Introduction
The reconstruction of large-sized bone defects,
caused by trauma, infection or tumor ablative surgery, remains a
significant clinical challenge and the existing approaches exhibit
multiple drawbacks including donor site morbidity, lack of
sufficient autografts, risk of disease transmission and immune
rejection (1,2). In the past few years a tremendous
effort has been made to improve bone tissue engineering methods to
overcome problems associated with existing grafts (1–3). It
is well known that stem cells, especially mesenchymal stem cells
(MSCs), have been used for bone tissue engineering for a
considerable amount of time and bone mesenchymal stem cells (BMSCs)
have been extensively investigated (3,4).
BMSCs can differentiate into adipocytes, chondrocytes and
osteocytes in different microenvironments and the appropriate
stimuli could enhance their ability of targeted differentiation
(5,6). However, the lineage commitment of
BMSCs is a tightly regulated and well-orchestrated complex event,
which has limited its clinical applications.
Multiple efforts have been devoted to modulate the
microenvironment supporting BMSC osteogenic differentiation,
including material surface functionalization by ECM analogues, drug
or growth factor administration, and sustained release of bioactive
molecules by gene modification, in which the well-controlled ROS
generation has been revealed to play an important role (7–10).
Substance P (SP), released predominantly by the peripheral
terminal, is a conserved undecapeptide and a member of the
tachykinin peptide family that acts as a sensory neurotransmitter
and neuromodulator. Similar to growth factors, increasing studies
have demonstrated that neuropeptides are critical for maintaining
tissue homeostasis, including SP (11,12).
Autophagy, an intracellular degradation and adaptive system, was
confirmed to play important roles in regulating intercellular ROS
levels and promoting neuropeptide function (13,14).
However, whether the interaction of SP and autophagy exists in
regulating the differentiation fate of BMSCs remains elusive.
In the present study, the profile of ROS generation
and autophagic activation was examined during BMSC osteogenic
differentiation and their roles in SP-promoted BMSC osteogenic
differentiation were further investigated. The results demonstrated
that both ROS level and autophagic activity were increased with
osteogenic induction. Furthermore, autophagic activity played an
important role in restricting the excessive ROS generation
mediating SP-enhanced BMSC osteogenic differentiation.
Materials and methods
Isolation and culture of rat
BMSCs
All animal experiments in this study were conducted
in accordance with the Guidelines for Animal Experimentation and
approved by the Ethics Committee of Xiangyang Central Hospital.
BMSCs were harvested from femora and tibiae of Sprague-Dawley rats
(six male, three months old, average weight 300 g), purchased from
the Laboratory Animal Center of Huazhong University of Science and
Technology (Wuhan, China). The rats had ad libitum access to
food and autoclaved water, and were housed at a constant
temperature (22–24°C), with 55% relative humidity, and a 12-h
light/dark cycle. All efforts were made to minimize suffering and
distress in this study. After being sacrificed by cervical
dislocation (six male), the rats were soaked in 75% alcohol for 5
min and the femora and tibiae were separated and the connective
tissue was removed. After cutting off both ends of the bones, the
bone marrow was flushed out from medullary cavities by a syringe
into complete culture media that consisted of Dulbecco's modified
Eagle's medium with F12 nutrient mixture with 15% fetal bovine
serum (FBS; both from Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.). The bone marrow suspension was then plated in 25 cm culture
bottles. The medium was changed every 2–3 days and cells were
passaged when 80% confluent.
BMSC identification and osteogenic
differentiation
After the second passage, the cells were collected
and identified using fluorescently-labeled antibodies for BMSC
markers: Allophycocyanin-conjugated CD29 (cat. no. 102225;
BioLegend, Inc.); FITC-conjugated CD44 (cat. no. 203906; BioLegend,
Inc.); FITC-conjugated CD45 (cat. no. 202205; BioLegend, Inc.); and
phycoerythrin-conjugated CD34 (ab223930; Abcam). Briefly, the cells
were incubated with corresponding antibodies for 30 min at 4°C in
the dark and then washed with PBS. The expression levels of
different cell surface markers were detected using FACSCalibur flow
cytometer and analyzed using CellQuestPro version 5.1.1 software
(BD Biosciences).
After detecting the purity, BMSCs from the second
passage were used in subsequent experiments. To induce osteogenic
differentiation, BMSCs were initially cultured in complete culture
media as aforementioned. When the cells reached 80% confluence,
osteogenic-inducing medium [α-MEM (Cyagen Biosciences) supplemented
with 10% FBS, 50 mg/ml ascorbate, 10 mM β-glycerophosphate, 100 nM
dexamethasone and 1% penicillin-streptomycin] was used. Lastly, the
plates were stained with alizarin red and alkaline phosphatase at
four time-points (0, 3, 7, and 14 days).
Treatment of rat BMSCs
Rat BMSCs were seeded on a 6-well culture plate.
When the culture confluence reached 80%, rat BMSCs were immediately
incubated with osteogenic-inducing medium with or without SP (10
nM), or pre-treated with 3-methyladenine (3-MA, 5 mM) and rapamycin
(100 nM) for 2 h.
Western blot analysis
The samples were lysed in cell lysis buffer for
western blotting and IP (Beyotime Institute of Biotechnology)
supplemented with protease inhibitors (Beyotime Institute of
Biotechnology), and the mixture was subjected to sonication at a
low frequency (20 kHz, 50W, for 36 sec on ice). After
centrifugation (at 13,500 × g for 10 min 4°C), the supernatant was
harvested and the protein concentration was assessed using a
bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology). Equal amounts of samples by weight (40 µg) from
each sample were electrophoresed on 10–20% SDS-PAGE and transferred
to nitrocellulose membranes (both from Bio-Rad Laboratories, Inc.).
