Introduction
Cancer is the second leading cause of human
mortality. In phototherapy, specific wavelengths of light are
adopted to treat diseases including both cancer and infection
(1–4). To date, photothermal therapy (PTT)
and photodynamic therapy (PDT) are the two most common phototherapy
methods for treating cancer (5).
In PTT, a photothermal (PT) agent is stimulated by both specific
band light and vibrational energy/heat release to selectively
target abnormal tissues and cells (6). In PDT, photosensitizer (PS) drugs
which are photoactivated molecules or materials, generate reactive
oxygen species (ROS) through a series of photochemical reactions.
As a result, the triggered oxidative stress in target cells is able
to induce intracellular lipid peroxidation, DNA injury and protein
damage, ultimately leading to cell death (7,8).
Recently, nanomaterials have garnered much attention and have been
extensively studied (9–11). Many effective photothermal and
photodynamic nanomaterials have been applied in the diagnosis and
treatment of cancer (12,13). The discrepancies, for example,
size, structure and morphology, existing between PTT and PDT
materials may affect the effectiveness of phototherapy. This review
predominantly summarizes the effects produced by different types,
formulations, morphologies and modifications on the photothermal or
photodynamic properties of materials. Moreover, we compared the
effectiveness of distinct nanomaterials in cancer phototherapy and
discussed the advantages and disadvantages of PTT and PDT. In
addition, we introduce the application of the assembly of
nanomaterials in cancer phototherapy. Thus, the present study aimed
to i) summarize PT and PD materials, ii) present their properties
and iii) discuss their relevance in cancer therapy.
Application of nanometer materials in
PTT
Precious metal nanomaterials
Recently, Au (gold) nanocages, a novel class of
nanomaterials, have been reported to be potential photothermal
transducers and drug carriers for mainstream clinical practice in
the near future (14). Au
nanocages have attracted great attention in regards to cancer
imaging, diagnosis and treatment (15,16).
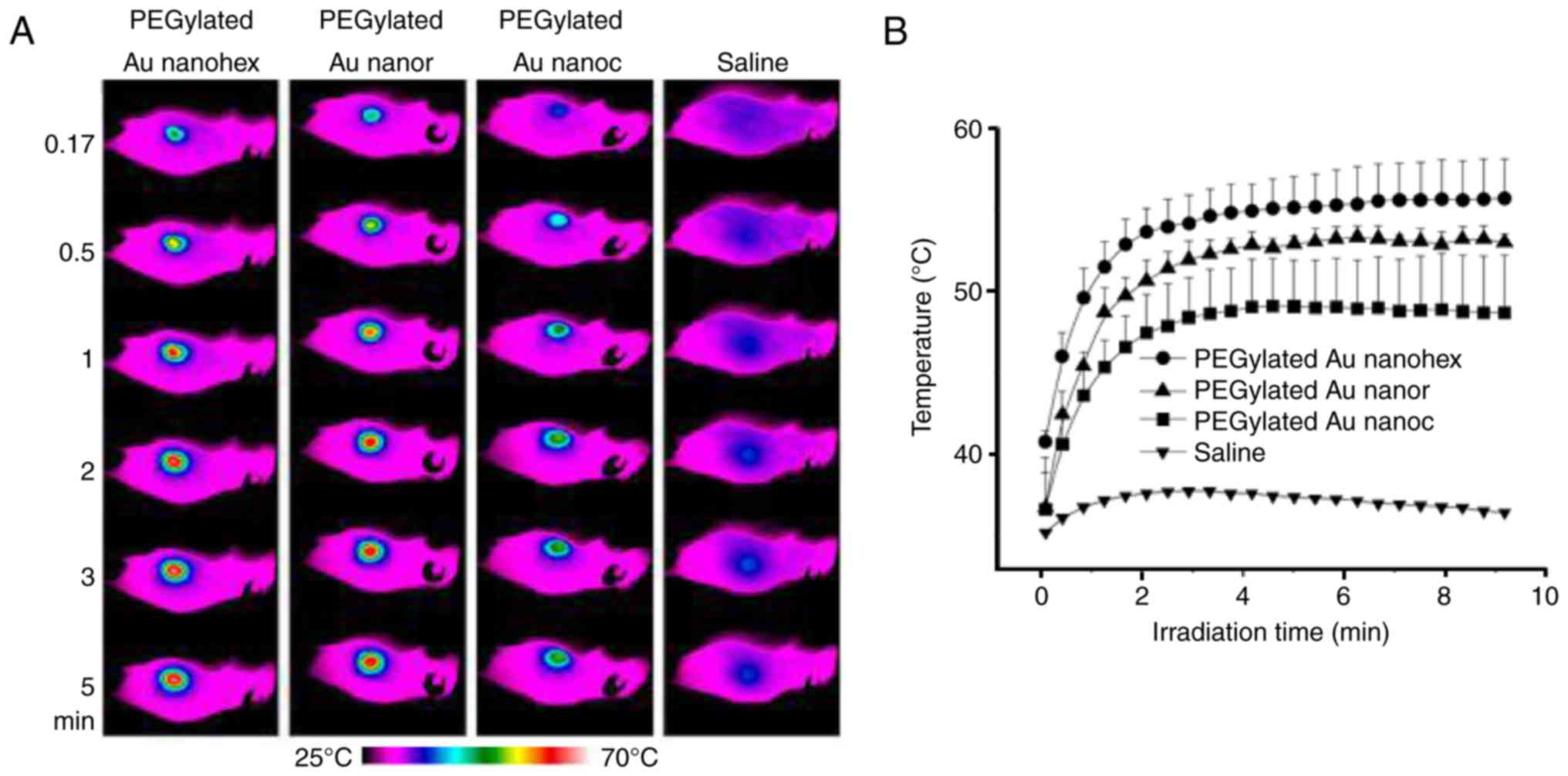
Wang et al (17,18) and Shrestha et al (19) showed that Au nanostructures were
able to absorb near infrared light and convert light to heat. Among
Au nanorods, Au nanocages and Au nanohexapods, the effect of Au
nanohexapods was found to be highly outstanding and it exhibited
the highest cell uptake and the lowest cytotoxicity in vitro
(18). Moreover, in athymic mice
(Nude-Foxn1nu nude) bearing breast tumors (the tumors were
generated through an subcutaneous injection of MDA-MB-435 cells in
the right flanks of mice), the PEGylated Au nanohexapods displayed
significant blood circulation. Additionally, this study also showed
that the accumulation of nanoparticles (passive target effect) in
the tumor site was elevated due to the enhanced penetration effect
of the nanoparticles (18). Thus,
heat was produced to dampen target cancer cells in PTT based on the
PEGylated Au nanostructures (18).
These results indicated the biocompatibility of the Au
nanostructures in vivo, pointing to the potential
application of Au nanostructures in the clinic. Furthermore, the
temperature around the tumor region was higher in the PEGylated Au
nanohexapods than temperatures in other PEGylated Au nanostructures
(Fig. 1A and B). In this way, the
tumors absorbed more heat through the PEGylated Au nanohexapods,
therefore helping realize the goal of detecting and treating the
cancer. Researchers have reported that as a new class of branched
Au nanostructures, Au nanohexapods are more effective in drug
loading and photothermal conversion in comparison to those with
smoother surfaces (20,21). Therefore, we conclude that Au
nanohexapods are a promising candidate material for the diagnosis
and treatment of cancer.
Transition metal sulfide
materials
Although precious metal nanomaterials have attracted
much attention in PTT due to their strong absorption of near
infrared light (22), transition
metal sulfide materials that have the effect of surface plasma
resonance are gaining increased attention for their advantages of
low-price, high efficiency of photothermal conversion and competent
biocompatibility (23,24). One study reported that copper
sulfide (CuS) nanoparticle-based drug delivery was effective in
cancer treatment (25). In this
study, the CuS-based drug was not only taken up by MCF-7 cells and
could effectively convert NIR light into heat, but also generated a
large amount of reactive oxygen species (ROS) for photodynamic
therapy. Moreover, the photothermal heating of Cu2-xSe
nanocrystals after 5 min of laser irradiation at 33
W/cm2 led to the cell destruction of human colorectal
cancer HCT-116 cells, pointing to the possible viability of
Cu2-xSe for PTT therapy (26). Furthermore, tungsten oxide has also
been confirmed to be pertinent to tumor CT imaging and PTT
(27).
