Introduction
Atopic dermatitis (AD) is a highly pruritic
cutaneous disease from an inflammatory background that affects up
to 10% of adults and 25% of children (1). The skin of AD-sufferers may feature
significantly disruption of the epithelial barrier, exacerbated
responsiveness to allergens and defective innate immune response to
pathogens (2). According to
guidelines from the American Academy of Dermatology, treatment
should be commenced using mild to moderate-potency topical
corticosteroids when emollients and careful skin care are not able
to keep AD under control. Should such an approach fail, then
calcineurin inhibitors ought to be considered. Calcineurin
inhibition reduces transcription factors that regulate cell
division, which in will turn exert an anti-inflammatory effect by
selectively preventing T-cell activation. Prolongued use of topical
corticosteroids is often associated with epithelial and skin
atrophy (3) and may occasionally
result in systemic adverse effects, e.g.,
hypothalamic-pituitary-adrenal suppression, particularly in
children (4). Undesirable
long-term risks associated with calcineurin inhibitors include
lymphoma and cutaneous carcinomas in animal studies (5).
The largest meta-analysis to date comparing the
efficacy between corticosteroids and calcineurin inhibitors on
6.954 children and adults with moderate to severe AD concluded that
calcineurin inhibitors and corticosteroids are just as effective
for managing AD, though the superiority of the former over
corticosteroid is yet to be demonstrated to justify routine use
(6). Calcineurin inhibitors are
expensive and show a greater range of adverse events, such as skin
burns and pruritus. There is therefore no consensus as to whether
calcineurin inhibitors would represent a superior option in the
management of AD. Since the range of drug-based options to treat AD
is limited, intense research is underway to develop new
pharmacological strategies to tackle AD.
Prostaglandins (PG) are the product of sequential
COXs-mediated reactions, which will eventually undergo spontaneous
dehydration to PGJ2 in vitro and be further
enhanced by albumin-induced catalysis, generating several other
derivatives, including 15-deoxy-Δ12,14-PGJ2
(15d-PGJ2) (7).
Similarly to other PGs, 15d-PGJ2 can be actively
transported into cells to promptly bind nuclear receptors and
modify intracellular signaling factors, thanks to a highly reactive
cyclopentenone ring (8). It has
been demonstrated that 15d-PGJ2 may be the basis for
promising strategies to tackle a variety of inflammatory diseases
(9,10). AD is also characterized by mast
cell migration into the epidermis to release paracrine mediators,
including PGD2, which in aqueous media, will
spontaneously dehydrate to yield biologically active cyclopentenone
PGs, such as 15d-PGJ2 (11).
Considering the anti-inflammatory potential of
15d-PGJ2, the aim of this study was to test the
effectiveness of topical thermoreversible
15d-PGJ2-poloxamer (PL) 407 hydrogel formulation in the
2,4-dinitrochlorobenzene (DNCB)-induced AD animal model.
Materials and methods
Preparation and physico-chemical
characterization of 15d-PGJ2 hydrogel
PL 407 hydrogels at 30% w/w were dispersed in
deionized water at 4°C by magnetic stirring (150 rpm) for 12 h
until complete dissolution. 15d-PGJ2 was then
solubilized in dimethyl sulfoxide (DMSO) and dispersed into the
hydrogel at 15 ng/µl. The final DMSO concentration into the
hydrogels was 0.015%, which is sufficiently low to avoid skin
toxicity.
The 15d-PGJ2-micelle interaction and
micellar self-assembly were investigated using dynamic light
scattering [(DLS; Nanoseries Zetasizer ZS-Malvern®
particle analyzer (Malvern Instruments, Ltd., Malvern, UK)] for
determining the micellar hydrodynamic diameter and mean
distribution size. For samples preparation, PL or
PL-PGJ2 systems (3% w/v) were filtered across a
polycarbonate membrane (pore 0.22 µm) and measurements acquired at
least three times for sample at a fixed 173° angle, at 25°C to
37°C.
Drug loading (DL, %) and entrapment efficiency (EE,
%) parameters were determined for 3% PL micellar formulation.
Aliquots (100 µl) were diluted in 0.02 M monobasic sodium phosphate
pH 3.5/acetonitrile (60/40% v/v) solution and analyzed by HPLC
method. DL, % (Eq. 1) and EE, % (Eq. 2) were determined as
follow:
(Eq.1)DL(%)=(CPGJ2in micellar phase/CPLin
micellar sample)x100
(Eq.2)EE(%)=(CPGJ2in micellar
phase/Ctotal)x100
where CPGJ2 is 15d-PGJ2
concentration, CPL is PL concentration, and
Ctotal is the total PGJ2 concentration into
the samples.
Differential Scanning Calorimetry (DSC) was
performed to determine temperature and enthalpy relative to
micellization. The hydrogels (30 mg) were placed in sealed aluminum
receptacles and underwent three heating-cooling cycles (0 to 50°C)
at 5°C/min in a DSC equipment (Q-200; TA Instruments, New Castle,
DE, USA). An empty receptacle was used as negative control. All
thermograms were described as heat flux (cal/g) against temperature
(°C).
The sol-gel transition temperature (Tsol-gel) and
gelation kinetics were determined by an oscillatory rheometer
(Kinexus Lab., Malvern Instruments, Ltd.) with a cone-plate
geometry, under a temperature range from 10 to 50°C and frequency
at 1 Hz. From the results, parameters related to the elastic (G′),
viscous modulus (G″) and viscosity (η) were obtained and data
analyzed by rSpace for Kinexus® software.
For investigating the 15d-PGJ2 release
mechanisms from PL hydrogel, in vitro assays were carried
out using a vertical two-compartment diffusion model Franz-type
cells (1.76 cm2 area, Microette Plus®, Hanson
Research, Chatsworth, CA, USA). An artificial membrane (cellulose
acetate sheets, MWCO 1000 Da, Spectrum Lab) was used as a barrier
for separating the two compartments. The donor compartment was
filled with 250 µl of 15d-PGJ2 (in ultrapure water) or
PL404-PGJ2. 15d-PGJ2 final concentration of
3.75 µg/250 µl for both formulations. Receptor compartment was
filled with 7.0 ml of 5 mM Hepes, 154 mM NaCl buffer (pH 7.4, at
37°C) and maintained under magnetic stirring (350 rpm). Aliquots of
1.0 ml were withdrawn from the receptor compartment at intervals
from 0.5 to 24 h. Samples were analyzed by HPLC. Data were
expressed as 15d-PGJ2 released percentage against time
(h).
Release profiles were then analyzed according to
Zero-order (Eq. 3), Higuchi (Eq. 4) and Hixson-Crowell (Eq. 5)
models, as described below:
Qt=Q0+K0t(Eq.3)
where Qt is the cumulative amount of drug released
at time t, Q0 is the initial amount of drug, K0 is the
zero-order release constant, and t is time.
Qt=KHt1/2(Eq.4)
where the rate of drug release is linear as a
function of square root of time and the drug is the only component
that diffuses through the medium, which the release mechanism is a
diffusion process dependent on Fick law. KH is the
release coefficient, and Qt is the drug released amount.
Q01/3-Qt1/3=KHCt(Eq.5)
Q0 is the initial amount of drug,
Qt is the cumulative amount of drug released,
KHC is the release constant and t is time.
HPLC method for 15d-PGJ2
quantification
The 15d-PGJ2 quantification was performed
by High Performance Liquid Chromatography (Ultimate 3000 with
Chromeleon 7.2 software; Dionex Corporation, Sunnyvale, CA, USA)
system composed of quaternary pump, DAD detector and C18 column
(150×4.6 mm, 5 µm-Phenomenex). Samples were detected at 216 nm, 0.6
ml/min flow rate (25°C) and mobile phase composed of 0.02 M
monobasic sodium phosphate pH 3.5/acetonitrile (60/40 v/v). Drug
retention time was 2.8 min. A calibration curve was obtained from
standard solutions (2.5, 5, 50, 250 and 300 ng/ml). The detection
(LOD) and quantification (LOQ) limits values were 0.063 and 0.189
ng/ml, respectively, obtained from the previously determined
equation (y=0.8378 × + 3.339, R2=0.989).
Animals
This study was performed on male Wistar rats
weighing 200 to 300 g (n=5/per group) and kept in cages (5 per
cage) in a temperature-controlled room (23±1°C), 12:12 light cycle,
with water and food ad libitum. All animals were obtained
from the Multidisciplinary Center for Biological Investigation on
Laboratory Animal Science (CEMIB-UNICAMP) and the experimentation
was approved by the Committee on Animal Research of the University
of Campinas (approval no. 4088-1), which followed the guidelines by
the Brazilian National Council for Control of Animal
Experimentation (CONCEA).
