Introduction
In the context of kidney transplantation, the
interactions between the innate and adaptive alloimmune responses
have not yet been fully investigated. Cytotoxic type 1 innate
lymphoid cells, NK cells and NKT cells may represent an interaction
between innate immune activity and adaptive alloimmune response, as
these cells have the ability to distinguish allogeneic cells from
self-cells. In previous studies, NK cells have also been
demonstrated to serve a paradoxical role in allograft acceptance
and dysfunction in solid organ transplantation through their effect
on the immune pathways involved in allograft tolerance and
rejection (1–3). Several studies have also indicated
that NK cells alone are not sufficient for direct rejection of a
solid allograft, but that they participate in the acute rejection
response by facilitating the action of alloreactive T cells,
supporting the maturation of immature recipient dendritic cells and
upregulating MHC class II expression on the graft endothelium by
producing interferon-γ (IFN-γ) (1). In addition, host NK cells have been
demonstrated to contribute to the induction of transplant tolerance
by limiting the persistence of donor-derived dendritic cells
through their killing of allogeneic antigen-presenting cells
(2,4).
Human NK cells are typically divided into two
phenotypic subsets, based on the level of neural cell adhesion
molecule 1 (CD56) and low affinity immunoglobulin gamma Fc region
receptor III (FcγRIII/CD16) expression:
CD56dimCD16+ and
CD56brightCD16−/+. The
CD56dimCD16+ population is cytotoxic and
forms at least 90% of all peripheral blood NK cells. By contrast,
CD56bright NK cells, which express low levels of CD16,
are less cytotoxic but produce immunoregulatory cytokines,
including IFN-γ, following activation in response to stimulation
with cytokines, including interleukin (IL)-2, IL-12 and IL-15, and
are less cytotoxic compared with CD56dim NK cells. By
contrast, NK cells from secondary lymphoid tissues and from other
tissues, including the liver and uterus, primarily exhibit the
CD56bright phenotype (5). Increasing evidence has indicated that
alloantibodies may trigger NK cell activation via FcγRIIIA and
contribute to antibody-mediated rejection via antibody-dependent
cell-mediated cytotoxicity and cytokine production (3). Additionally, lower NK cell numbers
and increased proportions of CD56bright NK cells were
identified in donor-specific antibody (DSA)-positive kidney
transplant recipients; this may indicate improved
antibody-dependent turnover following activation via CD16 (6). Similarly, increased percentages of
the CD56bright NK subset were identified in kidney
transplant recipients with progressive chronic allograft
dysfunction (7). However, studies
examining the frequency and phenotype of NK cells in acute
T-cell-mediated renal allograft rejection (ACR) is rare. Assessment
of the circulating NK cell subsets in renal allograft recipients
may contribute to defining signature alloreactive responses.
CD56bright NK cells are enriched in the
majority of human tissues, with the exception of blood, and
represent the majority of NK cells. CD56bright NK cells
appear to be outnumbered by CD56dim NK cells in the
lung, kidney, mammillary tissue, bone marrow and spleen, but this
may be a reflection of the high rate of blood perfusion in these
organs (8,9). In addition, unique subsets of
tissue-resident CD56bright NK cells have been described
in the lymphoid tissue, liver and uterus (9). Shin et al (10) demonstrated that CD56+
cell infiltration in kidney allografts is associated with poor
death-censored graft survival. However, studies concerning the
distribution of tissue-resident CD56+ NK cells in kidney
allografts with ACR are limited.
NKT cells constitute a conserved T cell sublineage
with unique properties, including reactivity against a synthetic
glycolipid presented by cluster of differentiation 1 (CD1)d,
expression of an invariant T cell antigen receptor α chain and
unusual requirements for thymic selection. NKT cells have been
indicated to serve key roles in the maintenance of allograft
tolerance by producing IL-10, and interacting with regulatory T
cells (Treg) cells by altering Treg cell function (2,11).
For example, Hongo et al (11) identified that IL-4 produced by NKT
cells may affect IL-10 production in Tregs (12–14).
However, there are few studies on the role of NKT-like cells in
ACR. As CD3+CD56+ NKT-like cells are not
classical invariant NKT cells, but represent a broader group of T
cells matching the original definition of NKT cells (15,16),
the present study measured the levels of
CD3+CD56+ NKT-like cells and considered them
to be indicative of the levels of NKT cells.
Acute rejection (AR) is an allograft-destructive
immune response that usually occurs in the first month following
transplantation, but may arise at any time during the life-span of
a renal transplant. Depending on the dominant mechanism,
morphological characteristics and the primary site of injury, AR is
sub-categorized into ACR and antibody-mediated allograft rejection
(AMR). Quite often, a combination of several mechanisms with
different types graft damage occur simultaneously or consecutively,
which result in AMR coexisting with ACR. The Banff classification
schemes have evolved for the assessment and grading of allograft
rejection: The diagnosis and grading of ACR is based on the
presence and degree of interstitial inflammation, tubulitis and
endothelialitis in the renal allograft. Present criteria require
the presence of all 3 of the following elements for a confirmed
diagnosis of AMR: i) Evidence of antibody interaction with vascular
endothelium, in particular complement 4 molecule C4d deposition;
ii) morphologic evidence of acute tissue injury (capillaritis,
fibrin thrombi and tubular injury/necrosis); and iii)
donor-specific antibodies (17,18).
In the present study, longitudinal changes in NK
cell and NKT-like cell frequency and phenotype in the blood and
kidney allograft tissue in the first year following transplantation
were assessed, and their associations with ACR were explored.
Furthermore, the serum concentrations of the NK- and NKT-associated
chemokines and cytokines C-C motif ligand (CCL) 19, CCL21, IFN-γ,
tumor necrosis factor-α (TNF-α), IL-2, IL-10, IL-12 and IL-15 were
assessed in patients at different stages of ACR and stable
controls, as CD56bright NK cells have been demonstrated
to produce high levels of the pro-inflammatory cytokines IFN-γ and
TNF-α, and notably, IL-12 (19,20).
In addition, IL-15 is a key cytokine involved in the expansion,
survival and function of NKT cells (21,22),
and IL-10 may function as an effector cytokine of NKT-cell-mediated
transplant tolerance (12).
Finally, CCL19 and CCL21 are ligands of C_C chemokine receptor type
7 (CCR7), which is homing receptor of CD56bright NK
cells.
Materials and methods
Patients
The present study was conducted on 142 renal
transplant recipients [72 patients with ACR, 9 patients with AMR, 3
patients with ACR and AMR, 52 recipients with stable renal
allograft function, 3 patients with ischemia reperfusion injury
(IRI) and 3 patients with calcineurin inhibitor (CNI) toxicity] who
underwent renal transplant procedures at The 309th Hospital of the
Chinese People's Liberation Army (Beijing, China) and 20 healthy
volunteers. The patients with ACR were divided as follows:
Early-stage ACR, when ACR occurred within the first month after
transplantation; mid-stage ACR, between 2 and 6 months after
transplantation; and late-stage ACR, between 6 and 12 months after
transplantation. Recipients with stable renal allograft function
were classified into two groups: Patients who were in the first
month following transplantation (n=47) and patients who were
between 2–6 months following transplantation (n=5). These were
recruited as the stable controls for the early-stage ACR and
mid-stage ACR groups, respectively (Fig. 1) (23). Healthy control subjects were
recruited from staff from The 309th Hospital of the Chinese
People's Liberation Army, and were age- and sex-matched to the
transplant cohort. All protocols were approved by the Ethics
Committee of The 309th Hospital of the Chinese People's Liberation
Army, and all patients and the healthy volunteers provided informed
consent for the use of samples for research. All donors provided
informed consent for kidney tissue donation. All patients received
standard triple therapy that consisted of cyclosporin A (CsA) or
FK506 and mycophenolate mofetil and steroids. CsA was initiated at
6–8 mg/kg/d and FK506 at 0.05–0.25 mg/kg/d. The drug dosage was
adjusted according to the plasma concentration. The target plasma
concentration for CsA and FK506 was 200–350 and 10–15 µg/l,
respectively, during the first month following transplantation;
150–300 and 8–15 µg/l, respectively, from the second to the third
month; 100–250 and 5–12 µg/l, respectively, from the fourth to the
twelfth month; and around 50 and 5–10 µg/l, respectively, after the
first year. MMF was started at 1.5–2 g/d for half a month and
maintained at 1 g/d thereafter. Methylprednisolone, 8–10 mg/kg/day
for 3 days and prednisone gradually reduced to 10 mg/day. Patient
characteristics are summarized in Table I.