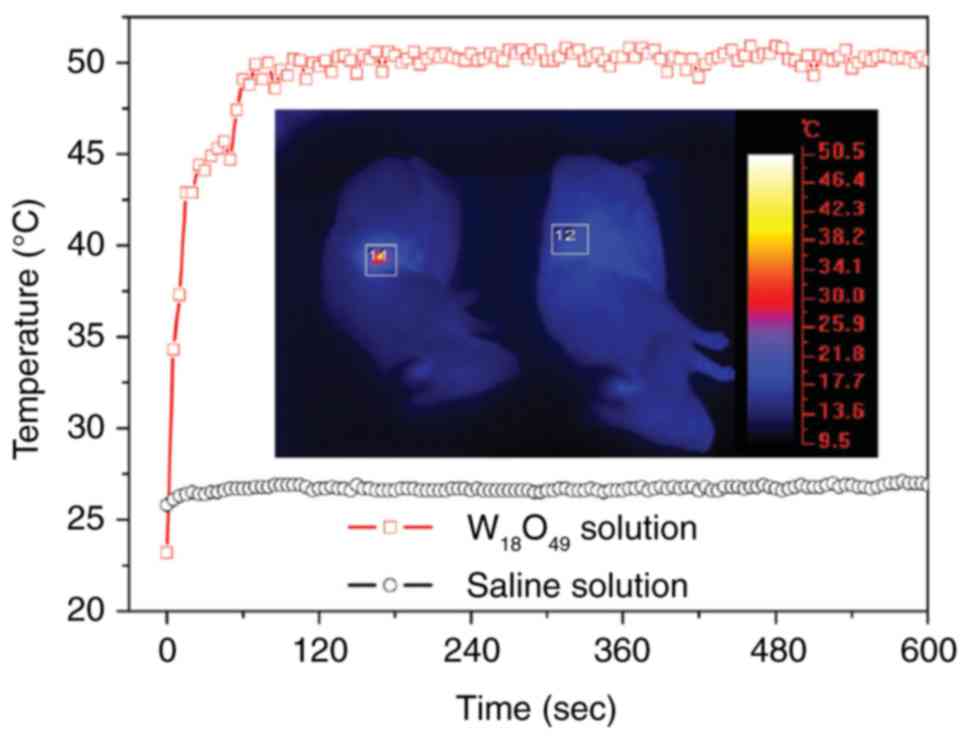
The effect of W18O49 nanowire
in PTT has been explored in a previous study (28). The results of the study showed that
ultrathin PEGylated W18O49 nanowire was
formulated by heating WCl6 with ethanol and PEG.
Following this treatment method, the prepared blue aqueous
dispersions with W18O49 nanowires were able
to enhance the absorption of near infrared light. Under the
irradiation of a 980-nm laser (which is safe for humans when the
power density is set to 0.72 W·cm2), the temperature of
aqueous dispersions with the W18O49 nanowires
(0.25–3.0 g/l) was increased by 12.2–41.2°C within 5 min (28). In the animal studies, severe
combined immunodeficiency (SCID) mice were inoculated with K7M2
cells and were grouped into control and treatment groups. Mice in
the treatment group were injected with W18O49
nanowires (100 µl, 2 g/l) at the central region of the tumor with a
depth of ~4 mm. Mice in the control and treatment groups were
simultaneously irradiated for 10 min at 0.72 W/cm2 by
two similar 980-nm laser devices. Full-body thermographic images
and temperature were recorded during the irradiation. It was
observed that the temperature of tumor tissues was quickly elevated
to 50.0±0.5°C within 120 sec of irradiation (28) (Fig.
2). Hence, as a near-infrared laser-induced photothermal agent,
the PEGylated W18O49 nanowires exhibited
superior efficiency in PTT and such an efficacy can be largely
explained by their high efficiency of photothermal conversion and
low cytotoxicity.
The influencing factors in PTT
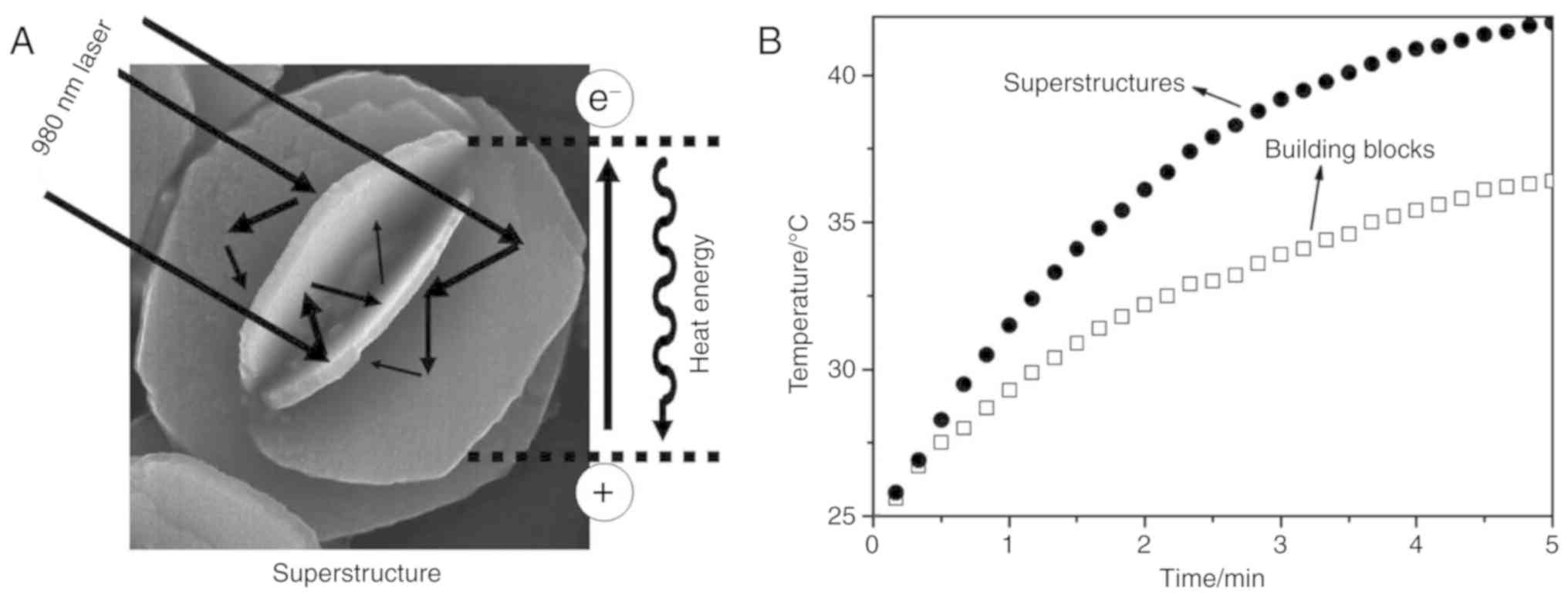
The morphology of a nanomaterial has significant
effects on its physical chemistry and biological properties
(29). The flower-like nano copper
sulphide can be prepared by the hydrothermal method as previously
described (30). The light can be
reflected in nano-flowered copper sulphide multiple times based on
the mechanism of light reflection (Fig. 3A). As a result, the photoabsorption
is increased and the photothermal conversion efficiency is
enhanced. Researchers have confirmed that the photothermal
conversion efficiency of flower-like nano copper sulphide is
elevated by 50% in comparison to ordinary hexagonal sulfide
nanoparticles (30). In addition,
the temperature of a superstructure nano-CuS aqueous solution was
found to be increased by 17.3°C within 5 min under irradiation with
a low power density of 0.51 W·cm2 by a 980-nm laser
in vitro (Fig. 3B). These
findings may inspire researchers to develop nanoparticles that can
provide high photothermal conversion efficiency in PTT.
As reported by Tian et al (31), the photothermal conversion
efficiency of Cu9S5 nanoparticles reached
25.7%, which was higher than that of as-synthesized Au nanorods
(23.7% from 980 nm laser) and that of (Cu2×Se)
nanocrystals (NCs) (22% from an 808-nm laser). The temperature of
Cu9S5 NCs (40 ppm) reached 15.1°C within 7
min under the irradiation condition of a 980-nm laser with a power
density of 0.51 W·cm2. Moreover, although semiconductor
nanocrystals containing copper exhibit low cost and low toxicity,
they have a high stability and high photothermal conversion
efficiency (32). Importantly, the
cancer cells can be killed by the photothermal effects of the
Cu9S5 NCs under 980-nm laser irradiation with
the conservative and safe power density over a short period (~10
min) (31). A previous study
demonstrated that the DNA-decorated Cu9S5
nanoparticles could be used as NIR light responsive drug carriers
in tumor chemo-phototherapy (33).
This indicated that the efficient photothermal effects produced by
nanoparticles may contribute to killing cancer cells.
