Introduction
Back pain-associated health care and psychosocial
problems are costly, placing a major burden on modern societies
(1); disc degeneration is regarded
as one of the primary contributors to back pain (2). Due to convenience and efficiency,
intradiscal injection of local anesthetics has been frequently used
for the diagnostic block and treatment of discogenic back pain.
Notably, the use of local anesthetics in managing patients who are
unwilling to receive surgery has markedly increased over the past
decade (3).
Clinically, bupivacaine has long been used in
intradiscal injections to control back pain symptoms (4,5).
Based on the mechanisms of relieving inflammation (6) and inhibiting the sensitization of
nerve endings in degenerative discs (7), the therapeutic effects of bupivacaine
on back pain have been well documented in clinical settings;
however, certain negative effects of bupivacaine have been reported
in vitro, particularly with regard to its cytotoxicity
towards intervertebral disc (IVD) cells (8). This observation was first
demonstrated by Quero et al (9), who reported that IVD cells exposed to
bupivacaine at clinically administered concentrations exhibit
decreased cellular viability. A subsequent study suggested that
these negative effects are dose- and time-dependent (10); however, the exact mechanisms
underlying the cytotoxicity of bupivacaine towards IVD cells remain
unclear.
Autophagy is an evolutionarily conserved process
that degrades cytoplasmic proteins and organelles. Under normal
conditions, basal autophagy is essential to maintain cellular
homeostasis by sequestrating damaged materials (11). Additionally, autophagy is activated
by harmful cellular conditions. Studies have demonstrated that
autophagic activation may be protective or harmful to cells under
stressful conditions (12,13); however, to the best of our
knowledge, the role of autophagy in bupivacaine-treated IVD cells
is yet to be determined.
Mammalian target of rapamycin (mTOR) protein is an
important contributor to autophagic induction, and is involved in
regulating the phosphorylation and activation of protein kinase B
(Akt) (14). It has previously
been illustrated that autophagy is negatively regulated by the
Akt/mTOR/S6 kinase (S6K) signaling pathway (15). Additionally, it was reported that
bupivacaine suppresses Akt and S6K activation in cells (13,16),
suggesting a potential function for bupivacaine in mediating
autophagy; however, the effects of bupivacaine on autophagic
activity in IVD cells via Akt/mTOR/S6K signaling remain
unclear.
In the present study, human IVD cells were treated
with bupivacaine at clinically relevant concentrations, and the
dose- and time-dependent cytotoxic effects of bupivacaine were
characterized. Subsequently, the potential roles of autophagy and
the Akt/mTOR/S6K signaling pathway were investigated. Furthermore,
the association between autophagy and apoptosis in IVD cells was
investigated to provide novel insight into the cytotoxic mechanisms
of bupivacaine.
Materials and methods
Human nucleus pulposus (NP) isolation
and culture
Human IVDs were collected from 10 patients (7 male
and 3 female patients; age, 33–55 years old) at the First
Affiliated Hospital, College of Medicine, Zhejiang University
(Hangzhou, China) during percutaneous lumbar discectomy between
June 2016 and October 2017 (Table
I). The inclusion criteria for the study were as follows: i)
Patients were diagnosed with lumbar disc herniation on the basis of
clinical symptoms and MRI findings; ii) the absence of a history of
lumbar spine disease, such as lumbar trauma or infection; and iii)
the absence of lumbar spondylolisthesis or scoliosis. Disc
degeneration was graded in patients using the Pfirrmann
classification system (17). This
study was approved by the Ethics Committee of the First Affiliated
Hospital, College of Medicine, Zhejiang University. Written
informed consent was obtained from all patients. The obtained IVDs
were washed in normal saline and then minced with a scalpel.
Freshly prepared digesting solution containing Dulbecco's Modified
Eagle's medium (DMEM; Sigma-Aldrich; Merck KGaA), 5% fetal bovine
serum (FBS; Gibco, Thermo Fisher Scientific, Inc.) and a
combination of 0.05% collagenase type II and type IV
(Sigma-Aldrich; Merck KGaA) was used for enzymatic digestion for 15
min at room temperature. Subsequently, IVDs were collected by
centrifugation at 250 × g for 5 min, and suspended and seeded in
DMEM supplemented with 10% FBS and 1% gentamicin at 37°C in a
humidified atmosphere containing 5% CO2. Primary
generation IVD cells at a confluence of 80–90% were used for all
experiments.
 | Table I.Patient data. |
Table I.
