Introduction
Breast cancer is one of the most widespread types of
cancer in females, and its incidence is increasing yearly. It is
expected that by 2018, the United States of America will have
~266,120 incident cases of invasive breast cancer and 63,960 cases
of in situ breast cancer. Furthermore, it is estimated that
40,920 women will succumb to breast cancer (1). Similarly, in China, the incidence of
breast cancer is the primary cause of mortality in women <45
years, followed by colorectal, liver and esophageal cancer
(2). Following systemic treatment,
patients with breast cancer continue to experience recurrence and
metastasis, and the majority of patients eventually succumb to
metastases. Therefore, it is important to identify diagnostic and
prognostic markers, and potential therapeutic targets (3,4). The
present study explored the mechanisms through which long non-coding
RNAs (lncRNAs) act as competing endogenous RNAs (ceRNAs) to
regulate target genes and participate in the prognosis and
pathogenesis of breast cancer.
Over previous decades, 70–90% of the transcribed
human genome has been identified. Relevant data indicate that the
proportion of genes encoding proteins in the genome is >2%, and
non-coding RNA accounts for the majority of the human transcriptome
(5). Non-coding RNAs are a large
class of RNA molecules that do not encode proteins, but which serve
regulatory roles. Non-coding RNAs may be divided into three
classes, by length: <50 nucleotides (nt), including microRNA
(miRNA), small interfering RNA and Piwi-interacting RNA; 50–500 nt,
including ribosomal RNA, transfer RNA, small nuclear RNA, small
nucleolar RNA and lncRNA; >500 nt, including long mRNA-like
non-coding RNAs and lncRNAs without polyA tails (6). lncRNA is a generic term for a class
of RNA molecules with lengths of >200 nucleotides, and is one of
the most active fields of study in molecular biology at present.
lncRNAs regulate the gene expression of tumor cells through
multiple modes of action, and therefore are widely involved in the
occurrence and metastasis of tumors (7–9).
lncRNAs are thought to serve an important role in the development
of cancer; however, only a few have been well-characterized
regarding their functional roles (10).
Differentially expressed ceRNAs have been identified
in a number of diseases, particularly in cancer. Previous studies
have demonstrated that ceRNAs affect the proliferation, growth,
differentiation, apoptosis and other biological behaviors of cancer
cells (11–13). miRNA response elements (MREs) are
partially complementary sequences located on mRNAs that bind to
miRNAs, and may inhibit the expression of miRNA target genes.
miRNAs regulate hundreds of mRNAs, and one miRNA may be regulated
by hundreds of mRNAs. Numerous types of RNA molecules constitute a
ceRNA regulatory network, and the more MREs that are shared between
them, the greater the potential for communication or co-regulation
of a target gene (14).
Theoretically, almost every RNA molecule possesses at least one MRE
binding site that binds to an miRNA, thereby forming a ceRNA
association with the corresponding miRNA (15). Among these, the interaction
mechanisms between lncRNA and miRNA include: miRNA binding to and
degrading lncRNA; lncRNA acting as miRNA sponge by binding to
adsorbed miRNA; and truncation of lncRNA to generate miRNA
(16). There is evidence that
lncRNAs interact with miRNAs and act as ceRNAs to regulate the
expression of miRNAs. CeRNAs serve important roles together with
cancer-associated genes, and they have demonstrated potential in
clinical tumor diagnosis and treatment (17,18).
As the largest database of cancer gene information
currently available, The Cancer Genome Atlas (TCGA) includes 33
types of cancers in situ, advancing the understanding of the
molecular basis of the onset of these diseases and improving the
ability to diagnose, treat and prevent cancer. TCGA comprises
multiple levels of tumor data, including genomic, transcriptomic,
proteomic and epigenetic data (19). In the present study, according to
the analysis of RNA expression profiles among 1,109 breast cancer
tumor and 113 non-tumor tissue samples, a lncRNA-miRNA-mRNA
regulatory network was successfully constructed. Furthermore, Cox
regression analysis was used to select the top 10 differentially
expressed lncRNAs (DElncRNAs), to understand the function of
lncRNAs and their potential mechanisms in breast cancer. Survival
analyses were also performed to identify prognostic genes.
Materials and methods
Study population
RNA sequencing data was downloaded from TCGA
(https://www.cancergenome.nih.gov/).
As of February 19, 2018, a total of 1,092 breast cancer cases were
collected in the database. Univariate Cox analysis was performed
using the library (survival) package in R software (version 3.4.3;
R: A Language and Environment for Statistical Computing) (20) from the TCGA database. The present
study adhered to the TCGA publication guidelines.
Screening differentially expressed
genes
Breast cancer mRNA and miRNA sequencing data were
derived from 1,222 samples, including 1,109 tumor samples (cohort
Tumor) and 113 normal samples (cohort Normal). Normal sample and
tumor sample data were then merged, and all expression values equal
to zero were removed. Comparing the data from normal tissues with
breast cancer samples, Perl language was used to extract the mRNA
matrix file data from the RNA sequencing data (lncRNA, mRNA)
downloaded by TCGA in the Windows Environment, running the program
with cmd.exe, and then the data were converted into ensemble ID to
obtain the gene symbol matrix file. The gene symbol matrix file
contained the lncRNA and mRNA matrix, and gets-lncRNA
symbol.pl and get-mRNA symbol.pl scripts were used to
obtain the lncRNA symbol and mRNA symbol matrix data, respectively.
DElncRNAs were measured using lncRNA symbol matrix and ‘edgeR’
package in R software (version 3.4.3), with thresholds of |log2
fold change (FC)| >2.0 and adjusted P<0.01. DEmiRNAs were
defined as those with |log2FC| >2.0 and adjusted P<0.01.
Constructing the ceRNA network in
breast cancer
DElncRNAs and DEmiRNAs were predicted using the
miRcode (http://www.miRcode.org/), starBase
(http://starbase.sysu.edu.cn/) (21), using miRTarBase online software to
perform target gene predictions on the screened differential miRNAs
(22). TargetScan (http://www.targetscan.org/vert_72/) and miRDB
(http://mirdb.org/) databases were used to predict the
targeting relationship between DEmiRNA and DEmRNA. The target
DEmiRNA is entered into the database, which then shows all the
target genes that may interact with the target microRNA, and then
further screens the genes involved by the construction of a ceRNA
network (23). To understand the
functions of miRNA and lncRNA with the ceRNA network, gene
co-expression network analysis was used, and the results were
visualized with Cytoscape 3.6.0 (24).
Top 10 aberrantly expressed lncRNAs in
breast cancer
The top 10 lncRNA were selected by receiver
operating characteristics (ROC) curve and area under the curve
(AUC) analysis. The prognostic roles of lncRNAs were examined with
Kaplan-Meier curve analysis, and the log-rank test was conducted to
distinguish survival time. P<0.05 was considered to indicate a
statistically significant difference.
Analysis the lncRNAs with gene
expression profiling interactive analysis (GEPIA)
Similarly, lncRNA expression levels in
para-noncancerous tissues and cancer tissues were compared using
the GEPIA database (http://gepia.cancer-pku.cn) (25), which was used to analyze the RNA
sequencing data of 33 types of cancers from TCGA database.
Survival analysis
Using the survival time data for breast cancer in
TCGA, the ‘Survival’ package in R software (version 3.4.3) was used
to analyze the specific lncRNA, miRNA and mRNA associated with
survival. Kaplan-Meier survival analysis was performed to analyze
the correlation between DERNAs signature and breast cancer patient
prognosis. P<0.05 was considered to indicate a statistically
significant difference.
Results
DElncRNAs, DEmRNAs and DEmiRNAs
RNA expression levels in 1,109 breast cancer tumor
and 113 normal tissue samples were investigated. Genes with |log2
FC| >1.5 and adjusted P<0.01 were considered as
differentially expressed. The significant DEmiRNAs and DEmRNAs was
identified in breast cancer samples. A total of 2,155 DEmRNAs, 89
DEmiRNAs and 1,028 lncRNAs were identified from TCGA data set using
the ‘edgeR’ package in R. Of these, 1,434 mRNAs, 68 miRNAs and 777
lncRNAs were upregulated and 721 mRNAs, 21 miRNAs and 251 lncRNAs
were downregulated in breast cancer tissues compared with normal
tissues. The RNAs hierarchical clustering analyses are presented in
Fig. 1A, and it was demonstrated
that the expression levels of these three types of RNAs were
significantly differentiated compared with the normal tissues.
Finally, volcano plots were generated, and differences between the
normal and tumor groups were identified (Fig. 1B).
