Introduction
Transcription intermediary factor 1γ (Tif1γ), also
known as tripartite motif containing 33 (TRIM33), is a ubiquitous
nuclear protein (1) that regulates
transforming growth factor-β (TGF-β)/Smad signaling. It is part of
the transcriptional intermediary factor 1 family, which includes
four identified Tif1 members in mammals (α to δ), with modulatory
roles in the innate immune response and inflammation processes.
Tif1γ was initially described a decade ago (2); however, this protein has recently
gained increasing interest in oncology due to its dual function as
an oncogene and a tumor suppressor gene (3,4).
Similar to its homolog transcriptional cofactors
(Tif1α and Tif1β), Tif1γ contains multiple domains, including the
RING finger, B boxes, coiled coil and a PHD-bromodomain that
stabilizes Tif1γ chromatin occupancy and enhances its E3 ligase
activity (5–7). Despite structural similarity with
other family members, Tif1γ exhibits very specific functions. Tif1γ
regulates TGF-β signaling pathways, potentially via SMAD family
member 4 (Smad4) mono-ubiquitination, targeting Smad4 for
degradation and inhibiting the TGF-β/Smad signaling pathway. TGF-β
signaling is initiated by the dimerization of TGF-β I and II
receptors, which results in Smad2 and Smad3 phosphorylation, and
binding with Smad4. As a complex, Smad4 migrates to the nucleus,
where it interacts with various transcription factors (8–11).
Activation of mitogen-activated protein kinases (MAPKs) is another
Smad-independent TGF-β signaling mechanism (12,13).
Certain studies have suggested that Tif1γ competes with Smad2/Smad3
for binding to Smad4 (14–16). Tif1γ interacts with the Smad1/Smad4
complex in vivo, inhibiting transcriptional activity of the
Smad1/Smad4 complex via its PHD domain. In blood stem cell lines,
Tif1γ interacts with the transcription factor stem cell leukemia
(SCL)/T-cell acute lymphocytic leukemia protein 1 to promote mRNA
transcript elongation. In other cells, Tif1γ forms repressive
complexes with SCL that inhibit transcriptional activation
(17–19).
A previous study has demonstrated that Tif1γ
expression is a biomarker of the response of chronic myelomonocytic
leukemia to demethylating agents, and that Tif1γ is an
epigenetically-regulated tumor suppressor gene (20,21).
Tif1γ is involved in the process of vertebrate hematopoietic
development by regulating the TGF-β1 receptor and mRNA elongation
during transcription (22), and
loss of Tif1γ expression or protein malfunction promotes
hematopoietic stem cells to differentiate to the myelomonocytic
lineage (17–23). In B cell neoplasms, Tif1γ acts as
an oncogene, as it suppresses the apoptosis of B lymphoblastic
leukemia cells. Notably, Tif1γ performs this function by
associating with a single DNA cis element (24–26).
As for solid tumors, in vitro studies
demonstrated that in benign and malignant pancreatic cell lines,
overexpression of Tif1γ was associated with a reduced level of
Smad4. However, both overexpression and knockdown of Tif1γ lead to
an inhibition in tumor growth, and Tif1γ knockdown reduces tumor
invasion. Tif1γ has also been reported to be a tumor suppressor in
non-small cell lung cancer (NSCLC), as mRNA and protein expression
in NSCLC cell lines is significantly decreased. By contrast, tissue
microarray analysis revealed that Tif1γ was overexpressed in
colorectal cancer and there was an absence of Smad4 expression in
neoplastic samples. The levels of Tif1γ overexpression were stage
dependent (higher in stage III compared with stage I and II)
(27–29).
Breast cancer is the most common non-cutaneous
cancer and the leading cause of cancer-associated mortality in
females worldwide (30–33). Despite advances in diagnostics and
therapeutics, breast cancer incidence rates are rising, due to
demographical aging, hormone replacement use, manifestation of
cancer risks in modern lifestyles and other factors (30,34).
Breast cancer is one of the most heterogeneous diseases and, thus,
the development of personalized cancer management is crucial.
Personalized treatment plans may use established predictive
factors, including receptor status, clinicopathological factors,
urokinase-type plasminogen activator/plasminogen activator
inhibitor 1 (PAI1), tumor size, lymph node stage, histological
grade or lymphovascular invasion, and novel prognostic factors to
determine the required therapy regimen (35–37).
Prognostic markers are essential for decision-making as they
attempt to foresee the outcome of patients, irrespective of the
treatment received. Efforts are made to develop novel markers that
are independently associated with the overall and disease-free
survival (38–41). Screening techniques remain a
crucial part of breast cancer prevention and reducing breast
cancer-associated mortality. Currently, novel biomarkers are
required for developing new treatment algorithms and prognosis
evaluation.
In breast cancer, the prognostic significance of
Tif1γ has not been established. TGF-β has been demonstrated to have
tumor suppressive (early stages) and oncogenic [later stages;
pro-metastatic and pro-epithelial-to-mesenchymal transition (EMT)]
effects (42–44). The isoform TGF-β1 is an inhibitor
of mammary gland epithelial cell proliferation and has a
particularly important role in breast carcinogenesis (45–48).
Certain studies have reported that lower levels of circulating
TGF-β1 were associated with a poor disease prognosis (49,50);
however, other studies have indicated the opposite (51–53).
It may be assumed that Tif1γ also has a paradoxical
role in breast cancer development and outcome, as Tif1γ is involved
in regulating TGF-β/Smad signaling. Tumor suppressor and
tumorigenic roles of Tif1γ have been suggested in various cancer
types (4,20,54).
Recent data demonstrated that Tif1γ reduces Smad4 activity and,
thus, inhibits TGF-β-induced EMT in mammary epithelial cells,
terminal differentiation of mammary alveolar epithelial cells and
lactation (55–58). Furthermore, Tif1γ binds to and
represses the PAI1 promoter, directly regulating TGF-β-dependent
gene expression. As a negative regulator of Smad4, Tif1γ is crucial
for the regulation of TGF-β signaling (58–61).
Kassem et al (62)
suggested that Tif1γ and the TGF-β1/Smad4 signaling have a
significant effect on the outcome of patients with operable breast
cancer, and if measured in combination they can be used for
prognostic evaluation.
The concept of circulating tumor cells (CTCs) was
first proposed in 1896; however, the isolation methods and
prognostic significance were established only recently (63–65).
CTCs are tumor cells that are released from a solid tumor (the
primary tumor or metastases) into the peripheral blood circulation,
spontaneously or during treatment, where they form a tumor
thrombus. CTCs have been suggested as biomarkers, as they are
precursors of breast cancer metastases (66–68).
In 2004, Cristofanilli et al (69) reported that the number of CTCs in
therapy-naïve patients with metastatic breast cancer is an
independent predictor of progression-free survival (PFS) and
overall survival (OS). Further reports soon confirmed these
findings and also demonstrated the prognostic significance in
non-metastatic breast cancer (70–72).
Despite advances in CTC capture technology, it remains an expensive
and difficult method, which limits its use as a daily clinical
diagnostic application.
The potential prognostic use of Tif1γ has not been
well investigated in breast cancer to date. Therefore, the
rationale of the current study was to elucidate the role of Tif1γ
in breast cancer tumorigenesis, cancer progression and metastasis,
and to determine whether its expression is an independent
prognostic marker. CTCs were also analyzed to compare the
prognostic use of CTCs with the prognostic value of Tif1γ, which
can be easily and rapidly detected, thus allowing direct
translation to the clinic without methodological constraints.
