTTCGGTGGGGTC | 345 |
| Bcl-2, forward |
CTGGTGGACAACATCGC |
|
| Bcl-2, reverse |
GGAGAAATCAAACAGAGGC | 317 |
| Survivin,
forward |
CCCTTTCTCAAGGACCACCGCATC |
|
| Survivin,
reverse |
GCCAAGTCTGGCTCGTTCTCAGT | 133 |
| Cyclin D1,
forward |
CTGGCCATGAACTACCTGGA |
|
| Cyclin D1,
reverse |
GTCACACTTGATCACTCTGG | 245 |
| p53, forward |
CTGAGGTCGGCTCCGACTATACCACTATCC |
|
| p53, reverse |
CTGATTCAGCTCTCGGAACATCTCGAAGCG | 360 |
| GAPDH, forward |
CCATCTTCCAGGAGCGAGAT |
|
| GAPDH, reverse |
TGCTGATGATCTTGAGGCTG | 222 |
Western blotting
The expression of SERPINC1, apoptosis-, cell
cycle-proteins, and phosphatidylinositol 3-kinase/protein kinase
B/mammalian target of rapamycin (PI3K/Akt/mTOR) signaling
pathway-associated proteins were determined via western blot
analysis; phosphorylation levels were also determined. Tissues and
cells were lysed using liquid nitrogen and radioimmunoprecipitation
assay buffer (Abmole Bioscience, Inc., Houston, TX, USA), and
subsequently subjected to cleavage and lysis using 1% phenylmethane
sulfonyl fluoride protease and phosphatase inhibitors (Abmole
Bioscience, Inc.), for 30 min at 4°C. The supernatant was collected
by centrifugation at 12,000 × g at 4°C for 15 min. Total protein
concentration was determined using the bicinchoninic acid method.
In total, 10 µg protein was loaded in each lane. Proteins were
separated by 10% SDS-PAGE. The separated proteins were transferred
onto a polyvinylidene difluoride membrane (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) using a Trans-Blot Transfer Slot (Bio-Rad
Laboratories, Inc.) and blocked with 5% skimmed milk for 2 h at
room temperature. The membranes were incubated with the following
primary antibodies obtained from Abcam (Cambridge, UK):
Anti-SERPINC1 (1:800; ab126598); anti-Bax (1:700; ab32503);
anti-Bcl-2 (1:900; ab32124); anti-survivin (1:700; ab76424);
anti-cyclin D1 (1:800; ab134175); anti-p53 (1:600; ab26); anti-PI3K
(1:700; ab125633); anti-phosphorylated (p)-PI3K (1:600; ab138364);
anti-Akt (1:800; ab8805); anti-p-Akt (1:800; ab38449); anti-mTOR
(1:800; ab2732); anti-p-mTOR (1:600; ab109268) and anti-GADPH
(1:800; ab8245). Following the application of primary antibodies,
membranes were agitated at room temperature for 2 h, and then