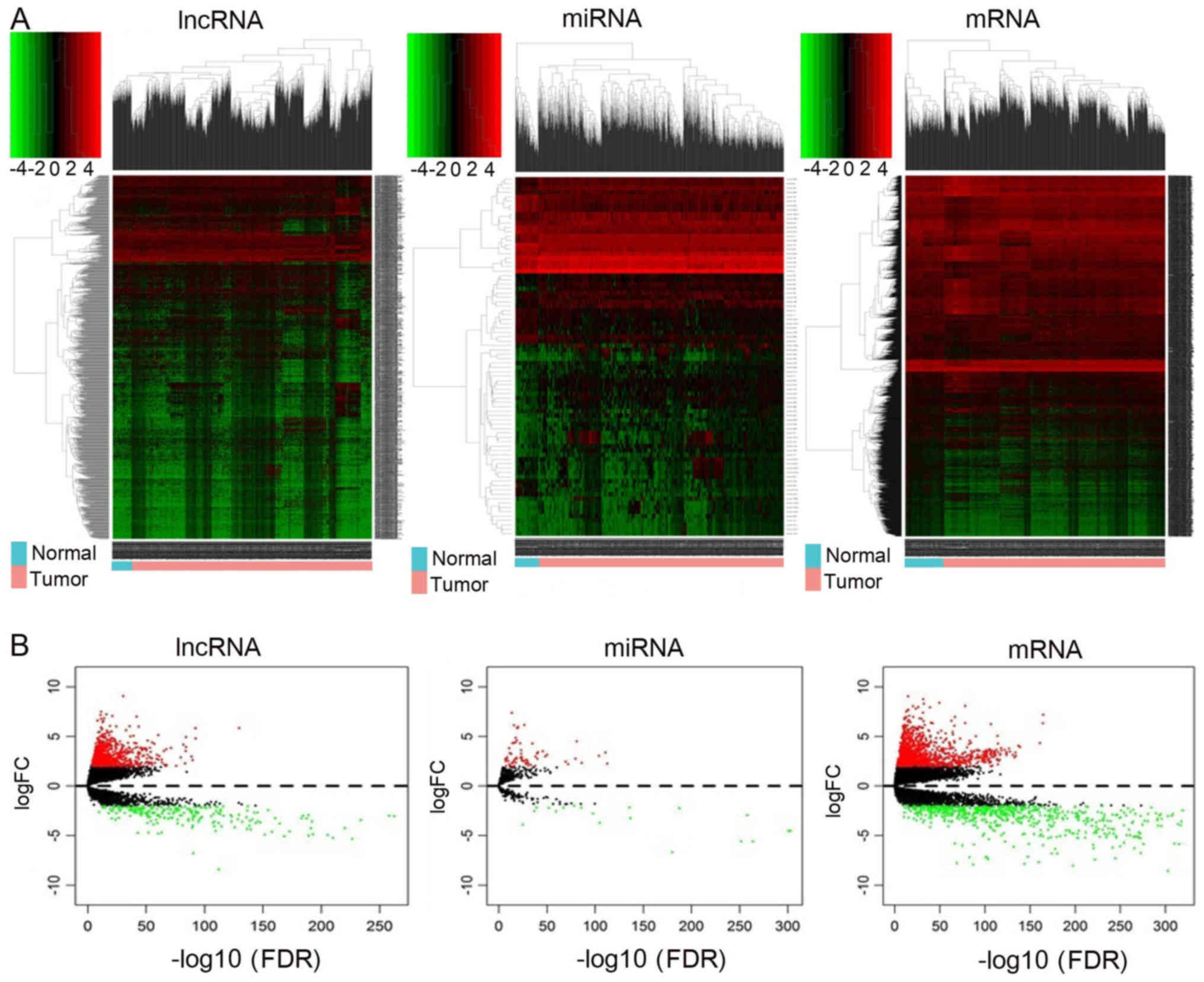 | Figure 1.Heatmap and volcano plots
demonstrating differential lncRNA, mRNA and miRNA expression
between normal and cancer samples. (A) Heatmap of DElncRNAs,
DEmiRNAs and DEmRNAs. Sample clusters are included above the
heatmap. Clusters of DElncRNAs, DEmiRNAs and DEmRNAs are noted on
the left of the heatmap. Red represents upregulated genes and green
represents downregulated genes. Normal samples (blue) are on the
left side of each heatmap, and tumor samples (pink) are on the
right. (B) Volcano plots reflecting number, significance and
reliability of differentially expressed lncRNAs, miRNAs and mRNAs.
Red dots indicate upregulation and green dots indicate
downregulation of lncRNAs, miRNAs and mRNAs. Black dots show the
lncRNAs, miRNAs and mRNAs with expression of |log2FC|<2. The
x-axis represents an adjusted FDR and the y-axis represents the
value of log2 FC. Aberrantly expressed lncRNAs were calculated by
DESeq R. In total, 777 upregulated and 251 downregulated lncRNAs,
68 upregulated and 21 downregulated miRNAs, and 1,434 upregulated
and 721 downregulated mRNAs were identified. lncRNA, long
non-coding RNA: miRNA, microRNA, DE, differentially expressed; FC,
fold change; FDR, false discovery rate. |
miRNA targeting lncRNAs predicted by
miRcode and starBase
A total of 89 breast cancer-associated miRNAs were
identified to be expressed in breast cancer and normal tissues. A
total of 19 miRNAs were selected from 89 breast cancer-associated
miRNAs in TCGA (|log2 FC| >3; P<0.001; Table I). It was identified that 19 miRNAs
interacted with 93 lncRNAs (Table
II).
 | Table I.Breast cancer-specific miRNAs in the
competing endogenous RNA network. |
Table I.
Breast cancer-specific miRNAs in the
competing endogenous RNA network.
| miRNA | Expression
change | log2 FC (T/N) | P-value | FDR |
|---|
| hsa-miR-141 | Upregulated | 2.31454976 |
2.06×10−88 |
6.36×10−87 |
| hsa-miR-200a | Upregulated | 2.197744863 |
5.86×10−74 |
1.32×10−72 |
| hsa-miR-145 | Downregulated | −2.240104939 |
4.89×10−190 |
3.59×10−188 |
| hsa-miR-182 | Upregulated | 2.45599701 |
2.74×10−71 |
5.75×10−70 |
| hsa-miR-206 | Downregulated | −3.892802244 |
1.7×10−26 |
9.3×10−26 |
| hsa-miR-204 | Downregulated | −2.548901757 |
2.02×10−61 |
3.29×10−60 |
| hsa-miR-21 | Upregulated | 2.26697984 |
5.18×10−115 |
2.54×10−113 |
| hsa-miR-375 | Upregulated | 2.657222381 |
8.83×10−30 |
5.36×10−29 |
| hsa-miR-183 | Upregulated | 3.040247685 |
3.28×10−106 |
1.28×10−104 |
| hsa-miR-122 | Upregulated | 7.377192392 |
8.45×10−15 |
2.46×10−14 |
| hsa-miR-96 | Upregulated | 3.37889678 |
5.04×10−113 |
2.28×10−111 |
| hsa-miR-187 | Upregulated | 3.256099283 |
1.11×10−24 |
5.49×10−24 |
| hsa-miR-301b | Upregulated | 2.966431803 |
7.76×10−40 |
7.59×10−39 |
| hsa-miR-429 | Upregulated | 2.767154505 |
9.75×10−82 |
2.49×10−80 |
| hsa-miR-210 | Upregulated | 3.146252439 |
5.59×10−52 |
7.62×10−51 |
| hsa-miR-144 | Downregulated | −2.790913655 |
4.88×10−100 |
1.59×10−98 |
| hsa-miR-137 | Upregulated | 2.533383379 |
6.50×10−11 |
1.48×10−10 |
| hsa-miR-184 | Upregulated | 4.310580766 |
1.02×10−23 |
4.84×10−23 |
| hsa-miR-100 | Downregulated | −2.005872118 |
3.19×10−80 |
7.8×10−79 |
 | Table II.DElncRNAs interacting with the 19
DEmiRNAs retrieved from the miRcode database. |
Table II.
DElncRNAs interacting with the 19
DEmiRNAs retrieved from the miRcode database.
| lncRNA | miRNAs |
|---|
| AGAP11 | hsa-miR-141,
hsa-miR-200a, hsa-miR-145, hsa-miR-182, hsa-miR-206, hsa-miR-204,
hsa-miR-21, hsa-miR-375 |
| C2orf48 | hsa-miR-183,
hsa-miR-204, hsa-miR-122 |
| SHANK2-AS3 | hsa-miR-96,
hsa-miR-145, hsa-miR-187, hsa-miR-204, hsa-miR-122 |
| C20orf166-AS1 | hsa-miR-301b,
hsa-miR-183, hsa-miR-429, hsa-miR-375 |
| C15orf54 | hsa-miR-301b,
hsa-miR-182, hsa-miR-206, hsa-miR-429, hsa-miR-375 |
| AC127496.1 | hsa-miR-96,
hsa-miR-182, hsa-miR-183, hsa-miR-204, hsa-miR-210,
hsa-miR-122 |
| MIR7-3HG | hsa-miR-145,
hsa-miR-204 |
| LINC00305 | hsa-miR-144,
hsa-miR-204 |
| C10orf91 | hsa-miR-429,
hsa-miR-204, hsa-miR-122 |
| WT1-AS | hsa-miR-96,
hsa-miR-141, hsa-miR-200a, hsa-miR-145, hsa-miR-182, hsa-miR-206,
hsa-miR-429, hsa-miR-375 |
| LINC00518 | hsa-miR-141,
hsa-miR-200a, hsa-miR-145, hsa-miR-206, hsa-miR-204,
hsa-miR-375 |
| LINC00221 | hsa-miR-301b,
hsa-miR-96, hsa-miR-182, hsa-miR-204, hsa-miR-21 |
| TCL6 | hsa-miR-301b,
hsa-miR-96, hsa-miR-137, hsa-miR-144, hsa-miR-145, hsa-miR-182,
hsa-miR-183, hsa-miR-187, hsa-miR-206, hsa-miR-204, hsa-miR-210,
hsa-miR-122, hsa-miR-375 |
| AC009093.1 | hsa-miR-183,
hsa-miR-122 |
| C17orf102 | hsa-miR-96,
hsa-miR-145, hsa-miR-206, hsa-miR-429, hsa-miR-21 |
| AC135178.1 | hsa-miR-122 |
| AF241725.1 | hsa-miR-145 |
| MUC2 | hsa-miR-145,
hsa-miR-182, hsa-miR-183, hsa-miR-184, hsa-miR-429, hsa-miR-210,
hsa-miR-122 |
| RMRP | hsa-miR-206,
hsa-miR-122 |
| C1orf137 | hsa-miR-204 |
| AL391421.1 | hsa-miR-137,
hsa-miR-144 |
| TDRG1 | hsa-miR-122 |
| C10orf126 | hsa-miR-141,
hsa-miR-200a, hsa-miR-206, hsa-miR-375 |
| MUC19 | hsa-miR-301b,
hsa-miR-96, hsa-miR-137, hsa-miR-144, hsa-miR-145, hsa-miR-182,
hsa-miR-184, hsa-miR-187, hsa-miR-206, hsa-miR-429, hsa-miR-204,
hsa-miR-122, hsa-miR-375 |
| UCA1 | hsa-miR-96,
hsa-miR-182, hsa-miR-184, hsa-miR-206, hsa-miR-122 |