Patient data.
| Patient number | Sex | Age (years) | Grade | Disc level |
|---|
| 1 | M | 54 | IV | L4/L5 |
| 2 | M | 52 | IV | L5/S1 |
| 3 | F | 43 | III | L4/L5 |
| 4 | F | 49 | III | L3/L4 |
| 5 | M | 51 | IV | L3/L4 |
| 6 | F | 33 | II | L4/L5 |
| 7 | M | 41 | III | L3/L4 |
| 8 | M | 47 | IV | L3/L4 |
| 9 | M | 55 | IV | L4/L5 |
| 10 | M | 37 | III | L4/L5 |
IVD cellular viability assay
IVD cellular viability was determined using an MTT
assay (Beyotime Institute of Biotechnology) according to a standard
protocol. Briefly, IVD cells (4×106/ml) were seeded in
24-well plates and cultured with 0.9% saline (control group), and
0.25% (8 mM) and 0.5% (17.5 mM) bupivacaine for 2, 6 and 12 h at
room temperature. As 3-methyladenine (3-MA; Sigma-Aldrich; Merck
KGaA) inhibits phosphatidylinositol 3-kinase to suppress autophagy
by inhibiting autophagosome formation (18), 3-MA (4 mM) was applied to IVD cells
at room temperature (with or without 8 or 17.5 mM bupivacaine) to
investigate the role of autophagy in IVD cellular viability. At the
appropriate time points following treatment, cellular viability was
monitored using MTT (0.5 mg/ml) at 37°C for 4 h. The formed
crystals were solubilized using dimethyl sulfoxide (Beyotime
Institute of Biotechnology) and quantified by spectrophotometry at
570 nm wavelength.
Transmission electron microscopy
For electron microscopy, IVD cells were fixed with
2.5% glutaraldehyde in 0.1 M phosphate buffer overnight at room
temperature, and then incubated in 1% osmium tetroxide and 2%
uranyl acetate for an additional 1 h at room temperature. Following
dehydration in a graded alcohol series, samples were embedded into
Araldite (Sigma-Aldrich; Merck KGaA) and cut into 1-µm sections,
which were stained with toluidine blue at room temperature for 1
min to locate cells. Finally, 70-nm sections were made and examined
using a transmission electron microscope (JEM-1220; JEOL,
Ltd.).
Western blot analysis
Total protein was extracted from IVD cells washed
with ice-cold PBS and lysed with RIPA lysis buffer (Sigma-Aldrich;
Merck KGaA). Cell lysates were extracted, and the protein
concentration was determined using a Bicinchoninic Acid Protein
Assay Reagent kit (Sigma-Aldrich; Merck KGaA) according to the
manufacturer's protocols. Protein samples (20 µg) were separated by
10% SDS-PAGE and then transferred to polyvinylidene difluoride
membranes. The membranes were blocked in 5% fat-free milk for 1 h
at room temperature, and then incubated overnight at 4°C with LC3
(1:500; cat. no. ab51520; Abcam), cleaved caspase-3 (1:1,000; cat.
no. ab32042; Abcam), cleaved caspase-9 (1:1,000; cat. no. ab2324;
Abcam), Beclin-1 (1:2,000; cat. no. MAB5295; R&D Systems,
Inc.), S6K (1:6,000; cat. no. ab9366; Abcam), phosphorylated
(p)-S6K (1:1,000; cat. no. ab9973; Abcam), Akt (1:5,000; cat. no.
ab179463; Abcam), p-Akt (1:2,000; cat. no. AF887; R&D Systems,
Inc.) and β-actin antibodies (1:10,000; cat. no. A1978;
Sigma-Aldrich; Merck KGaA). The membranes were then washed five
times with TBS-0.05% Tween-20 buffer, followed by incubation for 30
min at 37°C with horseradish peroxidase-conjugated anti-mouse
immunoglobulin G (IgG; 1:5,000; cat. no. HAF007; R&D Systems,
Inc.) and anti-rabbit IgG (1:5,000; cat. no. HAF008; R&D
Systems, Inc.). β-actin served as a loading control. Target
proteins were imaged using a FluorChem E Chemiluminescent Western
Blot Imaging system (ProteinSimple) and semi-quantified by
densitometric analysis using ImageJ software (version 1.48;
National Institutes of Health).
Statistical analysis
The experimental results from between three and five
independent experiments were quantified and presented as the mean ±
standard error of the mean. For analysis of time- and
dose-dependent effects of bupivacaine and signaling pathway
proteins, one-way ANOVA was conducted followed by post hoc
Bonferroni correction. For the inhibition of autophagic activity
experiments, control vs. 3-MA groups at each bupivacaine dose were
compared using independent-samples t-tests. The distribution of
normality was determined for all variables using a
Kolmogorov-Smirnov test. Statistical analysis was performed using
STATA version 12.0 software (StataCorp LP). P<0.05 was
considered to indicate a statistically significant difference.
Results
Evaluation of time- and dose-dependent
effects of bupivacaine on IVD cellular viability and apoptosis
To test the time- and dose-dependent effects of
bupivacaine on the viability of IVD cells, cells were exposed to a
control (0.9% saline) group, and 8 and 17.5 mM bupivacaine
treatment groups, for various periods of time.