Patients and methods
Study population
Patients with breast cancer (n=110) were
prospectively and randomly recruited between January 2008 and April
2016 at the Department of Breast Surgery, Yangpu Hospital, Tongji
University (Shanghai, China). The patients were aged 33–74 years,
with a median age of 51. An associated clinicopathological database
was established and long-term clinical follow-up was performed.
Written consent forms were obtained from all patients involved in
the current study (approval no. LL-2016-WSJ-002). The ethics review
board of Tongji University approved the study design a
priori. All patients underwent surgical tumor excision in which
tissue samples were collected. All participants provided their
consent for the use of tumor samples in academic research.
Preoperatively, neoadjuvant chemotherapy, endocrine therapy,
radiation or other therapies were received, as needed. As a control
group, blood samples were collected from 110 healthy subjects
between January 2008 and April 2016 at the Medical Examination
Center, Yangpu Hospital, Tongji University. Healthy volunteers
(30–67 years old; median age, 49 years) were females without breast
cancer, as indicated by pathological diagnostics. Individuals were
followed until the end of the follow-up period of 98 months.
Main reagents and instruments
Rabbit anti-Tif1γ antibody (cat. no. ab47062), mouse
anti-TGF-β1 (cat. no. ab64715), mouse anti-β-actin (cat. no.
ab8226) and goat anti-rabbit IgG horseradish peroxidase
(HRP)-conjugated antibody (cat. no. ab6721) were purchased from
Abcam (Cambridge, MA, USA). Human Tif1γ ELISA kit (cat. no.
GEN2413000) was purchased from Gentaur (Paris, France). The
ChemiDoc MP imaging system was purchased from Bio-Rad Laboratories,
Inc. (Hercules, CA, USA).
SDS-PAGE and western blot
analysis
For analysis of protein expression, four samples of
breast cancer tissue with paired adjacent normal breast tissue were
obtained from Yangpu Hospital of Tongji University (Shanghai,
China). Informed consent was provided by all patients and all
samples were histologically confirmed prior to analysis. To confirm
the expression of the protein of interest, western blot analysis
was performed as previously described (73). In brief, the protein concentration
of the crude tissue extracts was measured using the Bradford
method. SDS-PAGE was used to separate samples, with an equal amount
of total protein content loaded in each lane of 10% gels, then
transferred to polyvinylidene difluoride membranes using a semi-dry
apparatus. Nonspecific binding was blocked using PBS containing 3%
bovine serum albumin. Membranes were probed with Tif1γ and TGF-β1
primary antibodies (1:1,000). β-actin (1:1,000) served as a loading
control. The immune complexes were visualized using HRP-conjugated
secondary antibody (1:1,500), according to the manufacturer's
protocols. Blots were digitally imaged using the ChemiDoc MP
imaging system. ImageJ version 1.51p software (National Institutes
of Health, Bethesda, MD, USA) was used to quantify protein
expression.
ELISA detection of Tif1γ in the
plasma
Tif1γ levels were measured with the Human Tif1γ
ELISA kit (cat. no. GEN2413000) according to the manufacturer's
protocols. Serum was diluted in a 5- to 20-fold range to obtain
values falling within the linear range of the standard curve. The
qualitative absorbance analyses were performed using a Varioskan
Flash multifunctional microplate reader (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) at 450 nm minus 550 nm, according to the
manufacturer's guidelines.
Detection of CTCs in peripheral
blood
Peripheral venous blood of each patient was
collected (1 ml) and placed in a CellSave tube (containing EDTA and
cell preservative) at room temperature. The blood was diluted with
PBS and a Percoll density gradient-based method was used to
separate the mononuclear cell layer from the blood (74). Following removal of the mononuclear