| LINC00488 | hsa-miR-96,
hsa-miR-144, hsa-miR-206, hsa-miR-21, hsa-miR-122 |
| LINC00243 | hsa-miR-96,
hsa-miR-145, hsa-miR-182, hsa-miR-206, hsa-miR-122,
hsa-miR-375 |
| AL356479.1 | hsa-miR-429 |
| AL356310.1 | hsa-miR-206 |
| AC080037.1 | hsa-miR-429 |
| AL513123.1 | hsa-miR-141,
hsa-miR-200a, hsa-miR-183 |
| LGALS8-AS1 | hsa-miR-122 |
| SMCR2 | hsa-miR-145,
hsa-miR-183, hsa-miR-204 |
| PHEX-AS1 | hsa-miR-301b,
hsa-miR-96, hsa-miR-145, hsa-miR-182, hsa-miR-204, hsa-miR-122 |
| LINC00466 | hsa-miR-96,
hsa-miR-137, hsa-miR-141, hsa-miR-200a, hsa-miR-144, hsa-miR-183,
hsa-miR-206, hsa-miR-429, hsa-miR-204, hsa-miR-21 |
| CHL1-AS2 | hsa-miR-183 |
| TSSC1-IT1 | hsa-miR-137,
hsa-miR-141, hsa-miR-200a, hsa-miR-183 |
| LINC00337 | hsa-miR-145,
hsa-miR-182, hsa-miR-375 |
| LINC00113 | hsa-miR-145 |
| LINC00351 | hsa-miR-21,
hsa-miR-375 |
| ADIPOQ-AS1 | hsa-miR-144,
hsa-miR-145, hsa-miR-182, hsa-miR-183, hsa-miR-184, hsa-miR-122,
hsa-miR-375 |
| NAALADL2-AS2 | hsa-miR-183,
hsa-miR-206 |
| LINC00355 | hsa-miR-141,
hsa-miR-200a, hsa-miR-122 |
| LINC00392 | hsa-miR-183 |
| HOTAIR | hsa-miR-301b,
hsa-miR-206, hsa-miR-204, hsa-miR-21, hsa-miR-375 |
| SRGAP3-AS2 | hsa-miR-145,
hsa-miR-206 |
| HCG23 | hsa-miR-145 |
| LINC00200 | hsa-miR-183,
hsa-miR-204 |
| LINC00404 | hsa-miR-141,
hsa-miR-200a |
| SACS-AS1 | hsa-miR-144,
hsa-miR-187 |
| EMX2OS | hsa-miR-182,
hsa-miR-183, hsa-miR-184, hsa-miR-210 |
| ATXN8OS | hsa-miR-145,
hsa-miR-183, hsa-miR-204, hsa-miR-210, hsa-miR-122hsa-miR-375 |
| LINC00210 | hsa-miR-96,
hsa-miR-206, hsa-miR-204 |
| TLR8-AS1 | hsa-miR-182,
hsa-miR-187, hsa-miR-206, hsa-miR-204 |
| LINC00460 | hsa-miR-206,
hsa-miR-429 |
| POU6F2-AS2 | hsa-miR-137,
hsa-miR-187, hsa-miR-375 |
| BOK-AS1 | hsa-miR-184 |
| MAGI2-AS3 | hsa-miR-137,
hsa-miR-141, hsa-miR-200a, hsa-miR-144, hsa-miR-145, hsa-miR-429,
hsa-miR-204, hsa-miR-210, hsa-miR-122 |
| CHL1-AS1 | hsa-miR-137,
hsa-miR-187 |
| FNDC1-IT1 | hsa-miR-144 |
| DSCAM-AS1 | hsa-miR-137,
hsa-miR-141, hsa-miR-200a, hsa-miR-204, hsa-miR-122 |
| LINC00484 | hsa-miR-141,
hsa-miR-200a, hsa-miR-187, hsa-miR-206, hsa-miR-122 |
| ARHGEF7-AS2 | hsa-miR-187,
hsa-miR-210, hsa-miR-122, hsa-miR-375 |
| RBMS3-AS3 | hsa-miR-96,
hsa-miR-182, hsa-miR-204 |
| LINC00445 | hsa-miR-375 |
| NDP-AS1 | hsa-miR-145,
hsa-miR-206hsa-miR-122 |
| AC009121.1 | hsa-miR-141,
hsa-miR-200a |
| CLRN1-AS1 | hsa-miR-137,
hsa-miR-206, hsa-miR-429, hsa-miR-204 |
| PDZRN3-AS1 | hsa-miR-141,
hsa-miR-200a |
| AC080129.1 | hsa-miR-122 |
| AL109754.1 | hsa-miR-122 |
| MME-AS1 | hsa-miR-100,
hsa-miR-182, hsa-miR-429 |
| LSAMP-AS1 | hsa-miR-183,
hsa-miR-375 |
| ADAMTS9-AS1 | hsa-miR-301b,
hsa-miR-96, hsa-miR-144, hsa-miR-145, hsa-miR-182, hsa-miR-21 |
| SYNPR-AS1 | hsa-miR-96,
hsa-miR-182, hsa-miR-375 |
| ADAMTS9-AS2 | hsa-miR-301b,
hsa-miR-96, hsa-miR-137, hsa-miR-141, hsa-miR-200a, hsa-miR-144,
hsa-miR-145 |
|
| hsa-miR-182,
hsa-miR-183, hsa-miR-184, hsa-miR-204, hsa-miR-122,
hsa-miR-375 |
| AC007731.1 | hsa-miR-183 |
| AL139002.1 | hsa-miR-301b,
hsa-miR-210 |
| PEX5L-AS1 | hsa-miR-141,
hsa-miR-200a |
| AC061992.1 | hsa-miR-301b,
hsa-miR-204, hsa-miR-122 |
| AP000553.1 | hsa-miR-122 |
| LINC00461 | hsa-miR-96,
hsa-miR-137, hsa-miR-141, hsa-miR-200a, hsa-miR-144, hsa-miR-145,
hsa-miR-204, hsa-miR-210, hsa-miR-122 |
| ALDH1L1-AS2 | hsa-miR-301b,
hsa-miR-145, hsa-miR-210 |
| MAST4-IT1 | hsa-miR-204 |
| LINC00536 | hsa-miR-96,
hsa-miR-137, hsa-miR-182, hsa-miR-204 |
| OPCML-IT1 | hsa-miR-184,
hsa-miR-375 |
| C8orf49 | hsa-miR-301b,
hsa-miR-100, hsa-miR-184, hsa-miR-429, hsa-miR-122,
hsa-miR-375 |
| AL589642.1 | hsa-miR-141,
hsa-miR-200a, hsa-miR-145, hsa-miR-182, hsa-miR-183, hsa-miR-429,
hsa-miR-204, hsa-miR-210, hsa-miR-122 |
| AC040173.1 | hsa-miR-96,
hsa-miR-144, hsa-miR-182, hsa-miR-183, hsa-miR-429 |
| LINC00524 | hsa-miR-204 |
| LINC00052 | hsa-miR-145,
hsa-miR-187 |
| PWRN1 | hsa-miR-137,
hsa-miR-144, hsa-miR-145, hsa-miR-184, hsa-miR-21, hsa-miR-122 |
| LINC00261 | hsa-miR-301b,
hsa-miR-144, hsa-miR-145, hsa-miR-182, hsa-miR-183, hsa-miR-206,
hsa-miR-429, hsa-miR-204, hsa-miR-375 |
miRNA targets predicted by
miRTarBase
Based on the miRNAs described in Table I, miRNA-targeted mRNA were
identified by searching the TargetScan, miRDB and miRTarBase
databases. A total of 27 mRNAs were identified including titin,
chordin like 1, hydroxycarboxylic acid receptor 2, transforming
growth factor β receptor 2, A-kinase anchoring protein 12, sprout
RTK signaling antagonist 2, kelch like family member 40, sterile α
motif domain containing 5, transcription elongation factor A like
7, KIT proto-oncogene receptor tyrosine kinase, cyclin B1, cell
adhesion molecule L1 like, WAS protein family member 3, fibroblast
growth factor (FGF) 2, SHC binding and spindle associated 1, serine
rich and transmembrane domain containing 1, karyopherin α 2
(KPNA2), neurotrophic receptor tyrosine kinase 2 (NTRK2), cyclin
E2, secreted frizzled related protein 1 (SFRP1), par-6 family cell
polarity regulator β, ELAV like RNA binding protein 2, FGF receptor
3, cadherin 2, C-C motif chemokine ligand 20, solute carrier family
1 member 1 and homeobox B5 (Table
III).
 | Table III.Breast cancer-specific mRNAs in the
competing endogenous RNA network. |
Table III.