incubated at 4°C for 12 h. Subsequently, membranes were incubated
at room temperature for 1.5 h with the following secondary
antibodies: Fluorescein isothiocyanate-conjugated goat anti-mouse
IgG (1:8,000; ab6785; Abcam); horseradish peroxidase
(HRP)-conjugated mouse anti-rabbit IgG (1:9,000; ab99697; Abcam);
mouse anti-rabbit IgG (1:7,000; BA1034; Invitrogen; Thermo Fisher
Scientific, Inc.); NL557-conjugated donkey anti-rabbit IgG
(1:5,000; NL004; R&D Systems, Inc., Minneapolis, MN, USA);
HRP-conjugated rabbit anti-human IgG (1:10,000, ab6759; Abcam).
Protein bands were visualized using an enhanced chemiluminescence
reagent (Thermo Fisher Scientific, Inc.). The optical density was
quantified using ImageJ software (version 1.46; National Institutes
of Health, Bethesda, MD, USA).
Statistical analysis
Experimental data were presented as the mean ±
standard deviation. Data were analyzed using SPSS version 20.0 (IBM
Corporation, Armonk, NY, USA). All experiments were performed three
times. Differences between two groups were analyzed using paired
Student's t-tests. One-way analyses of variance were performed to
analyze differences between experimental groups, with Tukey's
multiple comparison test used as a post hoc test. Associations
between clinicopathological data and SERPINC1 expression were
analyzed using χ2 tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
SERPINC1 is upregulated in NPC
tissue
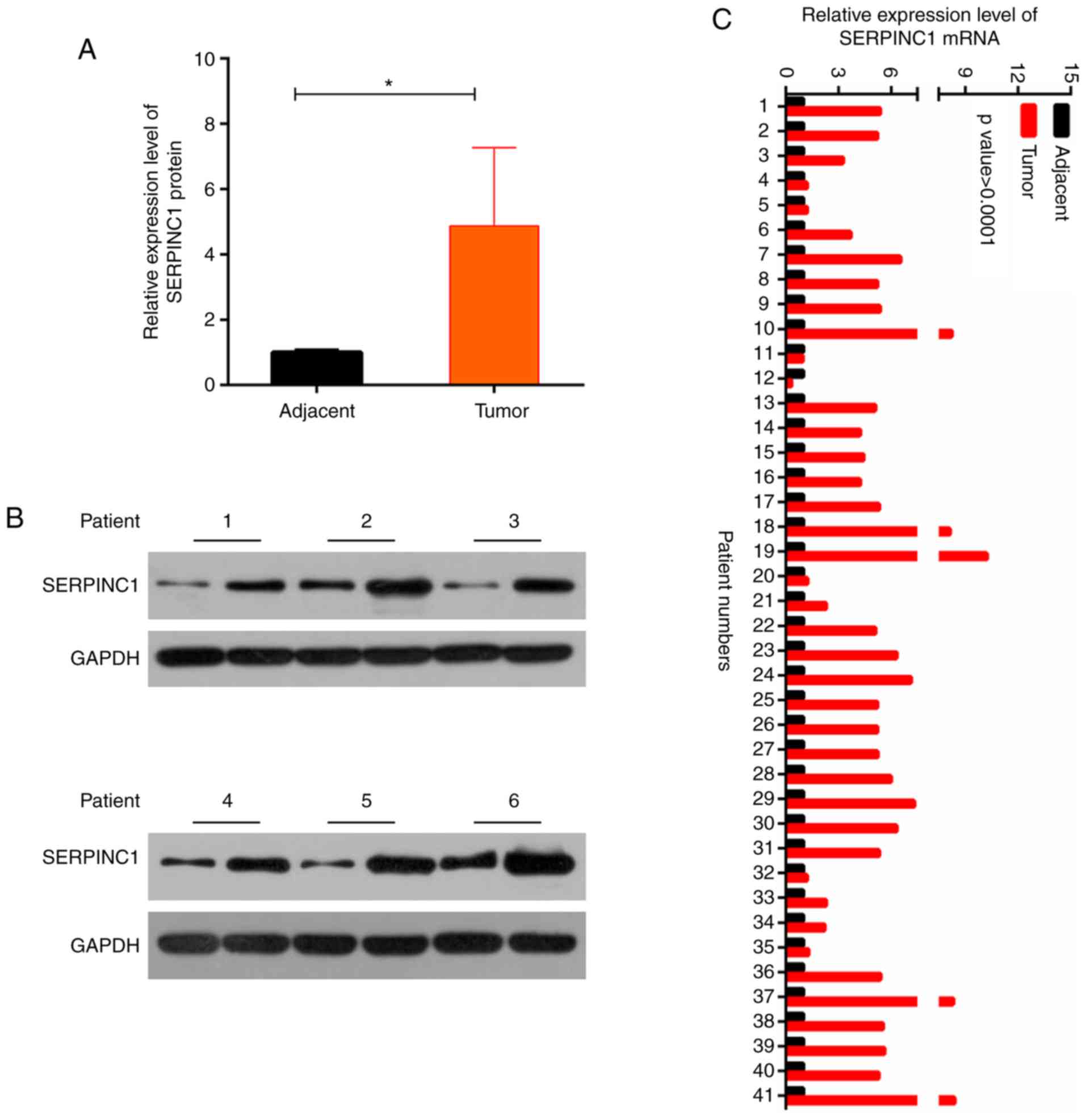
RT-qPCR and western blotting were performed to
analyze the levels of SERPINC1 expression in tumor and adjacent
healthy tissues from patients with NPC. It was revealed that
SERPINC1 protein and mRNA expression levels were significantly
increased in NPC tissues compared with in adjacent tissues
(P<0.05; Fig. 1A-C). As
presented in Table II, patients
were separated into low- and high-expression groups according to
the median value of SERPINC1 mRNA expression, and associations with
clinicopathological features were investigated. It was demonstrated
that increased SERPINC1 expression was significantly associated
with the metastasis of NPC. The results suggested that the SERPINC1
gene was overexpressed in NPC tissues.
 | Table II.Associations between
clinicopathological data of patients with nasopharyngeal carcinoma
and SERPINC1 expression. |
