Introduction
Colorectal cancer (CRC) is a common malignant tumor
with mortality ranking third among tumors. A recent study reported
that CRC mortality in European and American developed countries is
decreased (1). However, the
morbidity and mortality of CRC in China remains high (1). The national standard of living is
improving at present, along with accelerated population aging and
altered dietary structure (2).
These factors have resulted in markedly increased morbidity of CRC
in China, severely threatening human health (2). A study suggested that morbidity of
CRC, particularly that of distal colon cancer and rectal cancer,
increases gradually. Furthermore, it demonstrates a younger trend
(3). The pathogenesis of CRC has
not been completely elucidated (3). However, existing data suggest that
CRC incidence is closely associated with a number of factors,
including high fat/low cellulose diet, chronic colitis, colorectal
adenoma and heredity (3). Other
factors, including bilharziasis, pelvic radiation, environmental
factor and smoking, may additionally affect CRC incidence (3). The pathogenesis of CRC remains
unclear, with no early symptoms observed (3). This has contributed to the
difficulties in the early diagnosis and treatment of CRC (1).
MicroRNA (miRNA) is a class of endogenous small
non-coding RNA molecules, ~22 nucleotides in length (4). It is the single-strand endogenous
non-coding RNA that is extensively distributed in eukaryotes
(5). The majority of miRNAs are
transcribed by RNA polymerase II. The primary transcription product
is called pri-miRNA. Pri-miRNA forms the mature miRNA following
several steps of intranuclear and intracytoplasmic processing.
miRNAs directly bind with the 3′-untranslated region (UTR) of
target mRNAs to exert regulatory effect (6).
Virulence gene ataxin-3 (ATXN3) is located in the
short arm of chromosome 14. Its 3′-terminal protein-coding region
contains the cytosine-adenine-uracil trinucleotide repetitive
sequence (7). The repeat frequency
is 12–40 in healthy subjects, and can reach 51–86 in patients with
CRC. The ATXN3 gene contains the abnormally amplified CAG
repetitive sequence, which encodes ataxin-3 containing an expanded
poly-glutamine (polyQ) peptide chain (8). This is a toxic protein that is
extensively expressed in the human body (8,9). It
may selectively aggregate in specific regions of the nervous
system, including the cerebellum, brainstem and spinal cord, to
form neuronal intranuclear inclusion and may induce cell death
(7,9). The present study aimed to examine the
function of miRNA-25 (miR-25) on human colon cancer cell viability
and migration, and its potential mechanisms.
Materials and methods
Patients with CRC and survival
rate
Serum samples from patients with CRC (n=48) and
healthy volunteers (n=8) were collected at the Department of
Gastrointestinal Surgery, Yue Bei People's Hospital (Shaoguan,
China) between June 2011 to November 2011. The exclusion criteria
for patients included any history of inflammatory bowel disease.
Characteristics of patients with CRC and healthy volunteers are
presented in Table I. Serum
samples were collected and centrifuged at 1,000 × g for 10 min at
4°C. Every 3 months, patients were contacted by telephone for a
follow-up period of 5 years and the results were measured using a
log rank test. The present study was approved by the Ethics
Committee of the Yue Bei People's Hospital. Written informed
consent was obtained from all patients.
 | Table I.Characteristics of patients with CRC
and healthy volunteers. |
Table I.
Characteristics of patients with CRC
and healthy volunteers.
| Characteristic | CRC (n=72) | Healthy (n=8) |
|---|
| Age, years |
|
|
| ≤60 | 29 | 4 |
|
>60 | 43 | 4 |
| Sex |
|
|
|
Female | 25 | 3 |
| Male | 47 | 5 |
| Tumor size, cm |
|
|
| ≤3.0 | 31 | – |
|
>3.0 | 41 | – |
| TNM staging
system |
|
|
| I | 8 | – |
| II | 15 | – |
|
III–IV | 49 | – |
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the samples using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and cDNA synthesis was conducted with
RevertAid first strand cDNA synthesis kit (Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
qPCR was conducted using a SYBR-Green Master Mix (Takara
Biotechnology Co., Ltd., Dalian, China) and a CFX-96 qPCR system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The primers were
as follows: miR-25-3p, forward: 5′-CATTGCACTTGTCTCGGTCTGA-3′,
reverse: 5′-GCTGTCAACGATACGCTACGTAACG-3′ and U6, forward:
5′-CTCGCTTCGGCAGCACA-3′, reverse: 5′-AACGCTTCACGAATTTGCGT-3′. The
thermocycling conditions were as follows: Incubation at 95°C for 5
min, followed by 40 cycles of 95°C for 15 sec, 60°C for 30 sec and
72°C for 30 sec. The relative expression of miR-25 was calculated
using the 2−ΔΔCq method (10). Higher miR-25 expression was defined
as >2-fold the mean miR-25 expression of healthy volunteers and
lower miR-25 expression was defined as ≤2-fold the miR-25
expression of healthy volunteers.
Gene chip technology
A total of 500 ng RNA was hybridized to the
SurePrint G3 Mouse Whole Genome GE K Microarray G4852A platform
(G4852A; Agilent Technologies, Inc., Santa Clara, CA, USA). The
data was quantified using Agilent Feature Extraction software
version10.7.3.1 (Agilent Technologies, Inc.).
Cell culture and transfection
The human HCT116 colon cancer cell line was obtained
from the Shanghai Cell Bank of Chinese Academy of Sciences
(Shanghai, China) and cultured in RPMI-1640 medium (Invitrogen;
Thermo Fisher Scientific, Inc.) supplemented with 10% (v/v) fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) at 37°C
and 5% CO2. miR-25 (5′-GGCCAGTGTTGAGAGGCA-3′ and
5′-TGACAGTGCCGGCC-3′), anti-miR-25 (5′-CTCCCTCACAGGACAGCTGAACAC-3′
and 5′-CTGCCCCCCCACATCTGCAGT-3′) and negative mimics
(5′-CCCCCCCCCCCCC-3′ and 5′-CCCCCCCCCCCCC-3′) were purchased from
Sangon Biotech Co., Ltd. (Shanghai, China). In total, 100 nM
miR-25, anti-miR-25 and negative mimics were transfected into cells
using the X-tremeGENE small interfering RNA (si)RNA transfection
reagent (Roche Molecular Diagnostics, Pleasanton, CA, USA). A total
of 100 ng si-ATXN3 (sc-40359; Santa Cruz Biotechnology; sequence is
commercially unavailable) was transfected into cells using the
X-tremeGENE siRNA transfection reagent (Roche Molecular
Diagnostics) for 48 h.