Breast cancer-specific mRNAs in the
competing endogenous RNA network.
| mRNA | Gene ID | Expression
change | log2 FC (T/N) | P-value | FDR |
|---|
| TTN |
ENSG00000155657 | Downregulated | −4.950085607 | 0.001 | 0.001 |
| CHRDL1 |
ENSG00000101938 | Downregulated | −4.384645164 |
5.38×10−246 |
8.81×10−244 |
| HCAR2 |
ENSG00000182782 | Downregulated | −3.359595565 |
2.72×10−233 |
3.83×10−231 |
| TGFBR2 |
ENSG00000163513 | Downregulated | −2.148190475 |
4.53×10−182 |
4.04×10−180 |
| AKAP12 |
ENSG00000131016 | Downregulated | −2.630546355 |
1.93×10−180 |
1.68×10−178 |
| SPRY2 |
ENSG00000136158 | Downregulated | −2.373343848 |
3.08×10−179 |
2.63×10−177 |
| KLHL40 |
ENSG00000157119 | Downregulated | −7.140824495 |
5.06×10−150 |
3.06×10−148 |
| SAMD5 |
ENSG00000203727 | Downregulated | −2.921601554 |
1.42×10−134 |
5.06×10−150 |
| TCEAL7 |
ENSG00000182916 | Downregulated | −2.087672875 |
1.82×10−121 |
7.64×10−120 |
| KIT |
ENSG00000157404 | Downregulated | −2.904876101 |
5.47×10−120 |
2.24×10−118 |
| CCNB1 |
ENSG00000134057 | Upregulated | 2.641558633 |
5.60×10−119 |
2.22×10−117 |
| CHL1 |
ENSG00000134121 | Downregulated | −2.893484253 |
3.51×10−115 |
1.31×10−113 |
| WASF3 |
ENSG00000132970 | Downregulated | −2.100371097 |
1.12×10−111 |
3.94×10−110 |
| FGF2 |
ENSG00000138685 | Downregulated | −2.663380621 |
2.96×10−102 |
8.7×10−101 |
| SHCBP1 |
ENSG00000171241 | Upregulated | 2.674370272 |
3.03×10−87 |
6.84×10−86 |
| SERTM1 |
ENSG00000180440 | Downregulated | −3.596825432 |
3.8×10−81 |
7.75×10−80 |
| KPNA2 |
ENSG00000182481 | Upregulated | 2.232771491 |
8.08×10−81 |
1.64×10−79 |
| NTRK2 |
ENSG00000148053 | Downregulated | −2.532210134 |
3.89×10−70 |
6.46×10−69 |
| CCNE2 |
ENSG00000175305 | Upregulated | 2.495744882 |
6.12×10−70 |
1.01×10−68 |
| SFRP1 |
ENSG00000104332 | Downregulated | −2.49717591 |
1.76×10−46 |
1.59×10−45 |
| PARD6B |
ENSG00000124171 | Upregulated | 2.263852741 |
8.63×10−35 |
5.35×10−34 |
| ELAVL2 |
ENSG00000107105 | Upregulated | 2.693339772 |
2.05×10−34 |
1.25×10−33 |
| FGFR3 |
ENSG00000068078 | Upregulated | 2.472470941 |
5.52×10−32 |
3.06×10−31 |
| CDH2 |
ENSG00000170558 | Upregulated | 2.639030107 |
4.82×10−28 |
2.26×10−27 |
| CCL20 |
ENSG00000115009 | Upregulated | 2.568683156 |
1.40×10−24 |
5.69×10−24 |
| SLC1A1 |
ENSG00000106688 | Upregulated | 2.352249029 |
3.02×10−21 |
1.06×10−20 |
| HOXB5 |
ENSG000001200755 | Upregulated | 2.001121261 |
1.91×10−15 |
5.15×10−15 |
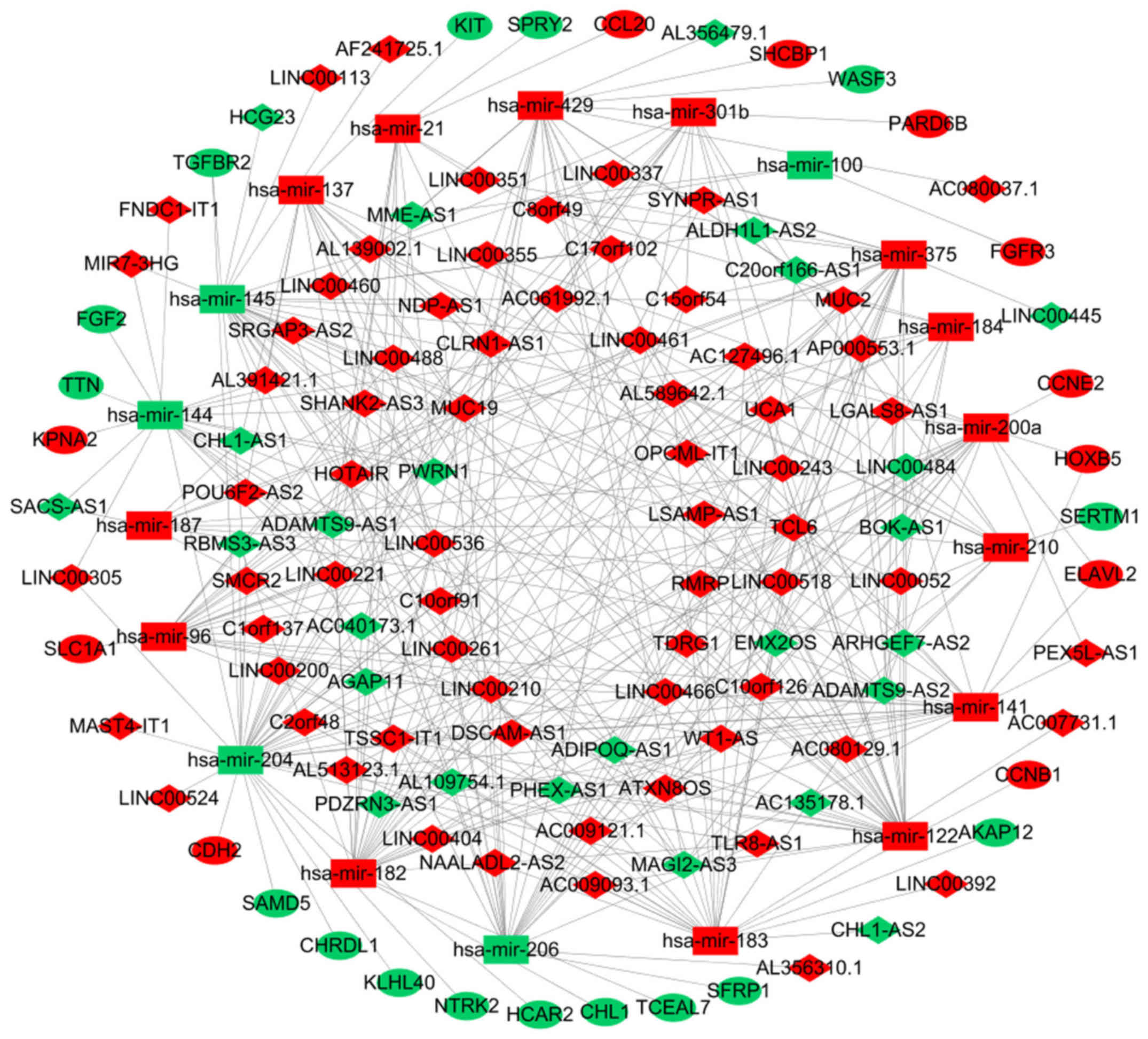
Construction of a ceRNA network in
breast cancer
To improve the understanding of the role of
DElncRNAs in breast cancer, a ceRNA network was constructed based
on co-expressed lncRNAs-miRNAs and miRNAs-mRNAs. As demonstrated in
Fig. 2, the ceRNA network was
composed of 93 lncRNAs, 27 mRNAs and 19 miRNAs.
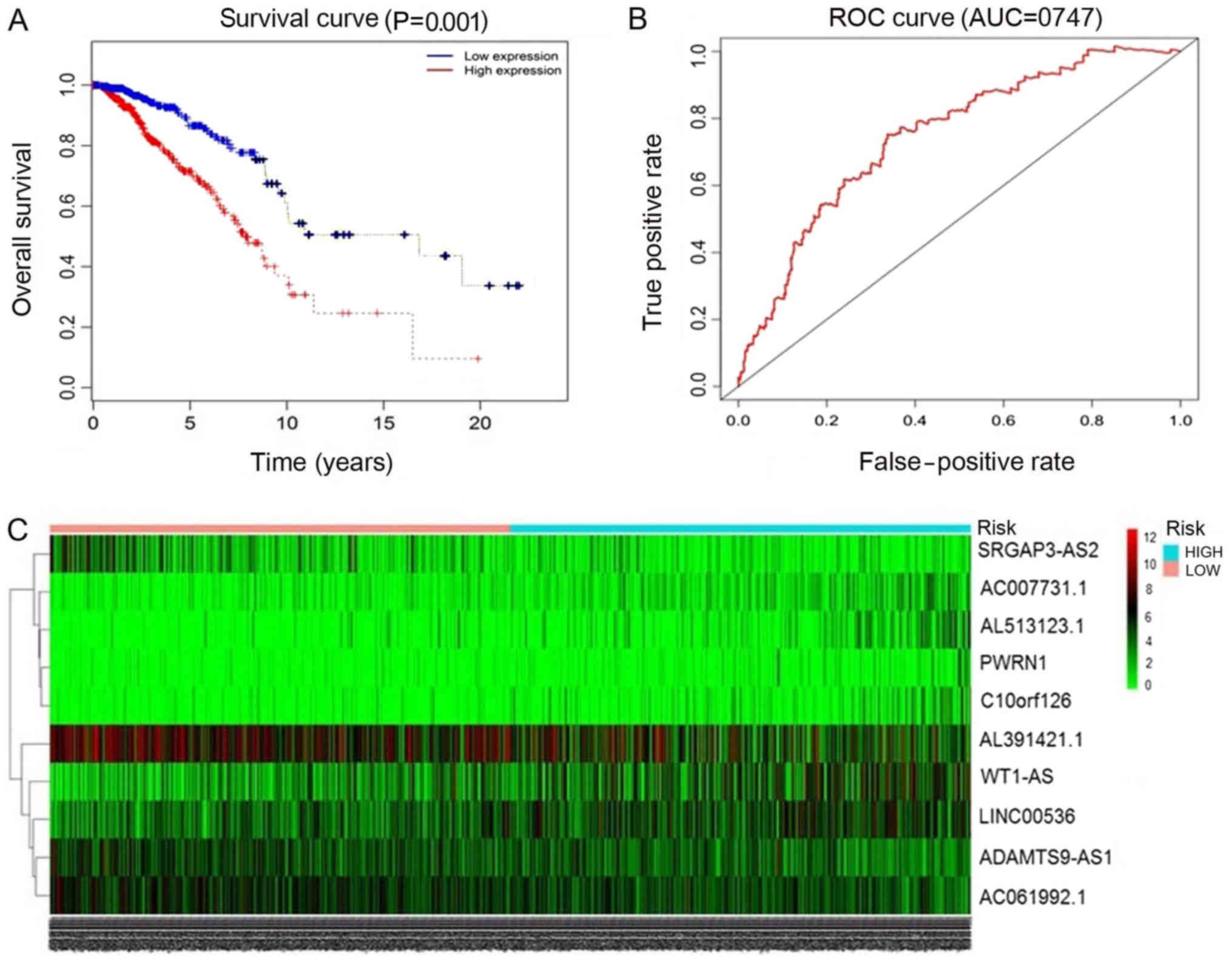
ROC analysis of breast cancer-specific
lncRNAs
The 93 miRNAs targeting lncRNAs in breast cancer
predicted by miRcode and starBase were selected. Then, Perl script
was used to obtain the survival analysis data, and the clinicalexp
file (lncRNA name, patient survival time, survival state) was
combined with the survival data for univariate Cox analysis. In the
univariate Cox analysis data, when the hazard ratio HR >1, this
indicated that the higher the gene expression, the higher the risk
of mortality, that is, the expression of the gene is contrary to
survival. HR <1, indicated that the higher the expression of
gene, the lower the risk of mortality, that is, the higher the
survival rate. Survival-associated genes were then listed in order
of ascending P-value, and the top 13 lncRNAs with P<0.05 were
selected. lncRNAs were selected subsequent to entering the
screening gene code in R software (version 3.4.3). Finally, the top
10 lncRNAs associated with breast cancer survival analysis were
screened (Table IV; Fig. 3). These included: ADAM
metallopeptidase with thrombospondin type 1 motif 9-antisense RNA 1
(ADAMTS9-AS1), AC061992.1, Wilms tumor 1 antisense RNA (WT1-AS),
long intergenic non-protein coding RNA 536 (LINC00536), AL391421.1,
SLIT-ROBO Rho GTPase activating protein 3 antisense RNA 2
(SRGAP3-AS2), Prader-Willi region non-protein coding RNA 1 (PWRN1),
family with sequence similarity 230 member G (AC007731.1),
chromosome 10 open reading frame 126 (C10orf126) and AL513123.1. Of
these, the levels of ADAMTS9-AS1 and PWRN1 were downregulated in
breast cancer tissues. The other 8 lncRNAs were upregulated in
cancer tissues compared with in normal tissues. A total of 6
lncRNAs exhibited high prognostic values for distinguishing tumor
tissues from normal tissues, with AUC values of >0.99.
