Introduction
Acute lung injury (ALI) is a disorder associated
with pulmonary inflammation that may lead to increased permeability
syndrome (1). Various factors that
may not be involved in the respiratory or in the cardiovascular
system are involved in the pathogenesis of ALI (2). ALI is characterized by pulmonary
edema and increased lung permeability caused by diffuse alveolar
capillary injury that may lead to acute and progressive hypoxic
respiratory failure (3). ALI is
caused by the extensive destruction of pulmonary vascular and
alveolar epithelial cells following severe inflammation (4). ALI exhibits significant morbidity and
mortality rates with poor outcomes in patients of all ages
(5). In addition, ALI is one of
the most common pediatric diseases and one of the most frequent
causes of neonatal mortality (6).
The estimated mortality rate of ALI is 38.6–65%, and previous
studies demonstrated that the mortality rate varied by geographical
region (7,8). Although the use of lung protective
strategies including ventilation, prolonged prone position,
neuromuscular blockade, treatment with inhalable vasodilators and
nonpulmonary treatments have improved the outcomes of ALI, the
treatment of ALI remains challenging (9,10).
Therefore, there is an urgent requirement to identify novel
effective therapies against ALI.
Lipopolysaccharides (LPS) are bacterial bioactive
components involved in various pathological conditions and are able
to promote the inflammatory cascade (11–13).
Previous studies used LPS for the establishment of animal models of
ALI (11–13). Therefore, in the present study, a
rat model of ALI was established by intraperitoneal injection of
LPS.
Propofol is used to induce or maintain anesthesia
(14). Nevertheless, accumulating
evidence demonstrated that propofol serve multiple functions,
including anticancer, antioxidant, neuroprotective and
anti-inflammatory activities (15–20).
The effect of propofol in lung injury has been previously
investigated; Yuan et al (21) observed that Propofol was able to
relieve ALI induced by liver transplantation by inhibiting gap
junction protein α 1. Liu et al (22) suggested that propofol increased the
effect of sevoflurane in relieving LPS-induced ALI in mice. A
recent study demonstrated that treatment with propofol
significantly increased the expression levels of heme oxygenase 1
in lung tissues and inhibited the lung morphological alterations
caused by ALI in rats (23).
Additionally, the p38 mitogen-activated protein kinase
(MAPK)/nuclear factor κ-light-chain-enhancer of activated B cells
(NF-κB) signaling pathway and the NLR family pyrin domain
containing 3 (NLRP3) inflammasome, which serve roles in the
regulation of inflammation, were identified to be regulated by
propofol (20,24). A number of previous studies
demonstrated that the p38 MAPK/NF-κB pathway was activated during
ALI (25,26). Furthermore, previous studies
suggested that the components of the NLRP3 inflammasome including
NLRP3, apoptosis-associated speck-like protein containing CARD
(ASC) and caspase-1, serve critical roles in ALI development and
progression by regulating the inflammatory response (27,28).
The mechanism underlying propofol effect on lung
injury remains unclear. Specifically, to the best of the authors'
knowledge, the effect of propofol on neonatal ALI has not been
examined. The present study aimed to investigate the role of
propofol on neonatal ALI and the molecular mechanism underlying its
effects.
Materials and methods
Establishment of the ALI model
A total of 30 male newborn Sprague-Dawley rats (age,
3–8 days; weight, 8–14 g) were obtained from the Animal
Experimental Center of Zhejiang University (Hangzhou, China). All
experimental procedures were performed according to the Recommended
Guidelines for the Care and Use of Laboratory Animals issued by The
Chinese Council on Animal Research (29). The present study was approved by
The Animal Ethics Committee of The Maternal and Child Health Care
Hospital of Qujing (Qujing, China). Rat pups were kept in
polypropylene cages with nursing mothers. The nursing mothers were
fed ad libitum Rats were housed at 25±5°C, with 50% humidity
and 12 h dark/light cycle conditions.
To induce ALI, rats were injected intraperitoneally
with LPS (3 mg/kg; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
as previously described (13) and
successful establishment of the model was confirmed by lung injury
scores. Then, 1 h after treatment with LPS, rats were additionally
treated by intraperitoneal injection of various concentrations of
propofol (Corden Pharma Caponago S.P.A., Milan, Italy) (1, 5 and 10
mg/kg) once a day for 7 consecutive days. Propofol working
concentrations were selected according to a previous study
(30). Rats were randomly divided
into six groups (n=5 per group): i) Control group, ii) LPS group (3
mg/kg), iii) LPS + propofol (1 mg/kg) group, iv) LPS + propofol (5
mg/kg) group and v) LPS + propofol (10 mg/kg) group.
Lung injury score assessment
To perform the assessment of the lung injury score,
lung tissues were collected from the control and the three
experimental groups. Lung tissues were fixed with 10% neutral
phosphate-buffered formalin at 4°C for 24 h, embedded in paraffin
and sectioned (4 µm). Subsequently, hematoxylin and eosin staining
was conducted using standard protocols. The pathological
alterations in the morphology of lung tissues were observed under a
light microscope (magnification, ×200). The lung injury score was
assessed to quantify the lung injury. Lung injury scores were
calculated by summing the degree of cell infiltration and the
severity score of tissue damage assessed from the lung sections
(31).
Lung edema assessment
Rats were anesthetized with 3% isoflurane. Blood
samples (200 µl) were collected from abdominal aorta, and an
automatic blood gas analyzer (Cobas B123; Roche Diagnostics, Basel,
Switzerland) was used to measure the partial pressure of oxygen
(PaO2). The rats were sacrificed and the right lungs
were collected from the control and the three experimental groups.