Thermal stability is a highly critical parameter for
photothermal materials (34). If
the heating rate far exceeds the cooling rate, the heat will
rapidly accumulate in the lattice. Therefore, a high temperature of
nanoparticles will be reached at a specific area over a short
period of time, and structural changes in terms of the shape or
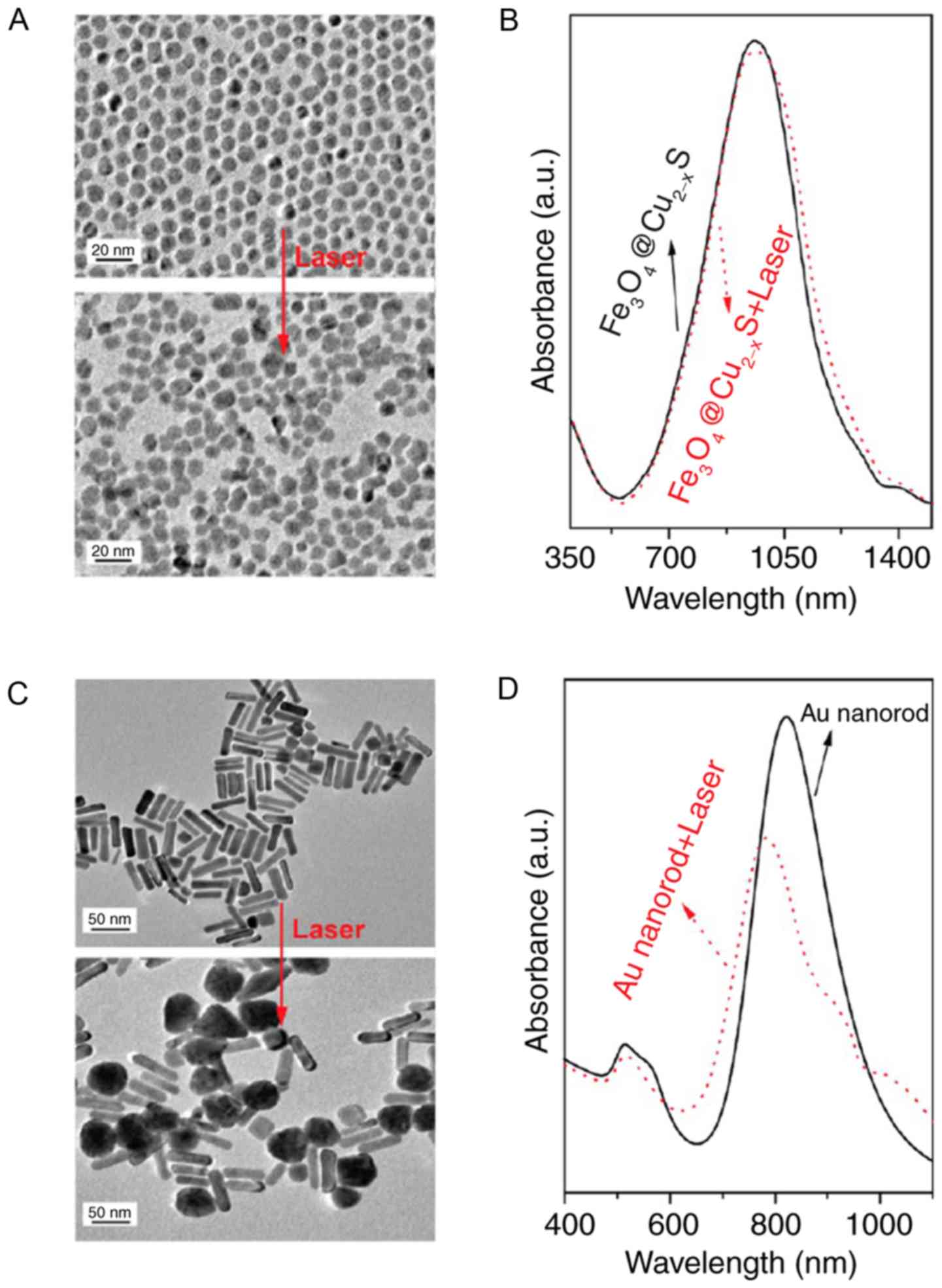
integrity of nanoparticles will result (35). A previous study showed that
core-shell
nanomaterial-Fe3O4@Cu2-xS has high
photothermal stability and super-paramagnetism (36). This previous study also confirmed
that as they had an intense absorption in the near infrared region
of 960 nm, these core-shell nanomaterials could serve as a magnetic
resonance imaging T2 contrast agent and were able to be employed in
infrared thermal imaging. Furthermore, the photothermal effect of
nanoparticles can be controlled by altering the content of Cu in
the core-shell nanomaterials. The synergistic effect of magnetic
and photothermal phenomena employed in this study may lay a solid
foundation for the development of nanoprobes in multimode
biomedicine application. In addition, the thermal stability of
core-shell nanomaterials was also improved in the same study. From
the transmission electron microscope laser scanning images, it was
clearly observed that the shape of core-shell nanomaterials and the
absorption of near infrared remained approximately the same after
administration of 980-nm laser irradiation for 30 min (Fig. 4). This suggested that the thermal
stability of nanomaterials is critical in biomedical
application.
Carbon nanomaterials
Carbon nanotubes are able to absorb near-infrared
light so as to efficiently convert light to heat (37). Thus, carbon nanotubes could be used
for thermal ablation, diagnosis and drug delivery in cancer for its
high aspect ratio, ultra-light weight, high mechanical strength,
high electrical conductivity and high thermal conductivity
(38). Ultra-small nano-reduced
graphene has been demonstrated to possess acceptable performance in
absorbing near-infrared light (39). The size and surface compositions of
graphene are in close relation to its thermal properties (40). The average transverse dimension of
graphene is approximately 20 nm. The modification with targeted
peptides can increase the specificity of graphene in killing target
cells (41). As demonstrated by
Yang et al (40), the
damage to cancer cells was dramatically augmented in raphene-based
PTT. Nevertheless, the power density of the laser used in this
study was 0.15 W·cm2, which is less than the power
density used (0.5–2 W·cm2) by most photothermal research
institutes.
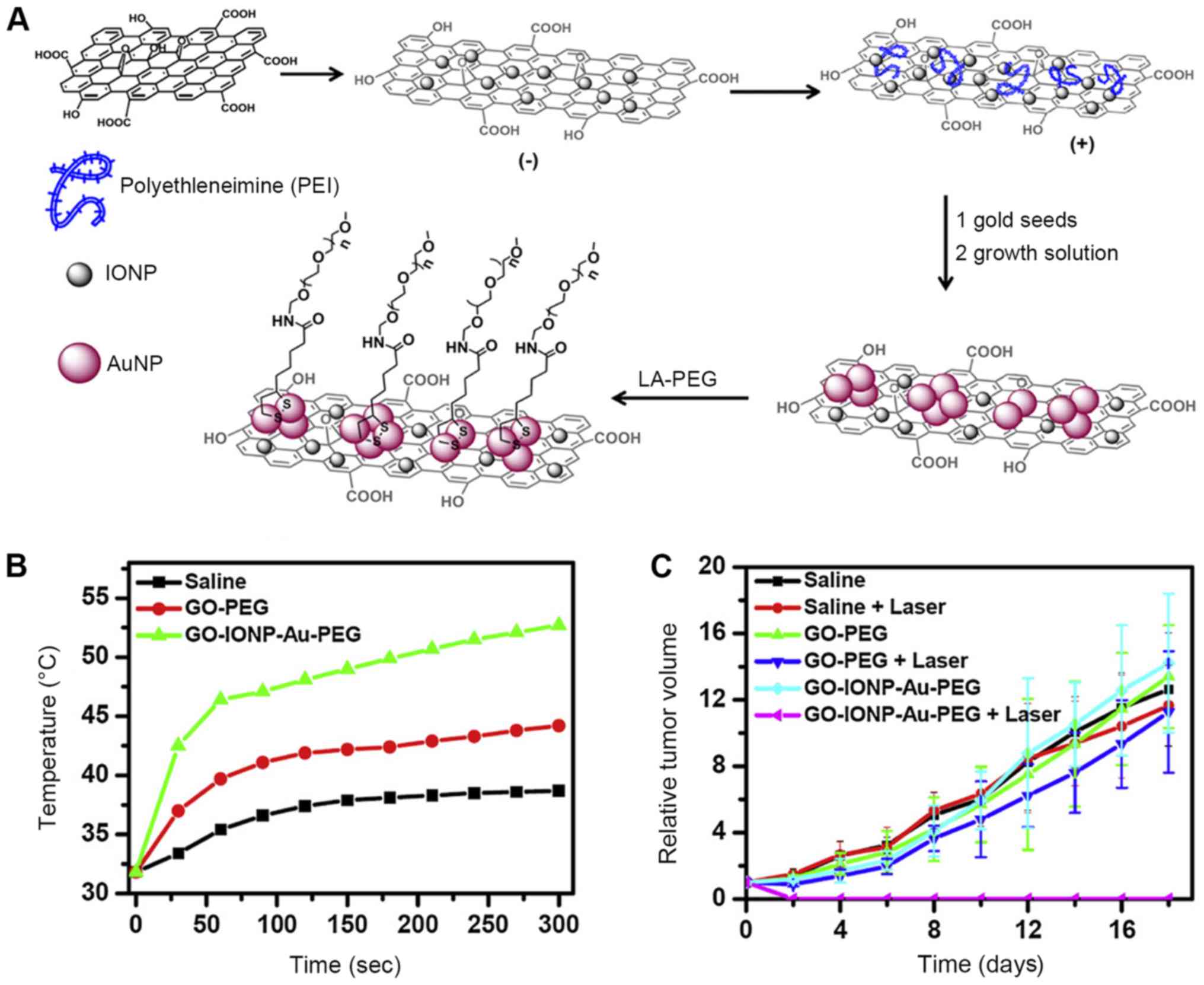
Liu et al (42) developed graphene-iron oxide-gold
nanoparticles (GO-IONP-Au), which efficiently combined the
photothermal properties of graphene (the magnetic properties of
iron oxide) and the properties of the surface plasma resonance of
gold nanoparticles. Moreover, the folate receptor on GO-IONP-Au
nanoparticles showed better performances in target cell killing and
damage. Thus, GO-IONP-Au acquired both passive and active targeting
prosperities. Moreover, GO-IONP-Au has magnetic properties and thus
could be employed as an imaging agent for nuclear magnetic imaging
in cancer therapy. Additionally, the surface plasma resonance
effect produced by gold nanoparticles in GO-IONP-Au increased its
photoabsorbtion and light-heat conversion efficiency. As an
effective PTT agent, graphene in combination with gold
nanoparticles also enhanced the effect of GO-IONP-Au in PTT.