cells, CTC capture was performed using non-antibody-dependent
specific magnetic beads (Fe3O4 inner cores)
(75).
Statistical analysis for overall
survival and prognostic calculations
Data were analyzed using SPSS (version 20.0; IBM
Corp., Armonk, NY, USA). The Kaplan-Meier method was used to
estimate OS and multivariate analysis was performed with the Cox
proportional hazards model. OS was calculated from the date of
diagnosis to the date of last follow-up. Analysis of the
differences between groups was calculated using a two-tailed
Student's t-test and χ2 test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Patient characteristics
Of the 110 recruited patients, none was missed
during follow-up or withdrew, and all successfully completed the
study. All patients were Chinese females. The patient
characteristics are summarized in Table I. Patients were followed for 98
months to assess the association between Tif1γ levels in serum and
OS. The majority of the patients presented with human epidermal
growth factor receptor 2 (Her2)-negative, grade 2 ductal type
carcinoma. The distribution of clinicopathological features was
representative and the median age at diagnosis was close to the
population median age.
 | Table I.Clinicopathologic variables of breast
cancer patient cohort. |
Table I.
Clinicopathologic variables of breast
cancer patient cohort.
| Variables | Number of
patients | % |
|---|
| Age |
|
|
| <50
years | 31 | 28.2 |
| ≥50
years | 79 | 71.8 |
| Tumor size |
|
|
| <2
cm | 46 | 41.8 |
| ≥2
cm | 64 | 58.2 |
| Tumor stage |
|
|
| T1 | 34 | 30.9 |
| T2 | 76 | 69.1 |
| Histologic
grade |
|
|
| G1 | 5 | 4.5 |
| G2 | 86 | 78.2 |
| G3 | 19 | 17.3 |
| Node status |
|
|
|
Negative | 68 | 61.8 |
|
Positive | 42 | 38.2 |
| Histologic
type |
|
|
|
Ductal | 93 | 84.5 |
|
Others | 17 | 15.5 |
| Molecular
subtypes |
|
|
|
Luminal | 30 | 27.3 |
|
Others | 80 | 72.7 |
| Her2 status |
|
|
|
Negative | 91 | 82.7 |
|
Positive | 19 | 17.3 |
| CTC |
|
|
|
Negative | 47 | 42.7 |
|
Positive | 63 | 57.3 |
| Tif1γ status |
|
|
|
Negative | 58 | 52.7 |
|
Positive | 52 | 47.3 |
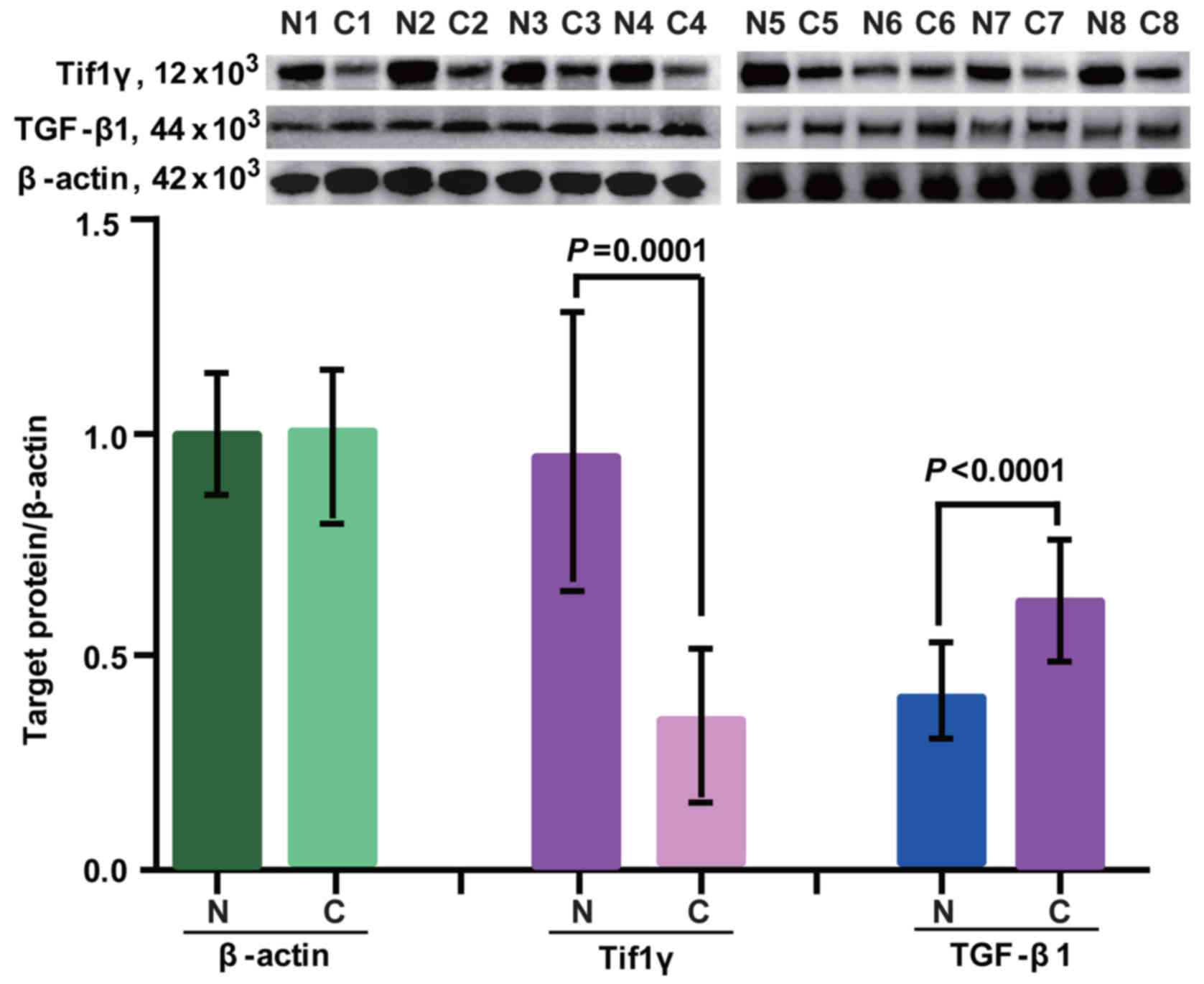
Expression of Tif1γ and TGF-β1 in
breast cancer tissues
Protein expression of Tif1γ and TGF-β1 in cancer and
healthy tissues was determined by western blot analysis. β-actin
was used as a loading control. The expression profiles are
presented in Fig. 1. Tif1γ and
TGF-β1 were detected in adjacent normal control tissues and cancer
tissues. In cancer tissues, the expression of Tif1γ was
significantly lower compared with adjacent normal control tissues
(Fig. 1). By contrast, TGF-β1
expression was higher in cancer tissues compared with the adjacent
control samples (Fig. 1).
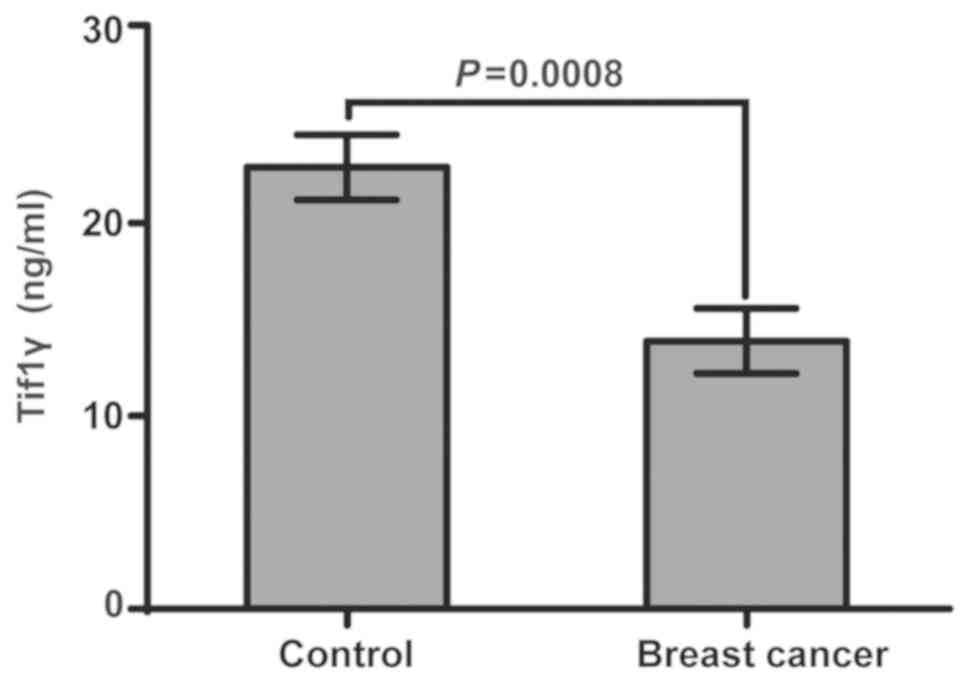
Tif1γ levels in plasma
Indirect ELISA was used to analyze the human Tif1γ
levels in plasma samples from 110 patients with breast cancer and
110 healthy controls. Tif1γ levels were significantly higher in the
healthy controls compared with the patients with breast cancer
(Fig. 2). The average Tif1γ value
in the breast cancer group was 13.89 ng/ml, while in the control
group it was 22.90 ng/ml. The average concentration of 18.40 ng/ml
was therefore used to divide the patients with breast cancer into
Tif1γ-positive (n=52) and Tif1γ-negative (n=58; P=0.0008) groups,
for subsequent analyses.
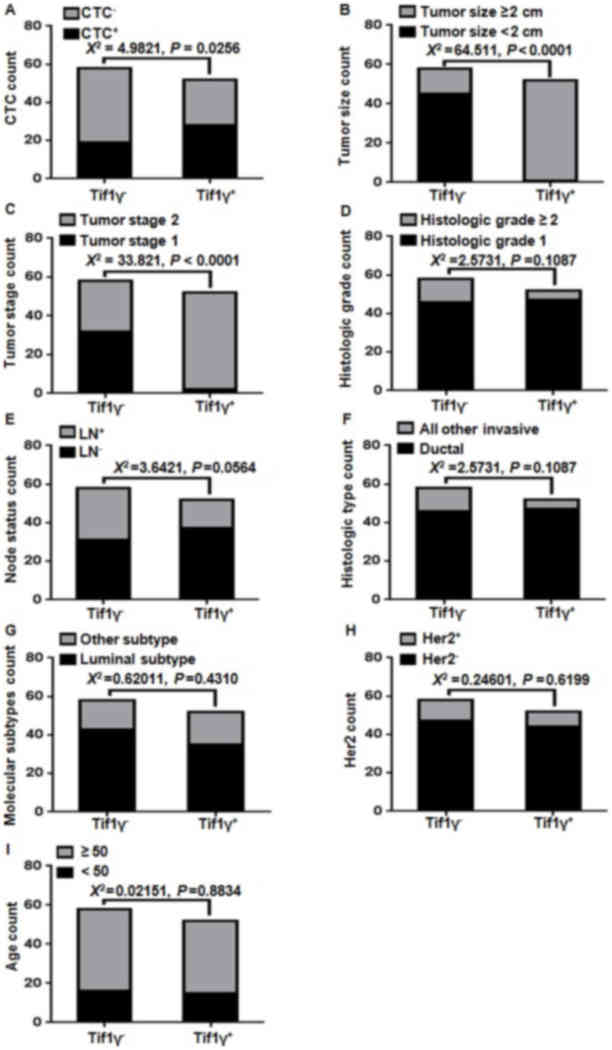
Comparing clinicopathological
characteristics and CTC count with the Tif1γ plasma level
Tif1γ plasma levels were measured by ELISA. CTC
detection was performed using non-antibody-dependent specific
magnetic beads. The clinicopathological characteristics were
collected upfront. χ2 correlations were performed and
presented in Fig. 3 Statistically
significant correlations were observed between the CTC count
(Fig. 3A), tumor size (Fig. 3B) and tumor stage (Fig. 3C). The number of CTC count-positive
patients was 67.2±6.2% in the Tif1γ-negative group and 46.2±7.0% in
the Tif1γ-positive group (Fig.
