Introduction
Acute myocardial infarction (MI) is a common cause
of chronic heart failure (HF), which is associated with impaired
quality of life and unfavorable long-term outcomes in patients
(1). Although
renin-angiotensin-aldosterone inhibitors, β-blockers, statins and
antiplatelet inhibitors have been widely used in the treatment of
HF, the risks of morbidity and mortality in these patients remain
high (1). A comprehensive
understanding of the pathogenesis and mechanisms underlying HF is
therefore crucial to develop novel therapeutic strategies for this
disorder.
Increasing evidence reported that gut microbes have
a crucial role in the pathogenesis of numerous cardiovascular
diseases including HF, thrombosis and atherosclerosis (2,3).
Trimethylamine N-oxide (TMAO), which is a gut-microbiota-derived
metabolite produced from dietary constituents, has emerged as a key
contributor to cardiovascular diseases (3). Dietary choline and
phosphatidylcholine (4) can be
metabolized to trimethylamine (TMA) by the gut microbiota.
Subsequently, TMA is rapidly converted to TMAO by enzymes from the
flavin monooxygenase (FMO) family, notably FMO3 in the liver. Under
physiological conditions, the kidneys rapidly clear circulating
TMAO via urinary excretion (4).
Changes in the composition of the intestinal microbiota known as
dysbiosis, or impaired renal function can lead to increased TMAO
synthesis (5,6). Clinical studies have reported an
association between increased circulating TMAO and increased risk
of adverse cardiovascular outcomes, including heart attack, stroke
and risk of mortality (2,3,7).
Experimental studies demonstrated that increased circulating TMAO
worsens pressure-overload-induced HF in mice (8), induces cardiac hypertrophy and
fibrosis in rats with transverse aortic constriction (9), and contributes to cardiac dysfunction
in mice with Western-diet-induced obesity (10). In addition, circulating TMAO is
elevated in patients hospitalized with MI and is associated with
poorer prognosis, but not with Global Registry of Acute Coronary
Events scores or other biomarkers of coronary artery disease,
including copeptin and natriuretic peptide, proenkephalin,
mid-regional proadrenomedullin and pro-substance P (7). Elevated circulating TMAO has been
suggested to contribute to cardiac dysfunction and fibrosis by
promoting the inflammatory response in high-fat-diet-induced
obesity (10).
MI is associated with systemic and cardiac
inflammation, as evidenced by increased proinflammatory cytokines
and chemokines (11). Although the
inflammatory response is critical for proper infarct healing,
excessive inflammation has been associated with progressive HF and
adverse outcomes (11). Recent
studies reported that circulating interleukin (IL)-8, which is a
prototypical chemokine primarily involved in the recruitment and
activation of neutrophils and monocytes, is elevated and associated
with large infarct size, impaired recovery of cardiac function and
adverse clinical outcome in patients with MI (12,13).
The biological effects of IL-8 are mediated by two the cell surface
receptors CXC chemokine receptor (CXCR)1 and CXCR2 (14).
The present study examined whether reductions in
circulating TMAO could improve heart function in rats with
MI-induced HF, and whether this potential effect could be
associated with decreased IL-8 secretion.
Materials and methods
Animals
A total of 36 male Sprague-Dawley rats (age, 6
weeks; weight, 200–250 g) were obtained from the Beijing Laboratory
Animal Research Center. All were housed at a controlled
temperature, under a 12-h light/dark cycle, and had access to
standard rat chow and water that were provided ad libitum.
All experimental procedures were approved by the Animal Care and
Welfare Committee of the Jining No. 1 People's Hospital.
Protocol
The rats underwent ligation of the left descending
coronary artery to induce MI or a sham operation (SHAM). One day
following MI or sham surgery, echocardiography was performed to
assess infarct size and cardiac function; 4 rats succumbed within
24 h of MI. Animals that survived after 24 h of MI and sham animals
were assigned to the following groups: i) MI rats treated with
vehicle (VEH; MI + VEH; n=9); ii) MI rats treated with the TMA
formation inhibitor 3,3-dimethyl-1-butanol (DMB; MI + DMB; n=7);
iii) sham rats treated with vehicle (SHAM + VEH; n=8); and iv) sham
rats treated with DMB (SHAM + DMB; n=8). These animals were treated
with either VEH (tap water) or 1.0% DMB (Sigma-Aldrich; Merck KGaA)
in drinking water for 8 weeks. The dose of DMB used in the present
study has been demonstrated to effectively inhibit TMA formation
and reduce plasma TMAO levels in rats (15). At termination, rats were weighed
and cardiac function of each animal was assessed by a second
echocardiography and left ventricle (LV) catheterization for
measurement of LV hemodynamics (16). The animals were then sacrificed,
plasma samples were collected from trunk blood by centrifugation at
1,500 × g for 15 min at 4°C, and subsequently stored at −80°C for
biochemical analysis. The heart and lungs were quickly removed,
blotted and weighed. The heart was frozen in liquid nitrogen and
stored at −80°C for molecular analysis. The lungs were weighed to
calculate the ratio of wet lung to body weight as an index of
pulmonary congestion in heart failure. The lungs were not
frozen.
Induction of MI
MI was induced by ligation of the left anterior
descending coronary artery as previously described (17). Briefly, rats were anesthetized with
a mixture of ketamine and xylazine [100:10 mg/kg; intraperitoneal
(ip)], intubated, ventilated with a rodent respirator, and laid on
a heating pad to maintain their body temperature at 37°C. The heart
was exposed via a left intercostal thoracotomy through the fourth
intercostal space and the pericardium was removed to expose the
left anterior descending coronary artery, which was ligated with a
5-0 polypropylene suture ~2 mm under the tip of the left atrium.
Successful ligation of the coronary artery was confirmed by a
sudden discoloration of the LV anterior wall. Sham animals were
subjected to the same procedure without ligation of the coronary
artery. The chest cavity was then closed and rats were allowed to
recover from the anesthesia.
Echocardiography
Transthoracic echocardiography was performed to
assess infarct size and cardiac function using a Sonos 5500R
ultrasonograph system (Philips Medical Systems) equipped with a 15
MHz-linear-array transducer, as previously described (18,19).
