Introduction
Oral squamous cell carcinoma (OSCC) is among the 10
most common cancer types worldwide and represents ~90% of malignant
tumours in the oral cavity (1).
Although considerable advances in oral cancer detection, prevention
and treatment have been made over the past 20 years, the 5-year
survival rate of OSCC remains ~50% (2,3).
OSCC treatment failures are primarily caused by local/regional
tumour recurrence, and postoperative tumour recurrence leads to
poor prognosis and poor quality of life. An improved understanding
of the mechanism of local recurrence may lead to the development of
new diagnostic and prognostic criteria and novel therapeutic
targets.
According to the World Health Organization (WHO)
lineage of origin classification, the transcription factor forkhead
box (FOX) protein M1 (FOXM1) belongs to the FOX protein family
(4,5). FOXM1 serves important roles in cell
cycle progression and mitosis during embryonic development and in
adult tissue homeostasis, and recent studies have indicated that
FOXM1 serves a pivotal role in the development and evolution of a
number of human cancer types (6–9).
FOXM1 is not only a key cell cycle regulator, but is also critical
to tumorigenesis, tumour aggressiveness and metastasis (4,10–12).
The upregulation of FOXM1 has been identified in cancer, including
lung cancer, basal cell carcinoma, pancreatic cancer, and head and
neck squamous cell carcinoma (HNSCC) (13,14).
Notably, in breast cancer, gastric cancer and laryngeal squamous
cell carcinoma, high expression of FOXM1 is associated with poor
prognosis (15–17). Although FOXM1 is markedly
upregulated in HNSCC tissues and cultured cells, the association of
FOXM1 expression with OSCC prognosis has yet to be fully
elucidated.
The present study examined the expression of FOXM1
in 119 primary OSCCs by immunohistochemistry (IHC) and analysed its
potential associations with clinical and pathological
characteristics.
Patients and methods
Patients and specimens
All 119 patients with stage I–IV OSCC included in
the present study were initially diagnosed and treated at the
Department of Oral and Maxillofacial Surgery, Affiliated
Stomatological Hospital of Nanjing Medical University (Nanjing,
China), between January 2005 and December 2008. None of the
patients received correlative treatment prior to the first
pathological examination. All patients were diagnosed as SACC
according to clinical and pathological examination and underwent
radical resection without any previous radiation or chemotherapy.
The tumours were diagnosed based on the International Union Against
Cancer tumour size, nodal metastasis and distant metastasis (TNM)
classification (18). The
histological results were confirmed based on the WHO definitions of
the tumour types. Following diagnosis, all the patients underwent
radical surgery. To evaluate postoperative conditions, the patients
were re-examined every 3 months for the first 2 years and every 6
months for the next 3 years. The present study was performed with
permission from the ethical committee of the Affiliated
Stomatological Hospital of Nanjing Medical University. Informed
consent was obtained from each patient. The follow-up period was
3–81 months (mean, 37.3 months). Local recurrence following surgery
was confirmed by pathological biopsy.
Assessment of FOXM1 expression in OSCC
by IHC
The tissues were fixed in formalin overnight at 4°C,
embedded in paraffin and cut into 4-µm sections. The sections were
deparaffinized through a graded alcohol series and incubated in 3%
H2O2 at room temperature for 20 min to block
endogenous peroxidases. The sections were microwaved in citrate
buffer (pH 6.0) for 20 min for antigen retrieval and subsequently
incubated with 1% bovine serum albumin (BSA; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) in phosphate-buffered saline (PBS) for 1
h at room temperature to block nonspecific binding. The sections
were incubated overnight at 4°C with anti-FOXM1 (rabbit polyclonal
antibody; 1:500; ab55006; Abcam, Cambridge, UK) and rinsed a number
of times with PBS, and sections incubated with 1% BSA were used as
negative controls. Following sequential incubation with the
corresponding anti-rabbit secondary antibodies (1:200; ab207298;
Abcam) for 30 min at 37°C and avidin-biotin-peroxidase reagent for
30 min, the signals were visualized using a diaminobenzidine
staining kit (DAKOCytomation; Agilent Technologies, Inc., Santa
Clara, CA, USA) for 3 min at room temperature. The OSCC tissue
sections were subsequently counterstained with 0.5% haematoxylin
for 5 min at room temperature.
The immunoreactivity of FOXM1 was assessed based on
the cytoplasmic and nuclear staining. The staining intensity was
categorized as follows: 0, negative; 1, weak; 2, moderate; and 3,
intense. A score based on the proportion of cells with positive
expression was obtained according to the following criteria: 0, 0;
1, 1–10; 2, 11–50; and 3, >50%. The staining intensity was
multiplied by the proportion scores to obtain a comprehensive
score. Comprehensive scores exceeding 5 represented high FOXM1
expression, whereas other scores indicated low FOXM1 expression.
The scores of the different cases were analysed independently by
two pathologists who had no knowledge of the clinical data. In
almost all cases, the differences between the scores of each
pathologist were <10%.
Cell culture
The human OSCC cell lines HN4, HN6, HN12, Tca-811
and SCC-22B (generously provided by Shanghai Ninth People's
Hospital, Shanghai, China), which had been used in previous study
(19–23). HN4, HN6, HN12, Tca-811 and SCC-22B
and Cal-27 (purchased from American Type Culture Collection,
Manassas, VA, USA) were incubated in Dulbecco's modified Eagle's
medium (DMEM, Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) containing 10% foetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in a 5% CO2 incubator.
Small interfering RNA and
transfection
The FOXM1 small-interfering RNA (siRNA) sequence was
designed according to a previous publication (24): 5-GGACCACUUUUCCCUACUUUDTDT-3 (sense)
and 5-AAAGUAGGGAAAGUGGUCCDTDT-3 (antisense). The sequences of the
scrambled siRNA were 5-UUCUCCGAACGUGUCACGUDTDT-3 (sense) and
5-ACGUGACACGUUCGGAGAADTDT-3 (antisense). RNA oligonucleotides were
purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China). 3
µl Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used for the transfection of the 100 pmol
siRNAs per six-well plate in accordance with the manufacturer's
protocol.