After blocking in 5% bovine serum albumin (Beyotime Institute of
Biotechnology) for 2 h at room temperature, the membranes were
incubated overnight at 4°C with primary antibodies for Runx2
(1:800; cat. no. ab76956; Abcam) and Osteocalcin (1:500; cat. no.
ab13420; Abcam), LC3 (1:500; cat. no. 3868T; Cell Signaling
Technology, Inc.), p62 (1:1,000; cat. no. 23214S; Cell Signaling
Technology, Inc.), AMPK (1:800; cat. no. 2532S; Cell Signaling
Technology, Inc.), p-AMPK (1:1,000; cat. no. 4184S; Cell Signaling
Technology, Inc.), mTOR (1:800; cat. no. 2972S; Cell Signaling
Technology, Inc.), p-mTOR (1:1,000; cat. no. 5536T; Cell Signaling
Technology, Inc.) and GAPDH (1:1,000; cat. no. ab37168; Abcam).
After rinsing with Tris-buffered saline supplemented with Tween,
four times, the membranes were incubated with horseradish
peroxidase-conjugated secondary antibodies goat anti-mouse and goat
anti-rabbit immunoglobulin G (1:2,000; cat. no. SA00001-1 and cat.
no. SA00001-2, respectively; ProteinTech Group, Inc.) for 1.5 h at
room temperature. The protein bands were visualized using an ECL
kit (Beyotime Institute of Biotechnology). Protein quantification
was performed using Glyco Bandscan 5.0 software (ProZyme; Agilent
Technologies, Inc.).
ROS assay
The intracellular total ROS levels were assessed by
2′,7′-dichlorofluorescin diacetate (DCFH-DA; Beyotime Institute of
Biotechnology) staining, which can be rapidly oxidized to a highly
fluorescent compound. The samples were stained with 10 µM DCFH-DA
at 37°C for 20 min, evaluated using FACSCalibur flow cytometer and
analyzed using CellQuestPro version 5.1.1 software (BD
Biosciences).
Alkaline phosphatase staining
To observe the osteogenic differentiation, the
levels of alkaline phosphatase were examined by BCIP/NBT Alkaline
Phosphatase Color Development Kit (Beyotime Institute of
Biotechnology). The plates were washed twice with PBS and fixed at
room temperature with 4% paraformaldehyde for 5 min. The plates
were then washed three times with ddH2O, stained at room
temperature with BCIP/NBT working solution for 30 min, washed three
times with ddH2O, and visualized using a fluorescence