Cell viability analysis and cell
migration assay
Cells were harvested at 0, 24, 48 and 72 h after
transfection. A total of 20 µl MTT solution (5 mg/ml;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added to the
cells and cultured for 4 h at 37°C. The supernatant was removed
using a pipette, and 150 µl dimethyl sulfoxide was added to the
cells and cultured for 20 min at 37°C. Optical density was measured
at 562 nm using a µQuant™ Microplate Spectrophotometer (BioTek
Instruments, Inc., Winooski, VT, USA).
Cells (1×104 cell/ml) at 24 h after
transfection were cultured in 100 µl media without serum in the
upper chamber of Millicells (pore size 8 µm; EMD Millipore,
Billerica, MA, USA) at 37°C for 24 h. The lower chamber contained
RPMI-1640 medium with 20% FBS. The migrated cells were fixed with
70% ethanol at room temperature for 30 min, stained with 5 mg/ml
crystal violet solution for 30 min at room temperature and counted
under a confocal microscope (Leica SP5; Leica Microsystems, Inc.,
Buffalo Grove, IL, USA).
Apoptosis rate analysis by flow
cytometry
Cells at 48 h after transfection were washed twice
with PBS, and 500 µl cell binding buffer (BD Pharmingen, San Diego,
CA, USA) was added. Cells were stained with 5 µl Annexin V-PE
conjugate and 5 µl propidium iodide for 15 min in the dark. The
apoptosis rate of the cells was subsequently determined using a
flow cytometer (C6; BD Pharmingen, San Diego, CA, USA) and analyzed
using Image Lab version 3.0 (Bio-Rad Laboratories, Inc.).
Western blot analysis
Total cell protein were collected and extracted 48 h
after transfection using radioimmunoprecipitation assay buffer
(Pierce; Thermo Fisher Scientific, Inc.) and quantified using a BCA
Protein Concentration kit (P0009; Beyotime Institute of
Biotechnology, Haimen, China). Total cell protein (50 µg) was
separated by SDS-PAGE on 10–12% gels and subsequently transferred
to nitrocellulose membranes (Pall Life Sciences, Port Washington,
NY, USA). The membranes were incubated with ATXN3 (ab175265,
1:1,000, Santa Cruz Biotechnology, Inc., Dallas, TX, USA), cyclin
D1 (sc-70899; 1:1,000; Santa Cruz Biotechnology, Inc.), apoptosis
regulator Bax (Bax; sc-6236; 1:1,000; Santa Cruz Biotechnology,
Inc.) and GAPDH (sc-293335; 1:5,000; Santa Cruz Biotechnology,
Inc.) at 4°C overnight following blocking with 5% skimmed milk in
Tris-buffered saline with Tween 20 (TBST) at room temperature for 2
h. The membranes were washed for 10 min in TBST and incubated with
goat anti-rabbit horseradish peroxidase-conjugated immunoglobulin G
(sc-2004; 1:5,000; Santa Cruz Biotechnology, Inc.) for 1 h at 37°C.
Proteins were visualized using enhanced chemiluminescent reagent
(Santa Cruz Biotechnology, Inc.) and analyzed using Image-Pro Plus
version 6.0 software (Media Cybernetics, Inc., Rockville, MD,
USA).
Caspase-3/9 activity
Total protein was extracted from cells using
radioimmunoprecipitation assay buffer (Pierce; Thermo Fisher
Scientific, Inc.) 48 h after transfection and quantified using BCA
Protein Concentration kit (P0009; Beyotime Institute of
Biotechnology). A total of 10 µg protein was used to measure
caspase-3/9 activity using Caspase-3/9 activity kits (cat. nos.
C1116 and C1158; Beyotime Institute of Biotechnology), according to
the manufacturer's instructions. Optical density at 405 nm was
measured using a µQuant™ Microplate Spectrophotometer (BioTek
Instruments, Inc.).
Luciferase reporter assay
The partial sequence of the ATXN3′-UTR was cloned
downstream of the firefly luciferase gene in the pGL3-Control
Vector. HCT116 cell was transfected with ATXN3 and microRNA-25 or
negative mimics using X-tremeGENE siRNA transfection reagent (Roche
Molecular Diagnostics). The cells were harvested and assayed with
the dual-luciferase assay (Promega, Madison, WI, USA) after 48 h of
transfection. The results are expressed as the relative luciferase
activity (Firefly LUC/Renilla LUC).
Statistical analysis
Experiments were repeated three times and the data
are presented as the mean ± standard deviation. Statistical
analysis was performed using SPSS version 17.0 (SPSS, Inc.,
Chicago, IL, USA). Statistical differences between two groups were
determined by Student's t-test. Statistical differences between
three groups were determined by one-way analysis of variance and
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference. Overall survival (OS) and
disease free survival (DFS) were analyzed using Kaplan-Meier
survival curves.