Furthermore, Liu et al also proved that PEG modifications
enabled the GO-IONP-Au to be more biocompatible. GO-IONP-Au
inhibited tumor growth and reduced the tumor size in vivo
(Fig. 5). In summary, as
graphene-based photothermal nanocomposites, GO-IONP-Au is a
powerful and promising PTT agent that can be applied in dual mode
imaging (nuclear magnetic imaging and thermal imaging) with its
multi-functional magnetic, surface plasma resonant effects. This
suggested that graphene-based multi-functional nanocomposite
materials have great potential in the diagnosis and treatment of
cancer.
For its suitable biocompatibility, the effect of
PEG-BPEI-rGO nanocomposites in gene transfection has already been
investigated (43). In this
previous study, the collapse of the endocytosis containing the
transfection gene was regulated by heat, thereby controlling the
time and site of gene release. This not only provided a simple,
practical and highly efficient strategy for developing possible
drugs and gene carriers, but also inspired a new insight for gene
therapy.
Application of nanometer materials in
PDT
Combining photosensitizers and light irradiation,
photodynamic therapy (PDT) is an emerging new treatment method for
treating various diseases, including cancers (such as lung, breast,
bladder and brain cancer) and non-cancer diseases (such as
bacterial and fungal infections, premalignant conditions and
inflammatory conditions) (44).
Nevertheless, most of the conventional PDT photosensitizers are
stimulated by visible light (VIS), which cannot penetrate thick
tissues or reach deep tumor tissue (45). Thus, VIS can only be employed in
treating skin or shallow tissues. A range of 700–1,100 nm (8,46)
has been accepted as the absorption window for most biomolecules.
Therefore, near-infrared light (NIR) was adopted in PDT as NIR can
penetrate deep tissue, eliminating cancer cells.
Upconversion nanoparticles
Upconversion nanoparticles are able to convert light
from long wavelengths to short wavelengths through the excitation
of NIR light (47). Upconversion
nanoparticles with fine crystallinity and monodispersity have been
successfully synthesized, with their sizes controlled within 100 nm
(48). A previous study reported
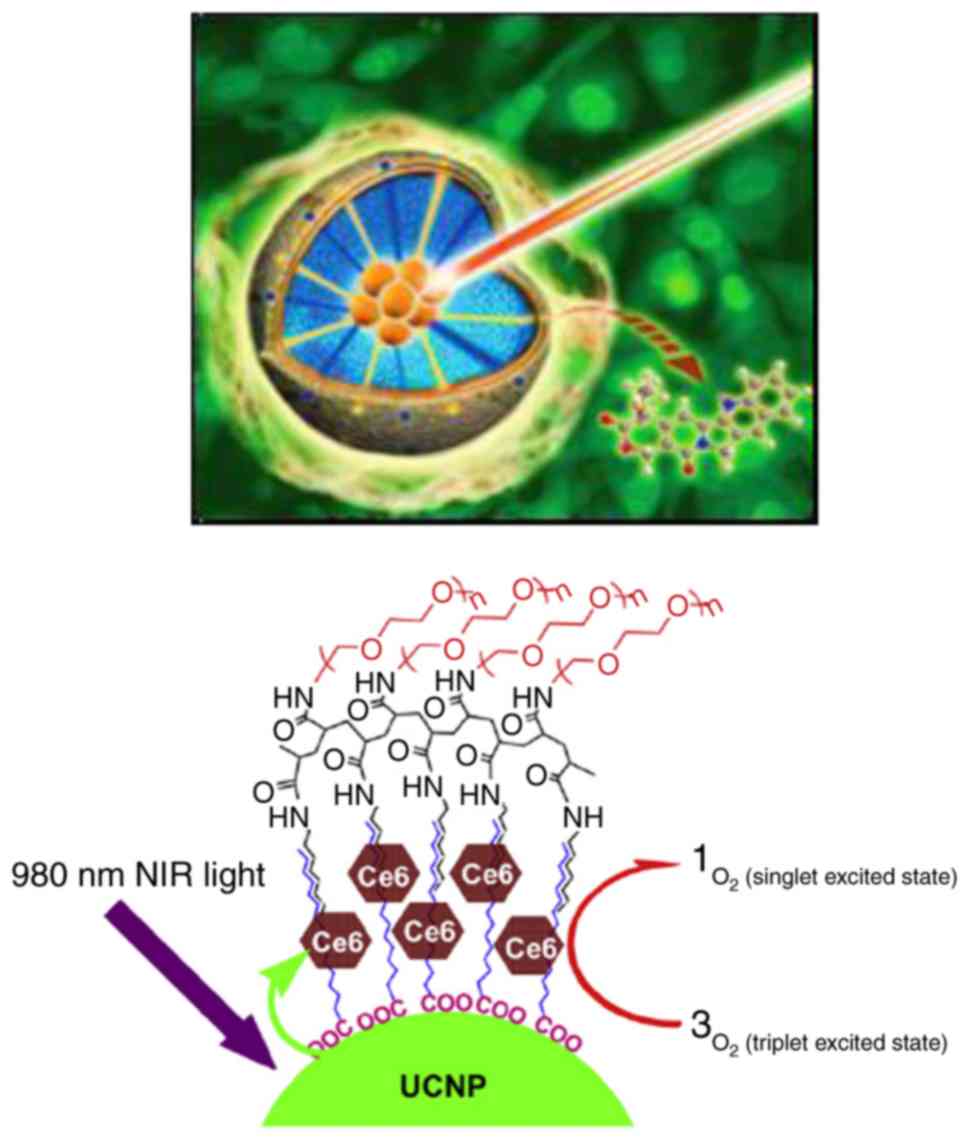
that penetrated NIR light is converted into VIS by upconversion
nanoparticles in a diseased site, therefore leading to the
absorption of VIS by photosensitizers (49). Finally, the cytotoxic reactive
oxygen species (ROS) produced by the photosensitizers would attack
the unwanted cells (Fig. 6)
(7,49). These results point to the
significance of upconversion nanoparticles in non-invasive deep
tissue imaging, drug delivery and photomodynamics (50,51).
Qian et al (52) and Chatterjee and Yong (53) explored the effect of upconversion
nanoparticles on PDT. Qian et al (52) proved that the effect of two
photosensitizers in combination produced better than that of the
use of a signal one. Noticeably, the combined photosensitizers did
not need the excitation of multiple wavelengths. In the two studies
(Fig. 7), multiple upconversion
nanomaterials (UCNs) with different colors and emissions were
irradiated under a 980-nm laser to activate two types of
photosensitizers, and therefore the effect of PDT was improved.
Upconversion nanoparticles effectively converted the near-infrared
light into visible light emission (53). The wavelength of emitted visible
light is matched with the maximum absorption wavelength of the
photosensitizer, therefore activating the photosensitizer so as to
produce the cytotoxic single line oxygen.