Table II.
Associations between
clinicopathological data of patients with nasopharyngeal carcinoma
and SERPINC1 expression.
|
|
| SERPINC1
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
factor | N | Low | High | P-value |
|---|
| Gender |
|
|
| 0.756 |
|
Female | 23 | 11 | 12 |
|
|
Male | 18 | 10 | 8 |
|
| Age |
|
|
| 0.756 |
|
<50 | 18 | 10 | 8 |
|
|
>50 | 23 | 11 | 12 |
|
| TNM stage |
|
|
| 0.536 |
|
I–II | 19 | 11 | 8 |
|
|
III–IV | 22 | 10 | 12 |
|
| Metastasis |
|
|
| 0.015a |
|
Yes | 11 | 2 | 9 |
|
| No | 30 | 19 | 11 |
|
Transfection with SERPINC1-siRNA
reduces SERPINC1 expression and the proliferation of HNE3
cells
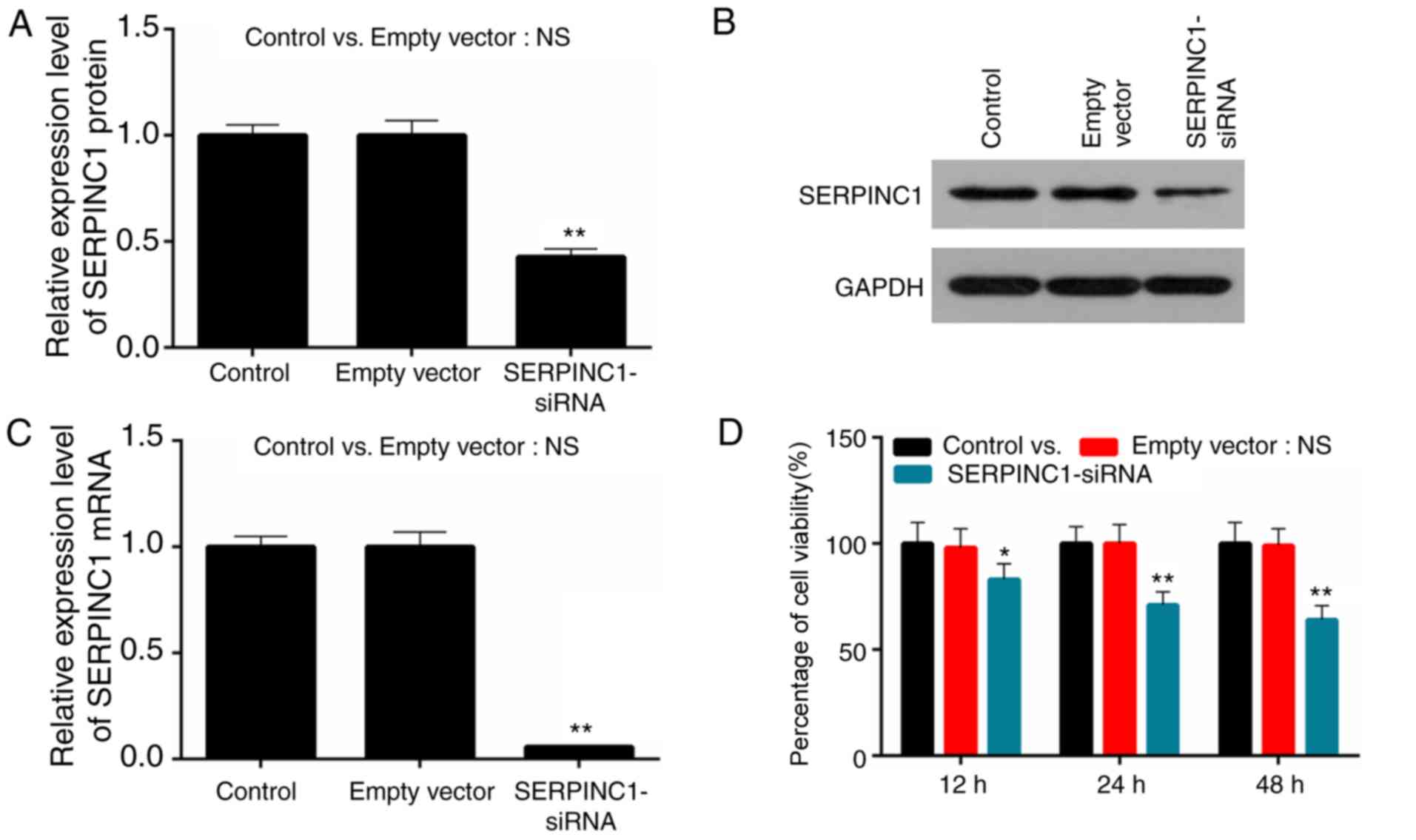
The human poorly-differentiated squamous cell
carcinoma NPC cell line HNE3 was used to study the role of SERPINC1
in NPC; si-SERPINC1 was transfected into HNE3 cells. The efficiency
of SERPINC1-siRNA transfection was determined via RT-qPCR and
western blot analysis. It was demonstrated that the expression
levels of SERPINC1 mRNA and protein were significantly decreased in
HNE3 cells following SERPINC1 knockdown compared with the negative
siRNA-transfected (empty vector) control group (P<0.01; Fig. 2A-C). The proliferation of HNE3
cells was determined via a CCK-8 assay; cell proliferation was
significantly reduced at 12, 24 and 48 h following transfection
with SERPINC1-siRNA compared with the empty vector control
(P<0.05, P<0.05 and P<0.01; Fig. 2D). The results indicated that
transfection of the SERPINC1-siRNA were successfully conducted, and
that downregulation of SERPINC1 expression induced a decrease in
the proliferation of NPC cells.
SERPINC1 gene silencing inhibits cell
proliferation and promotes apoptosis
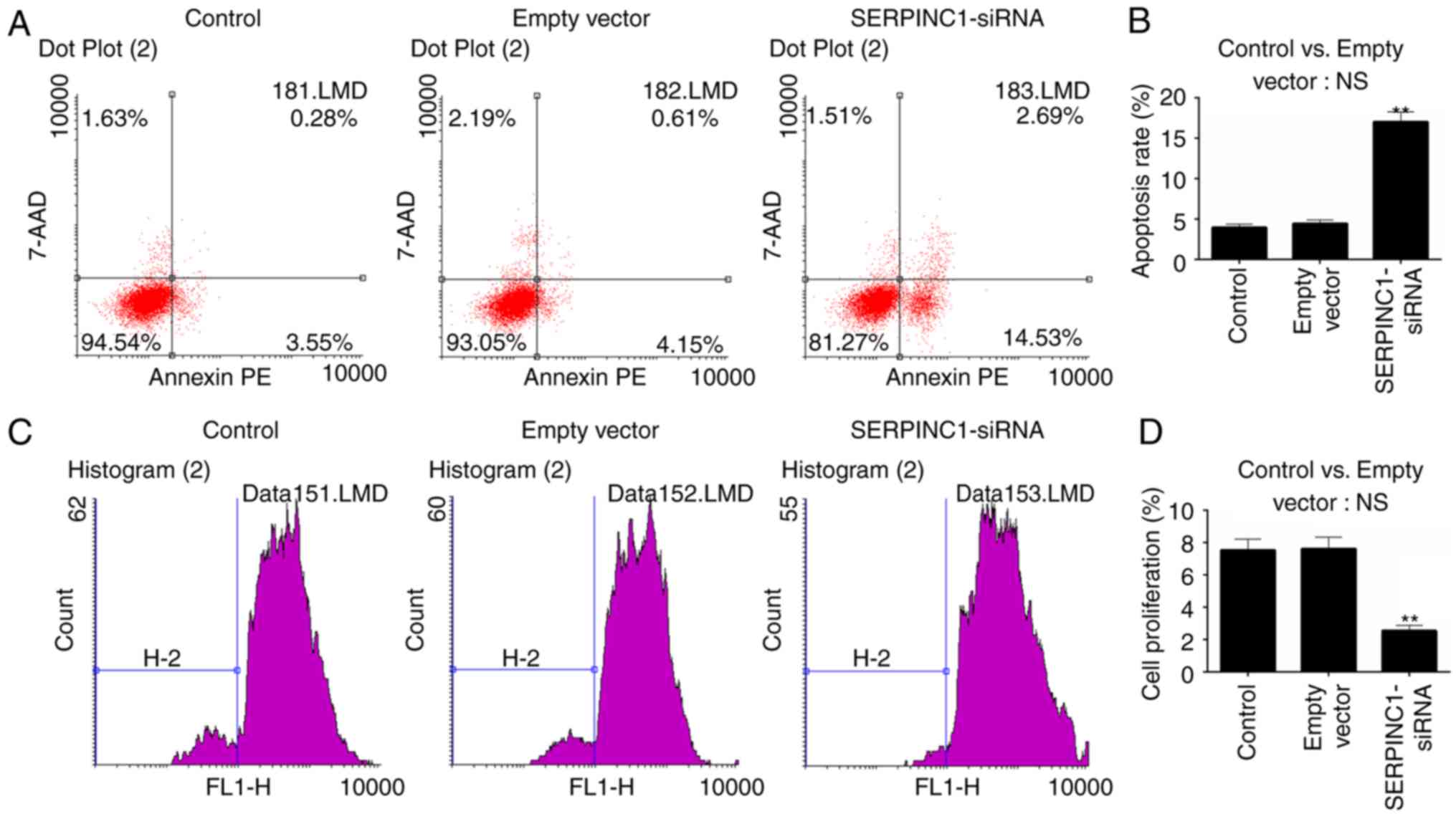
Flow cytometry was performed to investigate the
apoptosis and proliferation of HNE3 cells following SERPINC1-siRNA
transfection. No significant differences in apoptosis or
proliferation were reported between the empty vector and
non-transfected control groups. Conversely, the apoptotic rate of
the SERPINC1-siRNA group was significantly increased compared with
the empty vector group (P<0.01; Fig. 3A and B), whereas proliferation was
reduced following SERPINC1 knockdown compared with the empty vector
group (P<0.01; Fig. 3C and D).