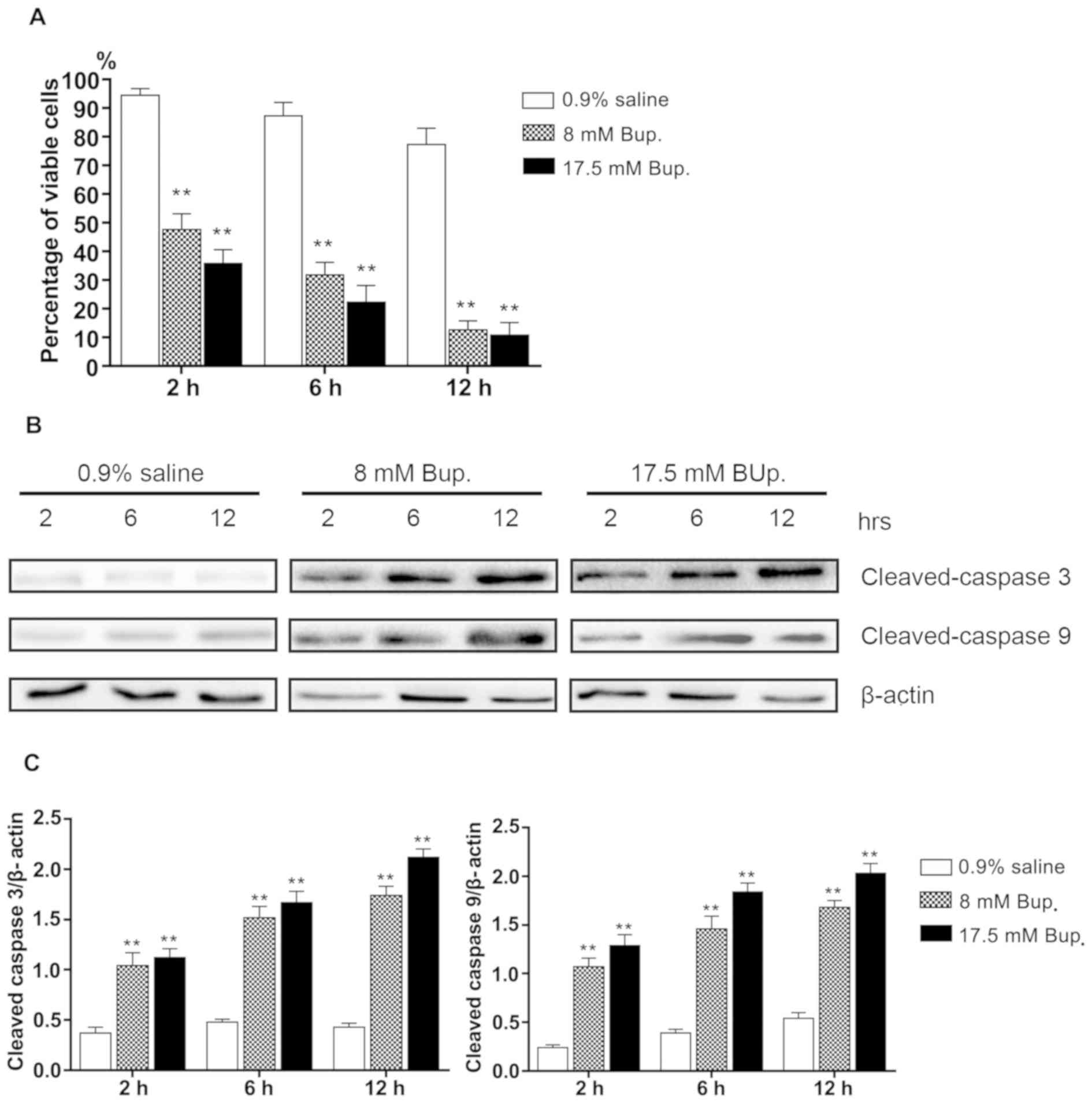
Following treatment of IVD cells with 8 and 17.5 mM
bupivacaine for 2 h, cellular viability was significantly reduced
compared with the control group (P<0.01; Fig. 1A). Additionally, the difference in
cellular viability between the control and bupivacaine treatment
groups markedly increased over time. Following treatment with
bupivacaine for 12 h, only 12.6±3.2 and 10.9±4.3% of the IVD cells
in the 8 and 17.5 mM-treated groups were viable, respectively,
whereas there were 77.4±5.6% viable IVD cells in the control group
after 12 h.
Subsequently, IVD cellular apoptosis was
investigated via western blot analysis. During the 12-h treatment
period, cells exposed to bupivacaine doses of 8 or 17.5 mM
exhibited upregulated expression of apoptotic proteins across the
12-h period, as indicated by a significant increase in cleaved
caspase-3 and cleaved caspase-9 expression compared with the
control (P<0.01; Fig. 1B and
C). The results indicated that bupivacaine induced apoptosis in
IVD cells compared with control-treated cells.