Results
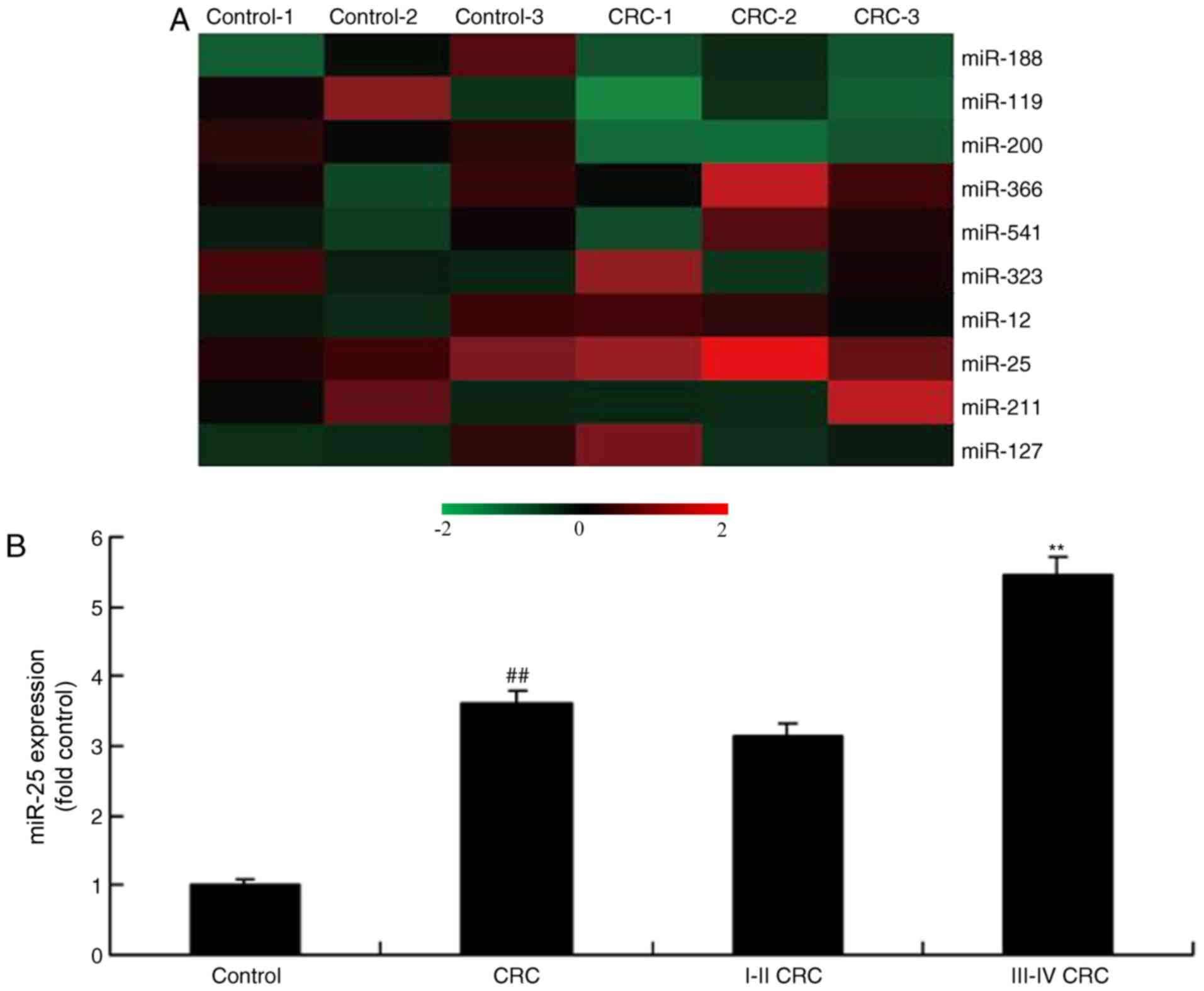
miR-25 expression in human colon
cancer
The alterations of miRNAs in human colon cancer
tissue were examined. As demonstrated in Fig. 1, miR-25 expression was upregulated
in patients with colon cancer, compared with the controls. In
addition, the expression of miR-25 in the serum of patients with
stage III–IV colon cancer was significantly increased compared with
patients with stage I–II colon cancer (Fig. 1B).
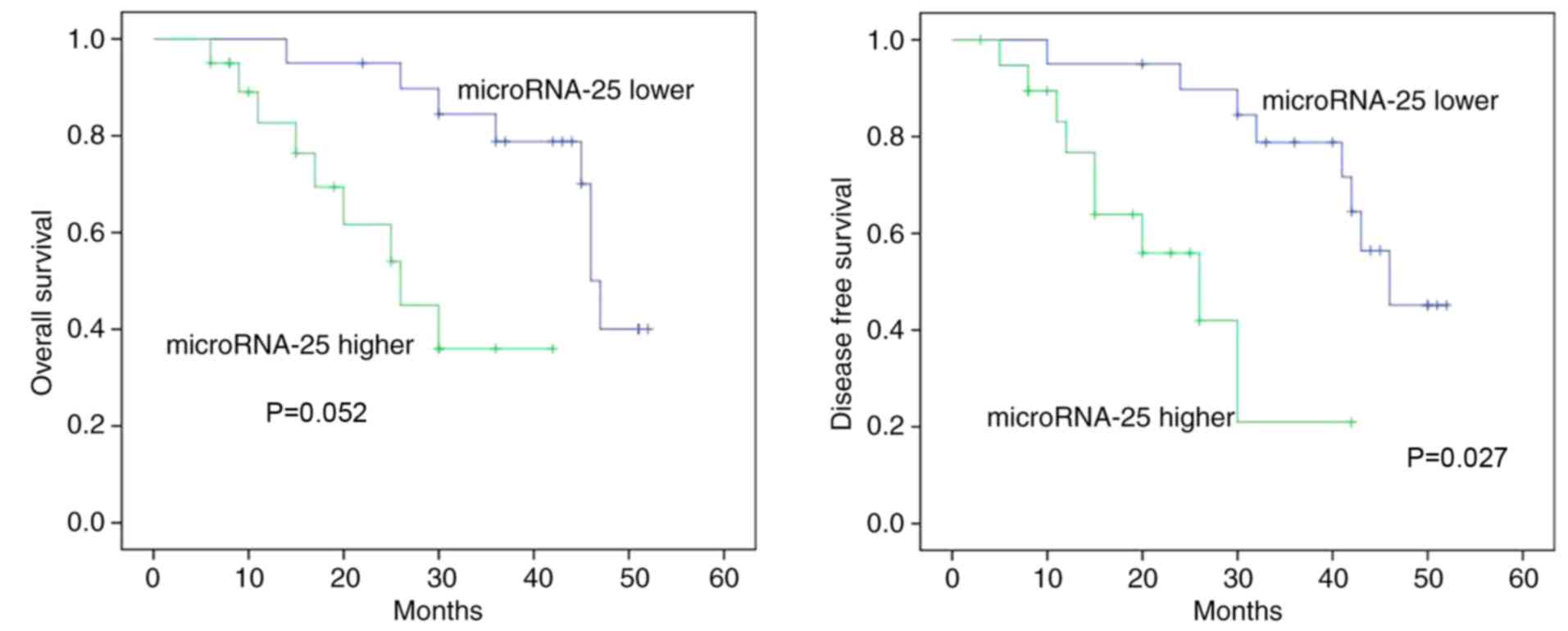
miR-25 expression is associated with
OS/DFS in human colon cancer
The association between miR-25 expression and OS/DFS
in human colon cancer was analyzed. As demonstrated in Fig. 2, the OS (P=0.052) and DFS (P=0.027)
of patients with human colon cancer with higher miR-25 expression
were markedly lower compared with lower miR-25 expression.
Overexpression of miR-25 promotes cell
viability and migration of colon cancer cells
To investigate whether the overexpression of miR-25
promoted cell viability and migration of colon cancer cells, colon
cancer cells were transfected with miR-25 mimics. As a result,
miR-25 expression was significantly upregulated in colon cancer
cells by miR-25 mimics compared with the control group (Fig. 3A; P<0.01). Furthermore,
overexpression of miR-25 significantly increased cell viability and
migration, decreased the apoptosis rate and decreased caspase-3/9
activity level of colon cancer cells compared with the control
group (Fig. 3B-E; P<0.01).