 | Table IV.Top 10 aberrantly expressed lncRNAs
and AUC values in breast cancer. |
Table IV.
Top 10 aberrantly expressed lncRNAs
and AUC values in breast cancer.
| lncRNA | Gene ID | Expression
change | log2 FC (T/N) | AUC | P-value |
|---|
| ADAMTS9-AS1 |
ENSG00000241158 | Downregulated | −2.746216755 | 0.888 | <0.05 |
| AC061992.1 |
ENSG00000266970 | Upregulated | 2.140133828 | 0.867 | <0.05 |
| WT1-AS |
ENSG00000183242 | Upregulated | 4.901360082 | 1.102 | <0.05 |
| LINC00536 |
ENSG00000249917 | Upregulated | 2.721025285 | 1.110 | <0.05 |
| AL391421.1 |
ENSG00000204049 | Upregulated | 3.149701497 | 0.940 | <0.05 |
| SRGAP3-AS2 |
ENSG00000228723 | Upregulated | 4.608297544 | 0.889 | <0.05 |
| PWRN1 |
ENSG00000259905 | Downregulated | −2.274243394 | 1.243 | <0.05 |
| AC007731.1 |
ENSG00000188280 | Upregulated | 2.840014701 | 1.182 | <0.05 |
| C10orf126 |
ENSG000002043655 | Upregulated | 3.688082405 | 1.183 | <0.05 |
| AL513123.1 |
ENSG00000236347 | Upregulated | 2.905568621 | 1.194 | <0.05 |
Additional analysis for the selected
lncRNAs expression
GEPIA results (Fig.
4A) revealed upregulation of ADAMTS9-AS1 in kidney renal clear
cell carcinoma (KIRC), kidney renal papillary cell carcinoma (KIRP)
and prostate adenocarcinoma (PRAD). As demonstrated in Fig. 4B, the results also identified that
WT1-AS levels were significantly increased in acute myeloid
leukemia, ovarian cancer (OV), uterine corpus endometrial carcinoma
(UCEC) and uterine carcinosarcomas compared with in normal tissues.
Notably, the increased expression of LINC00536 was demonstrated in
breast invasive carcinoma, while a decreased expression was
demonstrated in testicular germ cell tumors (TGCT) (Fig. 4C). Furthermore, increased
SRGAP3-AS2 expression was identified in adenoid cystic carcinoma,
cholangiocarcinoma, OV and UCEC, while significant downregulation
of SRGAP3-AS2 was observed in lung adenocarcinoma and lung squamous
cell carcinoma (LUSC) (Fig. 4D).
PWRN1 was observed to be downregulated in thyroid carcinoma and
upregulated in TGCT, pheochromocytoma and paraganglioma (Fig. 4E). A decreased expression of
C10orf126 was identified in KIRC, KIRP and liver hepatocellular
carcinoma (Fig. 4F).
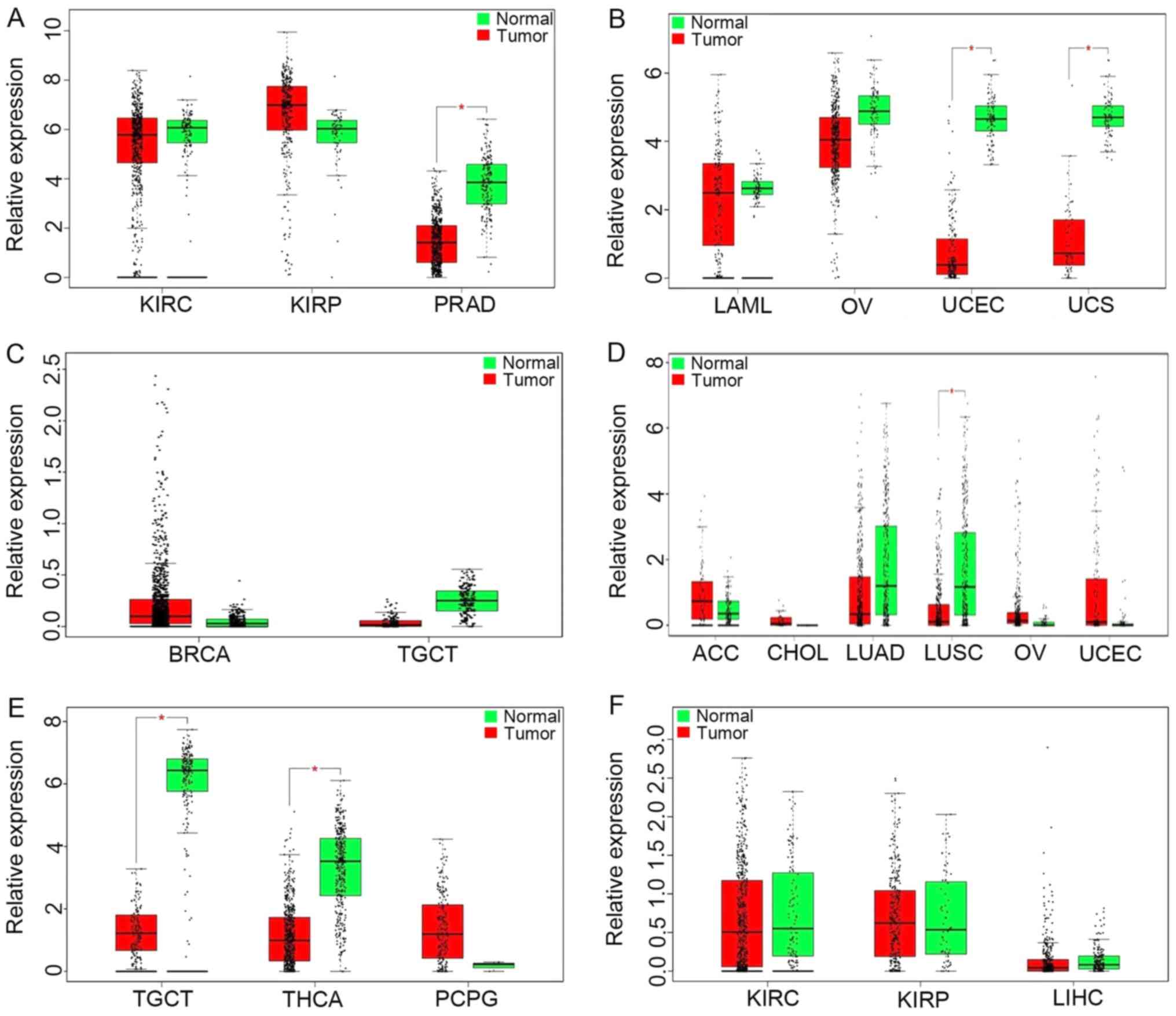 | Figure 4.lncRNAs expression among various
types of cancer involved in The Cancer Genome Atlas based on GEPIA.