Induction of autophagy in
bupivacaine-treated IVD cells via inhibition of the Akt/mTOR/S6K
signaling axis
Autophagy is important to cell survival and death
under pathological conditions (12). To determine the effect of
bupivacaine on autophagy in IVD cells, the autophagic activity of
IVD cells was evaluated following exposure to 0.9% saline or
bupivacaine for 6 h, at which point bupivacaine notably induced
cell apoptosis.
Following treatment with reagents, autophagy was
activated in the 8 and 17.5 mM treatment groups, as determined by
an increase in Beclin-1 expression and the ratio of LC3-II to
LC3-I, which are considered biomarkers of autophagy (Fig. 2A). The stimulatory effect of
bupivacaine on autophagy was verified under an electron microscope.
As presented in Fig. 2B,
autophagosomes clearly accumulated in bupivacaine-treated cells,
but not in the control group.
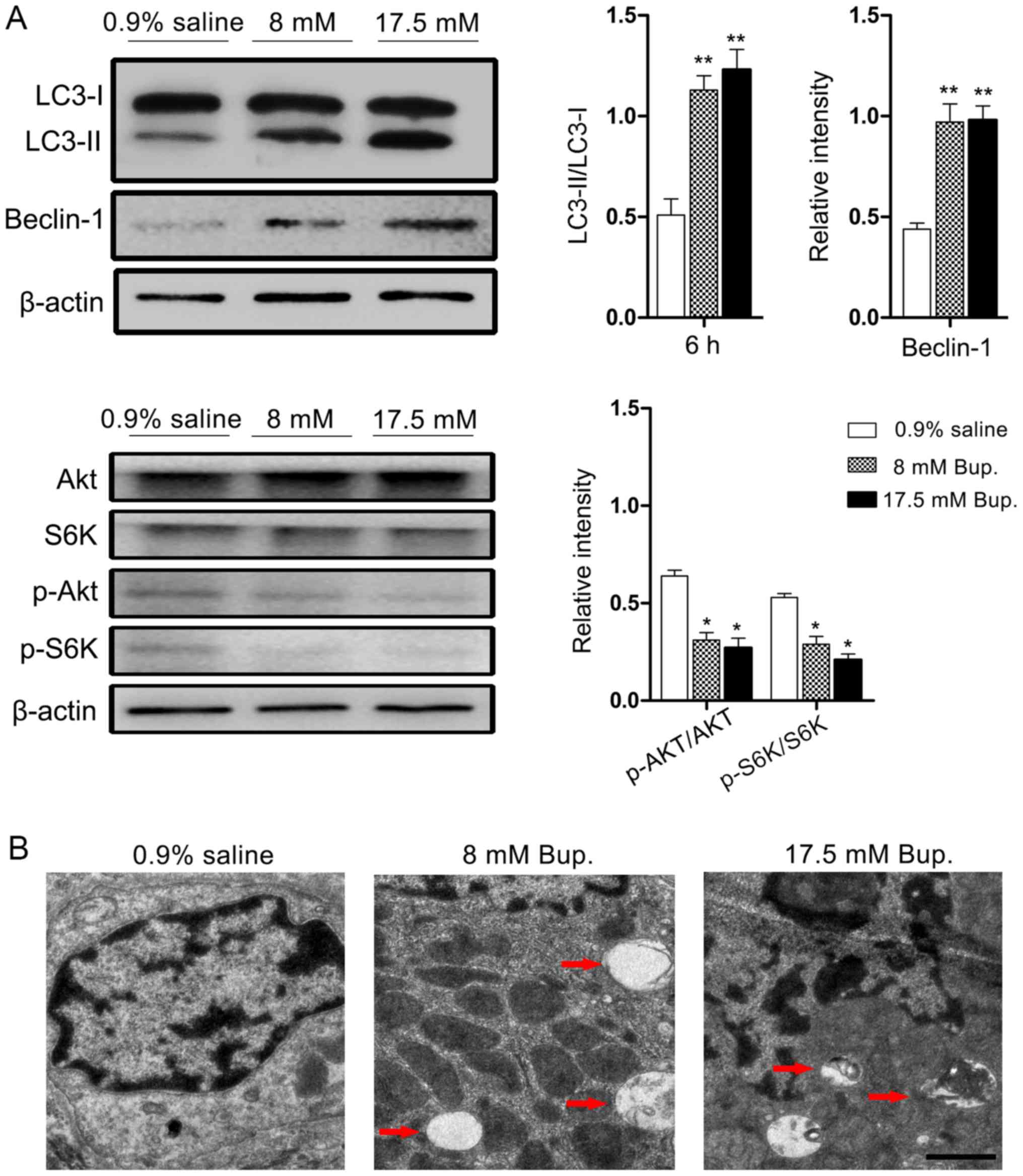 | Figure 2.Bupivacaine induces autophagic
responses in IVD cells via inhibition of the Akt/mTOR/S6K signaling
pathway. (A) Cells were treated with 0.9% saline and bupivacaine
for 6 h at the indicated concentrations. Western blot analysis
revealed that bupivacaine significantly increased LC3-II and
Beclin-1 levels, and decreased Akt and S6K phosphorylation in IVD
cells compared with in the control group. Beclin-1, p-Akt/Akt and
p-S6K/S6K were normalized to β-actin expression. *P<0.05,
**P<0.01 vs. 0.9% saline, n=3-4/group. (B) Ultrastructural
observations of autophagic vesicles deposited in IVD cells. Cells
treated with 0.9% saline displayed normal cell morphology. Cells
treated with bupivacaine for 6 h at the indicated concentrations
presented double-membrane autophagosomes indicated by red
arrowheads. Scale bar, 1 µm. Akt, protein kinase B; Bup.,
bupivacaine; IVD, intervertebral disc; mTOR, mammalian target of
rapamycin; S6K, S6 kinase. |
To assess the potential involvement of signaling
pathways in bupivacaine-induced autophagy in IVD cells, the
activation of Akt/mTOR/S6K signaling was investigated, as this
signaling pathway serves an important role in the negative
regulation of autophagy initiation. The results indicated that
bupivacaine treatment induced a significant reduction in the
p-Akt/Akt ratio by 51.6 and 57.8% at the doses of 8 and 17.5 mM,
respectively, in addition to a significant reduction in the
phosphorylation of S6K compared with in the control group
(P<0.01; Fig. 2A).