microscope (Olympus IX73; Olympus Corporation).
Alizarin Red staining
The mineralization of osteogenic induction was
detected by Alizarin red staining. The plates were washed twice
with PBS and fixed at room temperature with 4% formaldehyde for 10
min. The plates were then washed gently three times with
ddH2O, stained at room temperature with 1% Alizarin Red
(Sigma Aldrich) for 10 min, and visualized using a fluorescence
microscope (Olympus IX73; Olympus Corporation).
Statistical analysis
Data were analyzed using SPSS software, version 17.0
(SPSS, Inc.) and are reported as the mean ± standard deviation of
at least three independent experiments. The differences between two
groups were analyzed using a Student's t-test and comparisons among
multiple groups were performed using one-way analysis of variance
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Identification and characterization of
rat BMSCs
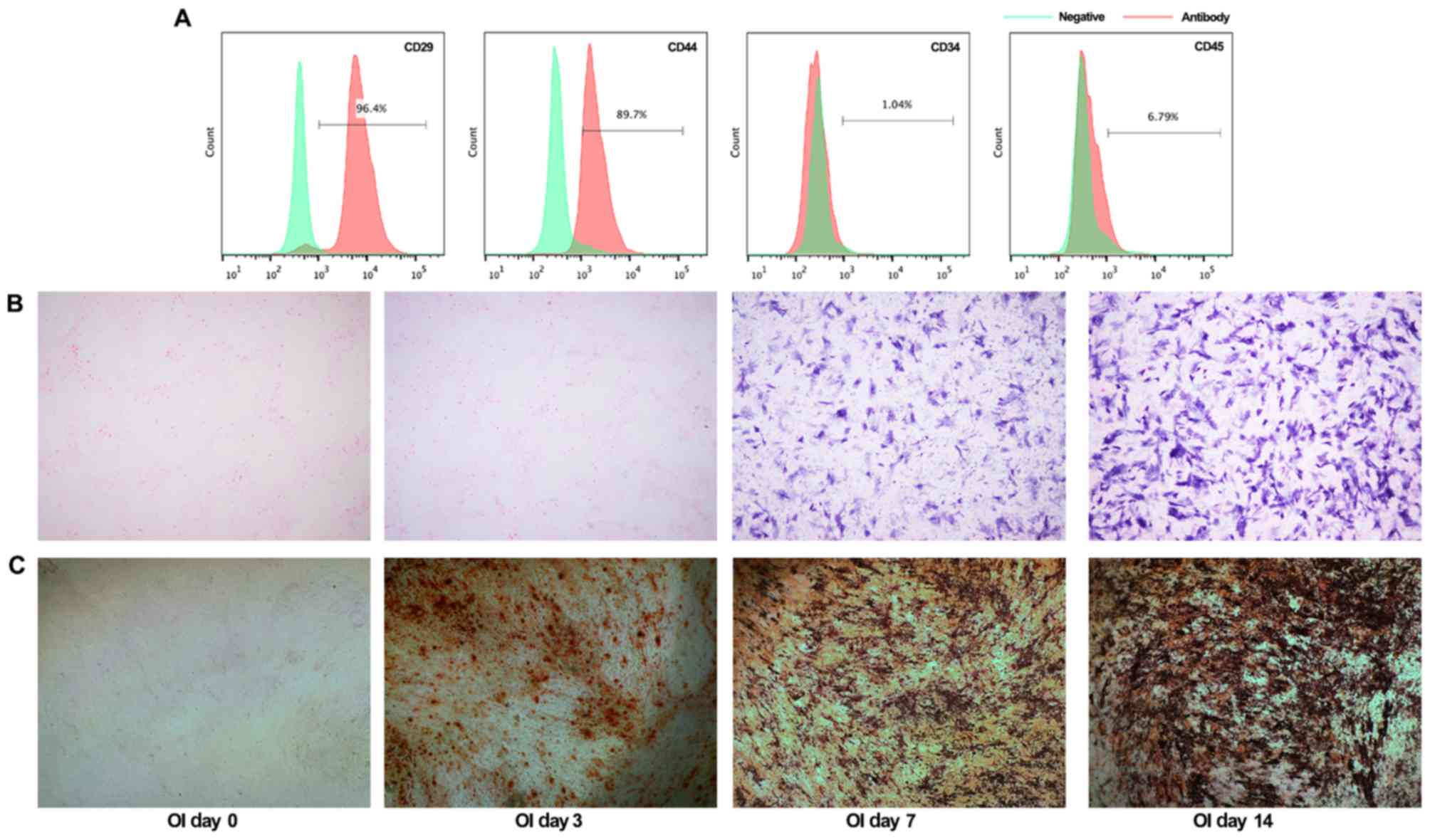
The BMSCs at second passage, were a homogenous
population and exhibited a spindle-shaped morphology. Furthermore,
BMSC phenotypes were identified using flow cytometric analysis. The
cells were positive for typical BMSC markers CD29 (96.4%) and CD44
(89.7%), with concomitant absence of CD34 (1.04%) and CD45 (6.79%)
(Fig. 1A). In addition, alkaline
phosphatase (ALP) staining and Alizarin red staining were performed
to detect osteogenic differentiation after culture with
osteogenic-inducing medium. As revealed in Fig. 1B, the NBT-formazan formation with
BCIP/NBT working solution staining was significantly increased at 7
and 14 days, indicating the ALP activity in BMSCs. Similarly, the
Alizarin Red staining results revealed that increased mineralized
nodules were formed along with the prolongation of
osteogenic-inducing term (Fig.
1C). Collectively, these data revealed the purity and
osteogenic ability of rat BMSCs isolated in our present
experiments.
Autophagy has a potential role in
reducing ROS production and promoting osteogenic
differentiation
While excessive ROS is deleterious to cell function,
moderate levels of ROS have been reported to participate in