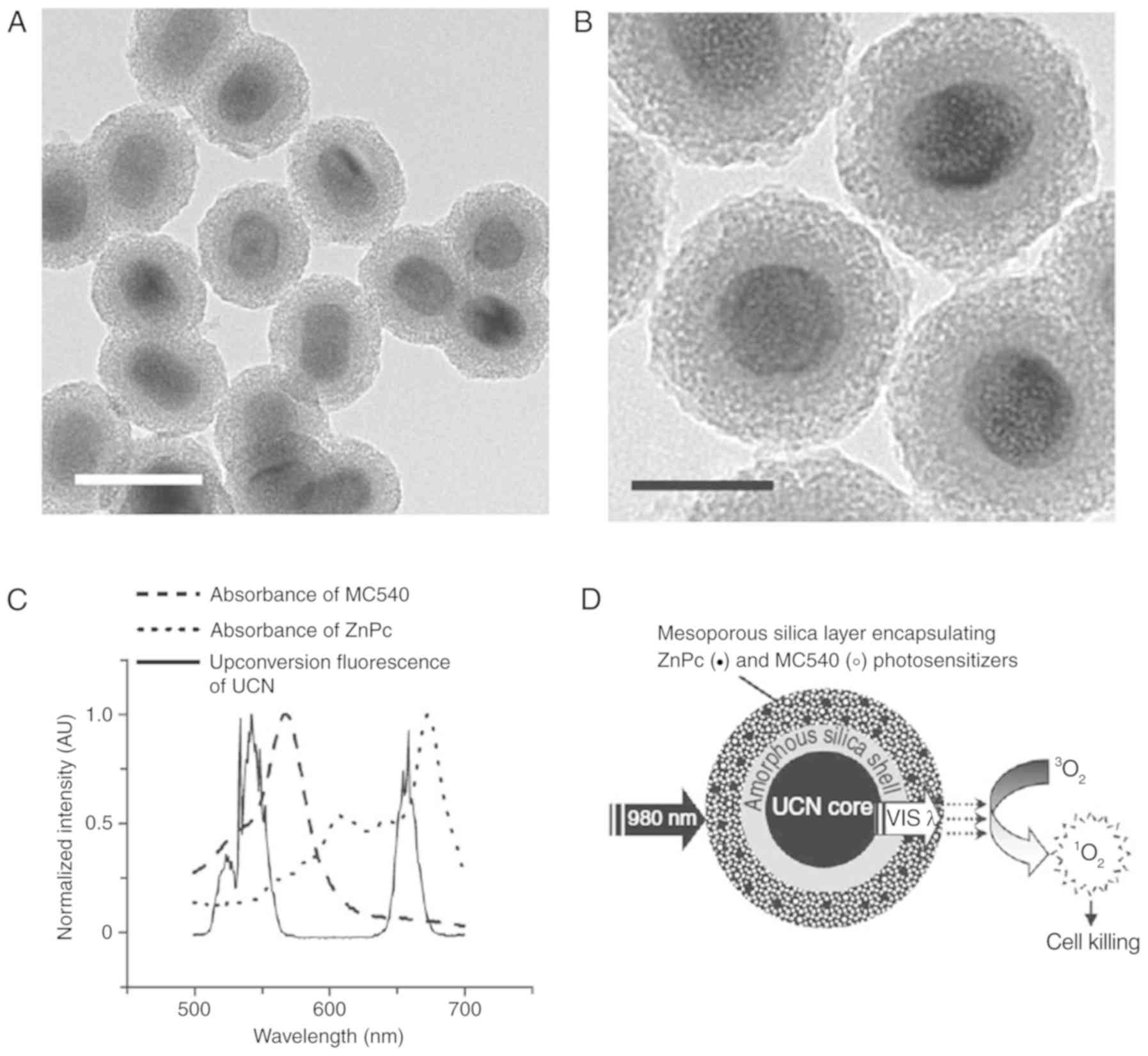
Mesoporous silica-coated upconversion nanoparticles
with photosensitizers were loaded in B16-F0 melanoma-bearing
C57BL/6 mice (54). The tumor
growth of the mice was examined under laser irradiation. Data from
this study showed that the exposure of upconversion nanoparticles
with 980-nm laser irradiation activated MC540 and ZnPc, which then
enhanced the therapy effect of PDT. Moreover, upconversion
nanoparticle modification by folic acid and PEG (FA-PEG-UCNs) has
also been developed to increase the bio-application values.
Furthermore, upconversion nanoparticles could be conjugated with
multiple dopants and employed for target labeling and imaging
(55). A previous study
demonstrated that FA-coupled up-converting nanophosphors (UCNPs)
effectively targeted folate-receptor over-expressing HeLa cells
in vitro and HeLa tumors in vivo (56). Recently, upconversion nanoparticles
have been coupled with fluorescence resonant energy transfer (FRET)
technology to form efficient biological labels that were used for
the diagnostics of diseases (55).
In this study, 7-nm gold nanoparticles, which were coupled with the
UC Na
(Y1.5Na0.5)F6:Yb3+,
Er3+ nanoparticles (energy donors), were formed into a
FRET biosensor whose strong absorption of gold nanoparticles
matches well with the upconversion emission. This suggested that
the efficiency of the FRET system based upon upconversion
nanoparticles was elevated. Such a finding will promote the
progression of fluorescence imaging. In addition,
Gd3+-based upconversion nanoparticles have been
formulated as magnetic resonance imaging (MRI) imaging agents.
NaGd4:Yb/Er nanoparticles may be used as probes for
bioimaging (57,58). Taken together, upconversion
nanoparticles, which can convert near-infrared light to visible
light in deep tissues, are promising in translating basic research
concepts into clinical practice (59).
Combination of PTT and PDT
Supramolecular polymers
Supramolecular polymers display great potentials for
applications in the biomedical field for its special structural and
physicochemical properties (60,61).
The application of PDT was restricted for its oxygen-dependent
characteristics (62). Porphyrins
belong to the class of four-pyrrole, which is a major component of
hemoglobin and myoglobin (63).
The biological activity of porphyrins is indispensable to living
organisms. These molecules are highly conjugated macrocyclic
compounds and may contain a central metallic atom such as
Mg2+ or Fe2+ (64). Porphyrins is considered as
long-wavelength-absorbing sensitizers (65). Therefore, for its application in
medical treatment (66,67), porphyrins have generated scientific
interest worldwide. Differences between the distribution and
photodegradation of hematoporphyrin can be used to distinguish
noncancerous from cancerous human breast tissue in Raman
spectroscopy (68).
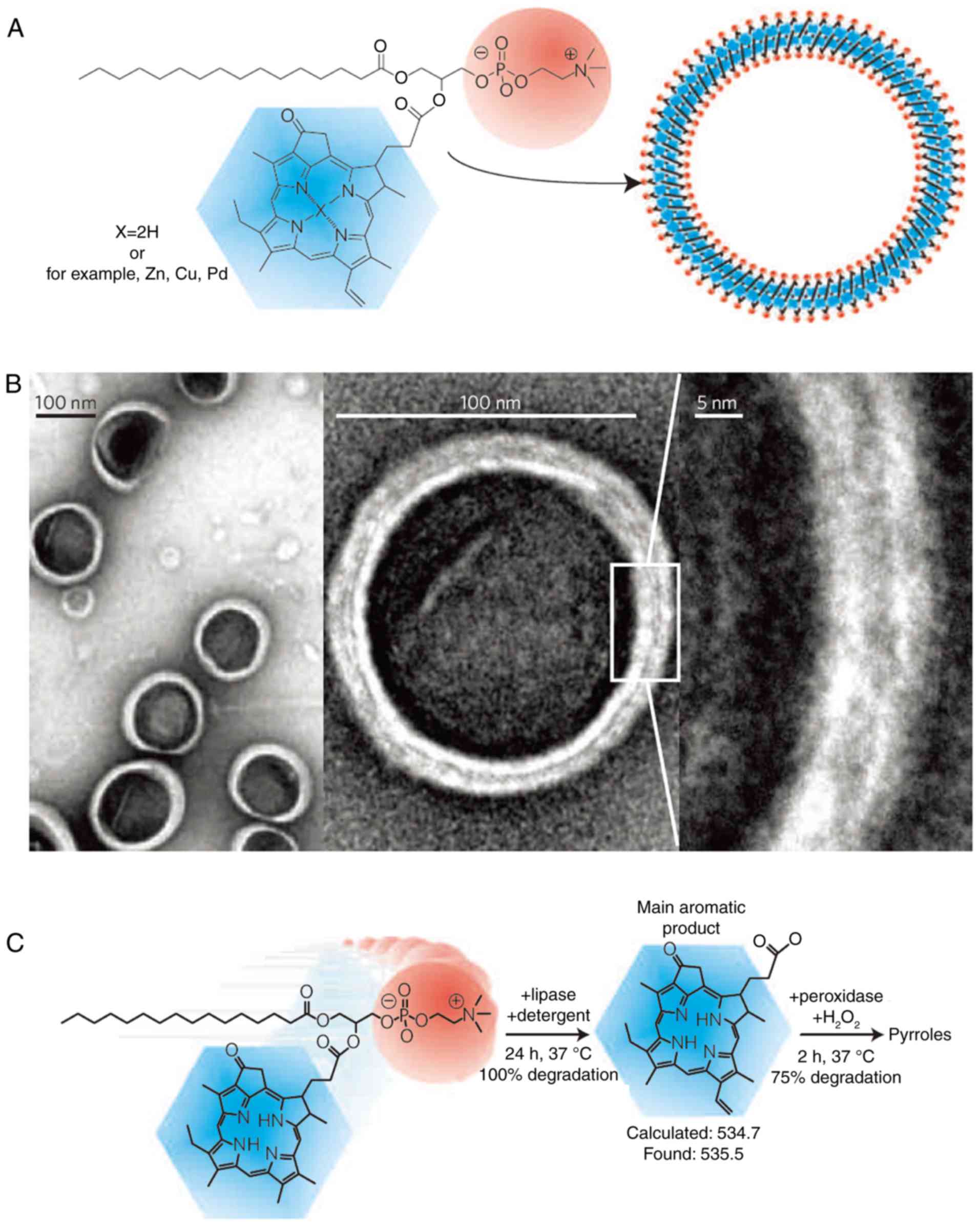
Porphysomes are similar to liposomes. The potential
applications of this material have been discussed (69). According to previous studies,
porphysomes could be formulated by exploiting the mechanism of
hydrophobic self-assembly (Fig.
8A) (70–72). Zheng et al (73) found that porphysomes, whose
monodisperse diameter is 100 nm (Fig.
8B), could enhance its passive accumulation in tumor tissues
through the osmotic cycle effect. Moreover, smaller nanoparticles
with 30 nm diameter can be obtained by ultrasonic treatment in
water. In addition, porphysomes with a diameter of 100 nm can be
loaded with approximately 8×104 porphyrin molecules.