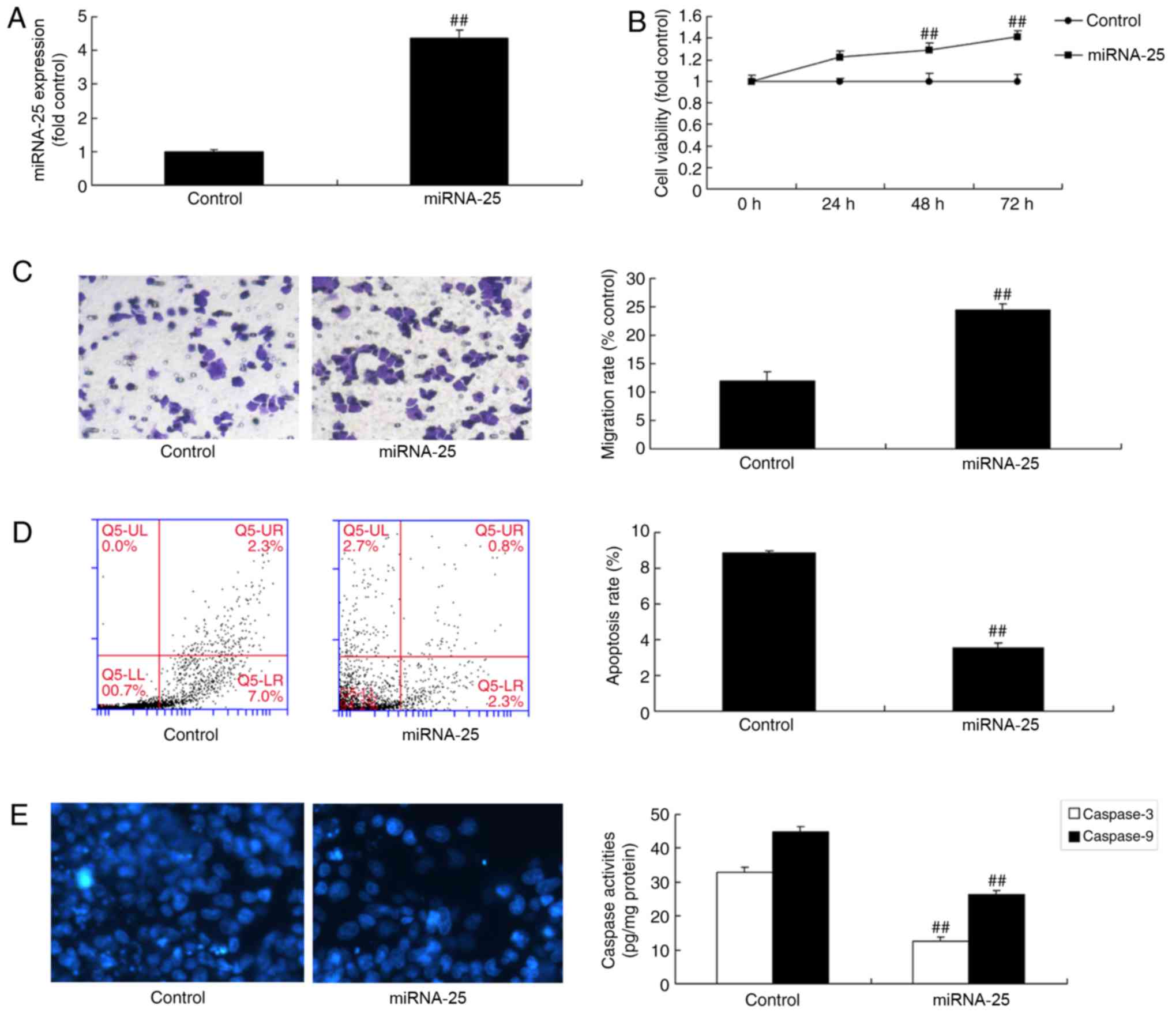 | Figure 3.Overexpression of miRNA-25 promotes
cell viability and migration of colon cancer cells. Cells were
transfected with miR-25 mimics and (A) miRNA-25 expression, (B)
cell viability, (C) cell migration (magnification, ×100), (D)
apoptosis rate and (E) caspase-3/9 activity level were determined.
Data are presented as the mean ± standard deviation.
##P<0.01 vs. respective control. Control, negative
control group; miRNA-25, microRNA-25; UL, upper left; UR, upper
right; LL, lower left; LR, lower right. |
Downregulation of miR-25 decreases
cell viability and migration of colon cancer cells
Additionally, the effects of miR-25 downregulation
on cell viability and migration of colon cancer cells were
examined. There was a significant inhibition of miR-25 expression
in colon cancer cells transfected with miR-25 inhibitor compared
with the control group (Fig. 4A;
P<0.01). Downregulation of miR-25 significantly decreased cell
viability and migration, and increased apoptosis and the
caspase-3/9 activity level of colon cancer cell, compared with the
control group (Fig. 4B-E;
P<0.01). Together, the present results demonstrated that miR-25
regulated colon cancer cell growth; however, its potential
mechanisms requires further investigation.
