Introduction
Sepsis is the leading cause of death for patients in
intensive care units (1). The
pathogenesis of sepsis is generally believed to be caused by severe
infection characterized by an overwhelming immune response. Sepsis
is frequently associated with the dysfunction of vital organs, most
commonly acute lung injury (ALI). ALI is associated with extreme
morbidity and a high mortality rate in critically ill patients
(2–4). The overactivation of inflammatory
signaling pathways, such as mitogen-activated protein kinase (MAPK)
and nuclear factor κB (NF-κB), leading to the excessive release of
inflammatory mediators, including tumor necrosis factor α (TNF-α),
interleukin-6 (IL-6) and high-mobility group box 1 protein (HMGB1),
appear to contribute to organ dysfunction and mortality in sepsis
(5–8). A variety of pharmacologic therapies
have been evaluated, including the neutralization of cytokines and
the activation of anti-inflammatory pathways. Despite decades of
basic and clinical studies, there is no specific therapy available
for this devastating disease. Therefore, the treatment of sepsis
and related organ injury or dysfunction remains largely focused on
supportive care (9,10).
Several lines of evidence have recently demonstrated
that the exacerbated release of pro-inflammatory cytokines can be
controlled by the cholinergic anti-inflammatory pathway via
cholinergic mediators or by electrical stimulation of the vagus
nerve in various experimental models, including lethal endotoxemia,
hemorrhagic shock and ischemia-reperfusion injury (11–13).
α7nAChR is an essential component of the cholinergic
anti-inflammatory pathway (14).
α7nAChR belongs to the family of acetylcholine-gated cation ion
channels formed by five subunits; it exhibits distinct biophysical
and pharmacological effects relative to other nAChR subtypes
(15,16). A previous study has shown that
α7nAChR presents on the reticuloendothelial system that targets
foreign pathogens in the lung, liver, spleen and other organs
(17).
Wang et al (13) found that nicotine decreased the
level of HMGB1 in the serum and improved survival in a murine
endotoxemia model. Although nicotine activates α7nAChR, it also
interacts with α4β2 nAChRs; thus, it is unclear whether α4β2
properties contribute to or detract from the effects of nicotine.
More recently, α7nAChR-selective ligands belonging to diverse
chemotypes have been reported to demonstrate high affinity and
efficiency, including PNU-282987 (18) and A585539 (19). PNU-282987 attenuates sterile
inflammation, including in ischemia/reperfusion-induced brain or
liver injury (20,21) and acid-induced ALI (22). Therefore, the aim of the present
study was to investigate the effects of PNU-282987 on polymicrobial
sepsis-induced ALI and examine its potential mechanism in
LPS-stimulated peritoneal macrophages.
Materials and methods
Animals
Male pathogen-free C57BL/6 mice were obtained from
the Laboratory Animal Research Center of Shanghai (SLAC, Shanghai,
China). Each male pathogen-free C57BL/6 mice (8–12 weeks of age and
weighing approximately 25 g) were raised in cages in an
air-conditioned room (20±1°C) with controlled 12 h light/dark and
maintained on standard laboratory food (Global Diet; Shanghai,
China) and water ad libitum at the Laboratory Animal Center
of Tongji (Shanghai, China). The total number of mice used in
experiments was 40 (8 mice per group).
All animal studies were conducted in accordance with
the National Institute of Health Guidelines on the use of
laboratory animals and approved by the Ethics Committee of the
University of Tongji (23).
Cell culture
Peritoneal macrophages were isolated from C57BL/6
mice as previously described (24). Peritoneal macrophages were treated
with PNU-282987 (20–100 µM, P6499; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany), and agents were added 60 min before the
challenge with lipopolysaccharides (LPS) (10 ng/ml,
Escherichia coli 055:B5; List Biological Laboratories, Inc.,
Campbell, CA, USA).
Experimental design
Male C57BL/6 mice (6–8 weeks old) were randomly
divided (n=8) into the sham group treated with vehicle (group
control), the sham group treated with PNU-282987 (group PNU), the
CLP group treated with vehicle (group CLP), and the group treated
with PNU-282987 (1 mg/kg) administered 1 h before or 2 h after CLP
(group CLP-Pre or CLP-Post). The surgical procedure to generate
CLP-induced sepsis was performed as previously described (24). In brief, mice were anesthetized
with sevoflurane, and a middle abdominal incision was made. The
cecum was mobilized, ligated, and punctured with a 22-gauge needle.
The bowel was repositioned and the abdomen was closed. The animals
were resuscitated with sterile saline subcutaneously immediately
after CLP surgery. The sham-operated control mice underwent the
same procedure, without ligation or puncture of the cecum. All mice
were sacrificed by cervical dislocation at 12 or 24 h after CLP.
Blood samples were collected in tubes containing heparin. BALF was
centrifuged immediately (at 4°C, 800 × g for 10 min) for harvesting
of the cells and the supernatant. The supernatant was used to
measure TNF-α and IL-6, and the deposits were collected for
neutrophil and macrophage counting by Wright-Giemsa staining.
Histopathological changes were examined in right lung tissues. Left
lung tissues were collected for real-time reverse transcriptase
polymerase chain reaction (RT-PCR).
Histopathological examination
For histological analyses, lung tissues were fixed
in 4% paraformaldehyde phosphate-buffered saline (PBS) for 48 h at
room temperature, embedded in paraffin, and sliced into 5-µm-thick
sections using a machine. After deparaffinization, slides were
stained with hematoxylin and eosin (H&E). Morphological
alterations in the lungs were examined by a light microscopy (Leica
DM6000 B, Leica, Wetzlar, Germany) and scored based on the extent
of pathology on a scale of 0 to 4 by measuring interstitial edema,
alveolar edema, hemorrhage and neutrophil infiltration (0, none, 4,
severe). Composite lung injury scores represent the sum of the mean
injury subtype scores for each condition on a scale of 0 to 16. All
histological studies were performed in a blinded fashion.