Inducing AD-Like Lesions and
15d-PGJ2 hydrogel treatment
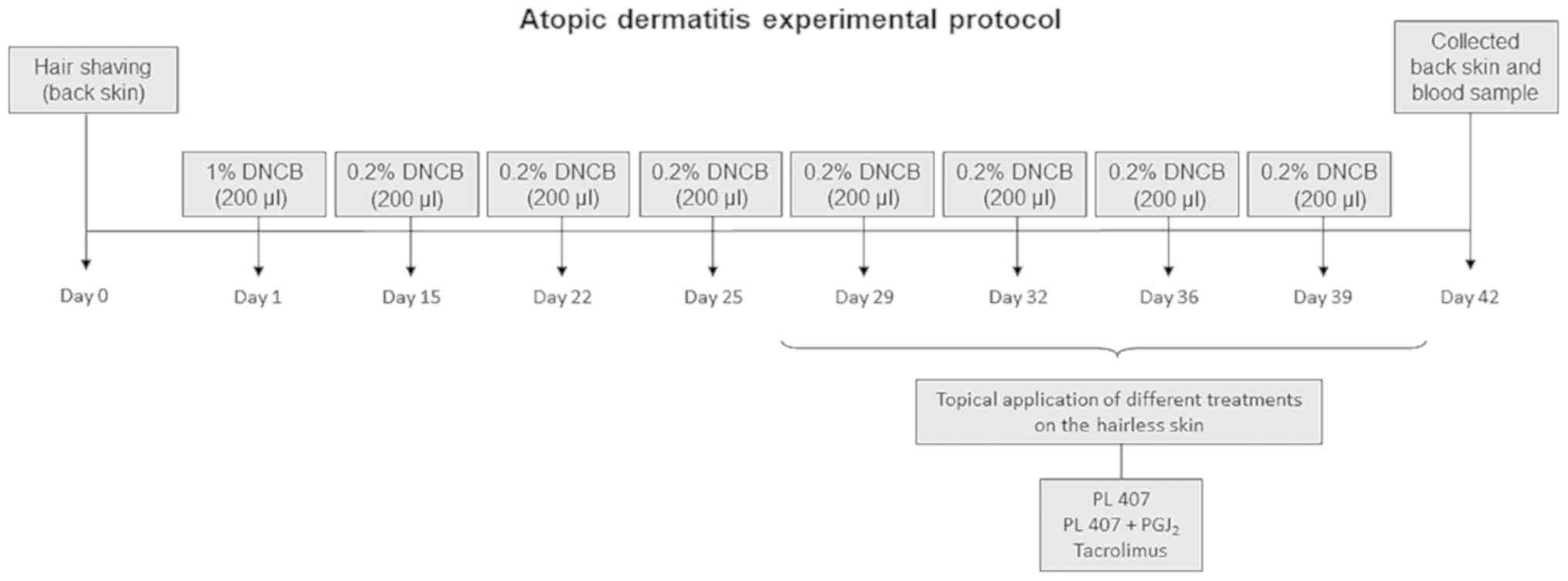
Induction of AD-like lesions was adapted from
previously published guidelines (12). The DNCB is an aromatic hydrocarbon
that when directly apply in the skin induce an inflammation. The
skin from the dorsum of the rats was shaved to an area of 1×1 cm
and painted once with 200 µl of 1% DNCB. Two weeks after
sensitization, the target area on the skin was challenged with 200
µl of 0.2% DNCB solution twice weekly for 2 weeks. Subsequently,
one of the following treatments was topically applied once daily
over 14 days: i) No treatment; ii) vehicle (PL-407, 3 µl); iii)
15d-PGJ2 hydrogel (75 ng/3 µl) or iv) Tacrolimus 0.1%
(Tarfic® 0.1%, Tacrolimus Monohydrate; Libbs
Pharmaceutics, São Paulo, Brazil). The treatments were maintained
at the site of lesion induction. When the experiment was complete,
the animals were sacrificed by CO2 inhalation and skin
biopsies were harvested. The AD-protocol is summarized in Fig. 1.
Histological analysis
A portion of the skin biopsies were fixed in neutral
formalin and paraffin-embedded. Seven-micrometer tissue sections
were taken and stained with toluidine blue for mast cell count.
Initial analysis of the toluidine blue sections were
performed by four examiners (ABS, NM, MJ, PA) using a multi-headed
microscope. Toluidine blue staining was evaluated both
qualitatively and quantitatively. Qualitative analysis was
performed via cell positivity in all areas of the section.
Quantitative analysis of mast cells was performed by positive cell
counting within the subepithelial connective tissue over 10 fields
per case at magnification, ×400 (×40 objective lens, field diameter
of 0.44 mm) using a CCD camera on a Nikon Eclipse Ci microscope.
The individual who performed the counting was blind to the
experimental groups.
Immunohistochemistry
Five-micrometer sections were immune-stained for
ROR-γt and TNF-α. following endogenous peroxidase activity
quenching in 3% hydrogen peroxide (Dinâmica, Diadema, SP, Brazil).
Antigen retrieval (AR) was performed in boiling citrate buffer (pH
6.0). The primary antibody was incubated overnight at 4°C, followed
by EnVision HRP and Envision+ (K1491; Dako; Agilent Technologies,
Inc., Santa Clara, CA, USA) at 37°C for one hour. The sections were
then stained with 3,3′-diaminobenzidine tetrahydrochloride (DAB,
Dako; Agilent Technologies, Inc.) for five min at 37°C and
counter-stained with hematoxylin.
ROR-γt and TNF-α expression was evaluated by
inflammatory positive cell counting within the subepithelial
connective tissue over 10 fields per case at magnification, ×400
(×40 objective lens, field diameter of 0.44 mm) using a CCD camera
on a Nikon Eclipse Ci microscope. Epithelial cells were not taken
into account for ROR-γt and TNF-α expression. The individual who
performed the counting was blind to the experimental groups.
Blood sample collection and IgE
quantification
Whole blood was collected by cardiac puncture to
quantify IgE levels following 15d-PGJ2 hydrogel
administration. The blood samples were stored in EDTA Vacutainer
tubes containing EDTA (BD Biosciences, Franklin Lakes, NJ, USA) and
blood plasma was then isolated. IgE measurements were obtained
using ELISA following the manufacturer's instructions (BD
Biosciences) via optical density (O.D.) measured at 450 nm and the
readings were expressed as pg/ml, according to the standard.
Statistical analysis
To determine if there were significant differences
(P<0.05) among groups, the data were analyzed using one-way
analysis of variance (ANOVA) with post hoc contrasts using the
Tukey's test. Data are presented in figures as mean ± standard
deviation (SD). All statistical calculations were performed on
GraphPad Prism 6® (GraphPad Software, Inc., La Jolla,
CA, USA).
Results
Physico-chemical characterization of
PL 407 micelles and hydrogel
The hydrodynamic diameter was the parameter used for
evaluating the PL 407 micelles formation in the presence or absence
of 15d-PGJ2. In general, there were no overall
significant changes on micellar hydrodynamic diameter for PL407
systems after 15d-PGJ2 incorporation. Micellar diameters
of ~60 nm (average distribution of 88.1±0.7%) and ~5 nm (12.1±0.2%)
were observed at 25°C, while at 37°C, micellar dimensions were
reduced to ~30 nm with 99.6±0.7% and polydispersion values of
~0.25, showing the influence of temperature variation on micellar
self-assembly, even in the presence of 15d-PGJ2. For DL
% and EE % parameters were obtained values of 44.1±0.2 and
98.0±0.3%, respectively, indicating that PL407 micelles are able to
carry high amounts of 15d-PGJ2.
Calorimetric analysis (Table I) showed that micelles formation is
an endothermic process (enthalpy values greater than zero), with
micellization temperature (Tm) at 17.8 and 15.2°C, before and after
15d-PGJ2 incorporation. Even slightly different Tm was
observed for 15d-PGJ2-PL407, this system presented high
enthalpy variation (ΔH°=0.31 cal/g) than that observed for PL407
isolated system (ΔH°=0.21 cal/g).
 | Table I.Hydrodynamic diameter, size
distribution, themodynamic and rheological parameters for
PGJ2-loaded PL407 hydrogels. |
Table I.