Suppression of bupivacaine-induced
autophagic activity increases IVD cellular viability
To determine whether inhibition of autophagic
activity affected viability and apoptotic processes in
bupivacaine-treated IVD cells, IVD cells were co-treated with the
autophagy inhibitor 3-MA and bupivacaine, and IVD cellular
apoptosis and viability were determined via western blotting and
MTT assay, respectively.
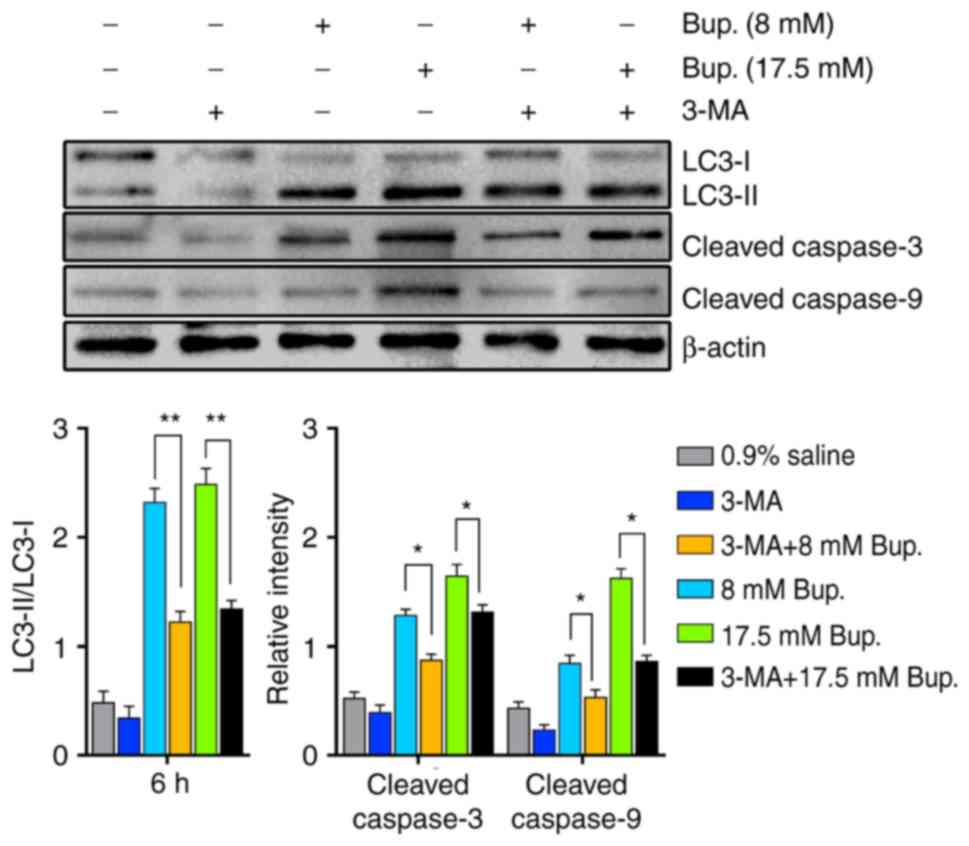
As shown in Fig. 3,
the expression levels of apoptotic biomarkers in IVD cells 6 h
after co-treatment with 3-MA and bupivacaine were significantly
reduced compared with in the bupivacaine treatment groups
(P<0.05). In addition, treatment with 3-MA alone resulted in a
decrease in the expression levels of autophagic and apoptotic
factors in IVD cells; however, there were no statistical
significance compared with in the control group (P>0.05;
Fig. 3). Additionally, cellular
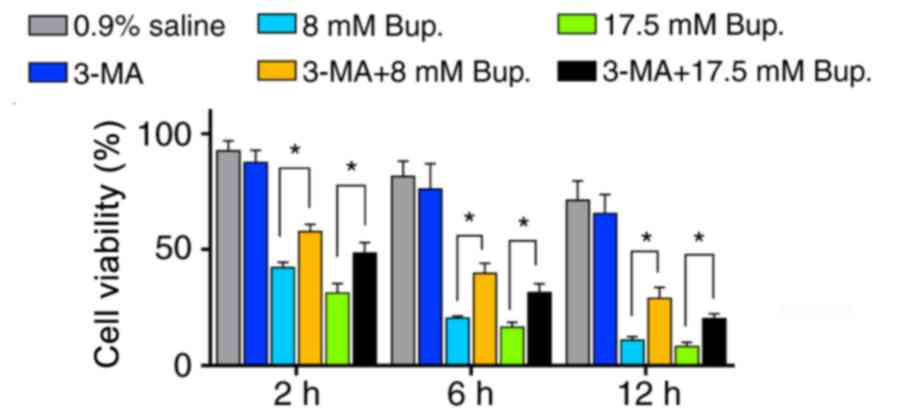
viability was evaluated over time using an MTT assay. Following
co-treatment with 3-MA and bupivacaine, cellular viability was
significantly increased compared with in the bupivacaine treatment
groups at 2, 6 and 12 h (Fig. 4).
This finding suggested that inhibition of autophagic activity
decreased apoptotic cell death and improved viability in
bupivacaine-treated IVD cells.
Discussion
Bupivacaine is widely used to manage patients with
discogenic back pain; however, the toxic effects of bupivacaine on
IVD cells have been documented in a number of studies (10,19).
To the best of our knowledge, this is the first study to report the
effects of bupivacaine at clinically relevant concentrations on
autophagy and apoptosis in human IVD cells in vitro. The
findings revealed that bupivacaine may activate autophagy in IVD
cells by inhibiting the Akt/mTOR/S6K pathway. Additionally, 3-MA
treatment inhibited autophagic activity and effectively protected
IVD cells from bupivacaine-induced apoptosis. Collectively, these
results suggested that bupivacaine exerted cytotoxic effects on IVD
cells via activation of cellular autophagy.
Discogenic back pain is a common and burdensome
problem in developed countries, the treatment options for which
remain limited (20). With the
advantages of minimal trauma and long duration, local anesthesia is
accepted as an important method to alleviate the symptoms of
patients with discogenic back pain (3). Previously, it was reported that
bupivacaine at clinically relevant doses induces toxic effects on