Subsequently, the wet weights (W) of the right lungs were measured.
Subsequently, following a 48-h incubation at 70°C, the dry weights
(D) of the right lungs were measured and the wet-dry weight ratio
(W/D) was calculated.
Measurement of inflammatory factors
and oxidative stress-associated factors
The bronchoalveolar lavage fluid (BALF) was obtained
by intratracheal instillation of sterile PBS, repeated three times.
The BALF supernatant was collected by centrifugation (800 × g) at
4°C for 10 min. ELISA kits (Wuhan Boster Biological Technology,
Ltd., Wuhan, China) were used to detect the levels in BALF of the
following pro-inflammatory cytokines: Tumor necrosis factor α
(TNF-α; cat. no. EK0526), interleukin (IL)-1β (cat. no. EK0393) and
IL-6 (cat. no. EK0412). The level of malondialdehyde (MDA) and the
activity of superoxide dismutase (SOD) in lung tissues were
determined using commercially available kits (cat. nos. A003-1 and
A001-3; Nanjing Jiancheng Bioengineering Institute, Nanjing, China)
according to the manufacturer's protocol.
Reverse transcription
quantitative-polymerase chain reaction (RT-qPCR)
Total RNA was isolated from lung tissues by using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) according to the manufacturer's protocol.
An equal amount of RNA from various groups was reversely
transcribed into cDNA using PrimeScript™ RT reagent kit (Takara Bio
Inc., Otsu, Japan) according to the manufacturer's protocol. qPCR
was performed using SYBR® Premix Ex Taq™ II (Takara Bio
Inc.). Thermocycling conditions were: 95°C for 5 min, 40 cycles at
95°C for 30 sec, 55°C for 30 sec, 72°C for 30 sec and then 72°C for
10 min. GAPDH was used as endogenous control. Primer sequences are
presented in Table I. Relative
mRNA expression levels were normalized to GAPDH using the
2−ΔΔCq method (32).
 | Table I.Primer sequences used for the reverse
transcription quantitative-polymerase chain reaction. |
Table I.
Primer sequences used for the reverse
transcription quantitative-polymerase chain reaction.
| Gene | Primer sequence
(5′→3′) |
|---|
| TNF-α | F:
CCTCTTCTCATTCCTGCTC |
|
| R: CTTCTCCTCCTTG
TTGGG |
| IL-1β | F:
TGTGAAATGCCACCTTTTGA |
|
| R:
TGAGTGATACTGCCTGCCTG |
| IL-6 | F:
CCGGAGAGGAGACTTCACAG |
|
| R:
CAGAATTGCCATTGCACA |
| NLRP3 | F:
GATCTTCGCTGCGATCAACAG |
|
| R:
CGTGCATTATCTGAACCCCAC |
| ASC | F:
GCAATGTGCTGACTGAAGGA |
|
| R:
TGTTCCAGGTCTGTCACCAA |
| Caspase-1 | F:
GCACAAGACCTCTGACAGCA |
|
| R:
TTGGGCAGTTCTTGGTATTC |
| GAPDH | F:
CTTTGGTATCGTGGAAGGACTC |
|
| R:
GTAGAGGCAGGGATGATGTTCT |
Western blot assay
Total proteins from whole tissue lysates were
extracted using radioimmunoprecipitation assay buffer (cat. no.
P0013B; Beyotime Institute of Biotechnology, Nanjing, China), and a
bicinchoninic acid assay kit was used to quantify protein
concentration. A total of 25 µg protein was loaded per lane, in a
volume of 20 µl. Proteins were separated by 12% SDS-PAGE, and
subsequently transferred onto polyvinylidene fluoride membranes
(EMD Millipore, Billerica, MA, USA), and blocked with 5% skim milk
for 1 h at room temperature. The membranes were incubated at 4°C
overnight with one of the following primary antibodies: TNF-α (cat
no. ab13597; 1:1,000; Abcam, Cambridge, MA, USA), IL-1β (cat no.
ab200478; 1:1,000; Abcam), IL-6 (cat no. ab9324; 1:1,000; Abcam),
phosphorylated (p-)p38 (cat no. ab45381; 1:1,000; Abcam), p38 (cat
no. ab31828; 1:1,000; Abcam), p-p65 (cat no. ab86299; 1:1,000;
Abcam), p65 (cat no. ab16502; 1:1,000; Abcam), NLRP3 (cat no.