MTT assay of cell viability
The effect of PNU-282987 on peritoneal viability was
measured using the standard MTT assay as previously described by
Wei et al (25). Briefly,
cells were seeded in 96-well culture plates at a density of 2×104
cells/well and allowed to attach overnight. Cells were washed twice
with PBS and subsequently treated with various concentrations of
PNU-282987 from 0.1 to 1 mM for 24 h. Then, 20 µl of MTT
(Sigma-Aldrich; Merck KGaA) was added to each well and incubated
for 4 h at 37°C. After removing the MTT solution, 200 µl of
dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA) was added to
each well. The absorbance was determined using a Synergy 2 Multiple
ELISA (BioTek Instruments, Inc., Winooski, VT, USA) at a wavelength
of 570 nm.
Enzyme linked immunosorbent assay
Levels of TNF-α and IL-6 were measured using
commercially available ELISA assay kits (Bio-Ray, Laguna Hills, CA,
USA) according to the manufacturer's instructions.
Western blot analysis
To detect the levels of p-P38MAPK (dilution 1:1,000;
cat. no. 4511), p-JNK (dilution 1:800; cat. no. 4668) and p-ERK
(dilution 1:1,200; cat. no. 4370; all from Cell Signaling
Technology, Danvers, MA, USA) in peritoneal macrophages,
immunoblotting was performed as previously described (21). Whole-cell lysates were prepared
using RIPA (Beyotime Institute of Biotechnology, Haimen, China)
containing protease inhibitor cocktail (Roche Diagnostics GmbH,
Mannheim, Germany) and 10 µg/ml phenylmethylsulfonyl
fluoride (PMSF). The protein concentration was
determined by the Bradford method (Bio-Rad Laboratories, Hercules,
CA, USA). Equal amounts (20 µg) of the lysate were boiled for 8 min
in equal volumes of 6X SDS buffer. The protein samples were
subjected to electrophoresis on a 10% sodium dodecyl sulfate
(SDS)-polyacrylamide and transferred to a polyvinylidene difluoride
(PVDF) membrane using a semi-dry transfer apparatus (Bio-Rad
Laboratories). Non-specific binding sites were blocked with 5% skim
milk in PBST (phosphate buffer solution with Tween-20) at room
temperature for 1 h. After washing three times with PBST buffer,
the membrane was incubated with the primary antibody overnight at
4°C. For total proteins, β-actin was used as a loading control. The
blots were washed three times with PBST and incubated with goat
anti-rabbit IRDye 800CW or goat anti-mouse IRDye 800CW-conjugated
secondary antibody (dilution 1:10,000; cat. no. P/N.925-32211 or
926–32210; LI-COR Biosciences, Lincoln, NE, USA) for 1 h at room
temperature. The blots were washed three times with PBST, and the
proteins were visualized using LI-COR Odyssey Infrared Imaging
System (LI-COR Biosciences, Lincoln, NE, USA).
Real-time polymerase chain reaction
analysis
Total RNA was extracted from lung tissue or
peritoneal macrophages by adding TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's instructions. The TNF-α, IL-6 and β-actin mRNA
levels were quantified in triplicate by SYBR Green two-step,
real-time RT-PCR. Total RNA (1 µg) from each sample was used for
reverse transcription with oligo-dT primers (Takara Bio, Inc.,
Otsu, Japan) and SuperScript II Reverse Transcriptase
(Takara Bio, Inc.) to generate the first-strand cDNA. The PCR
mixture was prepared using SYBR-Green PCR Master Mix (Takara) using
the following primers: TNF-α forward, AAGCCTGTAGCCCACGTCGTA and
reverse, AGGTACAACCCATCGGCTGG; IL-6 forward,
CCACTTCACAAGTCGGAGGCTTA and reverse, GCAAGTGCATCATCGTTGTTCATAC;
β-actin forward, GGCTGTATTCCCCTCCATCG and reverse,
CCAGTTGGTAACAATGCCATGT.
The mean fold-changes in the expression of TNF-α and
IL-6 mRNA in the experimental group compared with the control group
were calculated using the 2−ΔΔCq method (26).
Statistical analysis
All experiments were repeated at least three times
with nearly identical results, and the data are presented as means
± SEM. One-way analysis of variance (ANOVA) followed by
Newman-Keuls post hoc test was performed to assess significant
differences. Differences were considered statistically significant
at P<0.05.