human NP cells (9,19). The present results revealed that
bupivacaine exhibited cytotoxicity towards cultured human IVD cells
in a time- and dose-dependent manner. In addition, the expression
levels of apoptotic proteins, including cleaved caspase-3 and
cleaved caspase-9, in human IVD cells treated with bupivacaine were
increased compared with in the control group, suggesting that the
cytotoxic effects of bupivacaine may be mediated by apoptosis in
human IVD cells.
Apoptosis and autophagy frequently occur together in
response to cellular stress, and autophagic activity acts as both a
protector and promoter of cell death (21); however, the role served by
autophagy in the cytotoxicity of local anesthetic drugs and the
specific underlying mechanisms are yet to be determined. Since
LC3-II and Beclin-1 are important in the formation of
autophagosomes, the expression levels of Beclin-1 and the LC3-II/I
ratio are widely used as biomarkers to evaluate the activation of
autophagy (22–24). In the present study, the results
revealed that the levels of LC3-II/I and Beclin-1 in human IVD
cells treated with 8 and 17.5 mM bupivacaine were significantly
upregulated compared with in the control group. Electron microscopy
revealed that the formation of autophagosomes was significantly
increased in the bupivacaine groups. These results suggested that
autophagy was activated and closely associated with the
cytotoxicity of bupivacaine, which promoted autophagic marker
expression and autophagosome formation in human IVD cells.
It has been reported that activation of the
Akt/mTOR/S6K pathway serves as a negative regulator of autophagy
(25,26), whereas bupivacaine upregulates
autophagy by inhibiting Akt/mTOR/S6K signaling in cardiac and nerve
cells (27,28). In the present study, it was
observed that phosphorylation of Akt and S6K in human IVD cells
treated with bupivacaine was significantly decreased compared with
in the control group. These findings suggested that the
Akt/mTOR/S6K signaling pathway may be associated with
bupivacaine-induced autophagy. Furthermore, 3-MA, a selective
autophagy inhibitor, was applied to human NP cells in combination
with bupivacaine, revealing that the expression of autophagic
proteins decreased following the addition of 3-MA. Consistent with
previous studies, when the autophagy inhibitor 3-MA was applied,
the levels of the apoptosis effectors cleaved caspases-3 and −9
were significantly decreased (12,29).