Hydrodynamic diameter, size
distribution, themodynamic and rheological parameters for
PGJ2-loaded PL407 hydrogels.
|
| Hydrodynamic
diameter (nm) | Average
distribution (%) |
|
|
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Formulations | 25°C | 37°C | 25°C | 37°C | Tm (°C) | ΔH
(kJ.mol−1) | G′
(.104Pa) | G″
(.104Pa) | G′/G″ | η
(.103mPa.s) | Tsol-gel (°C) |
|---|
| PL407 | 59.7±2.1 | 31.4±1.7 | 88.1±2.1 | 94.1±1.6 | 17.8 | 0.21 | 1975 | 648.4 | 3.04 | 330900 | 20.4 |
|
|
|
| 12.1±0.2 |
6.8±0.9 |
|
|
|
|
|
|
|
|
PL407-PGJ2 | 61.2±0.2 | 29.8±1.3 | 87.9±1.2 | 99.6±0.8 | 15.2 | 0.31 | 9114 | 890.5 | 10.2 | 1457000 | 21.8 |
|
|
|
| 13.1±0.9 |
|
|
|
|
|
|
|
|
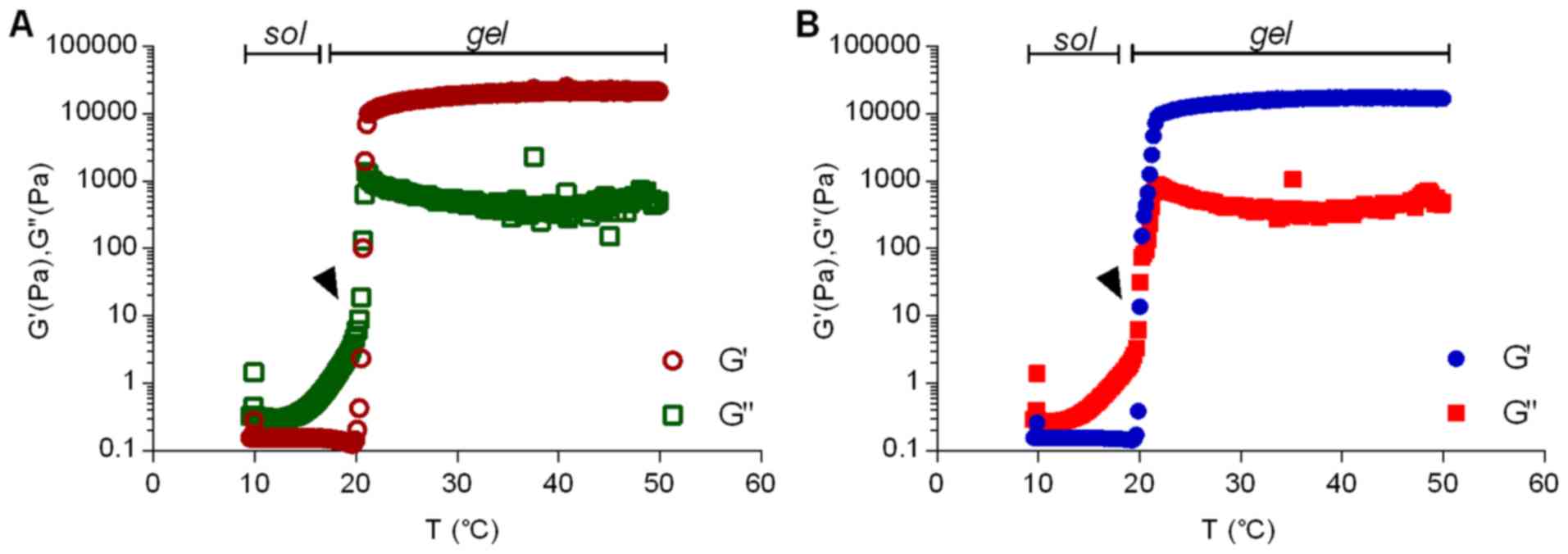
For PL-based formulations, the rheological behavior
provides essential information to study the hydrogel formation and
the influence of incorporated molecules into its structure. For
this reason, rheological parameters such as elastic (G′) and
viscous (G″) moduli, viscosity (η) and Tsol-gel (when the most
pronounced viscosity variation is observed) were determined before
and after 15d-PGJ2 insertion (Table I). Fig. 2 presents the rheograms for PL407
and PL407-15d-PGJ2 under temperature variation. The
incorporation of 15d-PGJ2 did not, significantly, shift
the Tsol-gel, but evoked pronounced changes on elastic modulus (G′)
reaching values ~10 times higher than that observed for G″.
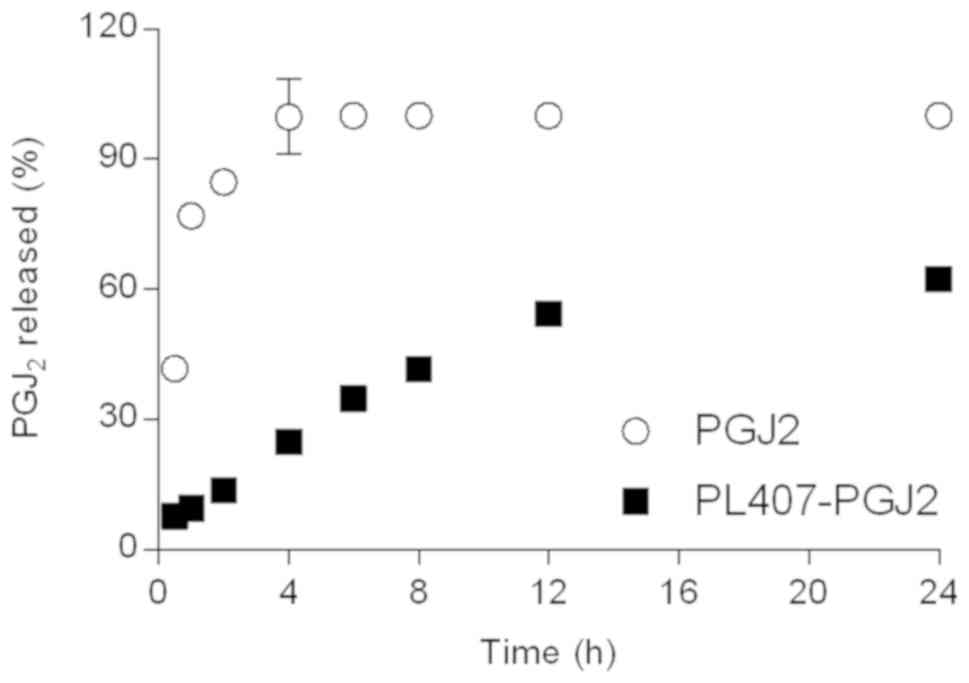
As demonstrated in Fig.
3 and Table II, the
15d-PGJ2 release profiles and their mathematical models.
The 15d-PGJ2 release from aqueous solution reached a
maximum release percentage after 4 h. On the other hand, the
15d-PGJ2 released from PL407 hydrogels was sustained and
lower drug release percentages were observed until 24 h (62.3%),
when compared to 15d-PGJ2 in solution (100%). In
general, low release constant (Krel) values were obtained for
PL407-PGJ2 (1.4%.h−1; 10.4%.h−1/2;
0.15%.h−1/3 for Zero Order, Higuchi and Hixson-Crowell
models, respectively) in relation to 15d-PGJ2. However,
the Hixson-Crowell mathematical model showed the highest
correlation coefficient value (R2=0.97) compared to Zero
Order (R2=0.95) and Higuchi (R2=0.91).
 | Table II.Release constants and determination
coefficients obtained for PGJ2 from PL407 (30% wt)
hydrogel. |
Table II.