ab214185; 1:1,000; Abcam), ASC (at no. sc-514414; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), caspase-1 (cat no. ab1872;
1:1,000; Abcam) and β-actin (cat no. 4970; 1:1,000; Cell Signaling
Technology, Inc., Danvers, MA, USA). Subsequently, the membranes
were incubated with the anti-rabbit immunoglobulin G horseradish
peroxidase-labeled secondary antibody (cat no. 7074; 1:2,000; Cell
Signaling Technology, Inc.) at room temperature for 2 h. Enhanced
chemiluminescence reagents (EMD Millipore) were used to visualize
the protein bands. The intensity of p-p38 and p-p65 was analyzed
using Gel-Pro-Analyzer software version 4.0 (Media Cybernetics,
Inc., Rockville, MD, USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
SPSS statistical software (version 16.0; SPSS, Inc., Chicago, IL,
USA) was used to conduct statistical analyses. One-way analysis of
variance followed by Student-Newman-Keuls post hoc test was
performed to determine differences between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
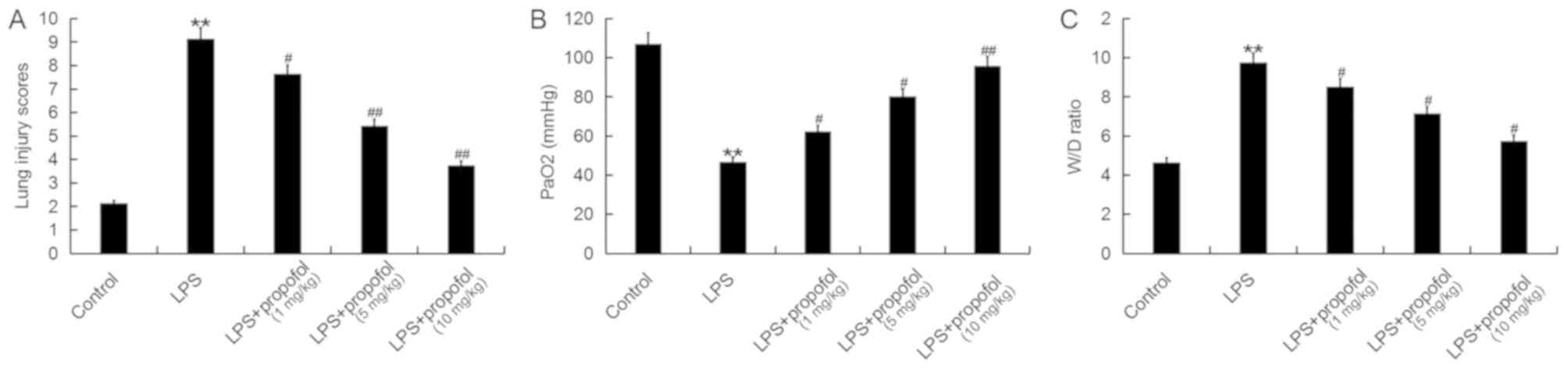
Propofol relieves the pulmonary edema
induced by LPS in neonatal rats
Treatment with propofol decreased the lung injury
score in a dose-dependent manner in ALI Model rats, which was
significantly increased upon treatment with LPS (Fig. 1A). Furthermore, treatment with LPS
significantly decreased the PaO2 and increased the W/D
ratio in neonatal rats (Fig. 1B and
C, respectively). However, treatment with propofol
significantly reversed the effects detected in LPS-associated ALI
in neonatal rats in a dose-dependent manner (Fig. 1B and C, respectively).
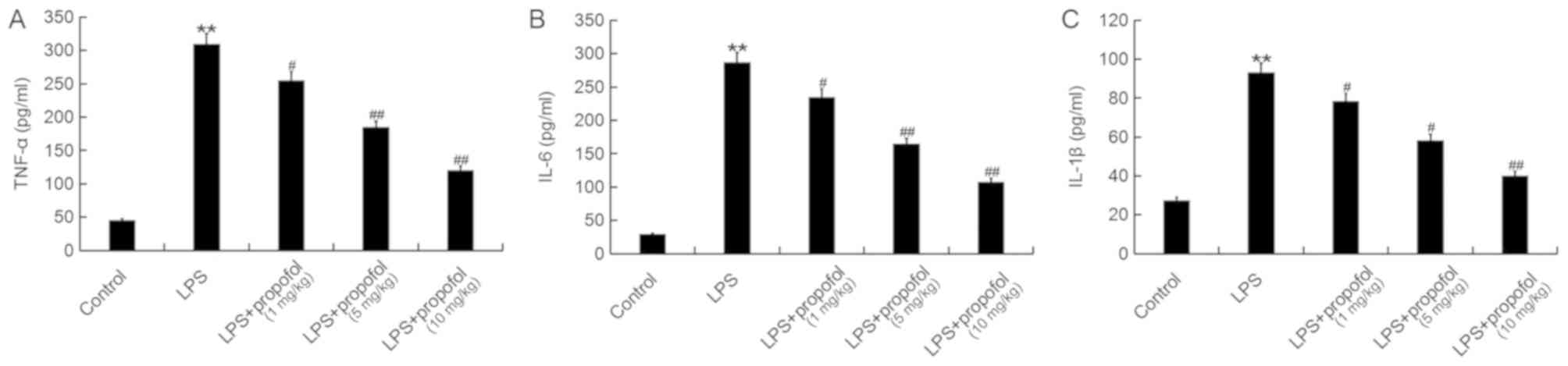
Propofol prevents the inflammatory
response induced by LPS in neonatal rats
Inflammation serves an important role in the
initiation and maintenance of ALI (33), and the principal feature of
ALI-associated inflammation is the upregulation of pro-inflammatory
factors, including TNF-α, IL-1β and IL-6 (34–36).
The present study investigated the effects of propofol on the
protein expression levels of TNF-α, IL-6 and IL-1β in the BALF of
neonatal rats by ELISA (Fig. 2).