(A) ADAMTS9-AS1, (B) WT1-AS, (C) LINC00536, (D) SRGAP3-AS2, (E)
PWRN1 and (F) C10orf126. There is no description of the genes
AC061992.1, AL391421.1, AC007731.1 and AL513123.1 in GEPIA. The
y-axis indicates the log2 (transcripts per million + 1) for lncRNA
expression. Red-colored bars represent the tumor tissue samples and
the green-colored bars indicate the normal tissue samples. These
charts were derived from GEPIA. lncRNAs; long non-coding RNAs;
GEPIA, Gene Expression Profiling Interactive Analysis; KIRC, kidney
renal clear cell carcinoma; KIRP, kidney renal papillary cell
carcinoma; PRAD, prostate adenocarcinoma; LAML, acute myeloid
leukemia; OV, ovarian cancer; UCEC, uterine corpus endometrial
carcinoma; UCS, uterine carcinosarcomas; BRCA, breast invasive
carcinoma; TGCT, testicular germ cell tumors; ACC, adenoid cystic
carcinoma; CHOL, cholangiocarcinoma; LUAD, lung adenocarcinoma;
LUSC, lung squamous cell carcinoma; THCA, thyroid carcinoma; PCPG,
pheochromocytoma and paraganglioma; LIHC, liver hepatocellular
carcinoma. ADAMTS9-AS1, ADAM metallopeptidase with thrombospondin
type 1 motif 9-antisense RNA 1; WT1-AS, Wilms tumor 1 antisense
RNA; LINC00536, long intergenic non-protein coding RNA 536;
SRGAP3-AS2, SLIT-ROBO Rho GTPase activating protein 3 antisense RNA
2; PWRN1, Prader-Willi region non-protein coding RNA 1; C10orf126,
chromosome 10 open reading frame 126. |
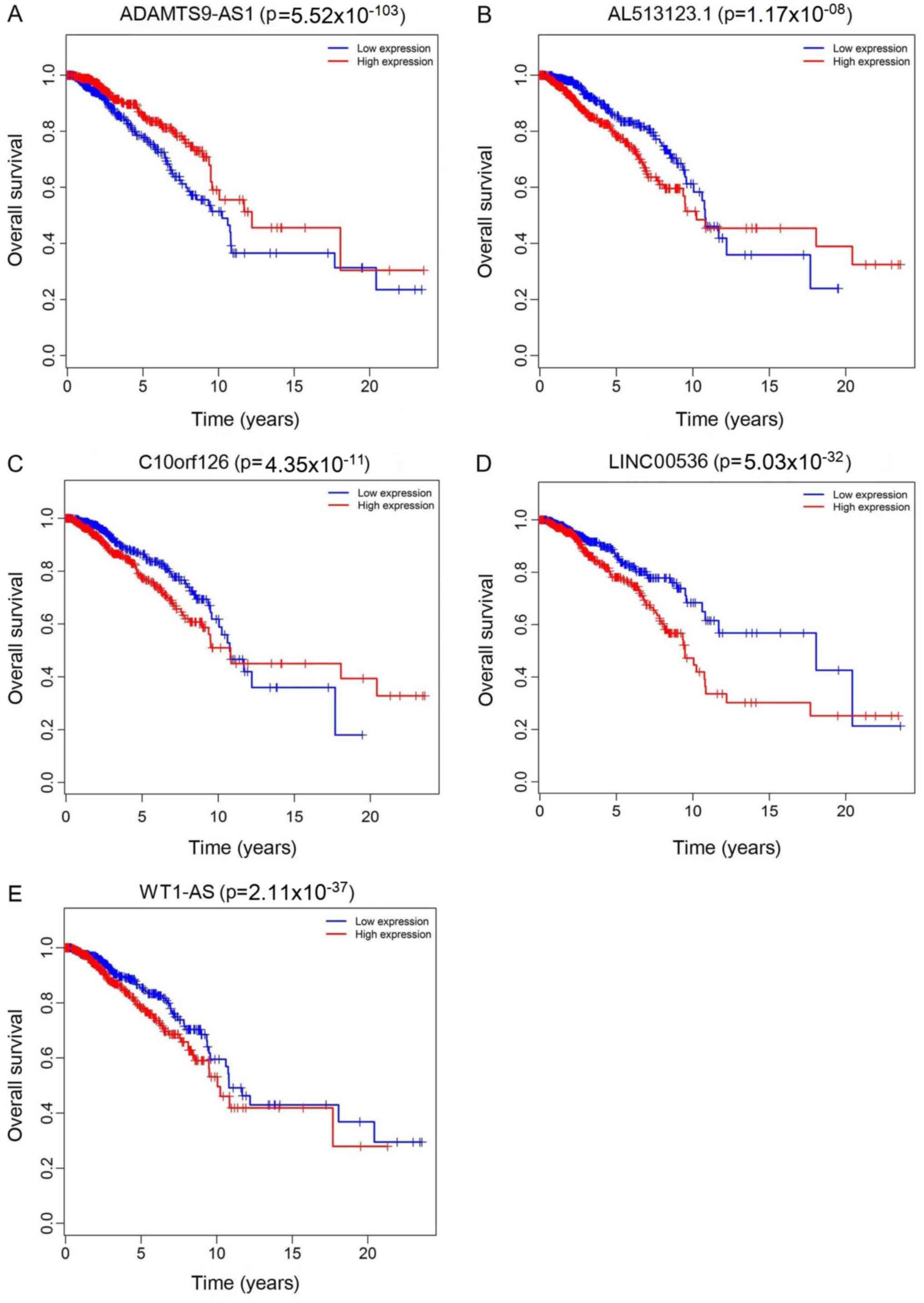
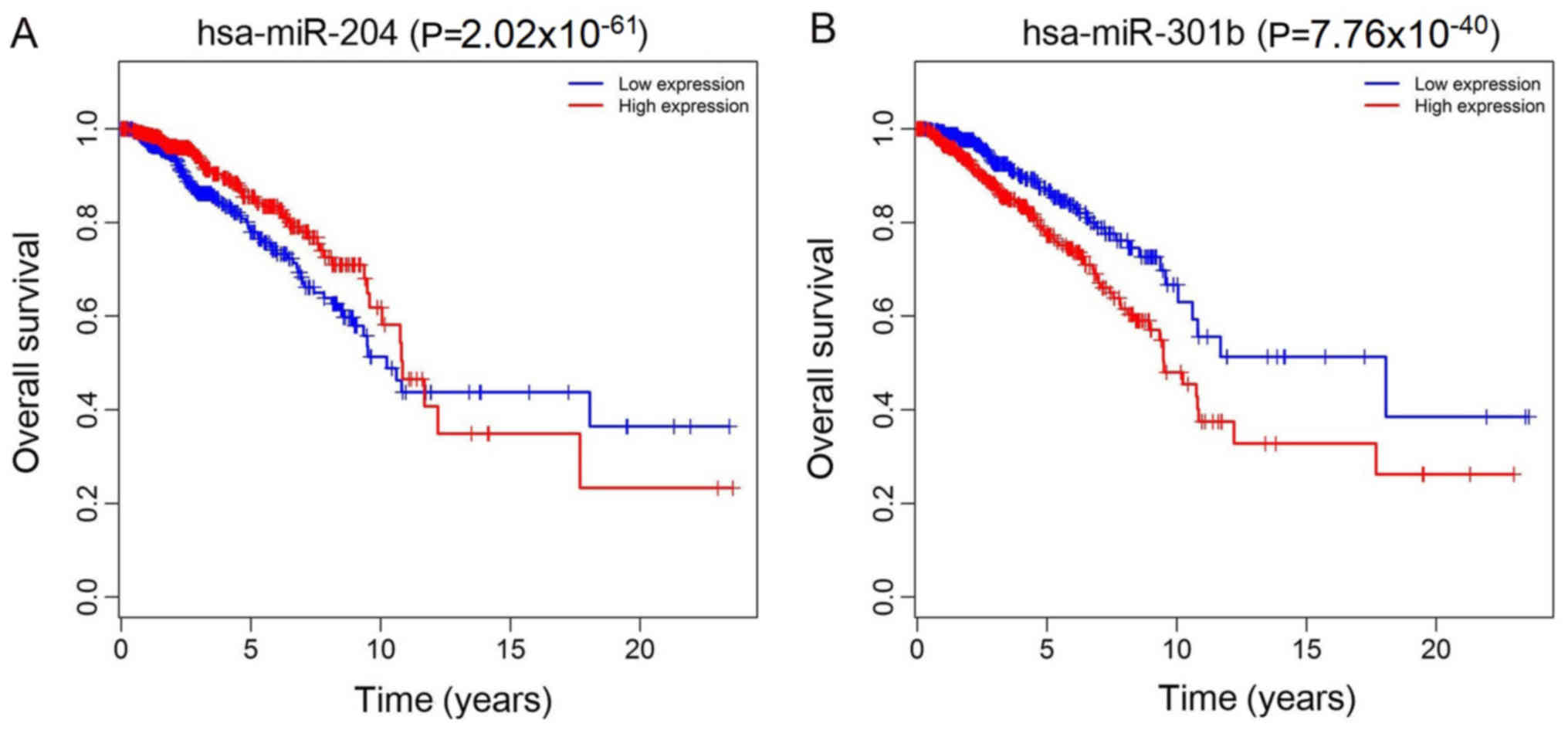
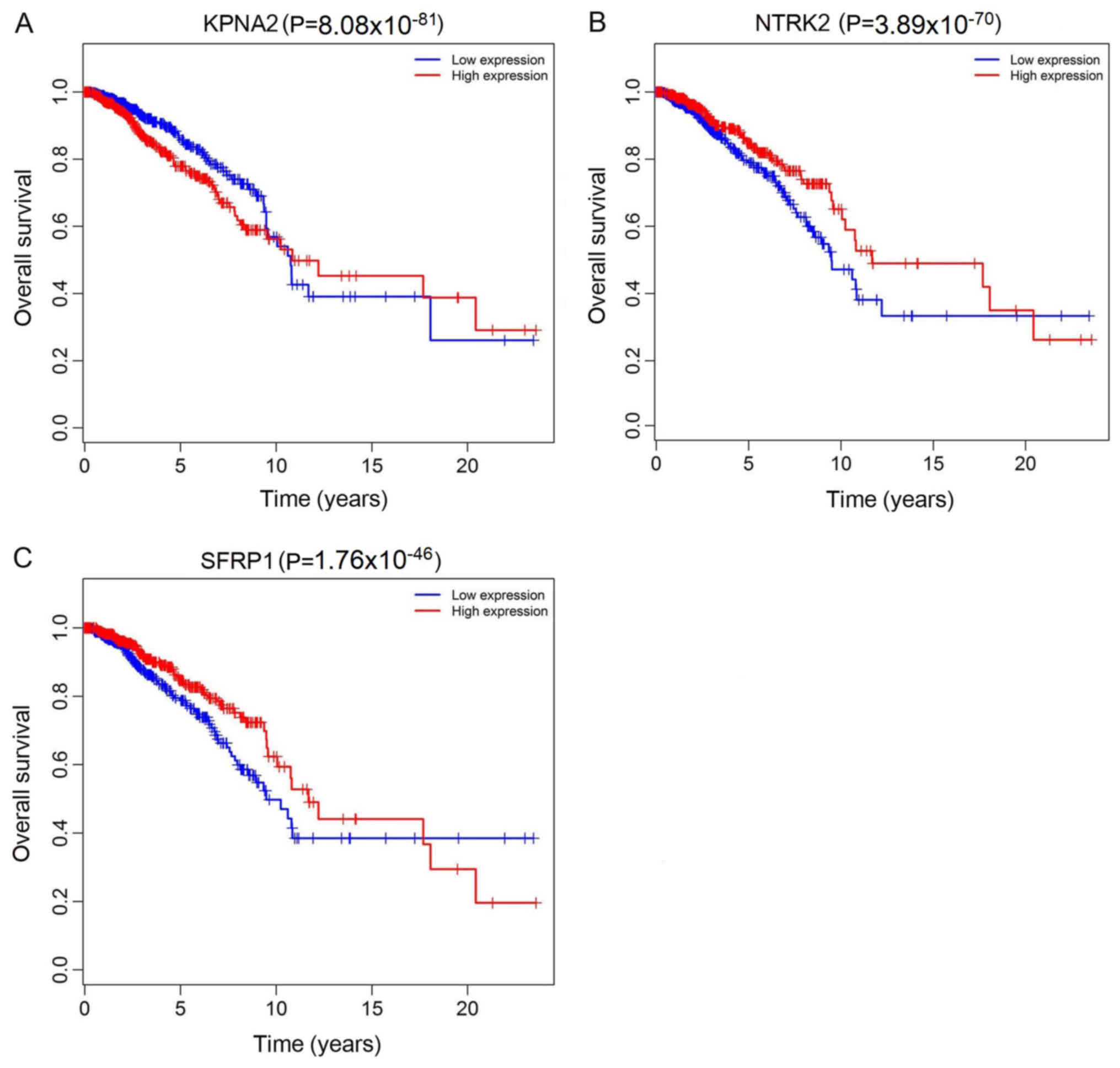
Survival analysis
The top 10 lncRNAs were selected using ROC analysis.