Release constants and determination
coefficients obtained for PGJ2 from PL407 (30% wt)
hydrogel.
|
| Zero Order | Higuchi | Hixson-Crowell |
|---|
|
|
|
|
|
|---|
| Formulations | K0
(%.h−1) | R2 | KH
(%.h−1/2) | R2 | KHC
(%.h−1/3) | R2 |
|---|
|
PGJ2 | 2.4±0.4 | 0.84 | 15.0±1.2 | 0.85 | 0.46±0.05 | 0.87 |
|
PL407-PGJ2 | 1.4±0.9 | 0.91 | 10.4±4.4 | 0.95 | 0.15±0.01 | 0.97 |
15d-PGJ2 hydrogel decreases
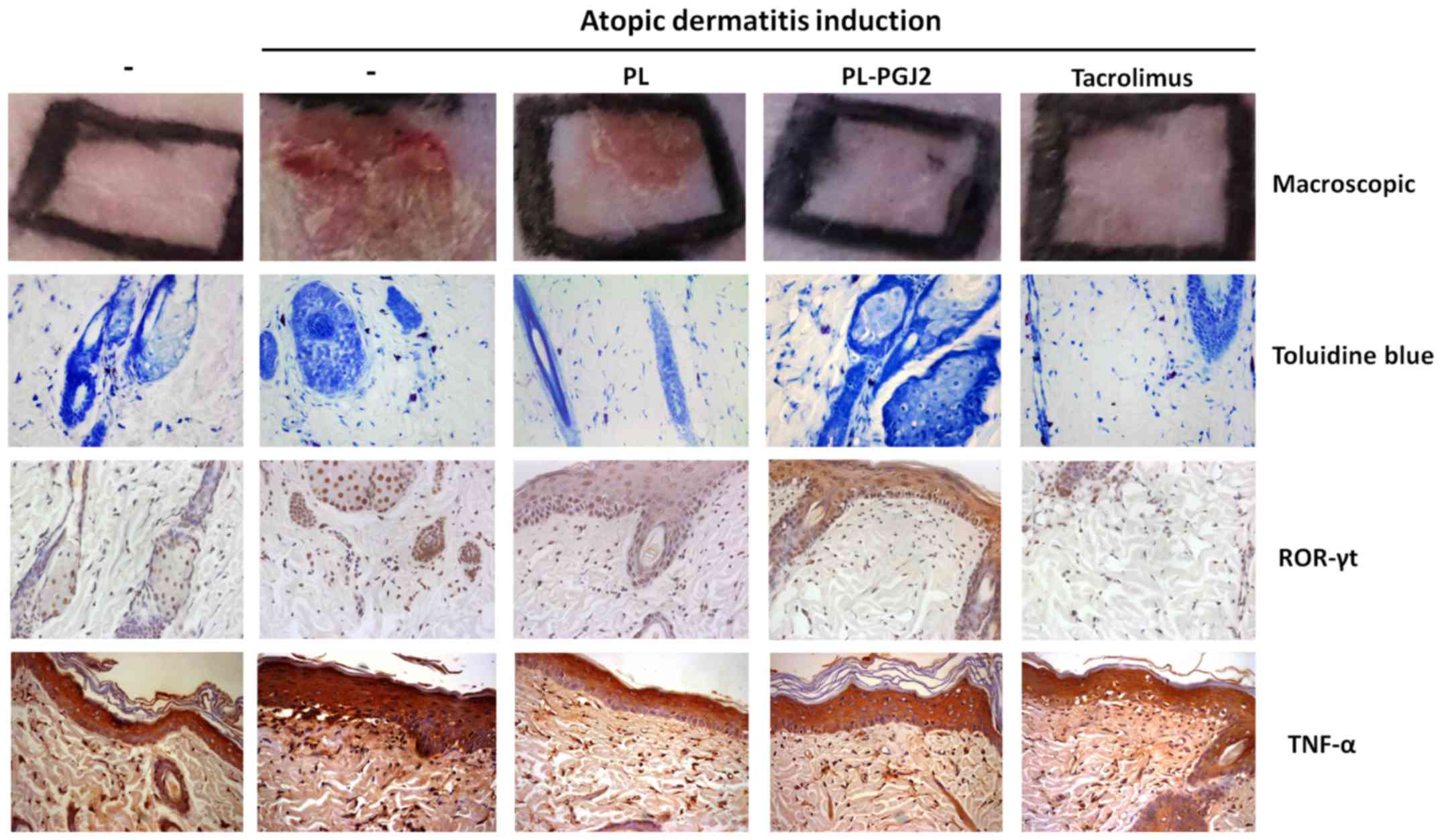
infiltration of mast cells into AD-like skin lesions
To establish whether 15d-PGJ2 hydrogel
reduces mast cell infiltration into AD-like skin lesions, toluidine
blue staining was performed on the skin biopsies following topical
administration of 15d-PGJ2 or vehicle (Fig. 4). Mast cell infiltration was
detected in the AD-like group and the AD-like group treated with
vehicle (PL-407), where the 15d-PGJ2 hydrogel
significantly decreased (P<0.05) such infiltration of mast cells
into the skin when compared with AD-like and AD-like + PL-407
(Fig. 5). Moreover, the group
treated with Tacrolimus 0.1% also decreased mast cell infiltration,
although no statistically significant difference was detected when
compared to both untreated groups. The data on mast cell counts is
shown in Fig. 5.
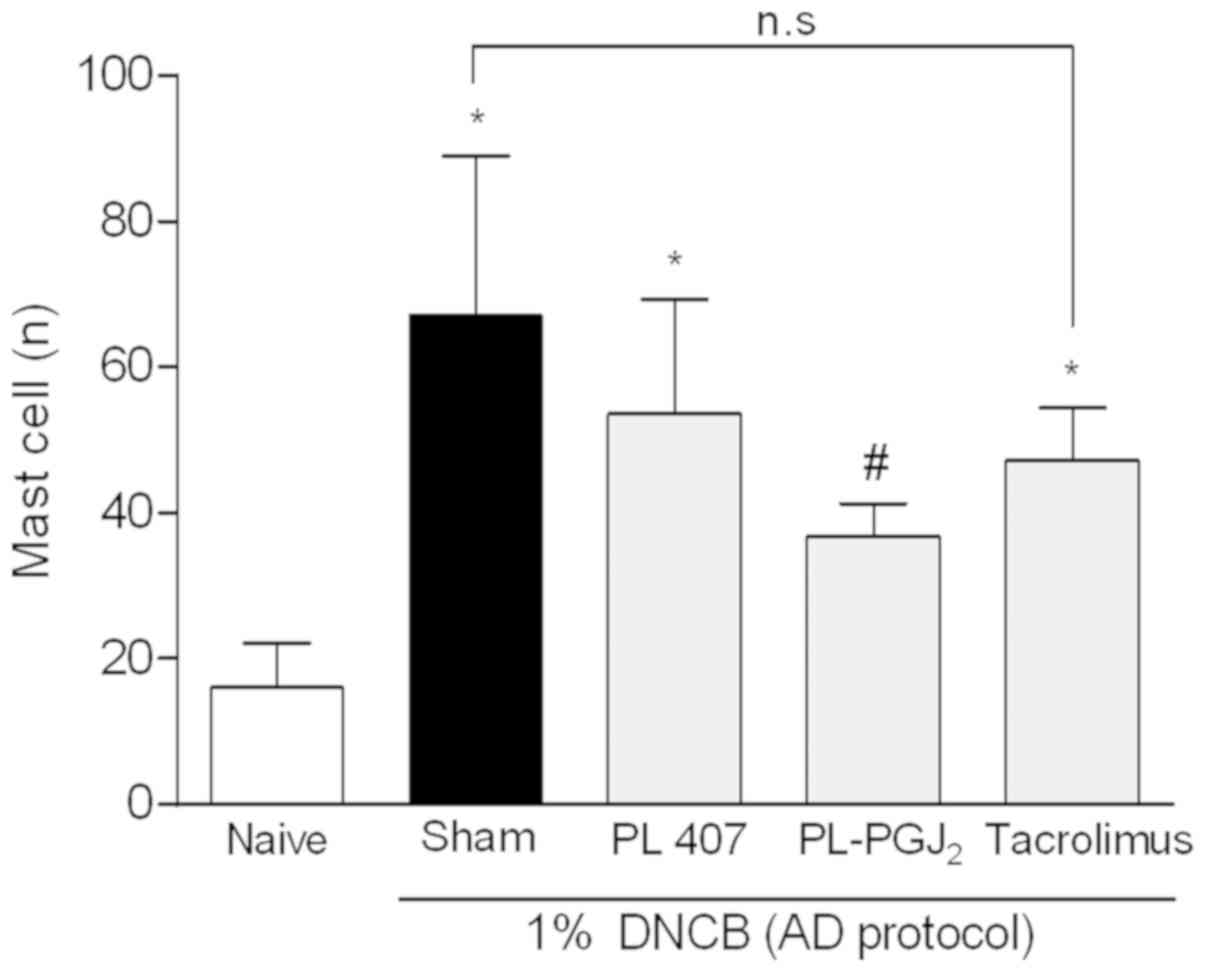 | Figure 5.Mast cell count in AD-like skin
lesions. The skin sections were stained with toluidine blue for
mast cells. Quantitative analysis of mast cells was performed by
positive cell counting within the subepithelial connective tissue
over 10 fields per case at magnification, ×400. The data are
presented as mean ± SD of 5 animals per group. The symbol (*)
indicates a mast cell counting significantly higher than Naïve
group (non AD-group) (P<0.05: ANOVA, Tukey's test). The symbol
(#) indicates a mast cell counting significantly lower than
AD-group group (P<0.05: ANOVA, Tukey's test). n.s, not
significant; AD, atopic dermatitis; SD, standard deviation; ANOVA,
one-way analysis of variance; PG, prostaglandin; PL, poloxamer;
DNCB, 2,4-dinitrochlorobenzene. |
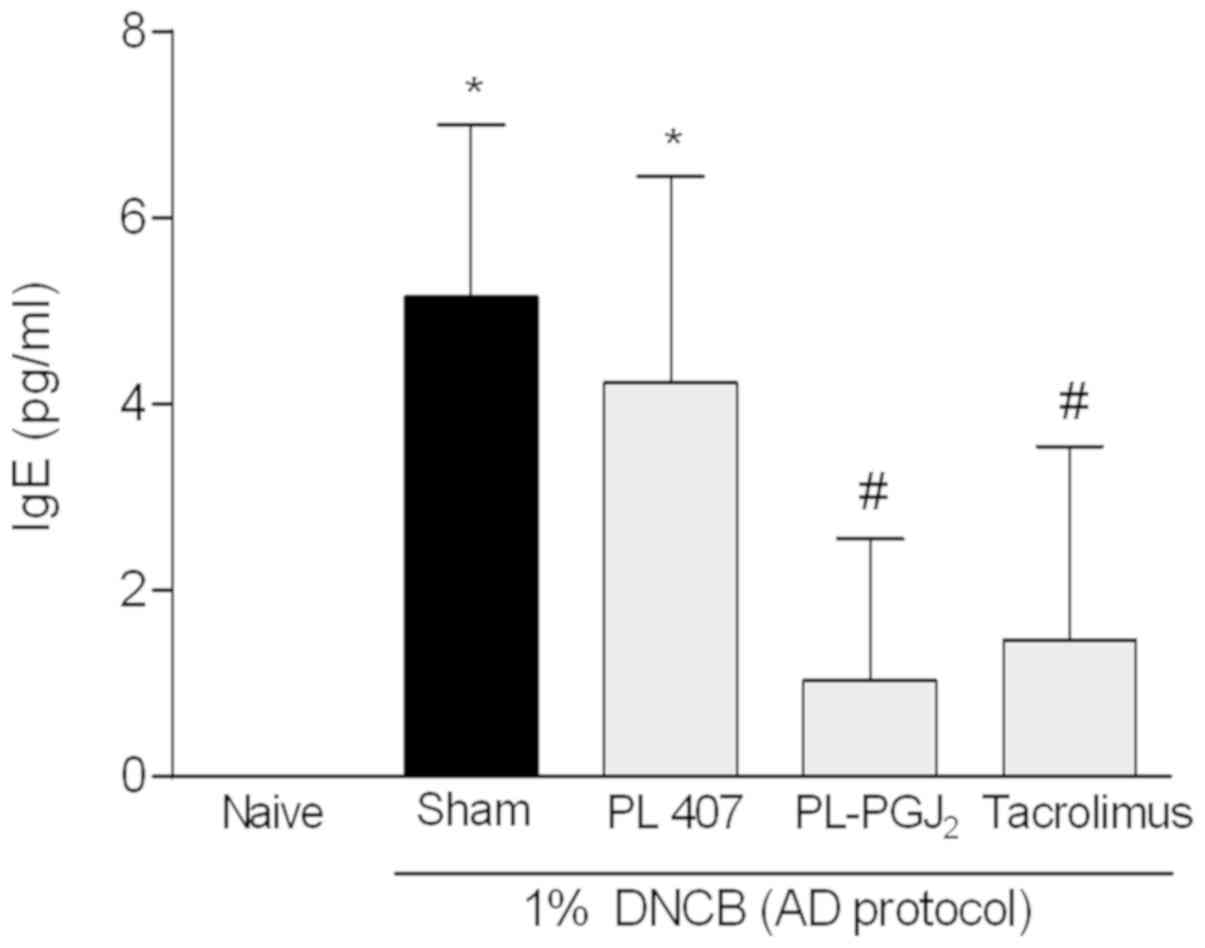
Measurement of total plasma IgE level
in AD-like skin lesion
High IgE levels are a major feature of AD and those
diagnosed with AD often exhibit high levels of total IgE and also