The results demonstrated that LPS significantly increased the
protein expression levels of TNF-α, IL-6 and IL-1β in the BALF of
neonatal rats, whereas, treatment with propofol decreased their
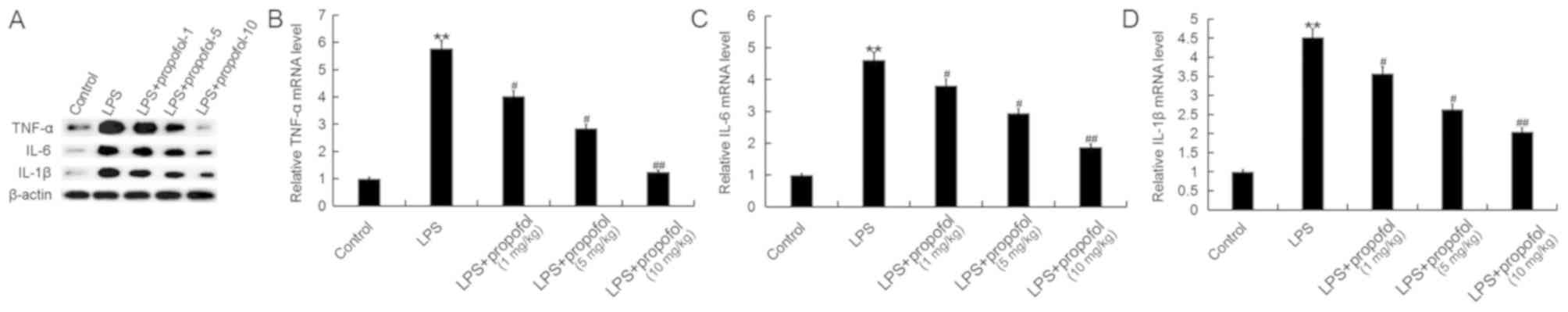
concentrations in a dose-dependent manner (Fig. 2). Additionally, the protein and
mRNA expression levels of TNF-α, IL-6 and IL-1β were measured by
western blot analysis and RT-qPCR, respectively. The protein and
mRNA expression levels of TNF-α, IL-6 and IL-1β in lung tissues of
neonatal rats were increased by treatment with LPS, and propofol
decreased their expression levels in a dose-dependent manner
(Fig. 3).
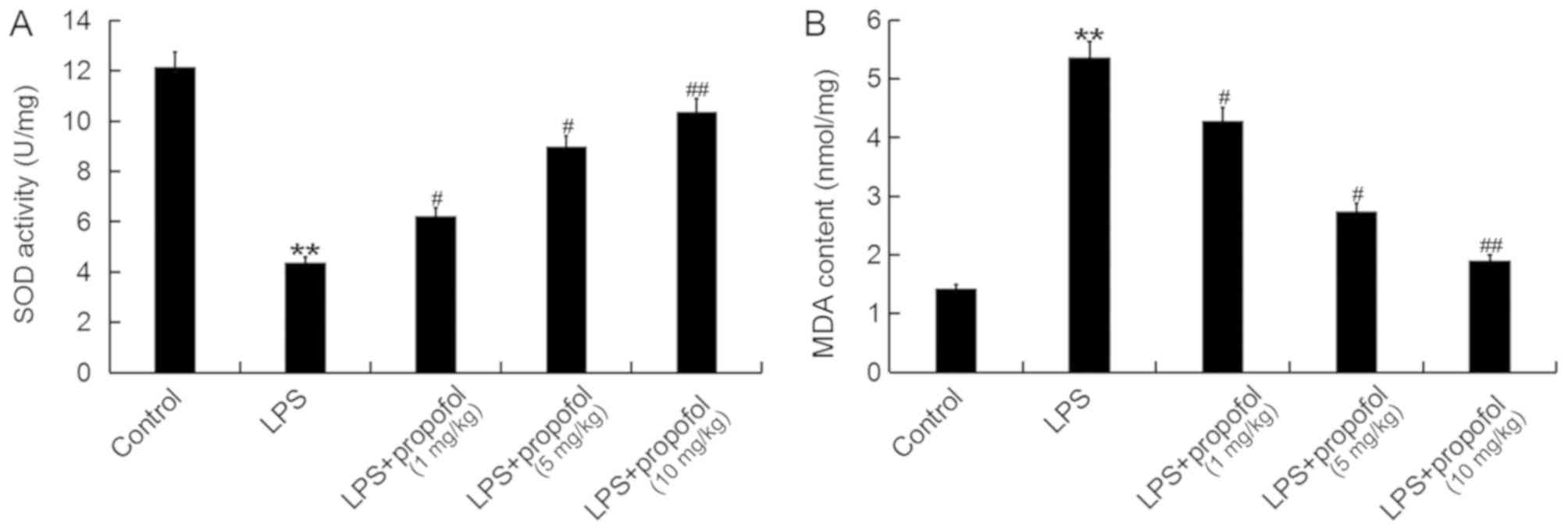
Propofol prevents the oxidative stress
induced by LPS in neonatal rats
The effects of propofol on the oxidative stress
induced by ALI were examined. Compared with the control group,
treatment with LPS significantly decreased the activity of SOD and
increased the level of MDA in lung tissues (Fig. 4A and B, respectively). The activity
of SOD was significantly increased and the level of MDA was
significantly decreased following treatment with propofol in
neonatal rats with ALI, compared with the Model ALI rats (Fig. 4). These results suggested that
propofol suppressed the oxidative stress detected in neonatal rats
with ALI.