regulating BMSC differentiation fate (15). It is well known that autophagy
plays an important role in maintaining cellular homeostasis,
including ROS generation. In the present study, we examined the
roles of autophagy and ROS in regulating BMSC differentiation fate.
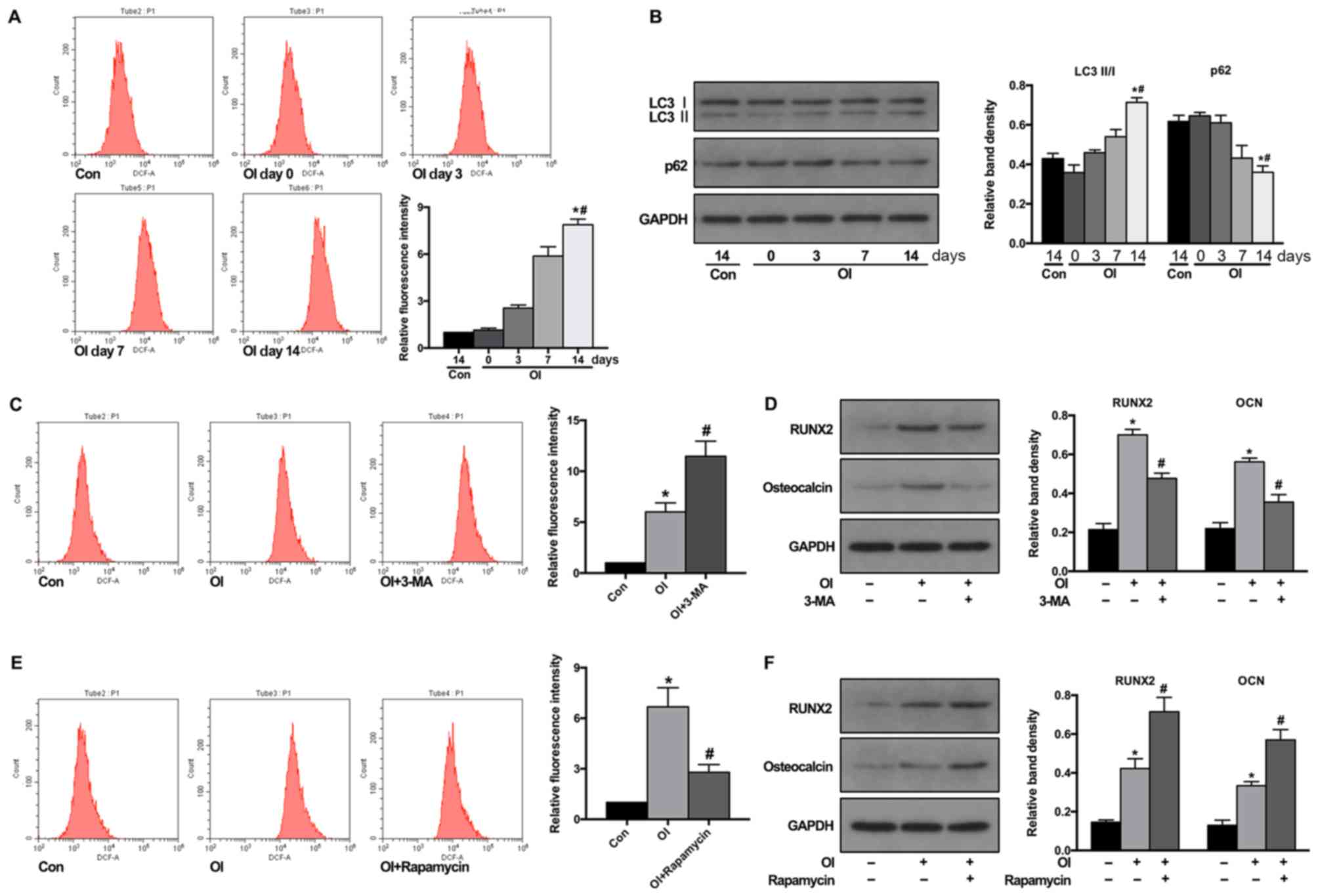
The ROS levels were first detected during osteogenic
differentiation. As anticipated, flow cytometric results revealed
that osteogenic-induction significantly increased the ROS level in
BMSCs (Fig. 2A). Next, whether
osteogenic induction could induce autophagy in BMSCs was examined.
LC3 II/LC3 I conversion is a well-known biomarker for indicating
the formation of autophagosomes and p62 for the degradation of
autolysosomes. As presented in Fig.
2B, the western blotting results revealed that osteogenic
induction significantly promoted the LC3 II conversion from LC3 I
and decreased the p62 levels, which indicated increased autophagic
activity.
To further examine whether autophagy participates in
regulating ROS production and the differentiation fate of BMSCs
during osteogenic-induction, BMSCs were pre-treated with autophagy
inhibitor 3-MA or activator rapamycin for 2 h. The results revealed
that pre-treatment with 3-MA prior to osteogenic induction
significantly increased ROS production in BMSCs (Fig. 2C) and decreased the protein levels
of Runx2 and osteocalcin, which are markers of osteogenesis
(Fig. 2D). Conversely,
pre-treatment with autophagy activator rapamycin attenuated ROS
production in BMSCs during osteogenic-induction (Fig. 2E) and further increased the protein
levels of Runx2 and osteocalcin (Fig.
2F). These results demonstrated that autophagic activity could
control the ROS level in BMSCs during osteogenic induction and
promote their osteogenic differentiation.
Substance P administration regulates
ROS generation in BMSCs via autophagic activation
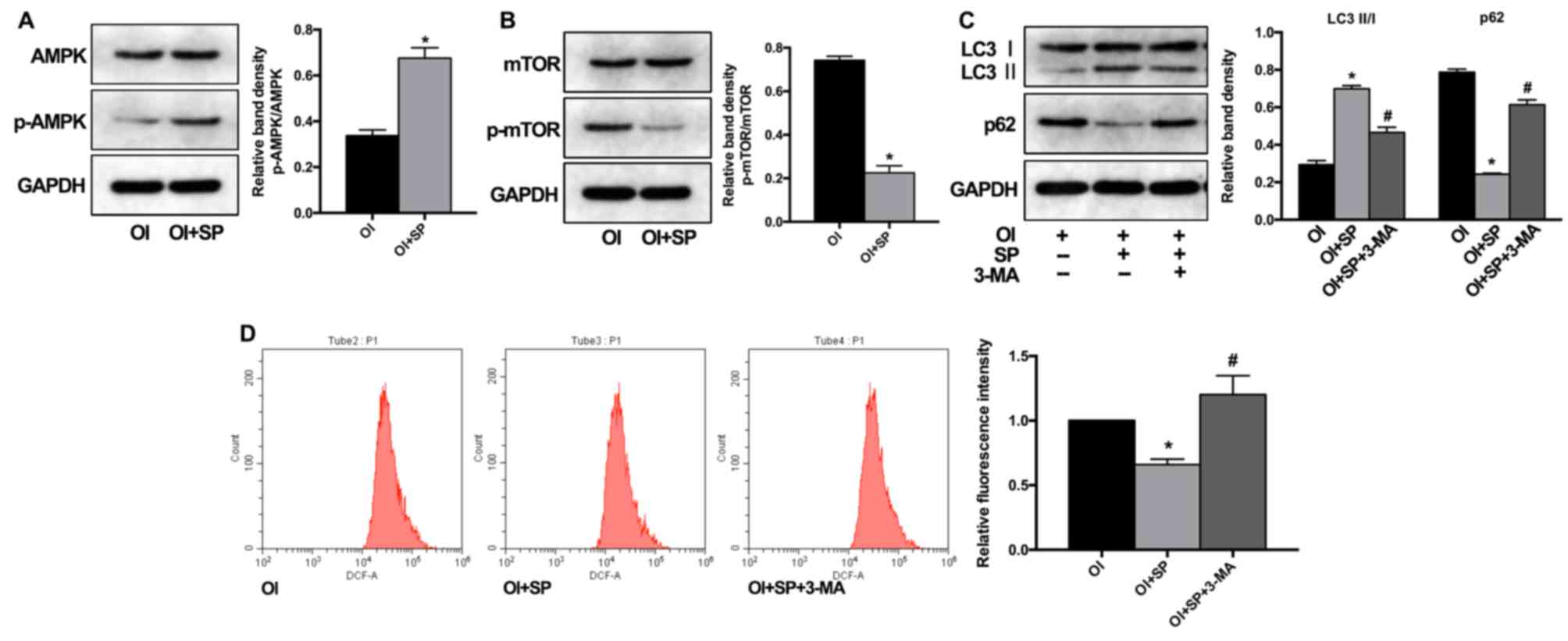
The neuropeptide SP has been demonstrated to have a
potentially protective role in multiple tissue disorders, partially
by regulating inflammatory response or ROS generation (16–18).