Rats were anesthetized in an induction chamber with 3% isoflurane
and maintained with 1.5% isoflurane during echocardiography
examinations, as previously described (20). Anesthetic depth was monitored by
testing the response to toe pinching, and ensuring that animals had
no retraction of the leg or withdrawing of the foot (21). Two-dimensional echocardiograms were
obtained from the parasternal long-axis and short-axis views of the
LV at the level of the papillary muscle tips. Two-dimensionally
targeted M-mode echocardiograms were applied to measure LV
dimensions in systole and diastole. All echocardiographic data were
recorded with a magneto-optical disk for off-line analysis. The
thickness of LV anterior and posterior walls and the LV dimensions
at end cardiac diastole and systole were measured in a blinded
manner, and the average of the three measurements for each
parameter was used. Left ventricular end systolic volume (LVESV),
left ventricular end diastolic volume (LVEDV), left ventricular
fractional shortening (LVFS), left ventricular ejection fraction
(LVEF) and left ventricular ischemic area (LVIA) were calculated as
previously described (18).
Cardiac hemodynamic measurements
LV hemodynamics were measured using a Millar
Microtip catheter (Millar Inc.) following catheterization of the
right carotid artery. Briefly, rats were anesthetized with a
mixture of ketamine and xylazine (100:10 mg/kg; ip). The right
carotid artery was exposed and the Millar microtip catheter was
inserted into the right carotid artery and advanced into the LV.
After stabilization for 15 min, the heart rate, LV peak systolic
pressure (LVSP), LV end-diastolic pressure (LVEDP), and
positive(+)/negative(−) change in pressure over time (dP/dt) were
recorded.
Western blotting
The non-infarct LV tissue samples from MI rats and
LV tissues samples from the sham rats were homogenized in RIPA
lysis buffer (R0278, Sigma-Aldrich; Merck KGaA) containing 0.1%
protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA). Protein
concentrations were measured with the Pierce bicinchoninic acid
protein assay (Thermo Fisher Scientific, Inc.). Equal amounts of
protein (30 µg) were separated on 12% SDS-polyacrylamide gels and
transferred onto polyvinylidene difluoride membranes (EMD
Millipore, Temecula, CA, USA). Membranes were blocked with 5% milk
in Tris-buffered saline containing 0.1% Tween-20 for 1 h at room
temperature. Membranes were then incubated overnight at 4°C with
rabbit polyclonal antibody against IL-8 (ab7747, 1:200; Abcam),
rabbit polyclonal antibodies against IL-8 receptors CXC chemokine
receptor 1 (CXCR1, ab14936, 1:200; Abcam) and CXCR2 (ab14935,
1:200; Abcam), and mouse monoclonal antibody against β-actin
(sc47778, 1:1,000; Santa Cruz Biotechnology, Inc.). Membranes were
washed with Tris-buffered saline containing 0.1% Tween-20 and
incubated with a mouse anti-rabbit horseradish
peroxidase-conjugated secondary antibody (sc-2357, 1:5,000; Santa
Cruz Biotechnology, Inc.) and a donkey anti-mouse horseradish
peroxidase-conjugated secondary antibody (sc-2318, 1:5,000; Santa
Cruz Biotechnology, Inc.) for 1 h at 4°C. Immunoreactive bands were
visualized with an enhanced chemiluminescence detection system (GE
Healthcare,), and band densities were analyzed using ImageJ
software version 1.49 (National Institutes of Health). Each band
was normalized to β-actin.
Circulating TMAO and IL-8
measurements
Plasma TMAO levels were determined using an liquid
chromatography-mass spectrometry (LC/MS) method that employed an
Agilent 6120 LC/MS model mass spectrometer (Agilent Technologies,
Inc.) and Phenomenex Kinetex Biphenyl Column (Phenomenex, Inc.) as
previously described (10). Plasma
IL-8 levels were measured using a rat IL-8 ELISA kit (MBS7606869,
MyBioSource, Inc.).
Statistical analysis
All data are expressed as the means ± standard error
of the mean. All samples were analyzed in duplicate. A two-way
analysis of variance followed by a Bonferroni's post hoc test was
applied for statistical analysis using GraphPad Prism 7 (GraphPad
Software, Inc.). Spearman's correlation analysis was used to
determine the correlation between circulating TMAO and IL-8 levels.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of MI and DMB treatment on
circulating TMAO levels
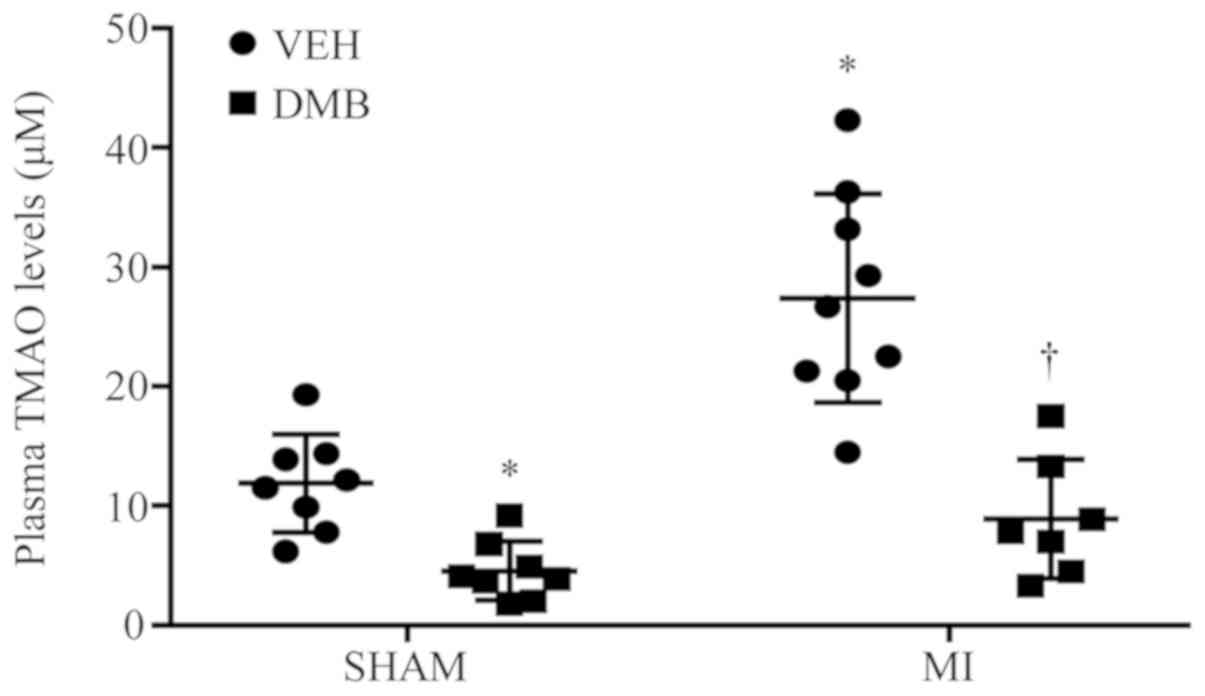
As presented in Fig.