The results indicated that SERPINC1 gene silencing inhibited the
proliferation and promoted the apoptosis of NPC cells.
Downregulation of SERPINC1 gene
affects cell cycle- and apoptosis-associated proteins
To further investigate the effects of SERPINC1
knockdown on the proliferation and apoptosis of HNE3 cells, RT-qPCR
and western blot analyses were conducted to determine the
expression of apoptosis-associated (Bax, Bcl-2 and survivin) and
cell cycle-associated (cyclin D1 and p53) genes and proteins,
respectively. It was revealed that the expression levels of Bax
mRNA were significantly upregulated in SERPINC1-siRNA-transfected
cells compared with the empty vector group, whereas those of Bcl-2
and survivin mRNA were downregulated (P<0.01; Fig. 4A-C). Additionally, the expression
levels of cyclin D1 mRNA were significantly decreased and those of
p53 increased following silencing of the SERPINC1 gene compared
with the empty vector group (P<0.01; Fig. 4D-G). Similar alterations in the
expression levels of the genes were also reported at the protein
level (P<0.01; Fig. 4F and G),
indicating that the suppression of SERPINC1 expression promoted the
expression of proapoptotic factors, inhibited the expression of
antiapoptotic factors and suppressed proliferation via regulation
of cell cycle-associated genes in NPC cells.
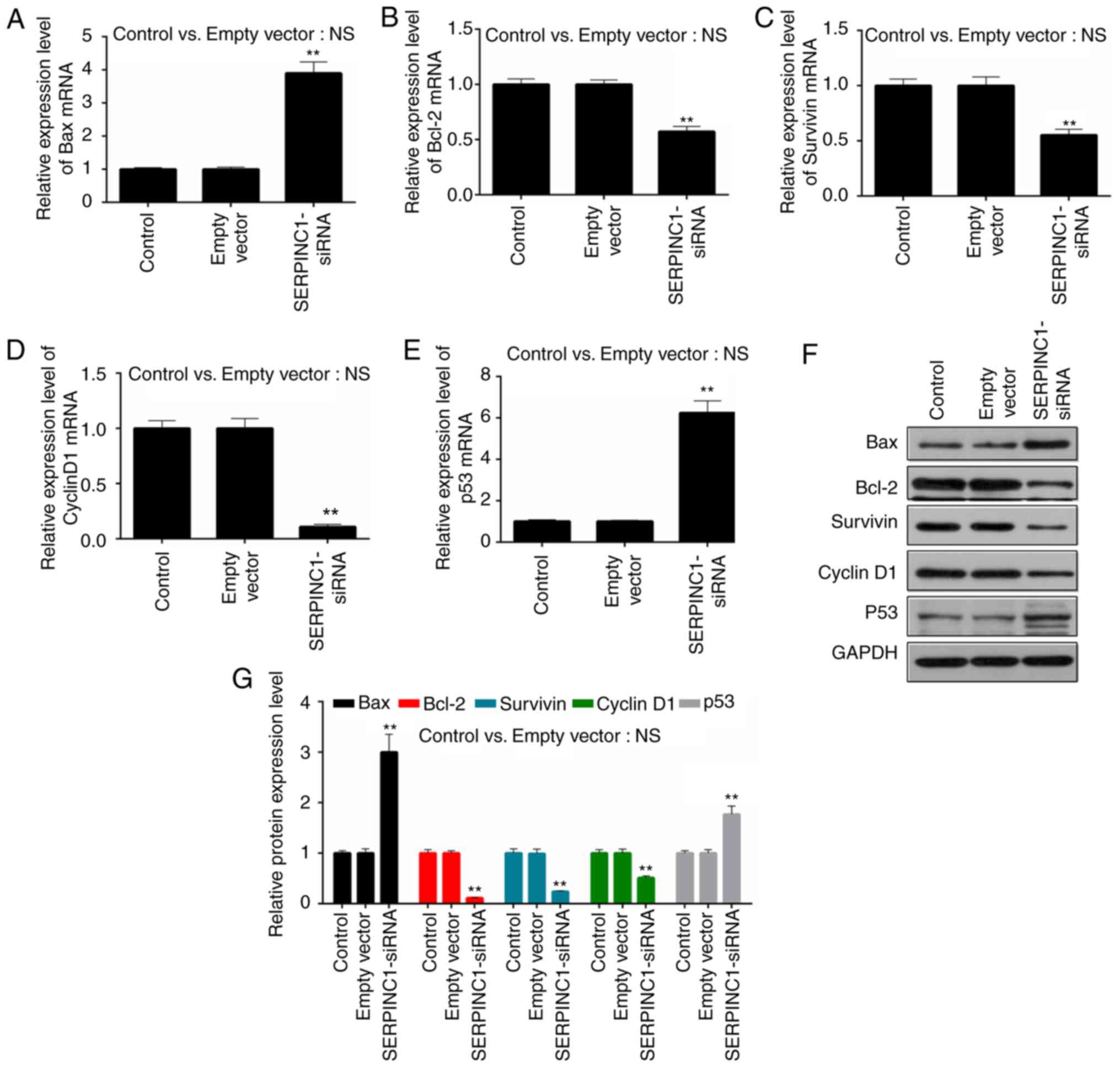 | Figure 4.Effects of SERPINC1-siRNA
transfection on the expression of apoptosis and cell
cycle-associated genes in HNE3 cells. Reverse
transcription-quantitative polymerase chain reaction was performed
to determine the expression levels of (A) Bax, (B) Bcl-2, (C)
survivin, (D) cyclin D1 and (E) p53 mRNA in non-transfected, empty
vector-transfected and SERPINC1-siRNA-transfected cells. (F and G)
Expression levels of Bax, Bcl-2, survivin, cyclin D1 and p53
proteins were determined via western blot analysis. Data are
presented as the mean ± standard deviation. **P<0.01 vs. the
empty vector group. Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated
X protein; empty vector, negative control siRNA; NS, not
significant; SERPINC1, serpin peptidase inhibitor clade C member 1;
siRNA, small interfering RNA. |
Knockdown of SERPINC1 inhibits the
phosphorylation of proteins involved in the PI3K/Akt/mTOR signaling
pathway
The mechanisms underlying the effects of SERPINC1 on
the proliferation and apoptosis of NPC cells were investigated by
evaluating the expression and phosphorylation levels of
PI3K/Akt/mTOR-associated proteins. It was revealed that the
phosphorylation levels of PI3K, Akt and mTOR were significantly
decreased in HNE3 cells following transfection with SERPINC1-siRNA
compared with the empty vector control group (P<0.01; Fig. 5A-D), suggesting that SERPINC1
knockdown inhibited the activity of the PI3K/Akt/mTOR pathway.