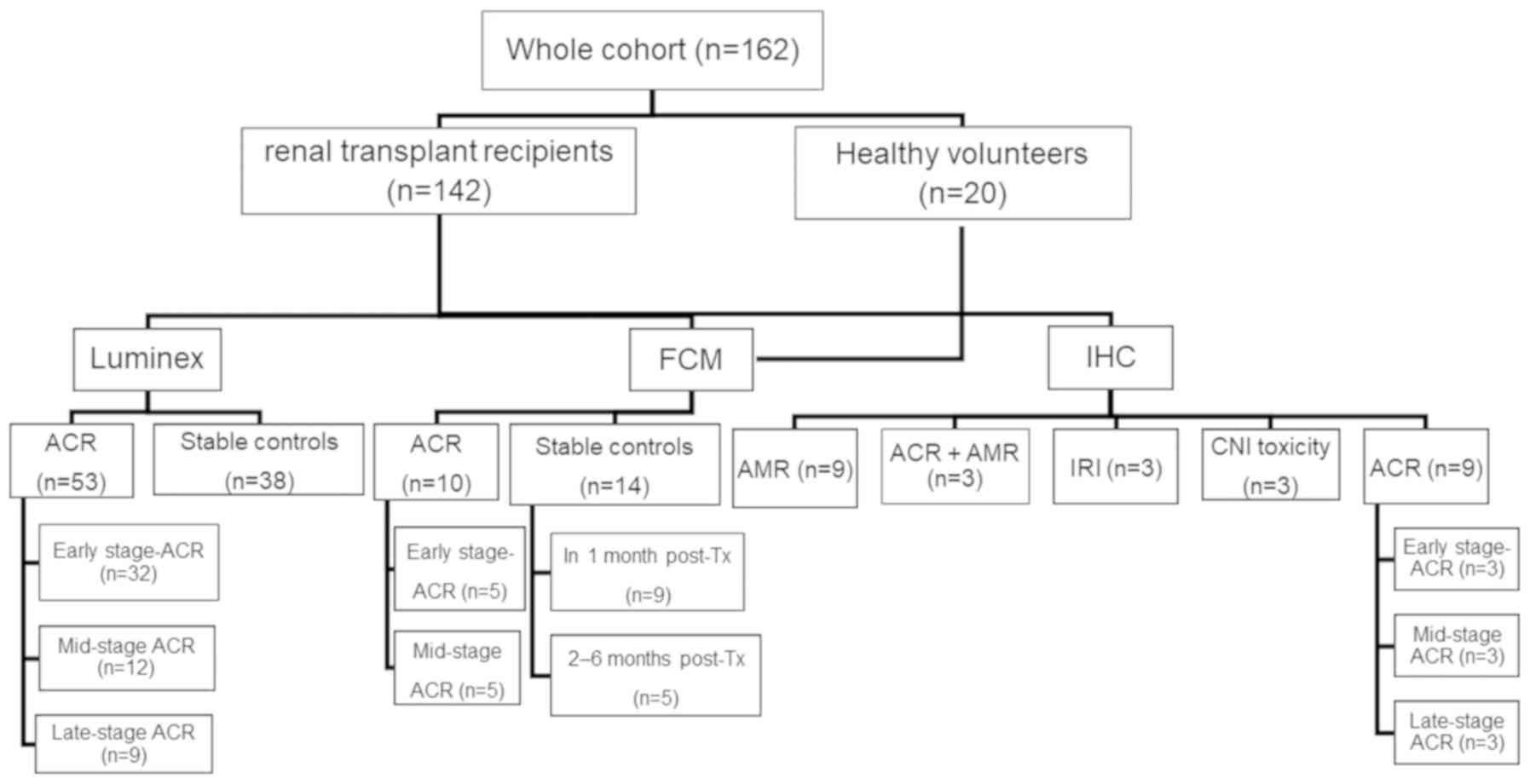 | Figure 1.Patient grouping and assessment. The
entire cohort consisted of 162 patients, divided into two groups:
Healthy volunteers (n=20); and renal allograft recipients (n=142).
The recipient group was divided into the following subgroups: The
ACR group (n=53) and the stable control group (n=38). Sera from 91
patients, including the patients with ACR (n=53) and the stable
controls (n=38), were used to assess cytokine expression profiles
by multiplex immunoassay. The ACR group was divided into the
early-stage ACR group (ACR occurred within the first month after
transplantation), the mid-stage ACR group (ACR occurred between 2–6
months after transplantation); and the late-stage ACR group (ACR
occurred between 7–12 months after transplantation). Peripheral
blood mononuclear cells from 44 subjects, including healthy
volunteers (n=20), patients with ACR (n=10) and stable controls
(n=14), were used for flow cytometric identification of the cell
subsets. Biopsy samples (n=27) from patients with ACR (n=9), AMR
(n=9), ACR and AMR (n=3), patients with IRI (n=3) and patients with
CNI toxicity (n=3) were used to determine the expression of CD56 by
immunohistochemistry. ACR, patients with acute cellular renal
allograft rejection; AMR, antibody-mediated allograft rejection;
IRI, ischemic reperfusion injury; CNI toxicity, calcineurin
inhibitor toxicity. |
 | Table I.Clinical characteristics and
parameters associated with renal transplantation. |
Table I.
Clinical characteristics and
parameters associated with renal transplantation.
|
Characteristics | ST | ACR | AMR | HC |
P-valuea |
|---|
| Number of patients,
n | 52 | 72 | 9 | 20 | – |
| Sex ratio
(F/M) | 23/29 | 21/51 | 3/6 | 10/10 | 0.101 |
| Age, years | 40.5±11.7 | 39.3±11.1 | 41±13.6 | 38.1±6.8 | 0.878 |
| Pre-sensitized
patients (PRA >10%) | 1 | 4 | 9 | 0 | 0.303 |
| Serum creatinine,
µM | 74.3±15.9 | 238.6±123.6 | 254.5±113.9 | 69.9±12.7 | 0.141 |
| CNI at the time of
biopsy |
|
|
|
| 0.537 |
|
Cyclosporine A | 25 | 38 | 6 | – |
|
FK506 | 27 | 34 | 3 | – |
Tissue sampling and patient
information
Tissues were obtained from patients with early-stage
ACR (n=3), mid-stage ACR (n=3), late-stage ACR (n=3), early-stage
AMR (n=3), mid-stage AMR (n=3), late-stage AMR (n=3), ACR and AMR
(n=3), IRI (within 1 month after kidney transplantation; n=3) and
CNI toxicity (2–6 months after kidney transplantation; n=3). Kidney
transplant biopsy samples were obtained from The 309th Hospital of
the Chinese People's Liberation Army between 2013 and 2016. Graft
rejection was assessed based on the Banff 2013 classification
(17).
Flow cytometry
Peripheral blood samples were obtained from patients
with early-stage ACR (n=5), mid-stage ACR (n=5), recipients with
stable renal allograft function within the first month after
transplantation (n=9), recipients with stable renal allograft
function between 2–6 months after transplantation (n=5) and healthy
volunteers (n=20). Peripheral blood mononuclear cells (PBMCs) were
separated from heparinized venous blood samples by density gradient
centrifugation (Centrifuged at 400 × g for 30–40 min at 18–20°C)
over Ficoll-Uropoline (Ficoll Paque Plus; GE Healthcare, Uppsala,
Sweden). Subsequently, cell viability was assessed with fixable
viability stain450 (FVS450; cat. no. 562247) and PBMCs were stained
for 30 min at 4°C extracellularly with anti-human CD3-fluorescein
isothiocyanate (1:5; cat. no. 340542), anti-human
CD56-allophycocyanin (1:5; cat. no. 555518), anti-human
CD16-phycoerythrin-cy7 (1:20; cat. no. 557744) or the respective
isotype control antibodies such as mouse IgG1-FITC (1:100; cat. no.
554679), mouse IgG1-APC (1:5; cat. no. 555751), and mouse
IgG1-PE-Cy™7 (1:20; cat. no. 557872; all from BD Pharmingen; BD
Biosciences, Franklin Lakes, NJ, USA). NK cell and NKT-like cell
subset distribution was then assessed by flow cytometry using
FACSCantoII Plus™ and FACSCalibur (BD Biosciences) and analyzed
with the FlowJo software (vX.0.7; Tree Star, Inc., Ashland, OR,
USA). The gating strategy used to identify subsets was performed
according to a previously described protocol (24). Representative images of the gating
strategies used to identify subsets are presented in Fig. 2. NK cells were identified as
CD3−CD56+ cells, and NKT-like cells were
identified as CD3+CD56+ cells. PBMCs from
healthy controls and the stable controls were analyzed again
independently from the ACR group using the same gating strategies
(Fig. 2). The frequency of NK and
NKT-like cells was expressed as the percentage of total cells by
sequential gating of lymphocyte populations.