1, at 8 weeks following coronary ligation (CL), MI + VEH rats
exhibited markedly increases in plasma TMAO levels compared with
SHAM + VEH rats. Furthermore, DMB treatment significantly reduced
plasma TMAO levels not only in MI rats, but also in sham rats.
Effects of MI and DMB treatment on
echocardiographic parameters
Echocardiographic assessment 1 day following CL
confirmed MI, as indicated by the LVIA in the two groups of MI
rats. LVIA was similar for the two MI groups 1 day after CL, but
was notably unaltered in the MI group 8 weeks following CL
(Fig. 2B). No significant
differences in LVESV (Fig. 2C),
LVEDV (Fig. 2D), LVFS (Fig. 2E) and LVEF (Fig. 2F) were observed between SHAM + VEH
and SHAM + DMB rats throughout the experimental protocol. Compared
with the two groups of sham rats, the two groups of MI rats
exhibited significant increases in LVESV and LVEDV and decreases in
LVFS and LVEF both at 1 day and 8 weeks following CL. There were no
significant differences in any of the echocardiographic parameters
between the two MI groups 1 day after CL. In addition, 8 weeks
after CL, MI + VEH rats, but not MI + DMB rats, presented further
significant increases in LVESV (P<0.01) and LVEDV (P<0.01),
and decreases in LVFS (P<0.05) and LVEF (P<0.05), compared
with those at 1 day after CL. Notably, LVESV and LVEDV were
significantly lower, while LVFS and LVEF were significantly higher
in MI + DMB rats compared with MI + VEH rats 8 weeks following
CL.
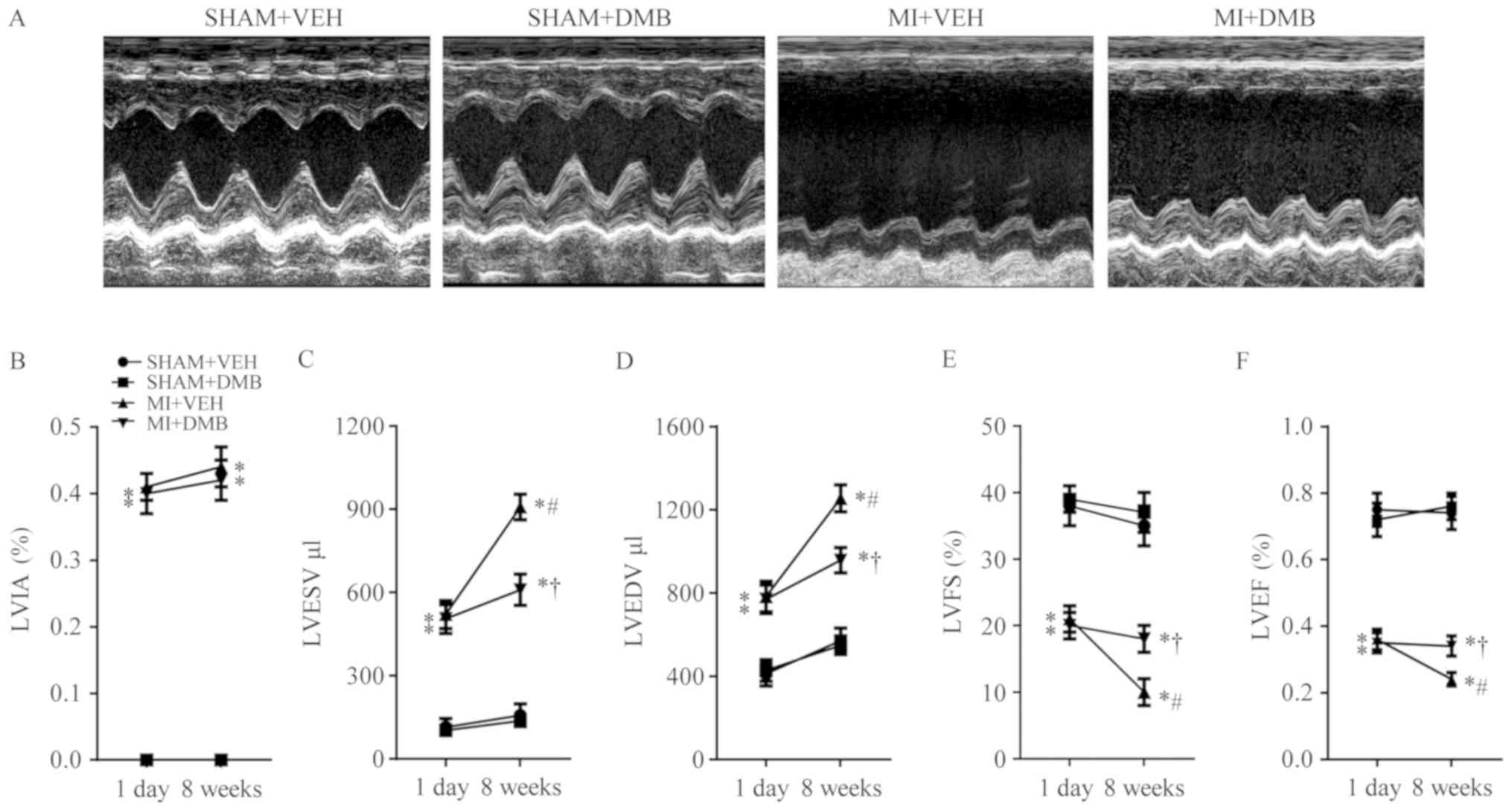 | Figure 2.Effects of MI and DMB treatment on
echocardiographic parameters. (A) Representative M-mode
echocardiographic recordings from each group at 8 weeks following
MI. (B) The percentage of LVIA remained similar at 8 weeks
following MI in DMB-treated and VEH-treated MI rats. Notably, (C)
LVESV and (D) LVEDV further increased, while (E) LVFS and (F) LVEF
decreased further in VEH-treated MI rats but not in DMB-treated MI
rats 8 weeks after MI. Data are expressed as the means ± standard
error of the mean (n=7–9 for each group). *P<0.05 vs. SHAM + VEH
at the same time-point; †P<0.05, MI + DMB vs. MI +
VEH at the same time-point; #P<0.05, 8 weeks vs. 1
day at the same group. DMB, 3,3-Dimethyl-1-butanol; MI, myocardial
infarction; VEH, vehicle; LVEF, left ventricular ejection fraction;
LVEDV, left ventricular end diastolic volume; LVESV, left
ventricular end systolic volume; LVFS, left ventricular fractional
shortening; LVIA, left ventricular ischemic area. |
Effects of MI and DMB treatment on
cardiac hypertrophy, lung congestion and LV hemodynamics
Body weight was similar across the four experimental
groups (Fig. 3A) 8 weeks following
CL. Compared with the SHAM + VEH rats, MI + VEH rats had
significantly increased heart weight/body weight (Fig. 3B) and lung weight/body weight
(Fig. 3C) ratios. MI + DMB rats
had significantly lower heart weight/body weight and lung
weight/body weight ratios compared with MI + VEH rats. DMB
treatment had no significant effects on heart weight/body weight
and lung weight/body weight ratios in the sham rats.