3A). No correlation was observed with histological grade, tumor
subtype, lymph node and Her2 status (Fig. 3D-I).
Association between Tif1γ plasma
levels and OS
The clinical prognostic value of the Tif1γ serum
levels in patients with breast cancer was investigated. Patients
were categorized into high and low serum level groups. The high
Tif1γ serum level group had improved OS compared with the low-level
group (Kaplan-Meier method; 98 months; P=0.0174; Fig. 4A). This was further confirmed using
univariate Cox analysis (Table
II). The Tif1γ serum level was significantly associated with
survival in patients with breast cancer [hazard ratio (HR)=0.125;
P=0.046; Table II]. Furthermore,
multivariate Cox analysis confirmed that high Tif1γ serum level was
a significant independent prognostic factor in the patients with
breast cancer (HR=0.046; P=0.011; Table II).
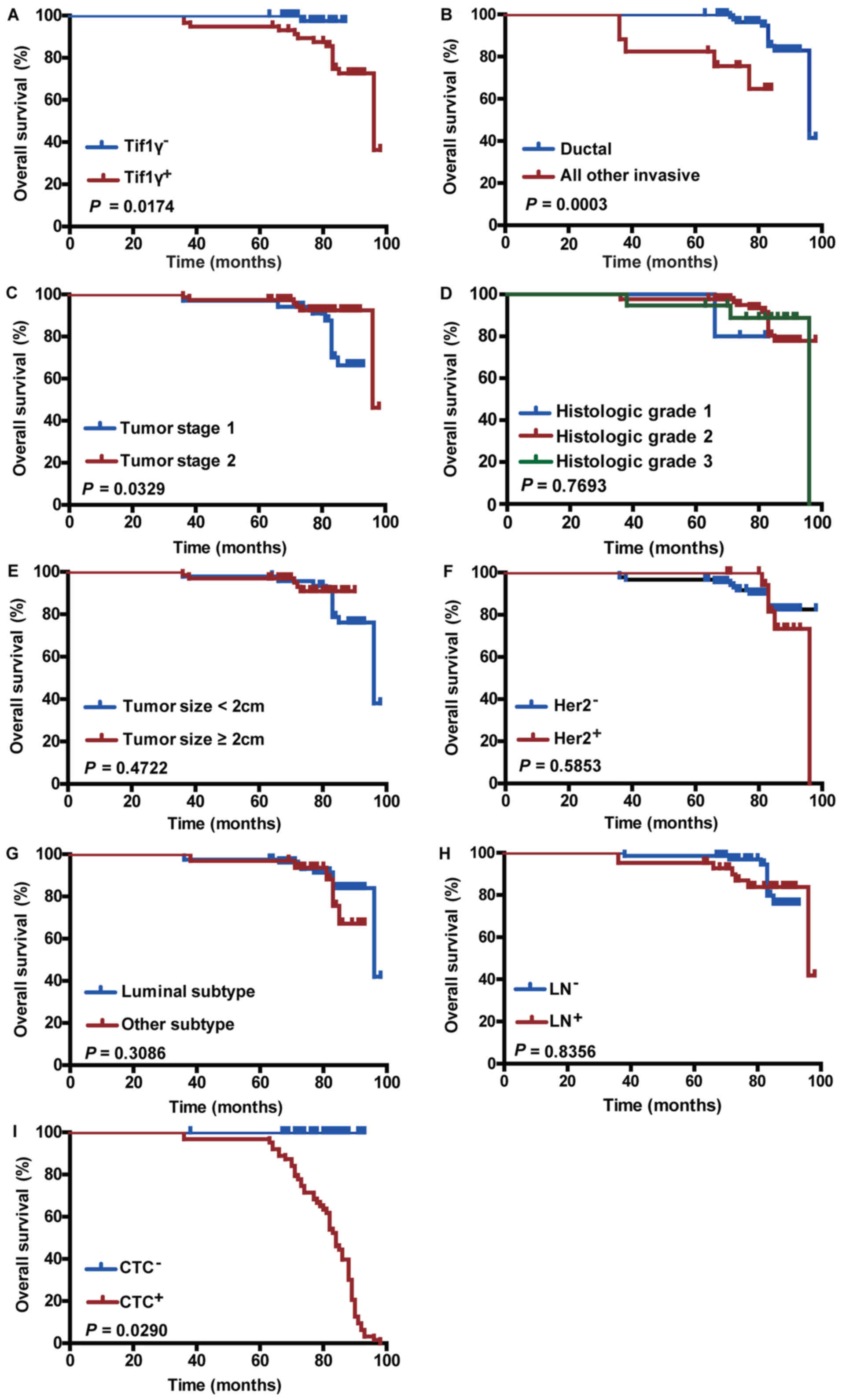 | Figure 4.Effect of Tif1γ plasma levels and
other clinicopathological factors on overall survival. Kaplan-Meier
survival analysis curves representing the association of overall
survival and (A) Tif1γ serum levels, (B) ductal or other invasive
types, (C) tumor stage, (D) histologic grade, (E) tumor size, (F)
Her2 status, (G) tumor subtype, (H) LN status and (I) CTC count.
Tif1γ, transcription intermediary factor 1 γ; Her2, human epidermal
growth factor receptor 2; LN, lymph node; CTC, circulating tumor
cell. |
 | Table II.Univariate and multivariate analyses
of overall survival by the Cox proportional hazards model. |
Table II.