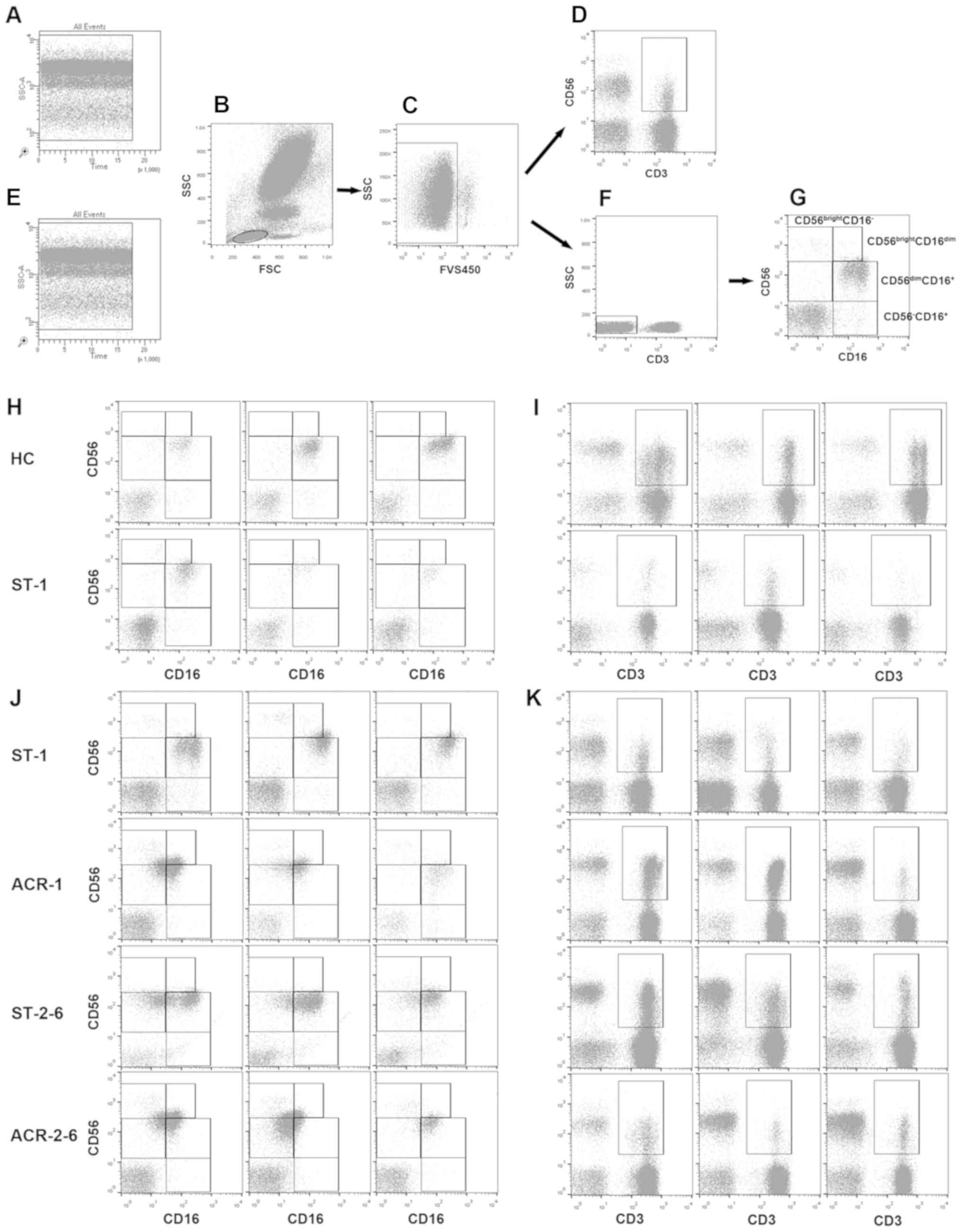 | Figure 2.Gating strategy for the
identification of NK cell subsets and NKT-like cells. The
peripheral blood mononuclear cells were stained with fluorescein
isothiocyanate-labelled anti-human CD3 antibody,
allophycocyanin-labelled anti-human CD56 antibody and
phycoerythrin-cy7-labeled anti-human CD16 antibody. (B) Lymphocyte
populations were identified based on FSC and SSC characteristics.
(A) Time gating and (E) single gating for the samples were
routinely performed using FACSCanto II Plus™; lymphocytes were
additionally gated as (C) FVS450− cells (live
lymphocytes), (D) CD3+CD56+ cells (NKT-like
cells) and (F) CD3− lymphocytes, which were then
subsequently gated according to (G) CD16 and CD56 expression, as
CD56bright CD16−, CD56bright
CD16dim and CD56dim CD16+. A total
of 3 representative plots of (H) CD16 and CD56 expression and (I)
NKT-like cells for HC and renal allograft recipients with stable
function using FACSCanto II Plus™ and for renal allograft
recipients with ACR and renal allograft recipients with stable
function using FACSCalibur of (J) CD16 and CD56 expression and (K)
NKT-like cells are presented. FSC, forward scatter; SSC, side
scatter; FVS450, fixable viability stain 450; HC, healthy controls;
ST-1, recipients with stable allograft function (occurring within
the first month after transplantation); ACR-1, early-stage acute
cellular rejection (occurring within the first month after
transplantation); ACR-2-6, middle-stage acute cellular rejection
(occurring between 2 and 6 months after transplantation); ST-2-6,
recipients with stable allograft function (occurring between 2 and
6 months after transplantation); CD3, cluster of differentiation 3;
CD56, neural cell adhesion molecule 1; CD16, low affinity
immunoglobulin gamma Fc region receptor III. |
Serum chemokine and cytokine
analysis
Serum samples were separated from peripheral blood
samples, which were collected from 38 recipients with stable graft
function, 32 patients with early-stage ACR, 12 patients with
mid-stage ACR and 9 patients with late-stage ACR. Peripheral blood
samples were taken at several time points and frozen at −80°C until
subsequent use. For the present study, pre-ACR samples prior to
rejection (serum creatinine, <103 µM), and at the time of ACR,
prior to the initiation of antirejection therapy, and following the
reversal of rejection (serum creatinine, <103 µM) were selected.
For patients with stable graft function, follow-up time points
matching the rejection time points were selected. Serum samples
were assayed to measure the levels of IL-4, IL-10, IL-12p70, IL-15,
CCL19, CCL21, IFN-γ and TNF-α using a human cytokine multiplex
immunoassay (MILLIPLEX® MAP kit; Merck KGaA, Darmstadt,
Germany) with the Luminex 200 system (Luminex Corp., Austin, TX,
USA), as described previously (25). The observed intensities of
duplicate samples were averaged and mapped to a fitted curve
derived from a serial dilution series of known cytokine standards
(26). In addition, recipients
with stable graft function were considered as negative
controls.
Immunohistological analysis
The renal cortex of freshly explanted allograft or
control kidneys was dissected and fixed in 10% neutral buffered
formalin for 24 h at room temperature, then embedded in paraffin.
Paraffin sections were each cut at a thickness of 2 µm and mounted
on positively-charged slides. All slides were stained with
hematoxylin (cat. no. ZLI 9608) and eosin (cat. no. ZLI 9613; both
OriGene Technologies, Inc., Beijing, China) for 3–5 min at room
temperature and used for grading of acute rejection and the
controls (IRI and CNI toxicity). Histopathological evaluations were
performed by two pathologists who specialized in rejection
diagnosis according to the Banff 2013 classification (17), by observation through an Olympus
CX51 light microscope (original magnification, ×100, ×200 or ×400,
Olympus Corporation, Tokyo, Japan). Indirect immunoperoxidase
staining was performed with the following primary antibodies: Mouse
anti-human protein tyrosine phosphatase, receptor type, C (clone
UCHL1; OriGene Technologies, Inc.), rabbit anti-human CD56 (clone
UMAB83; OriGene Technologies, Inc.) and rabbit anti-human C4d
(polyclonal; Biomedica Medizinprodukte GmbH & Co KG, Vienna,
Austria). Incubation with the primary antibody was followed by
single-antigen detection with a biotin-free polymer-based system
(PV-9000 Detection System OriGene Technologies, Inc. Negative
control experiments were performed by omitting the primary
antibody.
Statistical analysis
The levels of cytokines and chemokines were
expressed as the median and interquartile range (pg/ml). Receiver
operating characteristic (ROC) curves were created to determine the
diagnostic value of the ratios of CD56bright NK/NKT-like
cell and CD56bright/CD56dim cell in
distinguishing between patients with ACR and the control group. To
compare serum concentrations of cytokines and chemokines between
groups, the Kruskal-Wallis test followed by Mann-Whitney post-hoc
tests with a Bonferroni adjustment to the level of significance
(P=0.05/k=0.05/6=0.008, where k represents the number of pairwise
comparisons) was utilized (27).
For all other data in which statistics were performed, including
the proportions of the cell subsets and the ratios, two-tailed
nonparametric Mann-Whitney U-tests were used to evaluate the
differences in variables between patients with ACR and the control
groups. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using SPSS 13.0 software (SPSS Inc., Chicago, IL, USA).
Results
Clinical characteristics and
background variables
Patients with stable graft function and acute renal
allograft rejection did not differ with respect to age, sex or the
immunosuppressive protocol employed (Table I).