Furthermore, porphysomes can be degraded in living cells (Fig. 8C). The fluorescence quenching test
was performed to test the quenching property of porphysomes loaded
with numerous porphyrin molecules in the same study (Fig. 9). The results showed that the
quenching of porphysomes increased 1,200 times in comparison to the
standard liposomes, generating a considerably stronger quenching
than the common porphyrins. The fluorescence quenching could lead
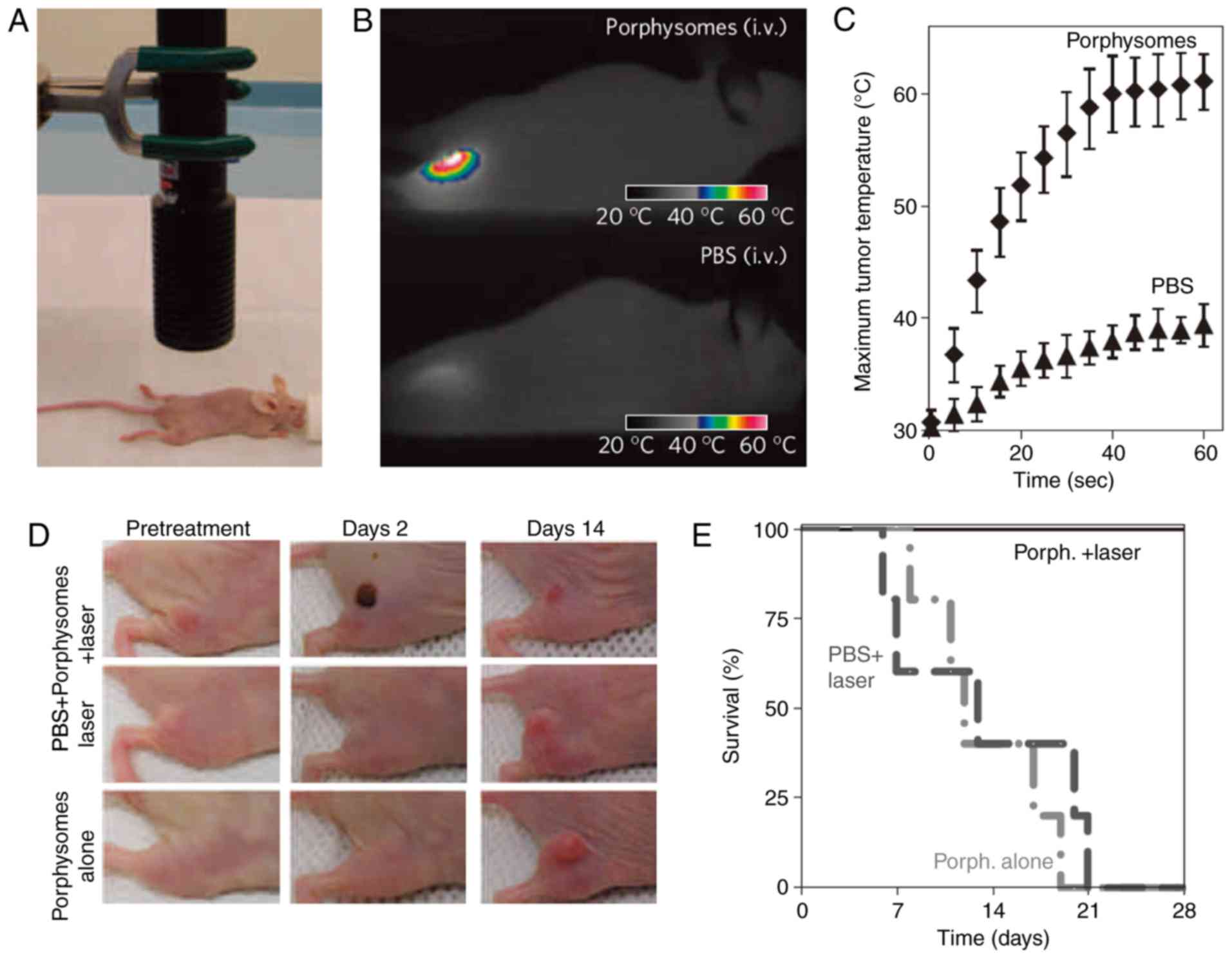
to the generation of heat and singlet oxygen production (69). Researchers suggested that
porphysomes have a high photothermal conversion efficiency. It was
also confirmed that porphysomes accumulated in tumors induced
photothermal tumor ablation under laser irradiation (74). In addition, based on the tissue
section and blood indicators, it can be observed that large doses
of porphyrins did not cause liver and kidney injuries (74), and that porphysomes were prone to
enzymatic degradation (74).
Therefore, porphyrins may be used as a biodegradable,
ultra-molecular photothermal therapy agents on account of their
minimal toxicity and high photothermal conversion efficiency.
The effect of porphyrins and porphysomes in
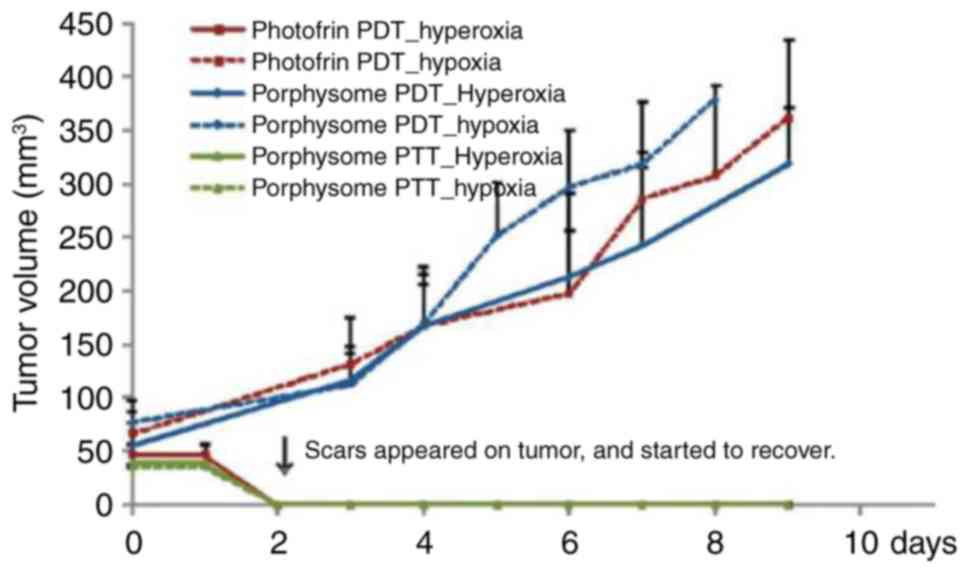
hypoxic/hyperoxic tumor tissues has previously been investigated.
Huynh and Zheng pointed out that porphyrins exhibited acceptable
performance in the treatment of hyperoxic tumors (69). This finding is consistent with the
mechanism of single line oxygen in PDT. Interestingly, porphysomes
exhibited excellent effects both in the treatment of hyperoxic
tumors and in the treatment of hypoxic tumors, and effectively
compensated for the defects in PDT (Fig. 10). This result may inspire
research associated with ultra-molecular assembly in PDT, which may
promote the quenching of the materials and produce more heat to
kill cancer cells.
Conclusions
As non-invasive methods of phototherapy, the
clinical value of photothermal therapy (PTT) and photodynamic
therapy (PDT) are of significance in the prevention of cancer.
Photothermal materials (such as precious metal nanomaterials,
transition metal sulfide, carbon nanomaterials and upconversion
nanoparticles) and photodynamic materials (such as phthalocyanas,
porphyrins and other dye molecules) have been extensively
investigated in recent years. The effect of PTT and PDT are largely
dependent on distinct materials, different preparation methods,
morphologies and modification methods. These parameters of
materials can be modified in terms of varied purposes and needs.
Additionally, PTT and PDT have their own advantages and defects.
However, the combination of PTT and PDT not only provides enough
time to achieve an effective treatment temperature for PTT, but
also overcomes the obstacle of oxygen dependence accompanied with
PDT. Therefore, such an combination could achieve a complementary
synergistic effect in cancer therapy. Thus, a natural progression
of this work is to practically transform the combination of PTT and
PDT from basic science to clinical application.
Acknowledgements
The authors express thanks for permission to reprint
the figures from the relevant publication organizations. The
permission and copyright are stated in the figure legends and
proper documentation has been provided.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81701894 and 81401583), the
Major Projects Foundation of General Logistics Department of PLA
(grant no. CNJ14L002), the Social Development Projects of Jiangsu
Province (grant no. BE2017720), the Jiangsu Provincial Medical
Youth Talent (grant nos. QNRC2016908, QNRC2016909) and the Peking
Union Farsighted Emergency Project (grant no. RE2016-002), the
Startup Fund of Wenzhou Institute of Biomaterials and Engineering
(grant no. WIBEZD2017001-03).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZY conceived and designed the review, and drafted
and revised the manuscript. ZS analyzed the previous research, and
drafted and revised the review. YR and XC contributed to the
literature search, data collection, and revisions. WZ, XZ, ZM and
JS analyzed the previous research, and ZM and JS also revised the
manuscript. SN designed and revised the review, and analyzed the
previous research.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Oh J, Yoon H and Park JH: Nanoparticle
platforms for combined photothermal and photodynamic therapy.