Univariate and multivariate analyses
of overall survival by the Cox proportional hazards model.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Clinicopathologic
variables | HR | CI | P-value | HR | CI | P-value |
|---|
| Age | 1.062 | 0.991–1.138 | 0.087 | 1.060 | 0.978–1.150 | 0.157 |
| Tumor size | 0.661 | 0.221–1.974 | 0.458 | 2.733 | 0.544–13.726 | 0.222 |
| Tumor stage | 0.329 | 0.111–0.976 | 0.045 | 0.477 | 0.107–2.117 | 0.330 |
| Histologic
grade | 0.837 | 0.262–2.672 | 0.763 | 1.122 | 0.402–3.131 | 0.826 |
| Node status | 0.773 | 0.365–1.639 | 0.502 | 0.556 | 0.171–1.800 | 0.327 |
| Histologic
type | 2.878 | 1.282–6.464 | 0.010 | 2.328 | 0.543–9.972 | 0.255 |
| Molecular
subtypes | 0.772 | 0.264–2.262 | 0.638 | 0.927 | 0.232–3.695 | 0.914 |
| Her2 status | 0.796 | 0.502–1.261 | 0.331 | 0.657 | 0.342–1.263 | 0.208 |
| CTC | 0.934 | 0.330–2.645 | 0.898 | 1.372 | 0.302–6.247 | 0.682 |
| Tif1γ status | 0.125 | 0.016–0.964 | 0.046 | 0.047 | 0.004–0.501 | 0.011 |
Association between
clinicopathological features and CTCs on OS
As expected, OS was significantly improved in
patients with ductal type cancer (P=0.0003; Fig. 4B) and early tumor stage (stage 1;
P=0.0329; Fig. 4C), and in
patients that were CTC count-negative (P=0.0290; Fig. 4I). In addition, these factors
displayed prognostic significance in univariate Cox analysis
(Table II). Her2 positivity,
tumor size (≥2 cm), tumor subtype, histological grade and lymph
node positivity were not significantly associated with the patient
survival outcome (Fig. 4D-H), and
had no prognostic significance (Table
II).
Discussion
Breast cancer is one of the most well-studied
diseases with constant novel insights into the molecular basis of
the disease and identification of novel therapy targets (40,76,77).
The subclassification of breast cancer is typically based on
estrogen receptor, progesterone receptor and Her2 status, which is
used for predicting prognosis and guiding treatment strategies
(78–82). Given the heterogeneity and
alterability of breast cancer, there is a constant need for novel
biological markers with predictive power, such as Tif1γ. The
findings of the current study demonstrated that Tif1γ is promising
as a simple, efficient and effective outcome predictor in patients
with breast cancer. Considering the increasing incidence of breast
cancer, easily applicable methods used for prognosis assessment and
early detection are crucial (83–86).
Furthermore, in the era of individualized cancer care, diagnostic
tumor markers allow oncologists to identify high-risk patients,
select and monitor treatment, and screen for disease recurrence
(40,76,87–90).
In the current study, the plasma levels of Tif1γ
were significantly higher in healthy controls than in patients with
breast cancer (Fig. 2). This
supports the general expectations, as Tif1γ is part of the
transcription intermediary factor 1 family and has been previously
reported to inhibit the TGF-β/Smad signaling pathway (60,62,91).
TGF-β itself is associated with tumor invasion and progression, as
it acts as a potent inducer of EMT; thus, lower levels or depletion
of Tif1γ increases the EMT process (pro-oncogenic) (55,58).
Previous microarray analysis has revealed that
certain Tif1γ target genes are associated with EMT. A deficiency in
Tif1γ expression has been reported in several cancer types,
including NSCLC and hepatocellular, pancreatic and colorectal
cancer. Thus, Tif1γ potentially has tumor suppressor gene activity
that is lost during cancer development. The majority of research
into Tif1γ in cancer has focused on the molecular functions and
interactions, whereas in vivo and clinical outcome data are
limited.
The aim of the present study was to confirm the
association between Tif1γ and breast cancer by examining the
expression of Tif1γ in the plasma of patients and healthy controls,
and to investigate the potential prognostic use of Tif1γ. Western
blot analysis was performed to determine the expression of Tif1γ,
compared with TGF-β, in adjacent noncancerous and cancerous
tissues. Tif1γ expression was reduced in breast cancer tissues
compared with adjacent noncancerous tissues. As expected,
reciprocal results were observed for TGF-β (Fig. 1). Serum Tif1γ levels were
significantly lower in the patients with breast cancer compared
with the healthy control samples (Fig.
2). This suggests that Tif1γ is active in normal cells,
exerting a tumor suppression role, and is less active in cancer
cells.
ELISA was used in order to quantify and to obtain
objective numerical values of the Tif1γ expression levels (Tif1γ
concentration), rather than biased, immunohistochemistry-based
observations. In addition, using plasma as the clinical sample
material has a multitude of benefits, the most important being the
simplicity and minimal invasiveness of sampling. Using the plasma
levels of Tif1γ, an average concentration was calculated, which was
then selected as a cut-off value (18.40 ng/ml) to divide patients
into Tif1γ-positive and Tif1γ-negative groups. The groups had a
representative number (52 Tif1γ-positive, 58 Tif1γ-negative), so
that a long-term follow-up (98 months) could be conducted. Patients
with low plasma Tif1γ had significantly shorter OS, while
Tif1γ-positive patients had improved OS compared with
Tif1γ-negative patients.
In order to further validate the clinical
significance of these results, the association of OS and
well-established clinicopathological characteristics, including
lymph node status or tumor grade, was assessed by univariate and
multivariate Cox proportional hazards analysis. The results
confirmed the prognostic significance of Tif1γ plasma levels.
In addition, the CTC count was measured, as CTC
number has been established as a strong independent prognostic
factor for OS and PFS in patients with breast cancer. Although not
all CTCs produce metastases, their spread is an important
prerequisite for clinical metastasis. CTCs with certain biological
characteristics are more prone to develop micrometastases. The
association between CTC number and poor OS was confirmed in the
cohort of the current study. Additionally, the results were similar
to those produced by measuring Tif1γ, which suggested that using
Tif1γ is as accurate as detecting CTCs for prognostic evaluation,
but simpler and more efficient.
Notably, the Tif1γ plasma levels maintained
significance in univariate and multivariate analyses, with low
Tif1γ being an independent negative prognostic factor for OS in
patients with breast cancer. The results are consistent with
reports in hepatocellular cancer, NSCLC and several other types of
cancer. However, the findings of the present study do not coincide
with those of Kassem et al (62), where high expression of TGF-β1 and
Tif1γ was associated with poorer outcome.
Overall, low Tif1γ was identified as an independent
and significant risk factor for survival following curative
resection in treatment-naïve patients. These results suggest that
the serum levels of Tif1γ can be used as an accessible and feasible
outcome predictor. To the best of our knowledge, this is the first
prospective, long-term study on the clinical impact of Tif1γ in
patients with breast cancer. The measurement of Tif1γ serum levels
is translatable into oncological practice as a simple,
cost-effective and rapid method, which may be implemented for
diagnosis, therapy decision-making, prognostic determination and
disease monitoring.
There are several limitations to the present study.
Although the patient cohort of 110 cases is the most comprehensive
data composition assembled in the existing literature thus far, it
is a relatively small sample. Further studies and longer follow-ups
are required to validate the findings. Additionally, investigating
the regulatory mechanisms of Tif1γ in tumor growth and metastasis
via inhibition of TGF-β/Smad signaling is crucial.
The current prospective study with a long-term
follow-up demonstrated that analyzing Tif1γ serum expression may be
useful for determining the prognosis of patients with breast
cancer. Further elaboration and validation are required to
establish Tif1γ as an easily detectable, non-invasive, novel
biomarker with predictive power that can be implemented in breast
cancer management and disease monitoring.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 30972633), the
Scientific Research Foundation for the Returned Overseas Chinese
Scholars, State Education Ministry (grant no. 2015-311), the
Shanghai Health and Family Planning Commission Project (grant nos.
20134298 and 201640253), the Shanghai Health and Family Planning
Commission Fund for Qing Nian Yi Shi Training Project (grant no.