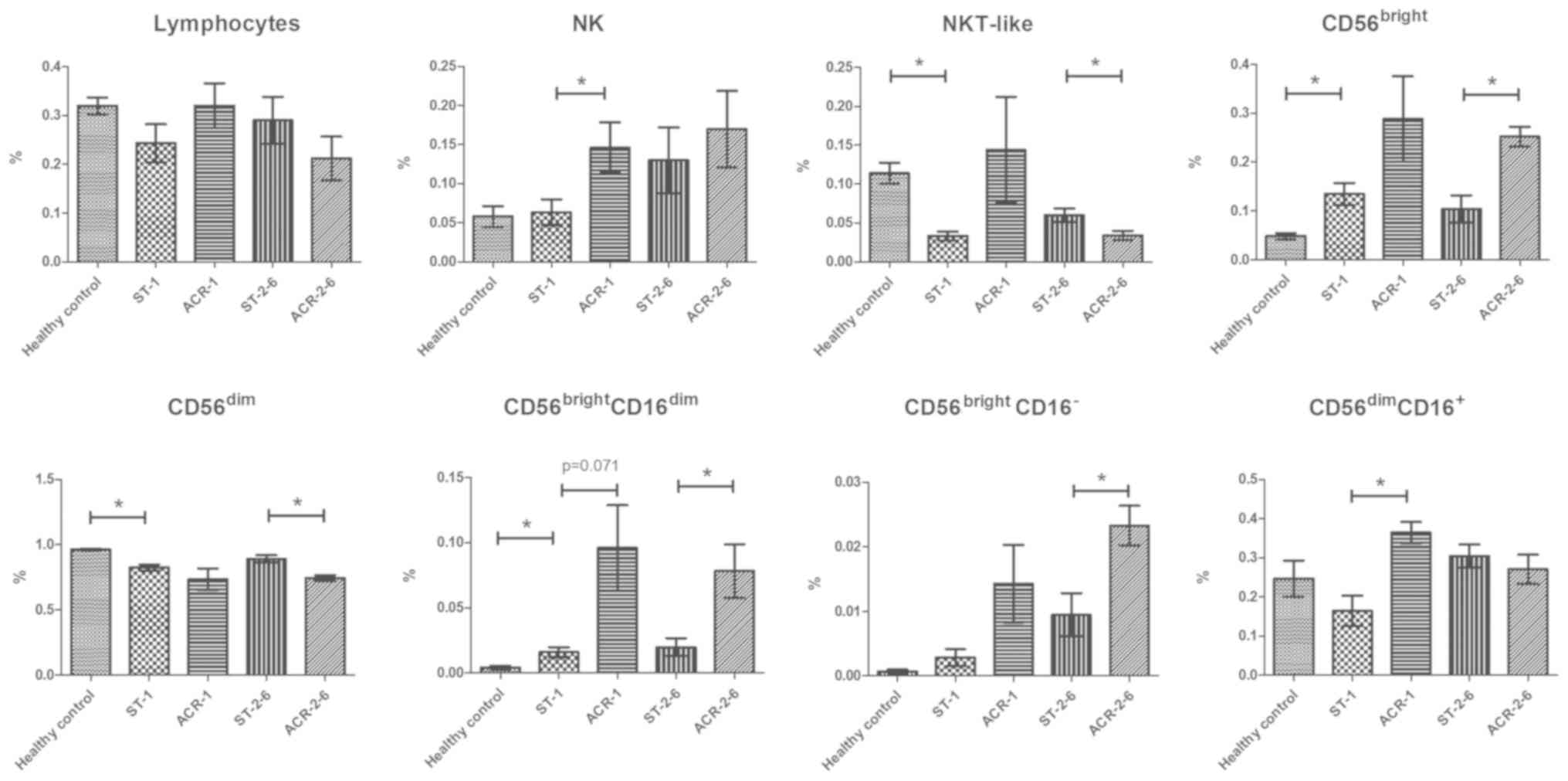
Increased proportions of
CD56bright cells and decreased proportions of
CD56dim cells in the peripheral blood of kidney
transplant recipients
To evaluate the immune status of kidney transplant
recipients, the proportions of lymphocytes, T cells and NK cell
subsets were analyzed. In contrast to the healthy controls, the
kidney transplant recipients exhibited increased proportions of
CD56bright subsets (P=0.018), in particular the
CD56brightCD16dim cells (P=0.029), and
decreased proportions of CD56dim subsets (P=0.0001).
However, the proportion of lymphocytes and
CD3−CD56+ NK cells was similar between the
two groups. In addition, the
CD56bright/CD56dim ratio was significantly
increased in the transplant recipients (P=0.002; Fig. 3).
Increase in the
CD3−CD56bright subset frequency and decrease
in the CD3−CD56dim subset frequency in PBMCs
from recipients with middle-stage ACR
The proportions of peripheral blood NK cell subsets
in the 24 kidney transplant recipients (5 patients with early-stage
ACR, 5 patients with middle-stage ACR, 9 recipients with stable
renal allograft function within 1 month after transplantation and 5
recipients with stable renal allograft function between 2–6 months
after transplantation) and 20 healthy volunteers were analyzed by
flow cytometric detection of CD56 and CD16 expression. Compared
with the recipients with stable graft function, patients with
early-stage ACR exhibited increased proportions of NK cells
(P=0.026). In addition, an increase in the percentage of
CD56brightCD16dim cells was observed in
transplant patients with early-stage ACR compared with the controls
(P=0.071), but the increase was not statistically significant
(Fig. 2). There was a significant
increase in the percentage of CD56dimCD16+
cells in transplant patients with early-stage ACR compared with the
controls (P=0.014). Notably, compared with the recipients with
stable graft function, patients with middle-stage ACR exhibited a
greater proportion of CD56brightCD16−
(P=0.016) and CD56brightCD16dim subsets
(P=0.045), which was associated with an increase in the
CD56bright/CD56dim ratio compared with the
controls (P=0.008). In addition, a decreased frequency of
CD56dimCD16+ cells (P=0.079) and
CD56−/CD16+ cells (P=0.071) was observed in
transplant patients with middle-stage ACR compared with the
controls (Fig. 3).
Decreased
CD3+CD56+ NKT-like cell proportions in
patients with middle-stage ACR
Analysis of the distribution of
CD3+CD56+ NKT-like cells in the PBMCs of
kidney transplant recipients indicated that there was a significant
decrease in the proportion of NKT-like cells in the PBMCs of kidney
transplant recipients compared with the healthy controls. In
addition, there was a trend towards increase in the percentage of
NKT-like cells (P=0.092) in transplant patients with early-stage
ACR compared with the controls. However, there was a significant
decrease in the percentage of NKT-like cells in transplant patients
with middle-stage ACR compared with recipients with stable graft
function (P=0.042; Fig. 3).