allergen-specific IgE. Serum levels of IgE in the AD-like group and
AD-like treated with vehicle were significantly higher than that in
the disease-free group. The administration of topical
15d-PGJ2 hydrogel or Tacrolimus 0.1% significantly
reduced the serum levels of IgE (P<0.05) compared to AD-like
groups (Fig. 6).
Effect of 15d-PGJ2 hydrogel
on ROR-γ expression in rat skin tissue
To determine whether 15d-PGJ2 hydrogel
decreases Th17 type lymphocyte, we performed immunohistochemistry
to quantify the transcription factor ROR-γt (Fig. 4). We found that topical
administration of 15d-PGJ2 hydrogel significantly
decreased the number of immunostained cells compared to the AD-like
group (P<0.05). No significant difference was observed between
the AD-like group and the Tacrolimus-treated group (P>0.05), nor
was it observed between the 15d-PGJ2 hydrogel and the
Tacrolimus groups (P>0.05). The data are shown in Fig. 7A.
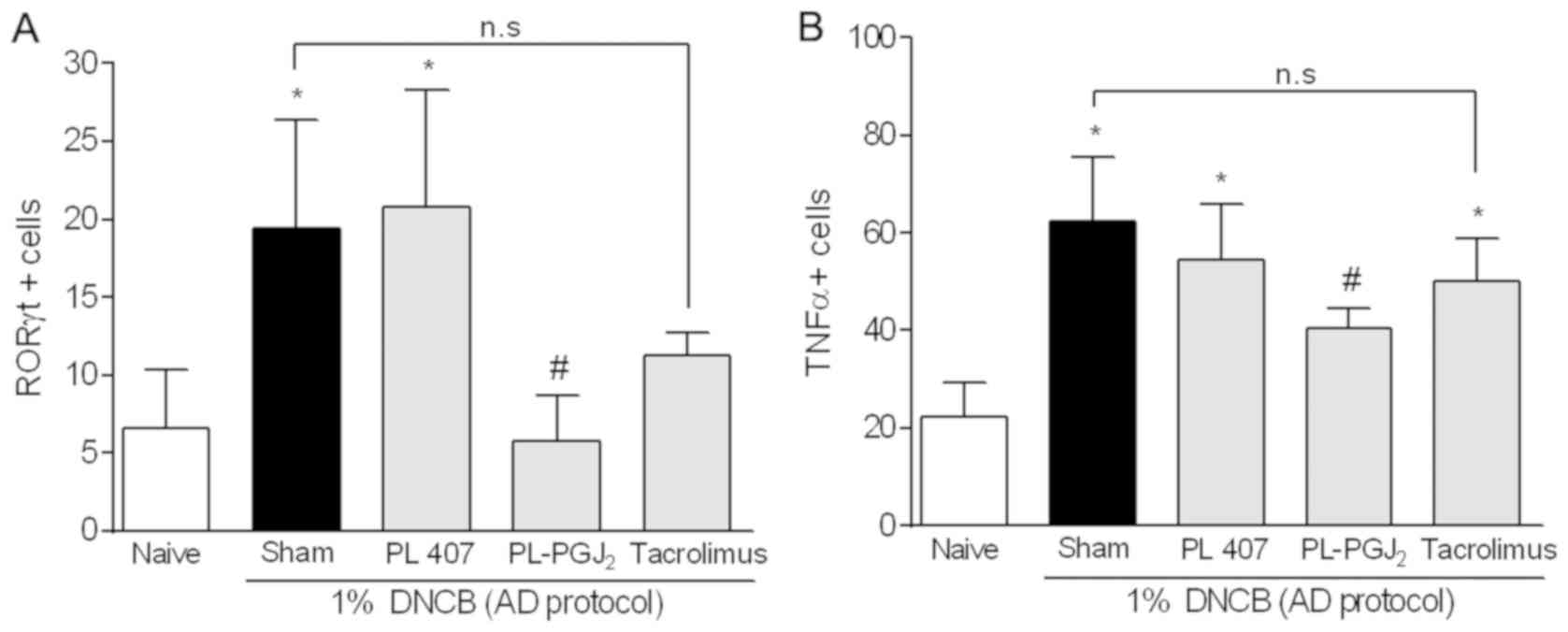 | Figure 7.Effect of 15d-PGJ2
hydrogel on the (A) ROR-γ and (B) TNF-α expression in rat skin
tissue measured by immunohistochemistry. The data are presented as
mean ± SD of 5 animals per group. The symbol (*) indicates a ROR-γ
or TNF-α expression significantly higher than Naïve group (non
AD-group) (P<0.05: ANOVA, Tukey's test). The symbol (#)
indicates a ROR-γ or TNF-α expression significantly lower than
AD-group (P<0.05: ANOVA, Tukey's test). 15d-PGJ2,
15-deoxy-Δ12,14-PGJ2; PG, prostaglandin; PL,
poloxamer; AD, atopic dermatitis; n.s, not significant; DNCB,
2,4-dinitrochlorobenzene; ANOVA, one-way analysis of variance; SD;
standard deviation. |
Effect of 15d-PGJ2 hydrogel
on TNF-α expression in rat skin tissue
Immunohistochemistry was performed to verify whether
15d-PGJ2 hydrogel would reduce TNF-α expression
(Fig. 4). The number of
immunostained cells in the AD-like group and the AD-like group
treated with vehicle significantly increased in comparison to the
disease-free specimens (P<0.05). Moreover, topical
administration of 15d-PGJ2 hydrogel significantly
reduced the TNF-α-positive cell count from the AD-like groups
(P<0.05). It is important to highlight that no significant
difference was detected between the AD-like group and the
Tacrolimus-treated group (P>0.05). The data are described in
Fig. 7B.