Cell proliferation assay
Briefly, the cells were transiently transfected with
scrambled siRNA and FOXM1 siRNA and, 24 h later, the cells were
plated in 96-well plates (5×103 cells/well) and
incubated for 72 h at 37°C. The cells were fixed with 50 µl of 50%
pre-cooled trichloroacetic acid per well at 4°C for 1 h and washed
five times with PBS. The cells were stained with 100 µl of 0.4%
sulforhodamine B (SRB) solution (Sigma-Aldrich; Merck KGaA) per
well for 15 min at room temperature and washed five times with 1%
acetic acid. Protein-bound SRB was mixed with 150 µl of 10 mmol/l
Tris-based solution and agitated for 5 min. The optical densities
at 560 nm of the samples in six parallel wells were measured using
a multi-well spectrophotometer (VERSAmax, Molecular Devices, LLC,
Sunnyvale, CA, USA).
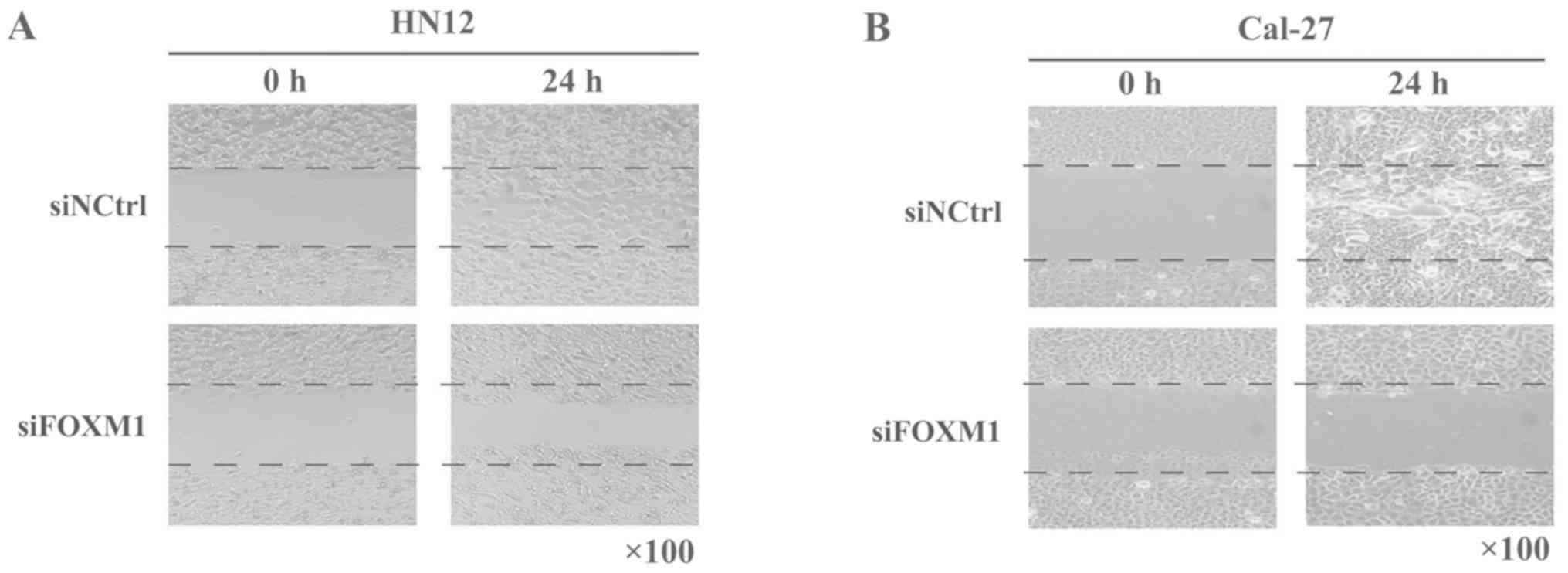
Wound healing assay
OSCC cells were plated in a six-well plate and FOXM1
was knocked down. After 24 h, the cells were seeded in 24-well
plates in 10% serum-containing media, incubated until they reached
100% confluence, serum-starved for 1 day and wounded with a 200-µl
plastic tip. The cells were washed three times with sterile PBS to
remove the floating cells and incubated for 24 h. The same wound
areas were observed and imaged under a light microscope
(magnification, ×200; Leica Microsystems GmbH, Wetzlar, Germany) at
0 and 24 h.
Cell migration assay
OSCC cells were plated in a six-well plate and FOXM1
was knocked down. After 24 h, cell suspensions were prepared and
counted. Subsequently, 5×104 cells in 100 µl of
serum-free media were seeded in each upper chamber of the Transwell
inserts (10-µm thickness and 8-µm pore size), and 600 µl of DMEM
with 10% FBS was added to each lower chamber. The cells were
cultured in 24-well Transwell chambers for 12 and 72 h at 37°C in
an atmosphere of 5% CO2, and the cells from the upper
chamber were gently removed. The cells that had invaded the lower
chamber were fixed with 5% glutaraldehyde 30 min at room
temperature and stained with 0.1% crystal violet for 3 min at room
temperature. The stained cells were observed under an inverted
microscope (magnification, ×200) and repeatedly counted three
times.
Western blot analysis
OSCC cells were plated in six-well plates and FOXM1
was knocked down. The cells were collected, suspended in lysis
buffer (50 mmol/l Tris-HCl, pH 6.8, 100 mmol/l DTT, 2% SDS, 0.1%
bromophenol blue and 10% glycerol) on ice for 5–10 min and
centrifuged at 4°C (12,000 × g, 15 min). The protein concentrations
were determined using a bicinchoninic acid protein assay. The 50 µg
protein samples per lane were separated by 10% SDS-PAGE and
transferred onto nitrocellulose filter membranes. The membranes
were blocked in freshly prepared PBS containing 5% non-fat dried
milk for 2 h at room temperature. The blots were subsequently
probed with primary antibodies specific for FOXM1, E-cadherin,
vimentin and β-actin (1:1,000; ab55006, ab1416, ab45939 and ab8226
respectively; Abcam,) overnight at 4°C. The membranes were washed
three times with PBS-0.05% Tween 20 and incubated with the
corresponding secondary antibodies (1:1,000; ab6940; Abcam) for 1 h
at room temperature. The blots were subsequently incubated in the
dark for enhanced chemiluminescence and visualized through exposure
to ECL reagents (GE Healthcare, Chicago, IL, USA).