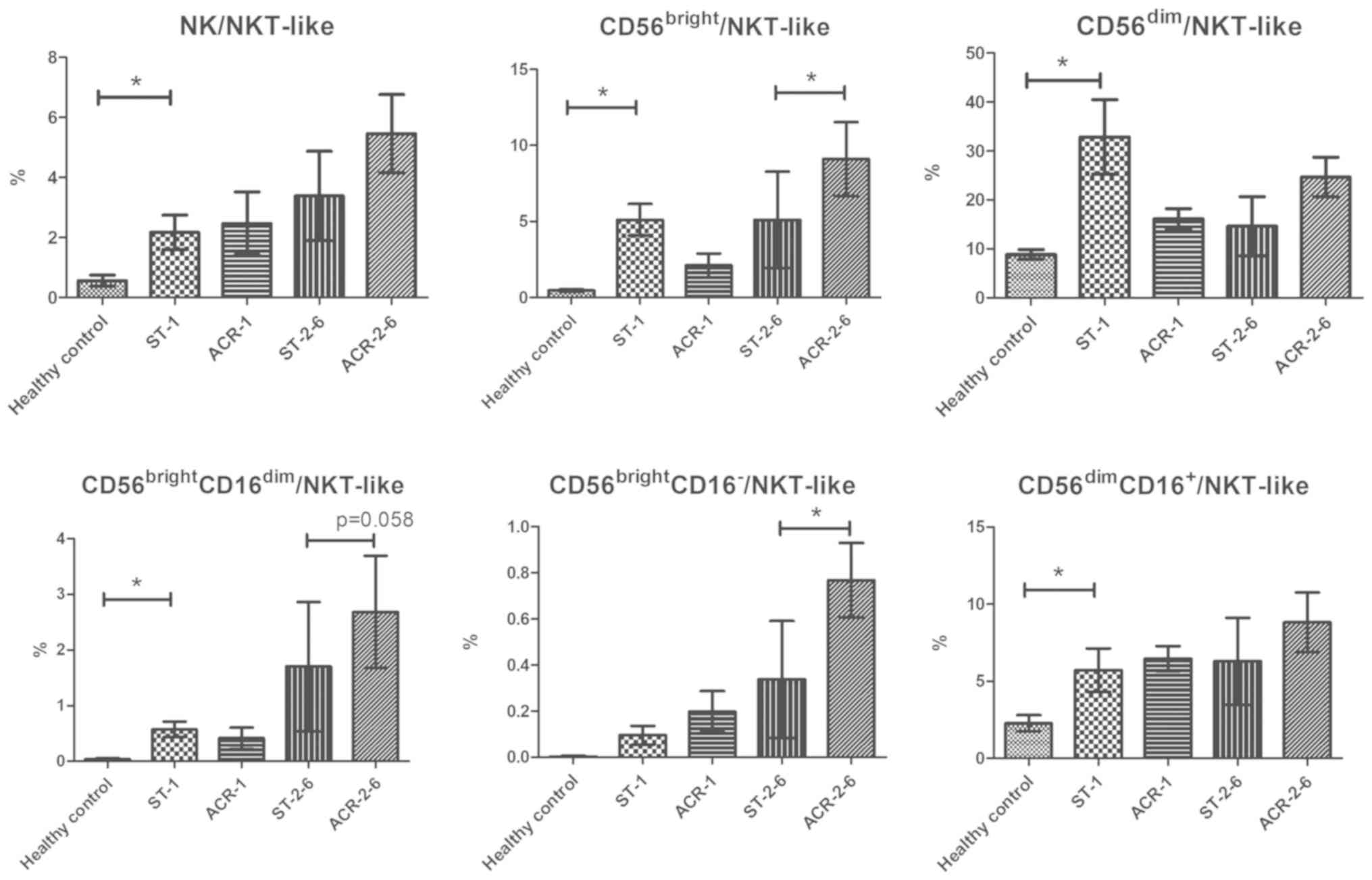
Increase in CD56bright
NK/NKT-like cell ratios in ACR
As it has been demonstrated that
CD56bright NK cells may be involved in destructive
immune reactions while NKT-like cells may participate in transplant
tolerance (10,12), the ratios of CD56bright
NK/NKT-like cells in PBMCs were analyzed to determine whether they
are useful indexes for the diagnosis or prognosis of ACR. The ratio
of NK/NKT-like cells (P=0.025), CD56brightNK/NKT-like
cells (P=0.002), CD56brightCD16dimNK/NKT-like
cells (P=0.005), CD56dimNK/NKT-like cells (P=0.040), and
CD56dimCD16+NK/NKT-like cells (P=0.045) in
kidney transplant recipients was markedly increased compared with
that in the healthy controls. However, there was no significant
difference in the ratios between patients with early-stage ACR and
recipients with stable allograft function. Notably, the ratio of
CD56bright NK/NKT-like cells (P=0.043) and
CD56brightCD16− NK/NKT-like cells (P=0.014)
in the PBMCs of patients with middle-stage ACR exhibited a
significant increase compared with the stable controls. In
addition, there was a significant increase in the ratio of
CD56brightCD16dim NK/NKT-like cells in the
PBMCs of patients with middle-stage ACR compared with the stable
controls (P=0.058; Fig. 4).
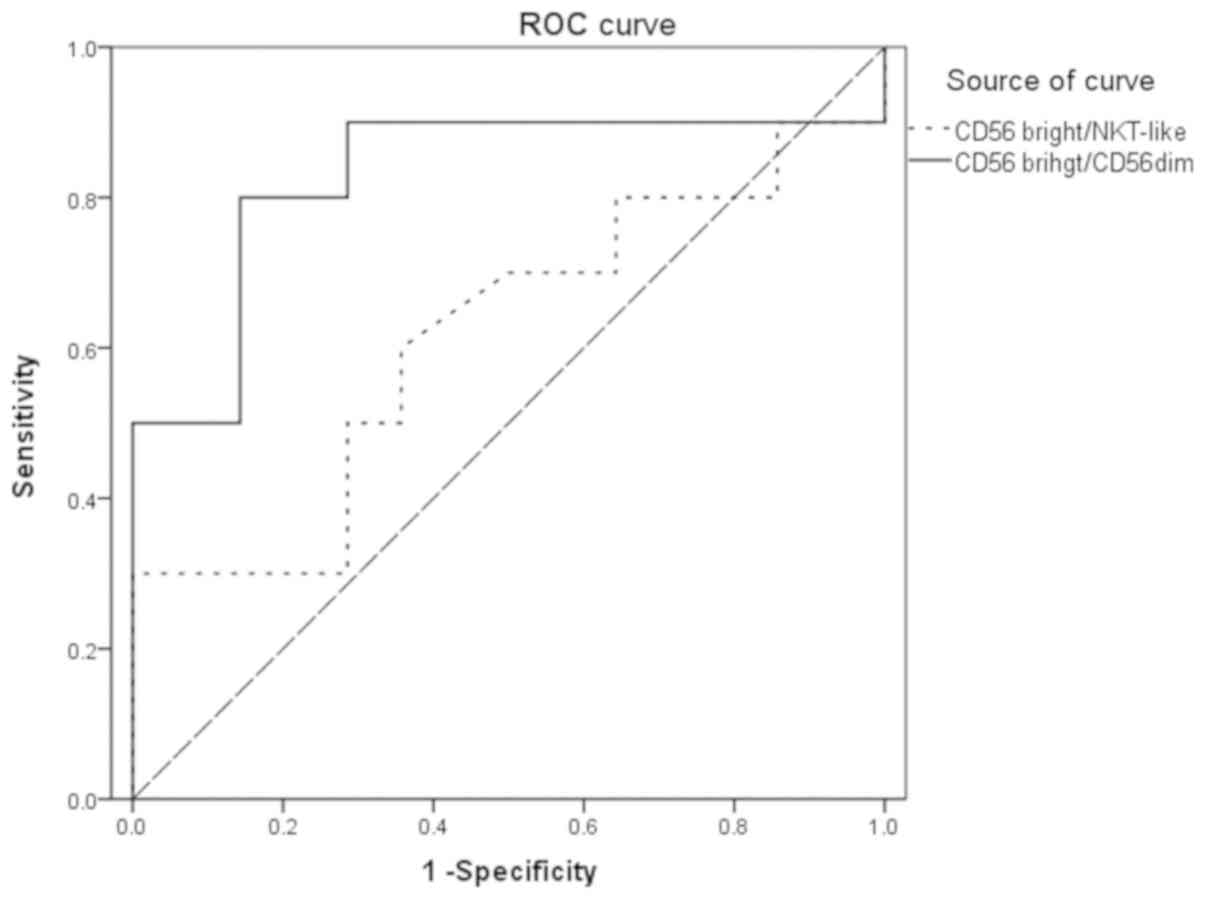
Finally, the area under the ROC curve (AUC) for the
CD56bright NK/NKT-like cell ratios was 0.614, which is
decreased compared with that for the
CD56bright/CD56dim cell ratio (AUC=0.829;
Fig. 5).