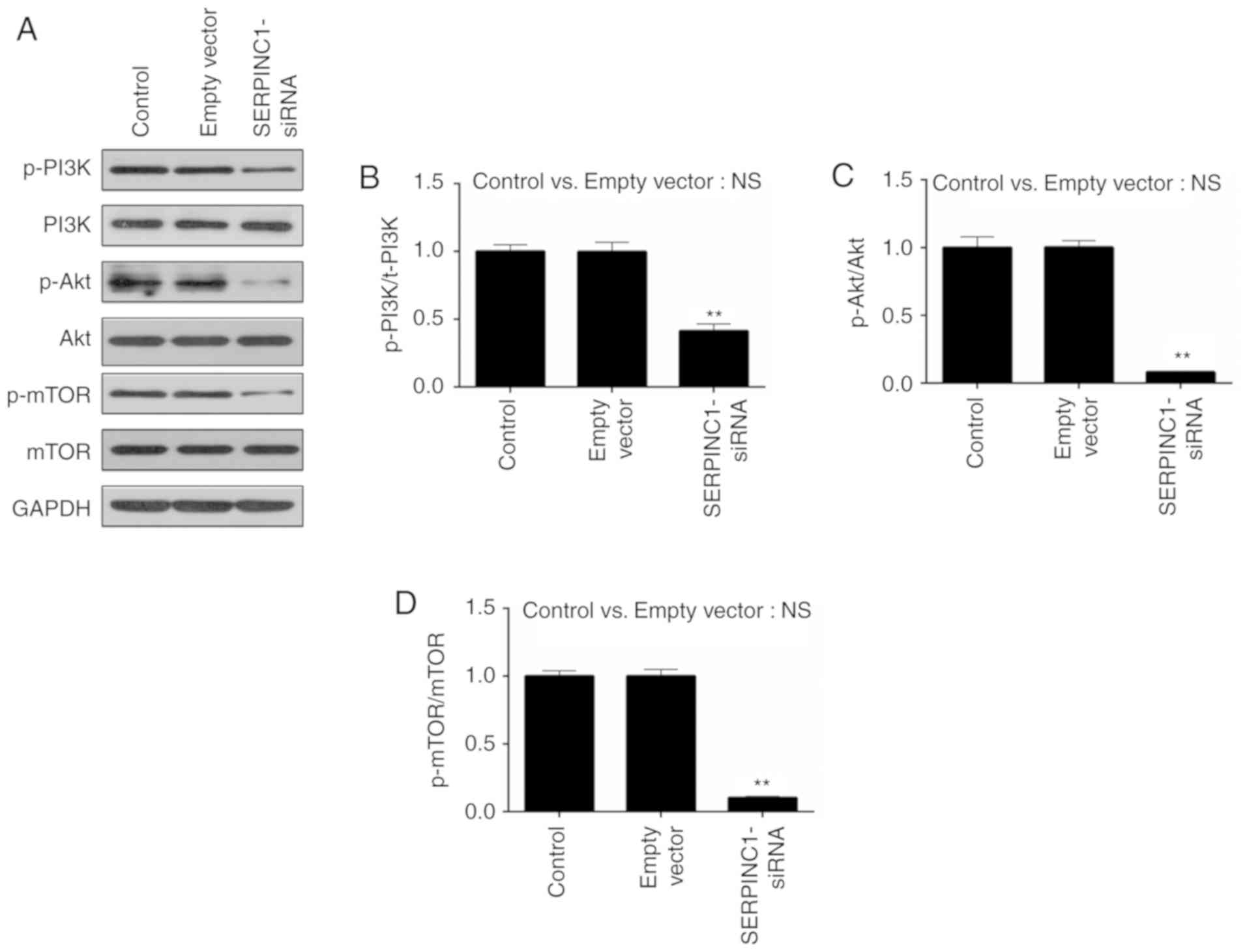 | Figure 5.Effects of SERPINC1-siRNA
transfection on PI3K/Akt/mTOR pathway. (A) Western blot analysis
was performed to determine the expression and phosphorylation of
PI3K, Akt and mTOR proteins. (B-D) p-PI3K/PI3K, p-Akt/Akt and
p-mTOR/mTOR ratios were calculated for non-transfected, empty
vector-transfected and SERPINC1-siRNA-transfected cells. Data are
presented as the mean ± standard deviation. **P<0.01 vs. the
empty vector group. Akt, protein kinase B; empty vector, negative
control siRNA; mTOR, mammalian target of rapamycin; NS, not
significant; p, phosphorylated; PI3K, phosphatidylinositol
3-kinase; SERPINC1, serpin peptidase inhibitor clade C member 1;
siRNA, small interfering RNA. |
Discussion
NPC is a malignant tumor prevalent in Southeast Asia
that occurs at the top of the nasopharyngeal cavity, and seriously
affects the survival and quality of life of patients (29). In addition, >50% of patients
with NPC are diagnosed at an advanced stage as the initial clinical
symptoms of NPC and the site of lesions are difficult to identify
(30). NPC is sensitive to
chemotherapy and radiotherapy, but surgery is an effect method for
treating NPC (31); however,
treatment outcomes are usually poor due to late diagnosis. It was
reported that the recurrence rate of NPC was 10–15% and the 5-year
survival rate was ~60% (30,31).
NPC exhibits high sensitivity to radiotherapy; however, patients
commonly experience strong adverse reactions and side effects
following treatment. Radiation brain injury was reported in 28.5%
of patients treated with radiotherapy, whereas 38.1% of patients
exhibited severe hearing loss, 40.6% patients possessed nasal
pharyngeal mucosal injuries and >50% patients succumbed to
mortality following severe radiation damage (32–34).