Discussion
The prostaglandin known as 15d-PGJ2 is an
endogenous PG that binds to PPAR-γ generated during the resolution
phase of inflammation following tissue injury (13) and it has shown a potent
anti-inflammatory action when administrated exogenously (10,14,15).
Moreover, improved bioavailability and efficiency of such compound
has been achieved from different strategies to couple the
15d-PGJ2 molecule to carrier systems (9,16,17).
In this study, we demonstrated that topical administration of
15d-PGJ2 hydrogel had a beneficial effect on AD
symptoms, suggesting a potentially useful role for this formulation
in the management of AD.
Considering that micellar dimensions were reduced at
physiological temperature, micelles can remain at the site of
administration for long periods of time and be small enough
(<100 nm) to avoid uptake by the reticuloendothelial system,
favoring the therapeutic efficacy of the drug carrier (18). In fact, for PL systems (such as PL
407), reductions on micellar hydrodynamic diameters in response to
temperature changes are well-described in the literature. This
phenomenon is attributed to the dehydration of PL polyoxypropylene
oxide units from micellar core, reducing the micellar dimensions
and promoting the formation of a colloidal system with spherical
and almost identical micelles (19–21).
PL407 is a relatively hydrophilic PL type with
hydrophilic lipophilic balance value of 22, due to differences on
its polyethylene oxide (PEO) and polypropylene oxide (PPG) units
number. The 1:3 units PEO:PPO relationship characterize its
chemical structure making possible the formation of both micellar
hydrophobic core and hydrophilic corona capable of
self-organization in a hydrogel supramolecular structure,
responding to the presence of different molecules according to
their chemical structure (22).
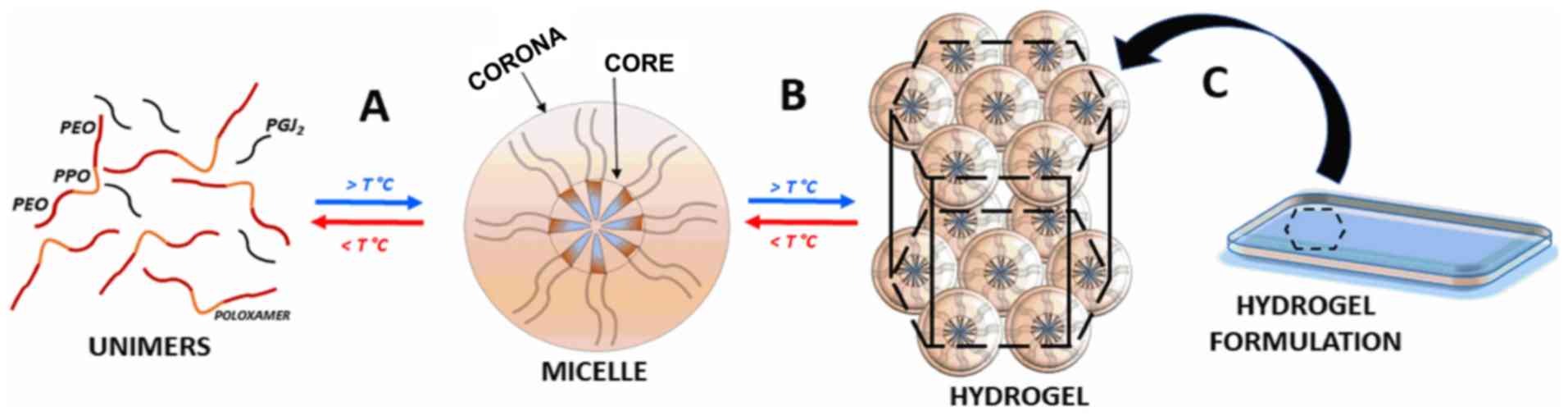
Fig. 8 presents the sol-gel
transition phenomena from PL unimers to micelles and their
self-assembly as hydrogels in response to concentration and
temperature, forming a final hydrogel formulation proposed
here.
Calorimetry analysis showed no significant shifts
regarding to temperature for micelles formation. However,
differences were observed for enthalpy variation value after
15d-PGJ2 incorporation, showing the drug interference on
micellar self-assembly possibly due to its insertion into the
system, as also described for different hydrophobic molecules
(23–25). One of the main advantages of this
system is the ability to incorporate hydrophobic molecules. Then,
the hydrophobicity of the PL micellar core (due to polypropylene
oxide units dehydration) and the 15d-PGJ2 chemical
structure, as a low molecular weight prostanoid derivative
(molecular weight of 316.4,
C20H28O3), are important features
for favoring its incorporation into PL407 micelles, explaining the
high 15d-PGJ2 DL and encapsulation efficiency
percentages. Additionally, no changes were observed on
thermoreversible properties, indicating the stability of the
hydrogel systems after 15d-PGJ2 incorporation.
Rheological analysis provided important information regarding the
sol-gel process kinetics, showing the formation of a structurally
ordered and viscous hydrogel due to the predominance of elastic
over viscous properties and increased viscosity values after
15d-PGJ2 incorporation. The 15d-PGJ2-loaded
hydrogels showed low release constant value determined by the drug
dissolution rate and its permeation during the hydrogel polymeric
matrix erosion. This mechanism contributes to the formation of
hydrated matrices due to the water penetration across the polymer
chains (23,26).
In this study, we presented the development of a
topical hydrogel formulation for AD based on
15d-PGJ2-loaded PL407 hydrogel. The in vitro
assays showed an extended release profile, being possible to
predict that low 15d-PGJ2 concentrations could be in
contact to the site of application. According to the
pharmacological daily scheme proposed here, a hydrogel volume of 3
µl was applied providing a 15d-PGJ2 final concentration
of 75 ng in contact to the skin area lesions. Since ~60% of
encapsulated 15d-PGJ2 was quantified after 24 h, that
concentration was sufficiently released from hydrogels formulation
explaining the formulation efficiency in terms of available drug
concentration, despite the differences between in vitro and
in vivo studies.
Regarding to molecular mechanism for AD treatment,
there are two main concerns: the first is that AD is a chronic
disease and long-term topical steroid use may lead to skin atrophy
(27) and the second is that
calcineurin inhibitors do not affect skin thickness but carry an
increased risk of inducing skin cancer (28). We have demonstrated that topical
administration of 15d-PGJ2 hydrogel significantly
reduced mast cell infiltration into the skin, reinforced by the
fact that the outer aspect of the epidermis on the treated animals
looked visibly normal (Figs. 4 and
5). A previous study showed that
subcutaneous administration of 15d-PGJ2 suppressed
Bleomycin-induced skin sclerosis, despite no significant
suppression of mast cell infiltration, but significant inhibition
of the mast cell activation process (29). Furthermore, 15d-PGJ2 was
found to inhibit a series of fibroblast-associated processes, such
as TGF-β stimulation of collagen gene expression,
transdifferentiation of myofibroblasts (30) as well as the Smad-dependent
promoter activity (31).