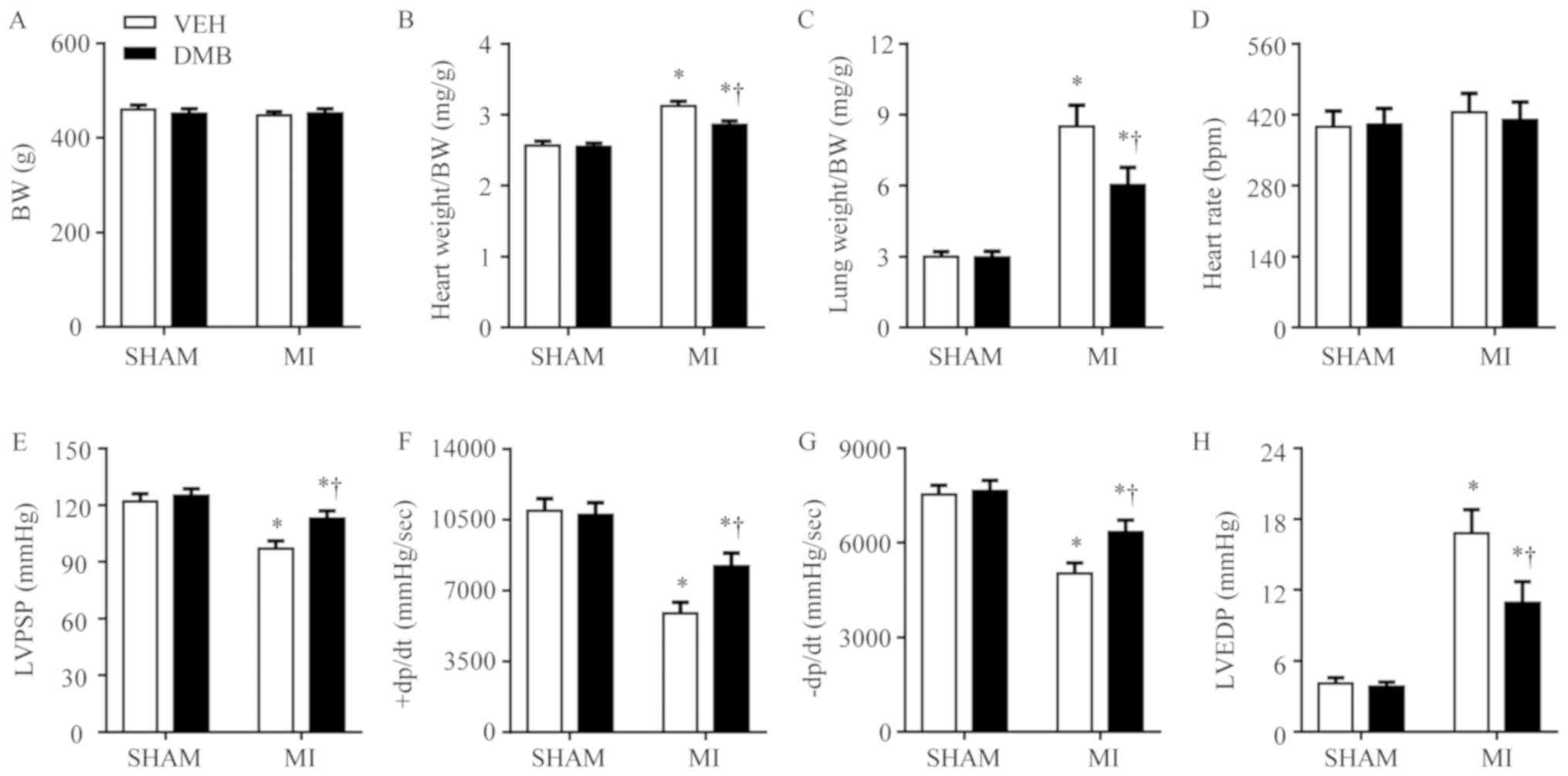 | Figure 3.Effects of MI and DMB treatment on
cardiac hypertrophy, lung congestion and LV hemodynamics. At 8
weeks following MI, (A) BW and (D) heart rate were similar in all
groups. However, (B) heart weight/BW and (C) lung weight/BW ratios,
and (H) LVEDP increased significantly, whereas (E) LVPSP, (F)
+dP/dt, and (G) -dP/dt further decreased in VEH-treated MI rats
than in VEH-treated SHAM rats. The factors observed in VEH-treated
MI rats had improved in DMB-treated MI rats. Data are expressed as
the means ± standard error of the mean (n=7–9 for each group).
*P<0.05 vs. SHAM + VEH; †P<0.05, MI + DMB vs. MI +
VEH. +dP/dt, positive change in pressure over time; -dP/dt,
negative change in pressure over time; BW, body weight; DMB,
3,3-dimethyl-1-butanol; LVEF, left ventricular ejection fraction;
LVEDV, left ventricular end diastolic volume; LVESV, left
ventricular end systolic volume; LVFS, left ventricular fractional
shortening; LVIA, left ventricular ischemic area; LVPSP, left
ventricular peak systolic pressure; MI, myocardial infarction; VEH,
vehicle. |
LV hemodynamics analyses revealed no notable
differences in heart rate across the four experimental groups
(Fig. 3D). LVPSP (Fig. 3E), maximum positive rate of LV
developed pressure (+LVdP/dt) (Fig.
3F) and maximum negative rate of LV developed pressure
(-LVdP/dt) (Fig. 3G) were
significantly lower, whereas LVEDP (Fig. 3H) was higher in MI + VEH rats
compared with SHAM + VEH rats. Compared with MI + VEH rats, MI +
DMB rats had significantly increased LVPSP, +LVdP/dt, and -LVdP/dt,
and decreased LVEDP. In particular, DMB treatment had no effect on
any of the hemodynamic parameters in sham rats.