Biomed Eng Lett. 3:67–73. 2013. View Article : Google Scholar
|
|
2
|
Cao J, An H, Huang X, Fu G, Zhuang R, Zhu
L, Xie J and Zhang F: Monitoring of the tumor response to
nano-graphene oxide-mediated photothermal/photodynamic therapy by
diffusion-weighted and BOLD MRI. Nanoscale. 8:10152–10159. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin J, Wang S, Huang P, Wang Z, Chen S,
Niu G, Li W, He J, Cui D, Lu G, et al: Photosensitizer-loaded gold
vesicles with strong plasmonic coupling effect for imaging-guided
photothermal/photodynamic therapy. ACS Nano. 7:5320–5329. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiong LQ, Chen ZG, Yu MX, Li FY, Liu C and
Huang CH: Synthesis, characterization, and in vivo targeted imaging
of amine-functionalized rare-earth up-converting nanophosphors.
Biomaterials. 30:5592–5600. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shibu ES, Hamada M, Murase N and Biju V:
Nanomaterials formulations for photothermal and photodynamic
therapy of cancer. J Photochem Photobiol C: Photochem Rev.
15:53–72. 2013. View Article : Google Scholar
|
|
6
|
Liu J, Han J, Kang Z, Golamaully R, Xu N,
Li H and Han X: In vivo near-infrared photothermal therapy and
computed tomography imaging of cancer cells using novel
tungsten-based theranostic probe. Nanoscale. 6:5770–5776. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang C, Tao H, Cheng L and Liu Z:
Near-infrared light induced in vivo photodynamic therapy of cancer
based on upconversion nanoparticles. Biomaterials. 32:6145–6154.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dolmans DE, Fukumura D and Jain RK:
Photodynamic therapy for cancer. Nat Rev Cancer. 3:380–387. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wen J, Xu Y, Li H, Lu A and Sun S: Recent
applications of carbon nanomaterials in fluorescence biosensing and
bioimaging. Chem Commun (Camb). 51:11346–11358. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu X, Ji C, Jin T and Fan X: The effects
of size and surface modification of amorphous silica particles on
biodistribution and liver metabolism in mice. Nanotechnology.
26:1751012015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luo M, Shen C, Feltis BN, Martin LL,
Hughes AE, Wright PF and Turney TW: Reducing ZnO nanoparticle
cytotoxicity by surface modification. Nanoscale. 6:5791–5798. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chow EK and Ho D: Cancer nanomedicine:
From drug delivery to imaging. Sci Transl Med. 5:216rv42013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jaque D, Martinez Maestro L, del Rosal B,
Haro-Gonzalez P, Benayas A, Plaza JL, Martin Rodriguez E and García
Solé J: Nanoparticles for photothermal therapies. Nanoscale.
6:9494–9530. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thakor AS, Jokerst J, Zavaleta C, Massoud
TF and Gambhir SS: Gold nanoparticles: A revival in precious metal
administration to patients. Nano Lett. 11:4029–4036. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bazán-Díaz L, Mendoza-Cruz R,
Velázquez-Salazar JJ, Plascencia-Villa G, Romeu D, Reyes-Gasga J,
Herrera-Becerra R, José-Yacamán M and Guisbiers G: Gold-copper
nanostars as photo-thermal agents: Synthesis and advanced electron
microscopy characterization. Nanoscale. 7:20734–20742. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pissuwan D and Niidome T:
Polyelectrolyte-coated gold nanorods and their biomedical
applications. Nanoscale. 7:59–65. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Liu Y, Luehmann H, Xia X, Brown P,
Jarreau C, Welch M and Xia Y: Evaluating the pharmacokinetics and
in vivo cancer targeting capability of Au nanocages by positron
emission tomography imaging. ACS Nano. 6:5880–5888. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Black KC, Luehmann H, Li W, Zhang
Y, Cai X, Wan D, Liu SY, Li M, Kim P, et al: Comparison study of
gold nanohexapods, nanorods, and nanocages for photothermal cancer
treatment. ACS Nano. 7:2068–2077. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shrestha R, Elsabahy M, Luehmann H,
Samarajeewa S, Florez-Malaver S, Lee NS, Welch MJ, Liu Y and Wooley
KL: Hierarchically assembled theranostic nanostructures for siRNA
delivery and imaging applications. J Am Chem Soc. 134:17362–17365.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hasan W, Stender CL, Min HL, Nehl CL and
Lee J: Tailoring the structure of nanopyramids for optimal heat
generation. Nano Lett. 9:1555–1558. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim DY, Yu T, Cho EC, Ma Y, Park OO and
Xia Y: Synthesis of gold nano-hexapods with controllable arm
lengths and their tunable optical properties. Angew Chem Int Ed
Engl. 50:6328–6331. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kumar A and Liang XJ: Gold nanomaterials
as prospective metal-based delivery systems for cancer treatment.
Kretsinger RH, Uversky VN and Permyakov EA: Encyclopedia of
Metalloproteins; Springer, New York, NY: pp. 875–887. 2013
|
|
23
|
Wu H, Yang R, Song B, Han Q, Li J, Zhang
Y, Fang Y, Tenne R and Wang C: Biocompatible inorganic
fullerene-like molybdenum disulfide nanoparticles produced by
pulsed laser ablation in water. ACS Nano. 5:1276–1281. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu T, Wang C, Gu X, Gong H, Cheng L, Shi
X, Feng L, Sun B and Liu Z: Drug delivery with PEGylated MoS2
nano-sheets for combined photothermal and chemotherapy of cancer.
Adv Mater. 26:3433–3440. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin H, Shan X, Hao L, Feng Q and Zhang Z:
Copper sulfide nanoparticle-based localized drug delivery system as
an effective cancer synergistic treatment and theranostic platform.
Acta Biomate. 54:307–320. 2017. View Article : Google Scholar
|
|
26
|
Hessel CM, Pattani VP, Rasch M, Panthani
MG, Koo B, Tunnell JW and Korgel BA: Copper selenide nanocrystals
for photothermal therapy. Nano Lett. 11:2560–2566. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou Z, Kong B, Yu C, Shi X, Wang M, Liu
W, Sun Y, Zhang Y, Yang H and Yang S: Tungsten oxide nanorods: an
efficient nanoplatform for tumor CT imaging and photothermal
therapy. Sci Rep. 4:36532014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Z, Wang Q, Wang H, Zhang L, Song G,
Song L, Hu J, Wang H, Liu J, Zhu M and Zhao D: Ultrathin PEGylated
W18O49 nanowires as a new 980 nm-laser-driven photothermal agent
for efficient ablation of cancer cells in vivo. Adv Mater.
25:2095–2100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song G, Shen J, Jiang F, Hu R, Li W, An L,
Zou R, Chen Z, Qin Z and Hu J: Hydrophilic molybdenum oxide
nanomaterials with controlled morphology and strong plasmonic
absorption for photothermal ablation of cancer cells. ACS Appl
Mater Interfaces. 6:3915–3922. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tian Q, Tang M, Sun Y, Zou R, Chen Z, Zhu
M, Yang S, Wang J, Wang J and Hu J: Hydrophilic flower-like CuS
superstructures as an efficient 980 nm laser-driven photothermal
agent for ablation of cancer cells. Adv Mater. 23:3542–3547. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tian Q, Jiang F, Zou R, Liu Q, Chen Z, Zhu
M, Yang S, Wang J, Wang J and Hu J: Hydrophilic Cu9S5 nanocrystals:
a photothermal agent with a 25.7% heat conversion efficiency for
photothermal ablation of cancer cells in vivo. ACS Nano.
5:9761–9771. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Knowles KE, Hartstein KH, Kilburn TB,
Marchioro A, Nelson HD, Whitham PJ and Gamelin DR: Luminescent
colloidal semiconductor nanocrystals containing copper: Synthesis,
photophysics, and applications. Chem Rev. 116:10820–10851. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liang S, Xie Z, Wei Y, Cheng Z, Han Y and
Lin J: DNA decorated Cu9S5 nanoparticles as
NIR light responsive drug carriers for tumor chemo-phototherapy.
Dalton Trans. 47:7916–7924. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stubenvoll M, Schäfer B, Mann K, Walter A
and Zittel L: Photothermal absorption measurements for improved
thermal stability of high-power laser optics. Journal 88851R.
2013.
|
|
35
|
Miokovic T, Schulze V, Löhe D and
Vöhringer O: Influence of heating rate, cooling rate and numbers of
pulses on the microstructure of AISI 4140 after
short-time-hardening. Int J Mater Prod Technol. 24:2005. View Article : Google Scholar
|
|
36
|
Tian Q, Hu J, Zhu Y, Zou R, Chen Z, Yang
S, Li R, Su Q, Han Y and Liu X: Sub-10 nm Fe3O4@Cu(2-x)S core-shell
nanoparticles for dual-modal imaging and photothermal therapy. J Am
Chem Soc. 135:8571–8577. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Murali VS, Wang R, Mikoryak CA, Pantano P
and Draper RK: The impact of subcellular location on the near
infrared-mediated thermal ablation of cells by targeted carbon
nanotubes. Nanotechnology. 27:4251022016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Madani SY, Naderi N, Dissanayake O, Tan A
and Seifalian AM: A new era of cancer treatment: Carbon nanotubes
as drug delivery tools. Int J Nanomedicine. 6:2963–2979.