In the present study, the role of autophagy in these processes was
further investigated. As revealed in Fig. 3A and B, SP treatment significantly
increased the ratio of p-AMPK/AMPK and decreased the ratio of
p-mTOR/mTOR, which are classic mediators of autophagy activity
(19). In addition, the LC3 II/LC3
I ratio was also enhanced by SP treatment, along with p62
degradation (Fig. 3C). These
results demonstrated that SP could induce autophagy by promoting
AMPK and suppressing mTOR activation. To further investigate the
role of SP and autophagy interaction in ROS production during
osteogenic induction, BMSCs were pre-treated with (3-MA). As a
result, the autophagic activation induced by SP treatment was
effectively inhibited by 3-MA (Fig.
3C). Subsequently, it was observed that SP treatment had a
suppressive effect on ROS generation during BMSC osteogenic
induction, which could be attenuated along with autophagic
inhibition (Fig. 3D).
Collectively, these findings indicated that SP administration could
regulate ROS generation during BMSC differentiation by inducing
autophagic activity.
Substance P administration promotes
BMSC osteogenic differentiation via autophagic activation
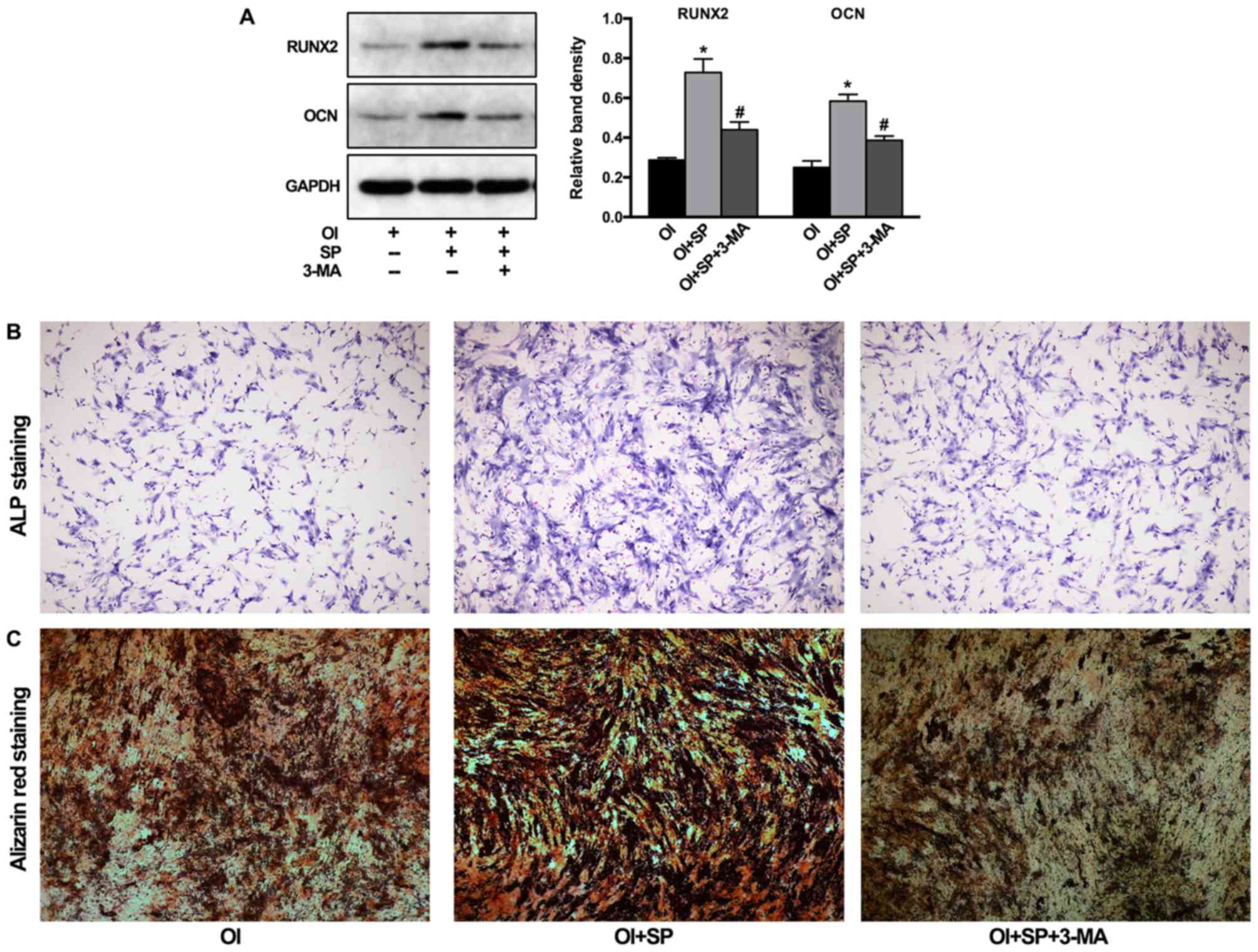
The effects of SP administration on BMSC
differentiation and the role of autophagy were further examined. As
revealed in Fig. 4A, the addition
of SP with osteogenic-inducing medium significantly increased the
protein levels of Runx2 and osteocalcin, which were attenuated by
3-MA pre-treatment. The results of ALP and Alizarin red staining
also confirmed that the enhanced osteogenic differentiation by the
addition of SP was decreased by 3-MA pre-treatment, visualized by
reduced formation of NBT-formazan and mineralized nodules (Fig. 4B).