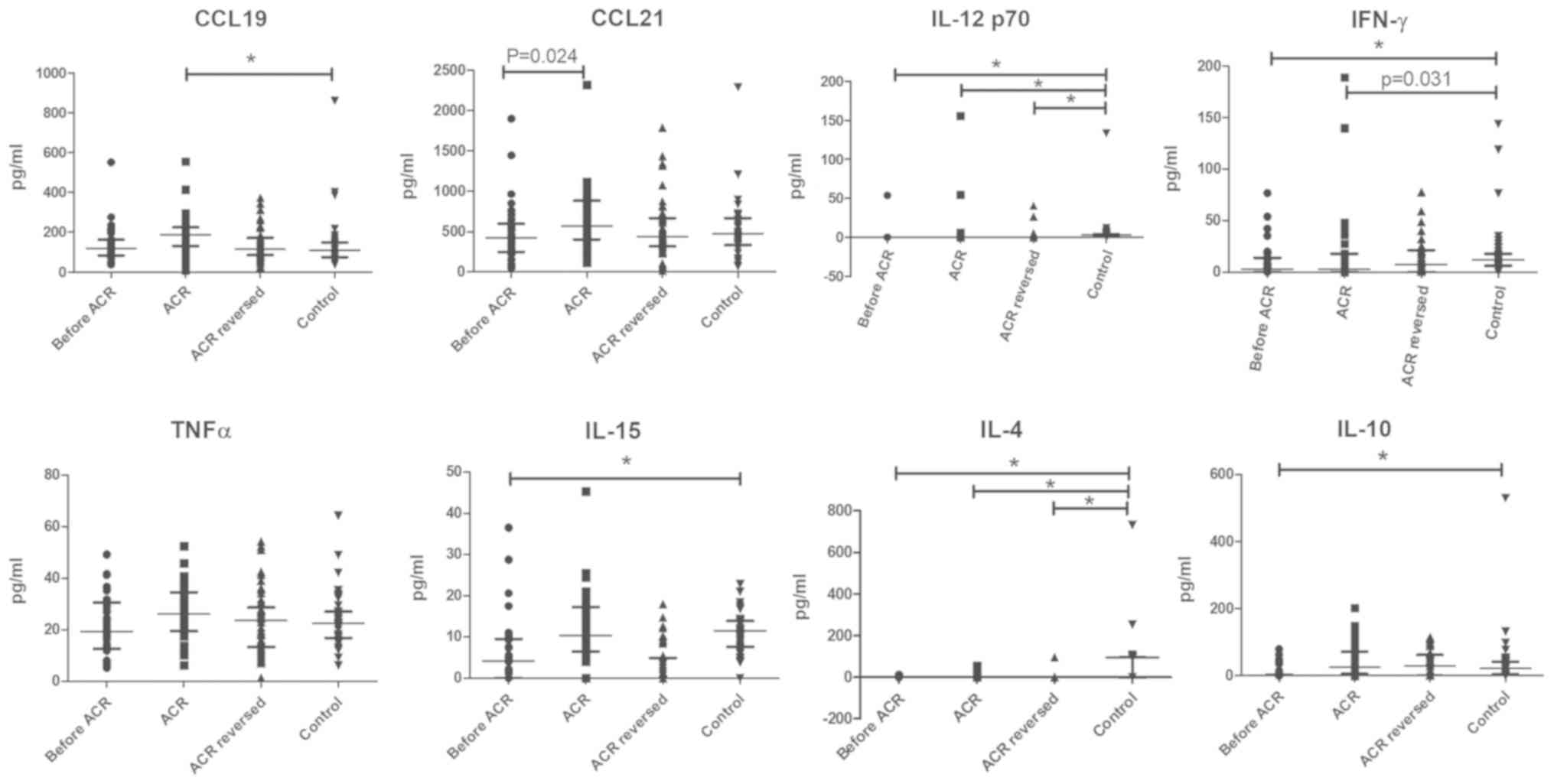
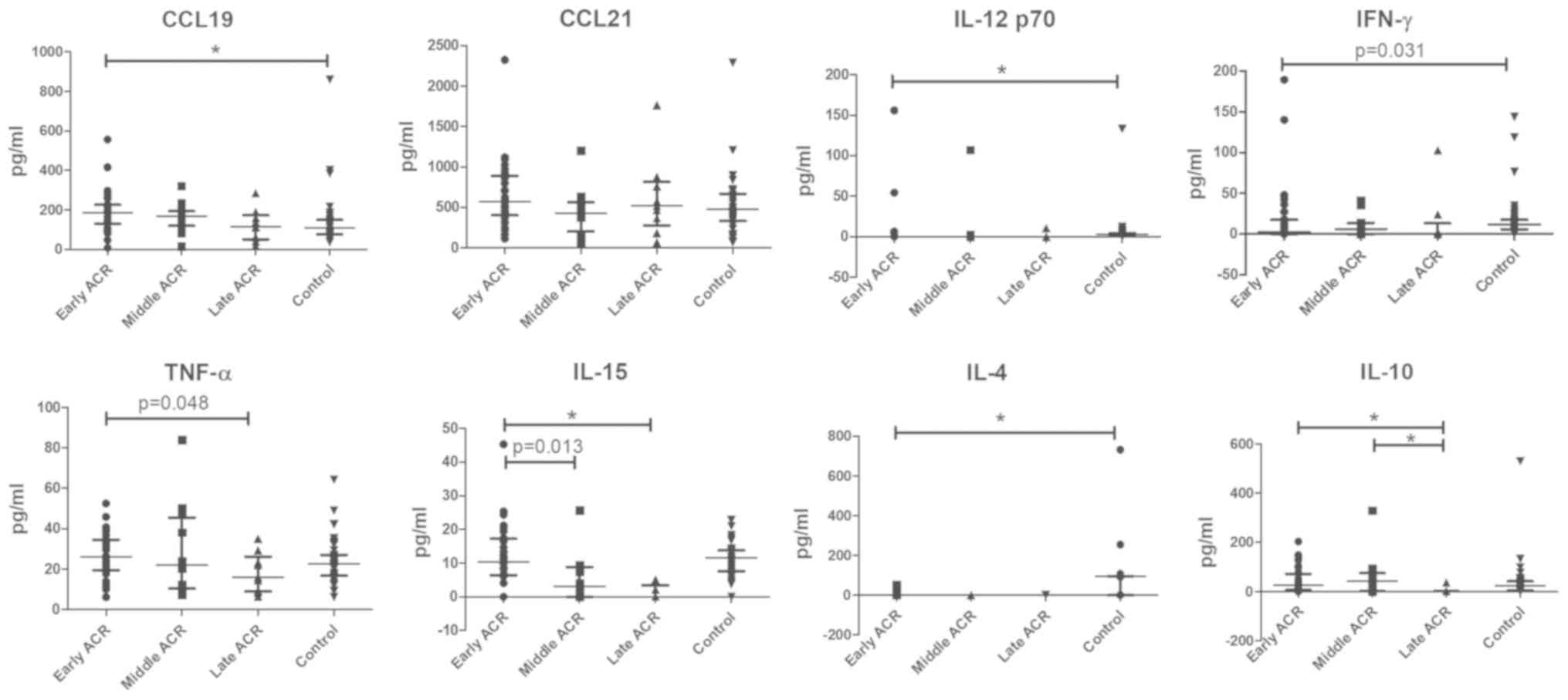
Altered serum concentrations of CCL19, IL-15 and
IL-10 in patients with ACR at different stages. The serum
concentrations of CCL19 in patients with early-stage ACR increased
significantly in contrast with the controls who had stable graft
function, while the serum concentrations of IL-12p70, IL-4 and
IFN-γ in patients with early-stage ACR exhibited a decrease
compared with the controls. No significant difference in the serum
concentrations of TNF-α and CCL21 between the ACR and control
groups was observed. Notably, there was a marked decrease in the
serum concentrations of IL-12p70, IFN-γ, IL-15, IL-4 and IL-10
prior to rejection compared with the control (Fig. 6). In addition, the IL-15 and IL-10
levels were markedly decreased in the late-stage ACR group. By
contrast, no differences in the serum levels of CCL19, CCL21,
IL-12p70 and IFN-γ among the ACR groups at different stages were
observed (Fig. 7).
Increase in CD56+ NK cell
infiltration in kidney allografts with early-stage ACR
To analyze the local expression of CD56 in renal
allografts, the expression levels of CD56 in graft biopsy specimens
were detected from the following groups of recipients: Early-stage
ACR; mid-stage ACR; late-stage ACR; early-stage AMR; mid-stage AMR;
late-stage AMR; ACR and AMR; IRI; and CNI toxicity. Biopsy tissues
from patients with early-stage ACR, early-stage AMR, late-stage ACR
and AMR were positive for CD56, while those from patients with
late-stage ACR, late-stage AMR, IRI and CNI toxicity exhibited a
decreased level of infiltration or complete absence of NK cells.
Furthermore, in comparison with biopsy tissue from patients with
early-stage AMR, samples from patients with early-stage ACR
exhibited increased levels of infiltration of CD56+ NK
cells (Fig. 8).