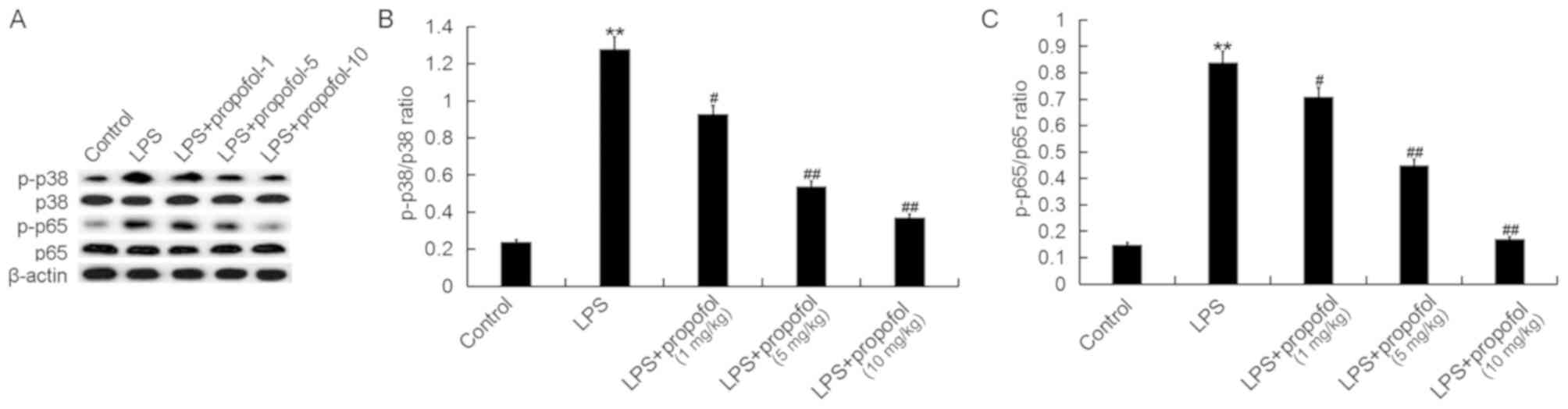
Propofol prevents the activation of
the p38 MAPK/NF-κB signaling pathway induced by LPS in neonatal
rats
The expression level of pro-inflammatory cytokines
is regulated at the transcriptional level by the MAPK and NF-κB
signaling pathways, and previous studies demonstrated that the p38
MAPK/NF-κB pathway is active during ALI (25,26).
In the present study, to investigate the molecular mechanism
underlying the effects of propofol on ALI, the p38 MAPK/NF-κB
signaling pathway was investigated. Treatment with LPS
significantly increased the protein expression levels of p-p38 and
p-p65 in the lung tissues of neonatal rats, which suggested that
the activity of the p38 MAPK/NF-κB signaling pathway was increased.
Furthermore, treatment with propofol was identified to decrease the
protein expression level of p-p38 and p-p65 in neonatal ALI model
rats in a dose-dependent manner (Fig.
5).
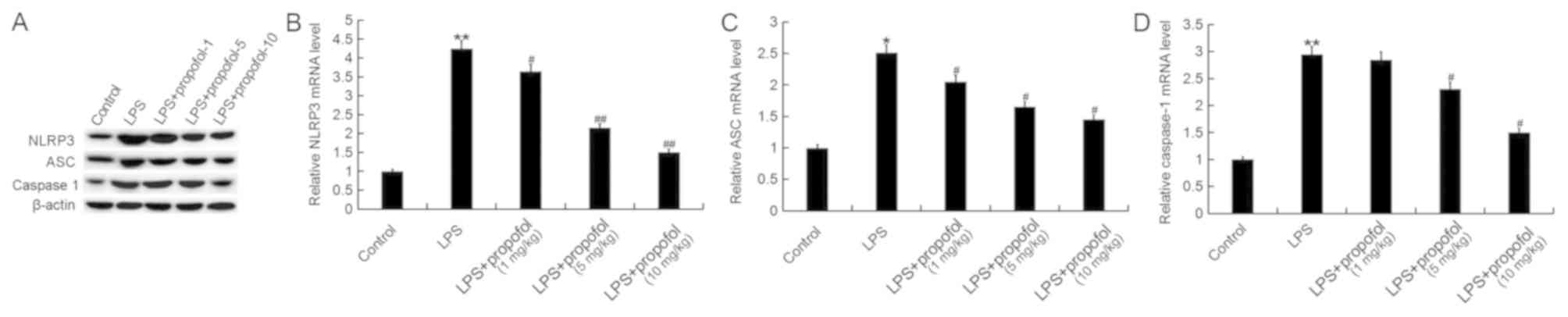
Propofol prevents the activation of
the NLRP3 inflammasome induced by LPS in neonatal rats
The NLRP3 inflammasome serves an important role in
regulating the inflammatory response and oxidative stress (37). Numerous previous studies
demonstrated that the NLRP3 inflammasome, consisting of NLRP3, ASC
and caspase-1, was activated during ALI and it may serve critical
roles in ALI development by promoting inflammation (27,28).
Therefore, the components of the NLRP3 inflammasome were
investigated in the present study. The protein and mRNA expression
levels of NLRP3, ASC and caspase-1 in lung tissues were
significantly increased following treatment with LPS, and these
effects were reversed by the treatment with propofol in a
dose-dependent manner (Fig.
6).
Discussion
In the present study, treatment with propofol was
identified to relieve LPS-induced pulmonary edema and to inhibit
LPS-mediated inflammatory response and oxidative stress in neonatal
rats. In addition, the present findings suggested that treatment
with propofol inhibited the activation of the p38-MAPK/NF-κB
pathway and NLRP3 inflammasome. These results suggested that
treatment with propofol may alleviate ALI induced by LPS
administration.