Therefore, identifying a safer and more effective treatment of NPC
is of great importance.
The SERPINC1 gene encodes ATIII, a serine protease
inhibitor involved in coagulation cascades (18). The main roles of ATIII have been
associated with the regulation of coagulation and hemostasis, and
the induction of anti-inflammatory processes (35). A previous study reported that the
activity of ATIII in the serum of patients with renal and bladder
cancer was significantly increased compared with in healthy
individuals (23). ATIII promotes
the inhibition of proteases via interactions with heparin-like
substances on the surface of endothelial cells (36). As NPC is a tumor that derives from
a malignant lesion of epithelial cells (37), the expression of SERPINC1 in NPC
was investigated. The results of the present study revealed that
SERPINC1 was significantly upregulated in NPC tissues and the
metastasis of NPC was highly associated with elevated expression of
SERPINC1.
The present study proposed that knockdown of
SERPINC1 may protect against the progression of NPC. The data
revealed that the proliferation of the NPC cell line HNE3 was
suppressed by SERPINC1-siRNA. To further investigate the effects of
SERPINC1 on NPC, flow cytometry analysis was performed on cells
following transfection with SERPINC1-siRNA. The results
demonstrated that silencing SERPINC1 promoted the apoptosis and
inhibited the proliferation of HNE3 cells. Therefore, the
expression of apoptosis- and cell cycle-associated genes following
SERPINC1-siRNA transfection was investigated. Cyclin D1 binds to
and activates cyclin-dependent kinase 4 during G1 to regulate G1/S
cell cycle transition, thereby promoting cell proliferation
(38). Conversely, the tumor
suppressor gene p53 inhibits cell proliferation (39). Bax directly regulates the activity
of proapoptotic target proteins to induce apoptosis (40), whereas Bcl-2 exhibits antiapoptotic
effects; overexpression of Bcl-2 is common in NPC (41). Survivin is expressed only in tumors
and embryonic tissues, and is associated with cellular immortality
(42). It was observed that the
downregulation of SERPINC1 increased the expression of Bax and p53,
but decreased that of Bcl-2, survivin and cyclin D1. Collectively,
SERPINC1 silencing promoted the apoptosis and inhibited the
proliferation of NPC cells by regulating the expression of
apoptosis-associated proteins and cyclin D1, suggesting that
SERPINC1 may contribute to the pathogenesis of NPC.
The PI3K/Akt/mTOR signaling pathway contributes to
the pathogenesis of numerous tumors (43,44).
Similar to SERPINC1, the PI3K/Akt pathway serves roles in
coagulation and anti-inflammatory processes (45,46).
Therefore, the activity of the PI3K/Akt/mTOR signaling pathway was
investigated following silencing of SERPINC1 in the present study.
The results revealed that the expression levels of PI3K, Akt and
mTOR proteins were markedly unaltered by transfection with
SERPINC1-siRNA; however, the levels of phosphorylation of each
protein were significantly downregulated. It was recently reported
that the PI3K-dependent activation of Akt induced the
phosphorylation of the Ser136/Ser112 residues of Bcl-2-associated
death promoter (Bad), promoting apoptosis via the separation of
Bad/Bcl-2 heterodimers (47).
Furthermore, activation of the PI3K-Akt pathway promotes the
phosphorylation of the Ser184 residue of Bax, inhibiting the
antiapoptotic effects of the protein (48). Additionally, PI3K/Akt/mTOR promotes
cell proliferation via regulation of cell cycle-associated proteins
(49–52). Collectively, it was proposed that
downregulation of SERPINC1 in NPC cells may suppress proliferation
and induce apoptosis by inhibiting the activation of the
PI3K/Akt/mTOR signaling pathway; however, the association between
this signaling pathway, and the proliferation and apoptosis of
cells require further investigation. Furthermore, due to the
crosstalk that occurs between signaling pathways, the possibility
that other pathways may also be involved in the effects of SERPINC1
downregulation cannot be excluded. Additionally, the findings of
the present study were obtained from a single cell line in
vitro; thus, performing similar experiments in additional NPC
cell lines or animals models may provide insight into the roles of
SERPINC1 in the pathogenesis of NPC. Finally, the use of specific
inhibitors of the PI3K/Akt/mTOR signaling pathway is required to
further demonstrate the role of this pathway in the effects of
SERPINC1 on NPC cells.
In conclusion, SERPINC1 was upregulated in tumor
tissues from patients with NPC. Knockdown of SERPINC1 suppressed
the proliferation and promoted the apoptosis of HNE3 cells by
regulating the expression levels of cell cycle- and
apoptosis-associated proteins. The activation of the PI3K/Akt/mTOR
signaling pathway was suppressed by silencing of the SERPINC1 gene.
Collectively, these findings suggested that SERPINC1 may be a
potential target for the treatment of patients with NPC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JX and YYi conceived and designed the present study.
GX, LL, QW, YYa acquired, analyzed and interpreted data. YYi, JX,
YYa drafted the article or critically revised it for important
intellectual content. All authors read and approved the final
manuscript. All authors agreed to be accountable for all aspects of
the work in ensuring that questions related to the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
All patients provided signed informed consent. The
present study was approved by The Ethics Committee of Tongde
Hospital of Zhejiang Province (Hangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chua MLK, Wee JTS, Hui EP and Chan ATC:
Nasopharyngeal carcinoma. Lancet. 387:1012–1024. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hsu MM and Tu SM: Nasopharyngeal carcinoma
in Taiwan. Clinical manifestations and results of therapy. Cancer.
52:362–368. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qi X, Li J, Zhou C, Lv C and Tian M:
MicroRNA-320a inhibits cell proliferation, migration and invasion
by targeting BMI-1 in nasopharyngeal carcinoma. FEBS Lett.