2014118), the Shanghai Yangpu District Science and Technology
Commission Project (grant nos. 2016-2017, YP17ZM02), the Shanghai
Yangpu District Health and Family Planning Commission Project
(grant nos. 2011-2013 and 2016–2017, YP17ZM02), the Shanghai Yangpu
District Health and Family Planning Commission Fund for Bai Yi Deng
Gao Training Project (grant no. 2014-2016), the Shanghai Yangpu
District Health and Family Planning Commission Fund for Hao Yi Shi
Training Project (grant no. 201742), the Academic Leader in
Climbing Program from Yangpu Center Hospital (grant nos. YE2201608
and YE2201703), the Science Program from Yangpu Center Hospital
(grant no. SE1201746), the Natural Science Foundation of Shanghai
(grant no. 18ZR1436000), and the Fundamental Research Funds for the
Central Universities (grant no. 22120180286).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
FC and XP made substantial contributions to the
design of the experiments and revised the manuscript. LC, ZZ and XP
performed the experiments, XP, MW, SC, MAFL, CC and EB analyzed and
interpreted the data.
Ethics approval and consent to
participate
Yangpu Hospital, Tongji University School of
Medicine approved the project under ‘Prognostic Significance of
Tif1γ Expression and Circulating Tumor Cells influence in Patients
with Breast Cancer (approval no. LL-2016-WSJ-002).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Herquel B, Ouararhni K and Davidson I: The
TIF1α-related TRIM cofactors couple chromatin modifications to
transcriptional regulation, signaling and tumor suppression.
Transcription. 2:231–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Venturini L, You J, Stadler M, Galien R,
Lallemand V, Koken MH, Mattei MG, Ganser A, Chambon P, Losson R and
de Thé H: TIF1gamma, a novel member of the transcriptional
intermediary factor 1 family. Oncogene. 18:1209–1217. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hatakeyama S: TRIM proteins and cancer.
Nat Rev Cancer. 11:792–804. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herquel B, Ouararhni K, Khetchoumian K,
Ignat M, Teletin M, Mark M, Béchade G, Van Dorsselaer A,
Sanglier-Cianférani S, Hamiche A, et al: Transcription cofactors
TRIM24, TRIM28, and TRIM33 associate to form regulatory complexes
that suppress murine hepatocellular carcinoma. Proc Natl Acad Sci
USA. 108:8212–8217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen T and Dent SY: Chromatin modifiers
and remodellers: Regulators of cellular differentiation. Nat Rev
Genet. 15:93–106. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xi Q, Wang Z, Zaromytidou AI, Zhang XH,
Chow-Tsang LF, Liu JX, Kim H, Barlas A, Manova-Todorova K,
Kaartinen V, et al: A poised chromatin platform for TGF-β access to
master regulators. Cell. 147:1511–1524. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Massagué J and Xi Q: TGF-β control of stem
cell differentiation genes. FEBS Lett. 586:1953–1958. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heldin CH and Moustakas A: A new twist in
Smad signaling. Dev Cell. 10:685–686. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moustakas A and Heldin CH: The regulation
of TGFbeta signal transduction. Development. 136:3699–3714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heldin CH and Moustakas A: Role of Smads
in TGFβ signaling. Cell Tissue Res. 347:21–36. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Attisano L and Lee-Hoeflich ST: The Smads.
Genome Biol. 2:REVIEWS30102001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Smith AP, Verrecchia A, Fagà G, Doni M,
Perna D, Martinato F, Guccione E and Amati B: A positive role for
Myc in TGFbeta-induced Snail transcription and
epithelial-to-mesenchymal transition. Oncogene. 28:422–430. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Padgett RW: TGFbeta signaling pathways and
human diseases. Cancer Metastasis Rev. 18:247–259. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dupont S, Zacchigna L, Cordenonsi M,
Soligo S, Adorno M, Rugge M and Piccolo S: Germ-layer specification
and control of cell growth by ectodermin, a Smad4 ubiquitin ligase.
Cell. 121:87–99. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morsut L, Yan KP, Enzo E, Aragona M,
Soligo SM, Wendling O, Mark M, Khetchoumian K, Bressan G, Chambon
P, et al: Negative control of Smad activity by ectodermin/Tif1gamma
patterns the mammalian embryo. Development. 137:2571–2578. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Agricola E, Randall RA, Gaarenstroom T,
Dupont S and Hill CS: Recruitment of TIF1g to Chromatin via Its PHD
finger-bromodomain activates its ubiquitin ligase and
transcriptional repressor activities. Mol Cell. 43:85–96. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kusy S, Gault N, Ferri F, Lewandowski D,
Barroca V, Jaracz-Ros A, Losson R and Romeo PH: Adult hematopoiesis
is regulated by TIF1γ, a repressor of TAL1 and PU.1 transcriptional
activity. Cell Stem Cell. 8:412–425. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bai RY, Koester C, Ouyang T, Hahn SA,
Hammerschmidt M, Peschel C and Duyster J: SMIF, a Smad4-interacting
protein that functions as a co-activator in TGFbeta signalling. Nat
Cell Biol. 4:181–190. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
He W, Dorn DC, Erdjument-Bromage H, Tempst
P, Moore MA and Massagué J: Hematopoiesis controlled by distinct
TIF1gamma and Smad4 Branches of the TGFbeta Pathway. Cell.
125:929–941. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aucagne R, Droin N, Paggetti J, Lagrange
B, Largeot A, Hammann A, Bataille A, Martin L, Yan KP, Fenaux P, et
al: Transcription intermediary factor 1γ is a tumor suppressor in
mouse and human chronic myelomonocytic leukemia. J Clin Invest.
121:2361–2370. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Emanuel PD: Juvenile myelomonocytic
leukemia and chronic myelomonocytic leukemia. Leukemia.
22:1335–1342. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Quéré R, Saint-Paul L, Carmignac V, Martin
RZ, Chrétien ML, Largeot A, Hammann A, Pais de Barros JP, Bastie JN
and Delva L: Tif1γ regulates the TGF-β1 receptor and promotes
physiological aging of hematopoietic stem cells. Proc Natl Acad Sci
USA. 111:10592–10597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lindley LE and Briegel KJ: Generation of
mice with a conditional Lbh null allele. Genesis. 51:491–497. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou Y, You MJ, Young KH, Lin P, Lu G,
Medeiros LJ and Bueso-Ramos CE: Advances in the molecular
pathobiology of B-lymphoblastic leukemia. Hum Pathol. 43:1347–1362.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang E, Kawaoka S, Roe JS, Shi J, Hohmann
AF, Xu Y, Bhagwat AS, Suzuki Y and Kinney JB: The transcriptional
cofactor TRIM33 prevents apoptosis in B lymphoblastic leukemia by
deactivating a single enhancer. Elife. 4:e063772015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yuan N, Song L, Zhang S, Lin W, Cao Y, Xu
F, Fang Y, Wang Z, Zhang H, Li X, et al: Bafilomycin A1 targets
both autophagy and apoptosis pathways in pediatric B-cell acute
lymphoblastic leukemia. Haematologica. 100:345–356. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jain S, Singhal S, Francis F, Hajdu C,
Wang JH, Suriawinata A, Wang YQ, Zhang M, Weinshel EH, Francois F,
et al: Association of overexpression of TIF1γ with colorectal
carcinogenesis and advanced colorectal adenocarcinoma. World J
Gastroenterol. 17:3994–4000. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jain S, Yu M, Singhal S, Francis F, Hajdu
C, Suriawinata S, Zhang M, Aladhamy N, Chiriboga L, Pan R, et al:
Overexpression of transcription intermediary factor 1 γ (TIF1γ) is
an early event in colorectal carcinogenesis and inversely related
to Smad4 inactivation in colorectal carcinoma. Lab Investig.