Statistical analysis
Data were tabulated, and statistical analyses were
performed using SPSS 22.0 statistical software (IBM Corp., Armonk,
NY, USA). The statistical significance between all comparisons of
clinicopathological features was examined using χ2
analysis. The time from the first diagnosis date to the date of
mortality or the last day of the follow-up assessment was plotted
as the overall survival time. The period from therapy to local
recurrence or metastasis was considered the period of disease-free
survival. The survival rate curves were analysed using the
Kaplan-Meier method, and the log-rank test was used to evaluate the
significance of the differences. The significance of variables for
survival rates was analysed using the Cox proportional hazards
model in univariate and multivariate analyses. The data from the
in vitro studies were assessed through unpaired Student's
t-tests. Each assay was conducted in triplicate and repeated three
times. All the cases were two-sided and presented as the mean ±
standard deviation, and P<0.05 was considered to indicate a
statistically significant difference.
Results
FOXM1 expression in OSCC and
clinicopathological features
To study the association between FOXM1 expression
and clinicopathological characteristics, the expression of FOXM1 in
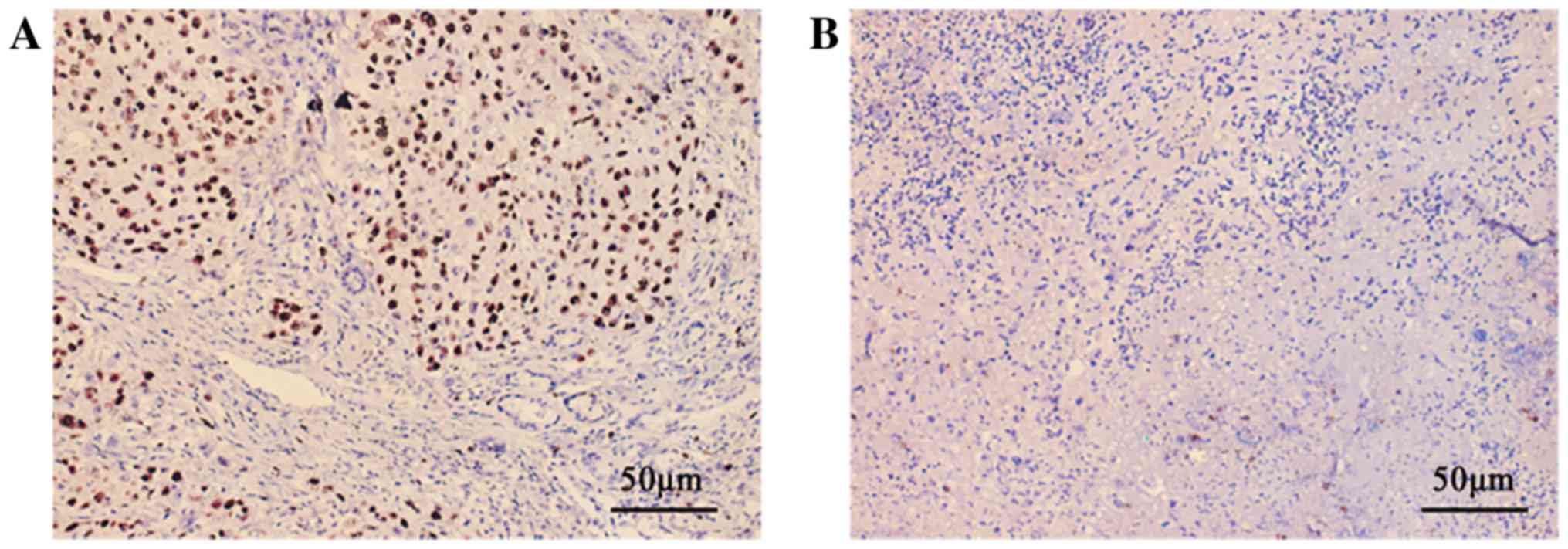
119 primary OSCC samples was assessed by IHC. All positive cases
demonstrated clear nuclear localization of FOXM1 protein (Fig. 1). Among the 119 tumours analysed,
63 (52.94%) demonstrated high FOXM1 expression and the others
exhibited low expression. Collectively, the levels of FOXM1
expression and their association with clinicopathological
characteristics are summarized in Table I. Among the 119 patients included
in the present study, 28 (23.7%) experienced local recurrence
following surgery, and 20 of the 28 (71.4%) patients who
experienced local recurrence exhibited high FOXM1 expression. Among
the 91 patients who did not experience recurrence, 47.25% (43/91)
had high expression levels of FOXM1. Statistically, high expression
of FOXM1 was significantly associated with recurrence following
surgery (P=0.025; n=119). No significant association was identified
between the FOXM1 protein level and other clinicopathological
characteristics, including age at diagnosis, sex, tumour size,
clinical TNM classification, pathological grade, cancer location,
or lymph and distant metastasis.
 | Table I.Association between FOXM1 and patient
characteristics. |
Table I.
Association between FOXM1 and patient
characteristics.