Results
PNU-282987 alleviates sepsis-induced
acute lung injury
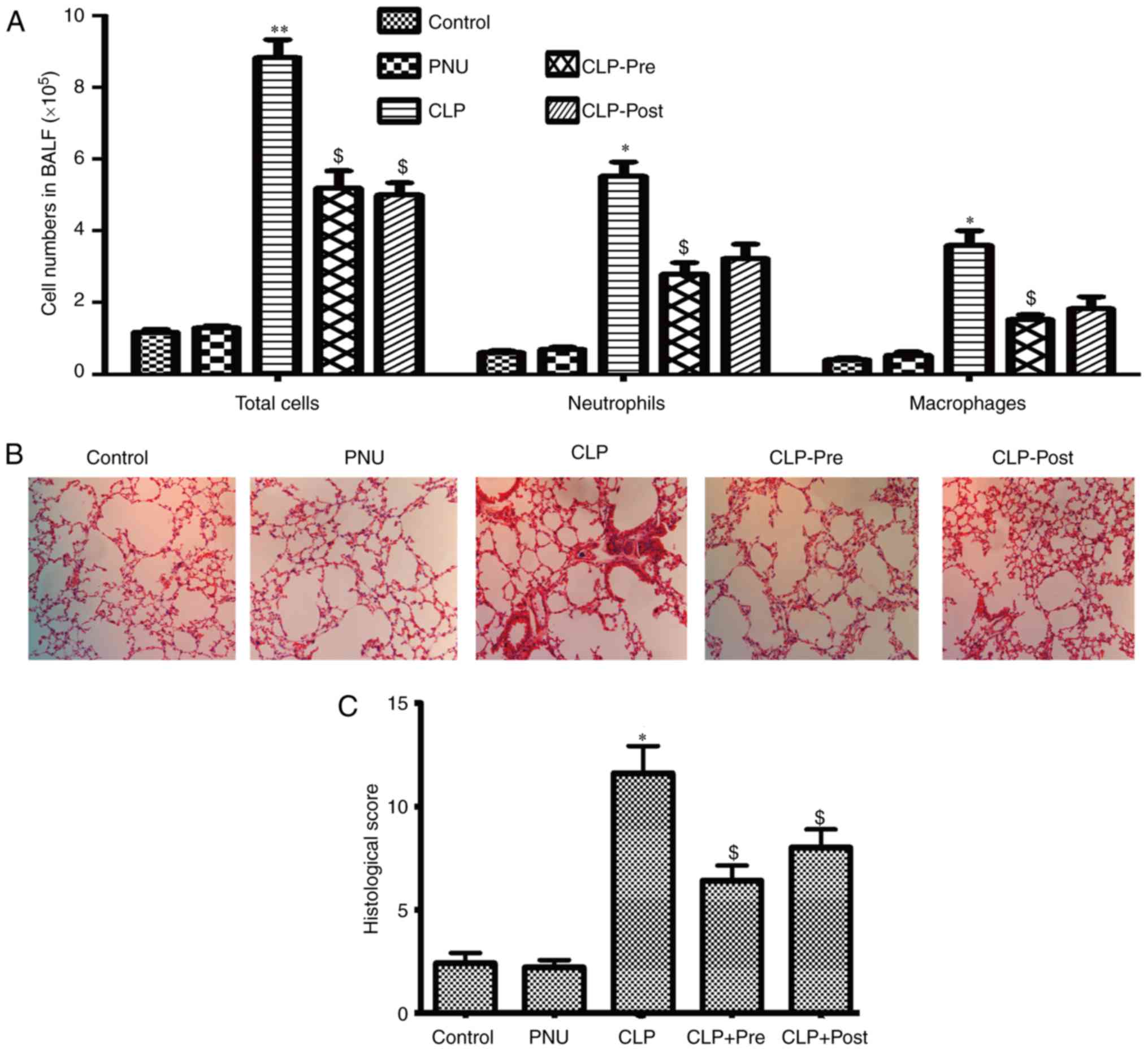
Among the vital organs in the body, the lung is
particularly susceptible to acute injury in CLP-induced
polymicrobial sepsis. Excessive inflammatory cell infiltration
plays a key role in pulmonary failure during sepsis. To determine
whether PNU-282987 could protect mice against sepsis-induced ALI,
we examined the quantity and type of cells in BALF and examined
morphological manifestations in the lung by H&E staining at 24
h after CLP. After the induction of sepsis by CLP, marked
elevations in total cells, neutrophils, and macrophages were
observed compared to the cell counts in the control group. The mice
that received pretreatment with PNU-282987 exhibited decreased
numbers of neutrophils and macrophages in BALF as well as the total
cells when compared to those in the CLP mice. The mice that
received post-treatment with PNU-282987 showed a decreased number
of neutrophils and macrophages but statistical difference was not
achieved (Fig. 1A).
Histopathological analysis of paraffin-embedded lung sections by
H&E staining showed that lung tissues from CLP mice exhibited
severe edema and infiltration of inflammatory cells. In contrast,
inflammatory cell infiltration and edema were obviously attenuated
in the lungs after treatment with PNU-282987 (1 mg/kg) both pre-
and post-surgery in mice (Fig.
1B). We then evaluated the lung injury using histological
sections by applying a semi-quantitative scale (described in detail
in Materials and methods). As shown in Fig. 1C, the total injury score in lungs
after CLP was significantly increased compared to that of the
control group, while the score was significantly reduced when
septic mice received PNU-282987.
PNU-282987 downregulates TNF-α and
IL-6 levels in sepsis