Pediatric ALI is one of the most common causes of
infant mortality, as newborns are more vulnerable than adults
(38,39). Unfortunately, diagnosis of ALI
remains challenging and no treatments are available, despite the
high morbidity and mortality rates of ALI. Therefore, there is an
urgent requirement to identify novel efficient strategies to treat
ALI. Propofol is frequently used as an anesthetic; however, it
possesses a variety of activities, including anti-inflammatory
activity (15–20). Although previous studies observed
the protective effects of propofol in ALI (21–23),
its mechanism of action requires further investigation, and the
role of propofol in neonatal ALI has not been previously examined,
to the best of the authors' knowledge. The present study aimed to
investigate the effects of propofol on neonatal ALI and to
investigate its mechanism of action.
As one of the early symptoms of multiple organ
failure, the onset of ALI is associated with increased circulation
levels of endotoxin or LPS (39).
LPS is considered the most important antigen for the development of
ALI (40). Furthermore, animal
models of LPS-induced ALI are frequently used to investigate ALI
(13,41–43).
Therefore, in the present study, LPS was used to establish an
animal model of ALI in neonatal rats, and various concentrations of
propofol were administrated by intraperitoneal injection. In a
previous studies, to assess lung edema, the lung W/D ratio of
neonatal rats was determined (44), and the level of arterial blood
PaO2 was examined to investigate the pulmonary gas
exchange function (44). Lung
injury scores were calculated in a previous study by summing the
degree of cell infiltration and the severity score of tissue damage
was assessed from observation of the lung sections (31). In the present study treatment with
propofol was able to relieve the pulmonary edema and to alleviate
the lung injury induced by LPS in neonatal rats. Accumulating
evidence demonstrated that inflammation serves a key role in the
initiation and maintenance of ALI (33), and inflammation in animal models of
ALI is characterized by an increase in the circulating levels of
inflammatory factors including TNF-α, IL-1β and IL-6 (34–36).
The effects of propofol on the protein expression
levels of certain inflammatory factors were examined in neonatal
rats with ALI. The present results suggested that propofol
decreased the protein expression levels of TNF-α, IL-6 and IL-1β
that were increased by LPS in neonatal rats, which is consistent
with a previous study (22).
Oxidative stress, which has been identified to be involved in
LPS-induced ALI (45,46), was also assessed in the present
study. Treatment with propofol was identified to significantly
suppress oxidative stress in neonatal rats with ALI.
To investigate the molecular mechanism underlying
the effects of propofol on neonatal ALI, the present study analyzed
the p38 MAPK/NF-κB pathway and NLRP3 inflammasome, which were
previously identified to serve important roles in the regulation of
the inflammatory response and to be regulated by propofol (20,21).
The present study results suggested that the p38 MAPK/NF-κB pathway
and NLRP3 inflammasome were activated by the treatment with LPS in
neonatal rats, and treatment with propofol significantly prevented
the activation of p38 MAPK/NF-κB signaling pathway and NLRP3
inflammasome.
However, due to its rapid onset and short
elimination half-life, propofol is considered an addictive
substance with sedative and relaxing effects, and the recreational
abuse of this drug has increased over the past years (47,48).
Although the concentration of propofol used in the present study is
not sufficient to induce addiction (48,49),
propofol is an addictive drug and treatment with propofol requires
to be monitored. Therefore, future studies are required to further
investigate the side effects of propofol prior to its clinical
use.
In summary, the present study suggested that
treatment with propofol may relieve LPS-induced pulmonary edema,
inhibit LPS-associated inflammatory response and oxidative stress
in neonatal rats by repressing the activation of the p38 MAPK/NF-κB
signaling pathway and the NLRP3 inflammasome. Propofol served a
protective role in neonatal ALI induced by LPS, and it may
represent a promising therapeutic agent for the treatment of
neonatal ALI.
Acknowledgements
The authors would like to thank Dr Deng Xingmei from
The Department of Paediatrics, Maternal and Child Health-Care
Hospital for his help.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XY was responsible for study design, data access and
analysis, interpretation of results and preparation of the
manuscript. CL was responsible for interpretation of results and
preparation of the manuscript.
Ethics approval and consent to
participate
All experimental procedures were performed according
to the Recommended Guidelines for the Care and Use of Laboratory
Animals issued by The Chinese Council on Animal Research. The
present study was approved by The Animal Ethics Committee of The
Maternal and Child Health-Care Hospital of Qujing (Qujing,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rubenfeld GD, Caldwell E, Peabody E,
Weaver J, Martin DP, Neff M, Stern EJ and Hudson LD: Incidence and
outcomes of acute lung injury. N Engl J Med. 353:1685–1693. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Butt Y, Kurdowska A and Allen TC: Acute
lung injury: A clinical and molecular review. Arch Pathol Lab Med.
140:345–350. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheifetz IML: Year in review 2015:
Pediatric ARDS. Respir Care. 61:980–985. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo Z, Gu Y, Wang C, Zhang J, Shan S, Gu
X, Wang K, Han Y and Ren T: Enforced expression of miR-125b
attenuates LPS-induced acute lung injury. Immunol Lett. 162:18–26.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De Luca D, Piastra M, Tosi F, Pulitanò S,
Mancino A, Genovese O, Pietrini D and Conti G: Pharmacological
therapies for pediatric and neonatal ALI/ARDS: An evidence-based
review. Curr Drug Targets. 13:906–916. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chakraborty M, McGreal EP and Kotecha S:
Acute lung injury in preterm newborn infants: Mechanisms and
management. Paediatr Respir Rev. 11:162–170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fujishima S, Gando S, Daizoh S, Kushimoto
S, Ogura H, Mayumi T, Takuma K, Kotani J, Yamashita N, Tsuruta R,
et al: Infection site is predictive of outcome in acute lung injury
associated with severe sepsis and septic shock. Respirology.