| Characteristic | Low FOXM1
expression (n=56) | High FOXM1
expression (n=63) | χ2 | P-value |
|---|
| Age at
diagnosis |
|
| 2.489 | 0.115 |
| <60
years | 33 | 28 |
|
|
| ≥60
years | 23 | 35 |
|
|
| Sex |
|
| 0.047 | 0.828 |
|
Female | 26 | 28 |
|
|
|
Male | 30 | 35 |
|
|
| Tumour size |
|
| 1.739 | 0.419 |
| <2
cm | 10 | 7 |
|
|
| >2
cm, <4 cm | 33 | 36 |
|
|
| >4
cm | 13 | 20 |
|
|
| TNM
classification |
|
| 0.988 | 0.320 |
| I or
II | 30 | 28 |
|
| III or
IV | 26 | 35 |
|
|
| Lymphatic
metastasis |
|
| 1.303 | 0.254 |
|
With | 20 | 29 |
|
|
|
Without | 36 | 34 |
|
|
| Distant
metastasis |
|
| 1.905 | 0.168 |
|
With | 3 | 8 |
|
|
|
Without | 53 | 55 |
|
|
| Local
recurrence |
|
| 5.023 | 0.025 |
|
With | 8 | 20 |
|
|
|
Without | 48 | 43 |
|
|
| Histological
grading |
|
| 0.930 | 0.628 |
| Grade
I | 33 | 37 |
|
|
| Grade
II | 18 | 23 |
|
|
| Grade
III | 5 | 3 |
|
|
| OSCC location |
|
| 1.895 | 0.595 |
|
Tongue | 18 | 27 |
|
|
|
Buccal | 16 | 14 |
|
|
|
Gingiva | 11 | 13 |
|
|
| Other
(e.g., hard palate or mouth floor) | 11 | 9 |
|
|
FOXM1 expression and survival
rates
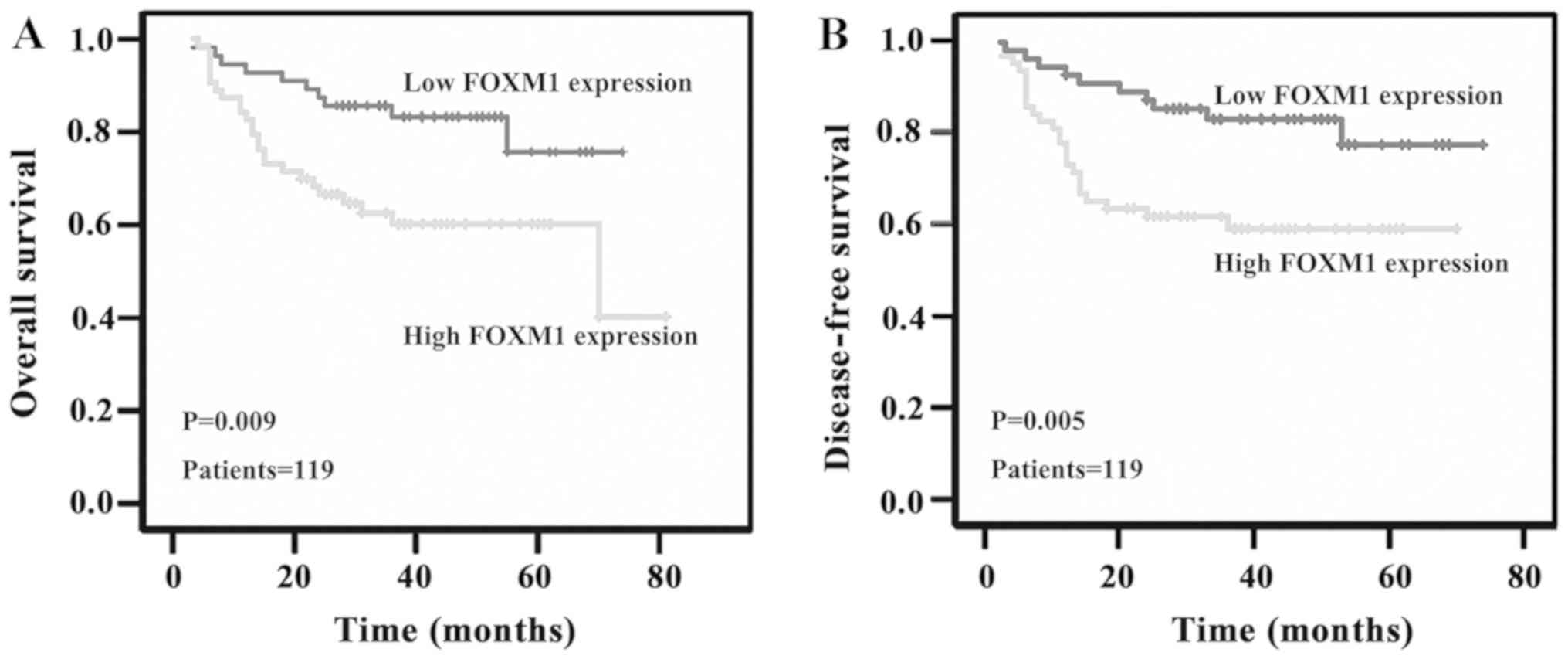
To determine the association between FOXM1 protein
expression and prognostic markers in patients with OSCC, the
overall and disease-free survival rates of patients with OSCC were
analysed using the log-rank test, and the trends were assessed
using the Kaplan-Meier method (Fig.
2). The survival rates of patients with OSCC following radical
surgery were computed. During a median follow-up time of 37.3
months (range, 3–81 months), 28 patients (23.53%) succumbed to
their diseases. Of these 28 patients who succumbed to OSCC, three
patients (10.7%) demonstrated low FOXM1 protein levels, whereas 25
patients (89.3%) had high FOXM1 protein levels. The survival rate
analysis demonstrated that FOXM1 expression was significantly
associated with the overall (log-rank, P=0.009) and disease-free
survival rates of patients with OSCC (log-rank, P=0.005).
Next, univariate survival rate analysis was
performed to evaluate the independent prognostic factors of tumour
recurrence, including TNM classification (P=0.033), lymphatic
metastasis (P=0.028), distant metastasis (P=0.002) and FOXM1
expression (P=0.027; Table II).
Multivariate survival rate analysis was performed to evaluate the
independent prognostic factors of tumour recurrence, and distant
metastasis and FOXM1 were identified to be independent prognosis
factors of tumour recurrence in patients with OSCC (Table III).
 | Table II.Summary of survival analysis by
univariate Cox regression analysis. |
Table II.
Summary of survival analysis by
univariate Cox regression analysis.
|
|
| Disease-free
survival overall |
|---|
|
|
|
|
|---|
| Parameter
category | Subcategory | P-value | Exp(B) | 95% CI |
|---|
| TNM
classification | I–II vs.
III–IV | 0.033 | 2.397 | 1.075–5.344 |
| Lymphatic
metastasis | with vs.
without | 0.028 | 2.342 | 1.094–5.013 |
| Distant
metastasis | with vs.
without | 0.002 | 4.181 | 1.671–10.462 |
| FOXM1
expression | Low vs. high | 0.027 | 2.788 | 1.121–6.937 |
 | Table III.Summary of survival analysis by
multivariate Cox regression analysis. |
Table III.