Effects of MI and DMB treatment on
circulating IL-8 levels
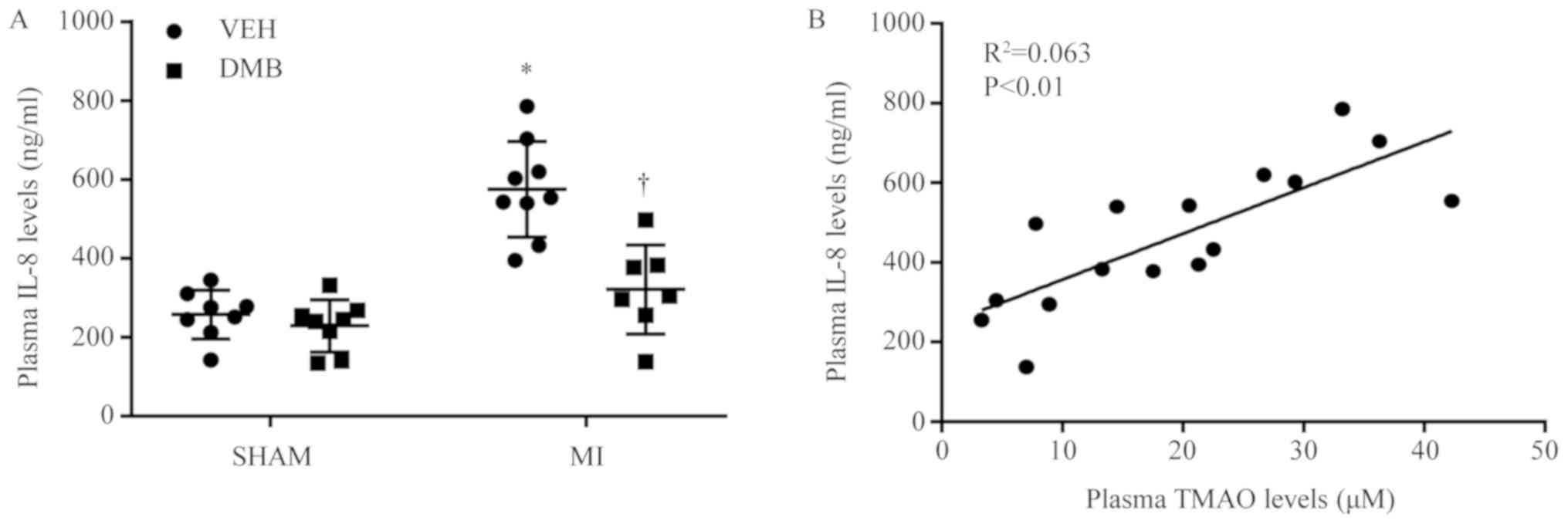
Compared with SHAM + VEH rats, MI + VEH rats had
significantly elevated plasma IL-8 levels 8 weeks following CL
(Fig. 4A). Plasma IL-8 levels were
restored near to the SHAM + VEH level following treatment with DMB
in MI rats. No differences in plasma IL-8 levels were observed
between SHAM + DMB and SHAM + VEH rats. In addition, elevated
plasma IL-8 levels were positively correlated with increased plasma
TMAO levels in MI rats (Fig.
4B).
Effects of MI and DMB treatment on
expression of cardiac IL-8 and its receptors
The expression levels of IL-8 (Fig. 5A and B) and its receptors CXCR1
(Fig. 5A and C) and CXCR2
(Fig. 5A and D) were significantly
increased in the non-infarct LV of MI + VEH rats compared with the
LV of SHAM + VEH rats; however, the expression of these proteins
was normalized by DMB treatment. DMB treatment did not alter the
expression of IL-8 (Fig. 5A and
B), CXCR1 (Fig. 5A and C), and
CXCR2 (Fig. 5A and D) in the LV of
the sham rats.
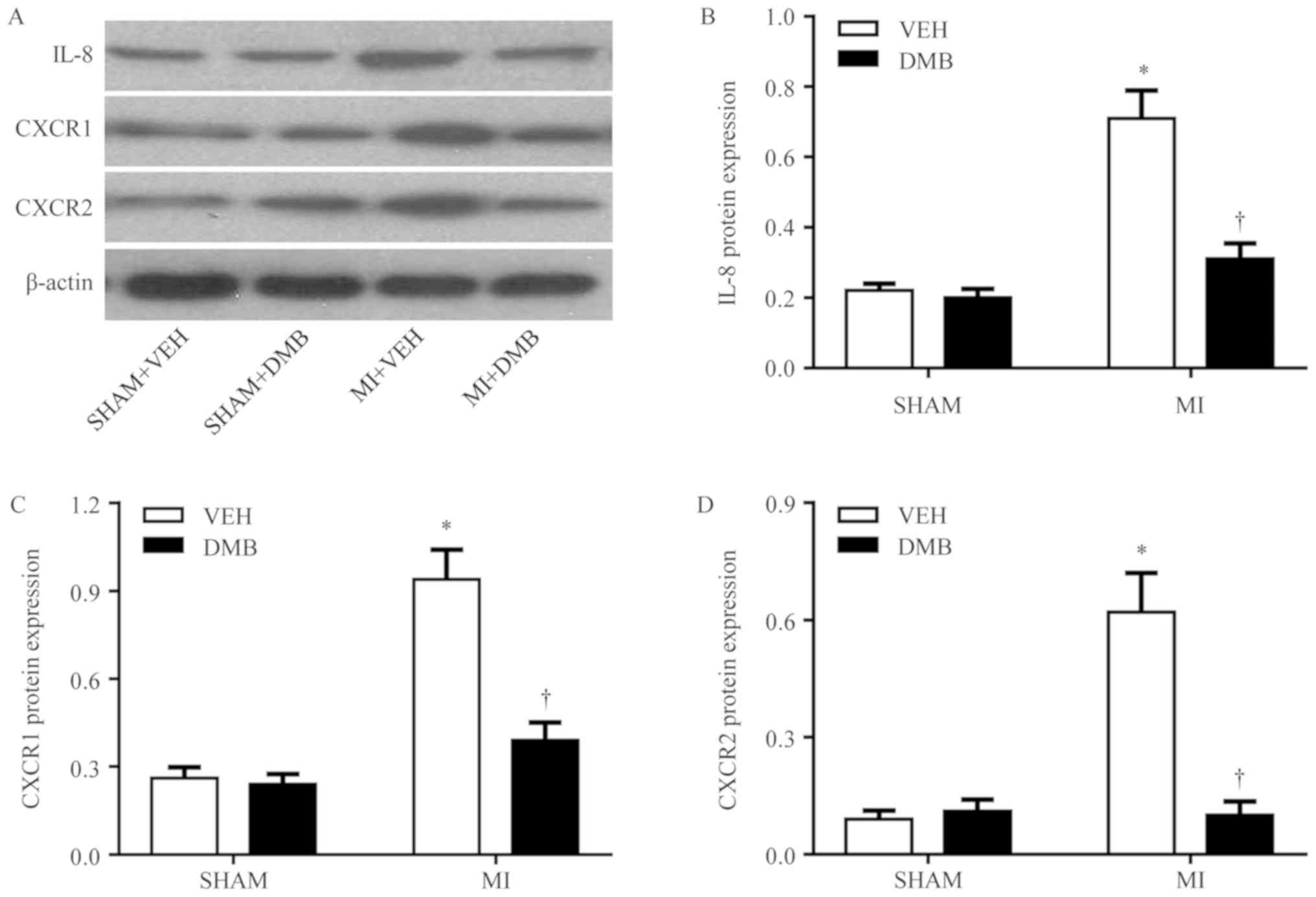 | Figure 5.Effects of MI and DMB treatment on
cardiac expression of IL-8 and its receptors CXCR1 and CXCR2. (A)
Representative western blot for each group. The expression of (B)
IL-8, and its receptors (C) CXCR1 and (D) CXCR2 was significantly
increased in the non-infarct LV of MI + VEH rats, compared within
the LV of SHAM + VEH rats; however, expression was normalized upon
DMB treatment. Data are expressed as the means ± standard error of
the mean (n=7–9 for each group). *P<0.05 vs. SHAM + VEH;
†P<0.05, MI + DMB vs. MI + VEH. CXCR, C-X-C motif
chemokine receptor; DMB, 3,3-dimethyl-1-butanol; IL, interleukin;
MI, myocardial infarction; TMAO, trimethylamine N-oxide; VEH,
vehicle. |
Discussion
The results from the present study demonstrated that
treatment with DMB reduced the plasma levels of TMAO, and prevented
the deterioration of cardiac remodeling and function, and lung
congestion in rats following MI. Furthermore, reductions in
circulating TMAO may have normalized the circulating levels of
IL-8, and cardiac expression of IL-8 and its receptors in rats
following MI. In addition, elevated circulating levels of IL-8 were
positively correlated with corresponding increases in circulating
levels of TMAO in rats following MI. Taken together, these results
indicated that inhibition of TMAO synthesis may attenuate the
development of HF following MI in rats, which may result from IL-8
downregulation.
Increasing evidence from experimental and clinical
studies (2,8,22,23)
revealed that TMAO could be an important contributor to the
pathogenesis of cardiovascular diseases, including HF (3). For example, increased circulating
level of TMAO has been identified as a prognostic biomarker in
patients with systolic HF (2).
Transverse aortic constriction in mice fed diets supplemented with
either choline or TMAO induces more severe pulmonary edema, cardiac
enlargement and LV ejection fraction (8). A large single-center study that
examined the incremental prognostic value of circulating TMAO
levels in patients with stable chronic systolic HF reported a
positive correlation between the levels of TMAO and B-type
natriuretic peptide (BNP), and negative correlation between TMAO
levels and the estimated glomerular filtration rate (eGFR)
(22). In addition, elevated