21:898–904. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Quasney MW, López-Fernández YM, Santschi M
and Watson RS; Pediatric Acute Lung Injury Consensus Conference
Group, : The outcomes of children with pediatric acute respiratory
distress syndrome: Proceedings from the pediatric acute lung injury
consensus conference. Pediatr Crit Care Med. 16 (5 Suppl
1):S118–S131. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pediatric Acute Lung Injury Consensus
Conference Group, : Pediatric acute respiratory distress syndrome:
Consensus recommendations from the pediatric acute lung injury
consensus conference. Pediatr Crit Care Med. 16:428–439. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matuschak GM and Lechner AJ: Acute lung
injury and the acute respiratory distress syndrome: Pathophysiology
and treatment. Mo Med. 107:252–258. 2010.PubMed/NCBI
|
|
11
|
Ding Q, Liu GQ, Zeng YY, Zhu JJ, Liu ZY,
Zhang X and Huang JA: Role of IL-17 in LPS-induced acute lung
injury: An in vivo study. Oncotarget. 8:93704–93711.
2017.PubMed/NCBI
|
|
12
|
Wang T, Hou W and Fu Z: Preventative
effect of OMZ-SPT on lipopolysaccharide-induced acute lung injury
and inflammation via nuclear factor-kappa B signaling in mice.
Biochem Biophys Res Commun. 485:284–289. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheng K, Yang A, Hu X, Zhu D and Liu K:
Curcumin attenuates pulmonary inflammation in lipopolysaccharide
induced acute lung injury in neonatal rat model by activating
peroxisome proliferator-activated receptor γ (PPARγ) pathway. Med
Sci Monit. 24:1178–1184. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rigouzzo A, Khoy-Ear L, Laude D, Louvet N,
Moutard ML, Sabourdin N and Constant I: EEG profiles during general
anesthesia in children: A comparative study between sevoflurane and
propofol. Paediatr Anaesth. Jan 4–2019.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vasileiou I, Xanthos T, Koudouna E, Perrea
D, Klonaris C, Katsargyris A and Papadimitriou L: Propofol: A
review of its non-anaesthetic effects. Eur J Pharmacol. 605:1–8.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang S, Liu Y, Huang L, Zhang F and Kang
R: Effects of propofol on cancer development and chemotherapy:
Potential mechanisms. Eur J Pharmacol. 831:46–51. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu XR, Cao L, Li T, Chen LL, Yu YY, Huang
WJ, Liu L and Tan XQ: Propofol attenuates
H2O2-induced oxidative stress and apoptosis
via the mitochondria- and ER-medicated pathways in neonatal rat
cardiomyocytes. Apoptosis. 22:639–646. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaur J, Flores Gutiérrez J and Nistri A:
Neuroprotective effect of propofol against excitotoxic injury to
locomotor networks of the rat spinal cord in vitro. Eur J Neurosci.
44:2418–2430. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zheng X, Huang H, Liu J, Li M, Liu M and
Luo T: Propofol attenuates inflammatory response in LPS-activated
microglia by regulating the miR-155/SOCS1 pathway. Inflammation.
41:11–19. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu S, Sun JY, Ren LP, Chen K and Xu B:
Propofol attenuates intermittent hypoxia induced up-regulation of
proinflammatory cytokines in microglia through inhibiting the
activation of NF-Bκ/p38 MAPK signalling. Folia Neuropathol.
55:124–131. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuan D, Su G, Liu Y, Chi X, Feng J, Zhu Q,
Cai J, Luo G and Hei Z: Propofol attenuated liver
transplantation-induced acute lung injury via connexin43 gap
junction inhibition. J Transl Med. 14:1942016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu W, Zhu H and Fang H: Propofol
potentiates sevoflurane-induced inhibition of nuclear
factor-κB-mediated inflammatory responses and regulation of
mitogen-activated protein kinases pathways via toll-like receptor 4
signaling in lipopolysaccharide-induced acute lung injury in mice.
Am J Med Sci. 354:493–505. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tan Z, Wang H, Sun J and Li M: Effects of
propofol pretreatment on lung morphology and heme oxygenase-1
expression in oleic acid-induced acute lung injury in rats. Acta
Cir Bras. 33:250–258. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma J, Xiao W, Wang J, Wu J, Ren J, Hou J,
Gu J, Fan K and Yu B: Propofol inhibits NLRP3 inflammasome and
attenuates blast-induced traumatic brain injury in rats.
Inflammation. 39:2094–2103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lv H, Zhu C, Liao Y, Gao Y, Lu G, Zhong W,
Zheng Y, Chen W and Ci X: Tenuigenin ameliorates acute lung injury
by inhibiting NF-κB and MAPK signalling pathways. Respir Physiol
Neurobiol. 216:43–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hu X, Shen H, Wang Y and Zhao M: Liver X
receptor agonist TO901317 attenuates paraquat-induced acute lung
injury through inhibition of NF-κB and JNK/p38 MAPK signal
pathways. Biomed Res Int. 2017:46526952017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grailer JJ, Canning BA, Kalbitz M,
Haggadone MD, Dhond RM, Andjelkovic AV, Zetoune FS and Ward PA:
Critical role for the NLRP3 inflammasome during acute lung injury.