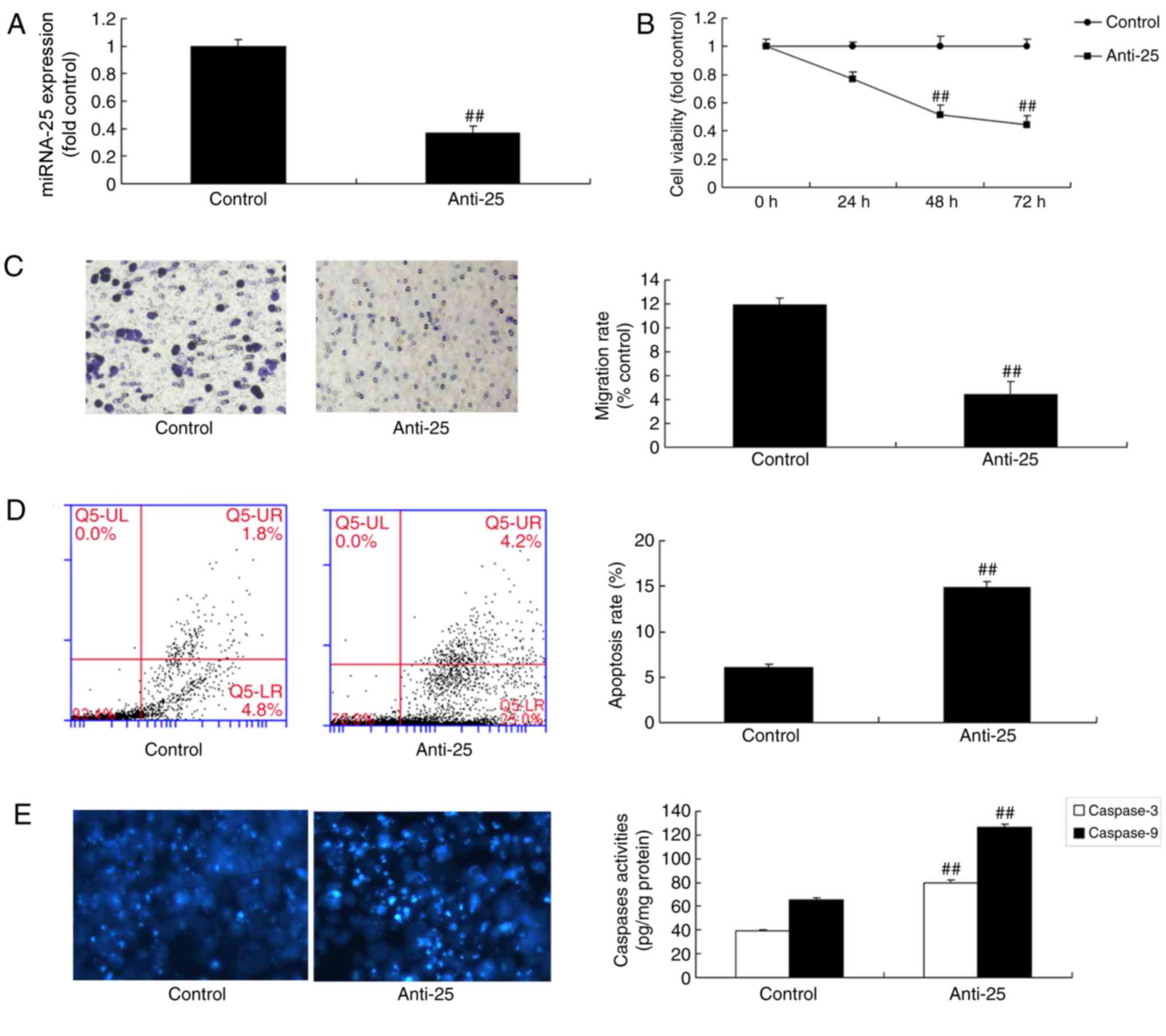 | Figure 4.Downregulation of miRNA-25 reduces
cell viability and migration of colon cancer cells. Cells were
transfected with miR-25 inhibitor oligos and (A) miRNA-25
expression, (B) cell viability, (C) migration, (D) apoptosis rate
and (E) caspase-3/9 activity level were determined. Data are
presented as the mean ± standard deviation. ##P<0.01
vs. respective control. Control, negative control group; miRNA-25,
microRNA-25; Anti-25, miR-25 inhibitor; UL, upper left; UR, upper
right; LL, lower left; LR, lower right. |
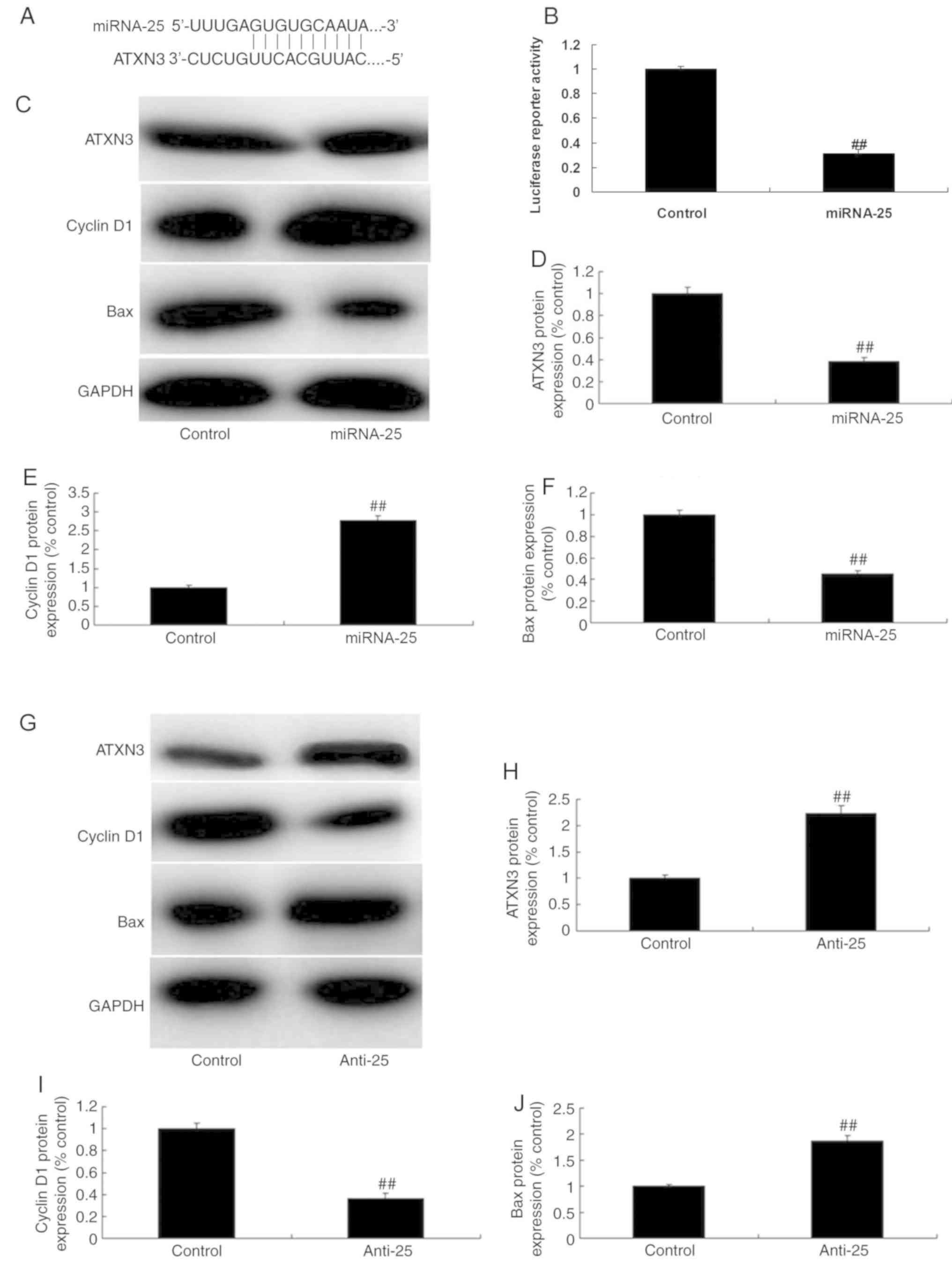
miR-25 targets ATXN3 in colon cancer
cell
The possible mechanisms of miR-25 in colon cancer
cells were investigated. Mutation analysis revealed an miR-25
binding site in the 3′-UTR of ATXN3 (Fig. 5A) and overexpression of miR-25
reduced luciferase reporter activity levels when compared with
negative group (Fig. 5B).