circulating TMAO levels indicated increased long-term mortality
independent of traditional biomarkers of risk in the HF population,
including BNP and eGFR (22).
Furthermore, a previous study that analyzed the predictive value of
TMAO in patients with acute decompensated HF reported that
circulating TMAO levels correlated with in-hospital mortality when
combined with clinical risk scores that include adjustments for
renal function (23). The results
from the present study reported that MI + VEH rats exhibited
cardiac hypertrophy, lung congestion, left ventricular remodeling
and impaired cardiac function 8 weeks following CL, according to
anatomical analysis, echocardiography and left ventricular
hemodynamics. These data indicated that rats could develop chronic
HF after MI. Furthermore, circulating TMAO levels were markedly
higher in MI + VEH rats compared with SHAM + VEH rats, which was
consistent with previous studies reporting that circulating TMAO
levels are increased in patients with MI or chronic HF (2,7). The
increase in circulating TMAO levels may be due to alterations in
gut microbiota composition, which has previously been reported in
mice following MI (24), or to
MI-induced cardiorenal syndrome that reduces the renal capacity for
excreting TMAO (5).
Reductions in circulating TMAO has been reported to
attenuate the development of dietary choline-enhanced
atherosclerosis (25), reduce the
risk of thrombosis (26), and
prevent cardiac dysfunction in mice fed with a Western diet
(10). However, whether reductions
in circulating TMAO have a beneficial effect on MI-induced HF
remains unknown. The present study employed DMB as a drug that
inhibits the formation of TMA from the gut microbiota; MI + DMB
rats had reduced plasma TMAO levels compared with MI + VEH rats,
which was accompanied with significant improvements in chronic HF
manifestations. These observations demonstrated that reductions in
circulating TMAO may ameliorate the development of chronic HF in
rats following MI.
MI is commonly accompanied with systemic and cardiac
inflammation, which is characterized by increases in the production
of proinflammatory cytokines and chemokines (11,27,28).
In addition, the inflammatory response serves a crucial role in the
development of chronic HF (11,29).
It has been suggested that increased TMAO can contribute to the
pathogenesis of numerous cardiovascular diseases, including
obesity-induced cardiac dysfunction and aging-associated
endothelial dysfunction by promoting inflammation (10,15).
IL-8, a prototypical chemokine primarily involved in the
recruitment and activation of neutrophils and monocytes, has
recently been reported to serve a crucial role in regulating
cardiac inflammation following MI (12). IL-8 is highly secreted by
macrophages, endothelial cells and smooth muscle cells. In
addition, circulating levels of IL-8 and cardiac expression of IL-8
and its receptors are increased in animal and human models of MI
(13,30–32).
Recent studies revealed that upregulated IL-8 expression is
associated with large infarct size, impaired recovery of LV
function and adverse clinical outcome in patients with MI (12,13);
however, attenuations in IL-8 production via treatment with
pterostilbene or a neutralizing monoclonal antibody is associated
with decreased infarct size, and reduced myocardial apoptosis and
necrosis in animals following MI (33,34).