90:149A2010.
|
|
29
|
Fearon ER and Vogelstein B: A genetic
model for colorectal tumorigenesis. Cell. 61:759–767. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics: 2011. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe:
Estimates for 40 countries in 2012. Eur J Cancer. 49:1374–1403.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sikov WM, Berry DA, Perou CM, Singh B,
Cirrincione CT, Tolaney SM, Kuzma CS, Pluard TJ, Somlo G, Port RT,
et al: Impact of the addition of carboplatin and/or bevacizumab to
neoadjuvant once-per-week paclitaxel followed by dose-dense
doxorubicin and cyclophosphamide on pathologic complete response
rates in stage II to III triple-negative breast cancer: CALGB 40603
(Alliance). J Clin Oncol. 33:13–21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Desantis C, Ma J, Bryan L and Jemal A:
Breast cancer statistics, 2013. CA Cancer J Clin. 64:52–62. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sharma P, Stecklein SR, Kimler BF, Sethi
G, Petroff BK, Phillips TA, Tawfik OW, Godwin AK and Jensen RA: The
prognostic value of BRCA1 promoter methylation in early stage
triple negative breast cancer. J Cancer Ther Res. 3:1–11. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Müller HM, Fiegl H, Widschwendter A and
Widschwendter M: Prognostic DNA methylation marker in serum of
cancer patients. Annals of the New York Academy of Sciences.
pp44–49. 2004. View Article : Google Scholar
|
|
37
|
Duffy MJ: Serum tumor markers in breast
cancer: Are they of clinical value? Clin Chem. 52:345–351. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cai FF, Kohler C, Zhang B, Wang MH, Chen
WJ and Zhong XY: Epigenetic therapy for breast cancer. Int J Mol
Sci. 12:4465–4487. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sharma S, Kelly TK and Jones PA:
Epigenetics in cancer. Carcinogenesis. 31:27–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kumar R: Breast cancer tumor markers. J
Solid Tumors. 2:432012. View Article : Google Scholar
|
|
41
|
Payne SJ, Bowen RL, Jones JL and Wells CA:
Predictive markers in breast cancer-the present. Histopathology.
52:82–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wakefield LM and Roberts AB: TGF-beta
signaling: Positive and negative effects on tumorigenesis. Curr
Opin Genet Dev. 12:22–29. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Giampieri S, Manning C, Hooper S, Jones L,
Hill CS and Sahai E: Localized and reversible TGFbeta signalling
switches breast cancer cells from cohesive to single cell motility.
Nat Cell Biol. 11:1287–1296. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ikushima H and Miyazono K: TGFbeta
signalling: A complex web in cancer progression. Nat Rev Cancer.
10:415–424. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Faure E, Heisterkamp N, Groffen J and
Kaartinen V: Differential expression of TGF-beta isoforms during
postlactational mammary gland involution. Cell Tissue Res.
300:89–95. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nguyen AV and Pollard JW: Transforming
growth factor beta3 induces cell death during the first stage of
mammary gland involution. Development. 127:3107–3118.
2000.PubMed/NCBI
|
|
47
|
Li M, Liu X, Robinson G, Bar-Peled U,
Wagner KU, Young WS, Hennighausen L and Furth PA: Mammary-derived
signals activate programmed cell death during the first stage of
mammary gland involution. Proc Natl Acad Sci USA. 94:3425–3430.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bierie B, Gorska AE, Stover DG and Moses
HL: TGF-beta promotes cell death and suppresses lactation during
the second stage of mammary involution. J Cell Physiol. 219:57–68.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Panis C, Herrera AC, Victorino VJ, Aranome
AM and Cecchini R: Screening of circulating TGF-beta levels and its
clinicopathological significance in human breast cancer. Anticancer
Res. 33:737–742. 2013.PubMed/NCBI
|
|
50
|
Paiva CE, Drigo SA, Rosa FE, Moraes Neto
FA, Caldeira JR, Soares FA, Domingues MA and Rogatto SR: Absence of
transforming growth factor-beta type II receptor is associated with
poorer prognosis in HER2-negative breast tumours. Ann Oncol.
21:734–740. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Richardsen E, Uglehus RD, Johnsen SH and
Busund LT: Immunohistochemical expression of epithelial and stromal
immunomodulatory signalling molecules is a prognostic indicator in
breast cancer. BMC Res Notes. 5:1102012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Desruisseau S, Palmari J, Giusti C, Romain
S, Martin PM and Berthois Y: Determination of TGFbeta1 protein
level in human primary breast cancers and its relationship with
survival. Br J Cancer. 94:239–246. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Koumoundourou D, Kassimatis T, Zolota V,
Tzorakoeleftherakis E, Ravazoula P, Vassiliou V, Kardamakis D and
Varakis J: Prognostic significance of TGFbeta-1 and pSmad2/3 in
breast cancer patients with T1-2,N0 tumours. Anticancer Res.
27:2613–2620. 2007.PubMed/NCBI
|
|
54
|
Vincent DF, Yan KP, Treilleux I, Gay F,
Arfi V, Kaniewsky B, Marie JC, Lepinasse F, Martel S, Goddard-Leon
S, et al: Inactivation of TIF1gamma cooperates with Kras to induce
cystic tumors of the pancreas. PLoS Genet. 5:e10005752009.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fattet L, Ay AS, Bonneau B, Jallades L,
Mikaelian I, Treilleux I, Gillet G, Hesling C and Rimokh R: TIF1γ
requires sumoylation to exert its repressive activity on TGFβ
signaling. J Cell Sci. 126:3713–3123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mascle XH, Germain-Desprez D, Huynh P,
Estephan P and Aubry M: Sumoylation of the transcriptional
intermediary factor 1beta (TIF1beta), the Co-repressor of the KRAB
multifinger proteins, is required for its transcriptional activity
and is modulated by the KRAB domain. J Biol Chem. 282:10190–10202.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kretzschmar M, Doody J, Timokhina I and
Massagué J: A mechanism of repression of TGFbeta/Smad signaling by
oncogenic Ras. Genes Dev. 13:804–816. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang L, Yang H, Lei Z, Zhao J, Chen Y,
Chen P, Li C, Zeng Y, Liu Z, Liu X and Zhang HT: Repression of
TIF1γ by SOX2 promotes TGF-β-induced epithelial-mesenchymal
transition in non-small-cell lung cancer. Oncogene. 35:867–877.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mikaelian I, Malek M, Gadet R, Viallet J,
Garcia A, Girard-Gagnepain A, Hesling C, Gillet G, Gonzalo P,
Rimokh R and Billaud M: Genetic and pharmacologic inhibition of
mTORC1 promotes EMT by a TGF-β-independent mechanism. Cancer Res.