Overexpression of miR-25 significantly decreased ATXN3 and Bax
protein expression, and increased cyclin D1 protein expression in
colon cancer cells compared with the control group (Fig. 5C-F; P<0.01). By contrast,
downregulation of miR-25 significantly increased ATXN3 and Bax
protein expression, and decreased cyclin D1 protein expression in
colon cancer cells, compared with the control group (Fig. 5G-J; P<0.01). Based on the
results, it was hypothesized that miR-25 targets ATXN3 in colon
cancer cells to promote colon cancer cell growth.
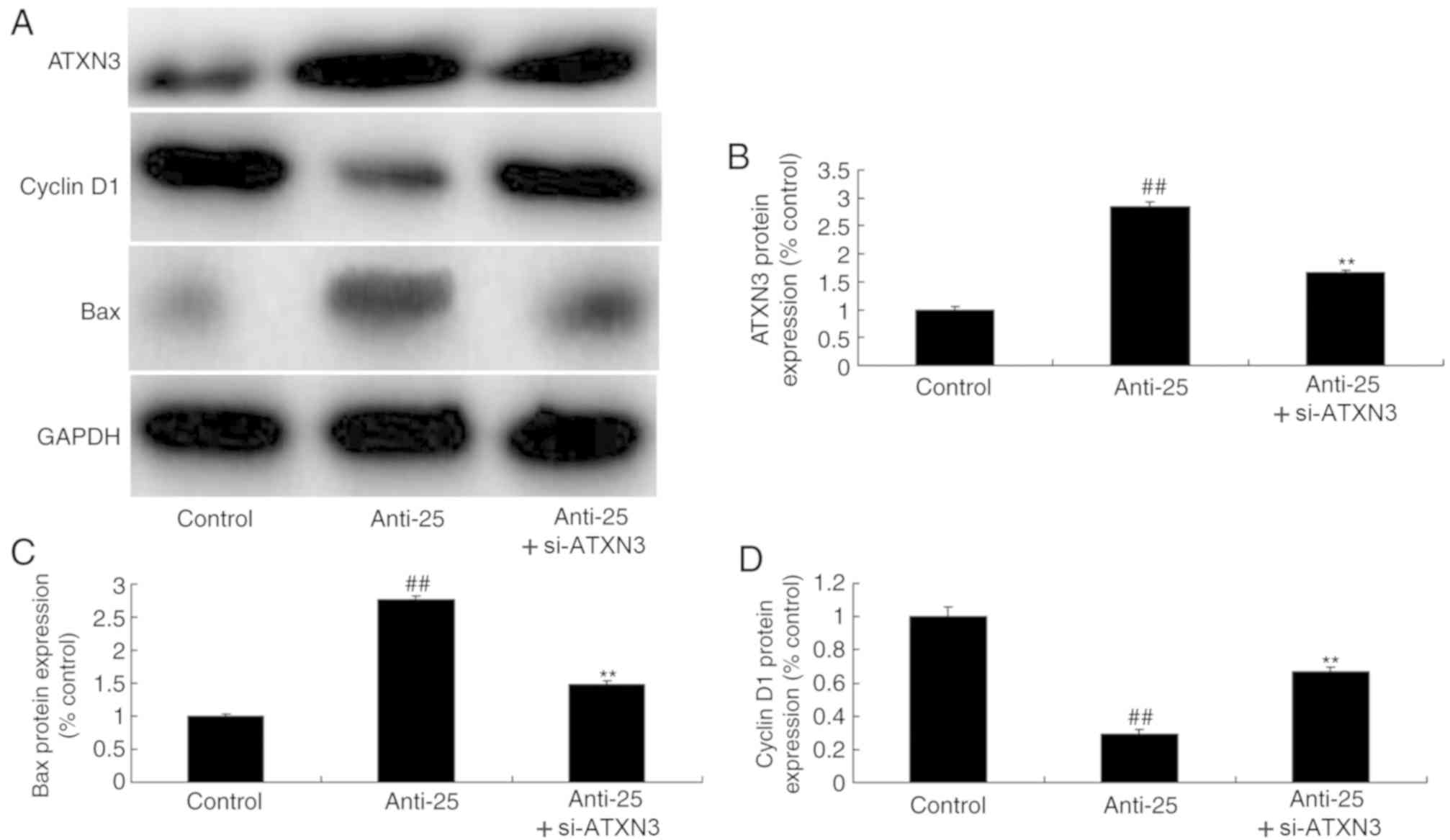
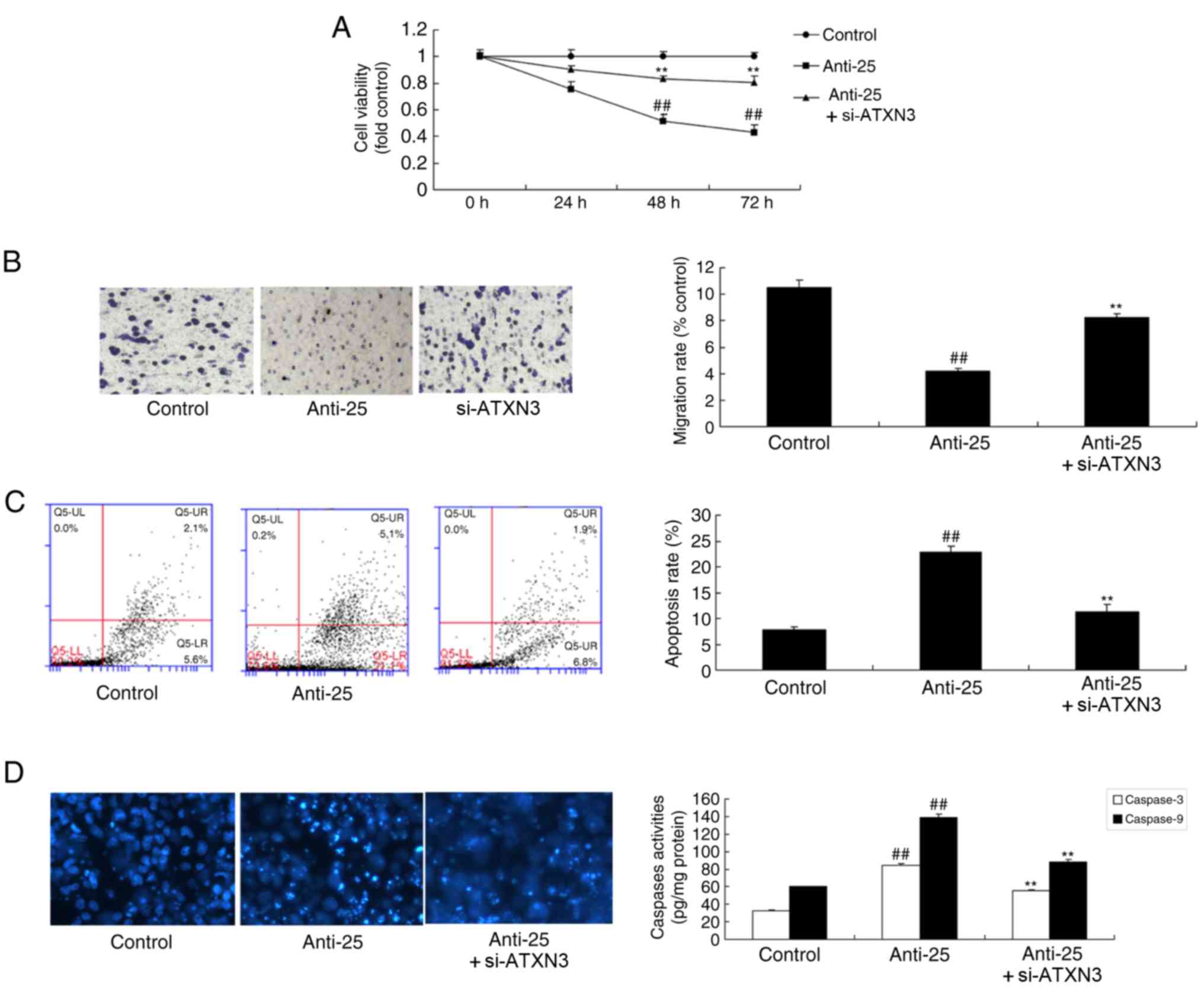
si-ATXN3 inhibits the anti-cancer
effects of miR-25 downregulation in colon cancer
To examine the role of ATXN3 in the anti-cancer
effects of miR-25 downregulation on colon cancer, si-ATXN3 and
anti-miR-25 oligos were co-transfected into colon cancer cells.