Summary of survival analysis by
multivariate Cox regression analysis.
|
|
| Disease-free
survival overall |
|---|
|
|
|
|
|---|
| Parameter
category | Subcategory | P-value | Exp(B) | 95% CI |
|---|
| TNM
classification | I–II vs.
III–IV | 0.532 | 1.521 | 0.409–5.657 |
| Lymphatic
metastasis | with vs.
without | 0.661 | 1.337 | 0.365–4.898 |
| Distant
metastasis | with vs.
without | 0.025 | 3.174 | 1.154–8.735 |
| FOXM1
expression | Low vs. high | 0.030 | 2.767 | 1.106–6.924 |
Knockdown of FOXM1 expression by siRNA
decreases the proliferation and migration of OSCC cells
To assess whether FOXM1 enhances the malignant
phenotypes of OSCC cells, the HN12 and Cal-27 cell lines were
selected as the cell models in our preliminary experiments because
they highly expressed FOXM1 among certain other cell lines,
including HN4, HN6, Tca-811 and SCC-22B (data not shown). FOXM1
siRNA was transiently transfected into two OSCC-derived cell lines,
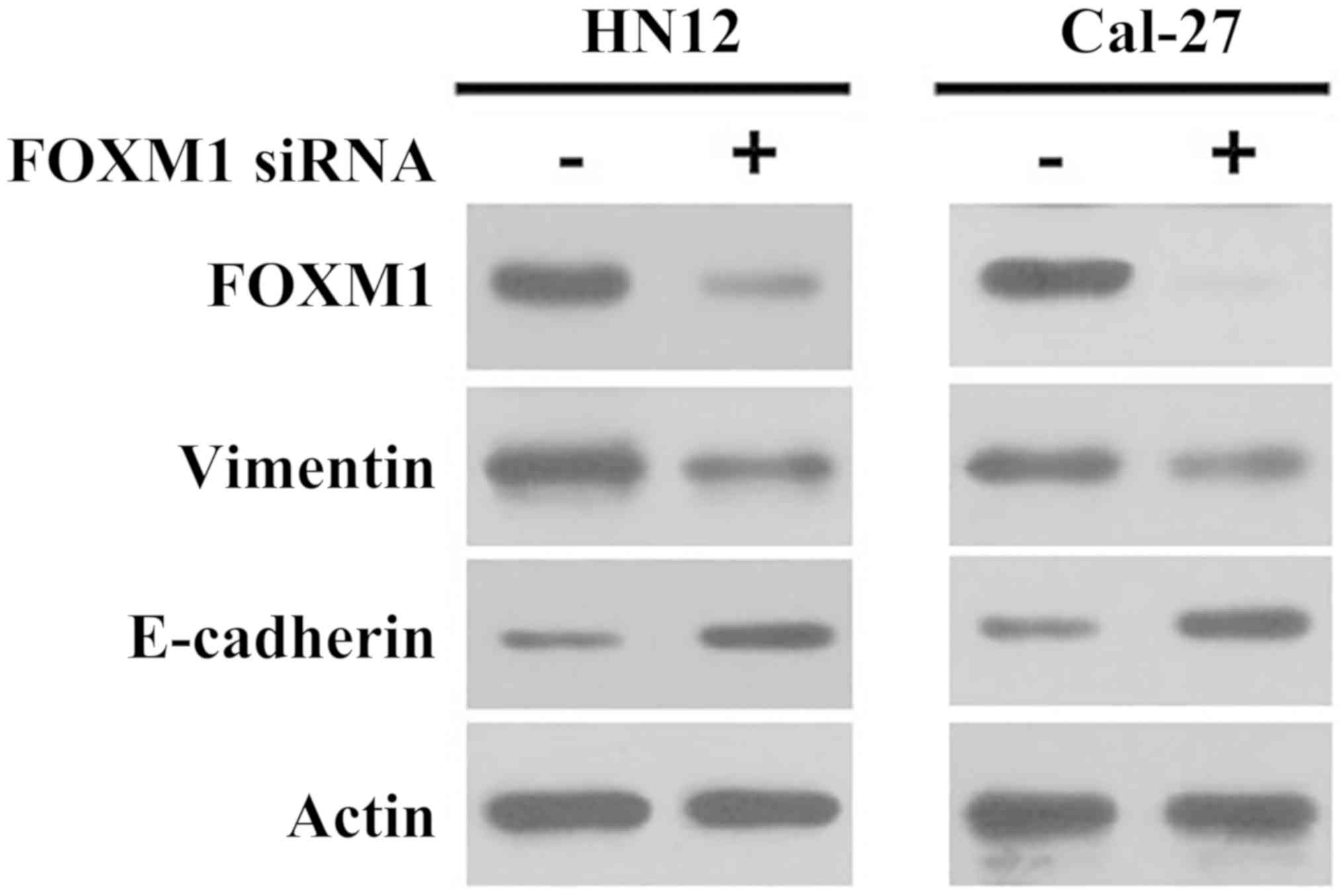
HN12 and Cal-27, the two of which express high FOXM1 protein
levels, as demonstrated by western blot analysis (Fig. 3). The transfection of FOXM1 siRNA
markedly decreased FOXM1 expression in HN12 and Cal-27 cells
(Fig. 3).