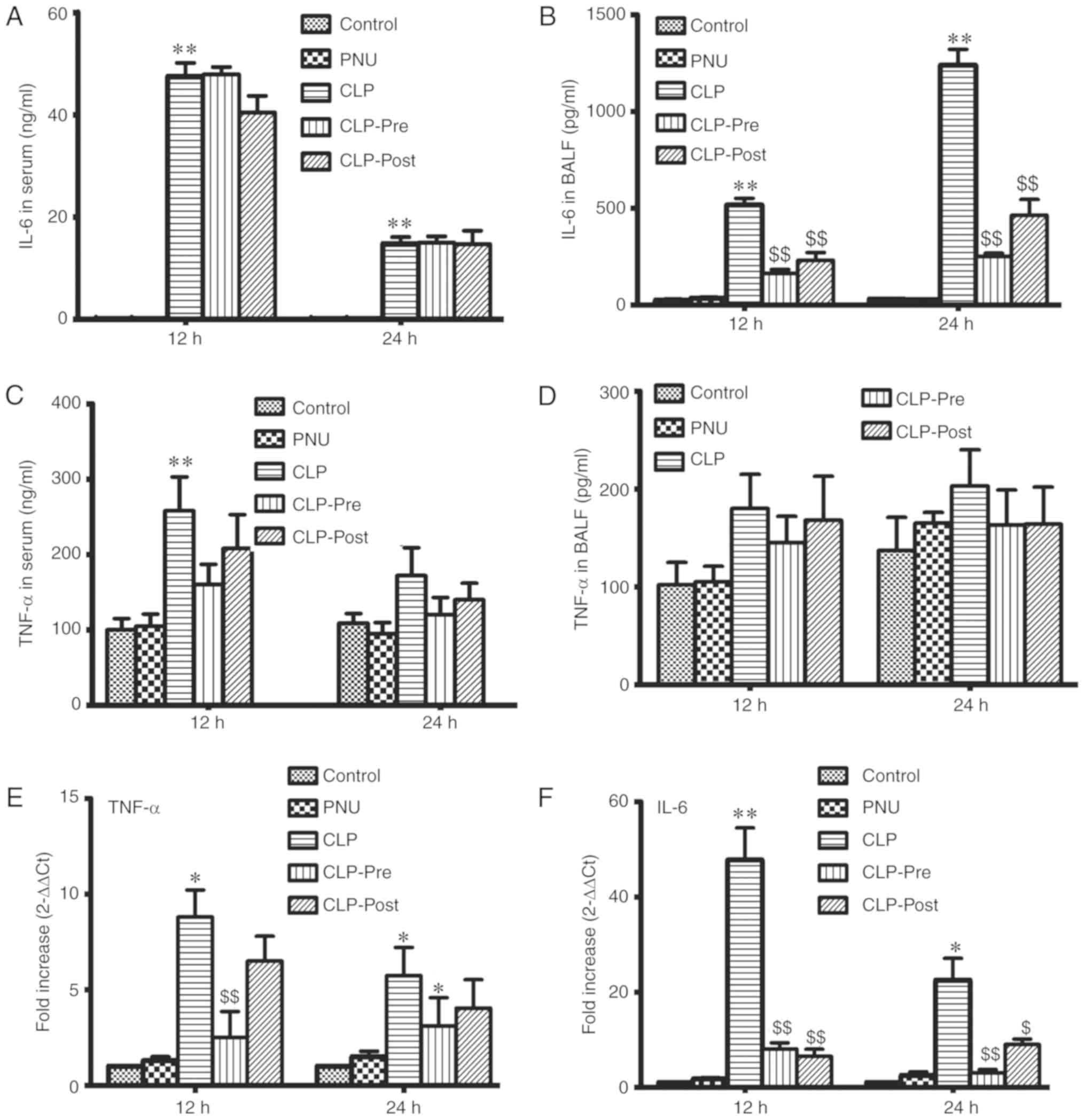
The inflammatory response is induced in sepsis.
Accordingly, we examined the levels of TNF-α and IL-6 in the serum
and BALF of septic mice by ELISA at 12 and 24 h after CLP. The
level of IL-6 was significantly increased in the CLP group compared
to that in the control group. Neither PNU-282987 pre- nor post-CLP
treatment significantly reduced IL-6 release in the serum (Fig. 2A). IL-6 levels in BALF were
significantly decreased in the CLP-pre and CLP-post groups compared
to those in the CLP group at 12 and 24 h (Fig. 2B). We did not observe significant
differences in the TNF-α level among CLP and CLP with PNU-282987
administration groups in serum and BALF (Fig. 2C and D), except for the TNF-α level
in the CLP group compared with the control which was significantly
increased at 12 h. We also examined changes in the mRNA levels of
TNF-α and IL-6 in the lung tissue by RT-PCR. In the lungs of septic
mice, mRNA expression levels of TNF-α and IL-6 were significantly
increased. The increases in the mRNA expression levels of IL-6 and
TNF-α were significantly suppressed by PNU-282987 pretreatment.
Post-treatment with PNU-282987 significantly inhibited IL-6 mRNA
expression, but resulted in only slight decreases in TNF-α
expression in the lung tissue (Fig. 2D
and E). Our results suggest that PNU-282987 treatment inhibits
the local inflammatory response in sepsis.
Effect of PNU-282987 on macrophage
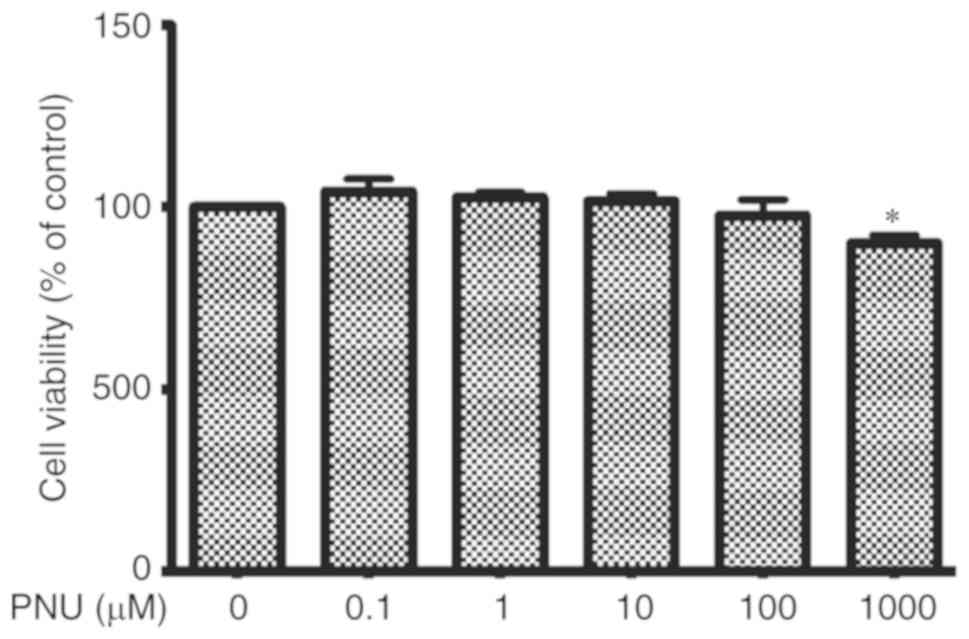
viability
Cell viability, essentially the mitochondrial
activity of living cells, was measured by a quantitative
colorimetric assay with MTT. In vitro, peritoneal
macrophages were treated with PNU-282987 for 24 h and no
significant differences in viability were found at concentrations
ranging from 0.1 to 100 µM when compared with viability in the
control cultures (Fig. 3).