J Immunol. 192:5974–5983. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang S, Zhao J, Wang H, Liang Y, Yang N
and Huang Y: Blockage of P2X7 attenuates acute lung injury in mice
by inhibiting NLRP3 inflammasome. Int Immunopharmacol. 27:38–45.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bayne K: Revised guide for the care and
use of laboratory animals available. American physiological
society. Physiologist. 39:199–208, 211. 1996.PubMed/NCBI
|
|
30
|
Wang X, Liu C and Wang G: Propofol
protects rats and human alveolar epithelial cells against
lipopolysaccharide-induced acute lung injury via inhibiting HMGB1
expression. Inflammation. 39:1004–1016. 2016.PubMed/NCBI
|
|
31
|
Wang C, Zeng L, Zhang T, Liu J and Wang W:
Casticin inhibits lipopolysaccharide-induced acute lung injury in
mice. Eur J Pharmacol. 789:172–178. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gando S, Kameue T, Matsuda N, Sawamura A,
Hayakawa M and Kato H: Systemic inflammation and disseminated
intravascular coagulation in early stage of ALI and ARDS: role of
neutrophil and endothelial activation. Inflammation. 28:237–244.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Azevedo ZM, Moore DB, Lima FC, Cardoso CC,
Bougleux R, Matos GI, Luz RA, Xavier-Elsas P, Sampaio EP,
Gaspar-Elsas MI and Moraes MO: Tumor necrosis factor (TNF) and
lymphotoxin-alpha (LTA) single nucleotide polymorphisms: Importance
in ARDS in septic pediatric critically ill patients. Hum Immunol.
73:661–667. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rabelo MAE, Lucinda LMF, Reboredo MM, da
Fonseca LMC, Reis FF, Fazza TF, Brega DR, de Paoli F, de Souza da
Fonseca A and Pinheiro BV: Acute lung injury in response to
intratracheal instillation of lipopolysaccharide in an animal model
of emphysema induced by elastase. Inflammation. 41:174–182. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen C, Shi L, Li Y, Wang X and Yang S:
Disease-specific dynamic biomarkers selected by integrating
inflammatory mediators with clinical informatics in ARDS patients
with severe pneumonia. Cell Biol Toxicol. 32:169–184. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Abderrazak A, Syrovets T, Couchie D, El
Hadri K, Friguet B, Simmet T and Rouis M: NLRP3 inflammasome: From
a danger signal sensor to a regulatory node of oxidative stress and
inflammatory diseases. Redox Biol. 4:296–307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Franco ML, Waszak P, Banalec G, Levame M,
Lafuma C, Harf A and Delacourt C: LPS-induced lung injury in
neonatal rats: Changes in gelatinase activities and consequences on
lung growth. Am J Physiol Lung Cell Mol Physiol. 282:L491–L500.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Miotla JM, Teixeira MM and Hellewell PG:
Suppression of acute lung injury in mice by an inhibitor of
phosphodiesterase type 4. Am J Respir Cell Mol Biol. 18:411–420.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shi D, Zheng M, Wang Y, Liu C and Chen S:
Protective effects and mechanisms of mogroside V on LPS-induced
acute lung injury in mice. Pharm Biol. 52:729–734. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun A, Wang W, Ye X, Wang Y, Yang X, Ye Z,
Sun X and Zhang C: Protective effects of methane-rich saline on
rats with lipopolysaccharide-induced acute lung injury. Oxid Med
Cell Longev. 2017:74301932017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang J, Li R, Peng Z, Zhou W, Hu B, Rao X,
Yang X and Li J: GTS-21 reduces inflammation in acute lung injury
by regulating M1 polarization and function of alveolar macrophages.
Shock. 30–Mar;2018.(Epub ahead of print).
|
|
43
|
Gao S, Li H, Zhou XQ, You JB, Tu DN, Xia
G, Jiang JX and Xin C: Withaferin A attenuates
lipopolysaccharide-induced acute lung injury in neonatal rats. Cell
Mol Biol (Noisy-le-grand). 61:102–106. 2015.PubMed/NCBI
|
|
44
|
Hua S, Liu X, Lv S and Wang Z: Protective
effects of cucurbitacin B on acute lung injury induced by sepsis in
rats. Med Sci Monit. 23:1355–1362. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dong Z and Yuan Y: Accelerated
inflammation and oxidative stress induced by LPS in acute lung
injury: Inhibition by ST1926. Int J Mol Med. 41:3405–3421.
2018.PubMed/NCBI
|
|
46
|
Du L, Hu X, Chen C, Kuang T, Yin H and Wan
L: Seabuckthorn paste protects lipopolysaccharide-induced acute
lung injury in mice through attenuation of oxidative stress. Oxid
Med Cell Longev. 2017:41309672017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Park HJ, Shin JY, Kim MH and Park BJ:
Increased use in propofol and reported patterns of adverse events
among anesthetics in Korea. Regul Toxicol Pharmacol. 71:478–483.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bonnet U and Scherbaum N: Craving
dominates propofol addiction of an affected physician. J
Psychoactive Drugs. 44:186–190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bonnet U: Assessment of the addictive risk
of propofol. Fortschr Neurol Psychiatr. 79:442–452. 2011.(In
German). View Article : Google Scholar : PubMed/NCBI
|