Subsequently, the activity of human IVD cells co-treated with 3-MA
and bupivacaine was evaluated, and changes in the levels of the
apoptosis-associated proteins cleaved caspase-3 and cleaved
caspase-9 were determined. It was demonstrated that the expression
levels of cleaved caspase-3 and cleaved caspase-9 were
significantly decreased, whereas the viability of IVD cells was
significantly increased following inhibition of autophagy by 3-MA
in the 8 and 17.5 mM bupivacaine-treated groups. These findings
suggested that autophagy serves an important role in mediating
bupivacaine-induced apoptosis in IVD cells.
The present study should be interpreted in the
context of certain limitations. For example, increased autophagy is
not only associated with the induction of autophagosome formation,
but also the inhibition of autophagosome degradation (30). In this study, only the activation
of important pathways in autophagy formation were explored, with
the mechanisms of autophagy clearance not investigated.
Additionally, bupivacaine can induce autophagy and inhibition of
the Akt/mTOR/S6K pathway; however, only 3-MA was used to determine
the effects of bupivacaine on the apoptosis of NP cells. In the
future, the specific protein expression of mTOR pathway components
should be regulated using gene knockout techniques, and alterations
in the levels of autophagy should be further demonstrated in disc
tissues, to provide further insight into cellular responses to the
effects of bupivacaine on the autophagy and apoptosis of IVD
cells.
In conclusion, the present study reported that the
levels of autophagic activity in human NP cells were significantly
increased following bupivacaine treatment, potentially via
inhibition of the Akt/mTOR/S6K signaling pathway. Additionally, the
expression of apoptotic effectors was decreased following
co-treatment with the autophagy inhibitor 3-MA. To the best of our
knowledge, this study is the first to report that autophagy induced
by bupivacaine is an upstream mechanism underlying apoptosis;
however, the specific pathways via which bupivacaine-induced
autophagy affects apoptosis remain unclear and require further
investigation. Collectively, the findings from this study may
provide novel insight to improve understanding of the specific
mechanisms underlying the cytotoxicity of bupivacaine.
Acknowledgements
Not applicable.
Funding
This study was supported by China Postdoctoral
Science Foundation (grant no. 2018M642459 and no. 2019M652773).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GY, ZYL and QT designed the experiments. GY, ZYL,
HBM, WHY, SXH and KL performed the experiments and analyzed the
data. GY, ZYL and QT drafted and revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First Affiliated Hospital, College of Medicine,
Zhejiang University. Written informed consent was obtained from all
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Deyo RA and Weinstein JN: Low back pain. N
Engl J Med. 344:363–370. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Schepper EI, Damen J, van Meurs JB,
Ginai AZ, Popham M, Hofman A, Koes BW and Bierma-Zeinstra SM: The
association between lumbar disc degeneration and low back pain: The
influence of age, gender, and individual radiographic features.
Spine (Phila Pa 1976). 35:531–536. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Friedly J, Chan L and Deyo R: Increases in
lumbosacral injections in the Medicare population: 1994 to 2001.
Spine (Phila Pa 1976). 32:1754–1760. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kotilainen E, Muittari P and Kirvelä O:
Intradiscal glycerol or bupivacaine in the treatment of low back
pain. Acta Neurochir (Wien). 139:541–545. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ohtori S, Inoue G, Orita S, Eguchi Y,
Ochiai N, Kishida S, Kuniyoshi K, Nakamura J, Aoki Y, Ishikawa T,
et al: No acceleration of intervertebral disc degeneration after a
single injection of bupivacaine in young age group with follow-up
of 5 years. Asian Spine J. 7:212–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang YH, Tsai PS and Huang CJ:
Bupivacaine inhibits COX-2 expression, PGE2, and cytokine
production in endotoxin-activated macrophages. Acta Anaesthesiol
Scand. 52:530–535. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yanagidate F and Strichartz GR:
Bupivacaine inhibits activation of neuronal spinal extracellular
receptor-activated kinase through selective effects on ionotropic
receptors. Anesthesiology. 104:805–814. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iwasaki K, Sudo H, Yamada K, Ito M and
Iwasaki N: Cytotoxic effects of the radiocontrast agent iotrolan
and anesthetic agents bupivacaine and lidocaine in
three-dimensional cultures of human intervertebral disc nucleus
pulposus cells: Identification of the apoptotic pathways. PLoS One.
9:e924422014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Quero L, Klawitter M, Nerlich AG, Leonardi
M, Boos N and Wuertz K: Bupivacaine-the deadly friend of
intervertebral disc cells? Spine J. 11:46–53. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai XY, Xiong LM, Yang SH, Shao ZW, Xie M,
Gao F and Ding F: Comparison of toxicity effects of ropivacaine,
bupivacaine, and lidocaine on rabbit intervertebral disc cells in
vitro. Spine J. 14:483–490. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hale AN, Ledbetter DJ, Gawriluk TR and
Rucker EB III: Autophagy: Regulation and role in development.