PNU-282987 inhibits LPS-induced TNF-α
and IL-6 release in peritoneal macrophages
To detect the effect of PNU-282987 on LPS-induced
macrophage activation, the macrophage inflammatory cytokine
production (TNF-α and IL-6) was examined after exposure to LPS (10
ng/ml) and PNU-282987 at various concentrations or time-points
in vitro. The levels of TNF-α and IL-6 in the culture
supernatant were significantly decreased by pretreatment with
PNU-282987 in a dose-dependent manner at 8 h (Fig. 4A and B). Pretreatment with
PNU-282987 at 100 µM inhibited LPS-induced TNF-α and IL-6 release
in a time-dependent manner (Fig. 4C
and D). PNU-282987 pretreatment also significantly decreased
TNF-α and IL-6 mRNA expression in macrophages at early times (4 and
8 h) (Fig. 4E and F). PNU-282987
had no inhibitory effect on the production of cytokines in resting
cells.
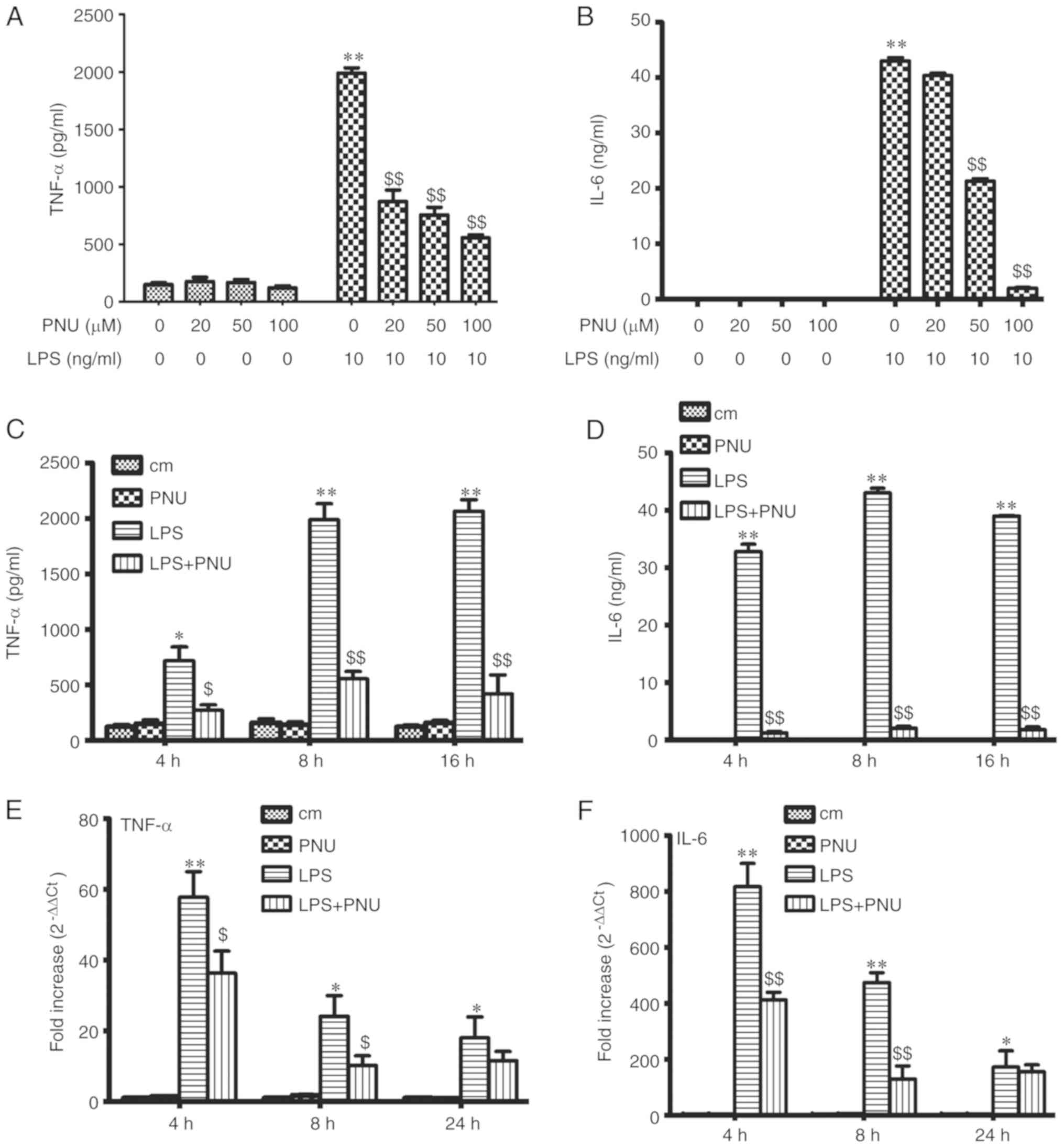 | Figure 4.Effects of PNU-282987 on the
secretion of TNF-α and IL-6 by LPS-stimulated peritoneal
macrophages. (A and B) The peritoneal macrophages were pretreated
with PNU-282987 (20, 50 and 100 µM) for 1 h before LPS (10 ng/ml)
stimulation. The medium was collected at 8 h. (C and D) Peritoneal
macrophages were pretreated with PNU-282987 (100 µM) for 1 h prior
to LPS (10 ng/ml). Medium was collected at 4, 8 and 16 h. Levels of
TNF-α and IL-6 secretion were measured by ELISA. (E and F) The
relative expression levels of TNF-α and IL-6 were measured by
RT-PCR. Data are presented as means ± SEM (n=3). *P<0.05, the
LPS group vs. the control group; **P<0.01, the LPS group vs. the
control group; $P<0.05, the LPS group vs. the LPS+PNU
group; $$P<0.01, the LPS group vs. the LPS+PNU group.