To further determine how reductions in circulating TMAO can
ameliorate MI-induced HF, the present study investigated the levels
of circulating IL-8, and the cardiac expression of IL-8 and its
receptors. Our results demonstrated that MI + VEH rats exhibited
significant increases in circulating levels of IL-8, and in the
cardiac expression of IL-8, CXCR1 and CXCR2, which was consistent
with previous studies (13,30–32).
In particular, reductions in circulating TMAO by DMB treatment led
to decreases in circulating IL-8, and cardiac expression of IL-8,
CXCR1 and CXCR2 in HF rats. Furthermore, circulating TMAO was
positively correlated with circulating IL-8 in HF rats. These
results indicated that the effects of reducing circulating TMAO on
MI-induced chronic HF may be mediated by IL-8 inhibition. Previous
studies have demonstrated that IL-8 release is mediated by the
proinflammatory cytokine IL-17 (35,36).
This cytokine dose-dependently increases IL-8 production in smooth
muscle cells (36). Furthermore,
IL-17 stimulation results in the activation of NF-κB and MAP
kinases, including p44/42 ERK, JNK and p38 MAP kinase in smooth
muscle cells (35). Blockade of
NF-κB or three MAP kinase pathways (ERK, JNK and p38 MAP kinase)
reduces IL-8 synthesis by smooth muscle cells (35). These observations indicate that
IL-17 induces IL-8 release via NF-κB and three MAP kinase pathways
(ERK, JNK and p38 MAP kinase). It has been demonstrated that
alterations in gut microbiota composition can induce IL-17
production (37). It is therefore
possible that alterations in gut microbiota composition in rats
following MI may lead to increases in circulating TMAO, which could
cause IL-8 release via regulation of IL-17. In the present study,
DMB treatment of MI rats inhibited TMAO synthesis, and normalized
plasma levels of IL-8 and cardiac expression of IL-8 and its
receptors. However, the cardiac functions of rats with MI-induced
HF improved only partially, which suggested that other mechanisms,
may also be involved in the pathogenesis of MI-induced HF. In
addition, DMB treatment of sham rats reduced the plasma levels of
TMAO, but had no effect on IL-8 plasma levels, and the expression
of IL-8, CXCR1 and CXCR2 or cardiac functions. Conversely,
increased circulating levels of TMAO could induce inflammatory
response in the heart, which may contribute to the development of
HF following MI.
As a limitation of this study, it should be noted
that the baseline plasma levels of TMAO and IL-8, and the LV
hemodynamics in all rats were not determined prior to and shortly
following MI induction or sham operation. Only measurements at 8
weeks post-MI induction were determined.
In conclusion, the present study demonstrated that
reductions in circulating TMAO ameliorated the development of
chronic HF in rats following MI, and that this beneficial effect
may result from inhibiting IL-8 synthesis. The results from this
study suggested that blocking the formation of the
gut-microbiota-derived metabolite TMAO may be considered as a novel
therapeutic approach to prevent or treat chronic HF in patients
following MI.
Acknowledgements
Not applicable.
Funding
The present study was supported by Jining No. 1
People's Hospital.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL, YS and JW conceived and designed the present
study. XL, YS and XZ performed the experiments. XL, YS and XZ
analyzed the data. XL, YS and JW wrote the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures were approved by the
Animal Care and Welfare Committee of the Jining First People's
Hospital.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hartman MH, Groot HE, Leach IM, Karper JC
and van der Harst P: Translational overview of cytokine inhibition
in acute myocardial infarction and chronic heart failure. Trends
Cardiovasc Med. 28:369–379. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Albert CL and Tang WH: Metabolic
biomarkers in heart failure. Heart Fail Clin. 14:109–118. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Subramaniam S and Fletcher C:
Trimethylamine N-oxide: Breathe new life. Br J Pharmacol.
175:1344–1353. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Velasquez MT, Ramezani A, Manal A and Raj
DS: Trimethylamine N-oxide: The good, the bad and the unknown.
Toxins (Basel). 8:E3262016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kitai T, Kirsop J and Tang WH: Exploring
the microbiome in heart failure. Curr Heart Fail Rep. 13:103–109.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu KY, Xia GH, Lu JQ, Chen MX, Zhen X,
Wang S, You C, Nie J, Zhou HW and Yin J: Impaired renal function
and dysbiosis of gut microbiota contribute to increased
trimethylamine-N-oxide in chronic kidney disease patients. Sci Rep.
7:14452017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suzuki T, Heaney LM, Jones DJ and Ng LL:
Trimethylamine N-oxide and risk stratification after acute
myocardial infarction. Clin Chem. 63:420–428. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Organ CL, Otsuka H, Bhushan S, Wang Z,
Bradley J, Trivedi R, Polhemus DJ, Tang WH, Wu Y, Hazen SL and
Lefer DJ: Choline diet and its gut microbe-derived metabolite,
trimethylamine N-oxide, exacerbate pressure overload-induced heart
failure. Circ Heart Fail. 9:e0023142016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Z, Wu Z, Yan J, Liu H, Liu Q, Deng Y,
Ou C and Chen M: Gut microbe-derived metabolite trimethylamine
N-oxide induces cardiac hypertrophy and fibrosis. Lab Invest.
99:346–357. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen K, Zheng X, Feng M, Li D and Zhang H:
Gut microbiota-dependent metabolite trimethylamine N-oxide
contributes to cardiac dysfunction in western diet-induced obese
mice. Front Physiol. 8:1392017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Westman PC, Lipinski MJ, Luger D, Waksman
R, Bonow RO, Wu E and Epstein SE: Inflammation as a driver of
adverse left ventricular remodeling after acute myocardial
infarction. J Am Coll Cardiol. 67:2050–2060. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shetelig C, Limalanathan S, Hoffmann P,
Seljeflot I, Gran JM, Eritsland J and Andersen GØ: Association of
IL-8 with infarct size and clinical outcomes in patients with
STEMI. J Am Coll Cardiol. 72:187–198. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zarrouk-Mahjoub S, Zaghdoudi M, Amira Z,
Chebi H, Khabouchi N, Finsterer J, Mechmeche R and Ghazouani E:
Pro- and anti-inflammatory cytokines in post-infarction left
ventricular remodeling. Int J Cardiol. 221:632–636. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Apostolakis S, Vogiatzi K, Amanatidou V
and Spandidos DA: Interleukin 8 and cardiovascular disease.