Autophagy. 9:951–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma KG, Shao ZW, Yang SH, Wang J, Wang BC,
Xiong LM, Wu Q and Chen SF: Autophagy is activated in
compression-induced cell degeneration and is mediated by reactive
oxygen species in nucleus pulposus cells exposed to compression.
Osteoarthritis Cartilage. 21:2030–2038. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li R, Ma H, Zhang X, Li C, Xiong J, Lu T,
Mao Y, Dai J, Liu L and Ding Z: Impaired autophagosome clearance
contributes to local anesthetic bupivacaine-induced myotoxicity in
mouse myoblasts. Anesthesiology. 122:595–605. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alers S, Löffler AS, Wesselborg S and
Stork B: Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy:
Cross talk, shortcuts, and feedbacks. Mol Cell Biol. 32:2–11. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang GE, Duan X, Lin D, Li T, Luo D, Wang
L and Lian K: Rapamycin-induced autophagy activity promotes bone
fracture healing in rats. Exp Ther Med. 10:1327–1333. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Beigh MA, Showkat M, Bashir B, Bashir A,
Hussain Mu and Andrabi KI: Growth inhibition by bupivacaine is
associated with inactivation of ribosomal protein S6 kinase 1.
Biomed Res Int. 2014:8318452014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pfirrmann CW, Metzdorf A, Zanetti M,
Hodler J and Boos N: Magnetic resonance classification of lumbar
intervertebral disc degeneration. Spine (Phila Pa 1976).
26:1873–1878. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hou H, Zhang Y, Huang Y, Yi Q, Lv L, Zhang
T, Chen D, Hao Q and Shi Q: Inhibitors of phosphatidylinositol
3′-kinases promote mitotic cell death in HeLa cells. PLoS One.
7:e356652012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee H, Sowa G, Vo N, Vadala G, O'Connell
S, Studer R and Kang J: Effect of bupivacaine on intervertebral
disc cell viability. Spine J. 10:159–166. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roelofs PD, Deyo RA, Koes BW, Scholten RJ
and van Tulder MW: Nonsteroidal anti-inflammatory drugs for low
back pain: An updated Cochrane review. Spine (Phila Pa 1976).
33:1766–1774. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gump JM and Thorburn A: Autophagy and
apoptosis: What is the connection? Trends Cell Biol. 21:387–392.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jain MV, Paczulla AM, Klonisch T, Dimgba
FN, Rao SB, Roberg K, Schweizer F, Lengerke C, Davoodpour P,
Palicharla VR, et al: Interconnections between apoptotic,
autophagic and necrotic pathways: Implications for cancer therapy
development. J Cell Mol Med. 17:12–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chaabane W, User SD, El-Gazzah M, Jaksik
R, Sajjadi E, Rzeszowska-Wolny J and Los MJ: Autophagy, apoptosis,
mitoptosis and necrosis: Interdependence between those pathways and
effects on cancer. Arch Immunol Ther Exp (Warsz). 61:43–58. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsunaga K, Saitoh T, Tabata K, Omori H,
Satoh T, Kurotori N, Maejima I, Shirahama-Noda K, Ichimura T, Isobe
T, et al: Two Beclin 1-binding proteins, Atg14L and Rubicon,
reciprocally regulate autophagy at different stages. Nat Cell Biol.
11:385–396. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Afanas'ev I: Signaling and damaging
functions of free radicals in aging-free radical theory, hormesis,
and TOR. Aging Dis. 1:75–88. 2010.PubMed/NCBI
|
|
26
|
Farrand L, Oh SW, Song YS and Tsang BK:
Phytochemicals: A multitargeted approach to gynecologic cancer
therapy. Biomed Res Int. 2014:8901412014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lv D, Bai Z, Yang L, Li X and Chen X:
Lipid emulsionreverses bupivacaine-induced apoptosis of h9c2
cardiomyocytes: PI3K/Akt/GSK-3β signaling pathway. Environ Toxicol
Pharmacol. 42:85–91. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fan YL, Li HC, Zhao W, Peng HH, Huang F,
Jiang WH and Xu SY: Curcumin attenuated bupivacaine-induced
neurotoxicity in SH-SY5Y cells via activation of the Akt signaling
pathway. Neurochem Res. 41:2425–2432. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang L, Jin Y, Wang H, Jiang Y and Dong
J: Glucosamine protects nucleus pulposus cells and induces
autophagy via the mTOR-dependent pathway. J Orthop Res.
32:1532–1542. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hsin IL, Sheu GT, Jan MS, Sun HL, Wu TC,
Chiu LY, Lue KH and Ko JL: Inhibition of lysosome degradation on
autophagosome formation and responses to GMI, an immunomodulatory
protein from Ganoderma microsporum. Br J Pharmacol. 167:1287–1300.
2012. View Article : Google Scholar : PubMed/NCBI
|