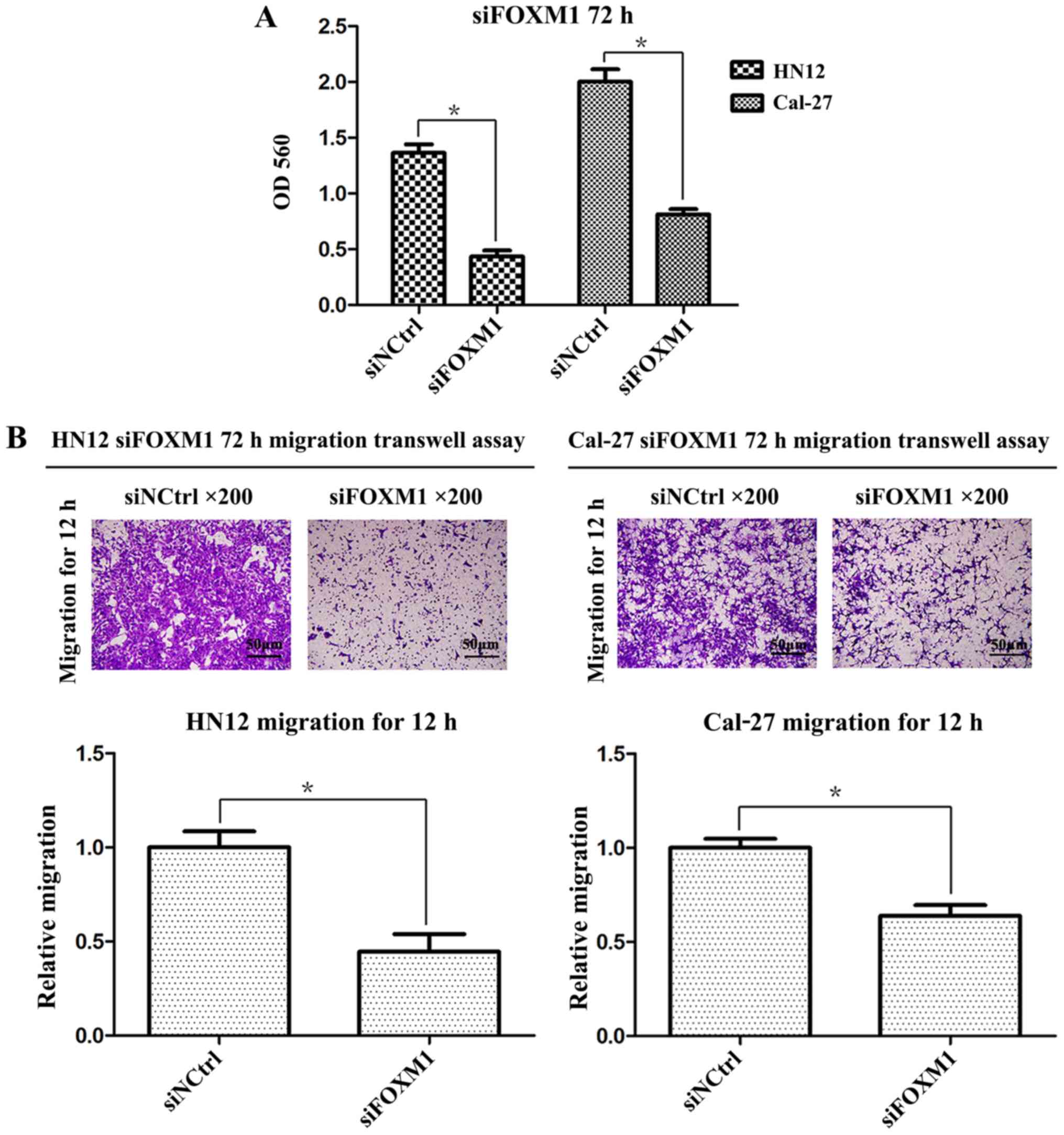
The inhibition level of FOXM1 expression was
associated with the proliferation of OSCC cells. The results of the
present study demonstrated that the silencing of FOXM1
significantly reduced the cellular proliferative capacity compared
with the negative control cells (Fig.
4A). The migratory capacity of OSCC cells following siRNA
transfection was further examined using in vitro migration
and wound-healing assays. As demonstrated in Fig. 4B, following FOXM1 knockdown,
significantly lower numbers of HN12 and Cal-27 cells migrated
through the Transwell membrane (P<0.01, respectively). The wound
healing assay demonstrated that the healing capacity of HN12 and
Cal-27 cells was reduced following transfection with FOXM1 siRNA
(Fig. 5). These data demonstrated
that the inhibition of FOXM1 led to decreased cancer cell
proliferation and migration.
FOXM1 inhibition alters the expression
of proteins involved in epithelial-mesenchymal transition (EMT) in
OSCC cells
To better understand the molecular mechanisms
through which FOXM1 contributes to cell migration, the expression
levels of E-cadherin and vimentin protein in FOXM1-knockdown HN12
and Cal-27 cells were determined by western blot analysis. The
knockdown of FOXM1 in OSCC cells by siRNA decreased the expression
of vimentin and increased the expression of E-cadherin (Fig. 3). As E-cadherin and vimentin are
the most important proteins associated with EMT, the results
suggested that FOXM1 may regulate the mesenchymal phenotype of OSCC
cells.
Discussion
FOXM1 serves an important regulatory role in a wide
range of biological processes, including cell proliferation, cycle
progression, differentiation, DNA damage repair, tissue
homeostasis, angiogenesis and apoptosis (25). The full gene expression profiles of
various cancer types have independently and consistently identified
FOXM1 as one of the most common highly expressed genes (13,14).
A previous study reported that FOXM1 forms part of the set of genes
that is upregulated in early tumour development (5). FOXM1 is associated with cancer
initiation, progression and metastasis, and drug resistance
(4,10–12).
FOXM1 is also involved in tumorigenesis by stimulating cell
proliferation and cell cycle progression. In oral cancer, FOXM1 and
its expression are markedly associated with the tumorigenesis and
prognosis of HNSCC (26).
FOXM1 induces tumour occurrence by accelerating
human epithelial stem cell regeneration and withstanding
differentiation. It has been reported that tobacco is a major risk
factor for OSCC development, since the nicotine in tobacco may
increase the differentiation of human oral keratinocytes induced by
FOXM1 (5). FOXM1B induces
correlated genomic variables, including genome-wide loss of
heterozygosity (LOH) and copy-number variations, and FOXM1 target
genes have been identified within recurrent LOH loci (27). It has been identified that FOXM1
may give methylome a tumour-like structure by prompting methylome
recombination in primary human keratinocyte cells (5). However, the clinicopathological
importance of FOXM1 in OSCC has not been sufficiently
investigated.