Cardiovasc Res. 84:353–360. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li T, Chen Y, Gua C and Li X: Elevated
circulating trimethylamine N-oxide levels contribute to endothelial
dysfunction in aged rats through vascular inflammation and
Oxidative stress. Front Physiol. 8:3502017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pacher P, Liaudet L, Mabley JG, Cziráki A,
Haskó G and Szabó C: Beneficial effects of a novel ultrapotent
poly(ADP-ribose) polymerase inhibitor in murine models of heart
failure. Int J Mol Med. 17:369–375. 2006.PubMed/NCBI
|
|
17
|
Hua Y, Chen H, Zhao X, Liu M, Jin W, Yan
W, Wu Y, Tan Z, Fan H, Wu Y, et al: Alda1, an aldehyde
dehydrogenase-2 agonist, improves longterm survival in rats with
chronic heart failure following myocardial infarction. Mol Med Rep.
18:3159–3166. 2018.PubMed/NCBI
|
|
18
|
Ram R, Mickelsen DM, Theodoropoulos C and
Blaxall BC: New approaches in small animal echocardiography:
Imaging the sounds of silence. Am J Physiol Heart Circ Physiol.
301:H1765–H1780. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iliopoulou I, Mourouzis I, Lambrou GI,
Iliopoulou D, Koutsouris DD and Pantos C: Timedependent and
independent effects of thyroid hormone administration following
myocardial infarction in rats. Mol Med Rep. 18:864–876.
2018.PubMed/NCBI
|
|
20
|
Morgan EE, Casabianca AB, Khouri SJ and
Kalinoski AL: In vivo assessment of arterial stiffness in the
isoflurane anesthetized spontaneously hypertensive rat. Cardiovasc
Ultrasound. 12:372014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zambricki EA and Dalecy LG: Rat sex
differences in anesthesia. Comp Med. 54:49–53. 2004.PubMed/NCBI
|
|
22
|
Tang WH, Wang Z, Fan Y, Levison B, Hazen
JE, Donahue LM, Wu Y and Hazen SL: Prognostic value of elevated
levels of intestinal microbe-generated metabolite
trimethylamine-N-oxide in patients with heart failure: Refining the
gut hypothesis. J Am Coll Cardiol. 64:1908–1914. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suzuki T, Heaney LM, Bhandari SS, Jones DJ
and Ng LL: Trimethylamine N-oxide and prognosis in acute heart
failure. Heart. 102:841–848. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Z, Liu HY, Zhou H, Zhan Q, Lai W, Zeng
Q, Ren H and Xu D: Moderate-intensity exercise affects gut
microbiome composition and influences cardiac function in
myocardial infarction mice. Front Microbiol. 8:16872017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Z, Roberts AB, Buffa JA, Levison BS,
Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, et al:
Non-lethal inhibition of gut microbial trimethylamine Production
for the treatment of atherosclerosis. Cell. 163:1585–1595. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roberts AB, Gu X, Buffa JA, Hurd AG, Wang
Z, Zhu W, Gupta N, Skye SM, Cody DB, Levison BS, et al: Development
of a gut microbe-targeted nonlethal therapeutic to inhibit
thrombosis potential. Nat Med. 24:1407–1417. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gong X, Zhou R and Li Q: Effects of
captopril and valsartan on ventricular remodeling and inflammatory
cytokines after interventional therapy for AMI. Exp Ther Med.
16:3579–3583. 2018.PubMed/NCBI
|
|
28
|
Meng C, Guo Z, Li D, Li H, He J, Wen D and
Luo B: Preventive effect of hesperidin modulates inflammatory
responses and antioxidant status following acute myocardial
infarction through the expression of PPAR-γ and Bcl-2 in model
mice. Mol Med Rep. 17:1261–1268. 2018.PubMed/NCBI
|
|
29
|
Na D, Aijie H, Bo L, Zhilin M and Long Y:
Gambogic acid exerts cardioprotective effects in a rat model of
acute myocardial infarction through inhibition of inflammation,
iNOS and NF-κB/p38 pathway. Exp Ther Med. 15:1742–1748.
2018.PubMed/NCBI
|
|
30
|
Lu L, Wei P, Cao Y, Zhang Q, Liu M, Liu
XD, Wang ZL and Zhang PY: Effect of total peony glucoside
pretreatment on NF-κB and ICAM-1 expression in myocardial tissue of
rat with myocardial ischemia-reperfusion injury. Genet Mol Res.
15:2016. View Article : Google Scholar :
|
|
31
|
Kukielka GL, Smith CW, LaRosa GJ, Manning
AM, Mendoza LH, Daly TJ, Hughes BJ, Youker KA, Hawkins HK, Michael
LH, et al: Interleukin-8 gene induction in the myocardium after
ischemia and reperfusion in vivo. J Clin Invest. 95:89–103. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Damås JK, Eiken HG, Oie E, Bjerkeli V,
Yndestad A, Ueland T, Tonnessen T, Geiran OR, Aass H, Simonsen S,
et al: Myocardial expression of CC- and CXC-chemokines and their
receptors in human end-stage heart failure. Cardiovasc Res.
47:778–787. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hu L, Cai N and Jia H: Pterostilbene
attenuates myocardial ischemia-reperfusion injury via the
phosphatidylinositol 3′-kinase-protein kinase B signaling pathway.
Exp Ther Med. 14:5509–5514. 2017.PubMed/NCBI
|
|
34
|
Boyle EM Jr, Kovacich JC, Hèbert CA, Canty
TG Jr, Chi E, Morgan EN, Pohlman TH and Verrier ED: Inhibition of
interleukin-8 blocks myocardial ischemia-reperfusion injury. J
Thorac Cardiovasc Surg. 116:114–121. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wuyts WA, Vanaudenaerde BM, Dupont LJ, Van
Raemdonck DE, Demedts MG and Verleden GM: Interleukin-17-induced
interleukin-8 release in human airway smooth muscle cells: Role for
mitogen-activated kinases and nuclear factor-kappaB. J Heart Lung
Transplant. 24:875–881. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vanaudenaerde BM, Wuyts WA, Dupont LJ, Van
Raemdonck DE, Demedts MM and Verleden GM: Interleukin-17 stimulates
release of interleukin-8 by human airway smooth muscle cells in
vitro: A potential role for interleukin-17 and airway smooth muscle
cells in bronchiolitis obliterans syndrome. J Heart Lung
Transplant. 22:1280–1283. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tedesco D, Thapa M, Chin CY, Ge Y, Gong M,
Li J, Gumber S, Speck P, Elrod EJ, Burd EM, et al: Alterations in
intestinal microbiota lead to production of interleukin 17 by
intrahepatic γδ T-cell receptor-positive cells and pathogenesis of
cholestatic liver disease. Gastroenterology. 154:2178–2193. 2018.
View Article : Google Scholar : PubMed/NCBI
|