15d-PGJ2 has also been shown to attenuate the
proliferation of keloid cells, inhibit collagen gel contraction as
well as to increase cleavage of caspase-3 (32). It has been demonstrated, however,
that 15d-PGJ2 and a prostanoid DP2 receptor agonist
(13,14-dihydro-15-keto-prostaglandin D2) had no clear effect on the
scratching behavior of rodents, thus suggesting that such
prostaglandin D2 suppressive effect ought to be mediated by the
prostanoid DP1 receptor instead (33).
A previous study demonstrated that
15d-PGJ2 is able to reduce IgE levels and inhibit the
proliferation of LPS-induced B cells in an asthma-like model
(34) Furthermore,
15d-PGJ2 was able to reduce FcERI expression, thus
bypassing IgE binding to cells, which in turn decreased the
secretion of important allergic inflammation activators (35). Additionally, previous reports have
shown that 15d-PGJ2 can inhibit the IgE-switch in
B-cells by suppressing the phosphorylation of STAT-6 (36). Corroborating with this previous
findings, we showed that both treatments (15d-PGJ2
hydrogel or Tacrolimus 0.1%) significantly reduced systemic IgE
levels when compared to the AD-like group. Beyond that,
15d-PGJ2 hydrogel or Tacrolimus 0.1% groups shown no
difference when compared each other.
The role of the Th17 pathway has recently been
extensively explored in chronic inflammatory illnesses. While a
fundamentally autoimmune role has been associated with activation
of the Th17 pathway (37,38), emerging data suggest that IL-17 and
Th17 participate in the pathogenesis of AD, where IL-17 expression
is upregulated in patients with acute AD lesions (39). Furthermore, Koga et al
(40) have shown a correlation
between circulating Th17 cells and the severity of acute AD.
Besides, AD is considered a biphasic inflammation, in which
Th2-mediated disease is predominant in the acute phase, switching
towards the Th1-Th17 environment in chronic disease (41). Activated immune cells (macrophages
and T cells) secrete TNF-α, which may also be produced by mast
cells in response to IgE (42).
Our data have shown that 15d-PGJ2 hydrogel was able to
decrease the Th17 population at the site of AD, whereas Tacrolimus
0.1% failed to do so. This is a highly relevant observation, since
IL-17 plays its part in modulating immune dysregulation and
affecting the integrity of the skin barrier (43). Furthermore, upregulation of IL-17
in situ and circulating interleukin levels in AD reiterates
the systemic inflammatory nature of such condition (44). Additionally, a significantly
decreased in TNF-α expression was observed in group treated with
15d-PGJ2 hydrogel but not with Tacrolimus 0.1%. This an
important finding since it is well-known hat TNF-α is a key
accessory mediator of T cell activation in AD.
As 15d-PGJ2 may have multiple effects on animal
models of AD, more studies are needed to fully elucidate its role
on treating allergic diseases. The PPAR-γ pathway may be one such
target, since previous studies have shown that 15d-PGJ2
activation of PPAR-γ has an anti-inflammatory effect in an asthma
model, which is yet another atopic condition (45).
The present study clearly shows that
15d-PGJ2 hydrogel was able to suppress the progression
of DNBC-induced AD. In addition, IgE levels decreased as well as
Th17 and TNF-α positive cells. The emerging of a possible new
treatment for AD that could be as effective as the current
available with potentially fewer side-effects and a wider spectrum
of action in the mechanism of atopic inflammation should be
evaluated in a clinical trial. In conclusion, this new formulation
of 15d-PGJ2 hydrogel may be a useful strategy in the
management of AD.
Acknowledgements
Not applicable.
Funding
The authors are grateful to the Brazilian National
Council for Scientific and Technological Development (CNPq), the
São Paulo Research Foundation (FAPESP) and the Coordenação de
Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for their
financial support. MHN, JTC-N and DRA were supported by a research
fellowship (grant nos. 303493/2016-0 and 309207/2016-9,
respectively).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
MHN, AJDPJ and DRDA designed the study. CGM and HBA
performed the animal model experiments. NMM, MEAJ, PHBCA, ABS and
MS acquired the data. MHN, JTCN, ABS, MS and DRDA analyzed and
interpreted the data, and drafted the manuscript. All authors
critically revised the manuscript, and read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
All animal experimental procedures and protocols
were approved by the Committee on Animal Research of the University
of Campinas (approval no. 4088-1) and are in accordance with
guidelines of CONCEA.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Veiga SP: Epidemiology of atopic
dermatitis: A review. Allergy Asthma Proc. 33:227–234. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
De Benedetto A, Kubo A and Beck LA: Skin
barrier disruption: A requirement for allergen sensitization? J
Invest Dermatol. 132:949–963. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berke R, Singh A and Guralnick M: Atopic
dermatitis: An overview. Am Fam Physician. 86:35–42.
2012.PubMed/NCBI
|
|
4
|
Dhar S, Seth J and Parikh D: Systemic
side-effects of topical corticosteroids. Indian J Dermatol.
59:460–464. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Margolis DJ, Abuabara K, Hoffstad OJ, Wan
J, Raimondo D and Bilker WB: Association between malignancy and
topical use of pimecrolimus. JAMA Dermatol. 151:594–599. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Broeders JA, Ahmed Ali U and Fischer G:
Systematic review and meta-analysis of randomized clinical trials
(RCTs) comparing topical calcineurin inhibitors with topical
corticosteroids for atopic dermatitis: A 15-year experience. J Am
Acad Dermatol. 75:410–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kikawa Y, Narumiya S, Fukushima M,
Wakatsuka H and Hayaishi O: 9-Deoxy-delta 9, delta
12–13,14-dihydroprostaglandin D2, a metabolite of prostaglandin D2
formed in human plasma. Proc Natl Acad Sci USA. 81:1317–1321. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Straus DS and Glass CK: Cyclopentenone
prostaglandins: New insights on biological activities and cellular
targets. Med Res Rev. 21:185–210. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Napimoga MH, da Silva CA, Carregaro V,
Farnesi-de-Assunção TS, Duarte PM, de Melo NF and Fraceto LF:
Exogenous administration of 15d-PGJ2-loaded nanocapsules
inhibits bone resorption in a mouse periodontitis model. J Immunol.
189:1043–1052. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Macedo CG, Napimoga MH, Rocha-Neto LM,
Abdalla HB and Clemente-Napimoga JT: The role of endogenous opioid
peptides in the antinociceptive effect of
15-deoxyΔ12,14-prostaglandinJ2 in the temporomandibular joint.
Prostaglandins Leukot Essent Fatty Acids. 110:27–34. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shibata T, Takahashi K, Matsubara Y,
Inuzuka E, Nakashima F, Takahashi N, Kozai D, Mori Y and Uchida K:
Identification of a prostaglandin D2 metabolite as a neuritogenesis
enhancer targeting the TRPV1 ion channel. Sci Rep. 16:212612016.
View Article : Google Scholar
|
|
12
|
Kim SR, Choi HS, Seo HS, Ku JM, Hong SH,
Yoo HH, Shin YC and Ko SG: Oral administration of herbal mixture
extract inhibits 2,4-dinitrochlorobenzene-induced atopic dermatitis
in BALB/c mice. Mediators Inflamm. 2014:3194382014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Surh YJ, Na HK, Park JM, Lee HN, Kim W,
Yoon IS and Kim DD:
15-Deoxy-Δ12,14-prostaglandin JZ, an
electrophilic lipid mediator of anti-inflammatory and pro-resolving
signaling. Biochem Pharmacol. 82:1335–1351. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Silva Quinteiro M, Henrique Napimoga M,
Gomes Macedo C, Furtado Freitas F, Balassini Abdalla H, Bonfante R
and Trindade Clemente-Napimoga J: 15-deoxy-Δ12,14-prostaglandin J2
reduces albumin-induced arthritis in temporomandibular joint of
rats. Eur J Pharmacol. 740:58–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Napimoga MH, Vieira SM, Dal-Secco D,
Freitas A, Souto FO, Mestriner FL, Alves-Filho JC, Grespan R, Kawai
T, Ferreira SH and Cunha FQ: Peroxisome proliferator-activated
receptor-gamma ligand, 15-deoxy-Delta12,14-prostaglandin J2,
reduces neutrophil migration via a nitric oxide pathway. J Immunol.