In the present study, the expression level of FOXM1
protein was determined in order to better understand the
association of FOXM1 expression in patients with OSCC with
clinicopathological characteristics and prognosis. The majority
(71.4%; 20/28) of the patients who experienced local tumour
recurrence had high expression levels of FOXM1, whereas a lower
proportion (43/91) of patients without recurrence demonstrated high
FOXM1 expression. This result revealed that FOXM1 is markedly
associated with local OSCC recurrence and survival rates in
patients with OSCC. In vitro, the knockdown of FOXM1 not
only decreased the proliferation, mobility and migration of OSCC
cells, but also altered the expression of proteins involved in
EMT.
EMT promotes the conversion of epithelial cells to
migratory mesenchymal phenotypic cells by breaking junctions among
cells, which leads to a low cell-cell adhesion capacity and high
cancer aggressiveness (28,29).
Tumour cells acquire migratory and invasive capabilities not only
by reducing cell adhesions, but also by recombining the
cytoskeleton and forming migratory protrusions (30). However, the formation of
protrusions, the function of certain organelles and the cellular
molecular mechanisms in the EMT process remain to be elucidated.
The regulation of the EMT process includes low expression of
E-cadherin, which is frequently identified in the majority of
epithelial cancers, accompanied by high expression of vimentin,
which serves as a marker of the mesenchymal phenotype (29). In the present study, OSCC cells
presented a lower E-cadherin level and a higher vimentin level,
indicating that patients with OSCC who require more radical
therapies may have a poor prognosis. It was previously reported
that grainyhead-like 2 regulates FOXM1 expression in OSCC, and
thereby regulates homeostasis and cell proliferation (31). Zinc-finger E-box binding homeobox 1
(ZEB1) and other transcriptional regulators, including ZEB2/SIP1,
snail family transcriptional repressor (Snail)1, Snail2/Slug, twist
family bHLH transcription factor 1 and transcription factor 3, have
been identified to be crucial for the EMT process (32). FOXM1 overexpression induces the EMT
process and acquisition of the cancer stem cell phenotype, and
these effects may be partly regulated by microRNA-200b and easily
attenuated by genistein (33).
Hypoxia induces EMT in OSCC cells by triggering Notch signalling,
and the inhibition of Notch signalling may result in enhanced
anti-tumour effects (34). The
inhibition of FOXM1 expression is able to directly downregulate the
expression of a variety of tumour-promoting factors, including
cyclin B1, cell division cycle 25 homologue B and survivin
(35). These factors are usually
the key factors in the signalling pathway, and the downregulation
of their expression blocks the signalling pathway. The simultaneous
inhibition of multiple signalling pathways to antagonize EMT may
exert synergistic effects, and may benefit the treatment and
prognosis of OSCC. The present study identified that FOXM1 may
regulate EMT, but no significant association between FOXM1
expression and metastasis was observed, which may be due to the
fact that FOXM1 expression was associated with local invasiveness
of the tumours. Possible omissions of occult lymphatic metastasis
in the pathology may also be a limitation. In addition, the sample
size was small, and a larger sample analysis may be required.
The present study identified that FOXM1 may mediate
the OSCC EMT phenotype. The siRNA-mediated knockdown of FOXM1
increased the E-cadherin level and decreased the vimentin level.
The effect of FOXM1 on the EMT phenotype may be associated with
local OSCC recurrence, and this finding warrants further
investigation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81600908), the
Jiangsu Provincial Medical Innovation Team (grant no. CXTDA2017036)
and the Jiangsu Provincial Medical Youth Talent (grant no.
QNRC2016854). The funders had no role in the study design, data
collection and analysis, decision to publish or preparation of the
manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XMZ and HMW conceived and designed the study and
proofread the manuscript. YDL and XD wrote the manuscript and
performed the experiments, statistical evaluations, and clinical
studies. HMD, YNW and HQL performed the clinical studies and
statistical evaluations. All the authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was performed with permission from
the ethical committee of the Affiliated Stomatological Hospital of
Nanjing Medical University. Informed consent was obtained from each
patient.
Patient consent for publication
All patients gave written informed consent before
participation in this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jerjes W, Upile T, Petrie A, Riskalla A,
Hamdoon Z, Vourvachis M, Karavidas K, Jay A, Sandison A, Thomas GJ,
et al: Clinicopathological parameters, recurrence, locoregional and
distant metastasis in 115 T1-T2 oral squamous cell carcinoma
patients. Head Neck Oncol. 2:92010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carvalho AL, Nishimoto IN, Califano JA and
Kowalski LP: Trends in incidence and prognosis for head and neck
cancer in the United States: A site-specific analysis of the SEER
database. Int J Cancer. 114:806–816. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ahmad A, Wang Z, Kong D, Ali S, Li Y,
Banerjee S, Ali R and Sarkar FH: FoxM1 down-regulation leads to
inhibition of proliferation, migration and invasion of breast
cancer cells through the modulation of extra-cellular matrix
degrading factors. Breast Cancer Res Treat. 122:337–346. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gemenetzidis E, Bose A, Riaz AM, Chaplin
T, Young BD, Ali M, Sugden D, Thurlow JK, Cheong SC, Teo SH, et al:
FOXM1 upregulation is an early event in human squamous cell
carcinoma and it is enhanced by nicotine during malignant
transformation. PLoS One. 4:e48492009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lam EW, Brosens JJ, Gomes AR and Koo CY:
Forkhead box proteins: Tuning forks for transcriptional harmony.