Consequently, si-ATXN3 significantly decreased ATXN3 and Bax
protein expression and increased cyclin D1 protein expression in
colon cancer cells compared with the miR-25 downregulation group
(Fig. 6). si-ATXN3 inhibited the
anti-cancer effects of miR-25 downregulation on colon cancer cell
proliferation and migration, apoptosis and caspase-3/9 activity
level; si-ATXN3 significantly increased cell viability and
migration, decreased the apoptosis rate and caspase-3/9 activity
level compared with the downregulated miR-25 group (Fig. 7; P<0.01).
Discussion
CRC is a glandular epithelium-derived malignant
tumor and its morbidity exceeds that of gastric cancer (11). It is the most common malignant
tumor in the digestive tract and its morbidity is the third highest
among all cancer types globally (11). The prevalence of CRC in populations
demonstrates (12). A previous
study suggested that a young patient population is associated with
poorer histopathological type and more dismal prognosis (12). At present, comprehensive treatment
is dominated by radical surgery supplemented with radiotherapy,
chemotherapy and traditional Chinese medicine treatment, with
biological immunotherapy is the principal treatment for CRC
(13). However, existing CRC
treatments cause various inconveniences for patients in their daily
life and reduces their quality of life (13). Consequently, the genesis and
development mechanism of CRC requires elucidation and novel
therapeutic targets require investigation (13). Research on miRNAs and their
associations with tumors has attracted increasing attention
(13). miRNAs can affect tumor
cell proliferation, apoptosis, migration, angiogenesis and
resistance (13). Collectively,
the present results demonstrated that miR-25 expression was
upregulated in patients with colon cancer, and the OS and DFS in
human colon cancer with high miR-25 expression were decreased
compared with in human colon cancer with low miR-25 expression. He
et al (14) demonstrated
that miR-25 contributes to cisplatin resistance by inhibiting
forkhead box O3a in gastric cancer cells.
Methylation of the ATXN3 gene results in
transcription silencing, affecting the expression level of polyQ
expansion mutant ataxin-2 protein (15). DNA methylation in the ATXN3 gene
promoter region may partially regulate its own expression (15). Other factors, including
post-transcriptional regulation, may affect the mRNA expression
level (16). Therefore, DNA
methylation in the promoter region may not be the principal
regulatory factor affecting expression of that gene. DNA
methylation is involved in regulating expression level, and also
involved in pathogenesis by affecting the interaction of DNA in
that region with associated DNA sequences and proteins (17). The present results suggested that
overexpression of miR-25 suppressed ATXN3 protein expression in
colon cancer cells. si-ATXN3 inhibited the anti-cancer effects of
miR-25 downregulation in colon cancer. Huang et al (18) demonstrated that miR-25 alleviates
polyQ-mediated cytotoxicity by silencing ATXN3.
Apoptosis is a gene-controlled, autonomic and
programmed suicidal cell death upon stimulation to maintain
homeostasis under adverse physiological conditions (19). Different from cell necrosis,
apoptosis is an initiative process as opposed to a passive one. It
involves the activation, expression and regulation of a series of
genes, including Bax, apoptosis regulator Bcl-2, caspase-3 and poly
[ADP-ribose] polymerase 1 (20).
Apoptosis is not a self-damage phenomenon under pathological
conditions; instead, it is a normal death process allowing cells to
better adapt to the surviving environment (20). Therefore, it was hypothesized that
overexpression of miR-25 suppressed Bax protein expression in colon
cancer cell. Zhang et al (21) observed that miR-25 overexpression
enhanced cell proliferation and reduced Bax protein expression in
human ovarian cancer.
Cyclin D1 is a cell cycle-associated nucleoprotein
that forms complexes with cyclin-dependent kinase (22). It serves a vital role in tumor
development, and tumor proliferation and differentiation (23). Typically, cyclin D1 serves a
critical role in the G1 stage of the cell cycle, which
may accelerate cell cycle progression (23). In the present study, it was
identified that overexpression of miR-25 increased cyclin D1
protein expression in colon cancer cells. Huo et al
(24) suggested that upregulation
of miR-25 mediates the migration of melanoma cells by targeting
Dickkopf-related protein 3 through effects on the expression of Myc
proto-oncogene protein and cyclin D1. A limitation of the study was
that only si-ATXN3 was used to downregulate the expression of
ATXN3. Other mechanisms that regulate ATXN3 expression, including
methylation, require investigation in future studies.
In conclusion, the present results demonstrate that
miR-25 promoted human colon cancer cell growth and migration, and
inhibited apoptosis via ATXN3 expression. The miR-25/ATXN3 axis
provides novel insight into the pathogenesis of human colon cancer,
particularly with respect to promote proliferation and metastasis
of CRC, and it represents a potential therapeutic target for human
colon cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DL designed the experiments. TZ, JL, JZ, TW, YL, SH
and ZH performed the experiments. DL and TZ analyzed the data. DL
wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Yue Bei People's Hospital (Shaoguan, China).