LPS, lipopolysaccharide; TNF-α, tumor necrosis factor α; IL-6,
interleukin-6. cm, control group. |
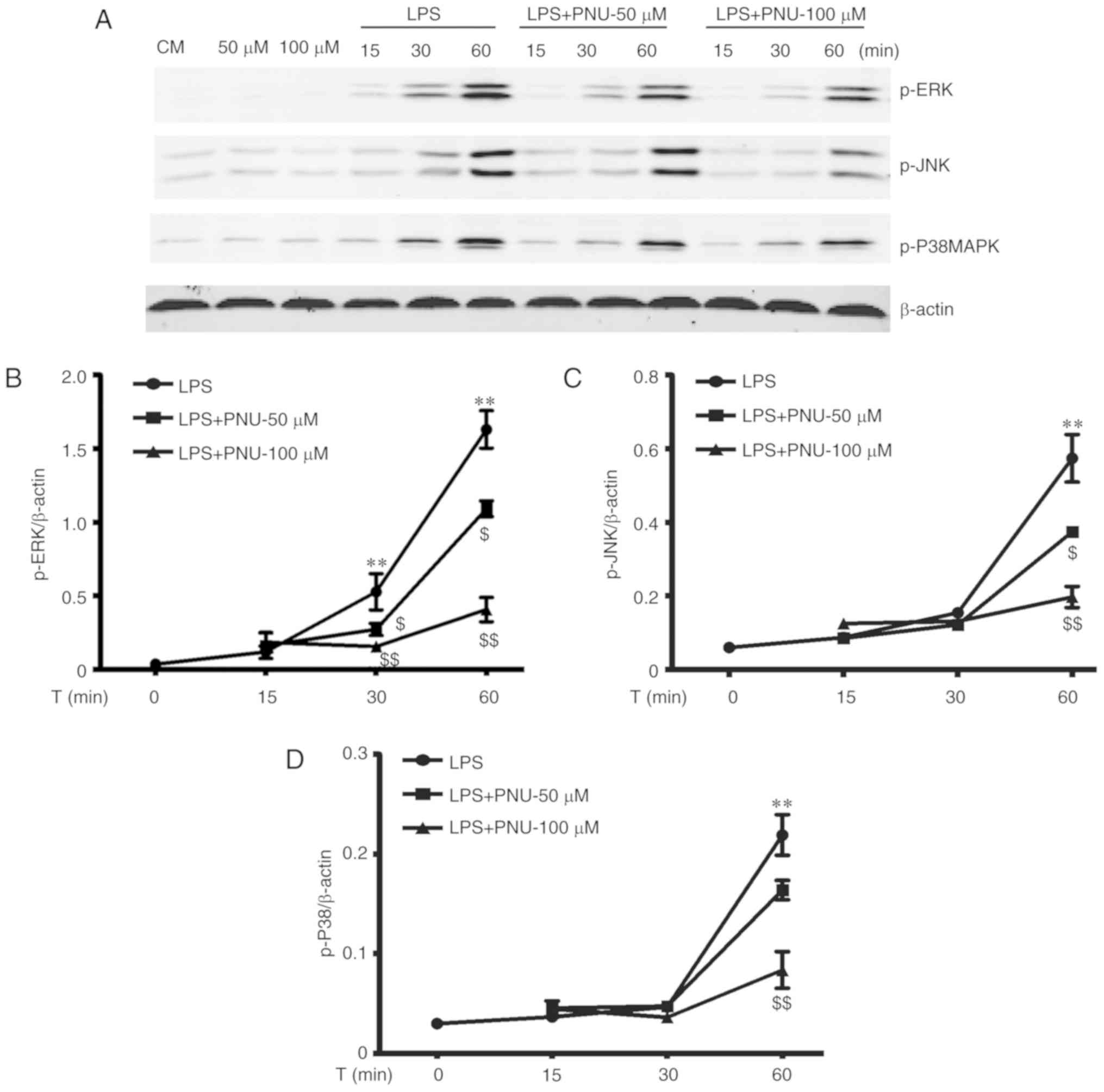
PNU-282987 inhibits LPS-activated MAPK
signaling in macrophages
The MAPK cascade is the key downstream pathway for
LPS-stimulated signaling events (7,27).
To further explore the intracellular mechanisms underlying the
anti-inflammatory effects of PNU-282987, we investigated whether
PNU-282987 could inhibit the LPS-induced activation of MAPK
pathways. The MAPK family involves three major subgroups, including
p38, ERK1/2 and JNK. The activation of p38, ERK1/2 and JNK were
assessed by their phosphorylation levels. LPS strongly activated
all three families of MAPKs in peritoneal macrophages in a
time-dependent manner. PNU-282987 pretreatment significantly
prevented LPS-induced increases in the levels of phosphorylated
p38, ERK1/2 and JNK in a time- and dose-dependent manner (Fig. 5).
Discussion
Sepsis and subsequent multiple organ failure remain
the leading cause of death in critically ill patients in intensive
care units (1,3). Inflammatory mediators are markedly
increased during the early phase. Recently, Huston et al
described a cholinergic anti-inflammatory pathway based on the
structure of the nervous system that restrains the production of
pro-inflammatory cytokines by immune cells (17). α7nAChR plays a key role in the
cholinergic anti-inflammatory pathway. In this study, the effects
of PNU-282987, an α7nAChR-selective agonist, were examined in a
highly clinically relevant mouse model of sepsis induced by cecal
ligation puncture (CLP).