588:3732–3738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qin DX, Hu YH, Yan JH, Xu GZ, Cai WM, Wu
XL, Cao DX and Gu XZ: Analysis of 1379 patients with nasopharyngeal
carcinoma treated by radiation. Cancer. 61:1117–1124. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jenkin RD, Anderson JR, Jereb B, Thompson
JC, Pyesmany A, Wara WM and Hammond D: Nasopharyngeal carcinoma-a
retrospective review of patients less than thirty years of age: A
report of Children's cancer study group. Cancer. 47:360–366. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Geara FB, Glisson BS, Sanguineti G, Tucker
SL, Garden AS, Ang KK, Lippman SM, Clayman GL, Goepfert H, Peters
LJ and Hong WK: Induction chemotherapy followed by radiotherapy
versus radiotherapy alone in patients with advanced nasopharyngeal
carcinoma: Results of a matched cohort study. Cancer. 79:1279–1286.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
OuYang PY, Xie C, Mao YP, Zhang Y, Liang
XX, Su Z, Liu Q and Xie FY: Significant efficacies of neoadjuvant
and adjuvant chemotherapy for nasopharyngeal carcinoma by
meta-analysis of published literature-based randomized, controlled
trials. Ann Oncol. 24:2136–2146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen L, Hu CS, Chen XZ, Hu GQ, Cheng ZB,
Sun Y, Li WX, Chen YY, Xie FY, Liang SB, et al: Adjuvant
chemotherapy in patients with locoregionally advanced
nasopharyngeal carcinoma: Long-term results of a phase 3
multicentre randomised controlled trial. Eur J Cancer. 75:150–158.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen YP, Tang LL, Yang Q, Poh SS, Hui EP,
Chan ATC, Ong WS, Tan T, Wee J, Li WF, et al: Induction
chemotherapy plus concurrent chemoradiotherapy in endemic
nasopharyngeal carcinoma: Individual patient data pooled analysis
of four randomized trials. Clin Cancer Res. 24:1824–1833. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chua DT, Sham JS, Kwong DL, Choy DT, Au GK
and Wu PM: Prognostic value of paranasopharyngeal extension of
nasopharyngeal carcinoma. A significant factor in local control and
distant metastasis. Cancer. 78:202–210. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan M, Kumachev A and Chan KKW: Is there
any benefit to adding adjuvant chemotherapy after concurrent
chemoradiotherapy for nasopharyngeal carcinoma? Eur J Cancer.
56:186–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nieder C: Influence of dose and
fractionation in intensity modulated re-irradiation of patients
with relapse of nasopharyngeal carcinoma: A randomized phase II
study. Strahlenther Onkol. 191:203–204. 2015.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Setton J, Han J, Kannarunimit D, Wuu YR,
Rosenberg SA, DeSelm C, Wolden SL, Jillian Tsai C, McBride SM, Riaz
N and Lee NY: Long-term patterns of relapse and survival following
definitive intensity-modulated radiotherapy for non-endemic
nasopharyngeal carcinoma. Oral Oncol. 53:67–73. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Solomon B, Wilner KD and Shaw AT: Current
status of targeted therapy for anaplastic lymphoma
kinase-rearranged non-small cell lung cancer. Clin Pharmacol Ther.
95:15–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sullivan RJ and Flaherty KT: Resistance to
BRAF-targeted therapy in melanoma. Eur J Cancer. 49:1297–1304.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Knoechel B, Roderick JE, Williamson KE,
Zhu J, Lohr JG, Cotton MJ, Gillespie SM, Fernandez D, Ku M, Wang H,
et al: An epigenetic mechanism of resistance to targeted therapy in
T cell acute lymphoblastic leukemia. Nat Genet. 46:364–370. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Riquelme I, Saavedra K, Espinoza JA, Weber
H, Garcia P, Nervi B, Garrido M, Corvalán AH, Roa JC and Bizama C:
Molecular classification of gastric cancer: Towards a
pathway-driven targeted therapy. Oncotarget. 6:24750–24779. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Caspers M, Pavlova A, Driesen J, Harbrecht
U, Klamroth R, Kadar J, Fischer R, Kemkes-Matthes B and Oldenburg
J: Deficiencies of antithrombin, protein C and protein S-practical
experience in genetic analysis of a large patient cohort. Thromb
Haemost. 108:247–257. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choay J, Petitou M, Lormeau JC, Sinay P,
Casu B and Gatti G: Structure-activity relationship in heparin: A
synthetic pentasaccharide with high affinity for antithrombin III
and eliciting high anti-factor Xa activity. Biochem Biophys Res
Commun. 116:492–499. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fukui H, Taniguchi A, Sakamoto S, Kawahara
S, Matsunaga T, Taira K, Tanaka S and Kamitsuji H: Antithrombin III
in children with various renal diseases. Pediatr Nephrol.
3:144–148. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Levy JH, Sniecinski RM, Welsby IJ and Levi
M: Antithrombin: Anti-inflammatory properties and clinical
applications. Thromb Haemost. 115:712–728. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zietek Z, Iwan-Zietek I, Kotschy M,
Wiśniewska E and Tyloch F: Antithrombin III activity in blood of
patients with renal cancer. Pol Merkur Lekarski. 2:191–192.
1997.(In Polish). PubMed/NCBI
|
|
23
|
Zietek Z, Iwan-Zietek I, Kotschy M,
Wiśniewska E and Tyloch F: Activity of antithrombin III in the
blood of patients with bladder cancer. Pol Merkur Lekarski.
2:268–269. 1997.(In Polish). PubMed/NCBI
|
|
24
|
Pal N, Kertai MD, Lakshminarasimhachar A
and Avidan MS: Pharmacology and clinical applications of human
recombinant antithrombin. Expert Opin Biol Ther. 10:1155–1168.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maeda A, Ohta K, Ohta K, Nakayama Y,
Hashida Y, Toma T, Saito T, Maruhashi K and Yachie A: Effects of
antithrombin III treatment in vascular injury model of mice.
Pediatr Int. 53:747–753. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gonzalez-Rodriguez A and Valverde AM: RNA
interference as a therapeutic strategy for the treatment of liver
diseases. Curr Pharm Des. 21:4574–4586. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: American joint committee on cancer staging
manual. 8th. Springer; New York, NY: 2017
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bei JX, Li Y, Jia WH, Feng BJ, Zhou G,
Chen LZ, Feng QS, Low HQ, Zhang H, He F, et al: A genome-wide
association study of nasopharyngeal carcinoma identifies three new
susceptibility loci. Nat Genet. 42:599–603. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Su SF, Han F, Zhao C, Huang Y, Chen CY,
Xiao WW, Li JX and Lu TX: Treatment outcomes for different
subgroups of nasopharyngeal carcinoma patients treated with
intensity-modulated radiation therapy. Chin J Cancer. 30:565–573.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin S, Lu JJ, Han L, Chen Q and Pan J:
Sequential chemotherapy and intensity-modulated radiation therapy
in the management of locoregionally advanced nasopharyngeal
carcinoma: Experience of 370 consecutive cases. BMC Cancer.