73:6621–6631. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hesling C, Fattet L, Teyre G, Jury D,
Gonzalo P, Lopez J, Vanbelle C, Morel AP, Gillet G, Mikaelian I and
Rimokh R: Antagonistic regulation of EMT by TIF1γ and Smad4 in
mammary epithelial cells. EMBO Rep. 12:665–672. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hesling C, Lopez J, Fattet L, Gonzalo P,
Treilleux I, Blanchard D, Losson R, Goffin V, Pigat N, Puisieux A,
et al: Tif1γ is essential for the terminal differentiation of
mammary alveolar epithelial cells and for lactation through SMAD4
inhibition. Development. 140:167–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kassem L, Deygas M, Fattet L, Lopez J,
Goulvent T, Lavergne E, Chabaud S, Carrabin N, Chopin N, Bachelot
T, et al: TIF1γ interferes with TGFβ1/SMAD4 signaling to promote
poor outcome in operable breast cancer patients. BMC Cancer.
15:4532015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kim MY, Oskarsson T, Acharyya S, Nguyen
DX, Zhang XH, Norton L and Massagué J: Tumor self-seeding by
circulating cancer cells. Cell. 139:1315–1326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Li P, Mao Z, Peng Z, Zhou L, Chen Y, Huang
PH, Truica CI, Drabick JJ, El-Deiry WS, Dao M, et al: Acoustic
separation of circulating tumor cells. Proc Natl Acad Sci USA.
112:4970–4975. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Dive C and Brady G: SnapShot: Circulating
tumor cells. Cell. 168:P742–P742.e1. 2017. View Article : Google Scholar
|
|
66
|
Dawson SJ, Tsui DW, Murtaza M, Biggs H,
Rueda OM, Chin SF, Dunning MJ, Gale D, Forshew T, Mahler-Araujo B,
et al: Analysis of circulating tumor DNA to monitor metastatic
breast cancer. N Engl J Med. 368:1199–1209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Scully OJ, Bay BH, Yip G and Yu Y: Breast
cancer metastasis. Cancer Genomics Proteomics. 9:311–320.
2012.PubMed/NCBI
|
|
68
|
Kuo AH and Clarke MF: Identifying the
metastatic seeds of breast cancer. Nat Biotechnol. 31:504–505.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Cristofanilli M, Budd GT, Ellis MJ,
Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ,
Terstappen LWMM and Hayes DF: Circulating tumor cells, disease
progression, and survival in metastatic breast cancer. N Engl J
Med. 351:781–791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Giuliano M, Giordano A, Jackson S, Hess
KR, de Giorgi U, Mego M, Handy BC, Ueno NT, Alvarez RH, De
Laurentiis M, et al: Circulating tumor cells as prognostic and
predictive markers in metastatic breast cancer patients receiving
first-line systemic treatment. Breast Cancer Res. 13:R672011.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Pierga JY, Bidard FC, Mathiot C, Brain E,
Delaloge S, Giachetti S, de Cremoux P, Salmon R, Vincent-Salomon A
and Marty M: Circulating tumor cell detection predicts early
metastatic relapse after neoadjuvant chemotherapy in large operable
and locally advanced breast cancer in a phase II randomized trial.
Clin Cancer Res. 14:7004–7010. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Bidard FC, Proudhon C and Pierga JY:
Circulating tumor cells in breast cancer. Mol Oncol. 10:418–430.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Pan X, Zeng SL, Yu D, Liang XL, Ji C, Pan
B, Cai J, Wang Y, Min Y, Fang W and Liao WQ: Variable domain of the
heavy chain of heavy-chain antibody of the Rv0733 antigen of
mycobacterium tuberculosis panned and identified from a nonimmune
llama VHH phage display library. Int J Clin Exp Pathol.
9:2869–2878. 2016.
|
|
74
|
Lin XY, Cai FF, Wang MH, Pan X, Wang F,
Cai L, Cui RR, Chen S and Ewelina B: Mammalian sterile 20-like
kinase 1 expression and its prognostic significance in patients
with breast cancer. Oncology Lett. 14:5457–5463. 2017.
|
|
75
|
Chen B, Le W, Wang Y, Li Z, Wang D, Ren L,
Lin L, Cui S, Hu JJ, Hu Y, et al: Targeting negative surface
charges of cancer cells by multifunctional nanoprobes.
Theranostics. 6:1887–1898. 2018. View Article : Google Scholar
|
|
76
|
Banin Hirata BK, Oda JM, Losi Guembarovski
R, Ariza CB, de Oliveira CE and Watanabe MA: Molecular markers for
breast cancer: Prediction on tumor behavior. Dis Markers.
2014:5131582014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Curtis C, Shah SP, Chin SF, Turashvili G,
Rueda OM, Dunning MJ, Speed D, Lynch AG, Samarajiwa S, Yuan Y, et
al: The genomic and transcriptomic architecture of 2,000 breast
tumours reveals novel subgroups. Nature. 486:346–352. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hah N, Danko CG, Core L, Waterfall JJ,
Siepel A, Lis JT and Kraus WL: A rapid, extensive, and transient
transcriptional response to estrogen signaling in breast cancer
cells. Cell. 145:622–634. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Xiong R, Zhao J, Gutgesell LM, Wang Y, Lee
S, Karumudi B, Zhao H, Lu Y, Tonetti DA and Thatcher GR: Novel
selective estrogen receptor downregulators (SERDs) developed
against treatment-resistant breast cancer. J Med Chem.
60:1325–1342. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Reis-Filho JS and Pusztai L: Gene
expression profiling in breast cancer: Classification,
prognostication, and prediction. Lancet. 378:1812–1823. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Assi HA, Khoury KE, Dbouk H, Khalil LE,
Mouhieddine TH and El Saghir NS: Epidemiology and prognosis of
breast cancer in young women. J Thorac Dis. 5 (Suppl 1):S2–S8.
2013.PubMed/NCBI
|
|
82
|
Hefti MM, Hu R, Knoblauch NW, Collins LC,
Haibe-Kains B, Tamimi RM and Beck AH: Estrogen receptor
negative/progesterone receptor positive breast cancer is not a
reproducible subtype. Breast Cancer Res. 15:R682013. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Cancer Research UK: Breast cancer
incidence statistics. Cancer Res UK. 2012.
|
|
84
|
Ban KA and Godellas CV: Epidemiology of
breast cancer. Surg Oncol Clin N Am. 23:409–422. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Cianfrocca M and Goldstein LJ: Prognostic
and predictive factors in early-stage breast cancer. Oncologist.
9:606–616. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ferlay J, Soerjomataram I, Ervik M,
Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D and
Bray F: GLOBOCAN 2012 v1.0, Cancer incidence and mortality
worldwide: IARC CancerBase. No. 11 [Internet]. Lyon: Fr Int Agency
Res Cancer. 11. http://globocan.iarc.f2013
|
|
87
|
Yoneda A, Lendorf ME, Couchman JR and
Multhaupt HA: Breast and ovarian cancers: A survey and possible
roles for the cell surface heparan sulfate proteoglycans. J
Histochem Cytochem. 60:9–21. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Esteva FJ and Hortobagyi GN: Prognostic
molecular markers in early breast cancer. Breast Cancer Res.
6:109–118. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
89
|
Prat A, Parker JS, Karginova O, Fan C,
Livasy C, Herschkowitz JI, He X and Perou CM: Phenotypic and
molecular characterization of the claudin-low intrinsic subtype of
breast cancer. Breast Cancer Res. 12:R682010. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Payne SJL, Bowen RL, Jones JL and Wells
CA: Predictive markers in breast cancer-the present.
Histopathology. 52:82–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Andrieux G, Fattet L, Le Borgne M, Rimokh
R and Théret N: Dynamic regulation of Tgf-B signaling by Tif1γ: A
computational approach. PLoS One. 7:e337612012. View Article : Google Scholar : PubMed/NCBI
|