Written informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guo J, Tao W, Tang D and Zhang J:
Th17/regulatory T cell imbalance in sepsis patients with multiple
organ dysfunction syndrome: attenuated by high-volume
hemofiltration. Int J Artif Organs. 40:607–614. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zivkovic AR, Tourelle KM, Brenner T,
Weigand MA, Hofer S and Schmidt K: Reduced serum cholinesterase
activity indicates splenic modulation of the sterile inflammation.
J Surg Res. 220:275–283. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang X, Luo B, Lu Y, Pang D, Zheng J, Mo
J, Huang H and Feng J: The triggering receptor expressed by myeloid
cells-1 activates TLR4-MyD88-NF-κB-dependent signaling to aggravate
ventilation-induced lung inflammation and injury in mice. Cell
Tissue Res. 374:137–148. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li JZ, Wang ZL, Xu WH, Li Q, Gao L and
Wang ZM: MicroRNA-495 regulates migration and invasion in prostate
cancer cells via targeting Akt and mTOR signaling. Cancer Invest.
34:181–188. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang H, Li XT, Wu C, Wu ZW, Li YY, Yang
TQ, Chen GL, Xie XS, Huang YL, Du ZW and Zhou YX: miR-132 can
inhibit glioma cells invasion and migration by target MMP16 in
vitro. Onco Targets Ther. 8:3211–3218. 2015.PubMed/NCBI
|
|
6
|
Zhang D, Sun G, Zhang H, Tian J and Li Y:
Long non-coding RNA ANRIL indicates a poor prognosis of cervical
cancer and promotes carcinogenesis via PI3K/Akt pathways. Biomed
Pharmacother. 85:511–516. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yao C, Shi X, Zhang Z, Zhou S, Qian T,
Wang Y, Ding F, Gu X and Yu B: Hypoxia-induced upregulation of
miR-132 promotes schwann cell migration after sciatic nerve injury
by targeting PRKAG3. Mol Neurobiol. 53:5129–5139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leinders M, Üçeyler N, Pritchard RA,
Sommer C and Sorkin LS: Increased miR-132-3p expression is
associated with chronic neuropathic pain. Exp Neurol. 283:276–286.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu T, Pang Q, Wang Y and Yan X: Betulinic
acid induces apoptosis by regulating PI3K/Akt signaling and
mitochondrial pathways in human cervical cancer cells. Int J Mol
Med. 40:1669–1678. 2017.PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yadav N and Chandra H: Suppression of
inflammatory and infection responses in lung macrophages by
eucalyptus oil and its constituent 1,8-cineole: Role of pattern
recognition receptors TREM-1 and NLRP3, the MAP kinase regulator
MKP-1, and NFκB. PLoS One. 12:e01882322017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van Kampen JGM, van Hooij O, Jansen CF,
Smit FP, van Noort PI, Schultz I, Schaapveld RQJ, Schalken JA and
Verhaegh GW: miRNA-520f Reverses epithelial-to-mesenchymal
transition by targeting ADAM9 and TGFBR2. Cancer Res. 77:2008–2017.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu XJ, Zheng XP, Zhang R, Guo YL and Wang
JH: Combinatorial effects of miR-20a and miR-29b on neuronal
apoptosis induced by spinal cord injury. Int J Clin Exp Pathol.
8:3811–3818. 2015.PubMed/NCBI
|
|
14
|
He J, Qi H, Chen F and Cao C: MicroRNA-25
contributes to cisplatin resistance in gastric cancer cells by
inhibiting forkhead box O3a. Oncol Lett. 14:6097–6102.
2017.PubMed/NCBI
|
|
15
|
Li W, Lu M, Zhang Y, Xia D, Chen Z, Wang
L, Yin N and Wang Z: Puerarin attenuates the daunorubicin-induced
apoptosis of H9c2 cells by activating the PI3K/Akt signaling
pathway via the inhibition of Ca2+ influx. Int J Mol Med.
40:1889–1894. 2017.PubMed/NCBI
|
|
16
|
Qiu ZK, Zhong DS, He JL, Liu X, Chen JS
and Nie H: The anxiolytic-like effects of puerarin are associated
with the changes of monoaminergic neurotransmitters and
biosynthesis of allopregnanolone in the brain. Metab Brain Dis.
33:167–175. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feng T, Zheng L, Liu F, Xu X, Mao S, Wang
X, Liu J, Lu Y, Zhao W, Yu X and Tang W: Growth factor progranulin
promotes tumorigenesis of cervical cancer via PI3K/Akt/mTOR
signaling pathway. Oncotarget. 7:58381–58395. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang F, Zhang L, Long Z, Chen Z, Hou X,
Wang C, Peng H, Wang J, Li J, Duan R, et al: miR-25 alleviates
polyQ-mediated cytotoxicity by silencing ATXN3. FEBS Lett.
588:4791–4798. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bahadori M, Baharara J and Amini E:
Anticancer properties of chrysin on colon cancer cells, in vitro
and in vivo with modulation of caspase-3, −9, Bax and Sall4. Iran J
Biotechnol. 14:177–184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
LeBlanc H, Lawrence D, Varfolomeev E,
Totpal K, Morlan J, Schow P, Fong S, Schwall R, Sinicropi D and
Ashkenazi A: Tumor-cell resistance to death receptor-induced
apoptosis through mutational inactivation of the proapoptotic Bcl-2
homolog Bax. Nat Med. 8:274–281. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang H, Zuo Z, Lu X, Wang L, Wang H and
Zhu Z: MiR-25 regulates apoptosis by targeting Bim in human ovarian
cancer. Oncol Rep. 27:594–598. 2012.PubMed/NCBI
|
|
22
|
Wang J, Li XM, Bai Z, Chi BX, Wei Y and
Chen X: Curcumol induces cell cycle arrest in colon cancer cells
via reactive oxygen species and Akt/GSK3β/cyclin D1 pathway. J
Ethnopharmacol. 210:1–9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li C, Peng W, Song X, Wang Q and Wang W:
Anticancer effect of icaritin inhibits cell growth of colon cancer
through reactive oxygen species, Bcl-2 and cyclin D1/E signaling.
Oncol Lett. 12:3537–3542. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huo J, Zhang Y, Li R, Wang Y, Wu J and
Zhang D: Upregulated MicroRNA-25 mediates the migration of melanoma
cells by targeting DKK3 through the WNT/β-catenin pathway. Int J
Mol Sci. 17(pii): E11242016. View Article : Google Scholar : PubMed/NCBI
|