10:392010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vargas C, Swartz D, Vashi A, Blasser M,
Kasareian A, Cesaretti J, Kiley K and Terk M: Long-term outcomes
and prognostic factors in patients treated with intraoperatively
planned prostate brachytherapy. Brachytherapy. 12:120–125. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tao CJ, Lin L, Zhou GQ, Tang LL, Chen L,
Mao YP, Zeng MS, Kang TB, Jia WH, Shao JY, et al: Comparison of
long-term survival and toxicity of cisplatin delivered weekly
versus every three weeks concurrently with intensity-modulated
radiotherapy in nasopharyngeal carcinoma. PLoS One. 9:e1107652014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feng HX, Guo SP, Li GR, Zhong WH, Chen L,
Huang LR and Qin HY: Toxicity of concurrent chemoradiotherapy with
cetuximab for locoregionally advanced nasopharyngeal carcinoma. Med
Oncol. 31:1702014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mekaj Y, Lulaj S, Daci F, Rafuna N,
Miftari E, Hoxha H, Sllamniku X and Mekaj A: Prevalence and role of
antithrombin III, protein C and protein S deficiencies and
activated protein C resistance in Kosovo women with recurrent
pregnancy loss during the first trimester of pregnancy. J Hum
Reprod Sci. 8:224–229. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Absher E, Labarrere CA, Carter C, Haag B
and Faulk WP: The endothelial heparan sulfate-antithrombin III
natural anticoagulant pathway in normal and transplanted human
kidneys. Transplantation. 53:828–834. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang PY, Zeng TT, Li MQ, Ban X, Zhu YH,
Zhang BZ, Mai HQ, Zhang L, Guan XY and Li Y: Proteomic analysis of
a nasopharyngeal carcinoma cell line and a nasopharyngeal
epithelial cell line. Tumori. 101:676–683. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Harper JW, Adami GR, Wei N, Keyomarsi K
and Elledge SJ: The p21 Cdk-interacting protein Cip1 is a potent
inhibitor of G1 cyclin-dependent kinases. Cell. 75:805–816. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yin Y, Tainsky MA, Bischoff FZ, Strong LC
and Wahl GM: Wild-type p53 restores cell cycle control and inhibits
gene amplification in cells with mutant p53 alleles. Cell.
70:937–948. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nomura M, Shimizu S, Sugiyama T, Narita M,
Ito T, Matsuda H and Tsujimoto Y: 14-3-3 Interacts directly with
and negatively regulates pro-apoptotic Bax. J Biol Chem.
278:2058–2065. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pan LL, Wang AY, Huang YQ, Luo Y and Ling
M: Mangiferin induces apoptosis by regulating Bcl-2 and Bax
expression in the CNE2 nasopharyngeal carcinoma cell line. Asian
Pac J Cancer Prev. 15:7065–7068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Satoh K, Kaneko K, Hirota M, Masamune A,
Satoh A and Shimosegawa T: Expression of survivin is correlated
with cancer cell apoptosis and is involved in the development of
human pancreatic duct cell tumors. Cancer. 92:271–278. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xu G, Zhang W, Bertram P, Zheng XF and
McLeod H: Pharmacogenomic profiling of the PI3K/PTEN-AKT-mTOR
pathway in common human tumors. Int J Oncol. 24:893–900.
2004.PubMed/NCBI
|
|
44
|
Ciruelos Gil EM: Targeting the
PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer.
Cancer Treat Rev. 40:862–871. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schabbauer G, Tencati M, Pedersen B,
Pawlinski R and Mackman N: PI3K-Akt pathway suppresses coagulation
and inflammation in endotoxemic mice. Arterioscler Thromb Vasc
Biol. 24:1963–1969. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xu YQ, Long L, Yan JQ, Wei L, Pan MQ, Gao
HM, Zhou P, Liu M, Zhu CS, Tang BS and Wang Q: Simvastatin induces
neuroprotection in 6-OHDA-lesioned PC12 via the PI3K/AKT/caspase 3
pathway and anti-inflammatory responses. CNS Neurosci Ther.
19:170–177. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bao RK, Zheng SF and Wang XY: Selenium
protects against cadmium-induced kidney apoptosis in chickens by
activating the PI3K/AKT/Bcl-2 signaling pathway. Environ Sci Pollut
Res Int. 24:20342–20353. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sui Y, Zheng X and Zhao D: Rab31 promoted
hepatocellular carcinoma (HCC) progression via inhibition of cell
apoptosis induced by PI3K/AKT/Bcl-2/BAX pathway. Tumour Biol.
36:8661–8670. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu W, Ren H, Ren J, Yin T, Hu B, Xie S,
Dai Y, Wu W, Xiao Z, Yang X and Xie D: The role of
EGFR/PI3K/Akt/cyclinD1 signaling pathway in acquired middle ear
cholesteatoma. Mediators Inflamm. 2013:6512072013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li Y, Qu X, Qu J, Zhang Y, Liu J, Teng Y,
Hu X, Hou K and Liu Y: Arsenic trioxide induces apoptosis and G2/M
phase arrest by inducing Cbl to inhibit PI3K/Akt signaling and
thereby regulate p53 activation. Cancer Lett. 284:208–215. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dey JH, Bianchi F, Voshol J, Bonenfant D,
Oakeley EJ and Hynes NE: Targeting fibroblast growth factor
receptors blocks PI3K/AKT signaling, induces apoptosis, and impairs
mammary tumor outgrowth and metastasis. Cancer Res. 70:4151–4162.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Prasad SB, Yadav SS, Das M, Modi A, Kumari
S, Pandey LK, Singh S, Pradhan S and Narayan G: PI3K/AKT
pathway-mediated regulation of p27(Kip1) is associated with cell
cycle arrest and apoptosis in cervical cancer. Cell Oncol (Dordr).
38:215–225. 2015. View Article : Google Scholar : PubMed/NCBI
|