As demonstrated in Fig. 5, the
expression levels of 5 DElncRNAs, including ADAMTS9-AS1,
AL513123.1, C10orf126, LINC00536 and WT1-AS, were associated with
overall survival (P<0.05). A total of 2 miRNAs (hsa-miR-204 and
hsa-miR-301b) were associated with overall survival (P<0.05;
Fig. 6). In addition, 3 mRNA
(KPNA2, NTRK2 and SFRP1) were associated with overall survival
(P<0.05; Fig. 7).
Discussion
In previous years, much attention has been paid to
breast cancer pathogenesis. Nevertheless, clinical outcomes remain
highly heterogeneous. Nik-Zainal et al (26) analyzed genome-wide data from 560
patients with breast cancer and identified that 93 oncogenes
involved in the encoded proteins may cause breast cancer. Prognosis
is an important indicator of disease treatment. Bioinformatics
methods have been used to investigate the prognostic significance
of lncRNAs in breast cancer. Several studies has also indicated
that the aberrant expression of lncRNAs contributes to the
development of different types of cancer: Xiao et al
(27) revealed that MALAT1 may be
used as a ceRNA to promote the expression of ZEB2 by expanding
miR-200s, and may be a therapeutic target for kidney cancer; Wang
et al (28) demonstrated
that UCA1 and AATBC are not only included in the ceRNA network, but
are also associated with overall survival in muscle-invasive
bladder cancer; Chen et al (25) suggested that the top 10 aberrantly
expressed lncRNAs identified in their study served important roles
in LUSC through an lncRNA-mRNA network; and Li et al
(29) described a novel gastric
cancer-specific ceRNA network including 11 lncRNAs, 9 miRNAs and 41
mRNAs.
ceRNA transcripts may be used to competitively bind
the same MRE with miRNAs, thereby relieving or mitigating the
inhibitory effects of miRNAs on target genes. MREs are the basis
for miRNA function, and other non-coding genes also interact with
miRNAs through MREs. Recently, Zhou et al (30) used a Pearson's correlation analysis
of miRNA-gene pairs to construct breast cancer-specific ceRNA
networks. Regulation of phosphatase and tensin homolog expression
in a 3′ untranslated region-and miRNA-dependent manner in breast
cancer has been identified in another study (31). Furthermore, ceRNA crosstalk may be
associated with regulatory mechanisms in breast cancer. Shen et
al (32) and Yang et al
(33) demonstrated that FAM83H-AS1
appeared to be a novel prognostic biomarker in breast cancer.
Cheang et al (34) revealed
that patients with breast cancer with high HER-2 protein expression
levels and Ki67 index exhibited poor recurrence and
disease-specific survival rates. Yuan et al (35) identified that the ceRNA crosstalk
network in triple negative breast cancer included the differential
expression of 22 hub mRNAs, 11 miRNAs and 14 lncRNAs. The present
study obtained lncRNA, mRNA and miRNA data from TCGA to construct
breast cancer-associated ceRNA networks, studied the regulatory
mechanisms of lncRNA as ceRNAs in the progression of cancer, and
also investigated their potential as prognostic biomarkers and
therapeutic targets in breast cancer. A ceRNA network with
differential expression of 27 mRNAs, 19 miRNAs and 93 lncRNAs was
constructed.
The present study investigated the lncRNA expression
profiles of a large cohort of patients in TCGA. A total of 93
DElncRNAs were identified in breast cancer samples compared with
the normal samples. Among these, the top 10 lncRNA were
significantly associated with overall survival. Furthermore, it was
observed that the lncRNAs ADAMTS9-AS1, AL513123.1, C10orf126,
LINC00536 and WT1-AS were included in the ceRNA network. Therefore,
we hypothesized that these lncRNAs may serve significant roles in
the pathogenesis and prognosis of breast cancer. Wang et al
(36) revealed that antisense
lncRNA ADAMTS9-AS1 was associated with the nearby coding gene
ADAMTS9, which was involved in ovarian cancer progression. Li et
al (37) identified that the
lncRNA ADAMTS9-AS1 served as a novel prognostic biomarker for
clinical application in esophageal squamous cell carcinoma. Crippa
et al (38) hypothesized
that LINC00536 disruption may have contributed to the onset of the
clinical trichorhinophalangeal syndrome-like phenotype. Wang et
al (39) suggested that WT1
was overexpressed in human hepatocellular carcinoma tissues, and
was negatively correlated with overall survival in patients with
hepatocellular carcinoma.
In the present study, a novel ceRNA network was
constructed to identify the associations between miRNAs and
lncRNAs. miRNAs also serve important roles in cell differentiation,
biological development and disease progression. Several previous
data have demonstrated that the mutual regulation between lncRNAs
and miRNAs and their downstream target genes are closely associated
with the occurrence and development of cancer (40–42).
Zhang et al (43) used
reverse transcription quantitative polymerase chain reaction and
in situ hybridization for breast cancer tissue samples and
cell lines. Cell phenotype experiments were performed to verify
that lncRNA-GAS5 exon 4 bound miR-21 and inhibited the occurrence
and development of breast cancer cells (43). The present study demonstrated that
hsa-miR-204 was downregulated and hsa-miR-301b was upregulated in
patients with breast cancer compared with healthy controls, and was
associated with overall survival. Yuan et al (44) revealed that miR-204 suppressed the
proliferation and metastasis of gastric cancer cells. Todorova
et al (45) identified that
miR-204 promoted prostate cancer-associated androgen-responsive
genes and androgen receptor (AR) target genes and AR co-regulated
molecules. Abmutalib et al (46) hypothesized that hsa-miR-301b may be
involved in regulating lymph node metastasis in papillary thyroid
carcinoma via interactions with hepatic leukemia factor,
hypoxia-inducible factor and REL/nuclear factor
kappa-light-chain-enhancer of activated B cells. Geng et al
(47) demonstrated that
overexpression of hsa-miR-301b significantly affected the cell
cycle of human lung adenocarcinoma A549 cells. ceRNAs have been
implicated in several biological processes, and abnormalities in
the ceRNA network may lead to tumorigenesis.
In summary, the present study investigated
lncRNA-mediated ceRNA interactions using miRNA, lncRNA and mRNA
expression profiles in cancer and normal tissues. The results
suggested that cancer-specific lncRNAs in breast cancer may be
involved in the regulation of a complex ceRNA network. These data
may provide novel insights into the clinical significance and
regulatory mechanisms of lncRNA-mediated ceRNA networks, and
identify novel lncRNAs as potential prognostic biomarkers and
therapeutic targets for the diagnosis and treatment of breast
cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from
National Natural Science Foundation of China (grant no. 81660324)
and the Key discipline Construction of the 13th Five-Year Plan in
Xinjiang, China - Plateau Discipline Project.
Availability of data and materials
All data analyzed during this study are included in
this published article.
Authors' contributions
SMF and LLL conceived and designed the manuscript.
TT and ZA analyzed the data. TT wrote the paper. All authors read
and approved the final manuscript
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
simpleBreastcancer.orgUS Breast Cancer Statistics.
http://www.breastcancer.org/symptoms/understand_bc/statisticsbreast
cancer org. February 18–2019
|
|
2
|
Linos E, Spanos D, Rosner BA, Linos K,
Hesketh T, Qu JD, Gao YT, Zheng W and Colditz GA: Effects of
reproductive and demographic changes on breast cancer incidence in
China: A modeling analysis. J Natl Cancer Inst. 100:1352–1360.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Higgins MJ and Baselga J: Targeted
therapies for breast cancer. J Clin Invest. 121:3797–3803. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Győrffy B, Hatzis C, Sanft T, Hofstatter
E, Aktas B and Pusztai L: Multigene prognostic tests in breast
cancer: Past, present, future. Breast Cancer Res. 17:112015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma L, Bajic VB and Zhang Z: On the
classification of long non-coding RNAs. RNA Biol. 10:925–933. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yuan SX, Yang F, Yang Y, Tao QF, Zhang J,
Huang G, Yang Y, Wang RY, Yang S, Huo XS, et al: Long noncoding RNA
associated with microvascular invasion in hepatocellular carcinoma
promotes angiogenesis and serves as a predictor for hepatocellular
carcinoma patients' poor recurrence-free survival after
hepatectomy. Hepatology. 56:2231–2241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu X, Feng Y, Zhang D, Zhao SD, Hu Z,
Greshock J, Zhang Y, Yang L, Zhong X, Wang LP, et al: A functional
genomic approach identifies FAL1 as an oncogenic long noncoding RNA
that associates with BMI1 and represses p21 expression in cancer.