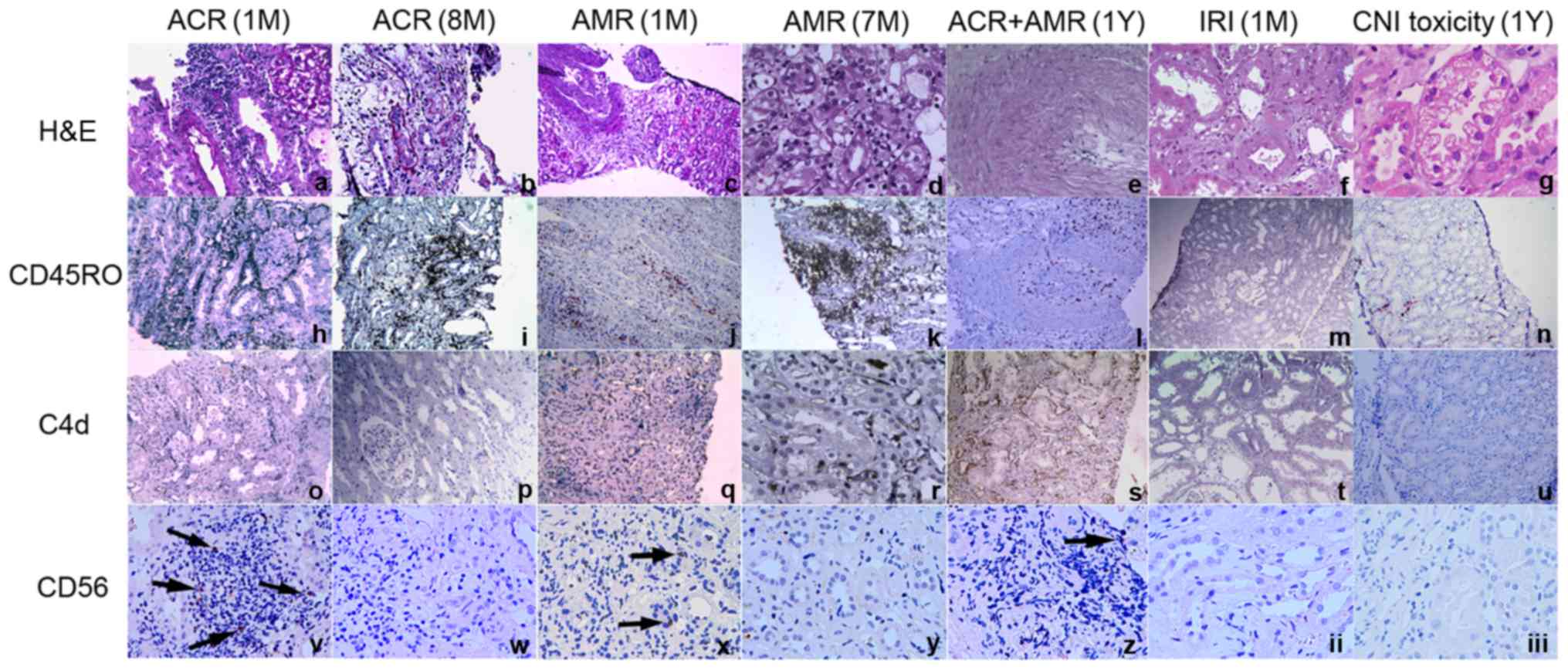 | Figure 8.CD56+ NK cell infiltration
in kidney allografts. Tissues from patients with early-stage ACR
(within the first month after transplantation), late-stage ACR (8
months after transplantation), early-stage AMR (within the first
month after transplantation), late-stage AMR (7 months after
transplantation), ACR and AMR (1 year after transplantation), IRI
(within 1 month after kidney transplantation) and CNI toxicity (1
year after kidney transplantation) are presented. Original
magnification ×100: c, h-q, s, u; ×200: a, b, f, r, t; ×400: d, e,
g, v-iii. The arrows indicate the CD 56 positive cells. ACR, acute
cellular rejection; AMR, antibody-mediated allograft rejection;
IRI, ischemia-reperfusion injury; CNI toxicity, calcineurin
inhibitor toxicity; CD56, neural cell adhesion molecule 1; H&E,
hematoxylin and eosin; CD45RO, protein tyrosine phosphatase,
receptor type, C. |
Discussion
The results from the present study indicated that
recipients with stable graft function had increased proportions of
CD56brightCD16dim subsets, and also an
increased CD56bright/CD56dim ratio compared
with the healthy controls. Similarly, patients with middle-stage
ACR exhibited increased proportions of NK cells, which were
associated with an increase in the
CD3−CD56bright subsets
(CD56brightCD16− and
CD56brightCD16dim), a decrease in the
CD3−CD56dim subsets
(CD56dimCD16+) and an increase in the
CD56bright/CD56dim ratio compared with the
controls. These data contrast those from that of Crespo et
al (6) who observed that DSA
and HLA non-DSA patients exhibited decreased proportions of NK
cells and increased proportions of CD56bright subsets
compared with patients without HLA antibodies (6). These results may indicate that there
are differences in the NK cell phenotype in the PBMCs of patients
with ACR and AMR.
Notably, transplant patients with early-stage ACR
did not exhibit a significant increase in the proportion of
CD3−CD56bright subsets or the
CD56bright/CD56dim ratio compared with stable
controls. In addition, more CD56+ NK cells infiltrated
the kidney allograft in patients with ACR and AMR in the first 1
month following transplantation, in contrast to stable controls or
patients with rejection after 1 month of transplantation. These
data imply that CD56bright NK cells may serve important
roles in the alloimmune response, which may be important for
allograft function in the initiation stage of ACR and AMR, and that
they primarily serve a role in the peripheral blood after 1 month
post-transplantation.
The specific chemotactic triggers for the movement
of NK cells to the kidney allograft remain a topic of study at
present. Peripheral CD56brightCD16dim/− NK
cells home into tissues including the peripheral lymph nodes and
the kidneys under certain physiological conditions via the homing
receptor CCR7, through which it responds to the chemokines CCL19
and CCL21 (28). CCR7 has been
demonstrated to localize in the blood vessel walls and tubular
epithelial cells of renal allografts and to be increasingly
expressed in patients with chronic/sclerosing allograft nephropathy
in comparison with the normal controls (29). Additionally, it has been suggested
that the IFN-γ/C-X-C chemokine receptor type 3 (CXCR3)/C-X-C
chemokine (CXCL)9/CXCL10/CXCL11 axis is important for the
recruitment of NK cells from storage organs including the bone
marrow into blood circulation (28). In the present study, these
CD56bright NK cell-associated cytokines were examined in
renal allograft recipients, and it was identified that the profiles
of patients with ACR exhibited increased serum concentrations of
CCL19 compared with those of recipients with stable graft function.
There were no significant differences in the serum concentrations
of CCL19 and CCL21 between patients at different stages of ACR. In
addition, our previous study demonstrated that the serum
concentrations of CXCL9, CXCL10 and CXCL11, the CXCR3-specific
ligands, measured during an episode of ACR, were significantly
increased compared with those of patients with stable function, and
that the number of CXCR3+ cells in the graft biopsy
specimens from patients with ACR was significantly increased
compared with the recipients with stable graft function (30). Therefore, the elevation in the
serum concentrations of CCL19, CXCL9, CXCL10, and CXCL11 is
consistent with the increase in the frequency of peripheral
CD56bright NK cells.
It has been demonstrated that CD56bright
NK cells often serve a regulatory role and are primary producers of
inflammatory and regulatory cytokines, including IFN-γ, TNF-α and
IL-12 (19,20). In the present study, the serum
concentrations of IL-12 and IFN-γ were decreased in patients with
ACR compared with recipients with stable graft function, but the
TNF-a concentration was similar. The reasons for the decreases in
the serum concentrations of IL-12 and IFN-γ are complicated, and
the present study was not able to determine whether they were
secreted by CD56bright NK cells. However, decreases in
the levels of IFN-γ secreted from Th1-like Treg cells in patients
with ACR may partially explain these data (23).
The frequency of CD3+CD56+
NKT-like cells in the PBMCs of kidney transplant recipients was
decreased compared with that of the healthy controls. In addition,
the percentage of NKT-like cells in patients with middle-stage ACR
was decreased compared with that in recipients with stable graft
function. Therefore, NKT-like cells may serve a protective role
from the alloimmune response, particularly 1 month after
post-translation. Several previous studies have described the
regulatory effects of antigen-presenting glycoprotein
CD1d-dependent NKT cells on alloantigens, and that these effects
were IL-10- or TGF-β-dependent (12,31,32).
In addition, NKT cells have been suggested to affect IL-10
production from Tregs via production of IL-4 (12). The results of the present study
indicated that there was a marked decrease in the serum
concentrations of IL-15, IL-4 and IL-10 prior to rejection compared
with the control. The IL-15 and IL-10 levels were also decreased
markedly in the late-stage ACR group. The decrease in IL-15 levels
prior to an episode of ACR appears to be consistent with the
decrease in the circulating proportions of NKT-like cells in
patients with ACR, and may potentially explain the decrease in the
proportion of NKT-like cells. Furthermore, the decrease in the
serum concentrations of IL-4 and IL-10 prior to an episode of ACR
may be the result of decrease in the frequency of NKT-like cells in
patients and also be responsible for the occurrence of ACR.
The NK/NKT-like, CD56bright NK/NKT-like
and CD56dim NK/NKT-like cell ratios in the PBMCs of
kidney transplant recipients were increased compared with those in
the healthy controls. Additionally, the
CD56brightNK/NKT-like cell ratios in the PBMCs of
patients with middle-stage ACR also exhibited a significant
increase compared with those in the stable controls. Finally, based
on the AUC values for the different cell ratios, it appears that
the CD56bright/CD56dim ratio and not the
CD56brightNK/NKT-like cell ratio may contribute to the
ACR occurrence.
In summary, the results of the present study
indicate that CD56brightCD16−/dim NK cells
promote the development of ACR, and that NKT-like cells may have
immunoregulatory function. In addition, the data indicate that the
CD56bright/CD56dim ratio may contribute to
the ACR patterns. However, as the sample size was small, the
results from the present study only provide a basis for subsequent
larger-scale and more in-depth studies on the role of NK subsets
and NKT cells in ACR.
Acknowledgements
The authors would like to thank Mrs. Yu Gao (Yianbo
Biotechnology Company, Henan, China) for her kind help with
analyzing certain flow cytometry data.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81571555) and the
Capital Characteristic Clinic Project (grant no.