Previously, Wang et al (14) reported that nicotine inhibits
high-mobility group box 1 protein (HMGB1) release induced by either
LPS or TNF-α in human macrophages. They also indicated that
treatment with nicotine attenuated the serum HMGB1 level and
improved survival in experimental models of sepsis. Although
nicotine activates α7nAChR, it also interacts with α4β2 nAChRs;
thus, it is unclear whether the properties of α4β2 contribute to or
detract from the effects of nicotine. Su et al (22) demonstrated that pretreatment with
PNU-282987, a highly specific α7nAChR agonist, attenuated
acid-induced ALI in mice. Different from our model, Pinheiro et
al (28) recently indicated
that PNU-282987 treatment reduced ALI generated by intratracheal
instillation of LPS via changes in the macrophage profile. He et
al (29) found that α7nAChR
activation attenuated intestine ischemia/reperfusion-induced lung
injury in rats. In our previous study, it was found that PNU-282987
pretreatment alleviated ischemia-reperfusion-induced liver injury
in mice (21). In this study, it
was found that PNU-282987 significantly reduced inflammatory cell
infiltration and lung injury, even when treatment was started 2 h
after the onset of CLP. The pro-inflammatory cytokines TNF-α and
IL-6 have been implicated in the pathogenesis of inflammatory lung
injury, particularly under conditions of sepsis (6). It was found that when PNU-282987 was
administered to septic mice, elevated levels of genes encoding
pro-inflammatory cytokines in the lungs and the secretion of IL-6
in BALF decreased substantially, implying that PNU administration
can reduce local inflammation in septic mice.
The anti-inflammatory property of PNU-282987 was
confirmed in vitro using peritoneal macrophages. PNU-282987
pretreatment markedlly inhibited pro-inflammatory cytokine
production in LPS-stimulated peritoneal macrophages. The mechanism
of PNU-282987 was further examined in LPS-stimulated macrophages.
The MAPK pathway is one of the most important signaling cascades
that regulates the LPS-induced inflammatory response (30). MAPK activity results in the
phosphorylation of substrates involved in inflammation.
Acetylcholine represses hypoxia-induced TNF-α production via the
regulation of MAPK phosphorylation in cardiomyocytes (31). According to a recent report,
nicotine suppressed p38, Erk1/2 and JNK MAPK activation induced by
MIA or IL-1β in chondrocytes (32). In the present study, the effects of
PNU-282987 on MAPK signaling were investigated during
LPS-stimulated peritoneal macrophages. As expected, LPS-induced
MAPK phosphorylation was attenuated by pretreatment with PNU-282987
before LPS stimulation, in macrophages in a time- and
dose-dependent manner. These results suggest that PNU-282987
inhibits LPS-induced inflammatory responses partially via the
blockade of the MAPK signaling pathways.
In conclusion, to the best of our knowledge, this is
the first study to investigate the effect of PNU-282987
administration in sepsis-induced lung injury via cecal ligation
puncture. A single dose of PNU-282987 administered by
intraperitoneal injection before or even after CLP inhibited IL-6
release, and this inhibition consequently resulted in the
alleviation of lung injury. Moreover, PNU-282987 inhibited
LPS-induced pro-inflammatory cytokine release, partially via the
blockade of MAPK signaling pathways in peritoneal macrophages.
Overall, these results suggest that PNU-282987 has potential
preventive and therapeutic functions for protection against early
inflammatory responses in sepsis-induced ALI.
Acknowledgements
The authors thank Dr Fuming Shen and Dr Junqi Yang
for help with the reagent preparation.
Funding
The present study was supported in part by grants
from the Science and Technology Program of Shanghai (no.
13411951700 to QL and no. SN81300004 to ZC).
Availability of data and materials
The materials used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
ZS and QL mainly performed the experiments and ZC
performed the statistical analysis. SW helped to complete the
additional experiment and revise the manuscript. ZC designed the
study and wrote this manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved
Ethics approval and consent to
participate
All animal studies were conducted in accordance with
the National Institute of Health Guidelines on the use of
laboratory animals and approved by the Ethics Committee of the
University of Tongji.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CLP
|
cecal ligation puncture
|
|
BALF
|
bronchoalveolar lavage fluid
|
|
ALI
|
acute lung injury
|
|
MAPK
|
mitogen-activated protein kinase
|
|
NF-κB
|
nuclear factor-κB
|
|
TNF-α
|
tumor necrosis factor α
|
|
IL-6
|
interleukin-6
|
|
PBS
|
phosphate- buffered saline
|
References
|
1
|
Winters BD, Eberlein M, Leung J, Needham
DM, Pronovost PJ and Sevransky JE: Long-term mortality and quality
of life in sepsis: A systematic review. Crit Care Med.
38:1276–1283. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ani C, Farshidpanah S, Bellinghausen
Stewart A and Nguyen HB: Variations in organism-specific severe
sepsis mortality in the United States: 1999–2008. Crit Care Med.
43:65–77. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Angus DC and Wax RS: Epidemiology of
sepsis: An update. Crit Care Med. 29 (7 Suppl):S109–S116. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seymour CW, Liu VX, Iwashyna TJ,
Brunkhorst FM, Rea TD, Scherag A, Rubenfeld G, Kahn JM,
Shankar-Hari M, Singer M, et al: Assessment of clinical criteria
for sepsis: For the third international consensus definitions for
sepsis and septic shock (Sepsis-3). JAMA. 315:762–774. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nathan C: Points of control in
inflammation. Nature. 420:846–852. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ulloa L and Tracey KJ: The ‘cytokine
profile’: A code for sepsis. Trends Mol Med. 11:56–63. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schuh K and Pahl A: Inhibition of the MAP
kinase ERK protects from lipopolysaccharide-induced lung injury.