Cancer Cell. 26:344–357. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feng S, Zhang J, Su W, Bai S, Xiao L, Chen
X, Lin J, Reddy RM, Chang AC, Beer DG and Chen G: Overexpression of
LINC00152 correlates with poor patient survival and knockdown
impairs cell proliferation in lung cancer. Sci Rep. 7:29822017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sang H, Liu H, Xiong P and Zhu M: Long
non-coding RNA functions in lung cancer. Tumor Biol. 36:4027–4037.
2015. View Article : Google Scholar
|
|
11
|
Zhong Y, Du Y, Yang X, Mo Y, Fan C, Xiong
F, Ren D, Ye X, Li C, Wang Y, et al: Circular RNAs function as
ceRNAs to regulate and control human cancer progression. Mol
Cancer. 17:792018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Lu T, Wang Q, Liu J and Jiao W:
Circular RNAs: Crucial regulators in the human body (Review). Oncol
Rep. 40:3119–3135. 2018.PubMed/NCBI
|
|
13
|
Wang Y, Mo Y, Gong Z, Yang X, Yang M,
Zhang S, Xiong F, Xiang B, Zhou M, Liao Q, et al: Circular RNAs in
human cancer. Mol Cancer. 16:252017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The Rosetta Stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo LL, Song CH, Wang P, Dai LP, Zhang JY
and Wang KJ: Competing endogenous RNA networks and gastric cancer.
World J Gastroenterol. 21:11680–11687. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Hou J, He D, Sun M, Zhang P, Yu Y
and Chen Y: The emerging function and mechanism of ceRNAs in
cancer. Trends Genet. 32:211–224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qi X, Zhang DH, Wu N, Xiao JH, Wang X and
Ma W: ceRNA in cancer: Possible functions and clinical
implications. J Med Genet. 52:710–718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng DL, Xiang YY, Ji LJ and Lu XJ:
Competing endogenous RNA interplay in cancer: Mechanism,
methodology, and perspectives. Tumor Biol. 36:479–488. 2015.
View Article : Google Scholar
|
|
19
|
Cline MS, Craft B, Swatloski T, Goldman M,
Ma S, Haussler D and Zhu J: Exploring TCGA pan-cancer data at the
UCSC cancer genomics browser. Sci Rep. 3:26522013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
R Core Team R, . A language and
environment for statistical computing. R Foundation for Statistical
Computing; Vienna, Austria: version 3.4.3. http://www.R-project.org/November 30–2017
|
|
21
|
Jeggari A, Marks DS and Larsson E:
miRcode: A map of putative microRNA target sites in the long
non-coding transcriptome. Bioinformatics. 28:2062–2063. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chou CH, Shrestha S, Yang CD, Chang NW,
Lin YL, Liao KW, Huang WC, Sun TH, Tu SJ, Lee WH, et al: miRTarBase
update 2018: A resource for experimentally validated
microRNA-target interactions. Nucleic Acids Res. 46(D1): D296–D302.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:Aug 12–2015.(Epub ahead of print). doi:
10.7554/eLife.05005. View Article : Google Scholar
|
|
24
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen WJ, Tang RX, He RQ, Li DY, Liang L,
Zeng JH, Hu XH, Ma J, Li SK and Chen G: Clinical roles of the
aberrantly expressed lncRNAs in lung squamous cell carcinoma: A
study based on RNA-sequencing and microarray data mining.
Oncotarget. 8:61282–61304. 2017.PubMed/NCBI
|
|
26
|
Nik-Zainal S, Davies H, Staaf J,
Ramakrishna M, Glodzik D, Zou X, Martincorena I, Alexandrov LB,
Martin S, Wedge DC, et al: Landscape of somatic mutations in 560
breast cancer whole-genome sequences. Nature. 534:47–54. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiao H, Tang K, Liu P, Chen K, Hu J, Zeng
J, Xiao W, Yu G, Yao W, Zhou H, et al: lncRNA MALAT1 functions as a
competing endogenous RNA to regulate ZEB2 expression by sponging
miR-200s in clear cell kidney carcinoma. Oncotarget. 6:38005–38015.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang H, Niu L, Jiang S, Zhai J, Wang P,
Kong F and Jin X: Comprehensive analysis of aberrantly expressed
profiles of lncRNAs and miRNAs with associated ceRNA network in
muscle-invasive bladder cancer. Oncotarget. 7:86174–86185. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li F, Huang C, Li Q and Wu X: Construction
and comprehensive analysis for dysregulated long non-coding RNA
(lncRNA)-associated competing endogenous RNA (ceRNA) network in
gastric cancer. Med Sci Monit. 24:37–49. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou S, Wang L, Yang Q, Liu H, Meng Q,
Jiang L, Wang S and Jiang W: Systematical analysis of lncRNA-mRNA
competing endogenous RNA network in breast cancer subtypes. Breast
Cancer Res Treat. 169:267–275. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tay Y, Kats L, Salmena L, Weiss D, Tan SM,
Ala U, Karreth F, Poliseno L, Provero P, Di Cunto F, et al:
Coding-independent regulation of the tumor suppressor PTEN by
competing endogenous mRNAs. Cell. 147:344–357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shen X, Xie B, Ma Z, Yu W, Wang W, Xu D,
Yan X, Chen B, Yu L, Li J, et al: Identification of novel long
non-coding RNAs in triple-negative breast cancer. Oncotarget.
6:21730–21739. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang F, Lv SX, Lv L, Liu YH, Dong SY, Yao
ZH, Dai XX, Zhang XH and Wang OC: Identification of lncRNA
FAM83H-AS1 as a novel prognostic marker in luminal subtype breast
cancer. Onco Targets Ther. 9:7039–7045. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheang MC, Chia SK, Voduc D, Gao D, Leung
S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, et al: Ki67
index, HER2 status, and prognosis of patients with luminal B breast
cancer. J Natl Cancer Inst. 101:736–750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yuan N, Zhang G, Bie F, Ma M, Ma Y, Jiang
X, Wang Y and Hao X: Integrative analysis of lncRNAs and miRNAs
with coding RNAs associated with ceRNA crosstalk network in triple
negative breast cancer. Onco Targets Ther. 10:5883–5897. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang H, Fu Z, Dai C, Cao J, Liu X, Xu J,
Lv M, Gu Y, Zhang J, Hua X, et al: lncRNAs expression profiling in
normal ovary, benign ovarian cyst and malignant epithelial ovarian
cancer. Sci Rep. 6:389832016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Z, Yao Q, Zhao S, Wang Y, Li Y and Wang
Z: Comprehensive analysis of differential co-expression patterns
reveal transcriptional dysregulation mechanism and identify novel
prognostic lncRNAs in esophageal squamous cell carcinoma. Onco
Targets Ther. 10:3095–3105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Crippa M, Bestetti I, Perotti M,
Castronovo C, Tabano S, Picinelli C, Grassi G, Larizza L, Pincelli
AI and Finelli P: New case of trichorinophalangeal syndrome-like
phenotype with a de novo t(2;8)(p16.1;q23.3) translocation which
does not disrupt the TRPS1 gene. BMC Med Genet. 15:522014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang N, Tan HY, Chan YT, Guo W, Li S and
Feng Y: Identification of WT1 as determinant of heptatocellular
carcinoma and its inhibition by Chinese herbal medicine Salvia
chinensis benth and its active ingredient protocatechualdehyde.
Oncotarget. 8:105848–105859. 2017.PubMed/NCBI
|
|
40
|
Cai C, Huo Q, Wang X, Chen B and Yang Q:
SNHG16 contributes to breast cancer cell migration by competitively
binding miR-98 with E2F5. Biochem Biophys Res Commun. 485:272–278.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kong Q and Qiu M: Long noncoding RNA
SNHG15 promotes human breast cancer proliferation, migration and
invasion by sponging miR-211-3p. Biochem Biophys Res Commun.
495:1594–1600. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang W, Luo P, Guo W, Shi Y, Xu D, Zheng H
and Jia L: lncRNA SNHG20 knockdown suppresses the osteosarcoma
tumorigenesis through the mitochondrial apoptosis pathway by
miR-139/RUNX2 axis. Biochem Biophys Res Commun. 503:1927–1933.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Z, Zhu Z, Watabe K, Zhang X, Bai C,
Xu M, Wu F and Mo YY: Negative regulation of lncRNA GAS5 by miR-21.
Cell Death Differ. 20:1558–1568. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yuan X, Wang S, Liu M, Lu Z, Zhan Y, Wang
W and Xu AM: Histological and pathological assessment of miR-204
and SOX4 levels in gastric cancer patients. Biomed Res Int.
2017:68946752017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Todorova K, Metodiev MV, Metodieva G,
Mincheff M, Fernández N and Hayrabedyan S: Micro-RNA-204
participates in TMPRSS2/ERG regulation and androgen receptor
reprogramming in prostate cancer. Horm Cancer. 8:28–48. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Abmutalib NS, Othman SN, Mohamad Yusof A,
Abdullah Suhaimi SN, Muhammad R and Jamal R: Integrated microRNA,
gene expression and transcription factors signature in papillary
thyroid cancer with lymph node metastasis. Peer J. 4:e21192016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Geng Y, Deng L, Su D, Xiao J, Ge D, Bao Y
and Jing H: Identification of crucial microRNAs and genes in
hypoxia-induced human lung adenocarcinoma cells. Onco Targets Ther.
9:4605–4616. 2016. View Article : Google Scholar : PubMed/NCBI
|