Z171100001017185).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LX designed the study and provided scientific
leadership to junior colleagues. BS analyzed the data, collected
clinical samples and patients' information, and revised the
manuscript. XX analyzed the data and wrote the paper. YH performed
the histopathological evaluation and rejection diagnosis. LB
performed the immunofluorescent staining and flow cytometry
analysis. HH performed the cytokine multiplex immunoassay, XK
performed the virus antigen detection assay and XM performed the
donor specific antibody detection assay. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of The 309th Hospital of the Chinese People's Liberation
Army, and all patients provided informed consent for the use of
their samples in this research. All donors provided informed
consent for kidney donation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NK
|
natural killer cells
|
|
ACR
|
acute T-cell-mediated renal allograft
rejection
|
|
early-stage ACR
|
ACR occurring within the first month
after transplantation
|
|
mid-stage ACR
|
ACR occurring between 2 and 6 months
after transplantation
|
|
late-stage ACR
|
ACR occurring between 7 and 12 months
after transplantation
|
|
AMR
|
antibody-mediated allograft
rejection
|
|
ST
|
stable control
|
|
IRI
|
ischemia-reperfusion injury
|
|
CNI toxicity
|
calcineurin inhibitor toxicity
|
|
PBMC
|
peripheral blood mononuclear cell
|
|
DSA
|
donor specific antibody
|
References
|
1
|
Kitchens WH, Uehara S, Chase CM, Colvin
RB, Russell PS and Madsen JC: The changing role of natural killer
cells in solid organ rejection and tolerance. Transplantation.
81:811–817. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pratschke J, Stauch D and Kotsch K: Role
of NK and NKT cells in solid organ transplantation. Transpl Int.
22:859–868. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
López-Botet M, Vilches C, Redondo-Pachón
D, Muntasell A, Pupuleku A, Yélamos J, Pascual J and Crespo M: Dual
role of natural killer cells on graft rejection and control of
cytomegalovirus infection in renal transplantation. Front Immunol.
8:1662017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu G, Xu X, Vu MD, Kilpatrick ED and Li
XC: NK cells promote transplant tolerance by killing donor
antigen-presenting cells. J Exp Med. 203:1851–1858. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Michel T, Poli A, Cuapio A, Briquemont B,
Iserentant G, Ollert M and Zimmer J: Human CD56bright NK cells: An
update. J Immunol. 196:2923–2931. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Crespo M, Yelamos J, Redondo D, Muntasell
A, Perez-Saéz MJ, López-Montañés M, García C, Torio A, Mir M,
Hernández JJ, et al: Circulating NK-cell subsets in renal allograft
recipients with anti-HLA donor-specific antibodies. Am J
Transplant. 15:806–814. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Assadiasl S, Sepanjnia A, Aghili B, Nafar
M, Ahmadpoor P, Pourrezagholi F, Parvin M, Shahlaee A, Nicknam MH
and Amirzargar A: Natural killer cell subsets and IL-2, IL-15 and
IL-18 genes expressions in chronic kidney allograft dysfunction and
graft function in kidney allograft recipients. Int J Organ
Transplant Med. 7:212–217. 2016.PubMed/NCBI
|
|
8
|
Carrega P, Bonaccorsi I, Di Carlo E,
Morandi B, Paul P, Rizzello V, Cipollone G, Navarra G, Mingari MC,
Moretta L and Ferlazzo G: CD56(bright)perforin(low) noncytotoxic
human NK cells are abundant in both healthy and neoplastic solid
tissues and recirculate to secondary lymphoid organs via afferent
lymph. J Immunol. 192:3805–3815. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Melsen JE, Lugthart G, Lankester AC and
Schilham MW: Human circulating and tissue-resident CD56 (bright)
natural killer cell populations. Front Immunol. 7:2622016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shin S, Kim YH, Cho YM, Park Y, Han S,
Choi BH, Choi JY and Han DJ: Interpreting CD56+ and CD163+
infiltrates in early versus late renal transplant biopsies. Am J
Nephrol. 41:362–369. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hongo D, Tang X, Dutt S, Nador RG and
Strober S: Interactions between NKT cells and Tregs are required
for tolerance to combined bone marrow and organ transplants. Blood.
119:1581–1589. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang X, Kojo S, Harada M, Ohkohchi N,
Taniguchi M and Seino KI: Mechanism of NKT cell-mediated transplant
tolerance. Am J Transplant. 7:1482–1490. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Godfrey DI, MacDonald HR, Kronenberg M,
Smyth MJ and Van Kaer L: NKT cells: What's in a name. Nat Rev
Immunol. 4:231–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peng LS, Mao FY, Zhao YL, Wang TT, Chen N,
Zhang JY, Cheng P, Li WH, Lv YP, Teng YS, et al: Altered phenotypic
and functional characteristics of CD3+CD56+ NKT-like cells in human
gastric cancer. Oncotarget. 7:55222–55230. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Golden-Mason L, Castelblanco N, O'Farrelly
C and Rosen HR: Phenotypic and functional changes of cytotoxic
CD56pos natural T cells determine outcome of acute hepatitis C
virus infection. J Virol. 81:9292–9298. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Diao H, He J, Zheng Q, Chen J, Cui G, Wei
Y, Ye P, Kohanawa M and Li L: A possible role for NKT-like cells in
patients with chronic hepatitis B during telbivudine treatment.
Immunol Lett. 160:65–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Haas M, Sis B, Racusen LC, Solez K, Glotz
D, Colvin RB, Castro MC, David DS, David-Neto E, Bagnasco SM, et
al: Banff 2013 meeting report: Inclusion of c4d-negative
antibody-mediated rejection and antibody-associated arterial
lesions. Am J Transplant. 14:272–283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haas M, Loupy A, Lefaucheur C, Roufosse C,
Glotz D, Seron D, Nankivell BJ, Halloran PF, Colvin RB, Akalin E,
et al: The banff 2017 kidney meeting report: Revised diagnostic
criteria for chronic active T cell-mediated rejection,
antibody-mediated rejection and prospects for integrative endpoints
for next-generation clinical trials. Am J Transplant. 18:293–307.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fehniger TA, Shah MH, Turner MJ, VanDeusen
JB, Whitman SP, Cooper MA, Suzuki K, Wechser M, Goodsaid F and
Caligiuri MA: Differential cytokine and chemokine gene expression
by human NK cells following activation with IL-18 or IL-15 in
combination with IL-12: Implications for the innate immune
response. J Immunol. 162:4511–4520. 1999.PubMed/NCBI
|
|
20
|
Márquez ME, Millet C, Stekman H, Conesa A,
Deglesne PA, Toro F, Sanctis JD and Blanca I: CD16 cross-linking
induces increased expression of CD56 and production of IL-12 in
peripheral NK cells. Cell Immunol. 264:86–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bendelac A, Savage PB and Teyton L: The
biology of NKT cells. Annu Rev Immunol. 25:297–336. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin SJ, Huang YC, Cheng PJ, Lee PT, Hsiao
HS and Kuo ML: Interleukin-15 enhances the expansion and function
of natural killer T cells from adult peripheral and umbilical cord
blood. Cytokine. 76:348–355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu X, Huang H, Wang Q, Cai M, Qian Y, Han
Y, Wang X, Gao Y, Yuan M, Xu L, et al: IFN-γ-producing Th1-like
regulatory T cells may limit acute cellular renal allograft
rejection: Paradoxical post-transplantation effects of IFN-γ.
Immunobiology. 222:280–290. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Poli A, Michel T, Thérésine M, Andrès E,
Hentges F and Zimmer J: CD56bright natural killer (NK) cells: An
important NK cell subset. Immunology. 126:458–465. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu X, Huang H, Cai M, Qian Y, Li Z, Bai H,
Han Y, Xiao L, Zhou W, Wang X and Shi B: Combination of IL-1
receptor antagonist, IL-20 and CD40 ligand for the prediction of
acute cellular renal allograft rejection. J Clin Immunol.
33:280–287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cassano P, Bui E, Rogers AH, Walton ZE,
Ross R, Zeng M, Nadal-Vicens M, Mischoulon D, Baker AW, Keshaviah
A, et al: Inflammatory cytokines in major depressive disorder: A
case-control study. Aust N Z J Psychiatry. 51:23–31. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Curran-Everett D: Multiple comparisons:
Philosophies and illustrations. Am J Physiol Regul Integr Comp
Physiol. 279:R1–R8. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maghazachi AA: Role of chemokines in the
biology of natural killer cells. Curr Top Microbiol Immunol.
341:37–58. 2010.PubMed/NCBI
|
|
29
|
Zhou HL, Wang YT, Gao T, Wang WG and Wang
YS: Distribution and expression of fibroblast-specific protein
chemokine CCL21 and chemokine receptor CCR7 in renal allografts.
Transplant Proc. 45:538–545. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang H, Xu X, Yao C, Cai M, Qian Y, Wang
X and Shi B: Serum levels of CXCR3 ligands predict T cell-mediated
acute rejection after kidney transplantation. Mol Med Rep. 9:45–50.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oh K, Kim S, Park SH, Gu H, Roopenian D,
Chung DH, Kim YS and Lee DS: Direct regulatory role of NKT cells in
allogeneic graft survival is dependent on the quantitative strength
of antigenicity. J Immunol. 174:2030–2036. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang SH, Jin JZ, Lee SH, Park H, Kim CH,
Lee DS, Kim S, Chung NH and Kim YS: Role of NKT cells in allogeneic
islet graft survival. Clin Immunol. 124:258–266. 2007. View Article : Google Scholar : PubMed/NCBI
|