Biochem Pharmacol. 77:1827–1834. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Everhart MB, Han W, Sherrill TP, Arutiunov
M, Polosukhin VV, Burke JR, Sadikot RT, Christman JW, Yull FE and
Blackwell TS: Duration and intensity of NF-kappaB activity
determine the severity of endotoxin-induced acute lung injury. J
Immunol. 176:4995–5005. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Toussaint S and Gerlach H: Activated
protein C for sepsis. N Engl J Med. 361:2646–2652. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deutschman CS and Tracey KJ: Sepsis:
Current dogma and new perspectives. Immunity. 40:463–475. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tracey KJ: Physiology and immunology of
the cholinergic antiinflammatory pathway. J Clin Invest.
117:289–296. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ulloa L: The vagus nerve and the nicotinic
anti-inflammatory pathway. Nat Rev Drug Discov. 4:673–684. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H, Liao H, Ochani M, Justiniani M,
Lin X, Yang L, Al-Abed Y, Wang H, Metz C, Miller EJ, et al:
Cholinergic agonists inhibit HMGB1 release and improve survival in
experimental sepsis. Nat Med. 10:1216–1221. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang H, Yu M, Ochani M, Amella CA, Tanovic
M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, et al:
Nicotinicacetylcholine receptor7 subunit is an essential regulator
of inflammation. Nature. 421:384–388. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leonard S and Bertrand D: Neuronal
nicotinic receptors: From structure to function. Nicotine Tob Res.
3:203–223. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kalamida D, Poulas K, Avramopoulou V,
Fostieri E, Lagoumintzis G, Lazaridis K, Sideri A, Zouridakis M and
Tzartos SJ: Muscle and neuronal nicotinic acetylcholine receptors.
Structure, function and pathogenicity. FEBS J. 274:3799–3845. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huston JM, Ochani M, Rosas-Ballina M, Liao
H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ,
Foxwell B, et al: Splenectomy inactivates the cholinergic
antiinflammatory pathway during lethal endotoxemia and
polymicrobial sepsis. J Exp Med. 203:1623–1628. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bodnar AL, Cortes-Burgos LA, Cook KK, Dinh
DM, Groppi VE, Hajos M, Higdon NR, Hoffmann WE, Hurst RS, Myers JK,
et al: Discovery and structure-activity relationship of
quinuclidine benzamides as agonists of alpha7 nicotinic
acetylcholine receptors. J Med Chem. 48:905–908. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li J, Mathieu SL, Harris R, Ji J, Anderson
DJ, Malysz J, Bunnelle WH, Waring JF, Marsh KC, Murtaza A, et al:
Role of α7 nicotinic acetylcholine receptors in regulating tumor
necrosis factor-α (TNF-α) as revealed by subtype selective
agonists. J Neuroimmunol. 239:37–43. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duris K, Manaenko A, Suzuki H, Rolland WB,
Krafft PR and Zhang JH: α7 nicotinic acetylcholine receptor agonist
PNU-282987 attenuates early brain injury in a perforation model of
subarachnoid hemorrhage in rats. Stroke. 42:3530–3536. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li F, Chen Z, Pan Q, Fu S, Lin F, Ren H,
Han H, Billiar TR, Sun F and Li Q: The protective effect of
PNU-282987, a selective α7 nicotinic acetylcholine receptor
agonist, on the hepatic ischemia-reperfusion injury is associated
with the inhibition of high-mobility group box1 protein expression
and nuclear factor κB activation in mice. Shock. 39:197–203.
2013.PubMed/NCBI
|
|
22
|
Su X, Lee JW, Matthay ZA, Mednick G,
Uchida T, Fang X, Gupta N and Matthay MA: Activation of the alpha7
nAChR reduces acid-induced acute lung injury in mice and rats. Am J
Respir Cell Mol Biol. 37:186–192. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ding X, Wang X, Zhao X, Jin S, Tong Y, Ren
H, Chen Z and Li Q: RGD peptides protects against acute lung injury
in septic mice through Wisp1-integrin β6 pathway inhibition. Shock.
43:352–360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Z, Ding X, Jin S, Pitt B, Zhang L,
Billiar T and Li Q: WISP1-αvβ3 integrin signaling positively
regulates TLR-triggered inflammation response in sepsis induced
lung injury. Sci Rep. 6:288412016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei W, Dejie L, Xiaojing S, Tiancheng W,
Yongguo C, Zhengtao Y and Naisheng Z: Magnolol inhibits the
inflammatory response in mouse mammary epithelial cells and a mouse
mastitis model. Inflammation. 38:16–26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaminska B: MAPK signalling pathways as
molecular targets for anti-inflammatory therapy-from molecular
mechanisms to therapeutic benefits. Biochim Biophys Acta.
1754:253–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pinheiro NM, Santana FP, Almeida RR,
Guerreiro M, Martins MA, Caperuto LC, Câmara NO, Wensing LA, Prado
VF, Tibério IF, et al: Acute lung injury is reduced by the α7nAChR
agonist PNU-282987 through changes in the macrophage profile. FASEB
J. 31:320–322. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He Y, Ye ZQ, Li X, Zhu GS, Liu Y, Yao WF
and Luo GJ: Alpha7 nicotinic acetylcholine receptor activation
attenuated intestine-derived acute lung injury. J Surg Res.
201:258–265. 2106. View Article : Google Scholar
|
|
30
|
Bode JG, Ehlting C and Häussinger D: The
macrophage response towards LPS and its control through the
p38(MAPK)-STAT3 axis. Cell Signal. 24:1185–1194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Borovikova LV, Ivanova S, Zhang M, Yang H,
Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW and Tracey
KJ: Vagus nerve stimulation attenuates the systemic inflammatory
Response to endotoxin. Nature. 405:458–462. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li DL, Liu JJ, Liu BH, Hu H, Sun L, Miao
Y, Xu HF, Yu XJ, Ma X, Ren J and Zang WJ: Acetylcholine inhibits
hypoxia-induced tumor necrosis factor-α production via regulation
of MAPKs phosphorylation in cardiomyocytes. J Cell Physiol.
226:1052–1059. 2011. View Article : Google Scholar : PubMed/NCBI
|