180:609–617. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Clemente-Napimoga JT, Moreira JA, Grillo
R, de Melo NF, Fraceto LF and Napimoga MH:
15d-PGJ2-loaded in nanocapsules enhance the
antinociceptive properties into rat temporomandibular
hypernociception. Life Sci. 90:944–949. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alves C, de Melo N, Fraceto L, de Araújo D
and Napimoga M: Effects of 15d-PGJZ-loaded
poly(D,L-lactide-co-glycolide) nanocapsules on inflammation. Br J
Pharmacol. 162:623–632. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gaumet M, Vargas A, Gurny R and Delie F:
Nanoparticles for drug delivery: The need for precision in
reporting particle size parameters. Eur J Pharm Biopharm. 69:1–9.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Trong LC, Djabourov M and Ponton A:
Mechanisms of micellization and rheology of PEO-PPO-PEO triblock
copolymers with various architectures. J Colloid Interface Sci.
328:278–287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oshiro A, da Silva DC, de Mello JC, Moraes
VW, Cavalcanti LP, Franco MK, Alkschbirs MI, Fraceto LF, Yokaichiya
F, Rodrigues T and de Araujo DR: Pluronics f-127/l-81 binary
hydrogels as drug-delivery systems: Influence of physicochemical
aspects on release kinetics and cytotoxicity. Langmuir.
30:13689–13698. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Akkari ACS, Papini JZB, Garcia GK, Franco
MKKD, Cavalcanti LP, Gasperini A, Alkschbirs MI, Yokaichyia F, de
Paula E, Tófoli GR and de Araujo DR: Poloxamer 407/188 binary
thermosensitive hydrogels as delivery systems for infiltrative
local anesthesia: Physico-chemical characterization and
pharmacological evaluation. Mater Sci Eng C Mater Biol Appl.
68:299–307. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dumortier G, Grossiord JL, Agnely F and
Chaumeil JC: A review of poloxamer 407 pharmaceutical and
pharmacological characteristics. Pharm Res. 23:2709–2728. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Santos Akkari AC, Ramos Campos EV, Keppler
AF, Fraceto LF, de Paula E, Tófoli GR and de Araujo DR:
Budesonide-hydroxypropyl-β-cyclodextrin inclusion complex in binary
poloxamer 407/403 system for ulcerative colitis treatment: A
physico-chemical study from micelles to hydrogels. Colloids Surf B
Biointerfaces. 138:138–147. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mello JC, Moraes VW, Watashi CM, da Silva
DC, Cavalcanti LP, Franco MK, Yokaichiya F, de Araujo DR and
Rodrigues T: Enhancement of chlorpromazine antitumor activity by
Pluronics F127/L81 nanostructured system against human multidrug
resistant leukemia. Pharmacol Res. 111:102–112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nascimento MHM, Franco MKKD, Yokaichyia F,
de Paula E, Lombello CB and de Araujo DR: Hyaluronic acid in
Pluronic F-127/F-108 hydrogels for postoperative pain in
arthroplasties: Influence on physico-chemical properties and
structural requirements for sustained drug-release. Int J Biol
Macromol. 111:1245–1254. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Valero M and Dreiss CA: Modulating
Pluronics micellar rupture with cyclodextrins and drugs: Effect of
pH and temperature. J Phys Conf Ser. 549:0120102014. View Article : Google Scholar
|
|
27
|
Mills CM and Marks R: Side effects of
topical glucocorticoids. Curr Probl Dermatol. 21:122–131. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
FDA Post market Drug Safety, . http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm107845.htm
|
|
29
|
Kohno S, Endo H, Hashimoto A, Hayashi I,
Murakami Y, Kitasato H, Kojima F, Kawai S and Kondo H: Inhibition
of skin sclerosis by 15deoxy delta12,14-prostaglandin J2 and
retrovirally transfected prostaglandin D synthase in a mouse model
of bleomycin-induced scleroderma. Biomed Pharmacother. 60:18–25.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kon K, Ikejima K, Hirose M, Yoshikawa M,
Enomoto N, Kitamura T, Takei Y and Sato N: Pioglitazone prevents
early-phase hepatic fibrogenesis caused by carbon tetrachloride.
Biochem Biophys Res Commun. 291:55–61. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ghosh AK, Bhattacharyya S, Lakos G, Chen
SJ, Mori Y and Varga J: Disruption of transforming growth factor
beta signaling and profibrotic responses in normal skin fibroblasts
by peroxisome proliferator-activated receptor gamma. Arthritis
Rheum. 50:1305–1318. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mantel A, Newsome A, Thekkudan T, Frazier
R and Katdare M: The role of aldo-keto reductase 1C3
(AKR1C3)-mediated prostaglandin D2 (PGD2) metabolism in keloids.
Exp Dermatol. 25:38–43. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Arai I, Takano N, Hashimoto Y, Futaki N,
Sugimoto M, Takahashi N, Inoue T and Nakaike S: Prostanoid DP1
receptor agonist inhibits the pruritic activity in NC/Nga mice with
atopic dermatitis. Eur J Pharmacol. 505:229–235. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Farnesi-de-Assunção TS, Alves CF,
Carregaro V, de Oliveira JR, da Silva CA, Cheraim AB, Cunha FQ and
Napimoga MH: PPAR-γ agonists, mainly 15d-PGJ(2), reduce eosinophil
recruitment following allergen challenge. Cell Immunol. 273:23–29.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fujimura Y, Tachibana H and Yamada K:
Peroxisome proliferator-activated receptor ligands negatively
regulate the expression of the high-affinity IgE receptor Fc
epsilon RI in human basophilic KU812 cells. Biochem Biophys Res
Commun. 297:193–201. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miyazaki Y, Tachibana H and Yamada K:
Inhibitory effect of peroxisome proliferator-activated
receptor-gamma ligands on the expression of IgE heavy chain
germline transcripts in the human B cell line DND39. Biochem
Biophys Res Commun. 295:547–552. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Klotz L, Burgdorf S, Dani I, Saijo K,
Flossdorf J, Hucke S, Alferink J, Nowak N, Beyer M, Mayer G, et al:
The nuclear receptor PPAR gamma selectively inhibits Th17
differentiation in a T cell-intrinsic fashion and suppresses CNS
autoimmunity. J Exp Med. 206:2079–2089. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vieira SM, Cunha TM, França RF, Pinto LG,
Talbot J, Turato WM, Lemos HP, Lima JB, Verri WA Jr, Almeida SC, et
al: Joint NOD2/RIPK2 signaling regulates IL-17 axis and contributes
to the development of experimental arthritis. J Immunol.
188:5116–5122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Toda M, Leung DY, Molet S, Boguniewicz M,
Taha R, Christodoulopoulos P, Fukuda T, Elias JA and Hamid QA:
Polarized in vivo expression of IL-11 and IL-17 between acute and
chronic skin lesions. J Allergy Clin Immunol. 111:875–881. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Koga C, Kabashima K, Shiraishi N,
Kobayashi M and Tokura Y: Possible pathogenic role of Th17 cells
for atopic dermatitis. J Invest Dermatol. 128:2625–2630. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Di Cesare A, Di Meglio P and Nestle FO: A
role for Th17 cells in the immunopathogenesis of atopic dermatitis?
J Invest Dermatol. 128:2569–2571. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Theoharides TC, Alysandratos KD, Angelidou
A, Delivanis DA, Sismanopoulos N, Zhang B, Asadi S, Vasiadi M, Weng
Z, Miniati A and Kalogeromitros D: Mast cells and inflammation.
Biochim Biophys Acta. 1822:21–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Heo WI, Lee KE, Hong JY, Kim MN, Oh MS,
Kim YS, Kim KW, Kim KE and Sohn MH: The role of interleukin-17 in
mouse models of atopic dermatitis and contact dermatitis. Clin Exp
Dermatol. 40:665–671. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Batista DI, Perez L, Orfali RL, Zaniboni
MC, Samorano LP, Pereira NV, Sotto MN, Ishizaki AS, Oliveira LM,
Sato MN and Aoki V: Profile of skin barrier proteins (filaggrin,
claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic
dermatitis. J Eur Acad Dermatol Venereol. 29:1091–1095. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Coutinho DS, Anjos-Valotta EA, do
Nascimento CVMF, Pires ALA, Napimoga MH, Carvalho VF, Torres RC, E
Silva PMR and Martins MA: 15-Deoxy-delta-12,14-prostaglandin J2
inhibits lung inflammation and remodeling in distinct murine models
of asthma. Front Immunol. 8:7402017. View Article : Google Scholar : PubMed/NCBI
|