Nat Rev Cancer. 13:482–495. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sorlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tian S, Wang C and An MW: Test on
existence of histology subtype-specific prognostic signatures among
early stage lung adenocarcinoma and squamous cell carcinoma
patients using a Cox-model based filter. Biol Direct. 10:152015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu CP, Yu S, Shi L, Wang S, Li ZX, Wang
YH, Sun CJ and Liang J: FoxM1 promotes epithelial-mesenchymal
transition of hepatocellular carcinoma by targeting Snai1. Mol Med
Rep. 16:5181–5188. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park HJ, Costa RH, Lau LF, Tyner AL and
Raychaudhuri P: Anaphase-promoting complex/cyclosome-CDH1-mediated
proteolysis of the forkhead box M1 transcription factor is critical
for regulated entry into S phase. Mol Cell Biol. 28:5162–5171.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raychaudhuri P and Park HJ: FoxM1: A
master regulator of tumor metastasis. Cancer Res. 71:4329–4333.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leung TW, Lin SS, Tsang AC, Tong CS, Ching
JC, Leung WY, Gimlich R, Wong GG and Yao KM: Over-expression of
FoxM1 stimulates cyclin B1 expression. FEBS Lett. 507:59–66. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Myatt SS and Lam EW: The emerging roles of
forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 7:847–859.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koo CY, Muir KW and Lam EW: FOXM1: From
cancer initiation to progression and treatment. Biochim Biophys
Acta. 1819:28–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma J, Qi G, Xu J, Ni H, Xu W, Ru G, Zhao
Z, Xu W and He X: Overexpression of forkhead box M1 and
urokinase-type plasminogen activator in gastric cancer is
associated with cancer progression and poor prognosis. Oncol Lett.
14:7288–7296. 2017.PubMed/NCBI
|
|
16
|
Jiang LZ, Wang P, Deng B, Huang C, Tang
WX, Lu HY and Chen HY: Overexpression of Forkhead Box M1
transcription factor and nuclear factor-κB in laryngeal squamous
cell carcinoma: A potential indicator for poor prognosis. Hum
Pathol. 42:1185–1193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abdeljaoued S, Bettaieb I, Nasri M, Adouni
O, Goucha A, El Amine O, Boussen H, Rahal K and Gamoudi A:
Overexpression of FOXM1 Is a potential prognostic marker in male
breast cancer. Oncol Res Treat. 40:167–172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wittekind C and Oberschmid B: TNM
classification of malignant tumors 2010: General aspects and
amendments in the general section. Pathologe. 31:333–334, 336-338.
2010.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim HE, Krug MA, Han I, Ensley J, Yoo GH,
Forman JD and Kim HR: Neutron radiation enhances cisplatin
cytotoxicity independently of apoptosis in human head and neck
carcinoma cells. Clin Cancer Res. 6:4142–4147. 2000.PubMed/NCBI
|
|
20
|
Patel V, Ramesh A, Traicoff JL, Baibakov
G, Emmert-Buck MR, Gutkind JS and Knezevic V: Profiling EGFR
activity in head and neck squamous cell carcinoma by using a novel
layered membrane Western blot technology. Oral Oncol. 41:503–508.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiao C, Wang L, Zhu L, Zhang C and Zhou J:
Secreted frizzledrelated protein 2 is epigenetically silenced and
functions as a tumor suppressor in oral squamous cell carcinoma.
Mol Med Rep. 10:2293–2298. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu J, Wu YN, Zhang W, Zhang XM, Ding X,
Li HQ, Geng M, Xie ZQ and Wu HM: Monocarboxylate transporter 4
facilitates cell proliferation and migration and is associated with
poor prognosis in oral squamous cell carcinoma patients. PLoS One.
9:e879042014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu Z, Xu X, Yu Y, Graham M, Prince ME,
Carey TE and Sun D: Silencing heat shock protein 27 decreases
metastatic behavior of human head and neck squamous cell cancer
cells in vitro. Mol Pharm. 7:1283–1290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang IC, Chen YJ, Hughes D, Petrovic V,
Major ML, Park HJ, Tan Y, Ackerson T and Costa RH: Forkhead box M1
regulates the transcriptional network of genes essential for
mitotic progression and genes encoding the SCF (Skp2-Cks1)
ubiquitin ligase. Mol Cell Biol. 25:10875–10894. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nandi D, Cheema PS, Jaiswal N and Nag A:
FoxM1: Repurposing an oncogene as a biomarker. Semin Cancer Bilo.
52:74–84. 2018. View Article : Google Scholar
|
|
26
|
Hwang S, Mahadevan S, Qadir F, Hutchison
IL, Costea DE, Neppelberg E, Liavaag PG, Waseem A and Teh MT:
Identification of FOXM1-induced epigenetic markers for head and
neck squamous cell carcinomas. Cancer. 119:4249–4258. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Teh MT, Gemenetzidis E, Chaplin T, Young
BD and Philpott MP: Upregulation of FOXM1 induces genomic
instability in human epidermal keratinocytes. Mol Cancer. 9:452010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tosi GM, Marigliani D, Romeo N and Toti P:
Disease pathways in proliferative vitreoretinopathy: An ongoing
challenge. J Cell Physiol. 229:1577–1583. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Casaroli-Marano RP, Pagan R and Vilaró S:
Epithelial-mesenchymal transition in proliferative
vitreoretinopathy: Intermediate filament protein expression in
retinal pigment epithelial cells. Invest Ophthalmol Vis Sci.
40:2062–2072. 1999.PubMed/NCBI
|
|
30
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen W, Yi JK, Shimane T, Mehrazarin S,
Lin YL, Shin H, Kim RH, Park NH and Kang MK: Grainyhead-like 2
regulates epithelial plasticity and stemness in oral cancer cells.
Carcinogenesis. 37:500–510. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thierauf J, Veit JA and Hess J:
Epithelial-to-mesenchymal transition in the pathogenesis and
therapy of head and neck cancer. Cancers (Basel). 9(pii): E762017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bao B, Wang Z, Ali S, Kong D, Banerjee S,
Ahmad A, Li Y, Azmi AS, Miele L and Sarkar FH: Over-expression of
FoxM1 leads to epithelial-mesenchymal transition and cancer stem
cell phenotype in pancreatic cancer cells. J Cell Biochem.
112:2296–2306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ishida T, Hijioka H, Kume K, Miyawaki A
and Nakamura N: Notch signaling induces EMT in OSCC cell lines in a
hypoxic environment. Oncol Lett. 6:1201–1206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nakamura S, Hirano I, Okinaka K, Takemura
T, Yokota D, Ono T, Shigeno K, Shibata K, Fujisawa S and Ohnishi K:
The FOXM1 transcriptional factor promotes the proliferation of
leukemia cells through modulation of cell cycle progression in
acute myeloid leukemia. Carcinogenesis. 31:2012–2021. 2010.
View Article : Google Scholar : PubMed/NCBI
|