2011.PubMed/NCBI
|
|
39
|
Robinson JT, Tabakman SM, Liang Y, Wang H,
Casalongue HS, Vinh D and Dai H: Ultrasmall reduced graphene oxide
with high near-infrared absorbance for photothermal therapy. J Am
Chem Soc. 133:6825–6831. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang K, Wan J, Zhang S, Tian B, Zhang Y
and Liu Z: The influence of surface chemistry and size of nanoscale
graphene oxide on photothermal therapy of cancer using ultra-low
laser power. Biomaterials. 33:2206–2214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guo Y, Xu H, Li Y, Wu F, Li Y, Bao Y, Yan
X, Huang Z and Xu P: Hyaluronic acid and Arg-Gly-Asp peptide
modified Graphene oxide with dual receptor-targeting function for
cancer therapy. J Biomater Appl. 32:54–65. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu Z, Robinson JT, Sun X and Dai H:
PEGylated nanographene oxide for delivery of water-insoluble cancer
drugs. J Am Chem Soc. 130:10876–10877. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim H, Lee D, Kim J, Kim TI and Kim WJ:
Photothermally triggered cytosolic drug delivery via endosome
disruption using a functionalized reduced graphene oxide. ACS Nano.
7:6735–6746. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hu Z, Oleinick N and Hamblin MR:
Photodynamic therapy as an emerging treatment modality for cancer
and non-cancer diseases. J Anal Bioanal Tech. S1:e0012014.
View Article : Google Scholar
|
|
45
|
Cui S, Yin D, Chen Y, Di Y, Chen H, Ma Y,
Achilefu S and Gu Y: In vivo targeted deep-tissue photodynamic
therapy based on near-infrared light triggered upconversion
nanoconstruct. ACS Nano. 7:676–688. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ali SM and Olivo M: Mechanisms of action
of phenanthroperylenequinones in photodynamic therapy (review). Int
J Oncol. 22:1181–1191. 2003.PubMed/NCBI
|
|
47
|
Ge X, Liu J and Sun L: Controlled optical
characteristics of lanthanide doped upconversion nanoparticles for
emerging applications. Dalton Trans. 46:16729–16737. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li Y, Dong Y, Tuerxun·, Aidilibike, Liu X,
Guo J and Qin W: Growth phase diagram and upconversion luminescence
properties of NaLuF4: Yb3+/Tm3+/Gd3+ nanocrystals. RSC Adv.
7:44531–44536. 2017. View Article : Google Scholar
|
|
49
|
Wang F, Banerjee D, Liu Y, Chen X and Liu
X: Upconversion nanoparticles in biological labeling, imaging, and
therapy. Analyst. 135:1839–1854. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jin S, Zhou L, Gu Z, Tian G, Yan L, Ren W,
Yin W, Liu X, Zhang X, Hu Z and Zhao Y: A new near infrared
photosensitizing nanoplatform containing blue-emitting
up-conversion nanoparticles and hypocrellin A for photodynamic
therapy of cancer cells. Nanoscale. 5:11910–11918. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wei Y, Chen Q, Wu B, Zhou A and Xing D:
High-sensitivity in vivo imaging for tumors using a spectral
up-conversion nanoparticle NaYF4: Yb3+, Er3+ in cooperation with a
microtubulin inhibitor. Nanoscale. 4:3901–3909. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Qian HS, Guo HC, Ho PC, Mahendran R and
Zhang Y: Mesoporous-silica-coated up-conversion fluorescent
nanoparticles for photodynamic therapy. Small. 5:2285–2290. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chatterjee DK and Yong Z: Upconverting
nanoparticles as nanotransducers for photodynamic therapy in cancer
cells. Nanomedicine (Lond). 3:73–82. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Idris NM, Gnanasammandhan MK, Zhang J, Ho
PC, Mahendran R and Zhang Y: In vivo photodynamic therapy using
upconversion nanoparticles as remote-controlled nanotransducers.
Nat Med. 18:1580–1585. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang L, Yan R, Huo Z, Wang L, Zeng J, Bao
J, Wang X, Peng Q and Li Y: Fluorescence resonant energy transfer
biosensor based on upconversion-luminescent nanoparticles. Angew
Chem Int Ed Engl. 44:6054–6057. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xiong LQ, Chen ZG, Yu MX, Li FY, Liu C and
Huang CH: Synthesis, characterization, and in vivo targeted imaging
of amine-functionalized rare-earth up-converting nanophosphors.
Biomaterials. 30:5592–600. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhou J, Sun Y, Du X, Xiong L, Hu H and Li
F: Dual-modality in vivo imaging using rare-earth nanocrystals with
near-infrared to near-infrared (NIR-to-NIR) upconversion
luminescence and magnetic resonance properties. Biomaterials.
31:3287–3295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Shen X, He F, Wu J, Xu GQ, Yao SQ and Xu
QH: Enhanced two-photon singlet oxygen generation by
photosensitizer-doped conjugated polymer nanoparticles. Langmuir.
27:1739–1744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang Y, Yang G, Wang Y, Zhao Y, Jiang H,
Han Y and Yang P: Multiple imaging and excellent anticancer
efficiency of an upconverting nanocarrier mediated by single near
infrared light. Nanoscale. 9:4759–4769. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bakker MH, Lee CC, Meijer EW, Dankers PY
and Albertazzi L: Multicomponent supramolecular polymers as a
modular platform for intracellular delivery. ACS Nano.
10:1845–1852. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Dong R, Zhou Y, Huang X, Zhu X, Lu Y and
Shen J: Functional supramolecular polymers for biomedical
applications. Adv Mater. 27:498–526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Selvasekar CR, Birbeck N, McMillan T,
Wainwright M and Walker SJ: Photodynamic therapy and the alimentary
tract. Aliment Pharmacol Ther. 15:899–915. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Khorami HH: Preliminary study on porphyrin
derivatives as transfection reagents for mammalian cell.
Porphyrins-synthesis. Anim Cell Biotechnol. 2013.
|
|
64
|
Chen M and Scheer H: Extending the limits
of natural photosynthesis and implications for technical light
harvesting. J Porphyr Phthalocyanines. 17:1–15. 2013. View Article : Google Scholar
|
|
65
|
Stilts CE, Nelen MI, Hilmey DG, Davies SR,
Gollnick SO, Oseroff AR, Gibson SL, Hilf R and Detty MR:
Water-soluble, core-modified porphyrins as novel,
longer-wavelength-absorbing sensitizers for photodynamic therapy. J
Med Chem. 43:2403–2410. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cui L, Lin Q, Jin CS, Jiang W, Huang H,
Ding L, Muhanna N, Irish JC, Wang F, Chen J and Zheng G: A
pegylation-free biomimetic porphyrin nanoplatform for personalized
cancer theranostics. ACS Nano. 9:4484–4495. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Rossi F, Bedogni E, Bigi F, Rimoldi T,
Cristofolini L, Pinelli S, Alinovi R, Negri M, Dhanabalan SC,
Attolini G, et al: Porphyrin conjugated SiC/SiOx nanowires for
X-ray-excited photodynamic therapy. Sci Rep. 5:76062015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Brozek-Pluska B and Kopec M: Raman
microspectroscopy of Hematoporphyrins. Imaging of the noncancerous
and the cancerous human breast tissues with photosensitizers.
Spectrochim Acta A Mol Biomol Spectrosc. 169:182–191. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Huynh E and Zheng G: Porphysome
nanotechnology: A paradigm shift in lipid-based supramolecular
structures. Nano Today. 9:212–222. 2014. View Article : Google Scholar
|
|
70
|
Li H, Marotta DE, Kim S, Busch TM, Wileyto
EP and Zheng G: High payload delivery of optical imaging and
photodynamic therapy agents to tumors using
phthalocyanine-reconstituted low-density lipoprotein nanoparticles.
J Biomed Opt. 10:412032005. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Stefflova K, Chen J, Marotta D, Li H and
Zheng G: Photodynamic therapy agent with a built-in apoptosis
sensor for evaluating its own therapeutic outcome in situ. J Med
Chem. 49:3850–3856. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zheng G, Chen J, Li H and Glickson JD:
Rerouting lipoprotein nanoparticles to selected alternate receptors
for the targeted delivery of cancer diagnostic and therapeutic
agents. Proc Natl Acad Sci USA. 102:17757–17762. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zheng G, Chen J, Stefflova K, Jarvi M, Li
H and Wilson BC: Photodynamic molecular beacon as an activatable
photosensitizer based on protease-controlled singlet oxygen
quenching and activation. Proc Natl Acad Sci USA. 104:8989–8994.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lovell JF, Jin CS, Huynh E, Jin H, Kim C,
Rubinstein JL, Chan WC, Cao W, Wang LV and Zheng G: Porphysome
nanovesicles generated by porphyrin bilayers for use as multimodal
biophotonic contrast agents. Nat Mater. 10:324–332. 2011.
View Article : Google Scholar : PubMed/NCBI
|