Discussion
Bone tissue engineering and therapeutic strategies,
developed at the pre-clinical and clinical levels are largely
limited to treat small size defects based on novel functional
materials and are not capable of treating large-size and
compromised defects due to innate limitations in bone inductive
properties of the materials (20).
In view of this, biomolecule-based and cell-based strategies are
being continuously evaluated (3)
but are also particularly difficult to translate to clinical
applications due to complex mechanisms involved. The present study
demonstrated that autophagy played an important role in restricting
the excessive ROS generation and promoting BMSCs osteogenic
differentiation. Furthermore, it was revealed that the
administration of neuropeptide SP could enhance autophagic activity
and subsequently promote BMSC osteogenic differentiation.
Endogenous reactive oxygen species (ROS) are
produced as by-products of the mitochondrial electron transport
chain, and can be greatly elevated with increase in mitochondrial
respiration that is essential to meet the energy demand of
differentiating cells. ROS generation is however not just a
consequence of differentiation but plays a critical role in
governing the fate of stem cell differentiation (21,22).
Elevated ROS in BMSCs has been reported to promote adipogenesis,
and scavenging of the ROS restores the osteogenic capacity
(23). Notably in the present
study, an apparent increase of ROS levels was observed during
osteogenic-induction, which was an outcome contradictory to the
results reported in a previous study (24). It is considered that this
difference may mainly result from the addition of dexamethasone to
the osteogenesis-inducing medium (25,26).
This osteogenesis-inducing medium that could increase ROS levels,
mimicked most oxidative stress microenvironments that are
associated with bone-defect diseases (15,27),
which may provide a closer insight for investigating the regulatory
mechanisms of BMSC differentiation. Similarly, it has been reported
that the non-direct crosstalk between acute myeloid leukemia cells
and BMSCs mediated by the local cytokine network could alter the
global gene expression profile of BMSCs (28), which indicated that the
disease-specific microenvironments are worthy of more attention
when tissue engineering products are developed and used for
reconstructing bone defects.
Autophagy is a critical intracellular degradation
system for maintaining cellular homeostasis. It has been reported
that increased ROS levels stimulated autophagy that acted as a
feedback control for excessive ROS production (29). Similarly, in the present study, a
concomitant induction of autophagic activity was observed with
increased ROS levels and pharmacological activation/inhibition of
autophagy could attenuate/aggravate ROS generation. Furthermore, we
observed that autophagic activity was involved in the regulation of
BMSC osteogenic differentiation. Considering the importance of
redox status in stem cell differentiation, multiple studies have
emphasized the crucial role of well-orchestrated coordination of
cellular antioxidant systems (22,30,31),
which are not always in a favorable environment (32,33).
In the present study, it was observed that autophagy functioned
well as a candidate for maintaining redox homeostasis.
Growth factors, in increasing numbers, have forayed
into pre-clinical trials or clinical applications as a result of
extensive investigations (3).
Neuropeptides are also critical for maintaining tissue homeostasis
and SP has been demonstrated to have an osteogenic effect on BMSCs
(11,12,34),
whereas few studies have reported their clinical trials or
application due to the unclear working mechanisms. Previous studies
have demonstrated that autophagic activity was involved in
mediating the biological function of neuropeptides (13,14).
However, its role in SP function and their interaction in BMSC
differentiation has remained unclear. AMPK and mTOR are two classic
pathways that elicit a coordinated response to stimuli and
regulation of autophagic activity (19). In our present study, it was
revealed that SP administration increased the protein ratio of
p-AMPK/AMPK while reducing the protein ratio of p-mTOR/mTOR and
activated autophagy in BMSCs. Suppression of ROS generation
resulted in increased osteogenic differentiation. Furthermore,
pharmacological inhibition of autophagy ameliorated the osteogenic
effect of SP, indicating that SP could orchestrate autophagic
activity and thereby promote BMSC osteogenic differentiation.
However, the limitations that involved the
pharmacological modulation of autophagic activity cannot be
neglected. Multiple chemical agents that are currently available to
activate or inhibit autophagy have limited specificity for the
autophagic process, including 3-MA and rapamycin (35). Addressing these complex effects in
cell- and disease-specific models is key to developing clinical
strategies using autophagic modulators. Although the present study
revealed the autophagy-activated and osteogenic effects of SP
administration, more potential molecules that interact with SP and
the involved mechanisms require further in vivo
investigations in the future.
In summary, a well-regulated redox status is
involved in determining BMSC differentiation fate and autophagic
activity is essential for maintaining redox homeostasis.
Additionally, it was observed that SP administration promotes
autophagic activity by regulating the AMPK and mTOR pathways and
further enhances the osteogenic differentiation of BMSCs. Thus, we
not only propose the application of SP in bone tissue engineering
and other therapeutic strategies for bone-damaging diseases, but
also postulate a potential molecular mechanism by which SP imparts
its beneficial effects.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Scientific
Action Plans for the Prevention and Treatment of Major Diseases
National Health Commission of the People's Republic of China-Trauma
Repair (ZX-01-C2016156).
Availability of data and materials
All data used in the present study are included in
this published article.
Authors' contributions
WG and HMS designed the study protocol, conducted
experiments and wrote the first manuscript. XMZ, WT and YC helped
to design the study, and collected and analysed data. RCM,
contributed to study design, gave final approval of study protocol,
and extensively reviewed and revised manuscript. All authors read
and approved the manuscript and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
The animal experiments were performed following a
protocol approved by the Animal Experimentation Committee of
Xiangyang Central Hospital, Affiliated Hospital of Hubei University
of Arts and Science.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Petite H, Viateau V, Bensaïd W, Meunier A,
de Pollak C, Bourguignon M, Oudina K, Sedel L and Guillemin G:
Tissue-engineered bone regeneration. Nat Biotechnol. 18:959–963.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pobloth AM, Checa S, Razi H, Petersen A,
Weaver JC, Schmidt-Bleek K, Windolf M, Tatai AÁ, Roth CP, Schaser
KD, et al: Mechanobiologically optimized 3D titanium-mesh scaffolds
enhance bone regeneration in critical segmental defects in sheep.
Sci Transl Med. 10(pii): eaam88282018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ho-Shui-Ling A, Bolander J, Rustom LE,
Johnson AW, Luyten FP and Picart C: Bone regeneration strategies:
Engineered scaffolds, bioactive molecules and stem cells current
stage and future perspectives. Biomaterials. 180:143–162. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marolt D, Knezevic M and Novakovic GV:
Bone tissue engineering with human stem cells. Stem Cell Res Ther.
1:102010. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Man Z, Yin L, Shao Z, Zhang X, Hu X, Zhu
J, Dai L, Huang H, Yuan L, Zhou C, et al: The effects of
co-delivery of BMSC-affinity peptide and rhTGF-beta1 from coaxial
electrospun scaffolds on chondrogenic differentiation.
Biomaterials. 35:5250–5260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cai S, Tsui YP, Tam KW, Shea GK, Chang RS,
Ao Q, Shum DK and Chan YS: Directed differentiation of human bone
marrow stromal cells to Fate-committed schwann cells. Stem Cell
Reports. 9:1097–1108. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen W, Shen X, Hu Y, Xu K, Ran Q, Yu Y,
Dai L, Yuan Z, Huang L, Shen T and Cai K: Surface functionalization
of titanium implants with chitosan-catechol conjugate for
suppression of ROS-induced cells damage and improvement of
osteogenesis. Biomaterials. 114:82–96. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Z, Yuan X, Fernandes G, Dziak R,
Ionita CN, Li C, Wang C and Yang S: The combination of nano-calcium
sulfate/platelet rich plasma gel scaffold with BMP2 gene-modified
mesenchymal stem cells promotes bone regeneration in rat
critical-sized calvarial defects. Stem Cell Res Ther. 8:1222017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li J, Zhang J, Chen Y, Kawazoe N and Chen
G: TEMPO-conjugated gold nanoparticles for reactive oxygen species
scavenging and regulation of stem cell differentiation. ACS Appl
Mater Interfaces. 9:35683–35692. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kumar Y, Biswas T, Thacker G, Kanaujiya
JK, Kumar S, Shukla A, Khan K, Sanyal S, Chattopadhyay N,
Bandyopadhyay A and Trivedi AK: BMP signaling-driven osteogenesis
is critically dependent on Prdx-1 expression-mediated maintenance
of chondrocyte prehypetrophy. Free Radic Biol Med. 118:1–12. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dioufa N, Schally AV, Chatzistamou I,
Moustou E, Block NL, Owens GK, Papavassiliou AG and Kiaris H:
Acceleration of wound healing by growth hormone-releasing hormone
and its agonists. Proc Natl Acad Sci USA. 107:18611–18615. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lim JE, Chung E and Son Y: A neuropeptide,
Substance-P, directly induces tissue-repairing M2 like macrophages
by activating the PI3K/Akt/mTOR pathway even in the presence of
IFNγ. Sci Rep. 7:94172017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oh TS, Cho H, Cho JH, Yu SW and Kim EK:
Hypothalamic AMPK-induced autophagy increases food intake by
regulating NPY and POMC expression. Autophagy. 12:2009–2025. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aveleira CA, Botelho M, Carmo-Silva S,
Pascoal JF, Ferreira-Marques M, Nóbrega C, Cortes L, Valero J,
Sousa-Ferreira L, Álvaro AR, et al: Neuropeptide Y stimulates
autophagy in hypothalamic neurons. Proc Natl Acad Sci USA.
112:E1642–E1651. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Atashi F, Modarressi A and Pepper MS: The
role of reactive oxygen species in mesenchymal stem cell adipogenic
and osteogenic differentiation: A review. Stem Cells Dev.
24:1150–1163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hong HS and Son Y: Substance P ameliorates
collagen II-induced arthritis in mice via suppression of the
inflammatory response. Biochem Biophys Res Commun. 453:179–184.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim SJ, Kim JE, Kim SH, Kim SJ, Jeon SJ,
Kim SH and Jung Y: Therapeutic effects of neuropeptide substance P
coupled with self-assembled peptide nanofibers on the progression
of osteoarthritis in a rat model. Biomaterials. 74:119–130. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang L, Di G, Qi X, Qu M, Wang Y, Duan H,
Danielson P, Xie L and Zhou Q: Substance P promotes diabetic
corneal epithelial wound healing through molecular mechanisms
mediated via the neurokinin-1 receptor. Diabetes. 63:4262–4274.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Klionsky DJ, Abdelmohsen K, Abe A, Abedin
MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD,
Adeli K, et al: Guidelines for the use and interpretation of assays
for monitoring autophagy 3rd edition). Autophagy. 12:1–222. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roffi A, Krishnakumar GS, Gostynska N, Kon
E, Candrian C and Filardo G: The role of Three-dimensional
scaffolds in treating long bone defects: Evidence from preclinical
and clinical Literature-A systematic review. Biomed Res Int.
2017:80741782017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tormos KV, Anso E, Hamanaka RB, Eisenbart
J, Joseph J, Kalyanaraman B and Chandel NS: Mitochondrial complex
III ROS regulate adipocyte differentiation. Cell Metab. 14:537–544.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang K, Zhang T, Dong Q, Nice EC, Huang C
and Wei Y: Redox homeostasis: The linchpin in stem cell
self-renewal and differentiation. Cell Death Dis. 4:e5372013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen H, Liu X, Chen H, Cao J, Zhang L, Hu
X and Wang J: Role of SIRT1 and AMPK in mesenchymal stem cells
differentiation. Ageing Res Rev. 13:55–64. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen CT, Shih YR, Kuo TK, Lee OK and Wei
YH: Coordinated changes of mitochondrial biogenesis and antioxidant
enzymes during osteogenic differentiation of human mesenchymal stem
cells. Stem Cells. 26:960–968. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang S, Liu Y and Liang Q: Low-dose
dexamethasone affects osteoblast viability by inducing autophagy
via intracellular ROS. Mol Med Rep. 17:4307–4316. 2018.PubMed/NCBI
|
|
26
|
Shen C, Cai GQ, Peng JP and Chen XD:
Autophagy protects chondrocytes from glucocorticoids-induced
apoptosis via ROS/Akt/FOXO3 signaling. Osteoarthritis Cartilage.
23:2279–2287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang L, Zhao X, Wei BY, Liu Y, Ma XY, Wang
J, Cao PC, Zhang Y, Yan YB, Lei W and Feng YF: Insulin improves
osteogenesis of titanium implants under diabetic conditions by
inhibiting reactive oxygen species overproduction via the PI3K-Akt
pathway. Biochimie. 108:85–93. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reikvam H, Brenner AK, Hagen KM, Liseth K,
Skrede S, Hatfield KJ and Bruserud Ø: The cytokine-mediated
crosstalk between primary human acute myeloid cells and mesenchymal
stem cells alters the local cytokine network and the global gene
expression profile of the mesenchymal cells. Stem Cell Res.
15:530–541. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gómez-Puerto MC, Verhagen LP, Braat AK,
Lam EW, Coffer PJ and Lorenowicz MJ: Activation of autophagy by
FOXO3 regulates redox homeostasis during osteogenic
differentiation. Autophagy. 12:1804–1816. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim H, Lee YD, Kim HJ, Lee ZH and Kim HH:
SOD2 and Sirt3 control osteoclastogenesis by regulating
mitochondrial ROS. J Bone Miner Res. 32:397–406. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y, Chen G, Yan J, Chen X, He F, Zhu
C, Zhang J, Lin J, Pan G, Yu J, et al: Upregulation of SIRT1 by
kartogenin enhances antioxidant functions and promotes osteogenesis
in human mesenchymal stem cells. Oxid Med Cell Longev.
2018:13681422018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liao L, Su X, Yang X, Hu C, Li B, Lv Y,
Shuai Y, Jing H, Deng Z and Jin Y: TNF-α inhibits FoxO1 by
upregulating miR-705 to aggravate oxidative damage in bone
marrow-derived mesenchymal stem cells during osteoporosis. Stem
Cells. 34:1054–1067. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Abnosi MH and Yari S: The toxic effect of
gallic acid on biochemical factors, viability and proliferation of
rat bone marrow mesenchymal stem cells was compensated by boric
acid. J Trace Elem Med Biol. 48:246–253. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fu S, Mei G, Wang Z, Zou ZL, Liu S, Pei
GX, Bi L and Jin D: Neuropeptide substance P improves osteoblastic
and angiogenic differentiation capacity of bone marrow stem cells
in vitro. Biomed Res Int. 2014:5960232014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Galluzzi L, Bravo-San Pedro JM, Levine B,
Green DR and Kroemer G: Pharmaological modulation of autophagy:
Therapeutic potential and persisting obstacles. Nat Rev Drug
Discov. 16:487–511. 2017. View Article : Google Scholar : PubMed/NCBI
|